Abstract
Groundwater is a viable alternative when access to surface water resources is limited. Iron and manganese are known ions in soil and naturally in groundwater sources. However, human activities also are responsible. To identifying the best module for removing manganese and iron in the water treatment plant (WTP) of Mazandaran, 516 samples were taken from raw and treated water. The concentration of manganese, iron, was measured by atomic absorption spectrophotometry, and turbidity was used with the nephelometry method. The water pollution index (WPI) was applied for categorizing the status of pollution in treated water. The effect of seasonal temperature and backwashing (At flow rates of 3.5, 9.2, and 15.3 m h-1) on the sand filter efficiency was also investigated. The highest concentrations of manganese, iron, and turbidity in raw water were 0.744, 6.70 mg L-1, and 41.8 NTU, and in treated water were 0.67, 1.09 mg L-1, and 5.58 NTU, respectively. The mean concentration of manganese and iron in raw and treated water were 0.24 ± 0.1, 0.93 ± 0.91, 0.105 ± 0.06 and 0.18 ± 0.14 mg L-1 respectively. The WPI statuses in drinking water were excellent for manganese and iron in 95.74 and 53.88 % of the samples and very poor in 1.16 and 12.01 % of the samples, respectively, and its classification for drinking water for manganese and iron was excellent ˃ good ˃ extremely polluted ˃ polluted and the concentration of iron was more than manganese in treated water. The study of temperature’s effect on sand filters showed that the removal efficiency in warm seasons was higher than in cold seasons. Also, the turbulence in the backwash with the 9.2 m h− 1 rates, is lesser than other speeds, and in this flow, after 270 s, the turbidity decreases to less than 10 NTU. Spearman correlation comparison showed that the parameters amounts after filtration decreased significantly (p ≤ 0.0001) in comparison to raw water. The results showed that module #1 that used open-aeration and chlorine as oxidations, was most effective in removing iron and manganese. In the end, the WTP couldn’t diminish the parameters completely and need subsidiary units.
Keywords: Water, Manganese, Iron, WPI, Mazandaran
Introduction
Without exaggeration, after air, water is the most significant need of all creatures, especially humans, and it is necessary to perform all the vital functions of the body [1, 2].
Groundwater is one of the best resources available to humans, and on the other hand, the reduction of desirable surface water resources or sometimes the contamination of these resources is a strong reason to increase the tendency to extract groundwater [3–5].
A group of mineral ions such as iron and manganese, in specified doses, are essential and useful for human survival. But when their rate increases significantly, adverse effects occur. For example, too much manganese intake affects the central nervous system [6, 7]. Also excessive iron intake can lead to health problems such as affecting the digestive system (anorexia or diarrhea), disrupting other organs such as the spleen and nervous system, and in more severe cases, death [8, 9].
Contamination of groundwater with iron and manganese ions can be of natural or anthropogenic origin. As a result of human activities such as mining or improper disposal of wastewater, water sources become contaminated with these mineral metals [10, 11]. These ions are divalent in the absence of oxygen. After the necessary conditions for oxidation and the presence of sufficient oxygen, these ions increase to their higher capacities [12, 13].
Problems such as increased turbidity, discoloration, taste, and odor in water can be related to the presence of iron and manganese ions. They can also cause acute disruptions in various parts of the treatment plant or distribution network. For example; Due to the growth of bacteria, the quality of distribution network pipes decreases and gradually wears out. On the other hand, the chlorine depletion and the absence of sufficient free chlorine to the point of consumption are side effects of these ions presence that cause secondary contamination. Also, if water with a high concentration of mineral elements is used in industries with boilers, it will lead to dangerous accidents [14].
What has been said is only part of these ions consequences and problems in groundwater. Due to the widespread use of groundwater as a reliable source, increasing water use in human service and industrial activities, the lack of a proper and uniform understanding of the protection of groundwater aquifers, and the permanent dependence of the planet Earth on water, the study of these ions in groundwater in Mazandaran province is beneficial.
Considering the geographical and study conditions of this region, the aims of this study were;
In this study, the quality of drinking water that outs of treatment plants related to 10 cities out of 21 cities in Mazandaran province has been studied. These water supply systems direct the raw water from the underground wells to the treatment plant and ultimately provide the drinking water needed by 116,000 residents.
Identify the best water treatment layout to reduce the concentration of iron and manganese and the turbidity in drinking water
Measuring water quality with the help of water pollution index (WPI)
Investigation the effect of temperature and backwashing on the efficiency of sand filters in water treatment plant (WTP)
Materials and methods
Study area
Mazandaran province had ~ 23,756 km2 area and, it had 1.46 % of the total space of the Islamic Republic of Iran. This province is located in the northern part of Iran in the form of a green strip. According to the latest census of the Statistical Organization of Iran, this province has a population of 3,283,582 and has a common border with the Caspian Sea. The average annual rainfall in this province is 977 mm. regarding the temperature parameter, due to the high relative humidity and a large number of cloudy days, the air temperature is moderate and, the temperature range is limited. This situation leads to hot and humid summers and mild winters with occasional frosts [15]. Due to the high groundwater level in this province, the tendency to extract water from wells and use it for drinking is high. Out of 42 water supply systems in Mazandaran province, 19 systems were selected that had almost identical modules to reduce the amount of manganese, iron, and turbidity and supplied water to ten cities out of 3 cities in this region. The first, second, and third cities in this study had 11, 5, and 3 water supply systems, respectively, which were sampled from their treated water. On average, the centers of these cities have 15 km distant from each other.
These WTPs used groundwater wells to supply drinking water to 10 cities and ~ 760,000 people through 4 modules of treating and, the water treatment method is conventional in the studied WTPs.
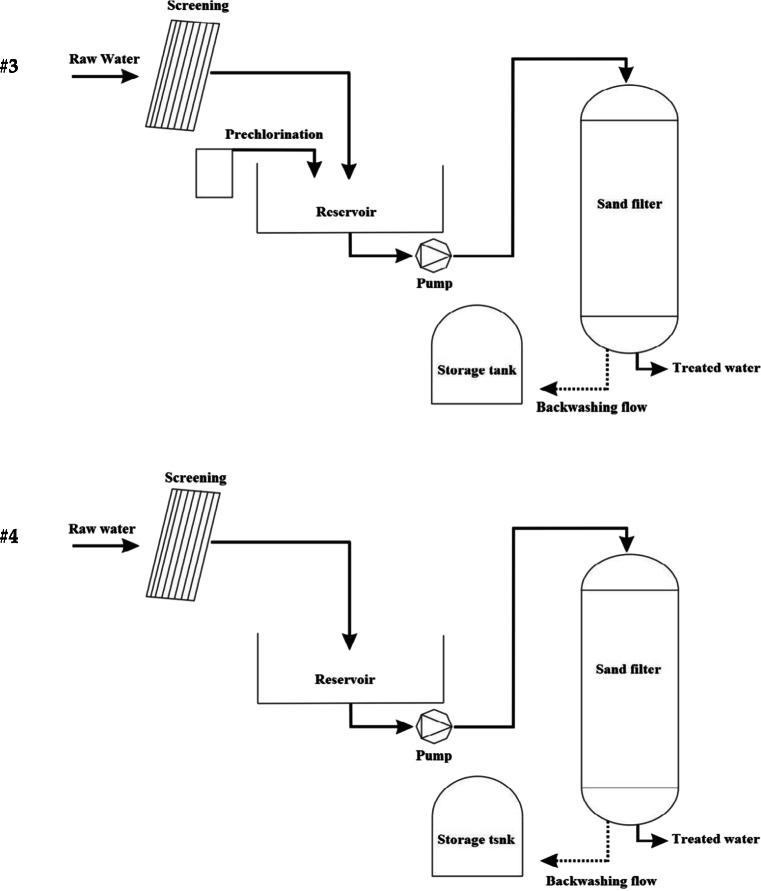
After water extraction from the well, groundwater enters the reservoir, and depending on the layout, pre-chlorination, aeration, filtration with sand filters (Rapid Sand Gravity Filter) and, final disinfection can be used.
The main lever to reduce the amount of iron and manganese in raw water in these WTPs are the use of oxidation and filtration (pressurized sand filter). The sand substrate used in these filters had three layers and its specifications are shown in Table 1.
Table 1.
Specifications of sand filter layers in WTP
| Parameter | First layer | Second layer | Third layer |
|---|---|---|---|
| D10 (mm) | 0.8 | 1.08 | 2.8 |
| D60 (mm) | 2 | 2.08 | 4.5 |
| D90(mm) | 2.28 | 2.6 | 4.9 |
| UC* | 2.5 | 1.93 | 1.6 |
| Specific gravity [g cm-3] | 2.3 | 2.3 | 2.3 |
| Height of filtration medium (mm) | 250 | 250 | 250 |
*UC is uniformity coefficient which is calculated from the ratio of D60/D10
Module of facilities
There are four modules for the removal of iron and manganese from raw water in Mazandaran’s WTP:
First module; steps of this module include; extracting water from the well, storing it in the reservoir. This layout has both open aeration and pre-chlorination. In the next stage, to reduce the concentration of iron and manganese ions, water enters the sand filters and in the last step, final disinfection is performed. For regulating the necessary pressure in the distribution network, water enters the elevated tanks and is transferred to the distribution network. It is also noted that in this modules, chlorine is injected into the water as the final disinfectant.
In the second module; after being extracted water from the well, it enters multi-story platter aerators. In the next step, water is stored in the reservoir. After the residence time, the water enters the sand filter, and finally, the post chlorination is done. Pre chlorination is removed in this method.
The third module; the only difference between this method and the first module is putting aside the aeration step.
Fourth module; this module includes; extracting water from the well, storage in the reservoir tank, and after the residence period, (depending on the volume of the reservoir), water enters the sand filter and, post disinfection is performed. The only source of oxygen in this is in contact with the air in the water reservoir. Pre-chlorination is not performed in this module either.
It is important to note that galvanized coatings are used to protect the treatment plant equipment from moisture. Sand filters are also placed inside uncovered concrete containers.
Figure 1 shows an illustration of the four modules described. All available modules have the backwashing effluent.
Fig. 1.
Overview of the four layouts in Mazandaran ‘s WTP
Sampling and analysis
Water quality was assessed by sampling raw groundwater at the entrance to the treatment plant and output flow from sand filters at a different season in 2016–2017. The number of samples taken before treatment was 258 samples and after treatment was 258 samples. The samples were transported in polyethylene bottles with a volume of 250 ml and maintained by the appropriate temperature conditions (0–4 °C) by the cold box to the reference laboratory. The method of atomic absorption spectrophotometry for manganese and iron detection was used. The model of the device was Hitachi U3900-3900 H. The nephelometric method and the turbidity meter model PCE - TUM 20 were used for turbidity measurement [15, 16].
Water pollution index (WPI)
When a (or some) contaminant exists in drinking water, we should examine its pollution status. There are many performances to indicate water quality [17] but one of them is a flexible approach that can use for measuring physical, chemical, and biological parameters. This approach is recognized as the WPI. Also, this method is most useful for water with domestic and drinking aims. For calculating this approach for treated water in this study, the concentration of parameters (Ci) has been estimated. The standard or highest permissible limit (Si) of manganese and iron are available (0.4 and 0.3 mg L− 1) and, the number of samples (n) after treatment was 258 cases. Finally, Eq. 1 and Eq. 2 was used.
| 1 |
Where pollution load (PLi) is;
| 2 |
Groundwater quality analysis based on the water pollution index is done by classifying sampling results into four different classes;
The water is excellent when WPI is ˂ 0.5; the quality of water is good if 0.5˂ WPI ≤ 0.75; when index value varied from 0.75 ˂WPI ≤ 1, water is moderately polluted and it is extremely polluted when WPI is greater than 1. The functions and instructions in this research extracted from study by Hossain et al. [18].
Water quality based on sand filters efficiency
In this study, to investigate the effect of temperature on the sand filter efficiency, the principle of identically distributed samples in different seasons was considered. In the warm seasons (20th Mar. − 21th Sep.), 129 samples of raw water and 129 samples of treated water were taken. This cycle was repeated for the cold seasons of the year (22th Sep. − 19th Mar.) [15].
Also, the effect of the backwash on sand filters in reducing turbidity was investigated by the sampling of sand filter effluent after backwashing at three speeds of 3.5, 9.2, and 15.3 m h− 1 .
Statistical analysis
First, the data of this study were examined for kurtosis and skewness, and in the next stage, the normality of the data was examined by Smirnov-Kolmogorov statistical test, and finally, the correlation of the data before and after treatment was analyzed. Statistical Package for the Social Sciences (SPSS – IBM version 20) and Excel 2016 software was used to calculate the descriptive statistics and for extracting the necessary tables or graphs.
Result
Lab results & efficiency of modules
According to the latest guidelines of the World Health Organization (WHO) and the standards of Iran, the permissible limit of iron in water is 0.3 mg L− 1, the maximum permissible limit and, the maximum contaminant level of manganese are 0.4, 0.1 mg L− 1, respectively and, the turbidity in drinking water should be 1 NTU (Nephelometric Turbidity Unit).
Table 2 summarized the comparison of water samples, before and after treatment (regardless of modules layout). According to this table, 250 (96.89 %), 211 (81.78 %) and, 257 (99.61 %) samples related to raw water, in compare with standards, had higher amounts of manganese, iron, and turbidity.
Table 2.
The comparison between understudy parameters in raw and treated water, 2016–2017
| Parameter | Raw water | Treated water | ||||
|---|---|---|---|---|---|---|
| Manganese | Iron | Turbidity | Manganese | Iron | Turbidity | |
| Min. | 0.036 | 0.12 | 0.94 | 0.01 | 0.01 | 0.31 |
| Max. | 0.74 | 6.70 | 41.8 | 0.67 | 1.09 | 5.58 |
| Mean ± SD | 0.24 ± 0.1 | 0.93 ± 0.91 | 5.92 ± 8.07 | 0.105 ± 0.06 | 0.18 ± 0.14 | 1.25 ± 0.82 |
| Higher than standard level (%) | 97.67* | 81.78 | 99.61 | 12.79 | 12.01 | 49.22 |
* The maximum contaminant level is considered for manganese
The highest concentrations of manganese and iron ions in raw water were 0.744, 6.70 mg L− 1, and turbidity was 41.8 NTU, respectively. Also, in raw water samples, the mean concentration of iron ion (0.93 ± 0.91 mg L− 1) was higher than the standard range and, the mean concentration of manganese ion (0.24 ± 0.1 mg L− 1) at these samples was higher than the maximum contaminant level. The mean of turbidity in raw water and treated water samples 5.92 ± 8.07 and 1.25 ± 0.82, respectively.
While the results of treated water samples show that the highest amount of manganese and iron ions after treatment were 0.67 and 1.09 mg L− 1, respectively, and the turbidity was 5.58 NTU. It is also important to note that none of the layouts were able to reduce the turbidity of all samples to the standard range, and 135 samples of treated water had turbidity above 1 NTU.
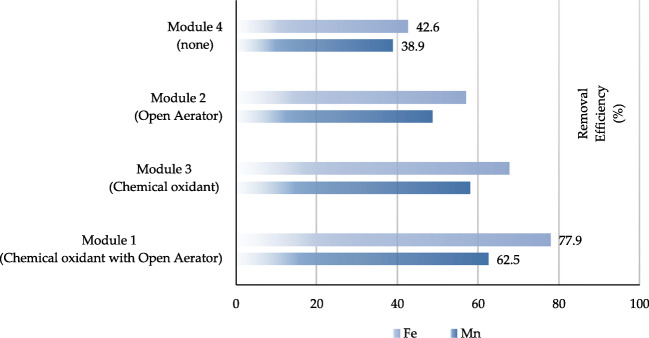
Different layouts of water supply systems showed; module #1 which used both open-aeration and chlorine oxidations were most effective in removing iron and manganese. The removal efficiencies of this method were 62.5 and 77.7 % for manganese and iron ions, respectively.
The second rank of the removal percentage belonged to module #3. In this method, chlorine, as the only oxidizing agent, reduces manganese and iron ions by 58 and 67.7 % and as a result, turbidity is reduced by decreasing the amount of these ions.
The module #2 has no chemical oxidants and, only tray aerators (Physical oxidation) have been used to reduce manganese and iron ions. However, a relatively good removal percentage was obtained for iron ions (57 %). This method had 48.7 % efficiency for manganese removal.
In the fourth module, neither air is used as an oxidizer nor chemical substances such as chlorine. In this method, water enters the sand filters after entering the storage tank and stays for a specific time. The removal efficiency of manganese and iron ions by this method was 38.9 and 42.6 %, respectively.
The status of WPI
The result of groundwater WPI in Mazandaran province showed that more than 53.87 and 95.73 % of samples had WPI ≤ 0.5 for manganese and iron elements. But some samples had the pollution index above 1. This index varied from 0.04 to 1.67 and 0.03–3.63 for manganese and iron respectively. On the next (Table 3), the number of samples categorized based on WPI.
Table 3.
The status of WPI of manganese and iron in treated water
| WPI | Number of samples | Percent (%) | |||
|---|---|---|---|---|---|
| Category | Status | Mn | Fe | Mn | Fe |
| ≤ 0.5 | Excellent | 247 | 139 | 95.74 | 53.88 |
| 0.5–0.75 | Good | 6 | 60 | 2.33 | 23.26 |
| 0.75-1 | Moderately polluted | 2 | 28 | 0.77 | 10.85 |
| ˃1 | Extremely Polluted | 3 | 31 | 1.16 | 12.01 |
Filters performance monitoring
The effect of temperature in the sand filter efficiency showed that the removal of manganese and iron ions in the warm seasons was higher than in cold seasons. Turbidity has also benefited from a concentration reduction of these ions and on average, the efficiency of filters in removing manganese and iron parameters in thermal seasons was higher than 50 %. In these seasons, the efficiency of filters to remove turbidity was 56.9 % and as result was better than the cold seasons of the year. Table 4 shows the effect of temperature on sand filters and the removal efficiency of the examined parameters.
Table 4.
The effect of temperature on the sand filters efficiency 2016–2017
| Sampling period | Efficiency of sand filters (%) | ||
|---|---|---|---|
| Manganese | Iron | Turbidity | |
| 20th Mar. − 21th Sep. | 56.8 | 69.7 | 56.9 |
| 22th Sep. − 19th Mar. | 48.2 | 61.7 | 50.8 |
Graph 1 also shows the effect of backwashing of sand filters at three speeds of 3.5, 9.2, and 15.3 m h− 1 on the turbidity of the water outlet.
Graph 1.
Comparing the turbidity of the backwashing outlet in different flow rates
According to this figure, with increasing backwashing speed, the time to reach the proper turbidity in the effluent is reduced and the maximum turbidity point is considerably higher at the backwashing rate of 15.3 m h− 1 than turbidity at the same point in the velocity of 3.5 m h− 1.
There was no significant difference in the turbidity of the effluent at 3.5 and 9.2 m h− 1. Only at the beginning of the flow at the rate of 3.5 m h− 1, the turbidity increased to 4000 NTU, which gradually decreases over time. But at 9.2 m h− 1 compared to other speeds, the current calm down sooner and, after 270 s, the turbidity decreases to less than 10 NTU.
Evaluation of WTP efficiency with statistical tests
The amount of kurtosis and skewness of the data showed that the amount of data scatter was outside the range of -2 to + 2. Smirnov-Kolmogorov test was used for more detailed examination and the significance of the recent test was < 0.05. As a result, non-parametric tests such as Spearman correlation were used to evaluate the efficiency of the filters and the reduction of the studied parameters.
The results of the Spearman correlation study for raw water and treated water parameters showed that manganese, iron, and turbidity were significantly reduced after treatment and Sig. (2-tailed) in this correlation for each parameters were 0.0001.
Discussion
Iron and manganese are common contaminants in drinking water sources but are more likely to be present in groundwater aquifers. These elements are normally found in water sources up to a concentration of 1 mg L− 1. However, in some regions such as Mazandaran, iron has been observed in concentrations greater than 6 mg L− 1 [19, 20, 21].
Consumption of water that contains high concentrations of these elements causes a wide range of problems. These problems start with the malfunction of the facilities, such as clogging of the filters and decreasing pipes diameter in the WTP, and continue until the clothes become stained and create an unpleasant taste at the point of use. It is also important to emphasize that long-term exposure to these elements with high doses can also pose health risks [22, 23].
The spectrophotometric atomic absorption experiments results showed that the raw groundwater samples had higher mean concentrations of iron and manganese than standards of drinking water. The highest levels of iron and manganese in Mazandaran groundwater were 6.7 and 0.744 mg L− 1.
After treatment, the amount of manganese in 225 and 136 samples was higher than the maximum permissible limit (0.4 mg L− 1) and maximum contaminant level (0.1 mg L− 1). Also, turbidity in 135 samples couldn’t pass the provisions. Because the water treatment method in this region was conventional and just had units such as; screening, aeration, sedimentation, filtration, disinfection. In these WTPs defined chemical material was not used for manganese/iron coagulating and removing and, the principal is open aeration or pre chlorination. Therefore, WTPs were not efficient in reducing iron, manganese, and turbidity [9, 25]. The existing layouts can utilize other useful methods such as zeolite Y to soften groundwater from manganese and iron [19].
The comparison between the types of layouts in diminishing iron and manganese elements is shown in Graph 2. According to this graph, when a disinfectant is used as an oxidizer at the beginning of the flow and aeration is performed at the same time in module #1, the removal efficiency is higher than other modules. This module can be one of the best choices to reduce the concentration of manganese and iron and, by its nature, the turbidity of drinking water.
Graph 2.
The efficiency comparison between different modules in WTP
As mentioned, WTP uses physical methods to reduce inorganic elements in the study area. While to increase the removal efficiency, more acceptable means, such as biological oxidation and efficiency of bacteria, activated carbon powder, and granules, oxidation with ozone can be used [24]. For example, the mean concentration of manganese and iron in groundwater in some parts of China was 1.58 and 1.12 mg L-1, and the use of inactivated filters containing penicillin and sodium chloride (NaCl) had a removal efficiency of 98 %. Also, in other region, the chemical oxidation had 81 % removal efficiency [25]. Another study showed that the biological bed of filters plays a significant role in reducing manganese and iron. In other words, the formation of a suitable substrate in the filters to remove these elements can depend on the quality of the raw water. So identifying effective microorganisms is a significant step in the removal process [26–28].
In this study, to find one or more basic solutions that are cost-effective and can have acceptable results to reduce the amount of manganese and iron in drinking water and reduce the side effects of their additional presence in the distribution network, the water pollution index (WPI) was used [29]. The WPI is based on the maximum permissible limit and its beneficial approach suggested by WHO. In this study the water quality categorized by concentrations of manganese and iron in treated water. The water pollution listed as excellent, good, moderately polluted and extremely polluted. In this study, this index proved that the quality of drinking water in most of the samples was acceptable. However, 11.62 % of total samples were moderately polluted and about 12.01 % of samples were extremely polluted, chiefly in iron samples. These results reflected that the quality of drinking water, especially outlet of module #2 and #4, request to extra or advanced treatment. The result of one WPI study in Indonesia for river site samples was consistent with our research. Its study expressed the WPI range was from 0.72 to 0.89 and, the quality of water was good [30]. Another study in India revealed that 63 % of total samples was drinkable and other samples needed to treatment processes [31].
Iron and manganese are minerals found in the soil in the form of oxides. Ferric (Fe3+) and manganese (Mn4+) oxides are very insoluble, but when water contains carbon dioxide or acidic conditions, these ions reduce to their lower capacities. As a result, divalent iron and manganese are dissolved in water. The levels of these ions in surface water are usually negligible, but in groundwater sources, they can cause problems for industries, distribution networks, and ultimately consumers. Due to the high amount of these ions in the drinking water samples of the study area, the maximum values of 0.74 and 6.7 mg / l for manganese and iron, from various removal methods such as (1) oxidation, (2) leaching and filtration (3) Ion exchange (4) Stabilization with separating agents and (5) Softening with lime used [32, 33].
The effect of temperature on the removal efficiency of the desired metals in this study showed that air temperature and warm seasons have contributed to the efficiency of sand filters. Most likely, the bacteriological colony was formed in the filter bed. The study of Kwakye-Awuah et al. [19] also showed that the efficiency of the sand filter column in summer was higher than the control column, which was in line with our results.
On the other hand, the viscosity of the flow in hot air is lower than in the cold seasons of the year, which leads to better fluid flow through the filter bed.
This study showed outlet water from filters that are cleaned by slow backwash can be of higher quality than other cleaning methods. Also, if the biological tissue is formed randomly, slow backwashing does not destroy the biological mass-produced.
This study showed that backwashing of WTPs filters in two flow rates, at 3.5 and 9.2 m h− 1, there was less turbulence and turbidity than 15.3 m h− 1 flow rate. But in the first flow at a speed of 3.5 m h− 1, in the first 60 s of backwashing, turbidity increased to 4000 NTU. Proper washing of the filters seems to require a specific surface velocity, which was seen at a flow rate with a 9.2 m h− 1 speed [27].
Numerous studies have shown that slow sand filtration reduces a wide range of pollutants, especially turbidity. As these filters are an acceptable option for wastewater treatment, they are suitable for treating groundwater has less pollution variability [34, 35].
Conclusion
About a third of the world’s population uses groundwater for various purposes. Due to the high water level in groundwater aquifers in Mazandaran province, one of the ablest choices is to extract groundwater for drinking. In summary, the results of this study in Mazandaran province showed that the concentrations of manganese and iron in groundwater are higher than national and international standards. The use of different WTP modules indicated that Module #1, which uses aeration and chemicals for pre-disinfection, was more efficient than other layouts and it was the best suitable module for diminishing manganese and iron from groundwater. The WPI for manganese and iron contaminants in drinkable water for 95.74 and 53.88 % of samples were excellent but in 1.16 % (Mn2+) and 12.02 % (Fe2+) of samples, WPI was extremely polluted. The index shows that existing modules only cannot lead to a significant reduction in pollutants and should to use other chemicals and equipment to reach the standard limits and requirements for drinking water. Also, the air temperature affected these modules and, in the warm season, the removal of manganese and iron has been higher than in the cold season. Backwashing of sand filters showed that the use of a constant surface velocity (in this research; 9.2 m h−1) can reduce turbidity in the output stream.
In this study, distorting inputs such as sampling on days of prolonged and disturbing rains, the service life of the filters, and their off-line time were eliminated, which is suggested to be considered as effective variables in future studies.
Acknowledgements
This research is the result of a master’s degree thesis. The authors of this study sincerely appreciate the consideration of Tehran University of Medical Sciences and the Water and Wastewater Engineering Organization of Mazandaran Province.
Declarations
Conflict of interest
The authors of this study explain that there is no conflict of interest with each other.
Footnotes
The original online version of this article was revised: The order of author(s) was incorrect in the original article.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/15/2021
A Correction to this paper has been published: 10.1007/s40201-021-00700-2
Contributor Information
Mahmood Alimohammadi, Email: m_alimohammadi@tums.ac.ir.
Abdollah Rashidi Mehrabadi, Email: a_rashidi@sbu.ac.ir.
References
- 1.Abolli S, et al. Comparing groundwater fluoride level with WHO guidelines and classifying at-risk age groups; based on health risk assessment. Int J Environ Anal Chem. 2020 doi: 10.1080/03067319.2020.1863389. [DOI] [Google Scholar]
- 2.Postawa A, et al. Best practice guide on the control of iron and manganese in water supply. London: IWA Publishing; 2013.
- 3.Singh KP, et al. Chemometric analysis of groundwater quality data of alluvial aquifer of Gangetic plain, North India. Anal Chim Acta. 2005;550(1–2):82–91. doi: 10.1016/j.aca.2005.06.056. [DOI] [Google Scholar]
- 4.Abolli S, et al. Survey of drinking water quality of household water treatment and public distribution network in Garmsar city, under the control of water safety plan. Iran J Health Environ. 2019;12(3):477–88. [Google Scholar]
- 5.Abolli S, et al. Water safety plan: A novel approach to evaluate the efficiency of the water supply system in Garmsar. Desalin Water Treat. 2021;211:210–220. doi: 10.5004/dwt.2021.26617. [DOI] [Google Scholar]
- 6.Fathabad AE, et al. Determination of heavy metal content of processed fruit products from Tehran’s market using ICP-OES: a risk assessment study. Food Chem Toxicol. 2018;115:436–46. doi: 10.1016/j.fct.2018.03.044. [DOI] [PubMed] [Google Scholar]
- 7.Gage B. Biological iron and manganese removal, pilot and full scale applications. in 2001 Ontario Water Works Association Conference, Toronto, Ontario, Canada; 2001.
- 8.Yavuz H, Say R, Denizli A. Iron removal from human plasma based on molecular recognition using imprinted beads. Mater Sci Eng C. 2005;25(4):521–8. doi: 10.1016/j.msec.2005.04.005. [DOI] [Google Scholar]
- 9.Namdeo M, Bajpai S. Chitosan–magnetite nanocomposites (CMNs) as magnetic carrier particles for removal of Fe (III) from aqueous solutions. Colloids Surf A. 2008;320(1–3):161–8. doi: 10.1016/j.colsurfa.2008.01.053. [DOI] [Google Scholar]
- 10.Marsidi N, Hasan HA, Abdullah SRS. A review of biological aerated filters for iron and manganese ions removal in water treatment. J Water Process Eng. 2018;23:1–12. doi: 10.1016/j.jwpe.2018.01.010. [DOI] [Google Scholar]
- 11.Radfard M, et al. Drinking water quality and arsenic health risk assessment in Sistan and Baluchestan, Southeastern Province, Iran. Hum Ecol Risk Assess Int J. 2019;25(4):949–65. doi: 10.1080/10807039.2018.1458210. [DOI] [Google Scholar]
- 12.Zakeri HR, et al. Chemical coagulation-electro fenton as a superior combination process for treatment of dairy wastewater: performance and modelling. Int. J. Environ. Sci. Technol. 2021 doi: 10.1007/s13762-021-03149-w. [DOI] [Google Scholar]
- 13.Yousefi M, et al. Data on corrosion and scaling potential of drinking water resources using stability indices in Jolfa, East Azerbaijan, Iran. Data in brief. 2018;16:724–31. [DOI] [PMC free article] [PubMed]
- 14.Diaz-Alarcón J, et al. Removal of iron and manganese in groundwater through magnetotactic bacteria. J Environ Manag. 2019;249:109381. doi: 10.1016/j.jenvman.2019.109381. [DOI] [PubMed] [Google Scholar]
- 15.Casalini LC, et al. Manganese removal efficiencies and bacterial community profiles in non-bioaugmented and in bioaugmented sand filters exposed to different temperatures. J Water Process Eng. 2020;36:101261.
- 16.Agency USEP, Method 180.1: Determination of turbidity by nephelometry. Environmental Monitoring Systems Laboratory office of Research and Development U.S. Environmental Protection Agency Cincinnati, Ohio 45268; 1993.
- 17.Suriadikusumah A, et al. Analysis of the water quality at Cipeusing river, Indonesia using the pollution index method. Acta Ecol Sin. 10.1016/j.chnaes.2020.08.001
- 18.Hossain M, Patra PK. Water pollution index–A new integrated approach to rank water quality. Ecol Ind. 2020;117:106668. doi: 10.1016/j.ecolind.2020.106668. [DOI] [Google Scholar]
- 19.Kwakye-Awuah B, et al. Adsorptive removal of iron and manganese from groundwater samples in ghana by zeolite Y synthesized from bauxite and kaolin. Water. 2019;11(9):1912.
- 20.Aziz HA, Smith PG. Removal of manganese from water using crushed dolomite filtration technique. Water Res. 1996;30(2):489–92. doi: 10.1016/0043-1354(95)00178-6. [DOI] [Google Scholar]
- 21.Jensen DL, et al. Speciation of dissolved iron (II) and manganese (II) in a groundwater pollution plume. Environ Sci Technol. 1998;32(18):2657–64.
- 22.Sarin P, et al. Iron release from corroded iron pipes in drinking water distribution systems: effect of dissolved oxygen. Water Res. 2004;38(5):1259–69. [DOI] [PubMed]
- 23.Aschner M, et al. Manganese and its role in Parkinson’s disease: from transport to neuropathology. Neuromol Med. 2009;11(4):252–66. [DOI] [PMC free article] [PubMed]
- 24.Barloková D, Ilavský J. Removal of iron and manganese from water using filtration by natural materials. Pol J Environ Stud. 2010;19(6):1117–22.
- 25.Yang H, et al. Removal of manganese from groundwater in the ripened sand filtration: Biological oxidation versus chemical auto-catalytic oxidation. Chem Eng J. 2020;382:123033.
- 26.Štembal T, et al. Removal of ammonia, iron and manganese from groundwaters of northern Croatia—pilot plant studies. Process Biochem. 2005;40(1):327–35.
- 27.de Souza ÁHC, et al. Backwash process in a sand filter prototype used in irrigation. Agric Eng Int CIGRJ. 2019;21(1):109–14.
- 28.Wołowiec M, et al. The properties of sludge formed as a result of coagulation of backwash water from filters removing iron and manganese from groundwater. SN Appl Sci. 2019;1(6):639.
- 29.Milanović A, et al. Assessment of polluting effects and surface water quality using water pollution index: a case study of Hydro-system Danube-Tisa-Danube. Serbia. Carpathian J Earth Environ Sci. 2011;6(2):269–277. [Google Scholar]
- 30.Effendi H. River water quality preliminary rapid assessment using pollution index. Procedia Environ Sci. 2016;33(1):562–7.
- 31.Adimalla N. Application of the Entropy Weighted Water Quality Index (EWQI) and the Pollution Index of Groundwater (PIG) to assess groundwater quality for drinking purposes: a case study in a rural area of Telangana State, India. Arch Environ Contam Toxicol. 2021;80:31–40 [DOI] [PubMed]
- 32.Saleh HN, et al. Carcinogenic and non-carcinogenic risk assessment of heavy metals in groundwater wells in Neyshabur Plain, Iran. Biol Trace Elem Res. 2019;190(1):251–61. [DOI] [PubMed]
- 33.Yousefi M, et al. Data on water quality index for the groundwater in rural area Neyshabur County, Razavi province, Iran. Data Brief. 2017;15:901–7. [DOI] [PMC free article] [PubMed]
- 34.Drechsel P, et al. Reducing health risks from wastewater use in urban and peri-urban sub-Saharan Africa: applying the 2006 WHO guidelines. Water Sci Technol. 2008;57(9):1461–6. doi: 10.2166/wst.2008.245. [DOI] [PubMed] [Google Scholar]
- 35.Sobsey MD, et al. Point of use household drinking water filtration: a practical, effective solution for providing sustained access to safe drinking water in the developing world. Environ Sci Technol. 2008;42(12):4261–67. [DOI] [PubMed]