Abstract
Sensor-augmented insulin pump therapy with a predictive low glucose suspend (SAP-PLGS) feature is a remarkably progressed modality for the glycemic management of patients with type 1 diabetes. This technology avoids nocturnal hypoglycemia and severe hypoglycemia. A Brazilian woman developed type 1 diabetes at age 11 and was treated with multiple daily insulin injections. At age 20, she was admitted to our internal medicine department for her first pregnancy. Her HbA1c was 7.9% in the 6 weeks of gestation. Although the combination of continuous subcutaneous insulin infusion and a sensor-augmented pump was introduced, she had a miscarriage in the next week. After 6 months, she became pregnant again. Despite an HbA1c of 7.2%, she had another miscarriage. Thereafter, she returned to multiple daily insulin injections and began using intermittently scanned continuous glycemic monitoring. At age 22, she had her third pregnancy. Her HbA1c was 7.3%. SAP-PLGS was then introduced, which reduced her frequent hypoglycemic events and blood glucose fluctuations. She gave birth to a 4137 g boy at 39 weeks without significant complications. Successful delivery can be obtained in women with type 1 diabetes following repeated miscarriages after introducing SAP-PLGS. We hypothesize that the modality might contributed to our patient’s miscarriage avoidance by reducing her glycemic fluctuations.
Keywords: SAP, PLGS, Type 1 diabetes, Pregnancy, Glycemic fluctuations
Introduction
The incidence of spontaneous miscarriage is high in pregnant women with type 1 diabetes [1]. Tight glycemic control during the perinatal period is essential to reduce the risk of perinatal and obstetrical complications [2–5]. Maternal glycemic fluctuations and severe hypoglycemia should be avoided [6, 7]. A recent report showed that the ideal glycemic achievement ratio in type 1 diabetes during pregnancy is 70% or more time in range (TIR) measured as follows: 63–140 mg/dL; 4% or less time below range measured as less than 63 mg/dL; 1% time below range at less than 53 mg/dL; and less than 25% time above range at greater than or equal to 140 mg/dL [8]. However, achieving this target is challenging for pregnant women with type 1 diabetes. Striving for euglycemia inevitably elicits both rebound hyperglycemia and hypoglycemia, resulting in glycemic fluctuations.
A combination of an insulin pump with a glucose sensor, namely a sensor-augmented pump (SAP), is a potentially promising modality that may enable intensive glycemic control in pregnant women with type 1 diabetes [9]. The SAP system provides a corresponding amount of insulin to treat transient hyperglycemia. However, ordinary SAP systems sometimes induce insulin over-compensation resulting in severe hypoglycemia and glycemic fluctuations. SAP systems have evolved and can now provide a predictive low glucose suspend (SAP-PLGS) feature. SAP-PLGS makes it possible to avoid severe hypoglycemia following the compensation of insulin to mitigate transient hyperglycemia [10, 11]. SAP-PLGS may help prevent the vicious hyperglycemia–hypoglycemia cycle and improve glycemic control; however, the clinical significance of SAP-PLGS has not been well validated. We present a case of a woman with type 1 diabetes who had experienced repeated miscarriages with an ordinary SAP but successfully gave birth when using SAP-PLGS.
Case report
Diagnosis and management of type 1 diabetes
A Brazilian woman emigrated to Japan at the age of 10 and subsequently suffered from bronchial asthma and mumps. Around 1 year later, she was admitted to our hospital for a mouth rash, polydipsia, and weight loss. Her blood glucose level was raised at 654 mg/dL, her HbA1c was 11.9%, and her anti-GAD and anti-IA-2 antibodies were positive. She was diagnosed with type 1 diabetes at the age of 11. Her physical findings at the time of diagnosis were as follows: height 169.0 cm, body weight 61.9 kg, body mass index (BMI) 21.7, blood pressure 100/50 mmHg, and heart rate 85 per minute. Except for one grandfather diagnosed with type 2 diabetes, she had no family history of diabetes. With her HbA1c levels maintained at 6–8%, the patient had no diabetic retinopathy, stage 1 nephropathy, and no neuropathy during junior high school. Multiple daily injections of insulin were introduced. Her clinical visits were irregular due to socioeconomic reasons.
First pregnancy
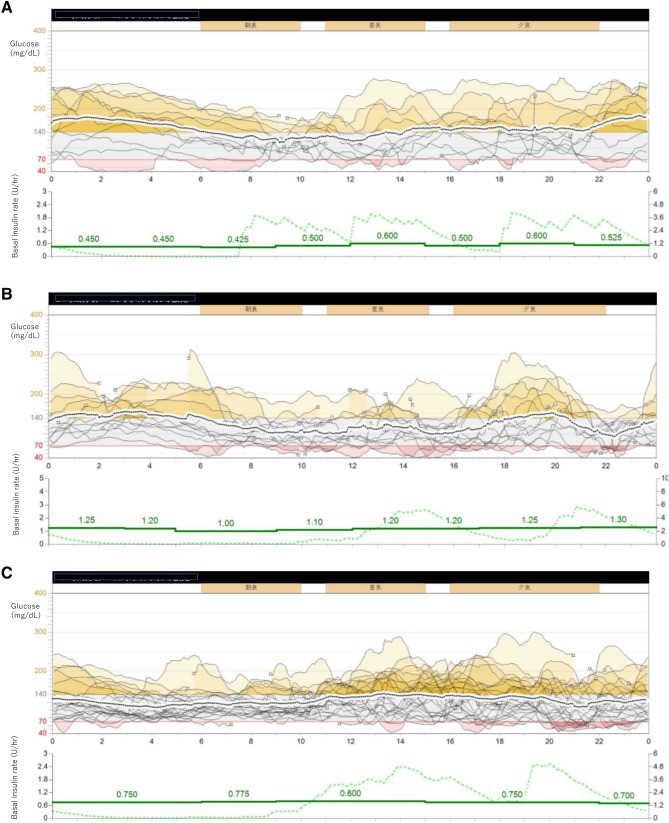
At the age of 20, the patient was admitted to our internal medicine department for her first pregnancy. As her HbA1c was 7.9% in the 6 weeks of gestation, she was hospitalized for glycemic management. She received 40 units of insulin glargine in the morning and eight units of insulin aspart before each meal. On the day of admission, symptomatic hypoglycemia with a blood glucose level of 55 mg/dL was observed. She experienced frequent episodes of nocturnal cold sweats and was unable to wake up early in the morning. The SAP system MiniMed 620G (Medtronic Inc., MN, USA) was introduced from the admission, and its record showed glucose fluctuations and severe nocturnal hypoglycemia. Furthermore, the pattern of its episode records suggested two possible reasons for the fluctuations. One was the administration of excessive insulin after hyperglycemia. The second was postprandial hyperglycemia with mismatched timing, dosage of insulin, and carbohydrate–insulin ratios. Although the insulin regimen was changed, repeated symptomatic hypoglycemia persisted. With basal insulin set at 21 units per day in total, the prescribed carbohydrates–insulin ratio was 0.7–0.9 U/exchange for each meal with an insulin sensitivity factor of 50–70 mg/dL/U. Unfortunately, in the 7 weeks of gestation, the patient had a miscarriage. Her 12-day sensor records through the fifth week showed a mean sensor glucose level of 149 ± 59 mg/dL. The average area under the curve (AUC) > 140 mg/dL was 29.7%, and the average AUC < 70 mg/dL was 1.1% (Fig. 1a, Table 1).
Fig. 1.
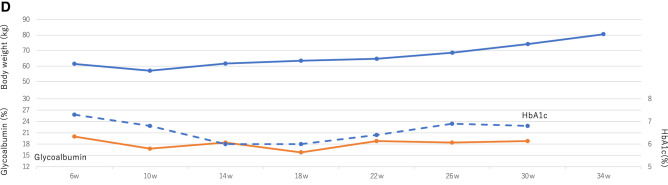
a−d Glycemic fluctuation shown through the demographic records of diurnal glycemic changes with intermittently scanned continuous glycemic monitoring for 72 h in three pregnancy periods; a first pregnancy, b second pregnancy, c third pregnancy, and d changes shown in body weight, HbA1c, and glycoalbumin during the third pregnancy
Table 1.
Summary of record obtained from SAP glycemic monitoring in three pregnancy periods
| First pregnancy | Second pregnancy | Third pregnancy | |
|---|---|---|---|
| HbA1c at the sixth week of the pregnancy (%) | 7.9 | 7.2 | 7.3 |
| Average of diurnal glucose level (mg/dL) | 149 ± 59 | 125 ± 51 | 125 ± 43 |
| Ratio of time range with average AUC above 140 mg/dL (%) | 29.7 | 15.0 | 11.3 |
| Hyperglycemic episodes (/day) | 4.2 | 3.3 | 2.9 |
| Hyperglycemic episodes due to the over-compensation of hypoglycemia (/day) | 0.8 | 0.8 | 0.8 |
| Ratio of time range with average AUC blow 70 mg/dL (%) | 1.1 | 1.4 | 0.5 |
| Hypoglycemic episodes (/day) | 2.5 | 2.1 | 1.4 |
| Hypoglycemic episodes following hyperglycemia (/day) | 0.5 | 0.5 | 0.3 |
| Total daily insulin (U/day) | 42.9 ± 5.7 | 46.3 ± 8.4 | 34.8 ± 8.3 |
| Basal daily insulin (U/day) | 21.7 | 27.2 | 13.5 |
| Bolus daily insulin (U/day) | 21.3 | 19.1 | 21.3 |
| Basal insulin to bolus insulin ratio | 50/50 | 59/41 | 39/61 |
| Low glucose suspension (min/day) | – | – | 11 |
| Predictive low glucose suspension (min/day) | – | – | 249 |
| Name of device | MiniMed 620G | MiniMed 620G | MiniMed 640G |
Data are means ± standard deviations or n (%) unless otherwise specified. Hyperglycemic episodes, blood glucose levels above 140 mg/dl for more than 30 min; Hypoglycemic episodes, blood glucose levels below 70 mg/dl for more than 30 min
SAP sensor-augmented pump therapy
Second pregnancy
After 8 months, the patient was found to be in the 6 weeks of gestation in her second pregnancy. Her HbA1c was 7.2%, glycoalbumin 20.4%, total basal rate 27.8 U/day, and carbohydrate–insulin ratio 0.8–0.9 U/exchange. In the 9 weeks of gestation, a spontaneous miscarriage was observed. Her HbA1c was 6.8% and glycoalbumin 18.2%. Her sensor data showed that a mean sensor glucose level of 125 ± 51 mg/dL, mean AUC > 140 mg/dL of 15.0%, mean AUC < 70 mg/dL of 1.4% (Fig. 1b, Table 1). After this miscarriage, she returned to multiple daily insulin injections and began using the intermittently scanned continuous glycemic monitoring because of the financial burden. Although the miscarriage was repeated, she was negative for anti-phospholipid autoantibody syndrome, and thyroid dysfunction.
Third pregnancy
At the age of 22, she was determined to be in the 6 weeks of gestation in her third pregnancy. Her treatment was 20 units of insulin degludec in the morning and 10 units of insulin aspart before each meal. Her HbA1c was 7.3% and glycoalbumin 20%. The SAP-PLGS system MiniMed 640G (Medtronic Inc., MN, USA) became commercially available in Japan just before her third pregnancy. The MiniMed 640G is practically the same as the MiniMed 620G except for the PLGS add-on. The introduction of the MiniMed 640G helped her avoid severe hypoglycemia due to hyperemesis. The SAP data showed a reduction in hypoglycemia. Although the mean sensor glucose level of 125 ± 43 mg/dL was the same as in her second pregnancy, the fluctuations improved dramatically with a mean AUC > 140 mg/dL of 11.3% and a mean AUC < 70 mg/dL of 0.5% (Fig. 1c, Table 1).
With the stabilization of her glycemic control, her appetite and bolus insulin doses increased. Until the 35 weeks of gestation, she gained 24 kg, blood pressure increased slightly, and lower limb edema was observed. Following hospitalization, her body weight and daily insulin doses were decreased (Fig. 1d).
At 39 weeks and 3 days, she delivered a baby boy weighing 4137 g (+ 3.2 SD) with a pH of 7.255, blood glucose level within the normal range of 69 mg/dL, and an Apgar score of 8/10 via a semi-urgent Caesarean section because of obstructed labor. The boy was admitted to the neonatal intensive care unit for observation for just 1 day without hypoglycemia and neonatal respiratory distress. The postpartum basal insulin dose was 10 units, and the carbohydrate–insulin ratio was changed to 0.9 U/exchange with frequent hypoglycemia. After the delivery, she returned to multiple daily injections at her request. Her HbA1c was stable at 5–6% with breastfeeding. She did not develop her diabetic retinopathy and nephropathy throughout the pregnancy periods.
Discussion
A woman with type 1 diabetes and twice history of spontaneous miscarriage gave birth following treatment with SAP-PLGS in her third pregnancy. Her SAP records showed a reduced average AUC and reduced glycemic fluctuations. Figure 1 showed a reduction in the AUC < 70 mg/dL and AUC > 140 mg/dL in the third pregnancy. TIR > 70% was achieved from the first to the second pregnancy. The SAP-PLGS also prevented the further deterioration of her hypoglycemia. As a result, the frequency of severe hypoglycemia (< 53 mg/dL) was significantly reduced. The ideal glycemic achievement ratio was attained through the course [8].
The moderate hypoglycemia (< 63 mg/dL) that occurred frequently after insulin supplementation suggested insulin over-compensation. The amount of basal insulin was reduced from the first pregnancy to the third. In parallel, rebound hyperglycemia elicited by hypoglycemia was reduced. Her severe hypoglycemia and glycemic fluctuations were resolved concomitantly following the introduction of SAP-PLGS, which is line with previous reports [12, 13].
We believe that an improvement in glucose fluctuations contributed to the continuation of our patient’s third pregnancy. Severe hypoglycemia in type 1 diabetes pregnancies has been found to be 15 times higher with intensive insulin treatment than conventional treatment [14, 15]. In diabetes pregnancies, placental expression and the activity of the glucose transporter (GLUT) is modified by an excessive supply of energy substrates to the fetus [16]. Placental GLUT overexpression during pregnancy is a reasonable response to preserve an intrauterine fetus. Since the fetus is resilient to maternal hypoglycemia, maternal hypoglycemia is generally ignored as it is considered harmless. Maternal hypoglycemia has not been reported to increase the risk of congenital disabilities, fetal death, fetal physiology (fetal heart, breathing, or body movement), umbilical artery Doppler waveforms, or neonatal intelligence [7]. However, hypoglycemia and its unawareness increase the risk of neonatal respiratory distress and pre-eclampsia [17]. The causes of recurrent miscarriages are multifactorial, chromosomal, anatomical, and immunological [18]. It has been proposed that abnormal changes in uteroplacental vascular development due to severe hypoglycemia play a role [19]. Severe hypoglycemia elicits vascular contractility and platelet activation [20], resulting in placental ischemia. Revealingly, hypoglycemia during pregnancy in the first trimester is teratogenic in animal models [21]. In other words, no case reports, no human studies, but several animal studies showed the association between glucose fluctuations and hypoglycemia to miscarriage. Avoidance of a similar incident with SAP-PLGS use could have resulted in the optimal outcome in our patient’s third pregnancy.
In the current case, the baby's birth weight exceeded 4000 g. Although the woman received repeated dietitians' nutrition guidance, she has ignored and kept excessive intakes resulting in 24 kg body weight gain during the third pregnancy. Although HbA1c levels in the third trimester present the most prominent risk, maternal BMI and weight gain during pregnancy contribute significantly to macrosomia [22]. Some components of macrosomia could be explained by the placental GLUT overexpression, and the macrosomia rate can be reduced with an increase in TIR during the second and third trimesters [23]. Glycemic fluctuations could, therefore, correlate significantly with macrosomia. Excess body weight gain in pregnant women increases the risk of macrosomia, too [24]. Both glycemic fluctuations and maternal weight gain strongly suggest underlying insulin over-compensation [25]. In our patient, the glycemic fluctuations were controlled with SAP-PLGS, even with the persistent insulin over-compensation. Given her morbid weight gain, the intervention against insulin over-compensation was the primary goal of management to prevent macrosomia.
Conclusion
The reported patient experienced two miscarriages with ordinary SAP; however, she could reach and achieve childbirth while using SAP-PLGS. More attention should be paid to glycemic fluctuations during pregnancy when managing patients with type 1 diabetes.
Acknowledgements
We express our sincere appreciation to Prof. Rimei Nishimura in Jikei University for his comments in clinical discussion. This work was supported by JSPS KAKENHI [Grant Number JP18K08505 and 20K10318]. The study sponsor was not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Abbreviations
- SAP
Sensor-augmented pump therapy
- PLGS
Predictive low glucose suspend feature
- TIR
Time in range
- BMI
Body mass index
- GAD
Glutamic acid decarboxylase
- IA-2
Anti-insulinoma-associated antigen-2
- AUC
Area under the curve
- GLUT
Glucose transporter
Compliance with ethical standards
Conflict of interest
The authors declare that they have nothing to disclose regarding conflicts of interest concerning this manuscript except Prof. Chujo (honoraria from Eli Lilly Japan K.K., Research funding from Sanofi and Novo Nordisk Pharm Ltd, and Subsides or Donations from Novo Nordisk Pharm Ltd) and Prof. Tobe (Honoraria from MSD K.K., Novo Nordisk Pharm Ltd, and Kowa Pharm Co. Ltd., and Subsides or Donations from Daiichi Sankyo Co. Ltd., Ono Pharm Co. Ltd., Takeda Pharm Co. Ltd., Nippon Boehringer Ingelheim Co. Ltd., MSD K.K., Mitsubishi Tanabe Pharm Co., Teijin Pharm Ltd., Eli Lilly Japan K.K., Asahi Kasei Pharm Co., The Mitsubishi Foundation, and Suntory Global Innovation Center Ltd.).
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and/or with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
Informed consent
Informed consent was obtained from the patient for this case report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dorman JS, Burke JP, McCarthy BJ, et al. Temporal trends in spontaneous abortion associated with Type 1 diabetes. Diabetes Res Clin Pract. 1999;43:41–47. doi: 10.1016/S0168-8227(98)00123-5. [DOI] [PubMed] [Google Scholar]
- 2.Akihisa R, Omori Y, Minei S, et al. Relationship between glycemic control and spontaneous abortion in diabetic women. J Japan Diab Soc. 1990;33:947–951. [Google Scholar]
- 3.Hanson U, Persson B, Thunell S. Relationship between haemoglobin A1C in early type 1 (insulin-dependent) diabetic pregnancy and the occurrence of spontaneous abortion and fetal malformation in Sweden. Diabetologia. 1990;33:100–104. doi: 10.1007/BF00401047. [DOI] [PubMed] [Google Scholar]
- 4.Omori Y, Minei S, Testuo T, et al. Current status of pregnancy in diabetic women. A comparison of pregnancy in IDDM and NIDDM mothers. Diabetes Res Clin Pract. 1994;24:273–278. doi: 10.1016/0168-8227(94)90261-5. [DOI] [PubMed] [Google Scholar]
- 5.Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for diabetes 2019. Diabetol Intern. 2020;11:165–223. doi: 10.1007/s13340-020-00439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy HR. Intensive glycemic treatment during type 1 diabetes pregnancy: a story of (mostly) sweet success! Diabetes Care. 2018;41:1563–1571. doi: 10.2337/dci18-0001. [DOI] [PubMed] [Google Scholar]
- 7.Feldman AZ, Brown FM. Management of type 1 diabetes in pregnancy. Curr Diab Rep. 2016;16:76. doi: 10.1007/s11892-016-0765-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuoka A, Hirota Y, Urai S, et al. Effect of switching from conventional continuous subcutaneous insulin infusion to sensor augmented pump therapy on glycemic profile in Japanese patients with type 1 diabetes. Diabetol Int. 2018;9:201–207. doi: 10.1007/s13340-018-0344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forlenza GP, Li Z, Buckingham BA, et al. Predictive low-glucose suspend reduces hypoglycemia in adults, adolescents, and children with type 1 diabetes in an at-home randomized crossover study: results of the PROLOG trial. Diabetes Care. 2018;41:2155–2161. doi: 10.2337/dc18-0771. [DOI] [PubMed] [Google Scholar]
- 11.Katayama A, Tone A, Watanabe M, et al. The hypoglycemia-prevention effect of sensor-augmented pump therapy with predictive low glucose management in Japanese patients with type 1 diabetes mellitus: a short-term study. Diabetol Int. 2020;11:97–104. doi: 10.1007/s13340-019-00408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishimura R. Evolution and prospect of sensor augmented pump—evolution and outlook of diabetes latest medical care. J Japan Diab Soc. 2018;61:805–808. [Google Scholar]
- 13.Zhong A, Choudhary P, McMahon C, et al. Effectiveness of automated insulin management features of the MiniMed(®) 640G sensor-augmented insulin pump. Diabetes Technol Ther. 2016;18:657–663. doi: 10.1089/dia.2016.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 15.Rosenn BM, Miodovnik M, Holcberg G, et al. Hypoglycemia: the price of intensive insulin therapy for pregnant women with insulin-dependent diabetes mellitus. Obstet Gynecol. 1995;85:417–422. doi: 10.1016/0029-7844(94)00415-A. [DOI] [PubMed] [Google Scholar]
- 16.Stanirowski PJ, Szukiewicz D, Pazura-Turowska M, et al. Placental expression of glucose transporter proteins in pregnancies complicated by gestational and pregestational diabetes mellitus. Can J Diabetes. 2018;42:209–217. doi: 10.1016/j.jcjd.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Perea V, Bertran B, Bellart J, et al. Impaired awareness of hypoglycaemia: A new risk factor for adverse pregnancy outcomes in type 1 diabetes. Diabetes Metab Res Rev. 2019;35:e3176. doi: 10.1002/dmrr.3176. [DOI] [PubMed] [Google Scholar]
- 18.Houwert-de Jong MH, Eskes TK, Termijtelen A, et al. Habitual abortion: a review. Eur J Obstet Gynecol Reprod Biol. 1989;30:39–52. doi: 10.1016/0028-2243(89)90092-0. [DOI] [PubMed] [Google Scholar]
- 19.Vuorela P, Carpen O, Tulppala M, et al. VEGF, its receptors and the tie receptors in recurrent miscarriage. Mol Hum Reprod. 2000;6:276–282. doi: 10.1093/molehr/6.3.276. [DOI] [PubMed] [Google Scholar]
- 20.Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care. 2010;33:1389–1394. doi: 10.2337/dc09-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ter Braak EW, Evers IM, Willem Erkelens D, et al. Maternal hypoglycemia during pregnancy in type 1 diabetes: maternal and fetal consequences. Diabetes Metab Res Rev. 2002;18:96–105. doi: 10.1002/dmrr.271. [DOI] [PubMed] [Google Scholar]
- 22.Cyganek K, Skupien J, Katra B, et al. Risk of macrosomia remains glucose-dependent in a cohort of women with pregestational type 1 diabetes and good glycemic control. Endocrine. 2017;55:447–455. doi: 10.1007/s12020-016-1134-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kristensen K, Ogge LE, Sengpiel V, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes: an observational cohort study of 186 pregnancies. Diabetologia. 2019;62:1143–1153. doi: 10.1007/s00125-019-4850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludwig DS, Currie J. The association between pregnancy weight gain and birthweight: a within-family comparison. Lancet. 2010;376:984–990. doi: 10.1016/S0140-6736(10)60751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kambara M, Yanagisawa K, Tanaka S, et al. Changes in insulin requirements during pregnancy in Japanese women with type 1 diabetes. Diabetol Int. 2019;10:102–108. doi: 10.1007/s13340-018-0369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]