Abstract
PBDEs are human-influenced chemicals utilized massively as flame retardants. They are environmentally persistent, not easily degraded, bioaccumulate in the biological tissue of organisms, and bio-magnify across the food web. They can travel over a long distance, with air and water being their possible transport media. They can be transferred to non-target organisms by inhalation, oral ingestion, breastfeeding, or dermal contact. These pollutants adsorb easily to solid matrices due to their lipophilicity and hydrophobicity; thus, sediments from rivers, lakes, estuaries, and ocean are becoming their major reservoirs aquatic environments. They have low acute toxicity, but the effects of interfering with the thyroid hormone metabolism in the endocrine system are long term. Many congeners of PBDEs are considered to pose a danger to humans and the aquatic environment. They have shown the possibility of causing many undesirable effects, together with neurologic, immunological, and reproductive disruptions and possible carcinogenicity in humans. PBDEs have been detected in small amounts in biological samples, including hair, human semen, blood, urine, and breastmilk, and environmental samples such as sediment, soil, sewage sludge, air, biota, fish, mussels, surface water, and wastewater. The congeners prevailing in environmental samples, with soil being the essential matrix, are BDE 47, 99, and 100. BDE 28, 47, 99, 100, 153, 154, and 183 are more frequently detected in human tissues, whereas in sediment and soil, BDE 100 and 183 predominate. Generally, BDE 153 and 154 appear very often across different matrices. However, BDE 209 seems not frequently determined, owing to its tendency to quickly breakdown into smaller congeners. This paper carried out an overview of PBDEs in the environmental, human, and biota niches with their characteristics, physicochemical properties, and fate in the environment, human exposure, and health effects.
Keywords: Polybrominated diphenyl ethers, Soxhlet extraction, Endocrine disruptor, Electron capture detector, Solid phase extraction
Introduction
The molecular structure of PBDEs has between 2 and 10 bromine atoms attached to two rings of phenyl connected by an ether bridge [1, 2].
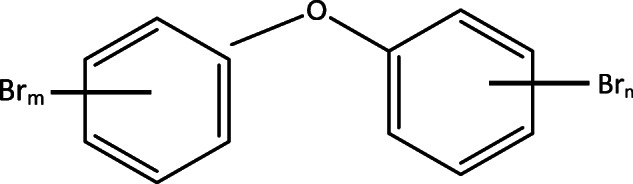
They are hydrophobic, organic halogenated, with long half-lives of 2–10 years, and are mainly generated by humans [3]. They are environmentally unrelenting [4, 5], bioaccumulate in biological tissue of organisms, and bio-magnify along with the food web [6]. Most PBDEs are not easy to degrade naturally [7], although their degradability notably reduces as the degree of halogenation decrease [8, 9]. They can travel over a long distance and infiltrate the food chain [10–12]. Despite their lipophilic and hydrophobic nature with a strong affinity to solid materials, they are still present in ground and surface water [2, 13]. They tend to store in sediment, mostly those with high organic content. Aquatic animals easily absorb them both across gill surfaces and from other diets. Proper species for observing PBDEs in the marine environment as given by OSPAR commission 1999 include oysters (ostrea sp or crassostrea sp), hake (merluccus), and sole (solea) [2].
PBDEs are human-influenced chemicals utilized massively as flame retardants in furnishing, building materials, airplanes, plastics, foods, polyurethane foams, vehicles, carpets, electronic devices, and printed circuit boards, among others [2, 14–16]. They are incorporated to lessen the plausibility of glowing during their utilization by meddling with polymeric materials’ burning. Most PBDEs are not bonded covalently to the polymeric substances to which they are attached. Hence, the probability of discharge from the material to which it is attached is a possibility [17, 18].
There are 209 congeners of PBDEs in existence and are utilized as additives to polymers and resins. The commercially formulated, PentaBDE (comprised of BDEs 47, 99, 100, 153) has been used predominantly in polyurethane foam, furniture, and upholstery, while the commercially formulated OctaBDE (comprised of primary BDEs 153, 183, 197, 207, and 196) is mainly used in casings for electronic products and plastics, and DecaBDE (consisting primarily of BDE-209) utilized mainly on textiles and polymer [2, 19–22] to improve their resistance against fire [4, 23]. Meanwhile, the octa, penta, and deca BDE products have been registered as persistent organic pollutants (POPs) at the Stockholm Convention Fourth Conference of the Parties (COP4) in 2009 [24].
Penta-BDE and Octa-BDE are no longer in use in the USA, and Deca-BDE is gradually being discontinued [25]. The concern for monitoring and regulating PBDEs became eminent by the rapidly rising level in human tissues such as human breastmilk [26]. The worldwide producers of PBDEs include France, Netherlands, France, Japan, Israel, and the U.S. [5]. Unfortunately, the ban and restrictions of deca-BDE in the USA and Europe have shifted the production to countries like China, India, Indonesia, Malaysia, the Philippines, Thailand, and Vietnam [27, 28], but most significantly China and India [29] as a consequence of rapid economic growth. Consequently, North America has exported 80% of e-waste to Asia [30], and the East China Sea regularly receives PBDEs driven by East Asian winter by deposition [31]. This reason and many more confirm the reason for the high rate of publication on PBDEs from China. Therefore, this paper aims to provide an overview of existing methods of investigation and detection of PBDEs in the environmental and biota samples, in relation to their characteristics, physicochemical properties, fate in the environment, human exposure, and health effects.
Highlights: Soxhlet Extraction has been routinely used for sediment samples, given its ease of application
Hexane or its mixture with dichloromethane are considered the most reliable solvents of recovery for extraction of PBDEs from water.
Solid Phase Extraction has verified to be the most efficient method of extracting PBDEs from water samples and also a means for aqueous samples clean-up
Electron Capture Detector is more appropriate and more sensitive for the detection of electromagnetic elements, e.g. (brominated analytes) compared to other detectors, though with a limited selectivity
Distribution and mobility
Therefore, PBDEs are ubiquitous and have been referred to as “omnipresent environmental contaminants” [16]. The congeners prevailing in environmental samples and most regularly detected in indoor dust, water, air, particulate matter, human tissues, sediments, soil, and the soil is the most important matrix are BDE 47, 99, and 100. They can be transferred to non-target organisms like humans by inhalation, oral ingestion, breastfeeding, or dermal contact [32, 33]. They bioaccumulate and get to humans through the food chain [34–37]. Jing et al. [38] documented BDE −209 to be the dominant congener from surface sediment. Similarly, the concentration of BDE −209 was observed to be higher, with BDE −183 and − 153 being dominated congeners in mangrove sediments [39]. Cheng et al. [40] also reported the decreasing trend of PBDEs to depth. This decreasing trend was evident by the higher concentration of BDE −209 observed at a depth of 0–10 cm than 0-20 cm in the soils of e-waste recycling areas. A Report elsewhere [41] also showed the frequency of determining various congeners in water, suspended particulate matter, and sediment at different seasons. BDE - 209 was also a dominant congener in municipal and industrial wastewater treatment plants in Ulsan city in Korea, though penta-BDE was higher in industrial sludge [42]. PBDEs appeared to be lower in the dry season than the wet season, with BDE 209 being the predominant congener. Furthermore, BDE - 209 was observed to be the principal congener in the sediment of sea determined in the coastal East China Sea, followed by BDE -99/100. The contamination is likely from electronic waste dismantling/recycling. This has made the coastal East China Sea a significant sink of PBDEs worldwide [43]. Consequently, PBDEs from coastal sediment of East China Sea was found to be majorly from uninterrupted release of indigenous anthropogenic activities (80.7%), then, surface runoffs of polluted soils (15.1%), photodegradation during atmospheric transportation (1.6%), and microbial degradation and sedimentation (2.6%) [44]. PBDEs are suspected to originate from atmospheric deposition as investigated in surface sediment, with BDE −47 being the dominant congener in winter [8]. Moreover, in their study, Wang et al. [45] observed that bioaccumulation of BDE −100 and − 154 was much higher than that of 99 and 153 respectively in ratio comparison, indicating that ortho-substituted isomers are more ubiquitous than meta-substituted isomers. PBDEs investigated in feather and muscle of the birds of prey in China by Yin et al. [46] showed that higher congeners BDE -209, −153, −207, and − 196 were the dominant congeners, while the lower brominated possibly reflected in the internal tissues of the birds of prey. PBDEs were also detected in marine fishes of Chinese Coastal waters as mono to hexa-substituted variants. The highest concentrations of PBDEs were found in yellow croakers and silver pomfrets species of fishes in Xiamen, where BDE −47 and − 154 were major congeners at a concentration of 4.29 ng/g, lipid wt. and 0.91 ng/g, lipid wt. respectively. The high concentration of BDE −154 was regarded as a consequence of the debromination of BDE −183 and − 209. Commonly detected congeners in marine fishes from Chinese coastal waters were BDE -47 > 99 > 100 > 153 > 154, which differed from the patterns usually found in fishes from other parts of the world [47]. Also, geographical dispersal of PBDEs in minor cetaceans from Asian waters as investigated by Kajiwara et al. [48] revealed the highest concentration in animals from Hong Kong, followed by Japan, and lower level from Philippines and India. Also, BDE −209, followed by BDE -183, −47, and − 99, were the key dominant congeners in China’s indoor dust, with toddlers having the highest exposure [49].
Microbial debromination gave the most effective procedure in the fate of hexa- and mono-BDE in the aerobic system. There was persistent adsorption for Deca-BDE to the sediments [50] and to agricultural soils [51]. Guan et al. [52] reviewed that environmental fate modeling such as air-water exchange, riverine runoff, dry and wet deposition, sedimentation, and degradation have been universally used to describe the course of organic pollutants across various sections. Tetra- and penta-BDE have a long life in the environment, and as such a growing concern [53], while deca-brominated adsorb strongly to soils and sediments [54]. BDE 209 has a half-life of debromination of >10 years in sediments [55, 56] but has a concise life in the human body, and this limits build-up of high concentration in the body [57]. Lower congeners have a half-life of <1 year [58]. Half-life of some PBDE congeners include: BDE 47–3 years, BDE 99–5.4 years, BDE 100–2.9 years, BDE 153–11.7 years, BDE 154–5.8 years [59], BDE 183–94 days, BDE 203–37 days, BDE 206–18 days, BDE 207–19 days, BDE 208–39 days, BDE 209–15 days [57].
Environmental matrices detected with PBDEs
Studies have revealed that PBDEs can be detected in all environmental matrices up to ppm range [60]. Their occurrence in deep oceans consequently shows that they are widespread and ubiquitous environmental contaminants [61–63]. PBDEs have been detected at significant concentrations in environmental matrices like air [64], suspended particulate matter [41, 65], hair [66] soil [67, 68], surface water [69], sediment [70, 71] and sewage sludge [72, 73] and also biological samples like biota [74, 75], food stuff [76], human blood [77, 78], and plasma [79, 80], indoor dust [81, 82], placenta [83, 84], adipose tissues [85, 86], liver [87], fish [71, 88], mussels [88], bird [89], human amniotic fluid [90], human serum [91, 92], urine [93], semen [78, 94] and breast milk [95, 96].
Whitehead et al. [97] reported that the detection of PBDEs in residential dust at elevated concentrations with quite many studies on U.S. homes revealing median concentrations for main PBDE congeners of at least 1 ppm. Owing to the State of California’s exceptional flammability standard, dust samples from California homes have been documented to have unusually high levels of PBDEs. Studies have established that PBDE levels in paired samples of human serum and residential dust are considerably concurrent; signifying that intake of dust is the key course of exposure to PBDEs in U.S. Moreover, current research indicates that dust may be a significant cause of contact accounting for 82% of daily exposure [98, 99].
Wastewater treatment plants (WWTPs) and sewage sludge
Wastewater treatment plant (WWTPs) effluents had been taken to be one of the major causes of pollutants to surface waters, and sludge contributes hugely to the pollution of agricultural land and other environmental matrices in the developed countries [100–102]. High measures of PBDEs in sewage sludge from WWTPs have been documented globally [103–106]. The majority of the accessible information on PBDEs in the marine environment is focused on the evaluation of sludge from WWTPs [42, 107, 108]. Sufficient data on PBDEs in a varied diversity of environment section in Korea exist [92, 109–114]; however, there is a paucity of information addressing PBDEs in WWTPs [115]. High volumes of WWTP effluent comprising concentrations of PBDEs within ~26 ng L−1 will possibly bring about a considerable PBDE unrest into receiving waters, exposing the provision of drinking water and fisheries resources to an impending risk [116].
Sewage sludge is an environmental intermediate that takes delivery of various domestic and industrial chemicals and consequently becomes a resulting source of lethal constituents formed from WWTPs to the environment as suggested by Lee et al. [117]. Similarly, sewage sludge is reflected as one of the PBDEs’ highest sources when applied as agricultural fertilizer [27]. This land application of sewage sludge signifying the main route of PBDEs and NBFRs to soils globally is to recover soil structure and nutrients [118]. PBDEs and NBFRs might flow into wastewater streams from industrial waste, manufacturing, and household sources and are frequently adsorbed in sludge in the course of municipal treatment procedures [103, 119, 120]. Understanding the likely impacts of such noxious waste upon its biota, the water cycle, soil fertility, human populations, and the food chain are essential to investigate satisfactory contaminants’ satisfactory levels within sludge intended for land relevance [121].
Soil and sediments
The lipophilic and hydrophobic properties of PBDEs and NBFRs are the reasons why they bind firmly to organic matter and persevere in soils having half-lives as long as ∼28 years [122]; hence, investigation of sediments can give facts on the current causes of PBDEs in the aquatic environment [123]. Indeed, soil matrix has become the significant hoard for organic contaminants due to its sorption value and holding capacity, and soil quality has turned out to be a good quality indicator of environmental contamination and risk for exposure of humans [124]. PBDEs and NBFRs go into soils through express transmission from flame-retarded products in dumpsites [125] or through landfill leachates [126]. PBDEs can get to the soil through wet/dry deposition during their LRAT and likely to be firmly absorbed in soil due to their persistence and lipophilicity. Thus, the soil has been considered a suitable contamination “snapshot” of the immediate atmospheric pollution of POPs [127]. Regrettably, several PBDE pollution studies in soil have not been able to measure BDE-209 due to definite challenges related to thermal instability and low solubility during the investigation [126, 128]. This possibly will indicate that total PBDE concentrations where BDE-209 is not represented are underestimated in evaluation [129–132].
Human samples (breast milk, urine, and blood)
Current investigations have shown that PBDEs have been discovered in samples of human biological tissue like breast milk, blood, and adipose collected from all over the world together with the United States [133], Canada [134], South Korea [135], and Japan [136]. The trend amongst United States residents showed that there had been growing concentrations of PBDE in humans blood serum from the mid-1980s [133], though some other reports revealed that PBDEs in human tissues are constant or decreasing [137, 138]. A report by Genius et al. [139] demonstrated that blood testing gives only an incomplete knowledge of human PBDE bioaccumulation; testing of both blood and perspiration gives a better understanding. He further showed that PBDE congeners were not detected in urine samples; discoveries’ focal point was on blood and sweat. Participants who tested positive in one or more body fluids for BDE −28 are 80%, 95% for BDE -99, 100% for BDE −47, and 90% for BDE −100 and − 153. Induced sweat made eliminating the five congeners easier, with diverse rates of elimination for diverse congeners.
Biota
The manufacture of congeners of PBDE (octa- and penta-BDE) and their commercial accessibility were prohibited in the European Union owing to their toxicological effects [140–142]. These limitations caused the promotion of a standard reduction of PBDE concentrations in soils in Europe [143]. Furthermore, a turndown of octa- and penta-mix PBDE concentrations has been seen in modern time sewage sludge. This actuality poses an extreme environmental unease, given that present proof recommends that some aquatic beings, as well as fish, have a capability of de-brominating BDE 209 to lesser-brominated congeners [144, 145], which have more significant toxicological and mobility properties. Moreover, it has been verified that benthic fauna can re-mobilize PBDEs buried from sediments [146]. Accordingly, sediments become a significant resultant source of these compounds.
Routes of exposure to PBDEs
There are various routes of access of PBDEs into the human body. Among them are indoor dust, indoor air, dermal uptake, and food. Of all these, entry via food is the key route, especially through the intake of contaminated fish [147] and seafood. Entry via food has been estimated with daily intake of 97 ng/day in Spain [148], 44 ng/day in Canada [149], 51 ng/day in Sweden [150], 50 ng/day in the U.S. [151], and 35 ng/day in Belgium [152]. Furthermore, occupational hazards due to PBDEs’ exposure are alarming, as reviewed by Kalantzi and Siskos [153]. Routes of exposure by dermal contact revealed by Jakobson et al. [154], in his research carried out on workers who worked with computers showed that technicians have higher concentrations of PBDEs in the blood related to clerks and cleaners, contributing to 35% of overall exposure [155]. Similarly, Ohajinwa et al. [156] documented in a research carried out on e-waste burning, dismantling, and repair sites that dermal contact was the key contact route. In contrast, contact through inhalation is insignificant for both carcinogenic and non-carcinogenic hazards. Also, exposure through inhalation of PBDEs contaminated air is evident, as confirmed by research carried out on workers in an electronic dismantling facility [157]. Toddlers are more exposed to PBDEs compared to older children. Major children’s exposure is via breast milk containing PBDEs and inhalation and ingestion of contaminated household dust and food [158, 159].
Health risk and toxicology
Many adverse effects of PBDE on humans with a growing concern have been documented. PBDEs have shown the possibility of causing several undesirable effects, together with neurologic, immunological, and reproductive disruptions and possible carcinogenicity in humans [160–162]. They have also been regarded as endocrine disruptors [163] because they can imitate or modify several vertebrate endocrine system pathways and actions of hormones [163–166]. Lower brominated PBDEs are more lethal than higher PBDEs, so International Agency for Research on Cancer (IARC) classified PBDEs as a group 3 carcinogen based on the inadequate indication of carcinogenicity in humans and inadequate proof in experimental animals [155]. PBDEs mixtures show low acute and chronic toxicity, and toxicity decreases with increasing bromination [167]. Evidence of exposure includes injuries [168, 169], infections of wounds, irritations, skin and eye injuries, respiratory problems [170, 171], noise pollution, and occupational stress [172]. Most reports on health risks are on animals. Adverse effects on women with a significant level of PBDEs range from a notable decline in the ability to conceive [18], pregnancy loss [173], breast cancer risk [86], teratogenicity, fetal toxicity, and non-Hodgkin’s lymphoma [153]. Sperm viability is also affected when their semen, reproductive, and thyroid hormones are affected by PBDEs [79]. There have also been cases of long-lasting behavioral abnormalities in children between 2 and 4 years exposed to a low level of PBDEs [79], disruption of thyroid hormone in male kids of 0–4 years [80], and residents exposed to e-waste [67]. Some low brominated congeners might result in developmental impacts in children [5, 160, 161]. Reports have revealed that PBDEs can transform the sex hormone and thyroid hormone physiology. Hence they have become a problem of global concern [174]. Following the reports on the potential hazards of exposure to PBDEs, proper measures are expected to be put in place because this contaminant is persistent in sediment. Yet, there is no guideline for toxicity on PBDEs detected in sediments [39]. PBDE metabolites may be more active biologically than the parent PBDE [175]. Reports showed that metabolites of PBDEs like hydroxylated halogenated diphenyl ethers bind strongly to thyroid hormone transport protein and hormone receptors with low affinity. Hydroxy PBDE (OH-PBDE) and methoxy PBDE (MeO-PBDE) have been detected at similar concentrations to major PBDE in salmon [58]. Muzikawa et al. [176] also detected OH-PBDE and MeO-PBDE at a higher concentration than parent PBDE in red algae and salmon, respectively, and even in marine animals and birds. These metabolites are capable of disrupting hormones [177], yet their toxicity is not known [58, 158].
Determination of PBDEs in environmental and biota samples
Sample collection and preparation
Sample collection, preparation, and analysis are done according to EPA 1614A [178]. Brominated flame retardants (BFRs) are present in almost every segment of the environment but detected in extremely low concentrations [179]; thus, contamination in the course of sample pre-treatment occurs effortlessly [180]. Dry solid samples are more efficiently homogenized and allow correct sub-sampling for a comparative investigation of other determinants (like organic carbon). The samples, void of water, prevent painstaking extraction from separation funnels and causes the sample matrix to be more available to organic solvents. Quite a lot of techniques can be useful for water-binding as a substitute for drying by evaporation. Grinding the samples with anhydrous Na2SO4 can be used to remove water. Crushing meticulously and adding an adequate amount of dehydrating salt to achieve a free-flowing powder are required for complete extraction and also to minimize cross-contamination and loss of analytes due to evaporation. Alternatively, water-adsorbing constituents (silica, alumina, etc.) can be used for drying. In this instance, water is not trapped permanently and can only be liberated readily when polar solvents are employed for extraction. The application of a blend of less polar solvents (e.g., dichloromethane, hexane) will facilitate the prevention of these problems. Lyophilisation (evaporating water under vacuum conditions at a temperature below 0 °C) can also dry the sample [181]. However, cross-contamination is unavoidable [180].
Storage
Water
Aqueous samples are maintained at <6 °C in the dark from the point of collection to the laboratory. Allowance is made for expansion if the sample will be frozen, after which storage is done in the dark at <6 °C [178], although most studies reported storage at <4 °C. [182–184]
Sediment
Storage, homogenization, and extraction of PBDE from sediments are easier when the samples are dried. Drying of sediment is usually done in a heated oven at (<40 °C) or at room temperature, although there might be losses and compound uptake during the process from the air [185]. Validation must be ensured, and the homogenized sediments should be stored below 0 °C, covered with a lid [2, 181]. Storage for sediment samples lasting several months should be kept frozen (−20 °C or less) [2].
Fish
Aluminum foil washed with solvent (like hexane/acetone or toluene for extraction) should be used to wrap fish that is not disemboweled. This can then be stored at below −20 °C. Plastic bags and boxes made use of should not be allowed to come in contact with the tissues of the fish but preferably used only as the outer container. For organ samples such as liver, they can also be wrapped in aluminum foil washed with solvent or stored in containers (made of steel, aluminum, or glass), washed with solvent. The samples can be stored at -20 °C until analysis. The sample must not be allowed to thaw if traveling a long distance. Trained personnel should also carry out the dissection [2].
Holding times
No holding time for PBDE in semi-solid, aqueous tissues or other matrices is assured. Nonetheless, aqueous samples can be put in storage for up to 1 year if preserved in the dark at <6 °C, while solids, multi-phase tissues, and semi-solid can stay for 1 year if maintained in the dark at < −10 °C. Sample extracts can also be put away in the dark at < −10 °C until analysis and up to 1 year [178].
Extraction techniques
Extraction methods generally in use for PBDEs extractions for sediments are ultra-sonication, accelerated solvent extraction (ASE), soxhlet extraction (SE), microwave-assisted extraction (MAE), and supercritical fluid extraction (SFE). The techniques mentioned above are typically employed with complicated instrumental techniques such as gas chromatography with electron capture detector (GC-ECD), gas chromatography-mass spectrometry (GC-MS), liquid chromatography-mass spectrometry (LC-MS), and other multi-task methods (e.g., gas chromatography - atomic emission detection) for the investigation of BFRs [186–189].
Several factors, including extraction time, ease of access of extraction solvent to the matrix, and solubility of analytes in the extraction mixture, determine the outcome of the extraction process. The extraction time and accessibility are more linked to the extraction’s kinetics. Simultaneously, the solubility of analytes ascertains the equilibrium that can be attained in a definite phase of the extraction [190]. BDE −209 is sensitive to UV light; therefore, exceptional measures have to be engaged to ensure that sunlight entering the laboratory, including probable UV light from fluorescent tubes, has to be restricted. To achieve this, UV filters are placed under the fluorescent lights and on the laboratory windows. Also, installing an ionizer that allows the attraction of tiny dust particles by stationary electricity and maintains clean air as much as possible is recommended since trivial dust particles in the laboratory are easily adsorbed by BDE 209 [178].
Additionally, the aluminum foil should be used to cover all open glassware to prevent the entering of dust particles into solutions or samples. The coolers have to be carefully rinsed before and after extraction with toluene when Soxhlet extraction is employed. There could be the presence of traces of BDE 209, which originates from a preceding extraction. This can lead to obtaining false-positive results, as blanks do not correct this problem because the level of contamination of the diverse coolers can vary [178]. Distribution, extraction, and analysis of PBDEs and the concentration in which they are detected from prior studies are shown in Table 1.
Table 1.
Extraction techniques of various congeners of polybrominated diphenyl ethers
| Congener | Sample matrix | Extraction mode | Analysis | Concentration | Reference |
|---|---|---|---|---|---|
| 28 | sediment | SE | b | 0.002 ng/g dw-0.251 ng/g dw | [70] |
| soil | SE | a | 0.78 ng/g dw-5.15 ng/g dw | [15] | |
| soil | PLE | b | <LOD-0.04 ng/g dw | [33] | |
| sewage sludge | UAE | c | 1.12 ± 0.03 ng/g | [191] | |
| human serum | UAE | d | <LOD-0.74 ng/g | [192] | |
| Fish and mussels | UAE | d | 0.04 ng/g ww-0.358 ng/g dw | [88] | |
| human semen | e | 2.282 pg/g ww-8.28 pg/g ww | [78] | ||
| human blood | centrifugation | e | 5.74 pg/g ww-15.1 pg/g ww | [78] | |
| 47 | sediment | SE | b | 0.012 ng/g dw-0.365 ng/g dw | [70] |
| sediment | USE | d | 190 ± 10–780 ± 60 pg/g dw | [193] | |
| sewage sludge | UAE | c | 38.6 ± 1.6 ng/g | [191] | |
| human serum | UAE | d | 0.84 ng/g-1.89 ng/g | [192] | |
| Water sample | SPE | d | 15–205 pg/L | [193] | |
| Soil | PLE | b | 0.23–0.39 ng/g dw | [33] | |
| human semen | e | 4.32 pg/g ww-9.32 pg/g ww | [78] | ||
| blood sample | centrifugation | e | 8.54 pg/g ww-18.0 pg/g ww | [78] | |
| fish and mussels | USE | d | 0.09 ng/g ww-1.73 ng/g dw | [88] | |
| wastewater | SPME | f | 134 ± 19 | [96] | |
| Milk | SPME | f | 12.7 ± 2.2–32.0 ± 3.2 | [96] | |
| Sludge from WWTP | USE | a | 8–90 ng/g dw | [147] | |
| 66 | sediment | SE | a | 0.80 ng/g dw | [15] |
| 71 | sediment | SE | a | 2.27 ng/g dw | [15] |
| soil | SE | a | 1.93 ng/g dw −16.1 ng/g dw | [15] | |
| 99 | sediment | SE | a | 6.87 ng/g dw | [15] |
| sediment | SE | b | 0.011 ng/g dw-0.491 ng/g dw | [70] | |
| sediment | USE | d | 340 ± 40–2990 ± 390 | [193] | |
| soil | SE | b | 13.3 ng/g dw-615 ng/g dw | [15] | |
| sewage sludge | UAE | c | 29.3 ± 0.7 | [191] | |
| human serum | UAE | d | <LOD-1.64 ng/g | [192] | |
| Water sample | SPE | d | 13–181 pg/L | [193] | |
| Soil | PLE | b | 0.26–0.55 ng/g dw | [35] | |
| human semen | e | 2.25 pg/g ww-7.47 pg/g ww | [78] | ||
| human blood | centrifugation | e | 1.52 pg/g ww-7.55 pg/g ww | [78] | |
| fish and mussels | USE | d | <LOD-0.402 ng/g dw | [88] | |
| wastewater | SPME | f | 155 ± 20 | [96] | |
| milk | SPME | f | nd −135 ± 11 | [96] | |
| Sludge from WWTP | USE | a | 18–33 ng/g dw | [147] | |
| 100 | sediment | SE | b | 0.005 ng/g dw | [70] |
| sediment | 92 ± 10–540 ± 80 pg/g dw | [193] | |||
| soil | SE | a | 2.70 ng/g dw-89.4 ng/g dw | [15] | |
| Soil | PLE | b | 0.09–0.16 ng/g dw | [35] | |
| fish and mussels | USE | d | 0.09 ng/g ww-0.387 ng/g dw | [88] | |
| sewage sludge | UAE | c | 8.1 ± 0,2 ng/g | [191] | |
| human serum | UAE | d | <LOD-1.48 ng/g | [192] | |
| Water sample | SPE | d | 4–29 pg/L | [193] | |
| human semen | e | <LOD-0.517 pg/g ww | [78] | ||
| human blood | centrifugation | e | <LOD-2.66 pg/g ww | [78] | |
| wastewater | SPME | f | 147 ± 10 | [96] | |
| milk | SPME | f | Nd-34.4 ± 5.2 | [96] | |
| 138 | soil | SE | a | 2.35 ng/g dw −9.91 ng/g dw | [15] |
| 139 | soil | SE | a | 3.56 ng/g dw-39.9 ng/g dw | [15] |
| 153 | sediment | SE | b | 0.001 ng/g dw-0.281 ng/g dw | [70] |
| sediment | USE | d | 70 ± 10–130 ± 30 pg/g dw | [193] | |
| soil | SE | a | 44.1 ng/g dw −210 ng/g dw | [15] | |
| fish and mussels | USE | d | <LOD-0.221 ng/g dw | [88] | |
| sewage sludge | UAE | c | 7.3 ± 0.4 ng g | [191] | |
| human serum | UAE | d | <LOD-2.61 ng/g | [192] | |
| Water sample | SPE | d | 6–38 pg/L | [192] | |
| Soil | PLE | b | 0.04–0,12 ng/g dw | [35] | |
| human semen | e | <LOD-6.37 pg/g ww | [78] | ||
| human blood | centrifugation | e | 10.7 pg/g ww-25.6 pg/g ww | [78] | |
| 154 | sediment | SE | b | 0.002 ng/g dw-0.541 ng/g dw | [70] |
| sediment | USE | d | 50 ± 10–100 ± 10 PG/G dw | [193] | |
| soil | SE | a | 32.0 ng/g dw-48.9 ng/g dw | [15] | |
| Soil | PLE | b | <LOD-0.08 | [35] | |
| fish and mussels | USE | d | <LOD-0.317 ng/g dw | [88] | |
| sewage sludge | UAE | c | 5.3 ± 0.2 ng/g | [191] | |
| human serum | UAE | d | <LOD-1.46 ng/g | [192] | |
| human semen | e | <LOD-0.734 pg/g ww | [78] | ||
| human blood | centrifugation | e | <LOD-2.45 pg/g ww | [78] | |
| wastewater | SPME | f | 215 ± 4 ng/L | [96] | |
| milk | SPME | f | Nd-225 ± 20 ng/L | [96] | |
| Water sample | SPE | d | 4–25 pg/g | [193] | |
| 183 | sediment | SE | b | 0.019 ng/g dw-0.911 ng/g dw | [70] |
| sediment | USE | d | 20 ± 10–90 ± 20 pg/g dw | [193] | |
| soil | SE | a | 22.3 ng/g dw- 824 ng/g dw | [15] | |
| Soil | PLE | b | <LOD-0.24 ng/g dw | [35] | |
| human semen | e | <LOD-1.06 pg/g ww | [78] | ||
| human blood | centrifugation | e | 0.193 pg/g ww −4.54 pg/g ww | [78] | |
| Water sample | SPE | d | 3–32 pg/L | [193] | |
| 209 | human semen | e | <LOD-18.2 pg/g ww | [78] | |
| human blood | centrifugation | e | 25.3 pg/g ww −61.3 pg/g ww | [78] | |
| Sludge from WWTP | USE | a | Nd- 1135 ng/g dw | [147] | |
| Water sample | SPE | d | 770–3810 pg/L | [193] | |
| sediment | USE | d | 2130 ± 270–10,600 ± 1200 | [193] | |
| sediment | soxhlet | a | 0.781 ng/g-8.95 ng/g dw | [70] |
a – GC-MS/MS; b – GC-MS; c – GC-ICP; d – GC-ICP-MS; e – GC-MS NCI with SIM; f - GC-ECD
Solid-phase extraction (SPE)
SPE allows for simultaneous extraction from numerous samples and is cost-effective. Its drawback is that it usually must precede the extraction of analytes with a solvent. SPE is a time-consuming method [194], has background interferences, plugging, and poor reproducibility between cartridges [195]. SPE is aimed to be a whole or extensive extraction technique for extracting the analyte absolutely from the entire sample volume passing through the sorbent. It is a pseudo equilibrium or non-equilibrium process [196]. The phase C18 suitable for retaining both non-polar and reasonably polar compounds is mostly used among SPE sorbents. Dichloromethane, acetone, hexane, acetonitrile, and tetrahydrofuran, are in most cases, utilized as elution solvents for PBDEs. The most reliable recovery standards are acquired when just hexane or its mixture with dichloromethane (3:2 v/v) is used [197].
Liquid-liquid extraction (LLE)
This technique allows target analytes to be extracted from the aqueous sample matrix into an organic solvent immiscible with water. The procedure’s driving force is the distribution coefficients of the target analytes between the organic solvent and the aqueous solution, and transport across the liquid-liquid interface is by diffusion. The procedure involves isolating the target analytes from the bulk sample, enriching the target analytes to a level where it can be detectable, and transferring the target analyte to the medium well-suited for the chromatography or electrophoretic system [198]. It is simple to operate and has high efficiency [198]. Its drawbacks include high solvent consumption, which is why it was replaced with SPE [199], being environmentally unfriendly, complicated time consuming, the difficulty of automation [193], limited flexibility in terms of extraction chemistry [198], and requiring pre-concentration of the extract [196]. It is essential to utilize the large volume of samples, even up to 1000 mL sometimes, owing to PBDEs’ hydrophobic nature and their small concentration in water samples, especially when using the liquid-liquid extraction (LLE) method. The most generally used solvents of extraction are tert-butyl ether, isooctane, and hexane [200].
Pressure liquid extraction (PLE)
The elution of analytes from the sample is usually carried out under significant temperature (80–130 °C) and pressure (100–120 bar). The equipment gives a proficient and rapid extraction, employing 2 or 3 extraction cycles in <30 min, devoid of mediation of the operator. However, the moderately elevated cost of equipment maintenance and application have to be considered [201–204]. Advantages of PLE include automation of the extraction method, moderate consumption of solvents, short extraction time, ability to extract thermally unstable analytes, the possibility of extraction from samples with high humidity, and favorable extraction kinetics. Its demerits include the high cost of procurement and low selectivity [190].
Ultrasound-assisted extraction (UAE)
UAE allows for concurrent extraction from numerous samples but has a long extraction time for PBDEs [190], compared to soxhlet extraction. Furthermore, Sonication employs ultrasonic vibration to bring the sample in contact with the solvent. Though the method is fast, it has low efficiency and may also lead to the decomposition of organophosphorus compounds. Consequently, before implementing the real sample, there is a need to try out first with the target analyte in reference standard [195]. There has not been much ultra-sonication application in PBDEs extraction from polyurethane foams utilized for air sampling in laboratories [205] or from high-impact polystyrene [206]. Also, this method has not been used widely in BFRs. It has yielded lesser extraction recoveries compared to Soxhlet extraction. The technique is painstaking for the reason that numerous consequent extractions are essential [180].
Pressurized hot water extraction (PHWE)
The analytes extracted are being stuck into a solid-phase trap (Tenax™ TA), and then from there, elution is done with a pentane/ethyl acetate mixture after the trap has been dried with nitrogen. Moreover, in this instance, it does not require any further clean up before GC analysis [180]. Its advantages include low-cost and environmental friendliness of water [207]. However, there could be degradation, hydrolysis, or oxidation of target analytes and reaction intensity at elevated temperatures [208, 209].
Magnetic-effervescent tablet-assisted ionic liquid-based dispersive liquid-liquid microextraction (META-IL-DLLME)
DLLME was introduced in 2006 by Assadi and his co-workers [210] and is centered on a ternary constituent solvent system such as Homogeneous liquid-liquid extraction (HLLE) and cloud point extraction (CPE). An applicable mixture of a solvent of extraction and disperser is injected into the aqueous sample by syringe. This promptly forms a cloud solution that accelerates the fast extraction of analytes from water samples [210]. The dispersing solvent has to be completely soluble in the water phase (commonly acetone, acetonitrile, and methanol). The extracting solvent must be capable of extracting analytes, soluble in dispersing solvent, and have low solubility in water. It is anticipated that phase separation be significantly different in the densities of extracting solvent and water. This method has been successfully utilized to extract water samples, few food samples, urine, and, recently, solid samples [211]. It is simple to operate, fast, cheap, has a high recovery and enrichment factor within a period (a few seconds) from a low volume of water samples [210]. However, it consumes a higher volume of dispersing solvent, not yet appropriate as a predictable applicable on-line pre-concentration procedure. Most importantly, it is not suitable for the extraction of analytes from biological samples because of the interaction between the organic solvents and matrix components. So, it does not produce an appropriate sediment phase for injection into an analytical instrument such as GC. This can only be achieved by diluting the samples, which also causes changes in the characteristic property of the matrices [212].
Microwave-assisted extraction (MAE)
MAE is a method, which combines microwave energy and traditional solvent extraction [213], to heat the interacting polar solvents and to partition the analyte of interest between sample and solvent in a way to reduce extraction time and solvent volume consumed in the extraction of the solid sample [214]. Characteristics of MAE are low demand for solvent and ease of implementation. It is not frequently utilized because microwaves’ effect on analytes and the need to make polar extraction solvents relevant is unknown. Furthermore, an additional clean-up step is required for the extracts obtained (e.g., filtration) [215]. To seek rapid and quality procedures for the analytical technique as a result of growing concern on the effect of PBDEs on the environment, MAE is employed due to its accuracy and precision with the capability of removing matrix interference. Small extraction volume and time (25 mL and 25 min respectively) compared to the larger volume and more time used in SE, and it has been able to detect as low as 0.01 ng/g for the analyzed samples [216]. MAE has the advantage of heating the whole sample simultaneously without heating the vessel, unlike classical heating. However, extraction conditions have to be optimized carefully, considering the matrix and analyte characteristics to prevent degradation of higher brominated congeners and enhance the yields of extraction [217]. Its drawback includes the necessity to separate the extract from the extraction residue and the utilization of only solvents that can absorb and propagate microwaves (a dipole moment different from zero) [190].
Accelerated solvent extraction (ASE) or pressurized fluid extraction (PFE)
ASE method was firstly developed in 1995. The process effectiveness is subject to the influence of temperature and pressure. Three steps are involved in the process- (1) desorption of solid particles, (2) diffusion via solvent situated inside a particle pore, and (3) relocation to the more significant part of the flowing fluid. It is operated at a temperature higher than the boiling point of the solvent. Implying that the pressure inside the extraction cell must be maintained high to conserve the solvent in a liquid state and advance the extraction proficiency by “pushing” the solvent into pores, thereby making the analytes accessible [218]. It has a wide range of solvent utilization though complicated with solid environmental samples and reduced extraction time. However, it is expensive and has low selectivity with environmental samples [218].
Supercritical fluid extraction (SFE)
Mitra [196] reviewed that SFE is fast (10–60 min), consuming less solvent (as small as 5–10 mL). CO2 is environmentally friendly, as it is non-toxic and non-flammable. Besides, there is no need for additional filtration because the extraction cell has frits. Nevertheless, it is very costly, and the analytical scale requires a small amount of analytes (< 10 g). SFE and solid-phase trapping have been utilized to extract PBBs and PBDEs and for PCBs and chlorinated benzenes extraction from sediment samples using CO2 as the supercritical fluid [219]. Copper powder and Na2SO4 would be used to mix the sediment before extraction. The use of CO2 with modifiers (acetone, diethylamine, and methanol) enhances results far better than when only CO2 or SE is used. Furthermore, the method does not require an additional clean-up step because the extracts would be much cleaner. There has been the extraction of PBDEs from sediment in other SFE applications [219] and shellfish [220], employing CO2 as a medium for extraction. Conversely, de Boer et al. [185] have made known that at least for sediments, regardless of elevated recoveries for BDE −47, the coefficient of variance was inadequately high, probably signifying that SFE is not as much of reproducibility as SE [180]. SFE has successfully extracted 97% of PBDEs from plastics with supercritical CO2 as solvents [221].
Soxhlet extraction (SE)
SE is broadly used in extracting PBDEs from solid samples because of the following advantages: low cost, simplicity of application, high efficiency, and the likelihood of finding an extract separated from the residue [190, 222]. To aid this, the mixture of polar and non-polar solvents is mostly used [222]. Wager et al. [223] successfully used a mixture of cyclohexane and acetone (1:1), which gave a better recovery. Its disadvantages include long extraction time, the need to use more solvents, the probability of extraction only from a single sample, and the requirement of thorough drying before extraction begins for moist samples [190].
Employing the Soxhlet apparatus for liquid-solid extraction is a generally utilized model method. Notwithstanding the current development in SFE, ASE, or MAE techniques, it is still preferred because of its robustness and low cost. For extracting BFRs from sediment, soil, dried biota, and sewage sludge, commonly used solvents are toluene, hexane/acetone mixtures, dichloromethane, or hexane [185].
Dispersive solid-phase extraction (dSPE)
dSPE allows for concurrent extraction from numerous samples. Other advantages include its low cost and ease of use. However, it commonly needs support, e.g., ultrasound or shaking and additional purification with the classic SPE technique. It can generally be used for pre-treatment of extract. It cannot be used as an independent and sufficient method in the samples’ pre-treatment [190].
Matrix solid-phase dispersion (MSPD)
This significantly shortens the sample preparation phase compared to the classic SPE technique; it allows for simultaneous purification of the extract and reduces organic solvents’ consumption. It is a less expensive method that allows for simultaneous extraction from quite many samples, with no filtration required. Its shortcomings are the difficulty in sustaining high and repeatable analytes’ recovery and the likelihood of co-elution with some matrix components [190].
Future trends in the extraction of PBDEs in complex matrices
Clean-up and pre-concentration
The crude extract needs a clean-up as there is a possibility of co-extraction of several other compounds (e.g., lipids, humic acids) with the analytes. There could be sulphur in the extracts samples from soil, sewage sludge, or sediments, which should be gotten rid of. Numerous other methods like Cu treatment [224] or the reaction with tetrabutylammonium sulphite [225] can also be utilized.
Final determination step and chromatographic analysis and detection
Various matrices have different analytical techniques for detection. This includes the usage of GC-MS for detection of PBDEs in sewage, air, animal tissues, and fish; capillary column GC-ECD for sediment and water samples; GC/high-resolution MS (HRMS) for fish tissue; and liquid chromatography (LC)-GC-MS/flame ionization detector (FID) for sediments [1]. Isotope dilution and internal standard high-resolution GC (HRGC)/HRMS were utilized by EPA Method 1614 to identify PBDEs in tissue, sediment, soil, and water [226].
Electron capture detector (ECD)
ECD is appropriate and more sensitive for the detection of electromagnetic elements, e.g. (brominated analytes) compared to other detectors, though with a limited selectivity [227], since identification is based on retention time [228]. Pietron and Malagocki [229] Based on their findings, countered this by saying that GC-ECD has a characterization of a low detection limit for halogenated compounds. Regardless of low selectivity and the need for validation, ECD has been used to measure PBDEs concentrations in the food of animal origin with a reported LOD range of 0.03–440 pg/g. ECD is not an actual substitute to MS in trace PBDEs investigation in food; so, the results acquired have to be confirmed.
Gas chromatography-inductively coupled plasma-mass spectrometry (GC–ICP-MS)
Capable extraction and clean-up processes must be applied before instrumental analysis by GC–ICP-MS to liberate hydrophobic PBDEs from such an intricate matrix. Thus, the consequence of diverse extracting agents and the extraction system on the particular PBDEs’ extraction effectiveness was analytically studied and the co-extracted lipids from the organic phase isolated by the clean-up process (using Florisil) that was developed earlier as reported by Novak et al. [191]. A sensitive analytical method that is simple, reliable, which requires small amounts of serum [192, 230], is in high demand owing to the impending undesirable impacts of PBDEs on humans, particularly neonates and children. Most of the reported techniques that have attained adequate detection limits and precision require between 2 and 5 mL of serum [231, 232], except the method developed by Yin et al. in which 0.5 mL of serum was utilized [233]. However, it has to be considered that this technique was appropriate for the analysis of degrees of PBDEs in North America, which are about ten times averagely higher than those experimented in the European countries. ICP-MS was found to be an extremely sensitive and selective detector in this case. Consequently, attainment of acceptable limits of detection (LODs) for the target PBDE congeners using only 1 mL of serum was found possible with the technique.
Gas chromatography-mass spectrometry and gas chromatography-mass spectrometry/mass spectrometry (GC-MS AND GC-MS2)
The generally utilized technique for the determination of PBDEs is GC–MS [198, 234, 235]. The trendiest ionization mode for investigating these compounds by low-resolution MS in negative chemical ionization (NCI). This method gives advanced sensitivity to electron ionization (EI), but the selectivity is lesser, given that it monitors only bromine ions. Moreover, since isotope dilution cannot be applied, less accuracy in quantification is acquired. GC–HRMS in EI mode is generally a selective and sensitive technique for investigating these compounds [236–239], and the USEPA suggests it for the investigation of PBDEs [178]. However, given that this technique is moderately costly and needs an experienced person and weighty maintenance to make specific appropriate performance, then low resolution-mass spectrometry would be ideal. GC coupled to ion trap-mass spectrometry working in tandem mode (GC–ITMS–MS) has proven to be an economical, quick, and consistent substitute to HRMS for the investigation of PBDEs in biological and environmental matrices as reported by [225]. Conventionally, MS is used as GC detectors for PBDEs. Electron capture negative ionization (ECNI) and electron ionization (EI) are the standard ionization techniques in GC-MS for the measurement of PBDEs. ECNI depends on the attachment of electrons to the electrophilic molecule, while EI formed ions based on several brominated atoms in the molecule. The mass spectrum dominated by ions for higher and lower brominated BDEs is (M-Br)+ and [M]+, respectively [240].
GC-MS has the advantage of providing fine chromatographic resolution for PBDE congeners. Other significant benefits include its excellent sensitivity, particularly when ECNI is used, although the monitored ions are merely bromine ions, making GC/ECNI-MS substandard. Also, EI is more specific with the formation of [M]+ and [M-Br2] + ions, though less sensitive, particularly for highly brominated PBDEs [200]. Furthermore, highly brominated PBDE degrade thermally [241].
Liquid chromatography-mass spectrometry and liquid chromatography-mass spectrometry/mass spectrometry (LC-MS and LC-MS2)
Using LC-MS with atmospheric pressure photo-ionization (APPI) is preferred over GC-MS because it gives exceptional sensitivity and specificity, allowing discrimination of signals, which could not be fixed on a triple quadrupole used as a reference. It also provides essential information for identifying these compounds together with specific ionization patterns, capable of monitoring parent compounds and detecting the key known PBDEs metabolites, namely hydroxylated PBDEs. This technique was developed by Marteau et al. [242], which allows the concurrent detection and identifying of PBDEs and their metabolites with distinct prominence on the bioactive OH-PBDEs for the first time. Other advantages of liquid-phase separation of the liquid chromatography with negative ion atmospheric pressure photo-ionization tandem mass spectrometry (LC/NI-APPI/MS/MS) method include the absence of thermal degradation higher PBDEs, particularly BDE-209, low detection limit, and congener specificity using select MRM transition [189].
Environmental remediation actions
Soil remediation of PBDE contaminated soil was done using Ni/Fe bimetallic properties, which could degrade BDE −209 at the efficiency of 72% [243]. The degradation increased with an increase in nano Ni/Fe particles [244]. Also, Wu et al. [245] achieved remediation using biochar Ni/Fe, which can reduce the uptake and translocation of PBDEs from the soil into Brassica chinensis, especially for lower brominated PBDEs. Synthesized nanoscale zerovalent iron also gave 90% removal of BDE −209 at 40 min favorable at acidic conditions [246]. Furthermore, BDE-209 and BDE-3 were adsorbed and degraded using microscale zerovalent Iron. About 10–20% and 15–30% of BDE-209 and BDE-3 respectively were adsorbed while 70% and 60% of BDE-209 and BDE-3 respectively were degraded [247].
Extraction techniques
Table 1 articulates the profile of several techniques for the extraction and analysis of PBDEs in various matrices in several studies. Generally, the soxhlet apparatus is widely used for sediment and soil because of its low cost and simplicity of application [190, 222], and few times USE employed. However, it gives lower extraction recoveries than soxhlet [198]. For sewage sludge, fish, and human serum, UAE is generally engaged, though with lower recoveries [198]. Also, SPE and SPME are used for water extraction. These have replaced LLE because they require less volume of solvent [198]. GC-MS/MS was employed to quantify fish and animal tissues because it provides a fine revolution with PBDEs congeners [228], while GC-ECD is used for water samples [226], though it has low selectivity. GC-ICP-MS is employed for human serum because it is simple, reliable, and sensitive and requires only 1 mL of human serum [192, 230]). It is observed from the table that BDE 28, 47, 99, 100, 153, 154, 183 are frequently detected, as they predominate in human tissue [248, 249]. BDE 209 was not often detected as it might have been broken down into smaller congeners [249]. A high concentration of these congeners was detected in humans showing the bioaccumulation of PBDEs in humans. BDE 28, 99, 209 were detected in high amount in human samples with human semen at the range of 2.25–8.28 pg/g ww, while BDE 100 and 183 were observed to be higher in sediment and soil at 0.89–824 ng/g ww, but low in human samples. BDE 153 and 154 were detected in most matrices at 0.317–210 ng/g ww.
Conclusion
The fact that PBDEs can travel over a long distance and be detected at non-point sources is threatening, given the health implication associated with exposure to PBDEs. They can be inhaled via air and incorporated in the food chain, naturally produced and influenced by human activities daily. Thus this calls for great concern. Several PBDE congeners have been banned, but they are still detected in various matrices, despite their probable hazards and endocrine disruption potentials. They are tenacious in the environment, having a long half-life, and bioaccumulate in the food chain. SE remains the widely employed technique for extracting PBDEs from solid materials because of the ease of their application, though UAE allows for simultaneous extraction from several samples. SPE is best for liquid samples like water and human samples, with hexane being the best solvent for recovery.
Acknowledgments
The authors specially thank the South African Medical Research Council (SAMRC) for funding support.
Authors’ contributions
Conceptualization and writing of the manuscript as part of Ph.D. research work—C.R.O.; review, editing, and revision of the manuscript—A.O.A., O.O.O., and A.I.O.; Ph.D. research supervision and funding—O.O.O. and A.I.O. The author(s) read and approved the final manuscript.
Funding
The research was funded by the South African Medical Research Council (grant number UFH/SAMRC/P790).
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.US Department of Health and Human Services, Agency for Toxic Substances and Disease Registry (ATSDR) Draft toxicological profile for Polybrominated diphenyl ethers (PBDEs) Atlanta: Public Health Service; 2015. [PubMed] [Google Scholar]
- 2.Webster L, Tronczynski J, Bersuder P, Vorkamp K, Lepom P. Determination of polybrominated diphenyl ethers (PBDEs) in sediment and biota. 2009. [Google Scholar]
- 3.Hooper K, McDonald TA. The PBDEs: an emerging environmental challenge and another reason for breast-milk monitoring programs. Environ Health Perspect. 2000;108(5):387. doi: 10.1289/ehp.00108387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alaee M, Arias P, Sjödin A, Bergman Å. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ Int. 2003;29(6):683–689. doi: 10.1016/S0160-4120(03)00121-1. [DOI] [PubMed] [Google Scholar]
- 5.Siddiqi MA, Laessig RH, Reed KD. Polybrominated diphenyl ethers (PBDEs): new pollutants–old diseases. Clin Med Res. 2003;1(4):281–290. doi: 10.3121/cmr.1.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng B, Zhao X, Ni X, Ben Y, Guo R, An L. Bioaccumulation characteristics of polybrominated diphenyl ethers in the marine food web of Bohai Bay. Chemosphere. 2016;150:424–430. doi: 10.1016/j.chemosphere.2016.01.110. [DOI] [PubMed] [Google Scholar]
- 7.Besis A, Samara C. Polybrominated diphenyl ethers (PBDEs) in the indoor and outdoor environments–a review on occurrence and human exposure. Environ Pollut. 2012;169:217–229. doi: 10.1016/j.envpol.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Nouira T, Risso C, Chouba L, Budzinski H, Boussetta H. Polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in surface sediments from Monastir Bay (Tunisia, Central Mediterranean): occurrence, distribution and seasonal variations. Chemosphere. 2013;93(3):487–493. doi: 10.1016/j.chemosphere.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Loganathan BG, Kannan K. Global organochlorine contamination trends: an overview. Ambio. 1994:187–91.
- 10.Covaci A, Harrad S, Abdallah MAE, Ali N, Law RJ, Herzke D, de Wit CA. Novel brominated flame retardants: a review of their analysis, environmental fate and behaviour. Environ Int. 2011;37(2):532–556. doi: 10.1016/j.envint.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 11.De Wit CA, Alaee M, Muir DC. Levels and trends of brominated flame retardants in the Arctic. Chemosphere. 2006;64(2):209–233. doi: 10.1016/j.chemosphere.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 12.Agrell C, ter Schure AF, Sveder J, Bokenstrand A, Larsson P, Zegers BN. Polybrominated diphenyl ethers (PBDES) at a solid waste incineration plant I: atmospheric concentrations. Atmos Environ. 2004;38(30):5139–5148. doi: 10.1016/j.atmosenv.2004.05.024. [DOI] [Google Scholar]
- 13.Shan H, Liu C, Wang Z, Ma T, Shang J, Pan D. A fluorescence-based method for rapid and direct determination of polybrominated diphenyl ethers in water. J Anal Methods Chem. 2015;2015:853085. doi: 10.1155/2015/853085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boon JP, Lewis WE, Tjoen-A-Choy MR, Allchin CR, Law RJ, de Boer J, ten Hallers-Tjabbes CC, Zegers BN. Levels of polybrominated diphenyl ether (PBDE) flame retardants in animals representing different trophic levels of the North Sea food web. Environ Sci Technol. 2002;36(19):4025–4032. doi: 10.1021/es0158298. [DOI] [PubMed] [Google Scholar]
- 15.Wang D, Cai Z, Jiang G, Leung A, Wong MH, Wong WK. Determination of polybrominated diphenyl ethers in soil and sediment from an electronic waste recycling facility. Chemosphere. 2005;60(6):810–816. doi: 10.1016/j.chemosphere.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 16.Abafe OA, Martincigh BS. Determination and human exposure assessment of polybrominated diphenyl ethers and tetrabromobisphenol A in indoor dust in South Africa. Environ Sci Pollut Res. 2016;23(7):7038–7049. doi: 10.1007/s11356-015-6031-0. [DOI] [PubMed] [Google Scholar]
- 17.Rahman F, Langford KH, Scrimshaw MD, Lester JN. Polybrominated diphenyl ether (PBDE) flame retardants. Sci Total Environ. 2001;275(1–3):1–17. doi: 10.1016/S0048-9697(01)00852-X. [DOI] [PubMed] [Google Scholar]
- 18.Daso AP, Fatoki OS, Odendaal JP. Development of analytical procedures for the simultaneous determination of tri-to heptabrominated diphenyl ethers and hexabrominated biphenyl (BB 153) in sediment samples. Water SA. 2011;37(3).
- 19.La Guardia MJ, Hale RC, Harvey E. Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-PBDE technical flame-retardant mixtures. Environ Sci Technol. 2006;40(20):6247–6254. doi: 10.1021/es060630m. [DOI] [PubMed] [Google Scholar]
- 20.Ma J, Qiu X, Zhang J, Duan X, Zhu T. State of polybrominated diphenyl ethers in China: an overview. Chemosphere. 2012;88(7):769–778. doi: 10.1016/j.chemosphere.2012.03.093. [DOI] [PubMed] [Google Scholar]
- 21.Ni K, Lu Y, Wang T, Kannan K, Gosens J, Xu L, Li Q, Wang L, Liu S. A review of human exposure to polybrominated diphenyl ethers (PBDEs) in China. Int J Hyg Environ Health. 2013;216(6):607–623. doi: 10.1016/j.ijheh.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Law RJ, Covaci A, Harrad S, Herzke D, Abdallah MAE, Fernie K, Toms LML, Takigami H. Levels and trends of PBDEs and HBCDs in the global environment: status at the end of 2012. Environ Int. 2014;65:147–158. doi: 10.1016/j.envint.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Batterman S, Godwin C, Chernyak S, Jia C, Charles S. Brominated flame retardants in offices in Michigan, USA. Environ Int. 2010;36(6):548–556. doi: 10.1016/j.envint.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdallah MA-E, Harrad S, Covaci A. Isotope dilution method for determination of polybrominated diphenyl ethers using liquid chromatography coupled to negative ionization atmospheric pressure photoionization tandem mass spectrometry: validation and application to house dust. Anal Chem. 2009;81(17):7460–7467. doi: 10.1021/ac901305n. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Environmental Protection Agency. Polybrominated Diphenyl ethers (PBDEs) Action Plan Summary. 2012. [Accessed in 2013]; Available from: http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/pbde.html.
- 26.Betts KS. Rapidly rising PBDE levels in North America. 2002. [DOI] [PubMed] [Google Scholar]
- 27.Hale RC, Alaee M, Manchester-Neesvig JB, Stapleton HM, Ikonomou MG. Polybrominated diphenyl ether flame retardants in the north American environment. Environ Int. 2003;29(6):771–779. doi: 10.1016/S0160-4120(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 28.Kwan CS, Takada H, Boonyatumanond R, Kato Y, Mizukawa K, Ito M, Zakaria MP, Santiago EC. Historical occurrences of polybrominated diphenyl ethers and polychlorinated biphenyls in Manila Bay, Philippines, and in the upper gulf of Thailand. Sci Total Environ. 2014;470:427–437. doi: 10.1016/j.scitotenv.2013.09.076. [DOI] [PubMed] [Google Scholar]
- 29.Chen SJ, Luo XJ, Lin Z, Luo Y, Li KC, Peng XZ, Mai BX, Ran Y, Zeng EY. Time trends of polybrominated diphenyl ethers in sediment cores from the Pearl River estuary, South China. Environ Sci Technol. 2007;41(16):5595–5600. doi: 10.1021/es070351e. [DOI] [PubMed] [Google Scholar]
- 30.Wong MH, Wu SC, Deng WJ, Yu XZ, Luo Q, Leung AOW, Wong CSC, Luksemburg WJ, Wong AS. Export of toxic chemicals–a review of the case of uncontrolled electronic-waste recycling. Environ Pollut. 2007;149(2):131–140. doi: 10.1016/j.envpol.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Lin T, Hu L, Feng J, Guo Z. Time trends of polybrominated diphenyl ethers in East China seas: response to the booming of PBDE pollution industry in China. Environ Int. 2016;92:507–514. doi: 10.1016/j.envint.2016.04.033. [DOI] [PubMed] [Google Scholar]
- 32.Rhind SM. Anthropogenic pollutants: a threat to ecosystem sustainability? Philos Trans R Soc London B Biol Sci. 2009;364(1534):3391–3401. doi: 10.1098/rstb.2009.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Z, Shanmugam M, Rhind SM. PLE and GC–MS determination of polybrominated diphenyl ethers in soils. Chromatographia. 2010;72(5–6):535–543. doi: 10.1365/s10337-010-1693-8. [DOI] [Google Scholar]
- 34.Pirard C, De Pauw E, Focant J-F. New strategy for comprehensive analysis of polybrominated diphenyl ethers, polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans and polychlorinated biphenyls by gas chromatography coupled with mass spectrometry. J Chromatogr A. 2003;998(1–2):169–181. doi: 10.1016/S0021-9673(03)00611-3. [DOI] [PubMed] [Google Scholar]
- 35.Chen ZJ, Liu HY, Cheng Z, Man YB, Zhang KS, Wei W, Du J, Wong MH, Wang HS. Polybrominated diphenyl ethers (PBDEs) in human samples of mother–newborn pairs in South China and their placental transfer characteristics. Environ Int. 2014;73:77–84. doi: 10.1016/j.envint.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Frederiksen M, Vorkamp K, Mathiesen L, Mose T, Knudsen LE. Placental transfer of the polybrominated diphenyl ethers BDE-47, BDE-99 and BDE-209 in a human placenta perfusion system: an experimental study. Environ Health. 2010;9(1):32. doi: 10.1186/1476-069X-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomara B, Herrero L, Ramos JJ, Mateo JR, Fernandez MA, Garcia JF, Gonzalez MJ. Distribution of polybrominated diphenyl ethers in human umbilical cord serum, paternal serum, maternal serum, placentas, and breast milk from Madrid population, Spain. Environ Sci Technol. 2007;41(20):6961–6968. doi: 10.1021/es0714484. [DOI] [PubMed] [Google Scholar]
- 38.Cheng JO, Ko FC. Occurrence of PBDEs in surface sediments of metropolitan rivers: sources, distribution pattern, and risk assessment. Sci Total Environ. 2018;637:1578–1585. doi: 10.1016/j.scitotenv.2018.05.075. [DOI] [PubMed] [Google Scholar]
- 39.Zhu H, Wang Y, Wang X, Luan T, Tam NF. Distribution and accumulation of polybrominated diphenyl ethers (PBDEs) in Hong Kong mangrove sediments. Sci Total Environ. 2014;468:130–139. doi: 10.1016/j.scitotenv.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 40.Cheng Z, Wang Y, Wang S, Luo C, Li J, Chaemfa C, Jiang H, Zhang G. The influence of land use on the concentration and vertical distribution of PBDEs in soils of an e-waste recycling region of South China. Environ Pollut. 2014;191:126–131. doi: 10.1016/j.envpol.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 41.Pei J, Yao H, Wang H, Li H, Lu S, Zhang X, Xiang X. Polybrominated diphenyl ethers (PBDEs) in water, surface sediment, and suspended particulate matter from the Yellow River, China: levels, spatial and seasonal distribution, and source contribution. Mar Pollut Bull. 2018;129(1):106–113. doi: 10.1016/j.marpolbul.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 42.Hwang IK, Kang HH, Lee IS, Oh JE. Assessment of characteristic distribution of PCDD/Fs and BFRs in sludge generated at municipal and industrial wastewater treatment plants. Chemosphere. 2012;88(7):888–894. doi: 10.1016/j.chemosphere.2012.03.098. [DOI] [PubMed] [Google Scholar]
- 43.Li Y, Lin T, Chen Y, Hu L, Guo Z, Zhang G. Polybrominated diphenyl ethers (PBDEs) in sediments of the coastal East China Sea: occurrence, distribution and mass inventory. Environ Pollut. 2012;171:155–161. doi: 10.1016/j.envpol.2012.07.039. [DOI] [PubMed] [Google Scholar]
- 44.Wang G, Peng J, Zhang D, Li X. Characterizing distributions, composition profiles, sources and potential health risk of polybrominated diphenyl ethers (PBDEs) in the coastal sediments from East China Sea. Environ Pollut. 2016;213:468–481. doi: 10.1016/j.envpol.2016.02.054. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Luo C, Li J, Yin H, Zhang G. Influence of plants on the distribution and composition of PBDEs in soils of an e-waste dismantling area: evidence of the effect of the rhizosphere and selective bioaccumulation. Environ Pollut. 2014;186:104–109. doi: 10.1016/j.envpol.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 46.Yin W, Zhang Y, Wang P, Zheng S, Zhu C, Han X, Zhang Q, Liang Y, Jiang G. Distribution of polybrominated diphenyl ethers (PBDEs) in feather and muscle of the birds of prey from Beijing, China. Ecotoxicol Environ Saf. 2018;165:343–348. doi: 10.1016/j.ecoenv.2018.08.056. [DOI] [PubMed] [Google Scholar]
- 47.Xia C, Lam JC, Wu X, Sun L, Xie Z, Lam PK. Levels and distribution of polybrominated diphenyl ethers (PBDEs) in marine fishes from Chinese coastal waters. Chemosphere. 2011;82(1):18–24. doi: 10.1016/j.chemosphere.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 48.Kajiwara N, Kamikawa S, Ramu K, Ueno D, Yamada TK, Subramanian A, Lam PK, Jefferson TA, Prudente M, Chung KH, Tanabe S. Geographical distribution of polybrominated diphenyl ethers (PBDEs) and organochlorines in small cetaceans from Asian waters. Chemosphere. 2006;64(2):287–295. doi: 10.1016/j.chemosphere.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 49.Zhu NZ, Liu LY, Ma WL, Li WL, Song WW, Qi H, Li YF. Polybrominated diphenyl ethers (PBDEs) in the indoor dust in China: levels, spatial distribution and human exposure. Ecotoxicol Environ Saf. 2015;111:1–8. doi: 10.1016/j.ecoenv.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 50.Zhu H. Distribution and fate of polybrominated diphenyl ethers (PBDEs) in Hong Kong mangrove wetland (Doctoral dissertation, City University of Hong Kong) 2013. [Google Scholar]
- 51.Andrade NA. Environmental fate of polybrominated diphenyl ethers in agricultural soils which have received biosolids application (doctoral dissertation) 2008. [Google Scholar]
- 52.Guan YF, Sojinu OS, Li SM, Zeng EY. Fate of polybrominated diphenyl ethers in the environment of the Pearl River estuary, South China. Environ Pollut. 2009;157(7):2166–2172. doi: 10.1016/j.envpol.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 53.Palm A, Cousins IT, Mackay D, Tysklind M, Metcalfe C, Alaee M. Assessing the environmental fate of chemicals of emerging concern: a case study of the polybrominated diphenyl ethers. Environ Pollut. 2002;117(2):195–213. doi: 10.1016/S0269-7491(01)00276-7. [DOI] [PubMed] [Google Scholar]
- 54.Ahn MY, Filley TR, Jafvert CT, Nies L, Hua I, Bezares-Cruz J. Photodegradation of decabromodiphenyl ether adsorbed onto clay minerals, metal oxides, and sediment. Environ Sci Technol. 2006;40(1):215–220. doi: 10.1021/es051415t. [DOI] [PubMed] [Google Scholar]
- 55.Tokarz Iii JA, Ahn MY, Leng J, Filley TR, Nies L. Reductive debromination of polybrominated diphenyl ethers in anaerobic sediment and a biomimetic system. Environ Sci Technol. 2008;42(4):1157–1164. doi: 10.1021/es071989t. [DOI] [PubMed] [Google Scholar]
- 56.Zhu H, Wang Y, Tam NF. Microcosm study on fate of polybrominated diphenyl ethers (PBDEs) in contaminated mangrove sediment. J Hazard Mater. 2014;265:61–68. doi: 10.1016/j.jhazmat.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 57.Thuresson K, Höglund P, Hagmar L, Sjödin A, Bergman Å, Jakobsson K. Apparent half-lives of hepta-to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environ Health Perspect. 2006;114(2):176–181. doi: 10.1289/ehp.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McDonald TA. A perspective on the potential health risks of PBDEs. Chemosphere. 2002;46(5):745–755. doi: 10.1016/S0045-6535(01)00239-9. [DOI] [PubMed] [Google Scholar]
- 59.Geyer HJ, Schramm KW, Darnerud PO, Aune M, Feicht EA, Fried KW, Henkelmann B, Lenoir D, Schmid P, McDonald TA. Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohalogen Compd. 2004;66(2004):3820–3825. [Google Scholar]
- 60.Rayne S, Ikonomou MG. Predicting gas chromatographic retention times for the 209 polybrominated diphenyl ether congeners. J Chromatogr A. 2003;1016(2):235–248. doi: 10.1016/j.chroma.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 61.de la Cal A, Eljarrat E, Barceló D. Determination of 39 polybrominated diphenyl ether congeners in sediment samples using fast selective pressurized liquid extraction and purification. J Chromatogr A. 2003;1021(1–2):165–173. doi: 10.1016/j.chroma.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 62.Dodder NG, Strandberg B, Hites RA. Concentrations and spatial variations of polybrominated diphenyl ethers and several organochlorine compounds in fishes from the northeastern United States. Environ Sci Technol. 2002;36(2):146–151. doi: 10.1021/es010947g. [DOI] [PubMed] [Google Scholar]
- 63.Eljarrat E, de la Cal A, Barceló D. Potential chlorinated and brominated interferences on the polybrominated diphenyl ether determinations by gas chromatography–mass spectrometry. J Chromatogr A. 2003;1008(2):181–192. doi: 10.1016/S0021-9673(03)00980-4. [DOI] [PubMed] [Google Scholar]
- 64.Guo J, Lin K, Deng J, Fu X, Xu Z. Polybrominated diphenyl ethers in indoor air during waste TV recycling process. J Hazard Mater. 2015;283:439–446. doi: 10.1016/j.jhazmat.2014.09.044. [DOI] [PubMed] [Google Scholar]
- 65.Wang XM, Ding X, Mai BX, Xie ZQ, Xiang CH, Sun LG, Sheng GY, Fu JM, Zeng EY. Polybrominated diphenyl ethers in airborne particulates collected during a research expedition from the Bohai Sea to the Arctic. Environ Sci Technol. 2005;39(20):7803–7809. doi: 10.1021/es051088p. [DOI] [PubMed] [Google Scholar]
- 66.Tadeo JL, Sánchez-Brunete C, Miguel E. Determination of polybrominated diphenyl ethers in human hair by gas chromatography–mass spectrometry. Talanta. 2009;78(1):138–143. doi: 10.1016/j.talanta.2008.10.054. [DOI] [PubMed] [Google Scholar]
- 67.Gao S, Hong J, Yu Z, Wang J, Yang G, Sheng G, Fu J. Polybrominated diphenyl ethers in surface soils from e-waste recycling areas and industrial areas in South China: concentration levels, congener profile, and inventory. Environ Toxicol Chem. 2011;30(12):2688–2696. doi: 10.1002/etc.668. [DOI] [PubMed] [Google Scholar]
- 68.Fontana AR, Silva MF, Martínez LD, Wuilloud RG, Altamirano JC. Determination of polybrominated diphenyl ethers in water and soil samples by cloud point extraction-ultrasound-assisted back-extraction-gas chromatography–mass spectrometry. J Chromatogr A. 2009;1216(20):4339–4346. doi: 10.1016/j.chroma.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 69.Moukas AI, Maragou NC, Thomaidis NS, Calokerinos AC. Determination of Polybrominated Diphenyl ether flame retardants in surface water by liquid chromatography–atmospheric pressure photoionization tandem mass spectrometry. Anal Lett. 2018;51(1–2):96–110. doi: 10.1080/00032719.2017.1339713. [DOI] [Google Scholar]
- 70.Da C, Wu K, Ye J, Wang R, Liu R, Sun R. Temporal trends of polybrominated diphenyl ethers in the sediment cores from different areas in China. Ecotoxicol Environ Saf. 2019;171:222–230. doi: 10.1016/j.ecoenv.2018.12.074. [DOI] [PubMed] [Google Scholar]
- 71.Shao Y, Han S, Ma L, Luo M, Yang G, Liu W, Xu D. Polybrominated diphenyl ethers in surface waters around Beijing: occurrence, distribution and sources. Appl Geochem. 2018;98:58–64. doi: 10.1016/j.apgeochem.2018.09.011. [DOI] [Google Scholar]
- 72.Meng XZ, Xiang N, Yu L, Zhang J, Chen L, Dai X. Exploring the bioaccessibility of polybrominated diphenyl ethers (PBDEs) in sewage sludge. Environ Pollut. 2015;207:1–5. doi: 10.1016/j.envpol.2015.08.043. [DOI] [PubMed] [Google Scholar]
- 73.Demirtepe H, Imamoglu I. Levels of polybrominated diphenyl ethers and hexabromocyclododecane in treatment plant sludge: implications on sludge management. Chemosphere. 2019;221:606–615. doi: 10.1016/j.chemosphere.2019.01.060. [DOI] [PubMed] [Google Scholar]
- 74.Oloruntoba K, Sindiku O, Osibanjo O, Balan S, Weber R. Polybrominated diphenyl ethers (PBDEs) in chicken eggs and cow milk around municipal dumpsites in Abuja, Nigeria. Ecotoxicol Environ Saf. 2019;179:282–289. doi: 10.1016/j.ecoenv.2019.04.045. [DOI] [PubMed] [Google Scholar]
- 75.Cruz R, Marques A, Casal S, Cunha SC. Fast and environmental-friendly methods for the determination of polybrominated diphenyl ethers and their metabolites in fish tissues and feed. Sci Total Environ. 2019;646:1503–1515. doi: 10.1016/j.scitotenv.2018.07.342. [DOI] [PubMed] [Google Scholar]
- 76.Wang J, Zhao X, Wang Y, Shi Z. Tetrabromobisphenol A, hexabromocyclododecane isomers and polybrominated diphenyl ethers in foodstuffs from Beijing, China: contamination levels, dietary exposure and risk assessment. Sci Total Environ. 2019;666:812–820. doi: 10.1016/j.scitotenv.2019.02.324. [DOI] [PubMed] [Google Scholar]
- 77.Guo LC, Yu S, Wu D, Huang J, Liu T, Xiao J, Huang W, Gao Y, Li X, Zeng W, Rutherford S. Disruption of thyroid hormone regulated proteins and gene expression by polychlorinated biphenyls, polybrominated diphenyl ethers and new flame retardants in residents of an e-waste region. Environ Pollut. 2019;254:112925. doi: 10.1016/j.envpol.2019.07.093. [DOI] [PubMed] [Google Scholar]
- 78.Liu PY, Zhao YX, Zhu YY, Qin ZF, Ruan XL, Zhang YC, Chen BJ, Li Y, Yan SS, Qin XF, Fu S. Determination of polybrominated diphenyl ethers in human semen. Environ Int. 2012;42:132–137. doi: 10.1016/j.envint.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 79.Ji H, Liang H, Wang Z, Miao M, Wang X, Zhang X, Wen S, Chen A, Sun X, Yuan W. Associations of prenatal exposures to low levels of Polybrominated Diphenyl ether (PBDE) with thyroid hormones in cord plasma and neurobehavioral development in children at 2 and 4 years. Environ Int. 2019;131:105010. doi: 10.1016/j.envint.2019.105010. [DOI] [PubMed] [Google Scholar]
- 80.Luan M, Liang H, Yang F, Yuan W, Chen A, Liu X, Ji H, Wen S, Miao M. Prenatal polybrominated diphenyl ethers exposure and anogenital distance in boys from a Shanghai birth cohort. Int J Hyg Environ Health. 2019;222(3):513–523. doi: 10.1016/j.ijheh.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 81.Gevao B, Al-Bahloul M, Al-Ghadban AN, Al-Omair A, Ali L, Zafar J, Helaleh M. House dust as a source of human exposure to polybrominated diphenyl ethers in Kuwait. Chemosphere. 2006;64(4):603–608. doi: 10.1016/j.chemosphere.2005.11.055. [DOI] [PubMed] [Google Scholar]
- 82.Harrad S, Ibarra C, Diamond M, Melymuk L, Robson M, Douwes J, Roosens L, Dirtu AC, Covaci A. Polybrominated diphenyl ethers in domestic indoor dust from Canada, New Zealand, United Kingdom and United States. Environ Int. 2008;34(2):232–238. doi: 10.1016/j.envint.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 83.Arita Y, Yeh C, Thoma T, Getahun D, Menon R, Peltier MR. Effect of polybrominated diphenyl ether congeners on placental cytokine production. J Reprod Immunol. 2018;125:72–79. doi: 10.1016/j.jri.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 84.Leonetti C, Butt CM, Hoffman K, Miranda ML, Stapleton HM. Concentrations of polybrominated diphenyl ethers (PBDEs) and 2, 4, 6-tribromophenol in human placental tissues. Environ Int. 2016;88:23–29. doi: 10.1016/j.envint.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vuong AM, Braun JM, Wang Z, Yolton K, Xie C, Sjodin A, Webster GM, Lanphear BP, Chen A. Exposure to polybrominated diphenyl ethers (PBDEs) during childhood and adiposity measures at age 8 years. Environ Int. 2019;123:148–155. doi: 10.1016/j.envint.2018.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.He Y, Peng L, Zhang W, Liu C, Yang Q, Zheng S, Bao M, Huang Y, Wu K. Adipose tissue levels of polybrominated diphenyl ethers and breast cancer risk in Chinese women: A case–control study. Environ Res. 2018;167:160–168. doi: 10.1016/j.envres.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 87.Covaci A, Voorspoels S, Roosens L, Jacobs W, Blust R, Neels H. Polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in human liver and adipose tissue samples from Belgium. Chemosphere. 2008;73(2):170–175. doi: 10.1016/j.chemosphere.2008.02.059. [DOI] [PubMed] [Google Scholar]
- 88.Novak P, Zuliani T, Milačič R, Ščančar J. Development of an analytical method for the determination of polybrominated diphenyl ethers in mussels and fish by gas chromatography—inductively coupled plasma mass spectrometry. J Chromatogr A. 2017;1524:179–187. doi: 10.1016/j.chroma.2017.09.059. [DOI] [PubMed] [Google Scholar]
- 89.Voorspoels S, Covaci A, Lepom P, Jaspers VL, Schepens P. Levels and distribution of polybrominated diphenyl ethers in various tissues of birds of prey. Environ Pollut. 2006;144(1):218–227. doi: 10.1016/j.envpol.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 90.Miller MF, Chernyak SM, Domino SE, Batterman SA, Loch-Caruso R. Concentrations and speciation of polybrominated diphenyl ethers in human amniotic fluid. Sci Total Environ. 2012;417:294–298. doi: 10.1016/j.scitotenv.2011.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hurley S, Goldberg D, Park JS, Petreas M, Bernstein L, Anton-Culver H, Neuhausen SL, Nelson DO, Reynolds P. A breast cancer case-control study of polybrominated diphenyl ether (PBDE) serum levels among California women. Environ Int. 2019;127:412–419. doi: 10.1016/j.envint.2019.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Choi G, Kim S, Kim S, Kim S, Choi Y, Kim HJ, Lee JJ, Kim SY, Lee S, Moon HB, Choi S. Occurrences of major polybrominated diphenyl ethers (PBDEs) in maternal and fetal cord blood sera in Korea. Sci Total Environ. 2014;491:219–226. doi: 10.1016/j.scitotenv.2014.02.071. [DOI] [PubMed] [Google Scholar]
- 93.Wang D, Chen T, Fu Z, Yang L, Li R, Sui S, Wang Y, Shi Z. Occupational exposure to polybrominated diphenyl ethers or decabromodiphenyl ethane during chemical manufacturing: occurrence and health risk assessment. Chemosphere. 2019;231:385–392. doi: 10.1016/j.chemosphere.2019.05.165. [DOI] [PubMed] [Google Scholar]
- 94.Yu YJ, Lin BG, Chen XC, Qiao J, Li LZ, Liang Y, Zhang GZ, Jia Y, Zhou XQ, Chen CR, Kan HD. Polybrominated diphenyl ethers in human serum, semen and indoor dust: effects on hormones balance and semen quality. Sci Total Environ. 2019;671:1017–1025. doi: 10.1016/j.scitotenv.2019.03.319. [DOI] [Google Scholar]
- 95.Chen T, Huang M, Li J, Li J, Shi Z. Polybrominated diphenyl ethers and novel brominated flame retardants in human milk from the general population in Beijing, China: occurrence, temporal trends, nursing infants’ exposure and risk assessment. Sci Total Environ. 2019;689:278–286. doi: 10.1016/j.scitotenv.2019.06.442. [DOI] [PubMed] [Google Scholar]
- 96.Wang JX, Jiang DQ, Gu ZY, Yan XP. Multiwalled carbon nanotubes coated fibers for solid-phase microextraction of polybrominated diphenyl ethers in water and milk samples before gas chromatography with electron-capture detection. J Chromatogr A. 2006;1137(1):8–14. [DOI] [PubMed]
- 97.Whitehead TP, Brown FR, Metayer C, Park JS, Does M, Petreas MX, Buffler PA, Rappaport SM. Polybrominated diphenyl ethers in residential dust: sources of variability. Environ Int. 2013;57:11–24. doi: 10.1016/j.envint.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lorber M. Exposure of Americans to polybrominated diphenyl ethers. J Expo Sci Environ Epidemiol. 2008;18(1):2. doi: 10.1038/sj.jes.7500572. [DOI] [PubMed] [Google Scholar]
- 99.Harrad S, de Wit CA, Abdallah MAE, Bergh C, Bjorklund JA, Covaci A, Darnerud PO, de Boer J, Diamond M, Huber S, Leonards P. Indoor contamination with hexabromocyclododecanes, polybrominated diphenyl ethers, and perfluoroalkyl compounds: an important exposure pathway for people? Environ Sci Technol. 2010;44(9):3221–3231. doi: 10.1021/es903476t. [DOI] [PubMed] [Google Scholar]
- 100.Cristale J, Lacorte S. PBDEs versus NBFR in wastewater treatment plants: occurrence and partitioning in water and sludge. AIMS Environ Sci. 2015;2(3):533–546. doi: 10.3934/environsci.2015.3.533. [DOI] [Google Scholar]
- 101.Adeniji AO, Okoh OO, Okoh AI. Analytical methods for the determination of the distribution of total petroleum hydrocarbons in the water and sediment of aquatic systems: A review. J Chem. 2017:1-13.
- 102.Adeniji AO, Okoh OO, Okoh AI. Analytical methods for polycyclic aromatic hydrocarbons and their global trend of distribution in water and sediment: a review. Recent Insights Petroleum Sci Eng. 2018;10:393–428. [Google Scholar]
- 103.Clarke B, Porter N, Symons R, Marriott P, Ades P, Stevenson G, Blackbeard J. Polybrominated diphenyl ethers and polybrominated biphenyls in Australian sewage sludge. Chemosphere. 2008;73(6):980–989. doi: 10.1016/j.chemosphere.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 104.Wang Y, Li X, Li A, Wang T, Zhang Q, Wang P, Fu J, Jiang G. Effect of municipal sewage treatment plant effluent on bioaccumulation of polychlorinated biphenyls and polybrominated diphenyl ethers in the recipient water. Environ Sci Technol. 2007;41(17):6026–6032. doi: 10.1021/es070913u. [DOI] [PubMed] [Google Scholar]
- 105.Yang C, Meng XZ, Chen L, Xia S. Polybrominated diphenyl ethers in sewage sludge from Shanghai, China: possible ecological risk applied to agricultural land. Chemosphere. 2011;85(3):418–423. doi: 10.1016/j.chemosphere.2011.07.068. [DOI] [PubMed] [Google Scholar]
- 106.Knoth W, Mann W, Meyer R, Nebhuth J. Polybrominated diphenyl ether in sewage sludge in Germany. Chemosphere. 2007;67(9):1831–1837. doi: 10.1016/j.chemosphere.2006.05.113. [DOI] [PubMed] [Google Scholar]
- 107.Kim UJ, Lee IS, Oh JE. Occurrence, removal and release characteristics of dissolved brominated flame retardants and their potential metabolites in various kinds of wastewater. Environ Pollut. 2016;218:551–557. doi: 10.1016/j.envpol.2016.07.037. [DOI] [PubMed] [Google Scholar]
- 108.Kupper T, de Alencastro LF, Gatsigazi R, Furrer R, Grandjean D, Tarradellas J. Concentrations and specific loads of brominated flame retardants in sewage sludge. Chemosphere. 2008;71(6):1173–1180. doi: 10.1016/j.chemosphere.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 109.Lee IS, Sim WJ, Oh JE, Kim CW, Chang YS, Yoon YS. Evaluation of removal efficiencies of micropollutants in wastewater treatment plants. J Korean Soc Environ Eng. 2007;29(2):214–219. [Google Scholar]
- 110.Lee HJ, Kim CJ, Hong GH, Hong SH, Shim WJ, Kim GB. Congener-specific accumulation and environmental risk assessment of polybrominated diphenyl ethers in diverse Korean sewage sludge types. Environ Sci Pollut Res. 2014;21(12):7480–7488. doi: 10.1007/s11356-014-2664-7. [DOI] [PubMed] [Google Scholar]
- 111.Moon HB, Kannan K, Choi M, Yu J, Choi HG, An YR, Choi SG, Park JY, Kim ZG. Chlorinated and brominated contaminants including PCBs and PBDEs in minke whales and common dolphins from Korean coastal waters. J Hazard Mater. 2010;179(1–3):735–741. doi: 10.1016/j.jhazmat.2010.03.063. [DOI] [PubMed] [Google Scholar]
- 112.Moon HB, Kannan K, Lee SJ, Choi M. Atmospheric deposition of polybrominated diphenyl ethers (PBDEs) in coastal areas in Korea. Chemosphere. 2007;66(4):585–593. doi: 10.1016/j.chemosphere.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 113.Hong SH, Kannan N, Jin Y, Won JH, Han GM, Shim WJ. Temporal trend, spatial distribution, and terrestrial sources of PBDEs and PCBs in Masan Bay, Korea. Mar Pollut Bull. 2010;60(10):1836–1841. doi: 10.1016/j.marpolbul.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 114.Jin X, Lee S, Jeong Y, Yu JP, Baek WK, Shin KH, Kannan K, Moon HB. Species-specific accumulation of polybrominated diphenyl ethers (PBDEs) and other emerging flame retardants in several species of birds from Korea. Environ Pollut. 2016;219:191–200. doi: 10.1016/j.envpol.2016.10.040. [DOI] [PubMed] [Google Scholar]
- 115.Lee HJ, Kim GB. Removal rate and releases of polybrominated diphenyl ethers in two wastewater treatment plants, Korea. Ocean Sci J. 2017;52(2):193–205. doi: 10.1007/s12601-017-0026-3. [DOI] [Google Scholar]
- 116.Rayne S, Ikonomou MG. Polybrominated diphenyl ethers in an advanced wastewater treatment plant. Part 1: concentrations, patterns, and influence of treatment processes. J Environ Eng Sci. 2005;4(5):353–367. doi: 10.1139/s04-071. [DOI] [Google Scholar]
- 117.Lee S, Song GJ, Kannan K, Moon HB. Occurrence of PBDEs and other alternative brominated flame retardants in sludge from wastewater treatment plants in Korea. Sci Total Environ. 2014;470:1422–1429. doi: 10.1016/j.scitotenv.2013.07.118. [DOI] [PubMed] [Google Scholar]
- 118.Kim M, Li LY, Gorgy T, Grace JR. Review of contamination of sewage sludge and amended soils by polybrominated diphenyl ethers based on meta-analysis. Environ Pollut. 2017;220:753–765. doi: 10.1016/j.envpol.2016.10.053. [DOI] [PubMed] [Google Scholar]
- 119.De la Torre A, Concejero MA, Martínez MA. Concentrations and sources of an emerging pollutant, decabromodiphenylethane (DBDPE), in sewage sludge for land application. J Environ Sci. 2012;24(3):558–563. doi: 10.1016/S1001-0742(11)60801-2. [DOI] [PubMed] [Google Scholar]
- 120.Zeng L, Yang R, Zhang Q, Zhang H, Xiao K, Zhang H, Wang Y, Lam PK, Jiang G. Current levels and composition profiles of emerging halogenated flame retardants and dehalogenated products in sewage sludge from municipal wastewater treatment plants in China. Environ Sci Technol. 2014;48(21):12586–12594. doi: 10.1021/es503510q. [DOI] [PubMed] [Google Scholar]
- 121.Andersen A. Disposal and recycling routes for sewage sludge part 4: economic report. Luxembourg: European Communities; 2002. [Google Scholar]
- 122.Andrade NA, McConnell LL, Torrents A, Ramirez M. Persistence of polybrominated diphenyl ethers in agricultural soils after biosolids applications. J Agric Food Chem. 2010;58(5):3077–3084. doi: 10.1021/jf9034496. [DOI] [PubMed] [Google Scholar]
- 123.Mai B, Chen S, Chen S, Luo X, Chen L, Chen L, Yang Q, Sheng G, Peng P, Fu J, Zeng EY. Distribution of polybrominated diphenyl ethers in sediments of the Pearl River Delta and adjacent South China Sea. Environ Sci Technol. 2005;39(10):3521–3527. doi: 10.1021/es048083x. [DOI] [PubMed] [Google Scholar]
- 124.Meijer SN, Harner T, Helm PA, Halsall CJ, Johnston AE, Jones KC. Polychlorinated naphthalenes in UK soils: time trends, markers of source, and equilibrium status. Environ Sci Technol. 2001;35(21):4205–4213. doi: 10.1021/es010071d. [DOI] [PubMed] [Google Scholar]
- 125.Hafeez S, Mahmood A, Syed JH, Li J, Ali U, Malik RN, Zhang G. Waste dumping sites as a potential source of POPs and associated health risks in perspective of current waste management practices in Lahore city, Pakistan. Sci Total Environ. 2016;562:953–961. doi: 10.1016/j.scitotenv.2016.01.120. [DOI] [PubMed] [Google Scholar]
- 126.Olukunle OI, Okonkwo OJ. Concentration of novel brominated flame retardants and HBCD in leachates and sediments from selected municipal solid waste landfill sites in Gauteng Province, South Africa. Waste Manag. 2015;43:300–306. doi: 10.1016/j.wasman.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 127.Wang Z, Na G, Ma X, Ge L, Lin Z, Yao Z. Characterizing the distribution of selected PBDEs in soil, moss and reindeer dung at Ny-Ålesund of the Arctic. Chemosphere. 2015;137:9–13. doi: 10.1016/j.chemosphere.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 128.Abdallah MAE, Drage D, Harrad S. A one-step extraction/clean-up method for determination of PCBs, PBDEs and HBCDs in environmental solid matrices. Environ Sci Process Impacts. 2013;15(12):2279–2287. doi: 10.1039/c3em00395g. [DOI] [PubMed] [Google Scholar]
- 129.Brits M, de Vos J, Weiss JM, Rohwer ER, de Boer J. Critical review of the analysis of brominated flame retardants and their environmental levels in Africa. Chemosphere. 2016;164:174–189. doi: 10.1016/j.chemosphere.2016.08.097. [DOI] [PubMed] [Google Scholar]
- 130.McGrath TJ, Ball AS, Clarke BO. Critical review of soil contamination by polybrominated diphenyl ethers (PBDEs) and novel brominated flame retardants (NBFRs); concentrations, sources and congener profiles. Environ Pollut. 2017;230:741–757. doi: 10.1016/j.envpol.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 131.Cetin B, Odabasi M, Bayram A. Wet deposition of persistent organic pollutants (POPs) in Izmir, Turkey. Environ Sci Pollut Res. 2016;23(9):9227–9236. doi: 10.1007/s11356-016-6183-6. [DOI] [PubMed] [Google Scholar]
- 132.Cousins AP, Holmgren T, Remberger M. Emissions of two phthalate esters and BDE 209 to indoor air and their impact on urban air quality. Sci Total Environ. 2014;470:527–535. doi: 10.1016/j.scitotenv.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 133.Sjödin A, Jones RS, Focant JF, Lapeza C, Wang RY, McGahee EE, 3rd, Zhang Y, Turner WE, Slazyk B, Needham LL, Patterson DG., Jr Retrospective time-trend study of polybrominated diphenyl ether and polybrominated and polychlorinated biphenyl levels in human serum from the United States. Environ Health Perspect. 2004;112(6):654–658. doi: 10.1289/ehp.112-1241957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Siddique S, Xian Q, Abdelouahab N, Takser L, Phillips SP, Feng YL, Wang B, Zhu J. Levels of dechlorane plus and polybrominated diphenylethers in human milk in two Canadian cities. Environ Int. 2012;39(1):50–55. doi: 10.1016/j.envint.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 135.Kim TH, Lim HJ, Won AJ, Ahn MY, Patra N, Chung KK, Kwack SJ, Park KL, Han SY, Choi WS, Han JY. Comparisons of polybrominated diphenyl ethers levels in paired south Korean cord blood, maternal blood, and breast milk samples. Chemosphere. 2012;87(1):97–104. doi: 10.1016/j.chemosphere.2011.11.074. [DOI] [PubMed] [Google Scholar]
- 136.Haraguchi K, Koizumi A, Inoue K, Harada KH, Hitomi T, Minata M, Tanabe M, Kato Y, Nishimura E, Yamamoto Y, Watanabe T. Levels and regional trends of persistent organochlorines and polybrominated diphenyl ethers in Asian breast milk demonstrate POPs signatures unique to individual countries. Environ Int. 2009;35(7):1072–1079. doi: 10.1016/j.envint.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 137.Sjödin A, Jones RS, Caudill SP, Wong LY, Turner WE, Calafat AM. Polybrominated diphenyl ethers, polychlorinated biphenyls, and persistent pesticides in serum from the National Health and nutrition examination survey: 2003–2008. Environ Sci Technol. 2013;48(1):753–760. doi: 10.1021/es4037836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zota AR, Linderholm L, Park JS, Petreas M, Guo T, Privalsky ML, Zoeller RT, Woodruff TJ. Temporal comparison of PBDEs, OH-PBDEs, PCBs, and OH-PCBs in the serum of second trimester pregnant women recruited from San Francisco general hospital, California. Environ Sci Technol. 2013;47(20):11776–11784. doi: 10.1021/es402204y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Genuis SK, Birkholz D, Genuis SJ. Human excretion of polybrominated diphenyl ether flame retardants: blood, urine, and sweat study. BioMed Res Intern. 2017;2017. [DOI] [PMC free article] [PubMed]
- 140.Council of the European communities (CEC). Directive 2003/11/EC of the European Parliament and of the Council of 6 February 2003. Amending for the 24th Time Council Directive 76/769/EEC Relating to Restrictions on the Marketing and Use of Certain Dangerous Substances and Preparations (Pentabromodiphenyl Ether, Octabromodiphenylether). Brussels: European Union; 2003. p. 45–6.
- 141.Council of the European Communities (CEC). Directive 76/769/EEC of the European Parliament and of the Council of 27 July 1976 on the Approximation of the Laws, Regulations and Administrative Provisions of the MemberStates RelatingtoRestrictionson theMarketingand UseofCertainDangerousSubstances andPreparations; European Union: Brussels, 1976.
- 142.Court of Justice of the European Union (CJEU). Court Proceeding 2008/c116/02. Kirchberg: European Union; 2008.
- 143.Schuster JK, Gioia R, Moeckel C, Agarwal T, Bucheli TD, Breivik K, Steinnes E, Jones KC. Has the burden and distribution of PCBs and PBDEs changed in European background soils between 1998 and 2008? Implications for sources and processes. Environ Sci Technol. 2011;45(17):7291–7297. doi: 10.1021/es200961p. [DOI] [PubMed] [Google Scholar]
- 144.Munschy C, Héas-Moisan K, Tixier C, Olivier N, Gastineau O, Le Bayon N, Buchet V. Dietary exposure of juvenile common sole (Solea solea L.) to polybrominated diphenyl ethers (PBDEs): part 1. Bioaccumulation and elimination kinetics of individual congeners and their debrominated metabolites. Environ Pollut. 2011;159(1):229–237. doi: 10.1016/j.envpol.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 145.Echols KR, Peterman PH, Hinck JE, Orazio CE. Polybrominated diphenyl ether metabolism in field collected fish from the Gila River, Arizona, USA–levels, possible sources, and patterns. Chemosphere. 2013;90(1):20–27. doi: 10.1016/j.chemosphere.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 146.Josefsson S, Leonardsson K, Gunnarsson JS, Wiberg K. Bioturbation-driven release of buried PCBs and PBDEs from different depths in contaminated sediments. Environ Sci Technol. 2010;44:7456–7464. doi: 10.1021/es100615g. [DOI] [PubMed] [Google Scholar]
- 147.Törnkvist A, Glynn A, Aune M, Darnerud PO, Ankarberg EH. PCDD/F, PCB, PBDE, HBCD and chlorinated pesticides in a Swedish market basket from 2005–levels and dietary intake estimations. Chemosphere. 2011;83(2):193–199. doi: 10.1016/j.chemosphere.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 148.Bocio A, Llobet JM, Domingo JL, Corbella J, Teixido A, Casas C. Polybrominated diphenyl ethers (PBDEs) in foodstuffs: human exposure through the diet. J Agric Food Chem. 2003;51(10):3191–3195. doi: 10.1021/jf0340916. [DOI] [PubMed] [Google Scholar]
- 149.Ryan JJ, Patry B. Body burdens and food exposure in Canada for polybrominated diphenyl ethers (BDEs) Organohalogen Compd. 2001;51:226–229. [Google Scholar]
- 150.Darnerud PO, Atuma S, Aune M, Bjerselius R, Glynn A, Grawé KP, Becker W. Dietary intake estimations of organohalogen contaminants (dioxins, PCB, PBDE and chlorinated pesticides, eg DDT) based on Swedish market basket data. Food Chem Toxicol. 2006;44(9):1597–1606. doi: 10.1016/j.fct.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 151.Schecter A, Haffner D, Colacino J, Patel K, Päpke O, Opel M, Birnbaum L. Polybrominated diphenyl ethers (PBDEs) and hexabromocyclodecane (HBCD) in composite US food samples. Environ Health Perspect. 2009;118(3):357–362. doi: 10.1289/ehp.0901345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Voorspoels S, Covaci A, Neels H, Schepens P. Dietary PBDE intake: A market-basket study in Belgium. Environ Int. 2007;33:93–97. doi: 10.1016/j.envint.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 153.Kalantzi OI, Siskos PA. Sources and human exposure to polybrominated diphenyl ethers. Global NEST J. 2011;13(2):99–108. [Google Scholar]
- 154.Jakobsson K, Thuresson K, Rylander L, Sjödin A, Hagmar L, Bergman Å. Exposure to polybrominated diphenyl ethers and tetrabromobisphenol A among computer technicians. Chemosphere. 2002;46(5):709–716. doi: 10.1016/S0045-6535(01)00235-1. [DOI] [PubMed] [Google Scholar]
- 155.Webster T, Vieira V, Schecter A. Estimating human exposure to PBDE-47 via air, food and dust using Monte Carlo methods. Organohalogen Compd. 2005;67:505–508. [Google Scholar]
- 156.Ohajinwa CM, van Bodegom PM, Osibanjo O, Xie Q, Chen J, Vijver MG, Peijnenburg WJGM. Health Risks of Polybrominated Diphenyl Ethers (PBDEs) and Metals at Informal Electronic Waste Recycling Sites. Intern J Environ Res Public Health. 2019;16(6):906. doi: 10.3390/ijerph16060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Thuresson K, Bergman Å, Jakobsson K. Occupational exposure to commercial decabromodiphenyl ether in workers manufacturing or handling flame-retarded rubber. Environ Sci Technol. 2005;39(7):1980–1986. doi: 10.1021/es048511z. [DOI] [PubMed] [Google Scholar]
- 158.Agency for Toxic Substances and Disease Registry (ATSDR) The ATSDR 2017 substance priority list. 2017. [Google Scholar]
- 159.Sahlström LM, Sellström U, de Wit CA, Lignell S, Darnerud PO. Brominated flame retardants in matched serum samples from Swedish first-time mothers and their toddlers. Environ Sci Technol. 2014;48(13):7584–7592. doi: 10.1021/es501139d. [DOI] [PubMed] [Google Scholar]
- 160.Gascon M, Vrijheid M, Martínez D, Forns J, Grimalt JO, Torrent M, Sunyer J. Effects of pre and postnatal exposure to low levels of polybromodiphenyl ethers on neurodevelopment and thyroid hormone levels at 4 years of age. Environ Int. 2011;37(3):605–611. doi: 10.1016/j.envint.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 161.Hertz-Picciotto I, Bergman Å, Fängström B, Rose M, Krakowiak P, Pessah I, Hansen R, Bennett DH. Polybrominated diphenyl ethers in relation to autism and developmental delay: a case-control study. Environ Health. 2011;10(1):1. doi: 10.1186/1476-069X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hallgren S, Sinjari T, Håkansson H, Darnerud P. Effects of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) on thyroid hormone and vitamin A levels in rats and mice. Arch Toxicol. 2001;75(4):200–208. doi: 10.1007/s002040000208. [DOI] [PubMed] [Google Scholar]
- 163.Wu L, Li Y, Ru H, Xie H, Yao F, Ni Z, Zhong L. Parental exposure to 2, 2′, 4, 4′ 5-pentain polybrominated diphenyl ethers (BDE-99) causes thyroid disruption and developmental toxicity in zebrafish. Toxicol Appl Pharmacol. 2019;372:11–18. doi: 10.1016/j.taap.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 164.Gorini F, Iervasi G, Coi A, Pitto L, Bianchi F. The role of polybrominated diphenyl ethers in thyroid carcinogenesis: is it a weak hypothesis or a hidden reality? From facts to new perspectives. Int J Environ Res Public Health. 2018;15(9):1834. doi: 10.3390/ijerph15091834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Heindel JJ, Blumberg B. Environmental obesogens: mechanisms and controversies. Annu Rev Pharmacol Toxicol. 2019;59:89–106. doi: 10.1146/annurev-pharmtox-010818-021304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ohoro CR, Adeniji AO, Okoh AI, Okoh OO. Distribution and chemical analysis of pharmaceuticals and personal care products (PPCPs) in the environmental systems: A review. Int J Environ Res Public Health. 2019;16(17):3026. doi: 10.3390/ijerph16173026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hardy ML. Regulatory status and environmental properties of brominated flame retardants undergoing risk assessment in the EU: DBDPO, OBDPO, PeBDPO and HBCD. Polym Degrad Stab. 1999;64(3):545–556. doi: 10.1016/S0141-3910(98)00141-4. [DOI] [Google Scholar]
- 168.Ohajinwa C, Van Bodegom P, Vijver M, Peijnenburg W. Health risks awareness of electronic waste workers in the informal sector in Nigeria. Int J Environ Res Public Health. 2017;14(8):911. doi: 10.3390/ijerph14080911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ohajinwa CM, van Bodegom PM, Vijver MG, Olumide AO, Osibanjo O, Peijnenburg WJ. Prevalence and injury patterns among electronic waste workers in the informal sector in Nigeria. Inj Prev. 2018;24(3):185–192. doi: 10.1136/injuryprev-2016-042265. [DOI] [PubMed] [Google Scholar]
- 170.Grant K, Goldizen FC, Sly PD, Brune MN, Neira M, van den Berg M, Norman RE. Health consequences of exposure to e-waste: a systematic review. Lancet Glob Health. 2013;1(6):e350–e361. doi: 10.1016/S2214-109X(13)70101-3. [DOI] [PubMed] [Google Scholar]
- 171.Chen A, Dietrich KN, Huo X, Ho SM. Developmental neurotoxicants in e-waste: an emerging health concern. Environ Health Perspect. 2010;119(4):431–438. doi: 10.1289/ehp.1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Burns KN, Sayler SK, Neitzel RL. Stress, health, noise exposures, and injuries among electronic waste recycling workers in Ghana. J Occup Med Toxicol. 2019;14(1):1. doi: 10.1186/s12995-018-0222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Choi G, Wang YB, Sundaram R, Chen Z, Barr DB, Louis GMB, Smarr MM. Polybrominated diphenyl ethers and incident pregnancy loss: the LIFE study. Environ Res. 2019;168:375–381. doi: 10.1016/j.envres.2018.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Holmes B, Ebitson M. Determination of PBDEs in river water utilizing automated SPE in conformance with EU standards at IRSA. 2017. [Google Scholar]
- 175.Zota AR, Mitro SD, Robinson JF, Hamilton EG, Park JS, Parry E, Zoeller RT, Woodruff TJ. Polybrominated diphenyl ethers (PBDEs) and hydroxylated PBDE metabolites (OH-PBDEs) in maternal and fetal tissues, and associations with fetal cytochrome P450 gene expression. Environ Int. 2018;112:269–278. doi: 10.1016/j.envint.2017.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mizukawa H, Nomiyama K, Nakatsu S, Yachimori S, Hayashi T, Tashiro Y, Nagano Y, Tanabe S. Species-specific differences in the accumulation features of organohalogen contaminants and their metabolites in the blood of Japanese terrestrial mammals. Environ Pollut. 2013;174:28–37. doi: 10.1016/j.envpol.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 177.Kojima H, Takeuchi S, Uramaru N, Sugihara K, Yoshida T, Kitamura S. Nuclear hormone receptor activity of polybrominated diphenyl ethers and their hydroxylated and methoxylated metabolites in transactivation assays using Chinese hamster ovary cells. Environ Health Perspect. 2009;117(8):1210–1218. doi: 10.1289/ehp.0900753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.EPA, U.S . Method 1614 brominated diphenyl ethers in water soil, sediment and tissue by HRGC/HRMS. 2010. [Google Scholar]
- 179.De Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46(5):583–624. doi: 10.1016/S0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- 180.Covaci A, Voorspoels S, de Boer J. Determination of brominated flame retardants, with emphasis on polybrominated diphenyl ethers (PBDEs) in environmental and human samples—a review. Environ Int. 2003;29(6):735–756. doi: 10.1016/S0160-4120(03)00114-4. [DOI] [PubMed] [Google Scholar]
- 181.Smedes F, de Boer J. Determination of chlorobiphenyls in sediments—analytical methods. TrAC Trends Anal Chem. 1997;16(9):503–517. doi: 10.1016/S0165-9936(97)00080-0. [DOI] [Google Scholar]
- 182.Komolafe O, Bowler B, Dolfing J, Mrozik W, Davenport RJ. Quantification of polybrominated diphenyl ether (PBDE) congeners in wastewater by gas chromatography with electron capture detector (GC-ECD) Anal Methods. 2019;11(27):3474–3482. doi: 10.1039/C9AY00266A. [DOI] [Google Scholar]
- 183.Byczkiewicz M, Jabłoński M. Determining polybrominated diphenyl ethers in surface waters of Western Pomerania using gas chromatography with electron capture detection. Pol J Environ Stud. 2015;24(3):961–968. doi: 10.15244/pjoes/34010. [DOI] [Google Scholar]
- 184.Swart C, Gantois F, Petrov P, Entwisle J, Goenaga-Infante H, Nousiainen M, Bílsel M, Binici B, Gonzalez-Gago A, Pröfrock D, Gören AC. Potential reference measurement procedures for PBDE in surface water at levels required by the EU water frame directive. Talanta. 2016;152:251–258. doi: 10.1016/j.talanta.2016.01.066. [DOI] [PubMed] [Google Scholar]
- 185.de Boer J, Allchin C, Law R, Zegers B, Boon JP. Method for the analysis of polybrominated diphenylethers in sediments and biota. TrAC Trends Anal Chem. 2001;20(10):591–599. doi: 10.1016/S0165-9936(01)00097-8. [DOI] [Google Scholar]
- 186.Zhou SN, Reiner EJ, Marvin C, Helm P, Riddell N, Dorman F, Misselwitz M, et al. Development of liquid chromatography atmospheric pressure chemical ionization tandem mass spectrometry for analysis of halogenated flame retardants in wastewater. Anal Bioanal Chem. 2010;396(3):1311–1320. doi: 10.1007/s00216-009-3279-6. [DOI] [PubMed] [Google Scholar]
- 187.Saito I, Onuki A, Seto H. Indoor organophosphate and polybrominated flame retardants in Tokyo. Indoor Air. 2007;17:28–36. doi: 10.1111/j.1600-0668.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 188.Johnson A, Olson N. Analysis and occurrence of polybrominated diphenyl ethers in Washington state freshwater fish. Arch Environ Contam Toxicol. 2001;41(3):339–344. doi: 10.1007/s002440010257. [DOI] [PubMed] [Google Scholar]
- 189.Lagalante AF, Oswald TD. Analysis of polybrominated diphenyl ethers (PBDEs) by liquid chromatography with negative-ion atmospheric pressure photoionization tandem mass spectrometry (LC/NI-APPI/MS/MS): application to house dust. Anal Bioanal Chem. 2008;391(6):2249–2256. doi: 10.1007/s00216-008-2156-z. [DOI] [PubMed] [Google Scholar]
- 190.Gevao B, Semple KT, Jones KC. Bound pesticide residues in soils: a review. Environ Pollut. 2000;108(1):3–14. doi: 10.1016/S0269-7491(99)00197-9. [DOI] [PubMed] [Google Scholar]
- 191.Novak P, Zuliani T, Milačič R, Ščančar J. Development of an analytical method for the determination of polybrominated diphenyl ethers in sewage sludge by the use of gas chromatography coupled to inductively coupled plasma mass spectrometry. Anal Chim Acta. 2016;915:27–35. doi: 10.1016/j.aca.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 192.Bergant M, Milačič R, Ščančar J. Determination of polybrominated diphenyl ethers in human serum by gas chromatography–inductively coupled plasma mass spectrometry. J Chromatogr A. 2018;1572:112–118. doi: 10.1016/j.chroma.2018.08.043. [DOI] [PubMed] [Google Scholar]
- 193.Labadie P, Tlili K, Alliot F, Bourges C, Desportes A, Chevreuil M. Development of analytical procedures for trace-level determination of polybrominated diphenyl ethers and tetrabromobisphenol A in river water and sediment. Anal Bioanal Chem. 2010;396(2):865–875. doi: 10.1007/s00216-009-3267-x. [DOI] [PubMed] [Google Scholar]
- 194.Śmiełowska M, Zabiegała B. Determination of polybrominated diphenyl ethers (PBDEs) in dust samples collected in air conditioning filters of different usage–method development. J Chromatogr A. 2018;1565:57–67. doi: 10.1016/j.chroma.2018.06.041. [DOI] [PubMed] [Google Scholar]
- 195.Magdic S, Pawliszyn JB. Analysis of organochlorine pesticides using solid-phase microextraction. J Chromatogr A. 1996;723(1):111–22. [DOI] [PubMed]
- 196.Mitra S. Sample preparation techniques in analytical chemistry, vol. 237: Wiley; 2004.
- 197.Liu X, Li J, Zhao Z, Zhang W, Lin K, Huang C, Wang X. Solid-phase extraction combined with dispersive liquid–liquid microextraction for the determination for polybrominated diphenyl ethers in different environmental matrices. J Chromatogr A. 2009;1216(12):2220–2226. doi: 10.1016/j.chroma.2008.12.092. [DOI] [PubMed] [Google Scholar]
- 198.Pedersen-Bjergaard S, Rasmussen KE. Electrical potential can drive liquid-liquid extraction for sample preparation in chromatography. TrAC Trends Anal Chem. 2008;27(10):934–941. doi: 10.1016/j.trac.2008.08.005. [DOI] [Google Scholar]
- 199.Covaci A, Voorspoels S, Ramos L, Neels H, Blust R. Recent developments in the analysis of brominated flame retardants and brominated natural compounds. J Chromatogr A. 2007;1153(1–2):145–171. doi: 10.1016/j.chroma.2006.11.060. [DOI] [PubMed] [Google Scholar]
- 200.Vonderheide AP. A review of the challenges in the chemical analysis of the polybrominated diphenyl ethers. Microchem J. 2009;92(1):49–57. doi: 10.1016/j.microc.2008.12.011. [DOI] [Google Scholar]
- 201.Labandeira A, Eljarrat E, Barceló D. Congener distribution of polybrominated diphenyl ethers in feral carp (Cyprinus carpio) from the Llobregat River, Spain. Environ Pollut. 2007;146(1):188–195. doi: 10.1016/j.envpol.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 202.Losada S, Santos FJ, Galceran MT. Selective pressurized liquid extraction of polybrominated diphenyl ethers in fish. Talanta. 2009;80(2):839–845. doi: 10.1016/j.talanta.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 203.Zhang Z, Ohiozebau E, Rhind SM. Simultaneous extraction and clean-up of polybrominated diphenyl ethers and polychlorinated biphenyls from sheep liver tissue by selective pressurized liquid extraction and analysis by gas chromatography–mass spectrometry. J Chromatogr A. 2011;1218(8):1203–1209. doi: 10.1016/j.chroma.2010.12.098. [DOI] [PubMed] [Google Scholar]
- 204.Barón E, Eljarrat E, Barceló D. Gas chromatography/tandem mass spectrometry method for the simultaneous analysis of 19 brominated compounds in environmental and biological samples. Anal Bioanal Chem. 2014;406(29):7667–7676. doi: 10.1007/s00216-014-8196-7. [DOI] [PubMed] [Google Scholar]
- 205.Sjödin A, Carlsson H, Thuresson KAJ, Sjölin S, Bergman Å, Östman C. Flame retardants in indoor air at an electronics recycling plant and at other work environments. Environ Sci Technol. 2001;35(3):448–454. doi: 10.1021/es000077n. [DOI] [PubMed] [Google Scholar]
- 206.Hamm S, Strikkeling M, Ranken PF, Rothenbacher KP. Determination of polybrominated diphenyl ethers and PBDD/Fs during the recycling of high impact polystyrene containing decabromodiphenyl ether and antimony oxide. Chemosphere. 2001;44(6):1353–1360. doi: 10.1016/S0045-6535(00)00363-5. [DOI] [PubMed] [Google Scholar]
- 207.Llop A, Borrull F, Pocurull E. Pressurised hot water extraction followed by simultaneous derivatization and headspace solid-phase microextraction and gas chromatography-tandem mass spectrometry for the determination of aliphatic primary amines in sewage sludge. Anal Chim Acta. 2010;665(2):231–236. doi: 10.1016/j.aca.2010.03.042. [DOI] [PubMed] [Google Scholar]
- 208.Andersson JM, Lindahl S, Turner C, Rodriguez-Meizoso I. Pressurised hot water extraction with on-line particle formation by supercritical fluid technology. Food Chem. 2012;134(4):1724–1731. doi: 10.1016/j.foodchem.2012.03.123. [DOI] [PubMed] [Google Scholar]
- 209.Plaza M, Turner C. Pressurized hot water extraction of bioactives. TrAC Trends Anal Chem. 2015;71:39–54. doi: 10.1016/j.trac.2015.02.022. [DOI] [Google Scholar]
- 210.Rezaee M, Assadi Y, Hosseini MRM, Aghaee E, Ahmadi F, Berijani S. Determination of organic compounds in water using dispersive liquid–liquid microextraction. J Chromatogr A. 2006;1116(1–2):1–9. doi: 10.1016/j.chroma.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 211.Zgoła-Grześkowiak A, Grześkowiak T. Dispersive liquid-liquid microextraction. TrAC Trends Anal Chem. 2011;30(9):1382–1399. doi: 10.1016/j.trac.2011.04.014. [DOI] [Google Scholar]
- 212.Rezaee M, Yamini Y, Faraji M. Evolution of dispersive liquid–liquid microextraction method. J Chromatogr A. 2010;1217(16):2342–2357. doi: 10.1016/j.chroma.2009.11.088. [DOI] [PubMed] [Google Scholar]
- 213.Hao JY, Han W, Xue BY, Deng X. Microwave-assisted extraction of artemisinin from Artemisia annua L. Sep Purif Technol. 2002;28(3):191–196. doi: 10.1016/S1383-5866(02)00043-6. [DOI] [Google Scholar]
- 214.Eskilsson CS, Björklund E. Analytical-scale microwave-assisted extraction. J Chromatogr A. 2000;902(1):227–250. doi: 10.1016/S0021-9673(00)00921-3. [DOI] [PubMed] [Google Scholar]
- 215.Tapie N, Budzinski H, Le Ménach K. Fast and efficient extraction methods for the analysis of polychlorinated biphenyls and polybrominated diphenyl ethers in biological matrices. Anal Bioanal Chem. 2008;391(6):2169–2177. doi: 10.1007/s00216-008-2148-z. [DOI] [PubMed] [Google Scholar]
- 216.Bayen S, Lee HK, Obbard JP. Determination of polybrominated diphenyl ethers in marine biological tissues using microwave-assisted extraction. J Chromatogr A. 2004;1035(2):291–294. doi: 10.1016/j.chroma.2004.02.055. [DOI] [PubMed] [Google Scholar]
- 217.Wang P, Zhang Q, Wang Y, Wang T, Li X, Ding L, Jiang G. Evaluation of Soxhlet extraction, accelerated solvent extraction and microwave-assisted extraction for the determination of polychlorinated biphenyls and polybrominated diphenyl ethers in soil and fish samples. Anal Chim Acta. 2010;663(1):43–48. doi: 10.1016/j.aca.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 218.Giergielewicz-Możajska H, Dąbrowski Ł, Namieśnik J. Accelerated solvent extraction (ASE) in the analysis of environmental solid samples—some aspects of theory and practice. Crit Rev Anal Chem. 2001;31(3):149–165. doi: 10.1080/20014091076712. [DOI] [Google Scholar]
- 219.Hartonen K, Bøwadt S, Hawthorne SB, Riekkola ML. Supercritical fluid extraction with solid-phase trapping of chlorinated and brominated pollutants from sediment samples. J Chromatogr A. 1997;774(1–2):229–242. doi: 10.1016/S0021-9673(97)00335-X. [DOI] [Google Scholar]
- 220.Rodil R, Carro AM, Lorenzo RA, Cela R. Multicriteria optimisation of a simultaneous supercritical fluid extraction and clean-up procedure for the determination of persistent organohalogenated pollutants in aquaculture samples. Chemosphere. 2007;67(7):1453–1462. doi: 10.1016/j.chemosphere.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 221.Peng S, Liang S, Yu M, Li X. Extraction of polybrominated diphenyl ethers contained in waste high impact polystyrene by supercritical carbon dioxide. J Mater Cycles Waste Manag. 2014;16(1):178–185. doi: 10.1007/s10163-013-0169-y. [DOI] [Google Scholar]
- 222.de Boer J. Chlorobiphenyls in bound and non-bound lipids of fishes; comparison of different extraction methods. Chemosphere. 1988;17(9):1803–1810. doi: 10.1016/0045-6535(88)90108-7. [DOI] [Google Scholar]
- 223.Wäger PA, Schluep M, Müller E, Gloor R. RoHS regulated substances in mixed plastics from waste electrical and electronic equipment. Environ Sci Technol. 2011;46(2):628–635. doi: 10.1021/es202518n. [DOI] [PubMed] [Google Scholar]
- 224.Covaci A, Gheorghe A, Redekker ES, Schepens P. Distribution of organochlorine and organobromine pollutants in two sediment cores from the Scheldt estuary (Belgium) Organohalogen Compd. 2002;57:329–332. [Google Scholar]
- 225.Jensen S, Renberg L, Reutergardh L. Residue analysis of sediment and sewage sludge for organochlorines in the presence of elemental sulfur. Anal Chem. 1977;49(2):316–318. doi: 10.1021/ac50010a033. [DOI] [PubMed] [Google Scholar]
- 226.EPA, U.S . Method 1614 brominated diphenyl ethers in water soil, sediment and tissue by HRGC/HRMS. 2007. [Google Scholar]
- 227.Alaee M, Backus S, Cannon C. Potential interference of PBDEs in the determination of PCBs and other organochlorine contaminants using electron capture detection. J Sep Sci. 2001;24(6):465–469. doi: 10.1002/1615-9314(20010601)24:6<465::AID-JSSC465>3.0.CO;2-U. [DOI] [Google Scholar]
- 228.Shao M, Jiang J, Li M, Wu L, Hu M. Recent developments in the analysis of polybrominated diphenyl ethers and polybrominated biphenyls in plastic. Rev Anal Chem. 2016;35(3):133–143. doi: 10.1515/revac-2016-0012. [DOI] [Google Scholar]
- 229.Pietroń WJ, Małagocki P. Quantification of polybrominated diphenyl ethers (PBDEs) in food. A review. Talanta. 2017;167:411–427. doi: 10.1016/j.talanta.2017.02.043. [DOI] [PubMed] [Google Scholar]
- 230.Xiao Q, Hu B, Duan J, He M, Zu W. Analysis of PBDEs in soil, dust, spiked lake water, and human serum samples by hollow fiber-liquid phase microextraction combined with GC-ICP-MS. J Am Soc Mass Spectrom. 2007;18(10):1740–1748. doi: 10.1016/j.jasms.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 231.Cequier E, Marcé RM, Becher G, Thomsen C. Determination of emerging halogenated flame retardants and polybrominated diphenyl ethers in serum by gas chromatography mass spectrometry. J Chromatogr A. 2013;1310:126–132. doi: 10.1016/j.chroma.2013.08.067. [DOI] [PubMed] [Google Scholar]
- 232.Thomsen C, Liane VH, Becher G. Automated solid-phase extraction for the determination of polybrominated diphenyl ethers and polychlorinated biphenyls in serum—application on archived Norwegian samples from 1977 to 2003. J Chromatogr B. 2007;846(1–2):252–263. doi: 10.1016/j.jchromb.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 233.Lin YP, Pessah IN, Puschner B. Simultaneous determination of polybrominated diphenyl ethers and polychlorinated biphenyls by gas chromatography–tandem mass spectrometry in human serum and plasma. Talanta. 2013;113:41–48. doi: 10.1016/j.talanta.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.de Boer J, Wells DE. Pitfalls in the analysis of brominated flame retardants in environmental, human and food samples–including results of three international interlaboratory studies. TrAC Trends Anal Chem. 2006;25(4):364–372. doi: 10.1016/j.trac.2006.02.001. [DOI] [Google Scholar]
- 235.Stapleton HM. Instrumental methods and challenges in quantifying polybrominated diphenyl ethers in environmental extracts: a review. Anal Bioanal Chem. 2006;386(4):807–817. doi: 10.1007/s00216-006-0400-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Luross JM, Alaee M, Sergeant DB, Cannon CM, Whittle DM, Solomon KR, Muir DC. Spatial distribution of polybrominated diphenyl ethers and polybrominated biphenyls in lake trout from the Laurentian Great Lakes. Chemosphere. 2002;46(5):665–672. doi: 10.1016/S0045-6535(01)00230-2. [DOI] [PubMed] [Google Scholar]
- 237.Moon HB, Kannan K, Lee SJ, Choi M. Polybrominated diphenyl ethers (PBDEs) in sediment and bivalves from Korean coastal waters. Chemosphere. 2007;66(2):243–251. doi: 10.1016/j.chemosphere.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 238.Pirard C, De Pauw E, Focant JF. Suitability of tandem-in-time mass spectrometry for polybrominated diphenylether measurement in fish and shellfish samples: comparison with high resolution mass spectrometry. J Chromatogr A. 2006;1115(1–2):125–132. doi: 10.1016/j.chroma.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 239.Tittlemier SA, Forsyth D, Breakell K, Verigin V, Ryan JJ, Hayward S. Polybrominated diphenyl ethers in retail fish and shellfish samples purchased from Canadian markets. J Agric Food Chem. 2004;52(25):7740–5. [DOI] [PubMed]
- 240.Sánchez-Brunete C, Miguel E, Tadeo JL. Determination of polybrominated diphenyl ethers in soil by ultrasonic assisted extraction and gas chromatography mass spectrometry. Talanta. 2006;70(5):1051–1056. doi: 10.1016/j.talanta.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 241.Debrauwer L, Riu A, Jouahri M, Rathahao E, Jouanin I, Antignac JP, Cariou R, Le Bizec B, Zalko D. Probing new approaches using atmospheric pressure photo ionization for the analysis of brominated flame retardants and their related degradation products by liquid chromatography–mass spectrometry. J Chromatogr A. 2005;1082(1):98–109. doi: 10.1016/j.chroma.2005.04.060. [DOI] [PubMed] [Google Scholar]
- 242.Marteau C, Chevolleau S, Jouanin I, Perdu E, De Sousa G, Rahmani R, Antignac JP, LeBizec B, Zalko D, Debrauwer L. Development of a liquid chromatography/atmospheric pressure photo-ionization high-resolution mass spectrometry analytical method for the simultaneous determination of polybrominated diphenyl ethers and their metabolites: application to BDE-47 metabolism in human hepatocytes. Rapid Commun Mass Spectrom. 2012;26(6):599–610. doi: 10.1002/rcm.6136. [DOI] [PubMed] [Google Scholar]
- 243.Xie Y, Fang Z, Cheng W, Tsang PE, Zhao D. Remediation of polybrominated diphenyl ethers in soil using Ni/Fe bimetallic nanoparticles: influencing factors, kinetics and mechanism. Sci Total Environ. 2014;485:363–370. doi: 10.1016/j.scitotenv.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 244.Fang Z, Qiu X, Chen J, Qiu X. Debromination of polybrominated diphenyl ethers by Ni/Fe bimetallic nanoparticles: influencing factors, kinetics, and mechanism. J Hazard Mater. 2011;185(2–3):958–969. doi: 10.1016/j.jhazmat.2010.09.113. [DOI] [PubMed] [Google Scholar]
- 245.Wu J, Yi Y, Fang Z, Tsang EP. Effects of biochar on phytotoxicity and translocation of polybrominated diphenyl ethers in Ni/Fe bimetallic nanoparticle-treated soil. Environ Sci Pollut Res. 2018;25(3):2570–2579. doi: 10.1007/s11356-017-0627-5. [DOI] [PubMed] [Google Scholar]
- 246.Shih YH, Tai YT. Reaction of decabrominated diphenyl ether by zerovalent iron nanoparticles. Chemosphere. 2010;78(10):1200–1206. doi: 10.1016/j.chemosphere.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 247.Peng YH, Chen MK, Shih YH. Adsorption and sequential degradation of polybrominated diphenyl ethers with zerovalent iron. J Hazard Mater. 2013;260:844–850. doi: 10.1016/j.jhazmat.2013.05.057. [DOI] [PubMed] [Google Scholar]
- 248.Drobná B, Fabišiková A, Čonka K, Gago F, Oravcová P, Wimmerová S, Feiler MO, Šovčíková E. PBDE serum concentration and preschool maturity of children from Slovakia. Chemosphere. 2019;233:387–395. doi: 10.1016/j.chemosphere.2019.05.284. [DOI] [PubMed] [Google Scholar]
- 249.McDonald TA. Polybrominated diphenylether levels among United States residents: daily intake and risk of harm to the developing brain and reproductive organs. Integr Environ Assess Manag. 2005;1(4):343–354. doi: 10.1002/ieam.5630010404. [DOI] [PubMed] [Google Scholar]


