Abstract
It has been demonstrated that sub-chronic exposure to air pollution containing nanoscale (˂100 nm) diesel exhaust particles (DEPs) may lead to excessive oxidative stress and neuro-inflammation in adult male mice. Hereby, we investigated the effects of DEPs on hippocampus-dependent spatial learning and neuro-inflammation and memory-related gene expression in male mice. In this study, we divided 48 adult NMRI male mice into control group VS. three exposure groups. Mice were exposed to 300-350 μg/m3 DEPs for 2, 5, and 7 h daily for 12 weeks. The Morris Water Maze (MWM) and Elevated Plus Maze device were used to examine anxiety, spatial memory and learning, respectively. The mRNAs expression of pro-inflammatory cytokines, N-methyl-D-aspartate (NMDA) receptor subunits, and glutaminase were studied in hippocampus (HI) by real-time RT-PCR. Besides, malondialdehyde (MDA) tests were used to determine the state of oxidative stress. After 5 and 7 h. of DEPs exposure, mRNA expression of NR2A and NR3B IL1α, IL1β, TNFα, NMDA receptor subunits and MDA levels increased significantly (P < 0.05). Also, DEPs exposed mice for 2, 5, and 7 h. showed diminished entrance into open arms with short time spent there. Indeed, 5 and 7 h/day exposed mice required a longer time to reach the hidden platform. Sub-chronic exposure to DEPs increased oxidative stress markers, neuroinflammation, anxiety, impaired spatial learning and memory.
Keywords: Air pollution, Diesel exhaust particles, Nanotoxicology, Neuroinflammation, Learning and memory
Introduction
Air pollution is a complex combination of solid particles, gases, metals, and organic compounds [1, 2]. Traffic-related air pollution is an important source of environmental pollution. It is estimated that 20 to 70% of environmental pollutions is due to the combustion of traffic which constitute 85% of particulate matter (PM) in urban air pollution [3]. Global estimates have depicted that daily particulate air pollution is related to an increased risk of various adverse health consequences [4, 5]. Besides, according to information derived from the World Health Organization (WHO), more than 7 million untimely deaths per year are associated with air pollution, making it the greatest environmental risk factor, worldwide. Diesel engine exhaust (DE) is an intricate combination of gases, sulfur, hydrocarbons, particulates, and heavy metals generated from combustion of diesel fuel [6]. DEPs include various combinations with potentially deleterious impacts on immune system and brain growth [7]. In 2013, the International Agency for Research on Cancer (IARC) identified DEPs as a human carcinogenic entity based on evidence originated from exposure to particulate with subsequent development of malignancies of lung and central nervous system (CNS) [8]. The precise composition of DE features depends on the operating conditions, the character of the engine, fuel composition, additives, lubricating oil, and emission control system [9]. Diesel engines are classified typically by their service requirements, and the operating conditions into heavy (such as trucks) and light-duty diesel engines (such as automobiles and light trucks) which vary regarding fuel composition, engine emission controls, expected load, and engine speed [10]. Typically, Light-duty vehicles operate at higher speeds than do heavy-duty vehicles. Regarding fuel composition and engine-control technology, light- and heavy-duty diesel engines can respectively emit 50 to 80 and 100 to 200 times as much particulate mass as typical catalyst-equipped gasoline engines [11, 12]. Almost 92% of DEPs diameter are less than 1.0 μm [13]. The elemental and organic carbon account for almost 80% of the total PM mass and the remaining is combined with sulfate (sulfuric acid) [14]. The major inorganic fraction of the DEPs is nuclei carbon particles in addition to some inorganic additives and components of motor oil and fuel [15]. Generally, the organic compounds identified in DE emissions include hydrocarbons and hydrocarbon derivatives, poly-aromatic hydrocarbons (PAHs) and PAH derivatives and PAHs multifunctional derivatives, aldehydes, heterocyclic compounds and heterocyclic derivatives and multifunctional derivatives [6]. The range of organic fraction of DEPs is often 20% to 30%, but it may be as high as 90% based on operating conditions and vehicle type [16]. DEPs could adsorb relatively major amounts of the organic material originated from lubricating oil, unburned fuel, and pyrosynthesis during fuel combustion due to their extensive surface area [17]. A variety of carcinogens and mutagens such as nitro-PAHs, and PAHs are adsorbed by DEPs [18]. Recently, it has been demonstrated that prenatal exposure to DEPs impair neurogenesis in the hippocampus of offspring adult male mice accompanied with learning and memory disorders [19]. Although DEPs have distinct chemical compositions, their sizes are within the nanometer range [20]. Such a small size allows their entry into multiple organs with subsequent development of adverse health effects. Indeed, DEPs can easily translocate into the CNS. Common routes for entrance of nano-sized particles entry into the CNS include their passage across blood-brain barrier (BBB) or retrograde transport along olfactory nerve [21, 22].
The hippocampus is crucial for spatial learning and memory acquisition, and this transmission is mainly mediated through glutamate receptors [23]. It has been reported that hippocampal NMDA-type glutamate receptors are vital for learning and memory and synaptic plasticity [24]. Transgenic studies have shown that missing NR2 subunit in Corn on Ammonis1 (CA1) of the hippocampal NMDA receptor resulted in disabled performance on the Morris water maze (MWM) test in knockout mice. Therefore, as assessed using the MWM task, it is believed that hippocampal NMDA receptor activity and NMDA receptor-associated synaptic plasticity are critical for learning and memory functions [21].
Glutaminase is an amino-hydrolyzate enzyme which produces glutamate from glutamine [25]. The Glutaminase is a tissue-specific isoenzymes that plays a vital role in glial cells. One of the most important functions of glutaminase is in the axonal ends of CNS neurons. Glutamate acts as an excitatory neurotransmitter in the CNS. After being released by presynaptic neurons, glutamate is rapidly absorbed by adjacent astrocytes and converted to glutamine. This glutamine is then returned to the presynaptic terminals of neurons, where the glutaminases turn then back to glutamate wit re-entrance into synaptic vesicles again [26]. In vitro studies using neuroglial culture also illustrated that DEPs can activate microglia and induce oxidative stress that leads to dopamine neurotoxicity [27]. Many reports indicate that intranasal instillation aerosols of nanoscale carbon black dependence of brain inflammation, and these particles capability to produce reactive oxygen species (ROS) and so have comprehensive toxicity [28].
The impacts of DEPs on memory functions and learning has not been throughout explored yet. Formerly, our laboratory has shown that sub-chronic exposure of DEPs affects oxidative stress, neuro-inflammation with subsequent induction of behavioral changes such as depression [21]. This study aimed to evaluate sub-chronic exposure to DEPs. In this work, we assumed that sub-chronic exposure to 300–350 μg/m3 of nanoscale (˂ 100 nm) DEPs would induce neuro-inflammation through the induction of pro-inflammatory cytokines in normal individuals. The neuroimmune system, especially the microglia, may be activated by these inflammatory signals. Activated microglia may release send toxic substances such as ROS, pro-inflammatory cytokines, and excitatory neurotransmitters (e.g., glutamate). Excessive amounts of glutamate may induce abnormal hippocampal NMDA receptor activation; which in turn may lead to impaired spatial memory and learning activity. Thus, in this study, we aimed to evaluate the effect of DEPs exposure for 2 h, 5 h, and 7 h per day during 12 weeks in adult male mice on anxiety and learning and memory function-related hippocampal gene expressions.
Materials and methods
DEPs collection and extraction
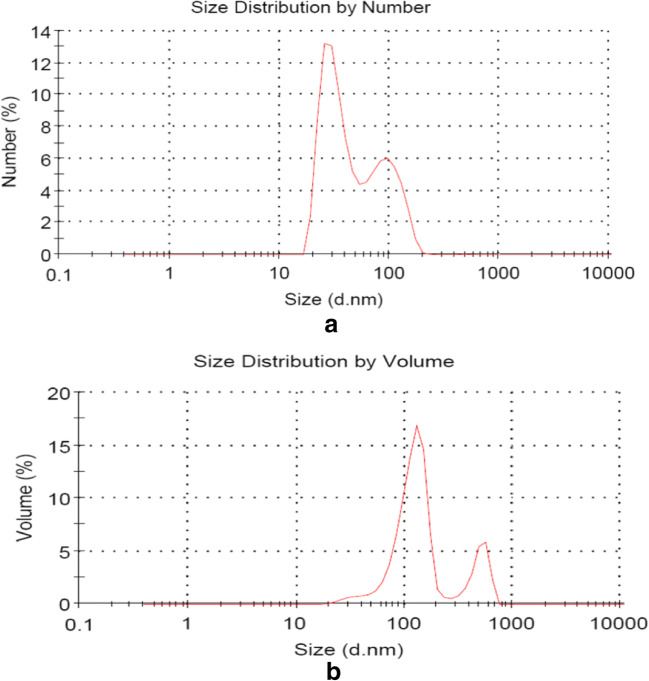
The engine used for collection of DEPs was a light-weight pickup truck (4-cylinder diesel engine, 2776 cc) that uses standard diesel fuel with a 10 kg/ m torque at a speed of 1500 rpm. The exhaust gas entered a dilution tunnel made of stainless steel (300 × 5800 mm). Dynamic light scattering (DLS) measurements to determine the particle size distribution of DEPs in the suspension were performed using a Zetasizer Nano-ZS system (Malvern Instruments Ltd., UK) (Fig. 1a and b). The derived DEPs were suspended at 1 mg/mL in an isotonic sodium chloride solution and then vortexed for 5 min and sonicated for 30 min before application [19, 21].
Fig. 1.
Peaks of the size distribution of DEPs were detected by the Zetasizer Nano-ZS system. a Size vs. number and (b) Size vs. volume
Animal exposure and ethics considerations
48- adult NMRI male mice (between 7 and 8-week-old) were purchased from animal laboratory facilities, Kashan University of medical sciences, Kashan, Iran. Mice were maintained with unlimited access to food and water in a room with 35–40% humidity at 23 °C temperature and a 12 h dark-light cycle (light on at 6:00 a.m.). All animal experiments were performed under the guidance of the National Council for Research and Care of Laboratory Animals, approved by the National Institutes of Health and the Comics Ethics Committee for research purposes. Mice were randomly assigned into case and control groups (n = 12 per group in a separate mesh cage). Control groups were just exposed to saline solution. The case mice were maintained in controlled environmental conditions (temperature, 21 ± 0.5 °C; humidity, 55–60%) in a closed systemic room (1 m3) and exposed to 300-350 μg/m3 DEPs for 2, 5, and 7 h. (8 AM to 3 PM)/day in a closed system chamber, connected to an ultrasonic nebulizer (NE-UO7; Omron Corporation, Tokyo, Japan) with an output of 1 mL/min.
At the end of the last behavioral tests, mice were sacrificed after induction of sedation using sodium pentobarbital (65 mg/kg body weight, intraperitoneal) and some of their brain regions such HI were dissected immediately, flash-frozen in liquid nitrogen, and stored at −80 °C for assessment of malondialdehyde, mRNA expression of inflammatory cytokine in HI, and mRNA expression in the HI.
Behavioral experiments
Elevated plus-maze
To investigate anxiety in mice, the elevated plus-maze was used. The elevated plus-maze device contains four 50 cm × 10 cm arms and a 10 cm × 10 cm central platform which rises 60 cm from the ground. Two opposing arms are open and the other two arms surrounded by a 40-cm wall. As a rule, an entrance is defined as having four paws on the arm. The tests were conducted in a room with moderate lighting. Each mouse was placed facing the open arm in the central platform and left for 5 min to explore the maze. The measured parameters are the time in open arms (OAT) and the number of entrances into open arms (OAE). The percentages of OAT and OAE are calculated as follow:
Spatial memory and learning performance
Spatial memory and learning were assessed using a MWM setup with a water maze (100 cm) and a platform (10 cm). The platform was placed at a fixed location in the center of one of the four maze quadrants. The starting point was set at the midpoint of the quadrant opposite to the platform quadrant. Milk powder was added to the water to form an opaque solution. The water temperature was set at 22 ± 1 °C. All mice were trained before DEPs administration, which consisted of 3 repeated trials per day (inter-trial interval 30–60 min) starting at 9 am for 4 consecutive days when mice were released at the starting point. Mice were allowed to spend the 90s to find out the hidden escape platform on water and stay there for 10s. If mice could not find the platform within 90s, the experimenter helped them locate this platform and the mice stayed there for 10s. Mice were placed in their cages after each trial. One day after the cessation of training experiments, mice began to receive DEPs administration. Next, another session of navigation tests was performed after DEPs administration (three times per day for 4 consecutive days), starting at 9 am. Mice were released from the starting point and allowed to swim to arrive at the platform or for up to 90s. Distance traveled, swim speed, and escape latency were recorded for each trial. On the fifth day, the probe trial was performed using the same procedure described above after the removal of the platform. Time spent in each quadrant, frequencies of entering the platform area, and swim speed were recorded using a fixed overhead camera and tracking system.
Measurement of MDA in the hippocampus
Frozen brain-region samples were thawed and homogenized in SBB. To obtain a 0.350 ml of tissue, the homogenate was added into 0.550 ml of 5% trichloroacetic acid (TCA) and 0.100 ml of 500 ppm butylated hydroxytoluene (BHT) in methanol. The sample in a boiling water bath was then heated for 30 min. The sample was centrifuged after cooling on ice. The supernatant fraction was mixed 1:1 with saturated thiobarbituric acid (TBA). The samples were again heated for 30 min in a boiling water tub. After cooling it on ice, 0.50 ml of each sample was extracted using 1 ml of n-butanol and centrifuged to facilitate the separation phase. MDA levels were measured using the Duo Sets ELISA test. The excitation and the emission wavelengths were set at 540 nm and 640 nm, respectively. The concentration of the TBA-MDA complex in the mixture was determined using a calibration curve obtained from a malondialdehyde bis (dimethyl acetal) standard solution.
Total RNA isolation
Immediately, after finishing the behavioral tests, the mice were eluted under deep pentobarbital anesthesia and their brain samples were taken and maintained at the temperature of −80 °C before analyzing their mRNA expression patterns. The RNA was extracted from the HI samples using RNX- Plus kits (SinaClon).
Quantification of the hippocampal mRNA expression levels
The purity and quantity of total RNA were assessed and calculated using the NanoDrop RNA Assay protocol (Thermo Scientific NanoDrop One C, USA). Then, the first-strand cDNA was synthesized per sample (one μg) using Takara Kit reverse transcriptase (Takara Bio USA, Inc.), per manufactured protocol. Afterward, the mRNA expression of IL-1α, IL-1β, IL-6, TNF-α, HO1, nNOS, NR2A, and NR2B in the HI was assessed. The expression level of target genes was estimated comparatively after normalization against the housekeeping gene, Hypoxanthine phosphoribosyltransferase (HPRT). The primer sequences used in this study are listed in Table 1. Quantitative real-time RT-PCR was performed using SYBR Green Real-Time PCR Master Mix (Toyobo Co., Ltd., Osaka Japan) in a BioRad Real-Time machine. The amplification of the target gene was monitored by measuring each sample’s fluorescence intensity during each cycle.
Table 1.
Primer sequences for qRT-PCR assays
| Gene | Sequence | Tm | |
|---|---|---|---|
| HPRT | Hypoxanthine phosphoribosyltransferase |
Forward: 5’GCT GGT GAA AAG GAC CTC T-3’ Reverse: CAC AGG ACT AGA ACA CCT GC-3’ |
60 |
| NR2A | N-methyl-D-aspartate receptor subunit 2A |
Forward: 5’-AGCCCCCTTCGTCATCGTAGA-3’ Reverse: 5’-ACCCCTTGCAGCACTTCTTCAC-3’ |
60 |
| NR2B | N-methyl-D-aspartate receptor subunit 2B |
Forward: 5’-GCCATGAACGAGACTGACCC-3’ Reverse: 5’-GCTTCCTGGTCCGTGTCATC-3’ |
60 |
| NR3B | N-methyl-D-aspartate receptor subunit 3B |
Forward: 5’-TCCTACTCCTCCGCTCTCAA-3’ Reverse: 5’-TGGATTCCAGACAGCTCCTC-3’ |
60 |
| Glutaminase | Glutamate metabolism |
Forward: 5’-GACTTCTCAGGGCAGTTTGC-3’ Reverse: 5’-TCCTTCTCTCCGAGGATCAA-3’ |
60 |
| TNF-α | tumor necrosis factor alpha |
Forward: 5’-GGCCTTCCTACCTTCAGACC-3’ Reverse: 5’-AGCAAAAGAGGAGGCAACAA-3’ |
58 |
| IL-1β | Interleukin 1β |
Forward: 5’-CAG CTC ATA TGG GTC CGA CA-3’ Reverse: 5’-CTG TGT CTT TCC CGT GGA CC-3’ |
60 |
| IL-1α | Interleukin 1α |
Forward: 5’-GCAACGGGAAGATTCTGAAG-3’ Reverse: 5’-TGACAAACTTCTGCCTGACG-3’ |
60 |
Nissl staining
Histological examinations after DEPs exposure was performed using an optical microscope (Nikon, Japan) after Nissl staining. For Nissl staining, 5-μm of coronal sections were first prepared. These sections were deparaffinized in xylene and then hydrated. After rinsing with tap water and then distilled water, sections were stained with 0.1% cresyl violet for 3 min. After dehydration, samples were finally placed under a glass coverslip. The Nissl body was dyed purple-blue.
Statistical analysis
Data are expressed as mean ± SEM unless indicated otherwise. The significance threshold was set at P- values<0.05. The 2-ΔΔCt method was used to analyze the qRT-PCR results. Statistical comparisons were performed using repeated-measurement ANOVA for behavioral tests and one-way ANOVA with Bonferroni correction for multiple comparisons. Statistical analysis was performed in Prism ver.7.3 software.
Results
There was no signifiant difference between control VS. case adult male mice exposed to 300–350 μg/m3 DEPs for 12 weeks regarding body length (P = 0.083), body weights (P = 0.071), vibrissae, and sensorimotor responses (p < 0.05).
Behavioral experimental
The impact of DEPs exposure on anxiety
The elevated plus-maze test was used to measure the anxiety of mice exposed to DEP. Anxiety levels are usually calculated based on the two factors of open arm entry and elapsed time in the arms. Our data demonstrated a significantly decreased ability to enter into the open arms accompanied with shorter elapse time in this state in male mice exposed to DEPs at 2, 5, and 7 h. compared to control mice (Fig. 2a, b), P < 0.05). Consequently, reduced number of open arm entrance and decrease time spent in this state, means development of more anxiety in this maze.
Fig. 2.
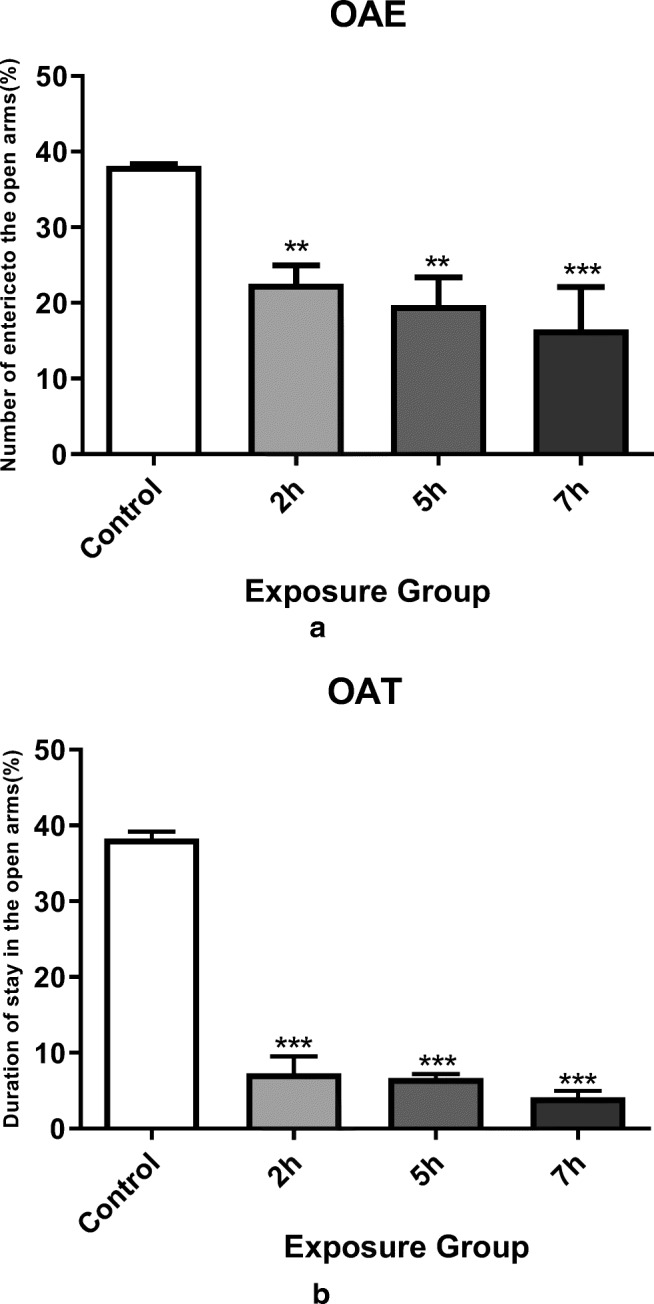
Elevated plus-maze navigation for different groups of mice. a Percentages of open arms entered by different groups of mice when searching for this maze. Exposure to DEPs in all exposure groups significantly reduced arm penetration. b Percentage of time spent in open arms while navigating the maze. All exposure groups showed a significant decrease in open arm command compared with control group. *P < 0.05, **P < 0.01, ***P < 0.001. Data are shown as mean 12 SEM
The impact of DEPs exposure on spatial learning ability
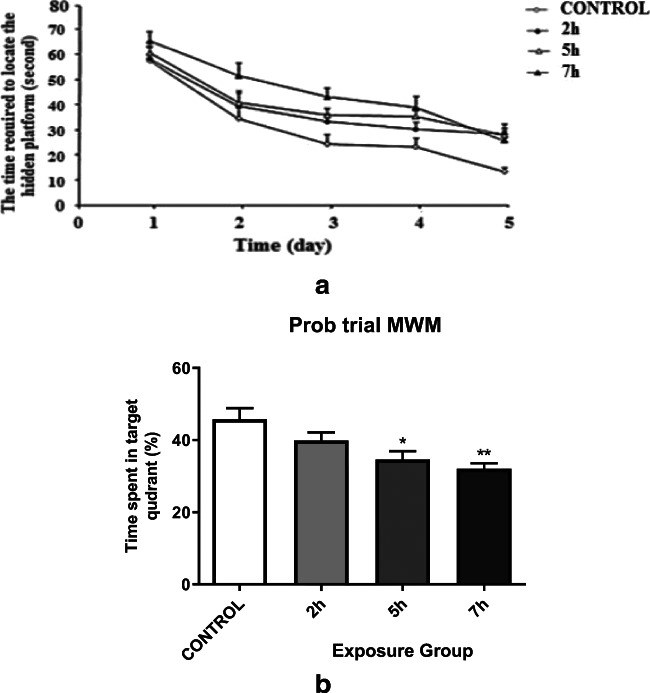
Through two maze navigation strategies, training and probe trial, spatial memory, and learning of mice exposed to DEPs were examined. To investigate hippocampal-dependent spatial memory and learning, we conducted a MWM test. Despite similar swimming speeds across groups (data not shown), four groups of mice introduced into the training phase showed different performance in maze task learning using analysis of variance (P < 0.0001). During 4 days of the experiment, all mice improved their performance. Follow- up data showed a significantly diminished behavioral performance in 5- and 7- h. exposure mice compared to control mice (P = 0.03 and P < 0.0001, respectively). Meanwhile, the 2-h. exposure group did not demonstrate significantly lower performance VS. control group (Fig. 3a). Probe experiment illustrated significantly altered reference memory in DEPs exposed mice compared to control mice (Fig. 3b). After platform removal, we have measured the time spent in the correct maze quadrant. Results showed that regarding the learning phase, the 5- and 8- h. groups had a smaller area in the correct quadrant compared to the control group (P = 0.002 and P < 0.0001, respectively). The overall changes were shown by statistical analysis between the exposure groups (P < 0.0001) in task. The values for the groups are 17.1 + 1.47 s and 22.2 + 1.31 s for group 5 h and group 7 h, respectively. Although the behavior of exposed mice in the 2-h. group was similar to the control group, there was a significant difference between the 2- h. compared to 5-h exposed groups (P < 0.015 and P < 0.0001, respectively).
Fig. 3.
a Spatial learning performance in the Morris water maze during the acquisition phase of adult male mice exposed to 2 h/day, 5 h/day, and 7 h/day for 12 weeks when compared with the control group (P < 0.038 and P < 0.001, respectively). b Memory retention during the probe trial of adult male mice exposed to Control, 2 h/day, 5 h/day, and 7 h/day for 12 weeks when compared with the control group. *P < 0.05, **P < 0.01. Data are shown as mean 10 SEM
The impacts of DEPs exposure on MDA in the hippocampus
MDA levels also increased immediately in all exposure groups (P < 0.05). A significant change in the MDA level was observed between the 2- and 7- h exposed groups as well as the 2- and 7- h. exposed groups (P < 0.001) (Fig. 4).
Fig. 4.
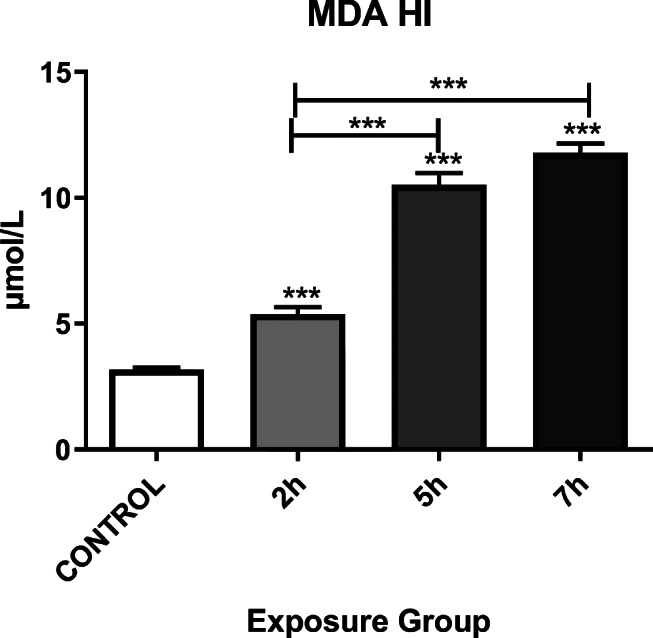
Alteration of the MDA in the hippocampi of mice exposed to Control, 2, 5, or 7 h. for 12 weeks. *P < 0.05, **P < 0.01, ***P < 0.001. Each bar represents the mean ± SE (n = 6)
The impacts of DEPs exposure on hippocampal NMDA receptor subunits
In addition to the impacts of DEPs exposure on spatial memory and learning, we also examined the impacts of DEPs exposure on hippocampal NMDA receptor subunit mRNA expression in adult mice. We observed significantly enhanced NR2A and NR3B mRNA expression in 5- and 7- h. exposed groups compared to the control group (Fig. 5a, c) (P < 0.05). However, the expression level of NR2B mRNA was not significantly different between control VS. exposed groups (Fig. 5b).
Fig. 5.
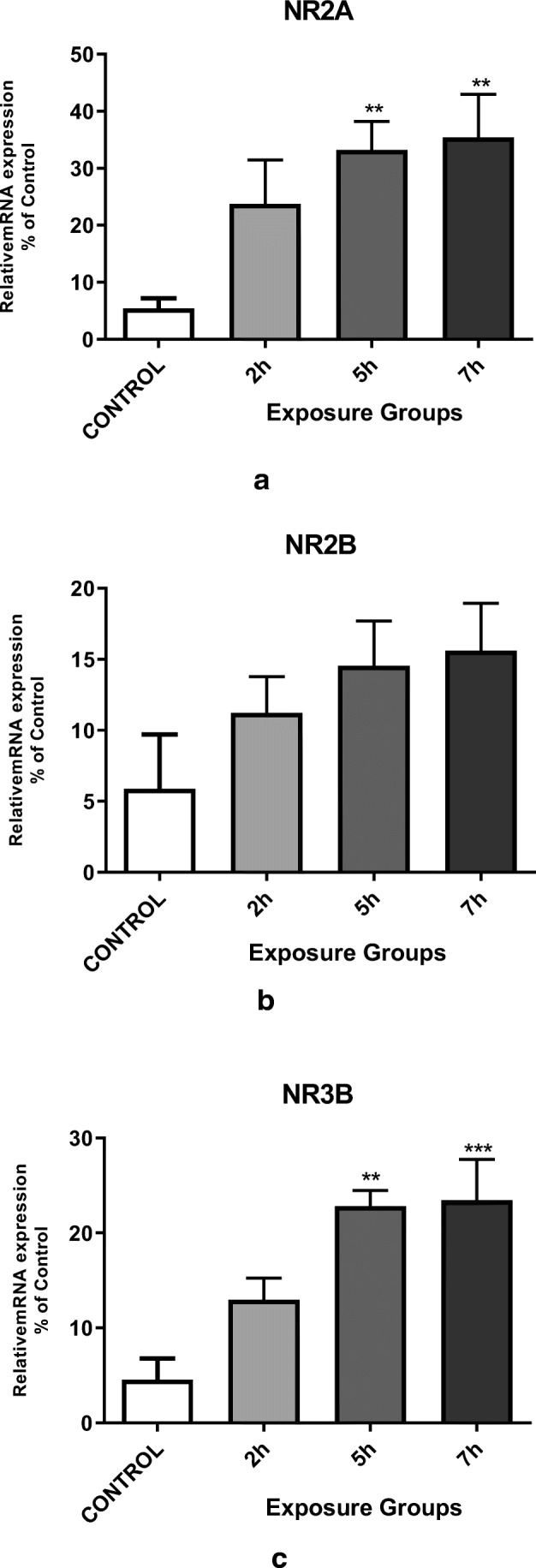
Messenger RNA expression of the hippocampal NMDA receptor subunits (a) NR2A, b NR2B, and c NR3B in the exposure groups (2, 5, or 7 h. per day for 12 weeks) and control mice. *P < 0.05, **P < 0.01, ***P < 0.001. Each bar represents the mean ± SE (n = 6)
The impacts of DEPs exposure on hippocampal mRNA expression of Glutaminase
We have evaluated the impacts of DEPs exposure on hippocampal glutamine mRNA expression in mice. There was a significantly increased hippocampal glutaminase mRNA expression in 5- and 7- h. groups than in the control group (P < 0.05). However, we did not observe any significant difference between the 5- and 7- h. groups (Fig. 6) (P < 0.05).
Fig. 6.
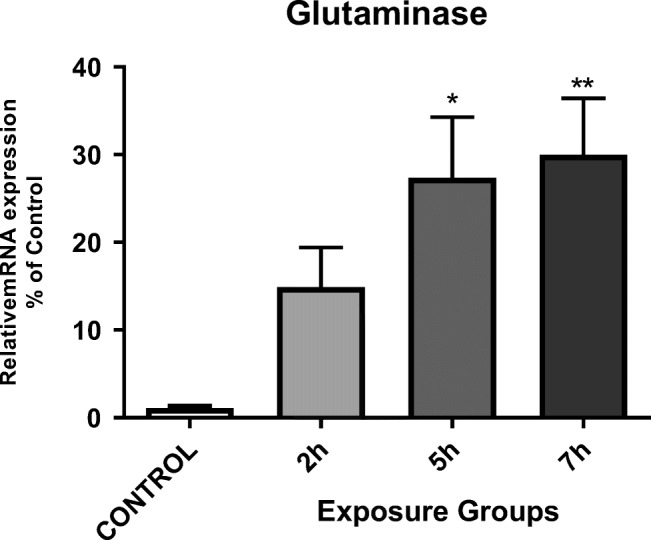
Messenger RNA expression of glutaminase in the hippocampi of mice exposed to DEPs (2, 5, or 7 h per day for 12 weeks) compared with control mice. *P < 0.05, **P < 0.01, ***P < 0.001. Each bar represents the mean ± SE (n = 6)
Effect of DEPs exposure on hippocampal cytokine mRNA expression
We have assessed the expression of pro-inflammatory hippocampal cytokines, such as IL1α, TNF-α, and IL1β, in adult male mice that were exposed to DEPs for 12 weeks. The mRNA levels of invasive cytokines such as TNF-α, IL1β, and IL1α were significantly higher in the 5- and 7- h. groups (P < 0.05). We observed a significant change in IL1α mRNA expression between 2- and 7- h. groups, as well as 2- and 5- h. groups. There was also a significant change in the expression levels of TNF-α and IL1β mRNA between the 2- and 7- h. groups (Fig. 7) (p < 0.001).
Fig. 7.
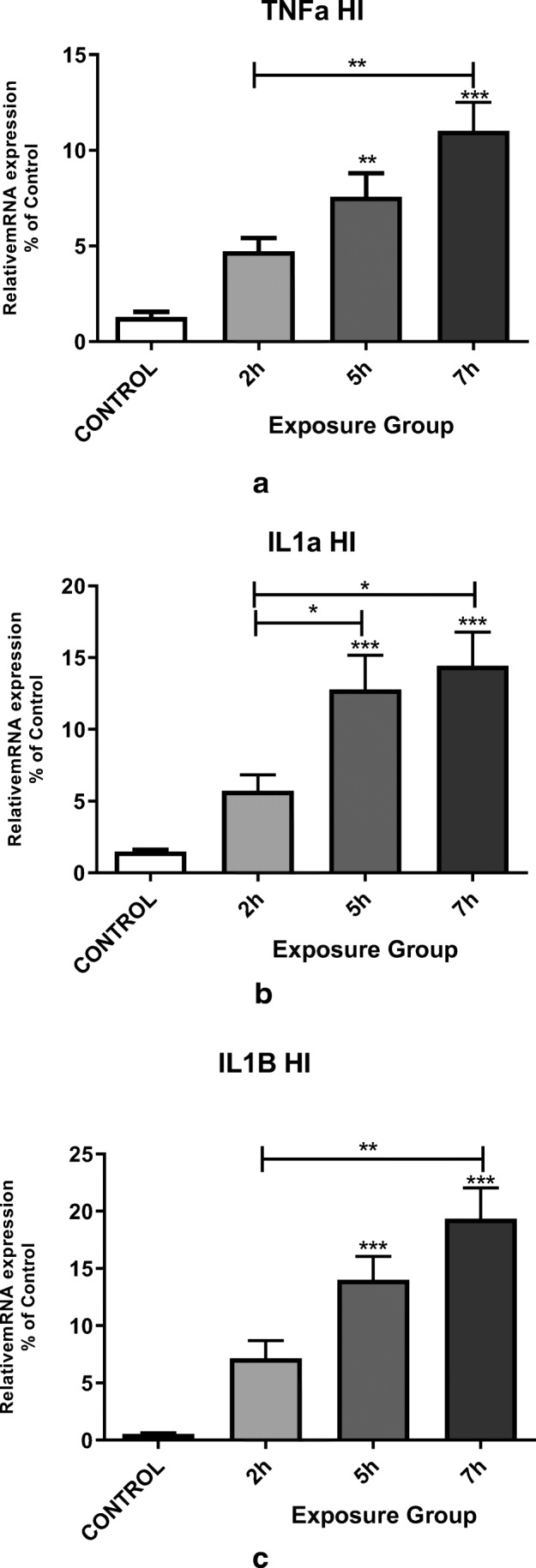
Messenger RNA expression of the pro-inflammatory cytokines (a) TNFα, (b) IL1α, and (c) IL1β in the hippocampi of mice exposed to DEPs (2, 5, or 7 h per day for 12 weeks) compared with control mice. *P < 0.05, **P < 0.01, ***P < 0.001. Each bar represents the mean ± SE (n = 6)
Hippocampal morphological analysis
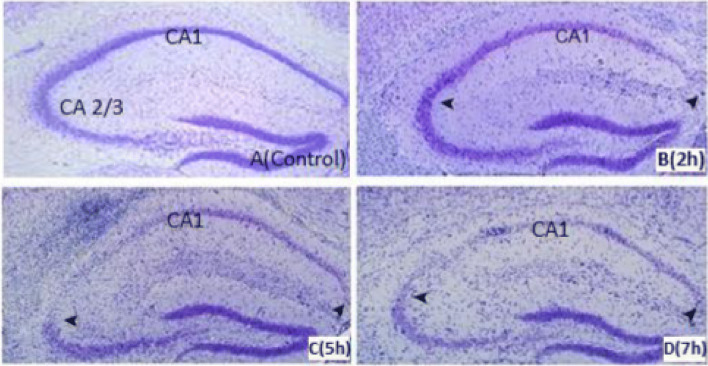
NISL staining in HI of control and mice exposed to DEPs 2-, 5- and 7- h. per day for 12 weeks indicated altered neuronal morphology in CA1 and CA3 regions of the hippocampus especially with more than two hrs. of exposure per day during long term period (Fig. 8) (P < 0.05) (data not shown).
Fig. 8.
Nissl staining in the hippocampus of the Control (a) and 2, 5, and 7 h/days exposure to DEPs for 5 days/week within 12 weeks. By 5 h/d exposure, neurons of CA1and CA3 region are lost in the mouse (c) but minimal neuronal dropout is evident in the 3 h/day exposure mouse (b). By 7 h/d exposure, the majority of CA1 and CA3 neurons are lost in mice (d). Hippocampal CA1 and CA3 neurons were loose and absent; nucleoli were missing or indistinct (arrows). In the control group, neurons were fairly well preserved and sparse in the CA1 and CA3 sub-region. Sections are representative of at least 4 mice of each group
Discussion
The main finding of this study was that 12 weeks of DEPs exposure would affect anxiety, spatial memory, and up-regulate NMDA receptor subunits of NR2A, NR3B, TNF-α, IL1α, IL1β, and glutamine Enzyme expression in the HI of adult male mice. Impaired learning and memory in male mice was also associated with enhanced expression of hippocampal NR2A and NR3B following exposure to DEPs. The dose used in this study did not significantly affect mice body weight. However, diminished performance in MWM test suggests impaired memory and learning ability.
Activated microglia secrete inflammatory cytokines and chemokines which contributes to development of chronic inflammatory brain diseases [29]. Upon inflammation, these resident CNS immune cells can modify synaptic connectivity essential for learning and memory processes [30]. Another study indicated that rodents exposed to DEPs and lipoteichoic acid (LTA) took longer time to attain the hidden plateau in the MWM test accompanied with upregulated mRNA expression of pro-inflammatory cytokines and NMDA receptor subunits. It was also shown that co-exposure of DEPs and LTA caused glutamate-induced neurotoxicity, followed by changes in the expression of related kinases, transcription factors, hippocampal NMDA receptor subunits and olfactory bulb in the mouse [31].
In line with these reports, our results have linked diminished learning and memory to increased inflammation, oxidative stress, and altered expression of NMDA receptor subunits in mice hippocampus upon DEPs exposure. In one study, it was demonstrated for the first time that co- exposure with bacterial cell wall and DEPs component LTA for one month correlated with altered expression level of NMDA receptor subunits in the hippocampus with subsequent poor spatial memory and learning in male mice [32]. In female rodents, estrogen acts on the brain to induce reproductive behaviors, including female-like behavioral properties such as lordosis, as well as non-reproductive behaviors, including anxiety and depression-like behaviors, and cognitive behavior [33, 34]. Thus in this study, we limited the experiment to male mice, during DEPs exposure. Morris water maze test indicate that learning and reference memory are impaired in male mice exposed to DEPs, which requires a fully functional HI. Besides, hippocampal NR2A and NR3B expression were significantly higher in adult male mice exposed to DEPs than in control mice. In rodents, the hippocampus has always been considered as a key structure for coding spatial information [35]. Oxidative stress can lead to stress-induced learning and memory disorders [36]. Oxidative damage induced by oxidative stress was associated with a significant decrease in glucocorticoid receptors in the CA1 region of the HI [37]. It has been suggested that oxidative stressors increase glucocorticoid levels and impair cognitive function due to damaging the HPA axis [38]. As several researchers have demonstrated, chronic stress exposure or glucocorticoids can lead to oxidative damage in the HI with subsequently impaired learning and memory [39]. In rats, the volume of dorsal hippocampal tissue injury is related to the extent of spatial learning deficits. Indeed, dorsal HI lesions cause more severe injury than ventral HI lesions and CA1 neurons are essential for learning [40]. In male mice hippocampus, treated with high-dose of DEPs for one month, the mRNA expression level of the TNF-α was elevated [32]. TNF-α is reported to be a key pro-inflammatory cytokine through autocrine [41]. The way to stimulate the release of a wide range of microglia glutamate up-regulates glutaminase, which results in excitation neurotoxicity [42]. In this study, we found an increase in IL6, IL1α, and TNFα mRNA levels in exposed mice that maybe due to chronic DEPs exposure related to the activation of different signal transduction pathways in NMDA receptor-associated memory. A significant change in the level of TNFα expression was also observed. Indeed, mRNA expression of cytokines related with DEPs exposure-induced neuro-inflammation was significantly increased in the 6- and 8- h groups. This finding suggests that mere DEPs exposure for 12 weeks may induce inflammation of the mouse hippocampus. We have reported that morphological alterations of hippocampal neurons in CA1 and CA3 regions were associated with up-regulation of inflammatory cytokines gene expression in the setting of sub-chronic DEPs exposure. Changes in the morphology of CA3 accompanied with disrupted learning and spatial memory have been previously reported in association with low-grade inflammation [43]. These findings are the same to our previous study in which significant pathological lesions in the hippocampal CA1 region of adult offspring male mice were observed due to maternal exposure to inhaled DE [19]. In the HI, NMDA receptors play an important role in spatial learning and memory tasks [44]. Significantly enhanced expression of NR1 subunits in the male mice hippocampus was demonstrated after one month of exposure to high doses of DEPs and bacterial cell wall components compared to the expression of NR2A and NR2B subunits [32]. Also, co-exposure with high-dose DEPs and bacterial cell wall components in the same month affected extracellular glutamate levels and expression of NR1, NR2A, and NR2B mRNA in the olfactory bulb in male mice [31]. In this study, DEPs exposure in adult male mice significantly increased hippocampal NR2A and NR3B expression, which may be related to the spatial memory and learning deficits. The NR2B subunit is required for fetal neuronal patterning and fetal survival. Although DEPs exposure did not significantly affect mRNA NR2B expression [45], NR2A subunit expression and synaptic binding gradually increased throughout development [40, 41]. In fact, synaptic NR plays a key role during brain development, plasticity, and pathology [46]. The insertion of NRs into synaptic sites follows a different mechanism, depending on the receptor subunit composition. With increasing levels of NR2B gene expression, synaptic insertion of receptors containing NR2B does not increase, while synaptic insertion of receptors containing NR2A, that is essential for synaptic activity, is raised by increased expression of the NR2A mRNA. Therefore, exposure to DEP may affect the insertion of NR2A and NR3B into hippocampal synapses since NR2A and NR3B expression is increased in the HI. According to the results derived from epidemiological studies, exposure to high concentration of DEPs might result in Alzheimer’s disease [47]. Our results highlight the need to identify measures to prevent and control the impacts of DEPs exposure on development of cognitive function. The living environment makes sense to prevent the development of DEPs. A rich environment can also prevent hippocampal impairment [48, 49]. In this study, it was found that the level of neurotransmitters was not determined. We believe that impairment of learning ability and spatial memory was due to excitatory neurons, especially glutamate, that induce neuronal damage to intercede by abnormal activation of specific NMDA receptor subunits in the HI. Up-regulation of mRNA expression of NMDA receptor subunits NR2A, NR3B, and glutaminase may be caused by a compensatory response to neuronal damage. Further investigations are needed to determine measures to prevent the impacts of DEPs exposure on recognition through early environmental enrichment or interventions.
Conclusions
In conclusion, DEPs exposure induced neuro-inflammation, and anxiety as well as altered NR2A and NR3B expression associated with hippocampus-dependent spatial learning and memory of adult mice. Somehow, we are aware that DEPs exposure time used in this study would jeopardize human cognitive function and further research is needed to elucidate the impacts of chronic DEPs exposure on NMDA receptor, memory, and learning function in rodents.
Acknowledgments
We thank Dr. Ehsanifar Lab. for technical assistance. This work was supported by Anatomical Sciences Research Center, Kashan University of Medical Sciences, Kashan, Iran.
Data availability
The dataset used in this study is available with the authors and can be made available upon request.
Compliance with ethical standards
Competing interests
The authors declared that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mojtaba Ehsanifar, Email: Ehsanifar@gmail.com.
Ahmad Jonidi Jafari, Email: ahmad_Jonidi@yahoo.com.
References
- 1.Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr, Whitsel L, Kaufman JD, American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism, Particulate matter air pollution and cardiovascular disease. Circulation, 2010. 121(21): p. 2331–2378. [DOI] [PubMed]
- 2.Xu F, et al. Investigation of the chemical components of ambient fine particulate matter (PM2. 5) associated with in vitro cellular responses to oxidative stress and inflammation. Environ Int. 2020;136:105475. doi: 10.1016/j.envint.2020.105475. [DOI] [PubMed] [Google Scholar]
- 3.Jonidi Jafari A, Ehsanifar M. The share of different vehicles in air pollutant emission in Tehran, Using 2013 traffic information. Caspian Journal of Health Research. 2016;2(2):28–36. [Google Scholar]
- 4.Burnett R, Chen H, Szyszkowicz M, Fann N, Hubbell B, Pope CA, III, Apte JS, Brauer M, Cohen A, Weichenthal S, Coggins J, di Q, Brunekreef B, Frostad J, Lim SS, Kan H, Walker KD, Thurston GD, Hayes RB, Lim CC, Turner MC, Jerrett M, Krewski D, Gapstur SM, Diver WR, Ostro B, Goldberg D, Crouse DL, Martin RV, Peters P, Pinault L, Tjepkema M, van Donkelaar A, Villeneuve PJ, Miller AB, Yin P, Zhou M, Wang L, Janssen NAH, Marra M, Atkinson RW, Tsang H, Quoc Thach T, Cannon JB, Allen RT, Hart JE, Laden F, Cesaroni G, Forastiere F, Weinmayr G, Jaensch A, Nagel G, Concin H, Spadaro JV. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci. 2018;115(38):9592–9597. doi: 10.1073/pnas.1803222115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Møller KL, Brauer C, Mikkelsen S, Bonde JP, Loft S, Helweg-Larsen K, Thygesen LC. Cardiovascular disease and long-term occupational exposure to ultrafine particles: a cohort study of airport workers. Int J Hyg Environ Health. 2020;223(1):214–219. doi: 10.1016/j.ijheh.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Steiner S, Bisig C, Petri-Fink A, Rothen-Rutishauser B. Diesel exhaust: current knowledge of adverse effects and underlying cellular mechanisms. Arch Toxicol. 2016;90(7):1541–1553. doi: 10.1007/s00204-016-1736-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki T, Oshio S, Iwata M, Saburi H, Odagiri T, Udagawa T, Sugawara I, Umezawa M, Takeda K. In utero exposure to a low concentration of diesel exhaust affects spontaneous locomotor activity and monoaminergic system in male mice. Particle and fibre toxicology. 2010;7(1):7. doi: 10.1186/1743-8977-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danysh HE, Mitchell LE, Zhang K, Scheurer ME, Lupo PJ. Traffic-related air pollution and the incidence of childhood central nervous system tumors: Texas, 2001–2009. Pediatr Blood Cancer. 2015;62(9):1572–1578. doi: 10.1002/pbc.25549. [DOI] [PubMed] [Google Scholar]
- 9.Ullman TL. Investigation of the effects of fuel composition on heavy-duty diesel engine emissions. SAE Trans. 1989:833–51.
- 10.Reşitoğlu İA, Altinişik K, Keskin A. The pollutant emissions from diesel-engine vehicles and exhaust aftertreatment systems. Clean Techn Environ Policy. 2015;17(1):15–27. [Google Scholar]
- 11.McClellan R. Toxicological effects of emissions from diesel engines. Dev Toxicol Environ Sci. 1986;13:3–8. [PubMed] [Google Scholar]
- 12.Pronk A, Coble J, Stewart PA. Occupational exposure to diesel engine exhaust: a literature review. Journal of exposure science and environmental epidemiology. 2009;19(5):443–457. doi: 10.1038/jes.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujita EM, Watson JG, Chow JC, Magliano KL. Receptor model and emissions inventory source apportionments of nonmethane organic gases in California's San Joaquin Valley and San Francisco Bay Area. Atmos Environ. 1995;29(21):3019–3035. [Google Scholar]
- 14.Offenberg JH, Baker JE. Aerosol size distributions of elemental and organic carbon in urban and over-water atmospheres. Atmos Environ. 2000;34(10):1509–1517. [Google Scholar]
- 15.Pierson WR, Brachaczek WW. Particulate matter associated with vehicles on the road. II Aerosol Science and Technology. 1982;2(1):1–40.
- 16.Dutcher J, et al. Generation and characterization of radiolabeled diesel exhaust. Am Ind Hyg Assoc J. 1984;45(7):491–498. doi: 10.1080/15298668491400142. [DOI] [PubMed] [Google Scholar]
- 17.Sturm R. Deposition of diesel exhaust particles in the human lungs: theoretical simulations and experimental data. Cancer. 2017;31:32. [Google Scholar]
- 18.Tokiwa H, Ohnishi Y, Rosenkranz HS. Mutagenicity and carcinogenicity of nitroarenes and their sources in the environment. CRC Crit Rev Toxicol. 1986;17(1):23–58. doi: 10.3109/10408448609037070. [DOI] [PubMed] [Google Scholar]
- 19.Ehsanifar M, Jafari AJ, Nikzad H, Zavareh MS, Atlasi MA, Mohammadi H, Tameh AA. Prenatal exposure to diesel exhaust particles causes anxiety, spatial memory disorders with alters expression of hippocampal pro-inflammatory cytokines and NMDA receptor subunits in adult male mice offspring. Ecotoxicol Environ Saf. 2019;176:34–41. doi: 10.1016/j.ecoenv.2019.03.090. [DOI] [PubMed] [Google Scholar]
- 20.Lankoff A, Brzoska K, Czarnocka J, Kowalska M, Lisowska H, Mruk R, Øvrevik J, Wegierek-Ciuk A, Zuberek M, Kruszewski M. A comparative analysis of in vitro toxicity of diesel exhaust particles from combustion of 1st-and 2nd-generation biodiesel fuels in relation to their physicochemical properties—the FuelHealth project. Environ Sci Pollut Res. 2017;24(23):19357–19374. doi: 10.1007/s11356-017-9561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehsanifar M, Tameh AA, Farzadkia M, Kalantari RR, Zavareh MS, Nikzaad H, Jafari AJ. Exposure to nanoscale diesel exhaust particles: oxidative stress, neuroinflammation, anxiety and depression on adult male mice. Ecotoxicol Environ Saf. 2019;168:338–347. doi: 10.1016/j.ecoenv.2018.10.090. [DOI] [PubMed] [Google Scholar]
- 22.Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roqué PJ. Neurotoxicity of traffic-related air pollution. Neurotoxicology. 2017;59:133–139. doi: 10.1016/j.neuro.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tombaugh GC, Rowe WB, Chow AR, Michael TH, Rose GM. Theta-frequency synaptic potentiation in CA1 in vitro distinguishes cognitively impaired from unimpaired aged Fischer 344 rats. J Neurosci. 2002;22(22):9932–9940. doi: 10.1523/JNEUROSCI.22-22-09932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeuchi T, Duszkiewicz AJ, Morris RG. The synaptic plasticity and memory hypothesis: encoding, storage and persistence. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369(1633):20130288. doi: 10.1098/rstb.2013.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newsholme P, Lima MMR, Procopio J, Pithon-Curi TC, Doi SQ, Bazotte RB, Curi R. Glutamine and glutamate as vital metabolites. Braz J Med Biol Res. 2003;36(2):153–163. doi: 10.1590/s0100-879x2003000200002. [DOI] [PubMed] [Google Scholar]
- 26.Botman D, Tigchelaar W, Van Noorden CJ. Determination of phosphate-activated glutaminase activity and its kinetics in mouse tissues using metabolic mapping (quantitative enzyme histochemistry) Journal of Histochemistry & Cytochemistry. 2014;62(11):813–826. doi: 10.1369/0022155414551177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Block M, et al. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. FASEB J. 2004;18(13):1618–1620. doi: 10.1096/fj.04-1945fje. [DOI] [PubMed] [Google Scholar]
- 28.Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang M, Oberley T, Froines J, Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003;111(4):455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shemer A, Erny D, Jung S, Prinz M. Microglia plasticity during health and disease: an immunological perspective. Trends in Immunology. 2015;36(10):614–625. doi: 10.1016/j.it.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y, Dissing-Olesen L, MacVicar BA, Stevens B. Microglia: dynamic mediators of synapse development and plasticity. Trends in immunology. 2015;36(10):605–613. doi: 10.1016/j.it.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Win-Shwe T-T, Mitsushima D, Yamamoto S, Fujitani Y, Funabashi T, Hirano S, Fujimaki H. Extracellular glutamate level and NMDA receptor subunit expression in mouse olfactory bulb following nanoparticle-rich diesel exhaust exposure. Inhal Toxicol. 2009;21(10):828–836. doi: 10.1080/08958370802538068. [DOI] [PubMed] [Google Scholar]
- 32.Win-Shwe T-T, Yamamoto S, Fujitani Y, Hirano S, Fujimaki H. Spatial learning and memory function-related gene expression in the hippocampus of mouse exposed to nanoparticle-rich diesel exhaust. Neurotoxicology. 2008;29(6):940–947. doi: 10.1016/j.neuro.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31(6):1097–1111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehsanifar M. Anxiety and depression following diesel exhaust Nano-particles exposure in male and female mice. J Neurophysiol Neurol Disord. 2020;8:1–8. [Google Scholar]
- 35.Kesner RP, Lee I, Gilbert P. A behavioral assessment of hippocampal function based on a subregional analysis. Rev Neurosci. 2004;15(5):333–352. doi: 10.1515/revneuro.2004.15.5.333. [DOI] [PubMed] [Google Scholar]
- 36.Sharma DR, Sunkaria A, Bal A, Bhutia YD, Vijayaraghavan R, Flora SJS, Gill KD. Neurobehavioral impairments, generation of oxidative stress and release of pro-apoptotic factors after chronic exposure to Sulphur mustard in mouse brain. Toxicol Appl Pharmacol. 2009;240(2):208–218. doi: 10.1016/j.taap.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 37.Woodward NC, Pakbin P, Saffari A, Shirmohammadi F, Haghani A, Sioutas C, Cacciottolo M, Morgan TE, Finch CE. Traffic-related air pollution impact on mouse brain accelerates myelin and neuritic aging changes with specificity for CA1 neurons. Neurobiol Aging. 2017;53:48–58. doi: 10.1016/j.neurobiolaging.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghadrdoost B, Vafaei AA, Rashidy-Pour A, Hajisoltani R, Bandegi AR, Motamedi F, Haghighi S, Sameni HR, Pahlvan S. Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur J Pharmacol. 2011;667(1–3):222–229. doi: 10.1016/j.ejphar.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Sato H, Takahashi T, Sumitani K, Takatsu H, Urano S. Glucocorticoid generates ROS to induce oxidative injury in the hippocampus, leading to impairment of cognitive function of rats. J Clin Biochem Nutr. 2010;47(3):224–232. doi: 10.3164/jcbn.10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris R, et al. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 41.Aljerf L, Aljurf M. Improvements in the ecological and nutritional aspects of Down's syndrome. 2020. [Google Scholar]
- 42.Takeuchi H, Mizuno T, Zhang G, Wang J, Kawanokuchi J, Kuno R, Suzumura A. Neuritic beading induced by activated microglia is an early feature of neuronal dysfunction toward neuronal death by inhibition of mitochondrial respiration and axonal transport. J Biol Chem. 2005;280(11):10444–10454. doi: 10.1074/jbc.M413863200. [DOI] [PubMed] [Google Scholar]
- 43.Fonken LK, Xu X, Weil ZM, Chen G, Sun Q, Rajagopalan S, Nelson RJ. Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Mol Psychiatry. 2011;16(10):987–995. doi: 10.1038/mp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morris R, et al. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319(6056):774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 45.Kutsuwada T, Sakimura K, Manabe T, Takayama C, Katakura N, Kushiya E, Natsume R, Watanabe M, Inoue Y, Yagi T, Aizawa S, Arakawa M, Takahashi T, Nakamura Y, Mori H, Mishina M. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor ε2 subunit mutant mice. Neuron. 1996;16(2):333–344. doi: 10.1016/s0896-6273(00)80051-3. [DOI] [PubMed] [Google Scholar]
- 46.Constantine-Paton M, Cline HT. LTP and activity-dependent synaptogenesis: the more alike they are, the more different they become. Curr Opin Neurobiol. 1998;8(1):139–148. doi: 10.1016/s0959-4388(98)80017-2. [DOI] [PubMed] [Google Scholar]
- 47.Calderón-Garcidueñas L, Vojdani A, Blaurock-Busch E, Busch Y, Friedle A, Franco-Lira M, Sarathi-Mukherjee P, Martínez-Aguirre X, Park SB, Torres-Jardón R, D'Angiulli A. Air pollution and children: neural and tight junction antibodies and combustion metals, the role of barrier breakdown and brain immunity in neurodegeneration. J Alzheimers Dis. 2015;43(3):1039–1058. doi: 10.3233/JAD-141365. [DOI] [PubMed] [Google Scholar]
- 48.Yokota S, Hori H, Umezawa M, Kubota N, Niki R, Yanagita S, Takeda K. Gene expression changes in the olfactory bulb of mice induced by exposure to diesel exhaust are dependent on animal rearing environment. PLoS One. 2013;8(8):e70145. doi: 10.1371/journal.pone.0070145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beauquis J, Pavía P, Pomilio C, Vinuesa A, Podlutskaya N, Galvan V, Saravia F. Environmental enrichment prevents astroglial pathological changes in the hippocampus of APP transgenic mice, model of Alzheimer's disease. Exp Neurol. 2013;239:28–37. doi: 10.1016/j.expneurol.2012.09.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used in this study is available with the authors and can be made available upon request.