Abstract
Background and aims
Gestational diabetes mellitus (GDM) has high prevalence worldwide. This study aimed to evaluate the fasting plasma glucose (FPG) cutoffs at first prenatal visit and at 24–28th of gestational weeks to avoid obtaining full oral glucose-tolerance test (OGTT) in the diagnosis of GDM.
Methods
This study was a cross-sectional study conducted in Tehran, Iran during October 2016 and November 2017. All pregnant women reporting for the first routine prenatal visit before 20th week of gestational age were included in this study. Participants without overt diabetes mellitus at first prenatal visit, underwent OGTT at 24–28th of gestational weeks.
Results
Totally 952 pregnant women with mean age of 26.4 ± 14.1 years took part in this study. The prevalence of GDM was 12.7% (mostly diagnosed based on the FPG alone). FPG cutoffs 75 and 80 mg/dL at first prenatal visit and at 24–28th of gestational weeks can rule out the GDM with high sensitivity and negative predictive value, respectively. FPG cutoffs 85 and 90 mg/dL at first prenatal visit and at 24–28th of gestational weeks had high capacity, excellent specificity and positive predictive value in diagnosing GDM, respectively.
Conclusions
Performing only the FPG and considering FPG cutoffs 75 and 80 mg/dL at first prenatal visit and at 24–28th of gestational weeks can be a useful tool predicting the incidence of GDM, respectively, and had similar diagnostic power.
Keywords: Diabetes, gestational; Pregnant women; Prenatal care; Glucose-tolerance test
Introduction
Gestational diabetes mellitus (GDM), defined as diabetes diagnosed during pregnancy in women without overt diabetes mellitus (DM), is one of the most important and common metabolic disorders during pregnancy [1]. GDM had wide range growing prevalence in different studies from 7.9 to 24.9% [2–5]. GDM can cause various complications for both mother and her offspring like increasing the risk of type 2 DM, premature cardiovascular disease in mother and macrosomia, obesity, hypoglycemia, diabetes, hypertension, and cardiovascular disease in youth and adult life in the offspring [6–12]. Therefore, early diagnosis of GDM is of paramount importance in preventing these complications. In 2010, International Association of Diabetes and Pregnancy Study Groups (IADPSG) recommended performing 2 h 75 g oral glucose-tolerance test (OGTT) for diagnosing GDM in the 24–28th of gestational weeks in all pregnant women who did not have history of overt diabetes [7].
OGTT had high potency of diagnosing GDM in different studies [13, 14]. However, some studies stated that OGTT is poorly acceptable because of complexity of test which requires prior appointments, taking oral glucose, prolonged waiting time and poorly cost-effectiveness, thus, leading to limited implementation. Physicians tried to choose an alternative and more acceptable strategy for diagnosing GDM to decrease the number of pregnant women who need to perform OGTT [5–7]. In this regard, some studies mentioned that fasting plasma glucose (FPG) has an excellent efficacy as screening diagnostic test for GDM especially in the low threshold which has great impact on ruling out GDM women [2, 3, 15–17]. The prevalence of GDM diagnosis with FPG alone was in the wide range from 24% in Bangkok and Thailand to 73% in Bellflower, California, and United States [5, 18].
Choosing the FPG cutoff point with the best sensitivity and specificity is still controversy. A study in 2018 on 6520 pregnant women in India showed that the prevalence of GDM was 18.3% and FPG cutoff 76 mg/dL showed high sensitivity and negative predictive value (NPV) and FPG cutoff 92 mg/dL showed high specificity and positive predictive value (PPV) for diagnosing GDM [5].
Due to the limited studies calculating the FPG cutoff point as high potent screening test with low false positive and false negative, the aim of this study was to evaluate the FPG cutoff point at first prenatal visit and at 24–28th of gestational weeks to avoid obtaining full OGTT in the diagnosis of GDM.
Materials and methods
Study design
This study was a cross-sectional study conducted at three obstetrics and gynecology clinics in the north, south, and center of Tehran, affiliated to Shahid Beheshti University of Medical Sciences, in Tehran, Iran between October 2016 and November 2017. The Ethics Committee of Ethic approval in Shahid Beheshti University of Medical Sciences approved the executive protocol of the study. The study was conducted in accordance with the Declaration of Helsinki (seventh revision 2013). Written consent form was taken from all participants prior to entering the study. This study did not impose any additional costs on the patients or on the health system.
Participants
All pregnant women reporting for the first routine prenatal visit before 20th week of gestational age were included in this study. Pregnant women with the failure to complete the glucose-tolerance test, receiving medications effective on the glucose metabolism, suffering from chronic liver disease or endocrine disorders like overt diabetes, and first prenatal visit after 20th weeks of gestation were excluded.
Data gathering
Participant were first asked to fill out a demographic and health surveys questionnaire at first prenatal visit prior to 20th week of pregnancy including age, past medical history (PMH) and family history of diabetes mellitus in first-degree relatives. The PMHs were as follows: history of abortion, still birth, polycystic ovary syndrome, macrosomia, low birth weight, congenital malformation, GDM in previous pregnancy, impaired fasting glycaemia or impaired glucose tolerance, hypertension, and gestational hypertension. The number of gravid and gestational age at first prenatal visit based on the last menstrual period or transabdominal uterus ultrasound were also assessed at first prenatal visit. The gestational weight gain was evaluated based on the difference of weight between the last measurement before pregnancy and childbearing. The researcher measured the weight, height and systolic blood pressure (SBP) and diastolic blood pressure of the participants using the standard clinic’s scale. The FPG and glycosylated hemoglobin (HbA1c) was measured in all participants. FPG level was measured on patients’ blood sample after 8 h of fasting. Overt DM was diagnosed according to the last American Diabetes Association (ADA) criteria with FPG ≥ 126 mg/dL or HgbA1c ≥ 6.5% at first prenatal visit [19].
All participants without overt DM at first prenatal visit underwent standard OGTT receiving 75 g of glucose oral solution during 24th and 28th of gestational weeks in pregnancy [19]. Prior to performing OGTT, the participants were asked to fast at least 8 h and the blood samples were taken at first and then 1 and 2 h after glucose solution consumption (1 h and 2 h). The laboratory tests were performed in a central laboratory using enzymatic glucose oxidase method by Man lab Kit. GDM was diagnosed based on the IADPSG criteria (FPG ≥ 92 mg/dL, 1-h post-glucose challenge ≥ 180 mg/dL or 2-h post-glucose challenge ≥ 153 mg/dL) [19].
Statistics
All data were analyzed with SPSS version 24.0 (SPSS Inc., Chicago, IL., USA). Categorical variables were described using frequency (percentage) of the data. Continuous variables were described using mean ± standard deviation of the data. An Independent Samples t test was applied to compare continuous variables between pregnant women with and without GDM. The association between categorical variables was assessed using Chi-square test or Fisher’s Exact test. The sensitivity, specificity, PPV, NPV, positive likelihood ratio (LR+), and negative likelihood ratio (LR-) of different range of FPG at first prenatal visit and at 24–28th of gestational weeks in diagnosing GDM were measured using statistical tests. ROC curve and the area under the curve (AUC) were also examined to calculate the strengths of the FPG and determine the best cutoff point for diagnosing GDM. Linear logistic regression analysis was performed to determine the relationship between predictor variables and FPG level at first prenatal visit. Binary logistic regression analysis was performed to determine the relationship between predictor variables and incidence of GDM. The results were expressed as an odds ratio with 95% confidence interval. P < 0.05 was considered statistically significant.
Results
In the present study, 1020 pregnant women reported for the first prenatal visit. Thirty-three (3.2%) had overt DM and 35 lost the follow-up and all were excluded from the study. Nine hundred and fifty-two pregnant women with maximum and minimum age of 16 and 43 years took part in this study.
One hundred and twenty-one pregnant women (12.7%) had GDM. GDM was diagnosed based on the FPG, 1-h post-glucose challenge, or 2-h post-glucose challenge above the cutoff point in 105 (86.8%), 81 (67%), or 38 (31.4%) of pregnant women, respectively. The GDM diagnosis based on one, two and three criteria were 46 (38%), 38 (31.4%), and 37 (30.6%), respectively.
Baseline characteristics and the difference between GDM and non-GDM women are shown in Table 1. The frequency of gravid one, three and more than three, past medical history of risk factors for GDM and family history of DM showed significant difference among category of GDM (P < 0.05). The mean of age, weight, body mass index (BMI), SBP, gestational weight gain, FPG level at first prenatal visit and HbA1c level was significantly higher in women with GDM (P < 0.05) (Table 1).
Table 1.
Baseline characteristics of studied population
| Variables | Total women (n = 952) | GDM (+) (n = 121) | GDM (−) (n = 831) | p valuea |
|---|---|---|---|---|
| Gravidity, N (%)c | ||||
| One | 451 (47.4) | 33 (27.3) | 418 (50.3) | < 0.001 |
| Two | 334 (35.1) | 38 (31.4) | 296 (35.6) | 0.359 |
| Three | 118 (12.4) | 32 (26.4) | 86 (10.3) | < 0.001 |
| More than three | 48 (5) | 18 (14.9) | 30 (3.6) | < 0.001 |
| Past medical history, N (%) | ||||
| Abortion | 105 (11) | 33 (27.3) | 72 (8.7) | < 0.001 |
| Still birth | 7 (0.7) | 5 (4.1) | 2 (0.2) | 0.001b |
| PCOS | 21 (2.2) | 9 (7.4) | 12 (1.4) | < 0.001 |
| Macrosomia | 6 (0.6) | 6 (5) | 0 | < 0.001b |
| LBW | 18 (1.9) | 8 (6.6) | 10 (1.2) | < 0.001 |
| Congenital malformation | 1 (0.1) | 0 | 1 (0.1) | 1.00b |
| GDM in previous pregnancy | 9 (0.9) | 8 (6.6) | 1 (0.1) | < 0.001b |
| IFG or IGT | 3 (0.3) | 3 (2.5) | 0 | < 0.002b |
| HTN | 3(0.3) | 2(1.7) | 1 (0.1) | 0.044b |
| Gestational HTN | 11 (1.2) | 9 (7.4) | 2 (0.2) | < 0.001b |
| Family history of DM in first-degree relatives | 87 (9.1) | 20 (16.5) | 67 (8.1) | 0.003 |
| Age (year), mean ± SD | 26.4 ± 14.1 | 28.5 ± 4.8 | 26.1 ± 3.9 | < 0.001 |
| Weight (kg), mean ± SD | 65.7 ± 8.5 | 72.8 ± 9.7 | 64.7 ± 7.8 | < 0.001 |
| BMI (kg/m2), mean ± SD | 25.3 ± 3.2 | 28 ± 3.6 | 24.9 ± 2.9 | < 0.001 |
| SBP (mmHg), mean ± SD | 112.9 ± 10.2 | 117.8 ± 11.3 | 112.3 ± 9.9 | < 0.001 |
| DBP (mmHg), mean ± SD | 70.9 ± 8 | 72.3 ± 8.8 | 70.7 ± 7.9 | 0.107 |
| Gestational age at first prenatal visit (week), mean ± SD | 12.5 ± 6 | 12.9 ± 5.3 | 12.4 ± 6.2 | 0.412 |
| Gestational weight gain (kg), mean ± SD | 5.6 ± 1.9 | 8.1 ± 2.1 | 5.2 ± 1.6 | < 0.001 |
| FPG level at first prenatal visit (mg/dL), mean ± SD | 78.7 ± 8.4 | 93.1 ± 8.7 | 76.7 ± 5.9 | < 0.001 |
| HbA1c (%), mean ± SD | 4.6 ± 0.7 | 5.5 ± 0.6 | 4.5 ± 0.6 | < 0.001 |
GDM gestational diabetes mellitus; DM diabetes mellitus; PCOS polycystic ovary syndrome; LBW low birth weight; IFG impaired fasting glucose; IGT impaired glucose-tolerance test; HTN hypertension; SD standard division; BMI body mass index; SBP systolic blood pressure; DBP diastolic blood pressure; FBS fasting plasma glucose; HbA1c glycosylated hemoglobin
ap value refers to the relationship of each variable and category of GDM
bp value refers to the relationship between variables based on Fisher’s Exact test
cRefers to one missing data in the category of gravid
Linear regression analysis showed that increasing 10 years of age can increase 5.91 and 4.14 mg/dL of FPG level at first prenatal visit (P < 0.001) and at 24–28th of gestational weeks (P < 0.001), respectively. Increasing 10 kg of weight can increase 2.94 and 2.8 mg/dL of FPG level at first prenatal visit (P < 0.001) and at 24–28th of gestational weeks (P < 0.001), respectively. Increasing one unit of BMI can increase 0.74 and 0.65 mg/dL of FPG level at first prenatal visit (P < 0.001) and at 24–28th of gestational weeks (P < 0.001), respectively.
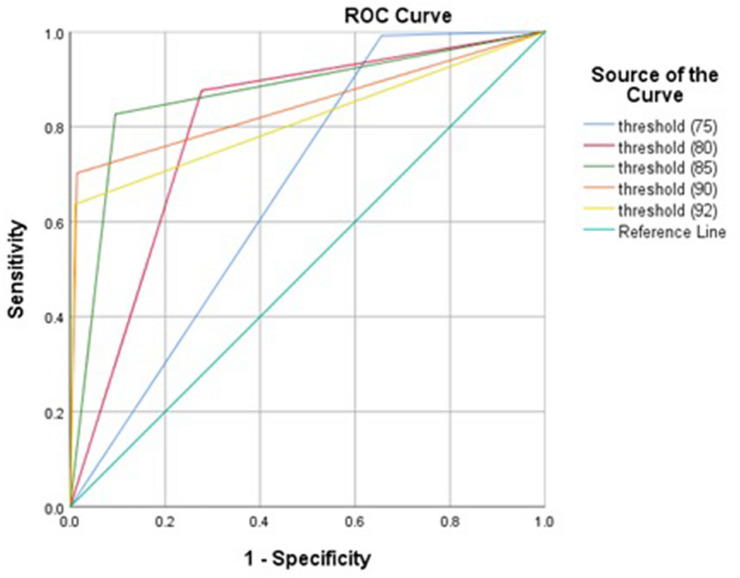
FPG cutoff 75 mg/dL at first prenatal visit can predict the incidence of GDM with highest sensitivity and NPV. FPG cutoff 85 mg/dL at first prenatal visit showed high capacity (AUC = 0.866, 95% CI 0.825–0.907) and excellent specificity in diagnosing GDM (Table 2 and Fig. 1). Two hundred and eighty-seven pregnant women were with FPG of less than 75 mg/dL at first prenatal visit and only one of them was diagnosed with GDM later. In addition, 616 pregnant women were with FPG of less than 80 mg/dL at first prenatal visit of whom 15 were diagnosed with GDM later. The clinical characteristics of those pregnant women are described in Table 3.
Table 2.
Statistic tests of FPG level at first prenatal visit in diagnosing GDM
| FPG level (mg/dL) at first prenatal visit | No of women < threshold, n (%) | Area | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | LR + | LR − |
|---|---|---|---|---|---|---|---|---|
| 75 | 287 | 0.668 | 99.2 | 34.4 | 18 | 99.7 | 1.51 | 0.02 |
| 80 | 616 | 0.800 | 87.6 | 72.3 | 31.5 | 97.6 | 3.16 | 0.17 |
| 85 | 773 | 0.866 | 82.6 | 90.5 | 55.9 | 97.3 | 8.69 | 0.19 |
| 90 | 855 | 0.844 | 70.2 | 98.6 | 87.6 | 95.8 | 50.14 | 0.30 |
| 92 | 867 | 0.813 | 63.6 | 99 | 90.6 | 94.9 | 63.6 | 2.72 |
FPG fasting plasma glucose; PPV positive predictive value; NPV negative predictive value; LR likelihood ratio
Fig. 1.
ROC curve of fasting plasma glucose level at first prenatal visit in diagnosing gestational diabetes mellitus
Table 3.
Clinical characteristics of pregnant women with FPG of less than 80 mg/dL at first prenatal visit who were diagnosed with GDM later
| FPG range | Age (years) | Gestational age at first prenatal visit, weeks | BMI (kg/m2) | Gravidity, No | Past medical history | Positive criteria of OGTT |
|---|---|---|---|---|---|---|
| 1a | 25 | 11 | 22.41 | 1 | N/A | FPG (97 mg/dL) |
| 2 | 27 | 6 | 23.88 | 3 | Abortion | 1 h OGTT (189 mg/dL) and 2 h OGTT (157 mg/dL) |
| 3 | 26 | – | 35.14 | 1 | N/A | FPG (100 mg/dL) and 1 h OGTT (182 mg/dL) |
| 4 | 24 | 8 | 28.76 | 2 | N/A | FPG (95 mg/dL), 1 h OGTT (189 mg/dL) and 2 h OGTT(154 mg/dL) |
| 5 | 20 | 18 | 28.72 | 1 | N/A | FPG (99 mg/dL) and 1 h OGTT (186 mg/dL) |
| 6 | 23 | 19 | 25.39 | 1 | N/A | FPG (94 mg/dL) and 1 h OGTT (189 mg/dL) |
| 7 | 29 | 19 | 27.92 | 3 | Abortion | FPG (97 mg/dL), 1 h OGTT (196 mg/dL) and 2 h OGTT(174 mg/dL) |
| 8 | 30 | 19 | 29.76 | 2 | Gestational hypertension | 1 h OGTT (187 mg/dL) |
| 9 | 25 | 19 | 28.69 | 1 | N/A | 1 h OGTT (187 mg/dL) |
| 10 | 26 | 18 | 26.64 | 2 | N/A | FPG (95 mg/dL) |
| 11 | 27 | 19 | 27.34 | 2 | N/A | FPG (96 mg/dL) and 1 h OGTT (187 mg/dL) |
| 12 | 27 | 10 | 31.96 | 2 | N/A | FPG (98 mg/dL) and 1 h OGTT (184 mg/dL) |
| 13 | 29 | 18 | 36.79 | 3 | Abortion, GDM and impaired fasting glycaemia or impaired glucose tolerance | FPG (117 mg/dL), 1 h OGTT (200 mg/dL) and 2 h OGTT(174 mg/dL) |
| 14 | 30 | 19 | 30.85 | 4 | Gestational hypertension, Abortion and family history of DM | FPG (94 mg/dL) and 1 h OGTT (185 mg/dL) |
| 15 | 34 | 19 | 34.05 | 3 | Gestational hypertension and GDM | FPG (92 mg/dL) and 1 h OGTT (185 mg/dL) |
aThe only woman with FPG of less than 75 mg/dL
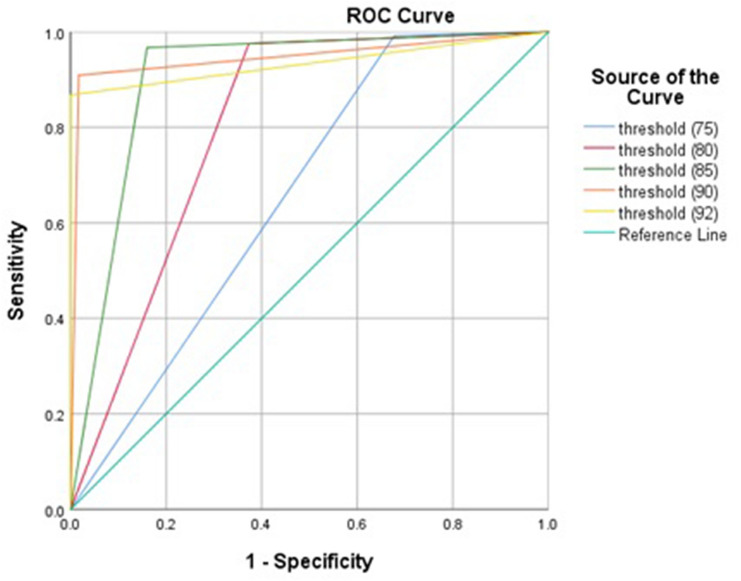
FPG cutoff 80 mg/dL at 24–28th of gestational weeks can predict the incidence of GDM with highest sensitivity and NPV. FPG cutoff 90 mg/dL at 24–28th of gestational weeks showed high capacity (AUC = 0.946, 95% CI 0.916–0.976), excellent specificity, PPV and LR- in diagnosing GDM (Table 4 and Fig. 2).
Table 4.
Statistic tests of FPG level at 24–28th of gestational weeks in diagnosing GDM
| FPG (mg/dL) at OGTT | No of women < threshold, n (%) | Area | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | LR + | LR − |
|---|---|---|---|---|---|---|---|---|
| 75 | 269 | 0.657 | 99.2 | 32.3 | 17.6 | 99.6 | 1.46 | 0.02 |
| 80 | 524 | 0.801 | 97.5 | 62.7 | 27.6 | 99.4 | 2.61 | 0.03 |
| 85 | 702 | 0.903 | 96.7 | 84 | 46.8 | 99.4 | 6.04 | 0.03 |
| 90 | 828 | 0.946 | 90.9 | 98.3 | 88.7 | 98.7 | 53.47 | 0.09 |
| 92 | 847 | 0.934 | 86.8 | 100 | 100 | 98.1 | – | 0.13 |
FPG fasting plasma glucose; PPV positive predictive value; NPV negative predictive value; LR likelihood ratio
Fig. 2.
ROC curve of fasting plasma glucose level at 24–28th weeks of gestation in diagnosing gestational diabetes mellitus
Table 5 shows the binary logistic regression and estimating the risk of GDM incidence in pregnant women with some risk factors and the results showed that the risk of GDM was significantly lower in pregnant women with gravid one and significantly higher in those with family history of DM, history of abortion, age range, BMI range and above all FPG threshold at first prenatal visit and at 24–28th of gestational weeks.
Table 5.
Logistic regression analysis as predictor of GDM in pregnant women
| Variablesa | GDM (+) (n = 121) | GDM (−) (n = 831) | p valuea | OR (CI) |
|---|---|---|---|---|
| Gravidityc | ||||
| One | 33 | 418 | < 0.001 | 0.37 (0.24–0.56) |
| More than one | 88 | 412 | ||
| Family history of DM | ||||
| Positive | 20 | 67 | < 0.001 | 2.25 (1.31–3.87) |
| Negative | 101 | 764 | ||
| History of abortion | ||||
| Positive | 33 | 72 | < 0.001 | 3.95 (2.47–6.30) |
| Negative | 88 | 759 | ||
| Age range (kg) | ||||
| ≥ 25 | 104 | 562 | < 0.001 | 2.92 (1.71–4.98) |
| < 25 | 17 | 269 | ||
| BMI range (kg/m2) | ||||
| ≥ 25 | 99 | 372 | < 0.001 | 5.55 (3.43–8.98) |
| < 25 | 22 | 459 | ||
| FPG at first prenatal visit | ||||
| ≥ 75 | 120 | 545 | < 0.001b | 62.97 (8.75–453.05) |
| < 75 | 1 | 286 | ||
| ≥ 80 | 106 | 230 | < 0.001 | 18.46 (10.53–32.38) |
| < 80 | 15 | 601 | ||
| ≥ 85 | 100 | 79 | < 0.001 | 45.32 (26.82–76.58) |
| < 85 | 21 | 752 | ||
| ≥ 90 | 85 | 12 | < 0.001 | 161.14 (80.79–321.42) |
| < 90 | 36 | 819 | ||
| ≥ 92 | 77 | 8 | < 0.001 | 180.03 (81.81–396.16) |
| < 92 | 44 | 823 | ||
| FPG at 24th–28th of gestational weeks | ||||
| ≥ 75 | 120 | 563 | < 0.001b | 57.12 (7.93–411.03) |
| < 75 | 1 | 268 | ||
| ≥ 80 | 118 | 310 | < 0.001b | 66.10 (20.83–209.7) |
| < 80 | 3 | 521 | ||
| ≥ 85 | 117 | 133 | < 0.001b | 153.5 (55.7–423.03) |
| < 85 | 4 | 698 | ||
| ≥ 90 | 110 | 14 | < 0.001 | 583.57 (258.46–1317.59) |
| < 90 | 11 | 817 |
OR odds ratio; CI confidence interval; GDM gestational diabetes mellitus; DM diabetes mellitus; BMI body mass index; FPG fasting plasma glucose
ap value refers to the relationship between each variables and incidence of GDM
bp value refers to the relationship between variables based on Fisher’s Exact test
cRefers to one missing data in the category of gravid
Discussion
The present study investigated the predictive value of FPG for diagnosing GDM at first prenatal visit and at 24–28th of gestational weeks. The results showed that diagnosis of GDM was mostly based on one criterion and that was FPG level above the cutoff point. FPG level under the threshold of 75 and 80 mg/dL at first prenatal visit and at 24–28th of gestational weeks can rule out the GDM with high sensitivity and NPV, respectively, and both thresholds showed similar diagnostic power. FPG cutoff 85 and 90 mg/dL at first prenatal visit and at 24–28th of gestational weeks showed high capacity and excellent specificity and LR- in predicting the incidence of GDM, respectively.
GDM is a first recognition hyperglycemia during pregnancy with high prevalence worldwide [8]. Physicians screen all pregnant women to avoid the complications both in mothers and offspring. Besides the recommendation of IADPSG, various studies reported large number of pregnant women who did not perform OGTT and found this recommendation very demanding. OGTT has many drawbacks and is expensive and hard for administration [5–7]. Therefore, finding an alternative test in all pregnant women is necessary to decrease the number of underdiagnosed GDM. Various studies applied FPG as an acceptable screening test to detect GDM. The sensitivity, specificity, PPV and NPV was different, which may be due to the different GDM criteria, time of test performance and FPG cutoffs. Finding the best FPG cutoff with excellent efficacy is of paramount importance to avoid performing full OGTT [5, 20, 21].
Agarwal et al. found that FPG showed high capacity (AUC: 0.907) in detecting GDM based on the IADPSG criteria and recommended the FPG cutoff of 80 mg/dL due to its excellent sensitivity (95.4%) [4]. Zhu et al. recommended FPG cutoff of 80 mg/dL as an excellent predictor due to the AUC of 0.836 and sensitivity of 87.8% [22]. A study in 6520 pregnant women in South Asia by Agarwal et al. showed that the prevalence of GDM was 18.3% of which 67.9% was diagnosed based on FPG criterion alone. FPG cutoff of 80 mg/dL showed sensitivity, specificity, PPV, NPV, LR + , and LR- of 92.6%, 55.7%, 31.9%, 97.1%, 2.09, and 0.13, respectively, based on the IADPSG criteria [5]. Reyes et al. showed that performing FPG for detecting GDM at cutoff point of 90 mg/dL helped avoiding unnecessary OGTT in 90.7% of pregnant women [6]. Different studies agreed with the high efficacy of FPG in detecting GDM, but the result of our study showed higher sensitivity and NPV in FPG cutoff point of 80 mg/dL.
Trujillo et al. investigated the prevalence of GDM in 4916 pregnant women at 24–28th of gestational weeks in Brazil. The results showed that 18% and 15.6% of patients had GDM based on the full IADPSG criteria and FPG criterion alone, respectively. FPG value showed excellent capacity (AUC: 0.96) to detect GDM. The FPG cutoff point of 80 mg/dL showed high sensitivity (96.9%) and NPV (98.8%) and cutoff point of 90 mg/dL showed high specificity (95.1%) and PPV (79.8%) for detecting GDM. This study emphasized on the efficacy of FPG alone for diagnosing GDM [7]. The results were consistent with ours.
Our study evaluated the efficacy of FPG both at first prenatal visit and at 24–28th of gestational weeks and showed that time of performing FPG had no priority in detecting GDM. However, a study by Sacks et al. stated that FPG can be a good predictor when performed later compared to earlier in pregnancy [23].
Our study showed that the frequency of gravid one, three and more than three, past medical history of risk factors for GDM, family history of DM, mean of age, weight, BMI, SBP, gestational weight gain, FPG level at first prenatal visit and HbA1c level had significant difference with incidence of GDM. In this regard, various studies investigated the possible risk factors associated with incidence of GDM. GDM was significantly higher in pregnant women with older age, poorer glycemic control, and higher BMI, which may be due to the reduction of insulin sensitivity in those women [1, 4, 6, 8].
Most of the studies assessed the diagnostic accuracy of lowest FPG level in diagnosing GDM and only few studies assessed the diagnostic accuracy of lowest FPG level in ruling out GDM. The FPG measurement was mostly once in the first prenatal visit and the limitation of one time measurements of FPG level, was the low accurate results. In this regard, the strengths of our study were the assessment of the predictive value of FPG for ruling out GDM both at first prenatal visit and at 24–28th of gestational weeks.
Limitations
This study evaluated the FPG diagnostic power for GDM in two stages at early pregnancy and third trimester in large population of pregnant women that may fade the effect of other associated variables like BMI and age. The results of our study may not be applicable to the general population with different races. This study did not investigate the possible associated factors such as going on special diets, exercise or other physicians’ recommendations for decreasing the FPG level, especially in patients with upper limit of normal FPG at first prenatal visit. The past medical history was self-reporting which might increase the bias. The association between FPG level and GDM was not assessed after adjusting the possible confounding factors. Therefore, it is highly recommended to investigate the prevalence and different diagnostic methods of GDM after adjusting all possible associated factors in a larger sample size at multi-center in future studies to increase the external validity of the results.
Conclusions
Performing only the FPG at first prenatal visit or at 24–28th of gestational weeks can be a useful tool for predicting the incidence of GDM with similar diagnostic power. FPG level under the threshold of 75 and 80 mg/dL at first prenatal visit and at 24–28th of gestational weeks can rule out GDM with high sensitivity and NPV, respectively. Therefore, the pregnant women with FPG level under the aforementioned thresholds would not require further tests such as OGTT for diagnosing GDM.
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contributions
All the authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MN, FH, FF and MA. The first draft of the manuscript was written by SB and EA and all the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector.
Human right statement
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration (seventh revision 2013). The implementation of the project was approved by the ethics committee of Shahid Beheshti University of Medical Sciences (Approval number: IR.SBMU.MCP.REC.1395.563) in 2016.
Informed consent
Informed consent for it was obtained from all patients for being included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Niroomand M, Afsar J, Hosseinpanah F, Afrakhteh M, Farzaneh F, Serahati S. Comparison of the International Association of Diabetes in Pregnancy Study Group Criteria with the Old American Diabetes Association Criteria for Diagnosis of Gestational Diabetes Mellitus. Int J Endocrinol Metab. 2019;17(4):e88343. doi: 10.5812/ijem.88343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal MM, Dhatt GS, Punnose J, Koster G. Gestational diabetes in a high-risk population: using the fasting plasma glucose to simplify the diagnostic algorithm. Eur J Obstet Gynecol Reprod Biol. 2005;120(1):39–44. doi: 10.1016/j.ejogrb.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal MM, Dhatt GS, Punnose J. Gestational diabetes: utility of fasting plasma glucose as a screening test depends on the diagnostic criteria. Diabetic Med. 2006;23(12):1319–1326. doi: 10.1111/j.1464-5491.2006.01987.x. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal MM, Dhatt GS, Shah SM. Gestational diabetes: simplifying the IADPSG diagnostic algorithm using fasting plasma glucose. Diabetes Care. 2010 doi: 10.2337/dc10-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agarwal MM, Punnose J, Sukhija K, Sharma A, Choudhary NK. Gestational diabetes mellitus: using the fasting plasma glucose level to simplify the International Association of Diabetes and Pregnancy Study Groups Diagnostic Algorithm in an Adult South Asian Population. Can J Diabetes. 2018;42(5):500–504. doi: 10.1016/j.jcjd.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Reyes-Muñoz E, Sandoval-Osuna NL, Reyes-Mayoral C, Ortega-González C, Martínez-Cruz N, Ramírez-Torres MA, et al. Sensitivity of fasting glucose for gestational diabetes mellitus screening in Mexican adolescents based on International Association of Diabetes and Pregnancy Study Groups criteria: a diagnostic accuracy study based on retrospective data analysis. BMJ Open. 2018;8(4):e021617. doi: 10.1136/bmjopen-2018-021617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trujillo J, Vigo A, Reichelt A, Duncan B, Schmidt M. Fasting plasma glucose to avoid a full OGTT in the diagnosis of gestational diabetes. Diabetes Res Clin Pract. 2014 doi: 10.1016/j.diabres.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Meek CL, Lewis HB, Patient C, Murphy HR, Simmons D. Diagnosis of gestational diabetes mellitus: falling through the net. Diabetologia. 2015;58(9):2003–2012. doi: 10.1007/s00125-015-3647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babaniamansour S, Aliniagerdroudbari E, Niroomand M. Glycemic control and associated factors among Iranian population with type 2 diabetes mellitus: a cross-sectional study. J Diabetes Metab Disord. 2020 doi: 10.1007/s40200-020-00583-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moosaie F, Davatgari R, Dehghani FF, Esteghamati S, Deravi N, Meysamie A, et al. Lp(a) and Apo-lipoproteins as predictors for diabetic retinopathy and its severity in patients with type 2 diabetes: a case-cohort study. Can J Diabetes. 2020 doi: 10.1016/j.jcjd.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Moosaie F, Dehghani FF, Abouhamzeh K, Esteghamati S, Meysamie A, Rabizadeh S, et al. Lp(a) and Apo-lipoproteins as predictors for micro-and macrovascular complications of diabetes: a case-cohort study. Nutr Metab Cardiovasc Dis. 2020 doi: 10.1016/j.numecd.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Rabizadeh S, Dehghani FF, Noshad S, Esteghamati S, Afarideh M, Ghajar A, et al. Beneficial effects of pentoxifylline plus losartan dual therapy in type 2 diabetes with nephropathy. Am J Med Sci. 2018 doi: 10.1016/j.amjms.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Rani PR, Begum J. Screening and diagnosis of gestational diabetes mellitus, where do we stand. J Clin Diagn Res. 2016;10(4):QE01–QE04. doi: 10.7860/JCDR/2016/17588.7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benhalima K, Van Crombrugge P, Moyson C, Verhaeghe J, Vandeginste S, Verlaenen H, et al. The sensitivity and specificity of the glucose challenge test in a universal two-step screening strategy for gestational diabetes mellitus using the 2013 world health organization criteria. Diabetes Care. 2018;41(7):e111. doi: 10.2337/dc18-0556. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal MM, Hughes PF, Punnose J, Ezimokhai M. Fasting plasma glucose as a screening test for gestational diabetes in a multi-ethnic, high-risk population. Diabetes Med. 2000;17(10):720–726. doi: 10.1046/j.1464-5491.2000.00371.x. [DOI] [PubMed] [Google Scholar]
- 16.Donovan L, Hartling L, Muise M, Guthrie A, Vandermeer B, Dryden DM. Screening tests for gestational diabetes: a systematic review for the US Preventive Services Task Force. Ann Internal Med. 2013;159(2):115–122. doi: 10.7326/0003-4819-159-2-201307160-00657. [DOI] [PubMed] [Google Scholar]
- 17.Wijeyaratne CN, Ginige S, Arasalingam A, Egodage C, Wijewardhena K. Screening for gestational diabetes mellitus: the Sri Lankan experience. Ceylon Med J. 2006;51(2):53–58. doi: 10.4038/cmj.v51i2.1353. [DOI] [PubMed] [Google Scholar]
- 18.Sacks DA, Hadden DR, Maresh M, Deerochanawong C, Dyer AR, Metzger BE, et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care. 2012;35(3):526–528. doi: 10.2337/dc11-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Classification and Diagnosis of Diabetes Standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S14–s31. doi: 10.2337/dc20-S002. [DOI] [PubMed] [Google Scholar]
- 20.Davidson MB. Status of research funded by the American Diabetes Association: year 2. Diabetes Care. 2001;24(5):952. doi: 10.2337/diacare.24.5.952. [DOI] [PubMed] [Google Scholar]
- 21.Kashi Z, Borzouei S, Akhi O, Moslemi ZN, Zakeri H, Mohammadpour TR, et al. Diagnostic value of fasting plasma glucose (FPG) in screening of gestational diabetes mellitus. ijdld. 2006;6(1):67–72. [Google Scholar]
- 22.Zhu W-W, Fan L, Yang H-X, Kong L-Y, Su S-P, Wang Z-L, et al. Fasting plasma glucose at 24–28 weeks to screen for gestational diabetes mellitus. Diabetes Care. 2013;36(7):2038. doi: 10.2337/dc12-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sacks DA, Chen W, Wolde-Tsadik G, Buchanan TA. Fasting plasma glucose test at the first prenatal visit as a screen for gestational diabetes. Obstet Gynecol. 2003;101(6):1197–1203. doi: 10.1016/s0029-7844(03)00049-8. [DOI] [PubMed] [Google Scholar]