Abstract
Purpose
Green approach to the nanoparticles, including metal oxides due to inevitable disadvantages of physical or chemical synthesis routes is attractive nowadays. Zink oxide (ZnO) nanoparticles play a key role in the medical and pharmaceutical fields. This research aimed to study the biologically synthesized ZnO nanoparticle using Bacillus subtilis, and evaluated its antibacterial properties.
Methods
Bacillus subtilis culture in a broth nutrient environment was used, followed by adding the Zinc acetate dehydrate. Biosynthesis of the nanoparticles was confirmed by the XRD, FTIR, and SEM imaging. The antibacterial effects of NPs on the expression of AdeB efflux pump genes and the AdeRS regulator were studied; clinical species of the Acinetobacter baumannii were collected from clinical samples of Khorramabad, using the phenotypic (MIC) and the genotypic methods through real-time PCR.
Results
X-ray diffraction pattern (XRD) result showed, that all of the peaks were related to the ZnO, and no other peaks were detected; it also demonstrated nanostructure nature with crystallite size of 25–50 nm. The results indicated, that the antibacterial properties of the nanoparticle increased the AdeRS expression and decreased the AdeB expression in 40% of the A. Baumannii. In addition, there was an increase in the AdeB expression in 60% of the species, indicating an increased probability for mutation.
Conclusion
Given the desirable inhibitory effects of biosynthesized ZnO NPs on the expression of AdeB and AdeRS, which play an important role in the pharmaceutical resistance of Acinetobacter species, it seems that ZnO NPs can be used as a medication candidate in pharmaceutical industry in the future.
Keywords: Znic oxide nanoparticle, Biological synthesis, Gene regulation, Multiple drug resistance, Real-time PCR
Introduction
The growing increase in the microbial resistance against present antibiotics, and decrease in the efficiency of antibiotics have led to the serious concerns about public health [1]. This issue is caused by genetic changes in the bacteria due to the excess use of antibiotics. Nowadays, one of the promising approaches to overcome microbial resistance is metal nanoparticles usage, whose application as antibacterial compounds is growing [2]. In addition to the inhibiting effect and due to the small size, the ratio of surface to volume is high in metal NPs, which leads to more outside contact and great antibacterial effects [3–7]. Recently, using the green synthesis of metal NPs has increased as a simple, low-cost, and environment-friendly method, compared to the physical and chemical methods. Another application of the zinc oxide (ZnO) NPs is to remove the environmental pollutants, as a newest methods in the world [8–10]. Moreover, bacteria are efficient organisms, since they are able to produce high amounts of enzymes, amino acids, polysaccharides, and vitamins, which act as reducing factors of metal ions [11, 12]. In addition, bacteria cells are able to chemically detoxify. Therefore, they can grow in high concentrations of toxic metals [13]. The dissemination and detoxification are performed using membrane proteins, which act as inverse carriers of cations and protons, or ATPase. Therefore, microbial systems can convert mineral ions of toxic solutions into the non-toxic and insoluble ions [14]. Synthesis of ZnO nanostructures using microorganisms is now highly considered; diverse microorganisms can be used to synthesize them [15]. Prrasad et al. biosynthesized ZnO NPs using Lactobacillus sporogens [16]. The main benefit of ZnO NPs is their ability to vigorously prevent the activity of pathogen microbes, when used in low concentrations [17]. The mechanism of ZnO action is through degradation of the bacteria’s cellular walls [18]. The difference between the negative charge of the microorganism and the positive charge of the nanoparticle acts as an absorbent electromagnet between the microbe and the nanoparticle, while the released ions from the NPs are likely to react with the thiol groups of SH of the surface proteins of the bacteria cells. Several proteins of the bacteria cells’ membrane are responsible for transferring minerals from the wall surface; however, NPs result in the membrane inactivation and impermeability [19, 20]. The inhibition of considerable growth ZnO against a wide spectrum of bacteria has been reported in previous studies. Lakshmi et al. studied the antibacterial activity of ZnO against the clinical isolates of Bacillus subtilis, Eschereshia coli, Klebsiella pneumonia, Pseudomonas aeruginosa, Salmonella typhi, and Staphylococcus aureus [21]. Nezamabadi.V et al. biosynthesized the ZnO nanoparticles by Artemisia aucheri extract and investigated its antibacterial effects on bacterial strains [22]. In addition, Gunalan et al. have shown that, green-synthesized ZnO has a stronger inhibitory effect than chemically-synthesized nanoparticles [23]. Tiwari. V et al. investigated the phenotypic effect of ZnO nanoparticles on carbapenem-resistant Acinetobacter [24]. Acinetobacter pathogen species is one of the most important bacteria, that are now widely resistant to the wide spectrum of antibiotics; it is known as a serious and global concern in the nosocomial infections incidence and mortality, especially in the ICUs. This has led to using a wide spectrum of antibiotics, especially carbapenems and third-generation cephalosporins [25]. This phenomenon can lead to the emergence of species with multiple drug resistance (MDR), and limitation of the therapeutic means around the world [26]. AdeABC is one of the most important efflux systems, belonging to the RND family in Acinetobacter, which plays an important role in the resistance to a broad group of antibiotics; its genes are chromosomal and encode three genes, i.e., AdeB, AdeA, and AdeC, forming an operon in the vicinity. In addition, the expression of AdeABC is done by a two-component system, which includes a response regulator (AdeR) and a sensor kinase (AdeS) [27]. In a study by Modarresi F. et al., iron oxide nanoparticles decreased the expression of efflux genes of AdeABC pump [28]. In addition, in a similar study, the inhibitory effect of silver nanoparticles on the expression of efflux genes of AdeABC pump was observed in Acinetobacter [29]. Considering that, this study is the first report of bacterial effects of the nanoparticles on the expression of pump efflux genes (AdeB, AdeRS) of clinical strains of Acinetobacter. We synthesized ZnO nanoparticles using green methods. After identifying and confirming the nanoparticle, we examined its antibacterial properties phenotypically and finally genotypically by examining the AdeABC pump efflux genes expression.
Materials and methods
Collecting the samples and antibiotic sensitivity assessment
Ten samples of Acinetobacter were collected from the clinical centers of Khorramabad city during 2020. The bacteria were collected from various clinical samples, such as wounds, blood, urine, bronchial lavage, etc. After transferring to the laboratory, were cultured on the Blood agar and Eosin Methylene Blue (EMB) culture mediums. Then all the bacteria samples were identified using diagnostic and biochemical tests. A. baumanii strains presented a large metabolic activity. They had the capacity to produce acid from glucose, xylose, galactose, manose, rhamnose and lactose. Acid production from maltose and the urea test were variable reactions. All strains were positive to Simmons citrate. The negative reactions were as follows: the acid production from mannitol and sucrose, esculin hydrolisis, Hydrogen sulfide gas (H2S) on Triple Sugar Iron Agar (TSI), nitrate reduction, methyl red and Voges-Proskauer [30, 31]. Then, they were assessed in terms of their resistance to antibiotics using the antibiotic sensitivity test, i.e. the Kirby-Bauer antibiotic sensitivity test. Antibiotics were purchased from various families based on the clinical characteristics and the table provided by the Laboratory Standards Institute (CLSI) from Padtan Teb Co. [32]. The disks included Amikacin 30 μg, Gentamycin 10 μg, cefazolin 30 μg, Ceftriaxone 30 μg, cefepime 30 μg, imipenem 10 μg, meropenem 5 μg, minocycline 10 μg, ciprofloxacin 5 μg, cotrimoxazole 25 μg, and Clarithromycin 15 μg.
Synthesis of ZnO nanoparticle
In order to biosynthesize the nanoparticles, the bacteria Bacillus subtilis, with the access code of IBRC-M10742 was used; it was purchased from the Iranian Genetic Supply. Lyophilized bacteria were inoculated to the primary growth in the nutrient-broth culture medium. After about an hour of incubation, the suspension was seeded in the blood agar culture medium. The bacteria colonies were inoculated in the nutrient-broth culture medium for 24 h; the medium was diluted four times by adding 75 ml of sterile broth. The diluted medium was placed at the temperature of 37 °C for 24 h under growth. After incubation, pH was regulated at 7 by 0.1 N of Sodium hydroxide(NaOH). Moreover, 50 nm of Zinc acetate dehydrate was infused into the bacterial suspension at the speed of 10 ml/min through a burette. The compound was stirred for 30 min, and then, 200 ml of 50 mm (Sodium borohydride); NaBH4 was gently added to the mixture as a reducing factor at the speed of 10 ml/min through a burette [33]. Then the final solution was stirred for 24 h at the room temperature. After the thorough reaction of the mixture, it was centrifuged and the emergence of white precipitates indicated the initiation of the nanoparticles reduction. Subsequently, the precipitate was collected. The obtained precipitate was washed three times with deionized water. The powdered form of the nanoparticles was produced by drying the plate at 50 °C in a hot oven for 6 h. Finally, the powder containing the nanoparticles was calcined at the temperature of 600 °C for 2 h, in order to preserve the bacterial residue and the organic compounds. The salt-free medium was considered as positive control, while the salty solution without the medium was considered as the negative control [33].
Analysis and assessment of the biosynthesized nanoparticles
After the biosynthesis of the nanoparticles, in order to confirm the synthesis of the nanoparticles infra-red spectroscopy was performed by Fourier-transform infrared spectroscopy (FTIR) (Bruker system, Model tensor 27, Germany); it was applied to identify the bonds and the involved factorial groups in the spectrum of 400-4000 cm−1 with a mean of 16 at the resolution of 4 cm−1. The X-ray Diffraction Spectrophotometer (XRD) (Bruker, Advance D8 Model, manufactured by Germany) with a copper anode material with a wavelength of about 1.5405 A° in 35 kw and 35 mA in the Bragg angle range of 80 < 2 θ < 10 was used; it determined the phases and detected the crystalline structure. Scanning electron microscope (SEM) analysis was performed to detect the presence of nanoparticles, and assess their size and morphology. Images and the sizes of the synthesized nanoparticles obtained from the bacteria were recorded.
Assessment of the antibacterial properties of the nanoparticles using the phenotypic method of broth microdilution
Minimum inhibitory concentration (MIC) was measured using broth microdilution in a sterile 96-well plate. Firstly, a stock was prepared with a value of 0.003 μg/ml from synthesized nanoparticles with a sterile medium of Mueller Hinton Broth containing 10% DMSO. Then, 50 μl of medium was added to the third to the twelfth rows of the sterile medium. Afterward, 100 μl of the prepared stock was added to the first and the second rows, and dilution was done from the second to the tenth rows. Finally, 50 μl, equal to Mcfarland semi-turbidity of 1.5*108 CFU/ml, of the 24-h culture of Acinetobacter baumannii was added to the second to the tenth rows. Plates were placed for 24–48 h in a shaker-incubator at a temperature of 37 °C and the humidity of 50%. Then, 2,3,5-triphenyltetrazolium chloride was used in order to determine the visual index for the growth of the microorganisms. Colorless wells were reported as MIC. The 1%-DMSO was considered as negative control, and the vancomycin and gentamicin solvent was used as positive control. Each of the above mentioned tests was repeated three times [34].
Genotypic assessment of the effects of the nanoparticle on the genes expression of Acinetobacter baumannii
Real-time PCR was used to assess the expression of the AdeB efflux pump gene and the AdeR and AdeS regulating genes. RNA extraction was performed using RNA extraction kit (Korea GeneAll Co.); it was done for both bacterial samples of Acinetobacter before and after the nanoparticles impressment under the identical conditions. Then, the quality of the extracted RNA was assessed in 1% agarose gel. In addition, the concentration of the extracted RNA was assessed using a nanodrop system. The construction of cDNA from the extracted RNAs of each sample was performed using cDNA synthesis kit, consisting of Script Reverse Transcriptase (Anacell, Tehran). Moreover, real-time PCR was performed using a master mix containing cyber green (SMOBIO Technology, Taiwan). Real time-PCR reaction resulted in the final volume of 25 μl, including 1 μL cDNA, 0.4 μL of forward and 0.6 μL of reverse primers for AdeB gene,.1 μL of forward and 1 μL of reverse primers for AdeRS genes, and 12 μl of master mix; the remaining volume was sterile distilled water. In addition, the gyrA gene was used as a housekeeping gene in the real-time PCR reaction. The heating cycle included primary denaturation at 95 °C for 10 min, followed by 40 cycles at 95 °C for 20 s at the annealing temperature, an extension at 72 °C for 20 s; the final was hold at 72 °C for one minute, and the final stage was drawing the melting curve at 55–95 °C for 15 s. Real-time PCR was performed using the CORBETT system (5 Plex HRM, manufactured by Australia) at Lorestan University of Medical Sciences. Specialized primers for the genes are presented in Table 1.
Table 1.
Sequence of primers used in RT-PCR
Results
Assessment of the synthesis of the NPs
Assessment of NPs using FT-IR
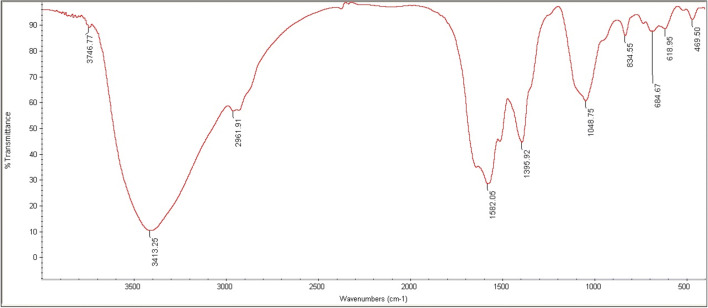
The results of the assessment of chemical structure and the type of chemical bonds of ZnO nanoparticles are presented in Fig. 1. ZnO nanoparticles synthesized at 3413.25 cm−1 have peaks for the bond of NH2, in adenine, cytosine, and guanine and H bonds of OH groups. In addition, they had peaks in 2961.91 cm−1 for CH bonds of aliphatic groups of cell walls (carbohydrates, fatty acids). In 1582.05 cm−1 for NH2, it had peaks for the side branches of the C=O C=N bonds (first-type amide bonds). The 1395.92 cm−1 peak was for the type III of amide bonds, and in 1048.75 cm−1; it also had peaks for the asymmetric bonds of C-O-C in the aliphatic esters. Stretching radiations of bonds of Zn-O-Zn in 684.67 cm−1 were thoroughly adapted with the available studies; this bond had a peak at the cited point in the current study. These peaks are combinatory properties of ZnO in the bacterial species (Fig. 1).
Fig. 1.
FT-IR spectrum of biosynthesized ZnO nanoparticles by Bacillus subtilis
Assessment of NPs by X-ray diffraction (XRD)
XRD was used to confirm the NPs to determine the phases, and detect the crystalline structure of the substance. Synthesized powder from ZnO NPs for obtained XRD was assessed at the scan speed of 0.02°s−1 and 2 θ. In addition, ZnO pattern varies from 20° to 80°. The observed peaks related to Bragg angel for ZnO were observed at the levels of 002, 102, and 200; they were adapted to the crystallography data of the reference pattern of the JCPD with the number of 89–7102, and did not show the secondary phase. Sharp peaks represented the appropriate crystal structure of the NPs, which have been shown in Fig. 2.
Fig. 2.
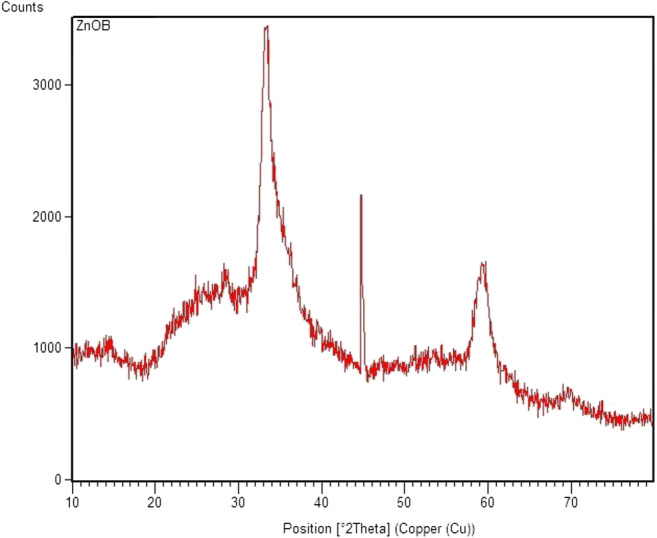
Analysis of XRD of biosynthesized ZnO NPs by Bacillus subtilis
Morphology and size assessment of NPs using SEM
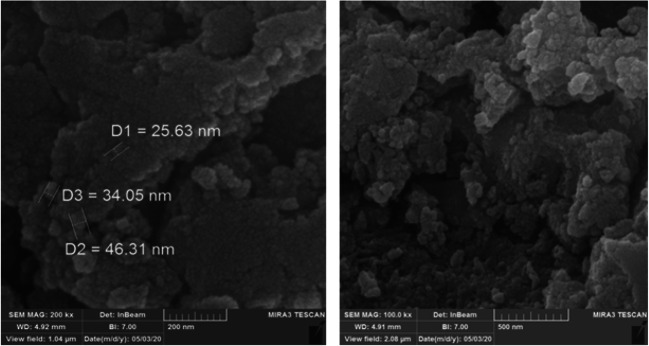
SEM analysis was performed in order to assess the morphology and the size of the NPs. Figure 3 shows the synthesized ZnO NPs using Bacillus subtilis. As observed in these figures, the produced morphology of NPs was as circular crystals with an average diameter of 25–50 nm.
Fig. 3.
SEM Images of biosynthesized ZnO NPs using Bacillus subtilis
Assessment of antibacterial properties of NPs using the MIC method
The results of the antibiogram test showed, that Acinetobacters were resistant to the various classes of antibiotic, and 100 species were MDR. Results obtained from the MIC test for the ZnO NPs were different after three repetitions for each species of Acinetobacter baumannii; all of them have been explained in Table 2.
Table 2.
Assessment of minimum inhibitory concentration of ZnO synthesized NPs by Bacillus subtilis against Acinetobacter Baumannii species
| Acintobacter Baumannii | MIC (μg/ml) |
|---|---|
| 1 | >1000 |
| 2 | >1000 |
| 3 | 31.2 |
| 4 | 125 |
| 5 | 62.5 |
| 6 | 250 |
| 7 | >1000 |
| 8 | >1000 |
| 9 | 500 |
| 10 | >1000 |
Expression of the AdeB gene and Genes regulating AdeS and AdeR
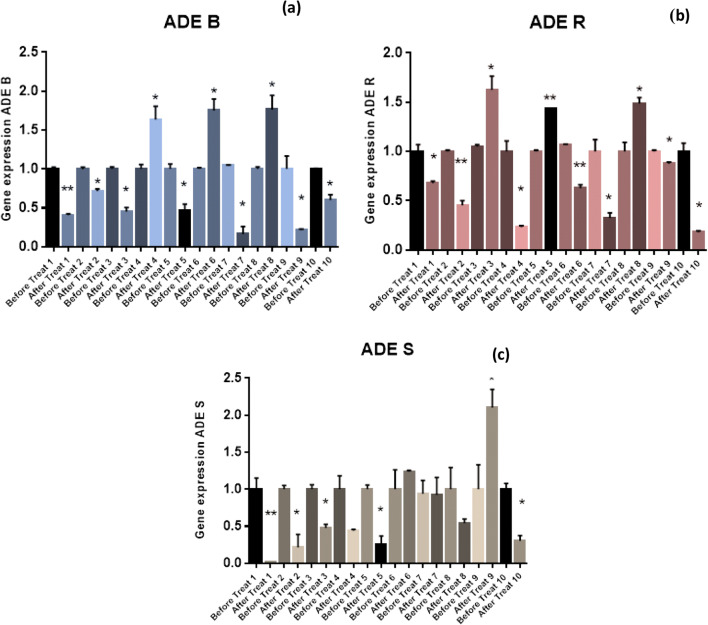
Rational reference formula of expression(2-ΔΔCt) was used to calculate the mount of gene expression. One-way ANOVA and Tukey tests (SPSS 22.0 Software) were applied to compare the significant differences of values obtained for the gene expression. Values lower than p value ≤0.05 were considered significant. The results have been explained in Figs. 4. PCR result confirmed the presence of the gyrA gene in 10 species of the Acinetobacter baumannii, as the housekeeping gene; it was present in all isolates. According to the previous studies, a mutation in AdeR gene will increase AdeR, and finally increase the AdeABC genes excessively; they have represented a direct association between cited genes expression. Moreover, based on the results of the present study, 40% of samples had inverse association regarding gene expression of AdeB and AdeR (in samples 3, 4, 5, 6) (Figs. 4a, b). In addition, 60% of samples had direct association in terms of AdeB and AdeR gene expression. So that, an increase in the AdeR expression also increased the AdeB expression (samples 1, 2, 7, 8, 9, 10), which can be due to the mutation in the AdeR and AdeS genes.
Fig. 4.
(a) Assessment of AdeB gene expression, (b) Assessment of AdeR regulatory gene expression, (c) Assessment of AdeS sensor kinase gene expression.
Discussion
This method successfully provided pure ZnO in nanoscale size distribution, and also in nanostructure type. The ZnO nanoparticle was biosynthesized using Bacillus subtilis. It is related to the Bacillus bacteria ability to grow under difficult and stressful conditions; they can also grow in the presence of toxic metals. Biosynthesized NPs of the present study had a diameter of approximately 25–50 nm, which is mostly considered from a nano-biotechnology perspective. In 2017, Mahdi ZS et al. biosynthesized ZnO NPs by Xanthomonas campestris; its approximate diameter was 300 nm [37]. In another study, the approximate diameter of biosynthesized nanoparticle via Aeromonas hydrophila was 57 nm [38]. Namazabadi.V et al. reported the diameter of ZnO biosynthesized NPs by Artemisia aucheri extract as 15–40 nm. This difference in reported results is probably due to differences in the type of selected species for biosynthesis and the spherical structure of the nanoparticle [22]. Also in a study in 2020, ZnO NPs were biosynthesized by L. lactis and Bacillus. Sp; the nanoparticle diameters were reported to be 55 and 99 nm, respectively [39]. After the biosynthesis of the ZnO nanoparticle, its antibacterial effects were assessed; at the first stage MIC was done. According to Table 2, minimum inhibitory concentration was reported in four cases of species as 31.2, 62.5, 125, and 250 (μg/ml), which represented the desirable inhibitory effect of ZnO nanoparticle on bacteria growth. In remaining species, the inhibitory effect of the nanoparticle was reported as 500 and > 1000 (μg/ml), which indicated resistance of the species to this concentration; it was an indicator of the genetic changes. Since expression of efflux genes of AdeABC pump is controlled by AdeS, AdeR genes, the stimulation of the second sensor of AdeS leads to ATP-dependent autophosphorylation in the hystidine kinase, transferring information using transforming phosphoryl to one root of the aspartate from AdeR, thus transferring information to the AdeR. Phosphorylated regulatory AdeR controls expression by bonding to the AdeABC promoter. Therefore, it seems that there is a reverse association between the increase in the expression of phosphorylated AdeR and the AdeABC genes expression [40]. Regulation through stimulation of the second sensor of AdeS leads to ATP-dependent autophosphorylation, and finally phosphorylated AdeR controls the expression of operon inversely by bonding to the promoter region. However, the signal of autophosphorylation of AdeS is not understood yet, and the ability and the region of the AdeR are not documented for bonding to the operon promoter [40]. The present study is the first report of the antibacterial effect of biosynthesized ZnO NPs on the expression of pump efflux genes of Acinetobacter species. Since many aspects of the effects of these genes on the expression of the AdeABC operon are not thoroughly and properly understood, more precise assessments and further studies are needed in this regard. The AdeRS gene is at the upstream of the AdeABC operon, and it is inversely transcribed [40]. According to the results of this study, after application of ZnO NPs, through the increase in AdeR regulatory gene, the AdeB gene expression decreased in 40% of the samples. Statistical analysis confirmed the gene expression decrement was statistically significant (P value ≤0.05). The results for the decrease in MIC in the species were thoroughly based on the decrease in gene expression after applying NPs on the species; as it was expected, by the decrease in MIC, we faced a decrease in the AdeB gene expression. It has been shown that mutation in the AdeRS increases AdeABC genes excessively [41]. Moreover, a study by Mahi M. et al. reported AdeB genes expression decrement in Acinetobacter baumannii after the effect of silver and chitosan NPs. Based on the previous studies, they represented the inhibitory effects of metal NPs on the expression of cited genes, which was in line with the findings of the current study [42]. There are two possible mechanisms of efflux pumps inhibitory activity of metal NPs. The first possible mechanism is the direct binding of metal NPs to the active site of efflux pumps, which blocks the extrusion of antibiotics outside the cells. Metal NPs may act here as a competitive inhibitor of antibiotics for the binding site of efflux pumps. Another possible mechanism is through the disruption of efflux kinetic [43]. In another study conducted by Behdad R., the inhibitory effect of silver nitrate NP on the AdeABC efflux genes expression decrement was evaluated; the results were consistent with the current study [29]. Banoee et al., suggested the novel efflux pump inhibitory role of ZnO NPs in NorA efflux pumps of Staphylococcus aureus. They reported 27% increment in the inhibition zone for Ciprofloxacin in the presence of ZnO NPs in S. aureus [44]. The results of the current study in 60% of remaining samples after applying ZnO NPs, indicated a direct association between the AdeR gene expression and the subsequent excess expression of the AdeB gene, which may be due to the mutation. MIC results in these species, which were reported at 500 and < 100 μg/ml, were in line with the results of gene expression of these species after applying ZnO NPs. In a study by Hornesy et al., conducted in 2010, one substitution mutation was found in AdeS of tigecycline-resistant Acinetobacter, which increases AdeB excessively [45]; it is somewhat in line with the results of this study. In addition, in Coyne and associates study in 2010, one substitution was observed in the second sensor of AdeS in the MDR Acinetobacter species, leading to the emergence of MDR species [46]. However, the exact interaction has not been identified [45]. In one study by Atasoy. AR in 2016, which conducted on aminoglycoside-resistant Acinetobacters, there was a significant association between the resistance of this species and the AdeRS genes mutation [47]; this finding was confirmed in the present study. These issues can be approximately in line with the increase in the direct expression of AdeRS and AdeB in some samples, which can be a probable reason for the occurrence of mutation in 60% of the samples of this study. It should be noted, that gene expression cannot be relied on alone, and further investigations such as RFLP or other analyses are needed to justify this issue. In this study, NP decreased MIC in 40% of studied samples, and increased AdeR expression, and also decreased AdeB expression, which plays an important role in transforming antibacterial compounds. In addition to being an environmentally-friendly process, the green synthesis of NPs especially ZnO, can be an alternative for antibiotics in the future; it can be a new way to solve the global concern of growing bacterial resistance including Acinetobacter to some various classes of antibiotics. Besides, based on the results of 60% of the samples, the increase in the direct expression of AdeB and AdeRS genes and the insufficient and ambiguous information about these genes expression and their associations, larger studies of the cited genes to analysis the mutations are recommended. However, further analysis of efflux pump genes at the protein level would help to verify the observed reductions in the genes expressions and regulation. So far, only one report is available on modulatory effects of AgNPs on AdeB and AdeRS genes expression in MDR Acinetobacter [29]. Thus, our study using biologically synthesized ZnO NPs was the first report on employing ZnONPs for the efflux pump gene inhibition. Given the increase in resistance against antibiotics, the world requires changes in the patterns of consuming and prescribing newer resources with more useful pharmaceutical value. If drug consumption remains as it is, even producing and developing new medications cannot prevent the increase in antibiotic-resistance. The aim of this study was to assess the biosynthesis of ZnO NPs, and its effects on the genes expression of the efflux pump of AdeABC in Acinetobacter, which plays a potential role in their resistance. Moreover, by producing such Nano substances, it was attempted to step toward advances in the therapeutic alternative strategies for bacteria; in a way, that using these appropriate inhibitors can disrupt the performance of these pumps to inhibit multi-drug resistance. However, further in vitro and in vivo experiments are required before conclusions can be drawn.
Conclusions
In the present study, ZnO NPs were synthetized successfully using Bacillus subtilis strain. This biosynthesis route is very low cost, fast and eco-friendly to the environment. The complete synthesis of nanostructure of ZnO NPs with crystallite size in the range of 25–50 nm was confirmed by the XRD, FTIR spectroscopy, and SEM. Antibacterial effect of NPs on the AdeABC pump efflux genes expression and the calculations performed by MIC and the statistical analysis test showed that ZnO NPs had inhibitory effect on the gene expression in 40% of Acinetobacter baumannii samples. In addition, based on the results of gene expression of 60% of the studied samples, and the probability of mutation in the AdeRS genes, assessment of other related factors should not be neglected to establish multi-drug resistance genotypes. The mechanisms of development, and their association results showed the remarkable potential of these NPs, especially against Acinetobacter. It could be used as an effective alternative for commonly used chemical drugs and covering drug resistance issues resulted from persistent use of chemical drugs.
Acknowledgments
The authors thank the head and staff of the Razi herbal medicines research center of Lorestan University of Medical Sciences.
Declaration
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
Not required.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zarei, M., A. Jamnejad, and E. Khajehali, Antibacterial effect of silver nanoparticles against four foodborne pathogens. Jundishapur journal of microbiology, 2014. 7(1). [DOI] [PMC free article] [PubMed]
- 2.Mohan S, et al. Biopolymers–application in nanoscience and nanotechnology. Recent advances in biopolymers. 2016;1(1):47–66. [Google Scholar]
- 3.Park E-J, Kim H, Kim Y, Yi J, Choi K, Park K. Inflammatory responses may be induced by a single intratracheal instillation of iron nanoparticles in mice. Toxicology. 2010;275(1–3):65–71. doi: 10.1016/j.tox.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Chaloupka K, Malam Y, Seifalian AM. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010;28(11):580–588. doi: 10.1016/j.tibtech.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Babu Nagati V, et al. Green synthesis and characterization of silver nanoparticles from Cajanus cajan leaf extract and its antibacterial activity. Intl J Nanomater Biostruct. 2012;2(3):39–43. [Google Scholar]
- 6.Azari A, et al. Efficiency of magnitized graphene oxide nanoparticles in removal of 2, 4-dichlorophenol from aqueous solution. J Mazandaran Univ Med Sci. 2017;26(144):265–281. [Google Scholar]
- 7.Kiani A, Ahmadloo M, Shariatifar N, Moazzen M, Baghani AN, Khaniki GRJ, et al. Method development for determination of migrated phthalate acid esters from polyethylene terephthalate (PET) packaging into traditional Iranian drinking beverage (Doogh) samples: a novel approach of MSPE-GC/MS technique. Environ Sci Pollut Res. 2018;25(13):12728–38. [DOI] [PubMed]
- 8.Rachel, R., et al. Surface layers of ore-leaching Bacteria and Archaea. In EMC 2008 14th European Microscopy Congress 1–5 September 2008, Aachen, Germany. 2008. Springer.
- 9.Gholami M, Shirzad-Siboni M, Yang J-K. Application of Ni-doped ZnO rods for the degradation of an azo dye from aqueous solutions. Korean J Chem Eng. 2016;33(3):812–822. doi: 10.1007/s11814-015-0218-4. [DOI] [Google Scholar]
- 10.Gharibzadeh F, Rezaei Kalantary R, Nasseri S, Esrafili A, Azari A. Reuse of polycyclic aromatic hydrocarbons (PAHs) contaminated soil washing effluent by bioaugmentation/biostimulation process. Sep Purif Technol. 2016;168:248–256. doi: 10.1016/j.seppur.2016.05.022. [DOI] [Google Scholar]
- 11.El Enshasy, H.A., et al., Medical and cosmetic applications of fungal nanotechnology: production, characterization, and bioactivity, in Fungal nanobionics: Principles and applications. 2018, Springer. p. 21–59.
- 12.Mohammadi F, Esrafili A, Kermani M, Farzadkia M, Gholami M, Behbahani M. Application of amino modified mesostructured cellular foam as an efficient mesoporous sorbent for dispersive solid-phase extraction of atrazine from environmental water samples. Microchem J. 2019;146:753–762. doi: 10.1016/j.microc.2019.01.049. [DOI] [Google Scholar]
- 13.Chokriwal A, Sharma MM, Singh A. Biological synthesis of nanoparticles using bacteria and their applications. Am J PharmTech Res. 2014;4(6):38–61. [Google Scholar]
- 14.Narayanan KB, Sakthivel N. Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interf Sci. 2010;156(1–2):1–13. doi: 10.1016/j.cis.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Azari A, et al. Nitrate removal from aqueous solution by carbon nanotubes magnetized with nano zero-valent iron. J Mazandaran Univ Med Sci. 2014;23(2):15–27. [Google Scholar]
- 16.Prasad K, Jha AK. ZnO nanoparticles: synthesis and adsorption study. Nat Sci. 2009;1(02):129. [Google Scholar]
- 17.Yunos a’b, M.Z., et al., Biosynthesis of zinc oxide nanoparticles by using fruits extracts of Ananas comosus and its antibacterial activity. 2019.
- 18.Esmaeilzade, H., et al., the effect of zno nanoparticles on the growth of bacillus subtilis and Escherichia coli o157:h7. journal of food technology and nutrition, 2014. 11(3 (43)): p. -.
- 19.Lin D, Xing B. Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut. 2007;150(2):243–250. doi: 10.1016/j.envpol.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Nabizadeh S, Shariatifar N, Shokoohi E, Shoeibi S, Gavahian M, Fakhri Y, et al. Prevalence and probabilistic health risk assessment of aflatoxins B 1, B 2, G 1, and G 2 in Iranian edible oils. Environ Sci Pollut Res. 2018;25(35):35562–70. [DOI] [PubMed]
- 21.Venkataraju JL, et al. Synthesis, characterization and evaluation of antimicrobial activity of zinc oxide nanoparticles. J Biochem Technol. 2014;3(5):151–154. [Google Scholar]
- 22.Nezamabadi V, et al. Biosynthesis and antibacterial activity of ZnO nanoparticles by Artemisia Aucheri extract. Iran J Biotechnol. 2020;18(2):82–91. doi: 10.30498/IJB.2020.151379.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunalan S, Sivaraj R, Rajendran V. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog Natural Sci Mater Intl. 2012;22(6):693–700. doi: 10.1016/j.pnsc.2012.11.015. [DOI] [Google Scholar]
- 24.Tiwari V, et al. Mechanism of anti-bacterial activity of zinc oxide nanoparticle against Carbapenem-resistant Acinetobacter baumannii. Front Microbiol. 2018;9:1–10. doi: 10.3389/fmicb.2018.01218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin M-F, Lin YY, Tu CC, Lan CY. Distribution of different efflux pump genes in clinical isolates of multidrug-resistant Acinetobacter baumannii and their correlation with antimicrobial resistance. J Microbiol Immunol Infect. 2017;50(2):224–231. doi: 10.1016/j.jmii.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Khosrishahi N, Sharifi M. Isolation of carbapenem resistant Acinetobacter baumannii(CRAB) strains from patients and equipments of intensive care units (ICUs) at Qazvin between 2005-2006. Iranian J Med Microbiol. 2007;1(3):33–38. [Google Scholar]
- 27.Goudarzi H, et al. Functional analysis of multidrug efflux pumps genes of Acinetobacter baumannii strains. Pejouhesh dar Pezeshki (Research in Medicine) 2013;37(2):107–112. [Google Scholar]
- 28.Modarresi F, Azizi O, Shakibaie MR, Motamedifar M, Valibeigi B, Mansouri S. Effect of iron on expression of efflux pump (adeABC) and quorum sensing (luxI, luxR) genes in clinical isolates of Acinetobacter baumannii. Apmis. 2015;123(11):959–968. doi: 10.1111/apm.12455. [DOI] [PubMed] [Google Scholar]
- 29.Behdad R, Pargol M, Mirzaie A, Karizi SZ, Noorbazargan H, Akbarzadeh I. Efflux pump inhibitory activity of biologically synthesized silver nanoparticles against multidrug-resistant Acinetobacter baumannii clinical isolates. J Basic Microbiol. 2020;60(6):494–507. doi: 10.1002/jobm.201900712. [DOI] [PubMed] [Google Scholar]
- 30.Bouvet PJ, Grimont PA. Taxonomy of the genus Acinetobacter with the recognition of Acinetobacter baumannii sp. nov., Acinetobacter haemolyticus sp. nov., Acinetobacter johnsonii sp. nov., and Acinetobacter junii sp. nov. and emended descriptions of Acinetobacter calcoaceticus and Acinetobacter lwoffii. Int J Syst Evol Microbiol. 1986;36(2):228–240. [Google Scholar]
- 31.Constantiniu S, et al. Cultural and biochemical characteristics of Acinetobacter spp. strains isolated from hospital units. J Prevent Med. 2004;12(3–4):35–42. [Google Scholar]
- 32.Weinstein, M.P. and J.S. Lewis, The Clinical and Laboratory Standards Institute Subcommittee on Antimicrobial Susceptibility Testing: Background, Organization, Functions, and Processes. J Clin Microbiol, 2020. 58(3). [DOI] [PMC free article] [PubMed]
- 33.Ali AA, et al. Green synthesis of ZnO nanoparticles using Bacillus subtilis and their catalytic performance in the one-pot synthesis of steroidal thiophenes. European Chemical Bulletin. 2014;3(9):939–945. [Google Scholar]
- 34.Wayne, P., Reference method for broth dilution antifungal susceptibility testing of yeasts, approved standard. CLSI document M27-A2, 2002.
- 35.Ahmed SH, et al. Multidrug resistant Egyptian isolates of Acinetobacter baumannii. J Am Sci. 2011;7(1):1013–1019. [Google Scholar]
- 36.Jassim KA, Ghaima KK, Saadedin SMK. AdeABC efflux pump genes in multidrug resistant Acinetobacter baumannii isolates. Avicenna J Clin Microbiol Infect. 2016;3(4):40898–8.
- 37.Mahdi, Z.S., et al., Biosynthesis of Zinc Oxide Nano-rods Using Xanthomonas campestris. Biol. J. Microorg., 2017: p. 22.
- 38.Jayaseelan C, Rahuman AA, Kirthi AV, Marimuthu S, Santhoshkumar T, Bagavan A, et al. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim Acta A Mol Biomol Spectrosc. 2012;90:78–84. [DOI] [PubMed]
- 39.Mahdi ZS, Talebnia Roshan F, Nikzad M, Ezoji H. Biosynthesis of zinc oxide nanoparticles using bacteria: a study on the characterization and application for electrochemical determination of bisphenol A. Inorganic Nano-Metal Chem. 2020:1–9.
- 40.Chang T-Y, Huang BJ, Sun JR, Perng CL, Chan MC, Yu CP, et al. AdeR protein regulates adeABC expression by binding to a direct-repeat motif in the intercistronic spacer. Microbiol Res. 2016;183:60–7. [DOI] [PubMed]
- 41.Peleg AY, Adams J, Paterson DL. Tigecycline efflux as a mechanism for nonsusceptibility in Acinetobacter baumannii. Antimicrob Agents Chemother. 2007;51(6):2065–2069. doi: 10.1128/AAC.01198-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madhi, M., et al., Impact of chitosan and silver nanoparticles laden with antibiotics on multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Archives of Clinical Infectious Diseases, (In Press).
- 43.Gupta D, Singh A, Khan AU. Nanoparticles as efflux pump and biofilm inhibitor to rejuvenate bactericidal effect of conventional antibiotics. Nanoscale Res Lett. 2017;12(1):1–6. doi: 10.1186/s11671-017-2222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Banoee M, et al. ZnO nanoparticles enhanced antibacterial activity of ciprofloxacin against Staphylococcus aureus and Escherichia coli. J Biomed Mater Res B Appl Biomater. 2010;93(2):557–561. doi: 10.1002/jbm.b.31615. [DOI] [PubMed] [Google Scholar]
- 45.Hornsey M, Ellington MJ, Doumith M, Thomas CP, Gordon NC, Wareham DW, et al. AdeABC-mediated efflux and tigecycline MICs for epidemic clones of Acinetobacter baumannii. J Antimicrob Chemother. 2010;65(8):1589–93. [DOI] [PubMed]
- 46.Coyne S, et al. Screening and quantification of the expression of antibiotic resistance genes in Acinetobacter baumannii with a microarray. Antimicrob Agents Chemother. 2010;54(1):333–340. doi: 10.1128/AAC.01037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atasoy AR, Ciftci IH, Petek M, Terzi HA. Investigation of mutations in adeR and adeS gene regions in gentamicine resistant Acinetobacter baumannii isolates. Biotechnol Biotechnol Equip. 2016;30(2):360–367. doi: 10.1080/13102818.2015.1135082. [DOI] [Google Scholar]