Abstract
Background
Parabens are esters of p-hydroxybenzoic acid and are widely used as preservatives in cosmetics, pharmaceuticals and foodstuffs. The presences of parabens in infant formulas raise concerns due to their potential to disrupt endocrine function in infants and cause reproductive toxicities.
Methods
In this study a new method was developed for extraction and determination of methylparaben in infant formulas using HPLC method and UV detector. Methanol and trichloroacetic acid were used for extraction and isocratic mobile phase comprising equal proportions of glacial acetic acid in water (50:850 v/v) and methanol was used for separation of methylparaben.
Results
Recovery of the extraction procedure was good and interferences between methylparaben and other ingredients peaks in HPLC chromatograms decreased. The average recoveries for methylparaben were about 88–108 %. The limit of detection and limit of quantitation for methylparaben were 0.2 and 0.5 µg/mL, respectively. Results of the method showed good reproducibility (relative standard deviation (RSD) 0.29–1.94 % for within day analysis and 0.84–2.18 % for between day analysis). Results were linear in range of 0.5–20 µg/mL methylparaben. The results of twenty real infant formula samples showed methylparaben was found only in one sample in concentration 0.3 µg/mL.
Conclusions
The new extraction and measurement method was a short-time method and could be applicable for large numbers of samples. This method was fast, sensitive and accurate and was capable of being used in legal laboratory references for determination of methylparaben content.
Keywords: Infant formula, Methylparaben, Iran market, HPLC, Validation
Background
The alkyl esters of p-hydroxybenzoic acid (parabens) are a group of a homologous series of chemicals that include methyl, ethyl, propyl, butyl, and benzyl parabens. Parabens are used as preservatives in cosmetics, pharmaceuticals, foodstuffs, including beverages [1–6]. Parabens exhibit several characteristics such as stability, water solubility and broad-spectrum antimicrobial activity which make them popular preservatives in consumer products [3]. Humans are exposed to parabens via ingestion, inhalation, and/or dermal absorption through a wide variety of sources [3, 5, 7]. Secretion of parabens in human fluid such as urine, serum, breast milk has been reported in several investigations. Furthermore, presence of parabens in breast tumors has been documented [8–15]. Studies conducted by the Centers for Disease Control and Prevention (CDC) in the United States showed the presence of methylparaben and propylparaben in concentrations about 100 ng/mL in more than 90 % of randomized urine sampling. Other paraben analogues including ethylparaben, butylparaben and benzylparaben were detected in lower concentrations (about half) in less than 50 % of sampling [8, 9, 11, 15]. Similar results were obtained by investigations conducted in Spain and Denmark [13, 14].
Concerns about the safety of parabens have been raised due to their potential to disrupt endocrine function, as have been shown in both in-vitro and in-vivo studies [3, 5, 16–21]. An in-vitro yeast-based assay suggested that the four most widely used parabens (methyl, ethyl, propyl and butyl parabens) had weak estrogenic properties and the most potent one (butylparaben) was approximately four fold weaker than the natural estrogen, 17β-estradiol [22]. The estrogenic activities of parabens also were reported in fish and rodent exposure studies [22, 23]. In male rats those consumed propylparaben and butylparaben, decrease in sperms content and adverse effects on reproductive system were reported [24, 25]. Due to their endocrine and reproductive toxicities and on the other hand, their widespread exposure, parabens have received considerable attention by national and international regulatory agencies [26, 27]. In 1974 the Joint FAO/ WHO Expert Committee on Food Additives (JECFA) recommended that an acceptable daily intake (ADI) for the sum of methylparaben, ethylparaben and propylparaben must be limited to 10 mg per kg of body weight per day [26]. Although, in Denmark utilization of propylparaben and butylparaben and also their isoforms and salts in children’s cosmetic products have been forbidden from march 2011 [27]. Given the widespread use of parabens in consumer products, the assessment of the safety of these chemicals is crucial and the estimation of exposure doses in humans is the first step toward the assessment of the potential risks that these compounds may pose to human health. Although the occurrence of parabens in humans has been reported, sources of human exposure are not well understood. In one study, they measured the concentrations of five parabens methyl, ethyl, propyl, butyl, and benzyl parabens in 267 food samples include beverages, dairy products, fats, milks, fish, shellfish, grains, meat, fruits and vegetables with the aims of establishing baseline concentrations and profiles of parabens in foodstuffs and estimating potential dietary exposure doses through food ingestion [28]. In literature there are many studies about determination of parabens in hygiene and cosmetic products [1, 4, 7] but there are few investigations about milk and infant formulae.
In the present study, in order to evaluate the safety of Iran market infant formulae from the aspect of being contaminated with parabens, we tried to assess sustainability of parabens residues in randomized samples gathered from Iran market.
According to the fact that in cocktails of paraben preservatives, methylparaben is an essential member and is used in higher concentrations, we considered it as a marker or indicator of presence of parabens in infant formulae.
At first and crucial step, it was necessary to develop a reliable and validated method for detection and determination of methylparaben in infant formulae.
Measurement of parabens in foodstuffs such as milk and infant formulae are essentially influenced by the method of extraction. Up to now parabens have been extracted by acetonitrile and analyzed by HPLC-MS/MS equipment that was time consuming and expensive method that need expert analysts for interpretation of the results [12, 28]. In our study, we changed extraction solvents to methanol and trichloro acetic acid and expected to have extractions with less impurity. Then we analyzed the resulting extracts by HPLC coupled to UV detector equipment. After confirming validity of the method, methylparaben residue was determined in the randomized selected samples.
Methods
Chemicals
Methanol, glacial acetic acid and trichloroacetic acid (TCA) were purchased from Merck (Darmstadt, Germany). Standard of methylparaben was purchased from Sigma Aldrich (St. Louis, MO, USA) and ultrapure water (Milipore-Q) was used where applicable.
Samples preparation
A total of twenty commercial samples from different brands collected randomly from local drug stores in food and drug organization, ministry of health and medical education in Tehran were used as samples.
For sample preparation, homogenized samples were prepared by dissolving 2.5 g of each infant formula in a mixture of 11 mL water, 4 mL trichloroacetic acid (TCA) solution 1 % (w/v in water) and 10 mL methanol. After sonication, the mixtures were centrifuged at 6000 rpm and temperature -4°C for 20 minutes. The centrifuged mixtures consisted of proteins precipitated at the bottom of the tubes; lipid layer appeared on the surface and the supernatant contained methylparaben extract of the infant formulae. After filtration, supernatants were used for the analysis of methylparaben by HPLC method.
Standard preparation of methylparaben
Standard stock solution
Accurately weighted quantity of methylparaben standard was dissolved in methanol to have a concentration of 1 mg/mL of methylparaben as standard stock solution. Then the solution was aliquoted and stored at -20°C.
Standard solutions
In the day of analysis, aliquoted of the standard stock solution was thawed at room temperature and then diluted by methanol to concentrations 0.5, 1, 2, 3, 5, 10, 20 µg/mL as standard solutions.
High‐performance liquid chromatography
Determination of methylparaben in infant formulae were performed by HPLC (Waters Alliance, United State) and separated on a 250 mm by 4.4 mm i.d. and 5 µm pore size AQ-C18 column, detected by a Waters 2487 dual wavelength UV detector at wavelength 256 nm and data were integrated using the Empower Pro software.
The methylparaben was separated by isocratic elution using a mobile phase comprising equal proportion of glacial acetic acid mixed with water (50:50 v/v) and methanol. The flow rate was adjusted to 1 mL/min. The injection volume and run time were 20 µL and 10 min, respectively.
Procedure
All standard and sample solutions filtered through 0.45 µm syringe nylon filters and injected into the HPLC column as previously described.
Calibration curve was plotted by the peak areas of different concentrations of standard solutions vs. respected concentrations of methylparaben. The linear regression equation calculated as y = ax ± b, where x was concentrations of methylparaben and y was the relative peak area.
Concentrations of methylparaben in different samples were determined by measuring the area of methylparaben in sample chromatograms and calculating the respected concentration base on the standard calibration curve.
Method validation
Selectivity
To evaluate selectivity of the extraction and HPLC methods, blank samples were extracted and analyzed as procedures mentioned above. In the first step water was considered as a blank sample that was extracted by the extraction method and obtained chromatogram was evaluated. Then the methylparaben free infant formula was evaluated as a blank. In this study the infant formula that we were ensure about no usage of methylparaben as ingredient was selected as methylparaben free infant formula. The chromatograms of blanks were compared to standard chromatogram of methylparaben.
Also the chromatogram of methylparaben free infant formula that was spiked by methylparaben standard was compared to that of standard and not spiked one.
Accuracy
Accuracy was determined by calculating the recovery of the extraction method. For this purpose, methylparaben-free infant formula was spiked by different amounts of standard stock solution of methylparaben in a way that the concentration of final extracted solutions be similar to the standard solutions of methylparaben (0.5, 5, 20 µg/mL). Concentrations of methylparaben in spiked samples were determined based on calibration curve. The recovery percent for each concentration was calculated as:
Calculated value/true value of methylparaben × 100.
In which, calculated value was concentration of methylparaben that was determined based on calibration curve and true value was the nominal concentration of the spiked sample.
Precision
Precision was assessed by determination of instrument precision, within day repeatability and intermediate precision (between days repeatability). To assess instrument precision or injection repeatability, spiked solution of methylparaben in concentration 3 µg/mL was analyzed for ten times by repeated injections to HPLC system. For within day repeatability, three different (low, Median and high) concentrations of spiked methylparaben solutions with concentrations 0.5, 5, 20 µg/mL were analyzed as triplicates in a day. And for between days precision, these analysis were repeated for three different days. Relative standard deviation of retention times and peak areas of methylparaben used to evaluate the precision in each step (instrument precision, within day and between days precision).
Linearity
Six different standard solutions of methylparaben in concentrations 0.5, 1, 2, 5, 10, 20 µg/mL were analyzed as triplicates. Average of the peak areas for each concentration was calculated. As described in the procedure, calibration curve was plotted and linearity of the results was evaluated.
Limit of detection (LOD) and limit of quantitation (LOQ)
The lowest concentration of methylparaben in infant formula that could be detected but not quantified accurately was defined as the detection limit (LOD). The lowest concentration that could be quantified with acceptable accuracy and precision was defined as limit of quantitation (LOQ). Signal to noise ratio was used to determine LOD and LOQ. The ratio 3:1 was used for LOD while the ratio 10:1 was used for LOQ. The spiked sample of methylparaben in the concentration of LOQ was injected to HPLC for five times and relative standard deviation of the retention times and peak areas of methylparaben were calculated.
Statistical analysis
The graph preparation was performed using Microsoft Excel 2010. The data were expressed as mean ± SD (standard deviation). A 0.05 level of probability was taken as the level of significance and 95 % level was considered for confidence intervals. All the analyses were carried out using the statistical software, MINITAB 14 (Minitab Inc., State College, PA, USA).
Results
In the present study, the new method for extraction and analysis of methylparaben in infant formula matrices were developed and twenty different brand infant formulae those were marketed in Iran were evaluated about the presence of methylparaben. Also the extraction method and analysis method (HPLC) were validated.
Selectivity
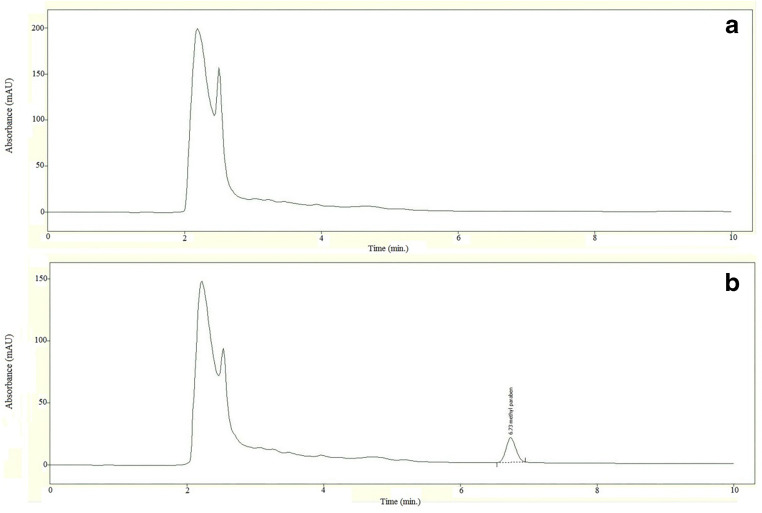
For evaluation of selectivity, water and methylparaben-free infant formula were analyzed as blanks. In the chromatograms of blanks, in the region of methylparaben retention time there were no interference peak (Fig. 1). It meant that dilution buffer and matrix had no interference with the method of analysis. The absence of any interfering peak in the region of methylparaben supported the good performance of the developed method for extraction and separation of methylparaben.
Fig. 1.
Chromatograms of methylparaben-free infant formula (a) and methylparaben-free infant formula spiked with methylparaben in concentration 5 μg/mL (b)
Accuracy
Accuracy was determined by evaluation of the recovery of the extraction method. As depicted in Table 1, recovery of the three different concentrations of methylparaben (0.5, 5, 20 µg/mL) were varied from 88 to 108 %.
Table 1.
Accuracy (recovery percent) of the method for three different concentrations of methylparaben (0.5, 5, and 20 µg/mL) in spiked samples (methylparaben-free infant formula), nine replicates for each concentration
| Concentration of methyl paraben in spiked samples (true value) (µg/mL) |
Concentration of methylparaben calculated in spiked samples (Calculated value) (µg/mL) |
Recovery (%) |
Mean Recovery ± SD* |
RSD** (%) |
|---|---|---|---|---|
| 0.5 | 0.44 | 88.11 | 89.20 ± 0.67 | 0.75 |
| 0.5 | 0.45 | 90.50 | ||
| 0.5 | 0.44 | 88.89 | ||
| 0.5 | 0.45 | 89.46 | ||
| 0.5 | 0.44 | 89.90 | ||
| 0.5 | 0.45 | 90.13 | ||
| 0.5 | 0.45 | 89.48 | ||
| 0.5 | 0.44 | 88.45 | ||
| 0.5 | 0.44 | 89.11 | ||
| 5 | 5.35 | 106.96 | 106.77 ± 0.33 | 0.31 |
| 5 | 5.35 | 107.40 | ||
| 5 | 5.35 | 107.01 | ||
| 5 | 5.35 | 107.80 | ||
| 5 | 5.34 | 106.81 | ||
| 5 | 5.35 | 106.95 | ||
| 5 | 5.32 | 106.47 | ||
| 5 | 5.31 | 106.11 | ||
| 5 | 5.32 | 106.50 | ||
| 20 | 18.98 | 94.89 | 97.28 ± 1.93 | 1.98 |
| 20 | 18.89 | 94.45 | ||
| 20 | 18.98 | 94.92 | ||
| 20 | 19.80 | 99.20 | ||
| 20 | 19.82 | 99.12 | ||
| 20 | 19.65 | 98.26 | ||
| 20 | 19.71 | 98.53 | ||
| 20 | 19.63 | 98.14 | ||
| 20 | 19.63 | 98.17 |
*SD: Standard deviation
**RSDs were calculated based on nine replicates for each concentration of methylparaben (RSD: relative standard deviation)
Precision
To determine precision of the method, at first instrument precision or injection repeatability was evaluated in concentration of 3 µg/mL of spiked methylparaben. For ten replicate injections of the solution to HPLC, relative standard deviation of the peak areas was 0.54 %.
Then method repeatability was assessed using three different concentrations 0.5, 5, and 20 µg/mL (low, medium and high concentrations, respectively) of spiked methylparaben and three replicates for each concentration in a day. The RSDs of replicates depicted in Table 2 were about 0.29–1.94 % (within day precision). To evaluate intermediate precision, variation of results in different days (three days, three concentrations of methylparaben and three replicates for each concentration) was calculated as RSD for replicates of each concentration (Table 2, between days precision). Variation of retention times in different days were negligible (RSD was about 0.19 %). Results showed that the method had good repeatability and intermediate precision.
Table 2.
Precision of the method in two levels (repeatability, Within day) and (intermediate precision, between days) for three different concentrations of methylparaben (0.5, 5, 20 µg/mL) spiked to the methylparaben-free infant formula
| Concentration of methylparaben | RSD* | |
|---|---|---|
| Within day (n:3) | 0.5 µg/mL | 0.41 % |
| 5 µg/mL | 0.29 % | |
| 20 µg/mL | 1.94 % | |
| Between days (n:3) | 0.5 µg/mL | 2.18 % |
| 5 µg/mL | 0.84 % | |
| 20 µg/mL | 1.94 % |
*RSDs were calculated based on three replicates for each concentration of methylparaben in within day precision and three replicates for between days precision (RSD: relative standard deviation)
Linearity
Linearity of the method was evaluated in the range of 0.5–20 µg/mL of methylparaben. The standard curve of peak areas against different concentrations of methylparaben plotted showed to be linear (Fig. 2).
Fig. 2.

Calibration curve for HPLC (high performance liquid chromatography) analysis. Peak areas were plotted against different concentrations (0.5–20 µg/mL) of methylparaben standard. Results were the averages of three replicates
LOD and LOQ
LOD (limit of detection) of the method was about 0.2 µg/mL and quantification limit was 0.5 µg/mL.
Finally, validated method was used to determine the amount of methylparaben in samples of twenty brand infant formulae marketed in Iran. In nineteen samples of brand infant formulae, methylparaben was under detectable limit (Not detected) and only one had methylparaben peak in its chromatogram in concentration below quantitation limit (about 0.3 µg/mL).
Discussion
Due to the importance of the target consuming group of infant formulae, it was necessary to develop a reliable, applicable, cost and time benefit analytical method for detecting and determining residues such as parabens in this matrices [29, 30]. According to this request, current method was implemented based on the professional use of solvents to extract methylparaben that eventuated the accurate and precise measurement of this residue in marketed infant formulae. Capability of the suggested method was demonstrated by tracing this residue in quantities as low as 0.2 ppm (LOD) and determination with valid results in the range of 0.5–20 µg/mL.
Up to now, because of the world wide application of methylparaben as preservative in non-edible materials, there are a lot of investigations about its extraction and measurement in hygienic and cosmetic products [3, 6, 7]. As well as non-edible materials, methylparaben has been monitored in foods such as fish and fish products, milk and dairy products and beverages [31, 32]. In contrast, restricted official utilization of methylparaben in infant formulae caused somewhat to be neglected the evaluation and tracing of methylparaben in these formulae. But it is possible this paraben be present in these products via illegal or indirect ways, ingredients impurity of infant formulae or feeding cows with feed contaminated methylparaben [33, 34].
In scarce studies about methylparaben in infant formulae, the common method was applied for milk, dairy products and infant formulae analysis. In Liao et al.. study, 31 different dairy products such as milk, infant formula, yogurt, cheese, and ice cream were analyzed by the same extraction method. Maybe low recovery of their analysis could be attributed to non-selectivity of the applied extraction method (63–112 % vs. 88–108 % in the present study) [28].
Since a wide variety of compounds with different polarities exist in infant formulae, finding an optimum extracting condition that could be selective for methylparaben extraction and removing other unwanted ingredients which interfere with methylparaben HPLC analysis is important and critical step in determination of methylparaben [29]. Therefore in the present study, improvement of extracting efficiency for less contaminated yield was considered at first step. For this purpose, instead of common extraction solvent (acetonitrile), methanol and trichloroacetic acid (TCA) were substituted. In contrast with acetonitrile, the resulted chromatograms baselines are obviously more flat, stable and with methylparaben sharp peak without interfering noises and peaks. As trichloroacetic acid induces protein precipitation, the resulting supernatants were more clear and in the relevant chromatograms, there were less interfering peaks [35]. In the present study the results were compared in three different concentrations of trichloroacetic acid (0.5, 0.75 and 1 %) in combination with methanol and water as extracting solvent and concluded that the concentration 1 % of TCA would be able to enhance the recovery of methylparaben (88–108 %); in three different concentrations (0.5, 5, 20 µg/mL) and three different days for three times.
After extraction by Methanol/TCA, methylparaben was detected and determined by HPLC in run time about 10 min. In HPLC analysis, long run times are time consuming and considered as limitation for large sample size analysis. On the other hand, results of very short run times may not be confidently reliable. The short but yet rational run time of the developed method (10 min.) could be suitable for large scale analysis in comparison to Liao et al. study (run time: 23 min.). Of course, fitness of the method for this purpose was fortified by the time saving properties of the extraction phase by its simplicity and reduction in steps. Besides, isocratic elution of HPLC separation sustained very stable condition with less variability in retention time and area under the peak during several analysis in a day or along several days.
Along with these issues, it should also be noticed that the proposed method was designed with more available facilities; HPLC with UV detector. Despite the valuable research analysis using high tech instruments such as HPLC MS-MS in the other studies, these methods are not considered applicable because of non-availability in most common laboratories. In the present study effort was focused on assembling the method with more available facilities and meanwhile the same quality. Results obtained by this investigation demonstrated that the proposed method could be efficient analytical choice for tracing methylparaben.
Conclusions
A new method for the extraction, separation and measurement of methylparaben in infant formulae was developed. In this method, methylparaben can be easily measured in the infant formula as a complex matrix by using available and inexpensive solvents. Recovery of the extraction procedure was good and interferences between methylparaben and other ingredients peaks in HPLC chromatograms decreased. The new extraction and measurement method was a short-time method and could be applicable for large numbers of samples. Contrary to some other detection methods, determination of methylparaben using HPLC method with UV detector showed to have a good dose-response correlation and proved to be a reliable, short-time, and budget-saving method. Capability of the method for measurement of methylparaben in real samples of infant formulae has been proved.
Acknowledgements
We are grateful Food and Drug Control Reference Laboratory, Iranian Food and Drug Administration for their assistance and providing some reagents.
Funding
The research was conducted with the internal funding of the Food and Drug Laboratory Research Center, Food and Drug Administration and no grants were used.
Data availability
The data will not be shared with a reason.
Code availability
Not applicable.
Declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human or animals.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rastogi SC, Schouten A, De Kruijf N, Weijland JW. Contents of methyl-, ethyl-, propyl-, butyl- and benzylparaben in cosmetic products. Contact Dermatitis. 1995;32(1):28–30. doi: 10.1111/j.1600-0536.1995.tb00836.x. [DOI] [PubMed] [Google Scholar]
- 2.Soni MG, Taylor SL, Greenberg NA, Burdock GA. Evaluation of the health aspects of methyl paraben: a review of the published literature. Food Chem Toxicol. 2002;40(10):1335–73. doi: 10.1016/S0278-6915(02)00107-2. [DOI] [PubMed] [Google Scholar]
- 3.Soni MG, Carabin IG, Burdock GA. Safety assessment of esters of p-hydroxybenzoic acid (parabens) Food Chem Toxicol. 2005;43(7):985–1015. doi: 10.1016/j.fct.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Q, Lian M, Liu L, Cui H. High-performance liquid chromatographic assay of parabens in wash-off cosmetic products and foods using chemiluminescence detection. Anal Chim Acta. 2005;537(1):31–9. doi: 10.1016/j.aca.2005.01.027. [DOI] [Google Scholar]
- 5.Andersen FA. Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int J Toxicol. 2008;27(Suppl 4):1–82. doi: 10.1080/10915810802548359. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson E, Andersen HR, Ledin A. Substance flow analysis of parabens in Denmark complemented with a survey of presence and frequency in various commodities. J Hazard Mater. 2008;156(1–3):240–59. doi: 10.1016/j.jhazmat.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 7.El Hussein S, Muret P, Berard M, Makki S, Humbert P. Assessment of principal parabens used in cosmetics after their passage through human epidermis-dermis layers (ex-vivo study) Exp Dermatol. 2007;16(10):830–6. doi: 10.1111/j.1600-0625.2007.00625.x. [DOI] [PubMed] [Google Scholar]
- 8.Ye X, Kuklenyik Z, Bishop AM, Needham LL, Calafat AM. Quantification of the urinary concentrations of parabens in humans by on-line solid phase extraction-high performance liquid chromatography-isotope dilution tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;844(1):53–9. doi: 10.1016/j.jchromb.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 9.Ye X, Bishop AM, Reidy JA, Needham LL, Calafat AM. Parabens as urinary biomarkers of exposure in humans. Environ Health Perspect. 2006;114(12):1843–6. doi: 10.1289/ehp.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye X, Tao LJ, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method for measuring environmental phenols and parabens in serum. Talanta. 2008;76(4):865–71. doi: 10.1016/j.talanta.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 11.Calafat AM, Ye X, Wong LY, Bishop AM, Needham LL. Urinary concentrations of four parabens in the U.S. population: NHANES 2005–2006. Environ Health Perspect. 2010;118(5):679–85. doi: 10.1289/ehp.0901560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlumpf M, Kypke K, Wittassek M, Angerer J, Mascher H, Mascher D, et al. Exposure patterns of UV filters, fragrances, parabens, phthalates, organochlor pesticides, PBDEs, and PCBs in human milk: correlation of UV filters with use of cosmetics. Chemosphere. 2010;81(10):1171–83. doi: 10.1016/j.chemosphere.2010.09.079. [DOI] [PubMed] [Google Scholar]
- 13.Casas L, Fernandez MF, Llop S, Guxens M, Ballester F, Olea N, et al. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environ Int. 2011;37(5):858–66. doi: 10.1016/j.envint.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Frederiksen H, Jorgensen N, Andersson AM. Parabens in urine, serum and seminal plasma from healthy Danish men determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS) J Expo Sci Environ Epidemiol. 2011;21(3):262–71. doi: 10.1038/jes.2010.6. [DOI] [PubMed] [Google Scholar]
- 15.Smith KW, Braun JM, Williams PL, Ehrlich S, Correia KF, Calafat AM, et al. Predictors and variability of urinary paraben concentrations in men and women, including before and during pregnancy. Environ Health Perspect. 2012;120(11):1538–43. doi: 10.1289/ehp.1104614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller D, Wheals BB, Beresford N, Sumpter JP. Estrogenic activity of phenolic additives determined by an in vitro yeast bioassay. Environmental health perspectives. 2001;109(2):133–8. doi: 10.1289/ehp.109-1240632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okubo T, Yokoyama Y, Kano K, Kano I. ER-dependent estrogenic activity of parabens assessed by proliferation of human breast cancer MCF-7 cells and expression of ERalpha and PR. Food Chem Toxicol. 2001;39(12):1225–32. doi: 10.1016/S0278-6915(01)00073-4. [DOI] [PubMed] [Google Scholar]
- 18.Byford JR, Shaw LE, Drew MG, Pope GS, Sauer MJ, Darbre PD. Oestrogenic activity of parabens in MCF7 human breast cancer cells. J Steroid Biochem Mol Biol. 2002;80(1):49–60. doi: 10.1016/S0960-0760(01)00174-1. [DOI] [PubMed] [Google Scholar]
- 19.Darbre PD, Harvey PW. Paraben esters: review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J Appl Toxicol. 2008;28(5):561–78. doi: 10.1002/jat.1358. [DOI] [PubMed] [Google Scholar]
- 20.Boberg J, Taxvig C, Christiansen S, Hass U. Possible endocrine disrupting effects of parabens and their metabolites. Reprod Toxicol. 2010;30(2):301–12. doi: 10.1016/j.reprotox.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Witorsch RJ, Thomas JA. Personal care products and endocrine disruption: a critical review of the literature. Crit Rev Toxicol. 2010;40(Suppl 3):1–30. doi: 10.3109/10408444.2010.515563. [DOI] [PubMed] [Google Scholar]
- 22.Routledge EJ, Parker J, Odum J, Ashby J, Sumpter JP. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153(1):12–9. doi: 10.1006/taap.1998.8544. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen KL, Pedersen SN, Christiansen LB, Korsgaard B, Bjerregaard P. The preservatives ethyl-, propyl- and butylparaben are oestrogenic in an in vivo fish assay. Pharmacol Toxicol. 2000;86(3):110–3. doi: 10.1034/j.1600-0773.2000.d01-20.x. [DOI] [PubMed] [Google Scholar]
- 24.Oishi S. Effects of propyl paraben on the male reproductive system. Food Chem Toxicol. 2002;40(12):1807–13. doi: 10.1016/S0278-6915(02)00204-1. [DOI] [PubMed] [Google Scholar]
- 25.Oishi S. Effects of butyl paraben on the male reproductive system in mice. Arch Toxicol. 2002;76(7):423–9. doi: 10.1007/s00204-002-0360-8. [DOI] [PubMed] [Google Scholar]
- 26.Toxicological evaluation of certain food additives with a review of general principles and of specifications. Seventeenth report of the joint FAO-WHO Expert Committee on Food Additives. 1974/01/01 ed1974. 1–40 p. [PubMed]
- 27.Scientific committee on consumer safety. SCCS/1446/11 Clarification on Opinion SCCS/1348/10 in the light of the Danish clause of safeguard banning the use of parabens in cosmetic products intended for children under three years of age. 10 October 2011.
- 28.Liao C, Liu F, Kannan K. Occurrence of and dietary exposure to parabens in foodstuffs from the United States. Environ Sci Technol. 2013;47(8):3918–25. doi: 10.1021/es400724s. [DOI] [PubMed] [Google Scholar]
- 29.Martin CR, Ling P-R, Blackburn GL. Review of infant feeding: key features of breast milk and infant formula. Nutrients. 2016;8(5):279. doi: 10.3390/nu8050279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemaire M, Le Huërou-Luron I, Blat S. Effects of infant formula composition on long-term metabolic health. J Dev Orig Health Dis. 2018;9(6):573–89. doi: 10.1017/S2040174417000964. [DOI] [PubMed] [Google Scholar]
- 31.Wu H, Wu L-H, Wang F, Gao C-J, Chen D, Guo Y. Several environmental endocrine disruptors in beverages from South China: occurrence and human exposure. Environ Sci Pollut Res Int. 2019;26(6):5873–84. doi: 10.1007/s11356-018-3933-7. [DOI] [PubMed] [Google Scholar]
- 32.Chiesa LM, Pavlovic R, Panseri S, Arioli F. Evaluation of parabens and their metabolites in fish and fish products: a comprehensive analytical approach using LC-HRMS. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2018;35(12):2400–13. doi: 10.1080/19440049.2018.1544721. [DOI] [PubMed] [Google Scholar]
- 33.Del Olmo A, Calzada J, Nuñez M. Benzoic acid and its derivatives as naturally occurring compounds in foods and as additives: Uses, exposure, and controversy. Crit Rev Food Sci Nutr. 2017;57(14):3084–103. doi: 10.1080/10408398.2015.1087964. [DOI] [PubMed] [Google Scholar]
- 34.Gao Y, Wang Y, Yan Y, Tang K, Ding CF. Self-assembly of poly(ionic liquid) functionalized mesoporous magnetic microspheres for the solid-phase extraction of preservatives from milk samples. J Sep Sci. 2020;43(4):766–73. doi: 10.1002/jssc.201900851. [DOI] [PubMed] [Google Scholar]
- 35.Sivaraman T, Kumar TK, Jayaraman G, Yu C. The mechanism of 2,2,2-trichloroacetic acid-induced protein precipitation. J Protein Chem. 1997;16(4):291–7. doi: 10.1023/A:1026357009886. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will not be shared with a reason.
Not applicable.