Abstract
Given the rapid loss of biodiversity as consequence of climate change, greater knowledge of ecophysiological and natural history traits are crucial to determine which environmental factors induce stress and drive the decline of threatened species. Liolaemus montanezi (Liolaemidae), a xeric-adapted lizard occurring only in a small geographic range in west-central Argentina, constitutes an excellent model for studies on the threats of climate change on such microendemic species. We describe field data on activity patterns, use of microhabitat, behavioral thermoregulation, and physiology to produce species distribution models (SDMs) based on climate and ecophysiological data. Liolaemus montanezi inhabits a thermally harsh environment which remarkably impacts their activity and thermoregulation. The species shows a daily bimodal pattern of activity and mostly occupies shaded microenvironments. Although the individuals thermoregulate at body temperatures below their thermal preference they avoid high-temperature microenvironments probably to avoid overheating. The population currently persists because of the important role of the habitat physiognomy and not because of niche tracking, seemingly prevented by major rivers that form boundaries of their geographic range. We found evidence of habitat opportunities in the current range and adjacent areas that will likely remain suitable to the year 2070, reinforcing the relevance of the river floodplain for the species’ avoidance of extinction.
Subject terms: Ecology, Zoology
Introduction
The consequences of global climate change on plants and animals have attracted a vast research endeavor on ecology and physiology over the last two decades as the rapid planetary warming becomes evident1–3. One of their main goals is the evaluation of physiological profiles on species and projections of how different climatic scenarios, derived from the monitoring of the climatological fluctuations and anomalies over the past century, might affect biodiversity. These ecophysiological models serve to perform long-term predictions of future climate as the Earth’s atmosphere changes by rapid accumulation of greenhouse gases4. Global warming indirectly leads to the alteration of other climatological events that also affect organismal population dynamics, such as shifts in seasonal rainfall patterns5,6, decreases in daily thermal amplitudes in some regions7, and more frequent climate anomalies or extremes8. At the same time, new advances and faster computational technologies have made possible more accurate predictions about future impacts of climate change on diverse biota9.
Current forecasts denote a substantial loss of biodiversity at multiple scales due to alterations in population distribution patterns and the exacerbation of environmental stressors. Such fluctuations might be extreme and lead organisms to their physiological limits and many populations might experience declines in size and local extinctions10–13. Therefore, one of the critical challenges for biologists is to identify which species and populations are likely to be critically affected by warming-driven changes and in which particular habitats. These results would provide to resource managers and policymakers an understanding of why species or habitats are or will become vulnerable to climate change and allow the development of strategies for mitigation, rehabilitation or rescue before the extinction spiral leads such taxa to extirpation or extinction14.
Distribution and abundance of most species could be altered as consequence of climate change15–18. However, species with limited dispersal capacities (low vagility) or confined to specialized habitats within restricted distribution ranges are particularly vulnerable. Hence, when these habitats develop conditions at the limits of species’ environmental envelopes, this scenario might irreversibly lead to the extirpation of such populations. The consequences might be worse if species under study are microendemics and such environmental extremes might lead to their extinction12,13,19. To respond to and mitigate such threats, current research efforts are focused on the identification of which species and populations are most vulnerable, and which aspects of their physiology and ecology might increase their vulnerability and what can be done (e.g., human translocation of founders groups to future suitable habitats). The implementation of conservation and management efforts of such vulnerable species and their habitats are one of the main outcomes of physiological ecology research11.
Vertebrate ectotherms, due to their marked dependence on environmental temperature (Ta), are particularly vulnerable to thermal fluctuations. These threats include heat waves that directly influence physiology and behavior of vulnerable taxa20–22 which affect their performance and fitness23,24. For example, body temperature (Tb) fluctuations in lizards affect several physiological and self-maintenance cornerstones including their digestion, metabolism, growth22,23, reproduction, and susceptibility to diseases25–27. Likewise, environmental temperature also influences locomotion and the ability to escape from predators, social interactions, feeding and reproductive behaviors27–32. In consequence, lizards have developed a variety of mechanisms to maintain their homeostasis affected by drastic changes in Tb as consequence of environmental thermal fluctuations33,34.
For instance, maintaining Tb within an optimal range for physiological and behavioral activities, will depend on the supply and quality of microhabitats available for thermoregulation, and also depends on the effectiveness of the individuals to exploit available resources35,36. Lizards can behaviorally regulate their Tb by moving among appropriate microhabitats, modifying the activity periods37–40, and adopting different thermoregulatory strategies39,41. The individual flexibility of making these physiological and behavioral adjustments depends on variability and phenotypic plasticity22,42. Therefore, if individuals fail in their behavioral or physiological responses to face environmental constraints, they will respond by spatial shifts to track for environments similar to those they are adapted (niche tracking) or afford the risk of extirpation43. Such negative impacts are amplified in species with limited dispersal capacities or confined to specialized habitats with small distribution ranges. Overall, these taxa are particularly vulnerable to rapid habitat degradation and are of special concern for conservation efforts (e.g., How do we prioritize or triage populations for conservation efforts?).
Many lizard species and populations are currently at risk of extinction due to an accelerated increase in the Earth’s temperature, mainly because rising ambient temperatures (Ta) that exceed organismal thermal tolerance margins13,44. This is particularly true in tropical lizards, whose populations already experience Ta values close to their physiological optimum45. When the individuals’ Tbs are close, or higher than the optimal temperatures (Topt), this results in physiological stress and consequently reduces the individuals’ performance13,46. Warmer temperatures also imply faster metabolism, which enhance aging leading to shorter lifespans47. Thus, increasing temperatures could be beneficial the near term, but if they reduce longevity over the long term, populations can become prone to extinction47,48. For example, if the projected Ta can increase 1.2 °C to 4 °C by 2050, many lizard populations could increase their metabolic rates49 in detriment of their longevity47. Likewise, if climatic disturbances prompt shifts in the thermal environments, many lizards may reduce the proportion of time outside their shelters performing social, reproductive, feeding or basking activities (hours of activity, ha) and increase the time spent in shelters (hours of restriction, hr; sensu Sinervo et al.13) having a negative impact on growth rates, and phenology of populations, resulting in an enhanced risk of extinction50,51. This conclusion has been documented in Mexican lizards and Sinervo et al.13 predicted that many other species have higher probabilities of extinction when forced to have fewer hours of activity to reach their optimal body temperatures.
High mountain environments, such as the Andes, constitute areas of great interest for biodiversity conservation because of the coexistence of different spatial units of vegetation, ecotones and natural gradients of elevation52,53. In particular, the central-western Andes (Argentina) have pronounced effects on temperature and humidity where the Puna and Monte biogeographic regions converge to form a heterogeneous mosaic of deserts54. This geospatial configuration contains several microendemic species of lizards including several taxa of the megadiverse Liolaemus55–57. For example, L. montanezi is a small-sized lizard (< 67 mm snout-vent length) with a unique life history58. For instance, these animals are oviparous and microendemic to a hyper-arid desert area with a restricted distribution range in Argentina (San Juan Province). Given its rarity, L. montanezi is categorized as “insufficient knowledge” by Abdala et al.59, consistent with the criteria for “Deficient Data” taxa by IUCN Red List of Threatened Species60. Unfortunately, this categorization makes L. montanezi a low priority for conservation as consequence of the limited studies on this taxon. However, based on our recent field work, we considered that L. montanezi population could be at an extreme risk of extinction by a combination of several factors. First, the geographical features of the species’ distribution are limited to a minute area of ~ 2 km2 limited by Blanco River to the east and its confluence with La Palca River to the southeast, and surrounded by the slopes of the Andes on the west and northwest sides. This reduced and confined habitat suggests an unsurpassable geographic restriction to overcome whenever habitat becomes too detrimental due to climate change for this species to shift in its distribution to a more suitable habitat. Secondly, the climate trends with strong warming and a substantial decrease of the precipitation during the period 1960 ‒ 2010 had impacted the biota in central-western Argentina which might have reduced the availability of shelter and food for L. montanezi61. Third, climate projections for this region based in long-term atmospheric conditions under RCP 4.5 and RCP 8.5 scenarios show a substantial increase of the temperature in the range of 2.0‒2.5 °C and 3.5 °C, respectively; and a drastic reduction of precipitation in northwestern Argentina by the year 210061. Such climatic projections suggest an accentuated thermal and hydric constraint imposed by the environment as they challenge the persistence of the current L. montanezi population.
We hypothesize that the long-term persistence of L. montanezi could be threatened by climate change considering its microendemic condition and the present thermal, humidity, and dispersion restraints of its natural habitat. Herein, we analyze its current thermal niche, use of microhabitat, and activity patterns to predict L. montanezi warming tolerance, thermal safety margins, and the potential hours of restriction of activity. Using ecophysiological, geographic, and climatic parameters in Species Distribution Models (SDM) we predict probabilities of persistence, dispersion, or extirpation of this narrowly distributed population under global warming 2050 and 2070 scenarios62. Finally, we propose strategies for present and future conservation of L. montanezi.
Results
Relationships of Tb, Tpref, VTmax, CTmax, CTmin with body size (SVL), body mass (BM) and body condition index (Mi)
Tb and Tpref of L. montanezi were not related with SVL, BM or Mi (Supplementary Table S1). However, CTmax was negatively related to BM and SVL, and VTmax was positively related to Mi (Supplementary Table S1).
Determination of the main thermal sources for thermoregulation, and relationship among Tb and climatic factors: wind velocity (Wd) and relative humidity (RH%)
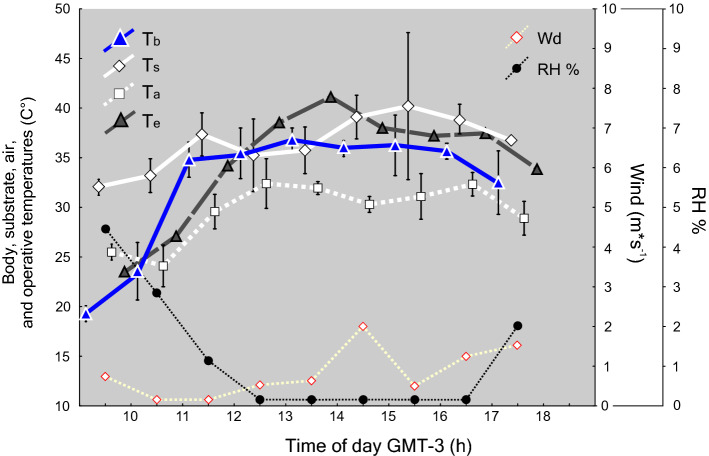
Tbs of L. montanezi were lower than Ts, while Tb and Ts were significantly higher than Ta (Supplementary Table S2). Tbs increased with Tas, and did not depend on Tss (Stepwise Regression, FTb-Ta (18) = 11.98, P < 0.001; F Tb-Ts (18) = 8.95, P > 0.3, Fig. 1).
Figure 1.
Variation during activity of mean body temperature (°C; Tb, blue triangles with white foreground), microenvironmental temperatures: substrate (C°, Ts, white diamond) and air (°C; Ta, white squares) and operative temperatures (°C; Te grey triangles with black foreground) along the day (hour) of Liolaemus montanezi. Polygons connect the mean ± s.e.m. Wind velocity is indicated in the Y-right axis (m*s−1, white diamond with red foreground—whitish dashed line polygon) and percentages of relative humidity are indicated in the doble Y-right axis (RH %, black circle—black dashed line polygon).
Tbs were not significantly related to wind velocity, but it was negatively related to RH% (Simple Regression, FTb-Wd (1; 21) = 0.393, t = 0.627, R2 = 0.02, P > 0.5; FTb-RH% (1; 21) = 55.37, t = − 7.442, R2 = 0.74, P < 0.001, Fig. 1).
Operative temperatures and thermal quality of the habitat
Overall, the Tes were different between models exposed to sun or shad conditions (Two-way ANOVA, Te-sun vs. Te-shade, F(1) = 786.4, P < 0.001), with the Te-sun higher than Te-shade (Supplementary Table S3, Fig. 2). Moreover, the hourly mean Te-sun was higher than the mean Te-shade (Two-way ANOVA, Te-sun vs. Te-shade * Hour F(8) = 12.5, P < 0.001, Supplementary Table S3, Fig. 2).
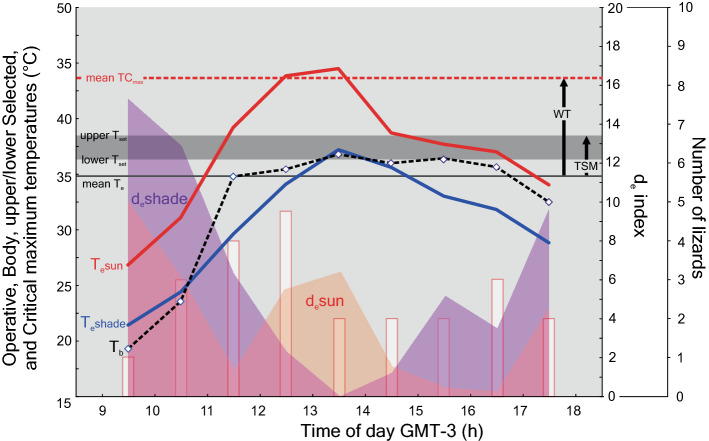
Figure 2.
Variation during the activity of the mean operative temperatures obtained from open (°C, Te-sun: red solid line) and shaded (°C, Te-shade: blue solid line) habitats, and the mean body temperature (°C, Tb: black dashed line with white diamonds—blue foreground) of Liolaemus montanezi. Mean operative temperatures (°C, Te: grey straight line), and critical thermal maximum (°C, CTmax: dashed straight red line). The grey vanished area represents the set-point range of the preferred body temperature in laboratory (°C, upper and lower Tset). The warming tolerance (WT) and the thermal safety margin for physiological performance (TSM) are indicated with arrows. The variation of the thermal quality during the activity calculated from open habitat (de-sun index: faded red area) and shaded (de-shade index: faded purple area) habitats are indicated in the Y-right axis. Number of lizards (faded white bars) are also indicated in the double Y-right axis.
Only 10% (N = 2) of the Tes records were included within Tset, whereas 65% of Tes (N = 13) were lower than Tset, and 25% (N = 5) of Te exceeded the Tset. However, the 38% (N = 8) of the operative temperatures in open habitat (Te-sun) exceeded the Tset from 11:00 to 15:00 h, whereas the operative temperatures from shaded habitat (Te-shade) were lower than the Tset of the species. Also, Te exceeded CTmax during two hours in sunny habitats (Fig. 2).
Thermal quality (de) was highly variable throughout the L. montanezi activity period, showing a trimodal distribution in open habitat (de-sun), and a bimodal pattern in shaded habitats (de-shade; Fig. 2). The mean thermal quality (de) of the environment was lower in the sun than in the shade condition (de-sun = 3.29 vs. de-shade = 6.20).
Preferred body temperatures, accuracy and effectiveness of thermoregulation
The mean preferred temperature for L. montanezi was 37.38 ± 1.53, whereas the lower and upper Tset obtained were 36.57 ± 1.94 °C and 38.40 ± 1.09 °C, respectively (N = 20; Fig. 2). A majority (65%, N = 13) of the Tbs of L. montanezi were lower than the minimum Tset, whereas 10% (N = 2) of the Tbs were higher than the maximum Tset. The remaining 25% of the Tb’ records (N = 5) were within the Tset range. Males, females and juveniles of this species exhibit negative values of E, indicating there are other constraints that prevent them from thermoregulating within their set-points of Tpref (Table 1).
Table 1.
Body size, thermal biology and thermal tolerance-related variables: Mean ± s.d. of body mass (BM, g), snout-vent length (SVL, mm), scaled mass index of body condition (Mi), body temperatures (Tb, °C), preferred body temperatures (Tpref, °C), absolute values obtained from the individual deviation of Tb from Tset (db), index of the average thermal quality of habitats (de), and index of effectiveness of thermoregulation (E), voluntary thermal maximum (VTmax), critical thermal maximum (CTmax) and critical thermal minimum (CTmin) between sexes and adult and juveniles of L. montanezi. The sample sizes N and (ranges) are also indicated.
| Variables | Class | |||
|---|---|---|---|---|
| Males N = 9 |
Females N = 7 |
Juveniles N = 5 |
Overall N = 21 |
|
| Body size | ||||
| BM |
8.00 ± 0.84 (6.7 ‒ 9.5) |
5.85 ± 0.47 (5.0 ‒ 6.4) |
3.65 ± 1.45 (2.2 ‒ 5.27) |
6.16 ± 1.97 (2.2 ‒ 9.5) |
| SVL |
63.8 ± 2.70 (58.0 ‒ 67.5) |
57.88 ± 1.02 (56.2 ‒ 59.0) |
48.4 ± 5.50 (43.0 ‒ 54.0) |
58.16 ± 6.88 (43.0 ‒ 67.5) |
| Mi |
5.83 ± 0.35 (5.26 ‒ 6.45) |
5.95 ± 0.62 (4.8 ‒ 6.7) |
6.09 ± 0.57 (5.39 ‒ 6.59) |
5.94 ± 0.50 (4.8 ‒ 6.7) |
| Thermal biology | ||||
| Tb |
31.98 ± 4.85 (20.0 ‒ 35.6) |
34.17 ± 6.62 (21.4 ‒ 40.0) |
32.62 ± 7.82 (19.3 ‒ 38.0) |
32.86 ± 5.98 (19.3 ‒ 40.0) |
| Tpref |
36.97 ± 1.59 (34.3 ‒ 38.8) |
37.61 ‒ 1.99 (33.6 ‒ 39.4) |
37.72 ± 0.50 (37.3 ‒ 38.5) |
37.38 ± 1.53 (33.6 ‒ 39.4) |
| db | 4.60 | 4.43 | 4.86 | 4.60 |
| de | 3.41 | 3.08 | 4.46 | 3.56 |
| E | −0.34 | −0.43 | −0.08 | −0.29 |
| Thermal tolerance | ||||
| VTmax |
38.43 ± 2.95 (32.5 ‒ 41.8) |
39.14 ± 2.57 (34.3 ‒ 41.8) |
39.67 ± 1.72 (36.7 ‒ 41.1) |
38.99 ± 2.49 (32.5 ‒ 41.8) |
| CTmax |
42.71 ± 1.61 (41.3 ‒ 46.1) |
43.64 ± 1.12 (41.8 ‒ 45.1) |
44.34 ‒ 1.17 (42.9 ‒ 46.2) |
43.44 ± 1.44 (41.3 ‒ 43.4) |
| CTmin |
12.16 ± 1.18 (10.8 ‒ 14.0) |
11.12 ± 1.16 (9.4 ‒ 12.8) |
12.01 ‒ 0.44 (11.6 ‒ 12.7) |
11.76 ± 1.10 (9.4 ‒ 14.0) |
Pairwise comparisons between Tb and Tpref, and comparisons between Tb, Tpref, CTmax, and CTmin among males, females and juveniles
The Tb of L. montanezi was significantly lower than Tpref (Wilcoxon, Paired rank test, T(1; 20) = 34.000, z = 2.65; P > 0.006; Table 1). Thermophysiological variables, Tb, Tpref, CTmax, and CTmin did not differ among males, females and juveniles (ANCOVA, Tb, F(2; 21) = 2.63, P < 0.1; Tpref, F(2; 20) = 0.97, P > 0.4; CTmax F(2; 20) = 2.01, P > 0.17; CTmin F(2; 20) = 2.32, P > 0.13; Table 1).
Daily activity and microhabitat use: bare soil exposed to sun (BS-sun), bare soil in shade (BS-shade), and rocks in shade (WR-shade)
Liolaemus montanezi (N = 21) was active for 9 h (09:00 to 18:00 h). Mean activity, [considered as the direction (vector) of the “Ɵ” angle and dispersion (± circular standard deviation) of the activity which is stretching within the origin to the end of the last observation] was at 12:58 h (Circular ± s.d. = 2 h 33 min = 38.261°, Fig. 3). Overall, the observed frequencies of individuals depicted a bimodal pattern of activity, with peaks occurring between 10:00 to 12:00 h in which 33% (N = 7) occurred and between 15:00 to 16:00 h, when 14% (N = 3) of lizards were seen during these time intervals (Fig. 3).
Figure 3.
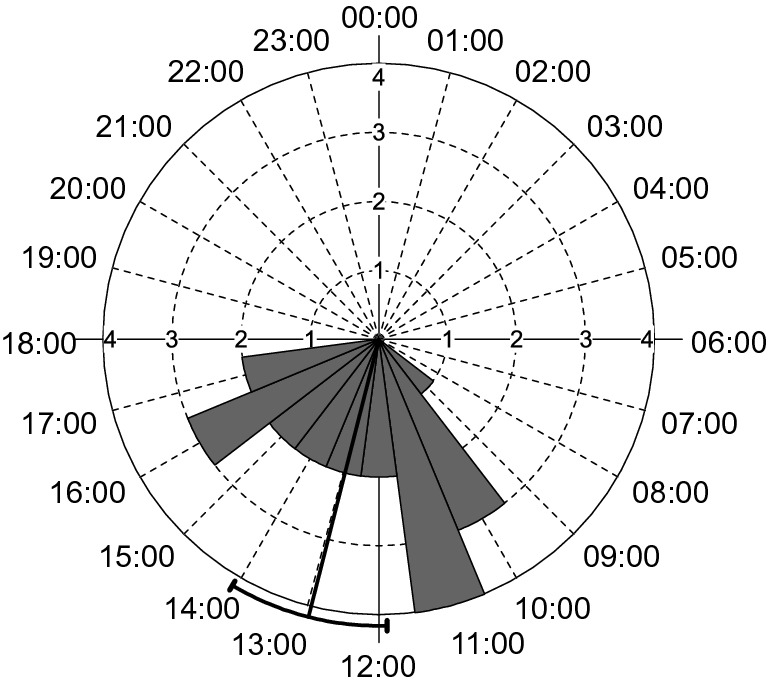
Circular histogram (rose diagram) representing the daily activity per 24-h of Liolaemus montanezi, corresponding to November 1, 2017. The bars represent the absolute frequencies of sighted lizards per hour. Vector and arc represent mean and the 95% confidence interval of the activity period.
Nearly 62% (N = 13) of the observed lizards were recorded using BS-shade, 14% (N = 3) occupying BS-sun, and 24% (N = 5) associated to WR-shade microhabitats (Supplementary Table S4). Observed frequencies were different than expected among the microhabitats (Chi-square χ2 Test2; 21 = 8.0, P > 0.01), and the deviation of the expected frequencies indicated that BS-shade was more frequently used than BS-sun or WR-shade (Supplementary Fig. S1). However, when combined frequencies of temporal and spatial activity, the observed frequencies were marginally significantly higher than expected in the BS-sun at the 10:00 ‒ 11:00 h, and WR-shade at the 17:00 ‒ 18:00 h (Pearson Chi-Square χ2 Test (16) = 29.47, P < 0.02; Multiple Comparison, zBS-sun; 10-11 h = 2.66, P < 0.005; z WR-shade;17-18 h, = 9.08, P < 0.002, Supplementary Table S4), suggesting that lizards observed at these hours preferably occupies BS-sun and WR-shade microhabitats, respectively.
Thermal tolerance, vulnerability to global warming, and extinction risk model
The mean critical thermal minimum, voluntary thermal maximum and critical thermal maximum were, CTmin = 11.76 ± 1.10, VTmax = 38.99 ± 2.49, and CTmax = 43.44 ± 1.44, respectively (Table 1). The indices to estimate the vulnerability to global warming were, WT = 8.8 °C and TSM = 3.57 °C (Fig. 2).
The ha and hr as a function of Ta, max of L. montanezi were explained by the logistic Richard’s curve (Table 2; Fig. 4). The parameters for the fitted functions of ha and hr for the present time and projected for the years 2050 and 2070 under RCP 4.5 and RPC 8.5 scenarios by months are shown in the Supplementary Table 5.
Table 2.
Values of the parameters of the first half of Richard’s curve fitted for the hours of activity (ha) and hours of restriction (hr) on air maximum temperature (Ta, max) of L. montanezi. A (asymptote), k (rate), i (inflection point) and m (shape) using nonlinear least squares (‘nls’ function R-stats) with ‘modpar’ and ‘SSposnegRichards’ of the R-package ‘FlexParamCurve’. The 95% c.i. were determined with the function ‘confint2’ of the R-package ‘nlstools’108. The m parameter is estimated from the data and fixed to allow others to behave as free parameters.
| Parameters | ha, c.i. 95% (lower–upper) | hr, c.i. 95% (lower–upper) |
|---|---|---|
| A | 15.136 (14.813–15.460) | 13.955 (13.558–14.352) |
| k | 0.169 (0.164–0.176) | − 0.134 (− 0.140 to − 0.128) |
| i | 17.883 (17.646–18.120) | 18.341 (17.929–18.752) |
| m* | 0.410 | 0.549 |
Figure 4.
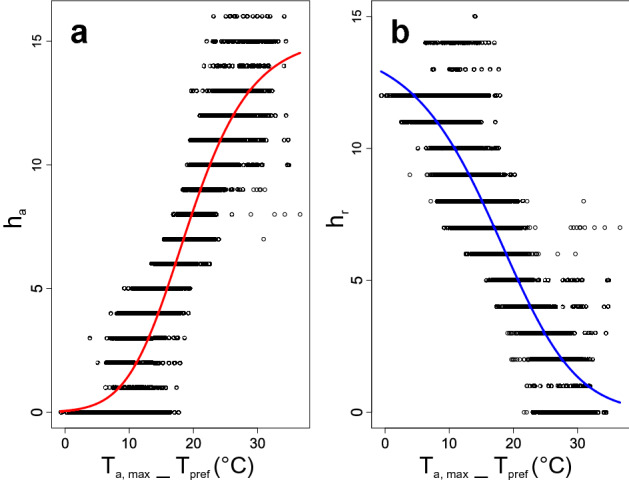
Richard´s Curve. Dependence of hours of activity (ha, panel a) and hours of restriction (hr, panel b) on daily maximum air temperature (Ta, max) of Liolaemus montanezi along the year.
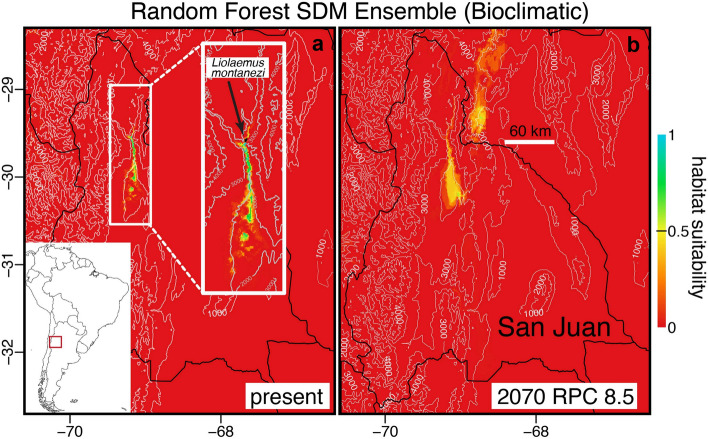
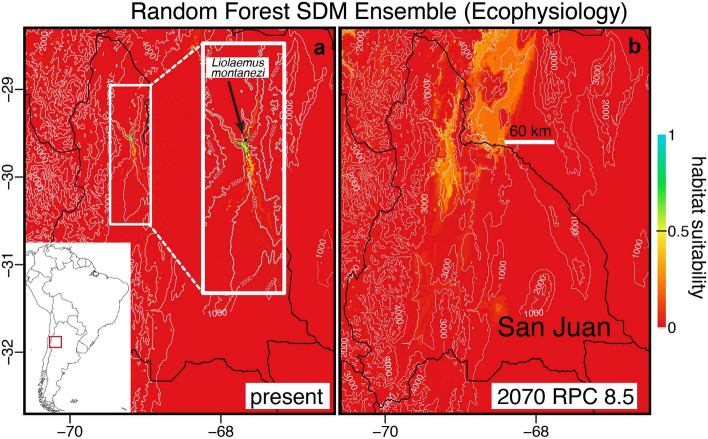
Most evaluation statistics (ROC, TSS, KAPPA) showed that most models derived from each algorithm were very good (all > 0.75; Supplementary Table S7) to predict the current known distribution of L. montanezi. However, SDMs based on GAM failed for ecophysiological predictors, as well as some based on ANN (Supplementary Table S7). For projections, most SDMs indicated the locality where L. montanezi exists (Supplementary Figs. S2, S3). However, the ensemble derived from the RF algorithm was considered the most accurate model as it has fewer over-projections that describe the contemporary and future locations of habitat suitability for L. montanezi (Figs. 5, 6). Overall, the occupancy likelihood and habitat suitability modeled based on RF shows that the probable potential area with suitable climatic properties for L. montanezi represents a narrow stretch located toward the south of the actual distribution in the junction of Blanco and La Palca rivers (San Juan, Argentina) by 2050 and 2070, under 4.5 and 8.5 RCP (Figs. 5, 6).
Figure 5.
Suitable habitat projections for L. montanezi under bioclimatic layers and random forest algorithm (RF) at present (panel a) and 2070 RCP 8.5 (panel b). The inlay (white square) represents a close-up of the known distribution of the species. Elevation curves are also shown. These maps were generated in the R environment (R Core Team109 ver. 3.6.1, URL: http://www.r-project.org/index.html) using the R-packages: biomod2 ver. 3.4.6 (Thuiller et al.110, URL: https://CRAN.R-project.org/package=biomod2), dismo v1.1-4 (Hijmans et al.111, URL: https://CRAN.R-project.org/package=dismo), and raster v3.3-13 (Hijmans112, URL: https://CRAN.R-project.org/package=raster) by J.C. Santos.
Figure 6.
Suitable habitat projections for L. montanezi under ecophysiological layers and random forest algorithm (RF) at present (panel a) and 2070 RCP 8.5 (panel b). The inlay (white square) represents a close-up of the known distribution of the species. Elevation curves are also shown. These maps were generated in the R environment (R Core Team109 ver. 3.6.1, URL: http://www.r-project.org/index.html) using the R-packages: biomod2 ver. 3.4.6 (Thuiller et al.110, URL: https://CRAN.R-project.org/package=biomod2), dismo v1.1-4 (Hijmans et al.111, URL: https://CRAN.R-project.org/package=dismo), and raster v3.3-13 (Hijmans112, URL: https://CRAN.R-project.org/package=raster) by J.C. Santos.
Discussion
Given the rapid decline and local extirpations of many lizards populations as consequence of climate change13, it has been emphasized that natural history traits and physiology are crucial to determine what critical environmental factors might be stressing and driving the decline in threatened species63. Liolaemus montanezi is a taxon that thermoregulates via a complex use of heat sources. Individuals were found basking directly exposed to the sun during the morning and retreat to shaded patches of Bulnesia retama and Larrea divaricata bushes to avoid overheating before midday during late spring. The mean Ts was significantly higher than Tb and Ta and, Tbs only increased according to the air temperature, suggesting the usage of air temperature on thermoregulation64,65. Cooling by convection is common in some psammophilous species of Liolaemus inhabiting sandy environments from Brazil (L. occipitalis66; L. lutzae67; L. arambarensis68), and Argentina (L. wiegmannii69; L. koslowskyi70; L. olongasta71; L. acostai72; L. sarmientoi73; L. ruibali74; L. chacoensis75; L. scapularis76). Indeed, downslope winds with strong convection are common phenomena observed along the eastern slopes at medium or high elevations in the Andes, especially from midday and thenafter77. In the case of L. montanezi, the population is located in alluvial fans in the western margin and riverbed of the Blanco river, an open gully with steep slopes that results in a wind corridor. The convection of heat, rather than radiation or conduction, appears to be the main mechanism of heat loss that explains the Tb of L. montanezi (Fig. 1).
In addition, body temperatures of L. montanezi varied according to the moisture level of the habitat. The inverse relationship between Tb and the percentage of relative humidity (RH %) suggests that the loss of environmental moisture at midday (12:00 h) might intensify the restriction of the activity, particularly in the sun-exposed microhabitats. Thus, when humidity decayed to near zero, lizards were frequently sighted using shaded habitats even when they still have high Tbs in the shade (Figs. 1, 2). Sheltering under the bushes probably helps them to avoid dehydration which could also affect the accuracy of thermoregulation78. Furthermore, Nicholson et al.79 studying populations of Anolis stratulus and Ameiva exsul had pointed out that in water-restricted locations the interaction of temperature and humidity has strong influence on the daily activity patterns. Such environmental conditions have a noticeable demographic impact in many lizards since juveniles are more vulnerable to water loss than adults. Nevertheless, we found there were no differences in the ways in which males versus females, or juvenile versus adult lizards interact with their environment. The Tb of L. montanezi were not different between sexes and age classes, nor did Tb depend on body size or body condition. But, the CTmax was lower in lizards of larger body size, leading to the assumption of a more accurate thermoregulation in adults. Thus, we hypothesize that the drastic decline of humidity at 12:00 h might entice lizards to refuge in more suitable habitats that provide hiding shelters in shaded sites. During these restriction hours, lizards will wait until milder heat and humidity conditions are given to resume their daily active behavior.
These activity patterns usually reflect the extent in which the behavioral thermoregulation buffers the environmental restrictions to maintain the Tb to optimize physiological processes80. Thus, changes in ambient temperature during the day could cause the frequency of active lizards to describe a distribution that is unimodal or bimodal. Likewise, these patterns of behavior might also vary among the seasons of the year80. Hence, during spring L. montanezi might be active during 9 h from 09:00 to 18:00 h with activity peaks occurred between 10:00 to 12:00 h and between 15:00 to 16:00 h showing a bimodal pattern of activity (Figs. 2, 3) with a retreat during the time when the Te max was higher than 44 °C (Fig. 2). When considering the operative temperatures of the models deployed over open habitats [i.e., those with high temperatures (Te-sun) or those placed in shaded habitats (Te-shade)], we found that they also support a pattern of activity likely associated with the thermal quality (de = de-sun + de-shade) of the environment (Fig. 2). Thus, we predict that the daily activity of these lizards starts when the environmental thermal quality is low (de-sun and de-shade exhibit the highest values) and increases while the thermal quality rises (de-sun and de-shade decrease). However, the number of active lizards outside shelters progressively decreases during the hotter hours and they are forced to retreat into shaded microhabitats where the thermal quality is optimal (de-shade tending to zero). Based on our observations, we predict that a second peak of activity occurs in the afternoon when the thermal quality in shaded habitats decreases while it improves in open habitats allows the lizards to resume active behaviors (Fig. 2). Therefore, when considered as trimodal and bimodal patterns of de-sun and de-shade, respectively, both describe scenarios in which lizards emerge in the morning, retreat before the midday, and emerge again at the afternoon. This pattern of activity of L. montanezi during spring support the ha and hr observed considering the model for global warming (Fig. 4, Supplementary Table S5). Our operative temperature models likely provide more accurate representations of available temperatures at the capture than measurements of ambient air temperature, they do not represent "instantaneous" measures of equilibrium temperatures as recommended by Bakken et al81 and therefore our analyses involving operative temperatures should be interpreted as such.
Microhabitat selection by individuals may allow populations to maximize the geographic range82. In this sense, shrubs play a significant role in thermal ecology of the species. Liolaemus montanezi maintain the Tb near the operative temperatures recorded in the shaded habitats (Fig. 2), behaving as a thermoconformers during the hottest hours of the day. Thus, in a well-identified habitat, most of lizards were seen using sandy, bare soil in shaded places, sheltered beneath shrubs. However, when combining such microhabitat use with their diel activity pattern, we predicted that lizards would occupy strictly sunny, bare soil in the morning and would perch on rocks only in the afternoon (Supplementary Table S4). The fact that the individuals might use sunny bare soil at the beginning of the activity period, shuttling to cooler microhabitats during the warmest hours suggests that the species could exhibit spatial and temporal flexibility in microhabitat use83 but their thermoconformity in the shady refuges point out their current vulnerability.
Preferred temperature of L. montanezi during spring was higher than Tb as shown for other Liolaemus species84, even when the mean Te was closer than Tset (Fig. 2). These results suggest that the species could obtain temperatures close to Tpref in their natural environment with low costs of thermoregulation. However, the effectiveness of thermoregulation (E = − 0.29) shows that L. montanezi behaves almost as a thermoconformer (E = 0). But, the negative value shows that this species is avoiding thermally optimal microenvironments probably to minimize the risk of overheating85 and dehydration (during the warmest hours of the day) selecting among the coolest and shaded microhabitats. At least a third (33%) of the recorded Te values were higher than the Tpref during the warmest hours which increases the risk for these lizards of attaining near-lethal body temperatures close to their CTmax. Accordingly, we found that their thermal safety margin (TSM = 3.57 °C) and warming tolerance index (WT = 8.8) were notably lower than reported in others liolaemid lizards from cold temperate environments such as Phymaturus tenebrosus (TSM = 15.58 and WT = 23.386) and P. calcogaster (TSM = 3.98 °C and WT = 10.67 °C87). Therefore, these observations suggest that L. montanezi has a narrow TSM range to prevent it from reaching lethal temperatures45. However, the thermal environments in the sub-Andean region characterized by high thermal variation could have a dramatic influence on such thermal tolerance. Thus, the mean breadth of thermal tolerance (CTmax − CTmin, sensu Litmer & Murray88) for L. montanezi was 31.68 °C showing that this species is likely eurythermic.
The east side of the Andes, inhabited by L. montanezi, is expected to experience thermal alteration during global warming. An increase of Ta and a reduction of rainfall are expected to impose droughts and habitat modification61,89. Added thermal stress in the habitat is expected to cause a decline in the water availability and reduce plant growth and recruitment61,90. Hence, lizards, like L. montanezi, that typically use shady microhabitats to thermoregulate might experience temperature-driven activity restrictions13, which might compromise population persistence and cause local extirpations and threaten extinction13,50 in particular in microendemic taxa.
The current known distribution of Liolaemus montanezi is explained by abiotic and ecophysiological factors (Supplementary Table S6). As we pointed out above, the small area occupied by this species is confined between rivers to the southeast and steep slopes to the west and northwest. Because the known population of L. montanezi occupies sandy patches interspersed between alluvial fans in the western margin and riverbed of Blanco river, the availability of moisture as indicated by evapotranspiration (one of the predictors in the ecophysiological models) may play a main role in L. montanezi habitat physiognomy. The role of evapotranspiration is more conspicuous in the model than hr and ha for L. montanezi (Supplementary Table S6). Contemporary bioclimatic and ecophysiological models have produced comparable results. Hence, the SDM projections show a narrow range of suitable habitat for L. montanezi located along their current riverine habitats (La Palca and Blanco Rivers) southeast of their current range (Figs. 5, 6). Future SDMs predictions based on RF (Supplementary Table S7), includes the current range of L. montanezi with a higher probability of persistence by 2070 under RCP 8.5 (Figs. 5, 6).
The SDM reinforces the hypothesis of the river floodplain south of their current range serving as the more suitable habitat for the species in the future. However, numerous surveys were done in the predicted area of occupation and confirm that L. montanezi is not currently occupying these southern locations. The predicted pattern of habitat suitability is probably influenced by a fluvial effect of moisture supply which promotes the recruitment and persistence of the dominant plants used by L. montanezi. One alternative interpretation is that the model forecasts increased rainfall as projected between 2081–2100 by Barros et al.61 for that area. The MaxEnt, GAM, GLM, and ANN SDMs showed unreliable projections by overestimating the present and future scenarios localizing the species in currently unoccupied environments (Supplementary Figs. S2, S3). Instead, the RF approach projected a low likelihood of occupancy in neighboring areas in which the reduced range of this species will be intensified by adverse changes to its current habitats. Therefore, the microendemic character plus the strong dependence of this species on sandy patches covered with shrubs of B. retama and L. divaricata, together with the actual habitat fenced by the rivers suggest high constraints on niche tracking. This study also suggests the vulnerability of other species present in the area such as L. eleodori, L. parvus, and Phymaturus punae. The assessment of population vulnerability to climate change is a powerful tool to highlight how this and other species could become threatened within a relatively short time (~ 30 or 60 years). Our projections should be interpreted with caution given our low physiological sampling which were only held at the beginning of the activity season in spring. Despite of our modelled Te obtained for 4123 days (see Supplementary material for details) the habitat suitability projections should be seen as preliminary. Additional work, with deeper sampling across more of the year, is necessary to fully understand the potential vulnerability to climate change in this species. This approach, although employing low sample size, provides enough species-specific ecophysiology and vulnerability information to prompt immediate reassessment of the conservation status of L. montanezi. Moreover, the SDM predictions can play an important role in support of a habitat conservation initiative. Although we cannot discard the notion of natural dispersal across the rivers to the south resulting in colonization of areas projected to be suitable in the future, prudent conservation would target new surveys of these areas to search for yet-undetected populations and to assess habitat quality for active translocation plans.
Materials and methods
Field work
Lizard sampling, study area, and climate
We captured a total of 21 specimens (9 males, 7 females, 5 juveniles) during one day in late spring (November) by noose in sandy environments in the riverbed at the NW margin of the junction of the Blanco and La Palca rivers, Iglesia Department, San Juan Province, Argentina (− 29.55 S; − 69.19 W, 2185 m asl). The study area belongs to the Monte phytogeographic region dominated by xerophilous plants such as shrubs of Bulnesia retama, Larrea divaricata, Prosopis alpataco, and Atriplex crenatifolia91. The climate corresponds to arid cold desert (BWk)92 with rainfall occurring mainly in the summer season and the annual mean temperature is < 18 °C.
Field data
Body temperatures (Tb) were taken in active lizards using a catheter probe TP-K01 (1.62 mm diameter) introduced ca. 3 mm into the cloaca. Individuals were handled by the head to avoid heat transfer and the temperature was recorded within 20 s of handling. In order to evaluate the main heating resources used by L. montanezi, microenvironmental temperatures were recorded at the exact site of capture for each lizard: substratum temperatures sand, rocks or beneath dwarf shrubs (Ts, TP-K03 substrate probe), and air temperature at 1 cm above the ground (Ta, TP-K02 gas probe). Probes were connected to a TES 1302 thermometer (TES, Electrical Electronic corp., Taipei, Taiwan, ± 0.01 °C). Thereafter, to determinate the relationship among environmental variables and lizard’s activity, we measured the wind velocity using an anemometer (Proster, ± 0.1 m/s) taken at 1 cm above the substrate at the exact first sighting of the lizard and we measured relative humidity (RH %; HOBO, Pro V2, HR%/T°C ± 2%). Snout-vent length (SVL, Mitutoyo, type Vernier digital caliper ± 0.01 mm) and body mass (BW, 10 g Pesola, spring scale ± 0.5 g) were also registered.
To determine the available spatiotemporal heterogeneity of the microenvironmental temperatures for thermoregulation, operative temperatures (Te, sensu Bakken93) were obtained using nine polyvinyl chloride (PVC) pipe models connected to external dataloggers (HOBO, U23-003, T°/T°C ± 1 °C) during the collection of lizards. The model with the greatest correspondence of thermal data to reflect the lizard Tb was a PVC pipe (80 mm length × 2.15 mm thickness) sealed at both ends with silicone Fastix (Regression: Adjusted R2 = 0.846, N = 2836, slope = 1.09, confidence interval = 1.05 ‒ 1.14)94,95. Subsequently, the models were deployed in the study area in the most representative microhabitats used by L. montanezi: (i) Bare soil with sunny, sandy substrate, (ii) Bare soil with shady, sandy substrate (beneath shrubs), and (iii) Weathered rocks in the shade beneath shrubs (3 microhabitats × 3 replicates). The Te was recorded every 5 min during the lizard activity from 8:00 to 19:00 h during one day of fieldwork.
Activity pattern and microhabitat use
Daily activity and microhabitat use were recorded during one day throughout randomly visual surveys in ~ 2 km2 area. The survey was done walking from 08:00 to 19:00 h at a very slow pace (5‒6 m/min) to provide enough time to scan all available habitat. Then, we registered the number of active lizards (frequencies of lizards) and time of day of each lizard sighting. All lizards seen were captured to avoid any possibility of temporal pseudoreplication.
Due to the complexity of the habitat structure, the microhabitat used by the individuals were registered according to three main categories also used to deploy the Te models, unifying all the suitable records as follows: (i) BS-sun or bare soil with sandy substrate exposed to sun when the lizards can be seen moving outside shelters; (ii) BS-shade or bare soil with a sandy substrate in the shade when the lizards can be seen perching in bushy-edges or sheltering beneath shrubs, and (iii) WR-shade or weathered rocks in the shade when the lizards were seen on shaded rocky substrate or on rocks in the shade.
Laboratory experiments
We brought the lizards (N = 20; 8 males, 7 females, 5 juveniles) to the laboratory in individual cloth bags. Lizards were placed individually in open-top terraria with a sand substrate (120 cm length, 25 cm width, 30 cm height). Experiments were performed 2 days after captures. Because the photoperiod may affect the thermal physiology96, all experiments were performed within restricted periods corresponding to the range of activity of the species from 09:00 to 19:00 h. Lizards were supplied with water ad libitum, and fed with Tenebrio sp. larvae every day after the experiments.
Estimation of preferred body temperatures (Tpref), and effectiveness of thermoregulation (E)
Lizards were provided with a thermal gradient produced by a 100-W incandescent lamp from 15° to 50 °C (taken 1 cm above the substrate) to thermoregulate during the experiments. Body temperatures were taken using ultra-thin (1 mm) catheter thermocouples located ca. 5 mm inside the cloaca and taped at the base of the lizard’s tail to prevent the thermocouple from being dislodged during the experiment. The temperature of each lizard was obtained every 5 min for 3 consecutive hours by connecting the thermocouple to an 8-channel data-logger (Measurement Computing 1.2 kHz Data Acquisition Device, OMEGA, TC-08 ± 0.5 °C, Stamford, CT, USA).
The preferred body temperatures registered for each individual during 3 h trial (Tpref-i) were used to obtain the mean and the interquartile of Tpref-i for each individual (Tpref and Tset-i, respectively). The accuracy of thermoregulation (db) of L. montanezi in their natural environment, was calculated as the mean of the absolute values obtained from the deviations of Tb-i from Tset-i (individual deviation; db-i). The index of the average thermal quality of the habitat from the organism’s perspective (de) was calculated as the mean of the absolute values of the deviations of mean Te (obtained within the hour of the capture for each lizard) from the Tset-i. The effectiveness of temperature regulation, E, was calculated as 1 − (mean db-i/mean de-i)38. The values of E range from − 1 to 1, and E ~ 0 represents thermoconformers, E ~ 0.5 moderate thermoregulators, and E ~ 1 effective thermoregulators. Negative E values occur when lizards avoid thermally high-quality habitats with Te near or within the range of Tpref85,97. We also calculated the de in the shade (de-shade) and de in the sun (de-sun) in order to determine their differences in thermal quality, and to examine their contribution to the lizard’s activity.
Thermal tolerance: Determination of critical thermal minimum and maximum, and voluntary thermal maximum
Critical thermal minimum (CTmin) and critical thermal maximum (CTmax) were determined by means of cooling and heating trails, respectively. For CTmin and CTmax, lizards were placed individually in a plastic transparent box (20 cm × 20 cm × 20 cm) with a layer (5 mm) of high-density Styrofoam covering the bottom of the box to prevent thermal conductance. Lizards were connected to ultra-thin K-type thermocouples (OMEGA, 5SC-TT-K-30-36; diameter = 0.076 mm, Norwalk, Connecticut, USA) introduced ca. ~ 5 mm inside the cloaca, and taped at the base of tail to prevent the thermocouple from being dislodged during the experiments, and Tb was recorded every 5 s using a data-logger (Measurement Computing 1.2 kHz Data Acquisition Device, OMEGA, TC-08 ± 0.5 °C, Stamford, CT, USA).
The lizards were placed individually in a 2 °C chest refrigerator with glass-top door cooled at constant rate (approximately, − 0.7 °C/30 s). During cooling, the lizards were turned onto their back (no more than four times per individual) until they reached CTmin, considered as the Tb at which the lizard was no longer able to right itself when placed on its back86.
After at least 48 h, the heating experiments were performed. Lizards were heated at a constant rate (0.7 °C/10 s) using a 150-W infrared lamp placed 30 cm overhead. In the same experimental system, the voluntary thermal maximum (VTmax) defined as the body temperature that induces a behavioral response seeking to cool down, was recorded98. Each lizard was monitored throughout the trials to record the critical thermal maximum (CTmax), defined as the temperature at the higher extreme of tolerance in which the lizard was no longer able to right itself when placed onto its back27,86,99. Finally, after the determination of CTmin, VTmax and CTmax, each lizard was promptly removed from cooling or heating systems and steadily returned to room temperature to prevent the death of the individuals under study.
Vulnerability to global warming
To estimate population vulnerability to global warming we calculated the Warming Tolerance index (WT), defined as the difference between mean CTmax and mean Te to show how close lizards are environmental temperatures to detrimental or lethal temperatures for physiological processes86,100. We also calculated the thermal safety margin (TSM) as the difference between mean Tpref max (upper Tpref) and mean Te (sensu Deutsch et al.45). Since most physiological processes are considered to occur at Tpref, the upper Tpref threshold is a good proxy of the upper optimal temperature (Topt) for most physiological functions (i.e., digestion, locomotion or embryo development) that could exhibit a large degree of plasticity within the population89.
Species distribution models (SDMs) using bioclimatic and ecophysiological predictors
We estimated the present and future habitat suitability derived from the known distribution locations for L. montanezi and its physiological parameters described above. For this purpose, we implemented two approaches for SDMs, first using generic bioclimatic method and secondly ecophysiological (species specific) predictors. The latter uses georeferenced grids (rasters) derived from predicted behavioral response of our focal species to environmental parameters and primary productivity estimates associated with the species’ habitats and community. We describe the SDM methods and their corresponding variable derivations in the Supplementary Material S1.
Statistical analyses
Variability in thermophysiological variables was described using descriptive statistics (mean ± standard deviation, minimum and maximum). Normality and variance homogeneity assumptions were tested using Kolmogorov–Smirnov’s test and Levene’s test, respectively. When normality or variance homogeneity assumptions were not met, non-parametric correlation, Mann–Whitney, and Kruskal–Wallis rank sum were used101. Data were analyzed using Sigma Plot, version 14.0 (Systat Software Inc., San José, CA), SPSS, version 20.0 (IBM, SPSS Statistics for Windows, Armonk, NY), and figures were produced using Statistica, version 10.0 (Statsoft Inc., Tulsa, OK) and optimized with Corel X8, version 18.0 (Cowpland Research Lab, OTT, Canada).
Daily activity patterns were described using circular statistics. We estimate the mean vector (hour) and standard deviation among the frequencies of individuals with Oriana 4.02102. We also summarized all records plotting the frequencies of lizards sighted per hour using an angular histogram with Oriana 4.02102. The use of microhabitats was analyzed by contrasting the observed versus expected frequencies of lizards using a Chi-square test (χ2) under the assumption of uniformity in the expected frequencies (H0 = all microhabitat categories are equally used by lizards)101. Then, if the differences between observed versus expected frequencies were significant, a diagram of the deviation of the occurrence of individuals from its expected frequency (− 1 to 1 scale) was drawn to represent the relative changes of individuals among the microhabitats103.
A third approach that combined the periods of activity (by time of day) and microhabitat use (by categories of microhabitat) allowed the contrast of these two types of lizard frequencies. We then analyzed a 9 × 3 contingency table containing the frequencies of lizards by time intervals (N = 9, from 09:00 to 18:00 h) and microhabitat categories (N = 3, BS-sun, BS-shade, and WR-shade). Pearson's Chi-square test (χ2) was performed to evaluate significant differences between observed versus expected frequencies under assumption of uniformity in the expected frequencies (H0 = all microhabitat categories are equally used in any hour by the lizards). Post hoc analysis was performed from the adjusted residuals to obtain z-scores for each combined case. Subsequently, P-values were calculated from the transformation of Chi-square values derived from multiple tests104,105. Correction of Bonferroni was applied dividing the standard significance level (α = 0.05) by the number of multiple tests (N = 27, derived from 9 × 3 contingency table). Thus, the adjusted significance P-level obtained for the whole model was P < 0.0018.
Snout-vent length (SVL) and body mass (BW) were included in the scaled mass index of body condition in each individual (Mi, sensu Peig and Green106) to determine the scaled mass index of condition as an indicator of the health or quality assumed to be related to fitness. The scale mass index was calculated as:
| 1 |
where Mi and SVLi are the BW and SVL of each individual, SVL0 is the arithmetic mean SVL of the population, and bSMA is the standardized major axis slope from the regression of ln(BW) on ln(SVL) for the population106. The scaling bSMA exponent was calculated directly using the software RMA v. 1.21107. We then tested for the influence of Mi on thermal traits (Tb, Tpref, CTmax, VTmax, CTmin) according to sex and age classes (males, females, and juveniles).
Ethical statement
Capturing and handling of the lizards were conducted in accordance with international standards on animal welfare (ASIH/HL/SSAR Guidelines for Use of Live Amphibians and Reptiles), being compliant with Argentinian regulations (Argentinean National Law #14.346). All individuals were collected under permits Exp. Number 1300-2643. Field and laboratory protocols were approved in the UNSJ-SEADS-2017-RBSG research engagement and in the CICITCA-UNSJ 21/E1101 plan (Universidad Nacional de San Juan).
Supplementary Information
Acknowledgements
This work was supported by the Universidad Nacional de San Juan—Secretaría de Estado de Medio Ambiente del Gobierno de San Juan (grant agreement FCEFN-SEADS 2017), by the CICITCA-UNSJ 21/E1101 granted to AL. NRI was supported by PICT 2017-0553 and Institute of Biodiversity and Environment Research (INIBIOMA-COMAHUE CONICET). JCS was supported by SJU starting funds and NSF-DEB # 2016372. AL and JEP thank Leonardo Ponce and Bibi Ontiveros (RBSG rangers) for their valuable help during the fieldwork and logistical support. We thank Yanina Ripoll (Secretaría de Estado de Medio Ambiente – Provincia de San Juan) for permitting the field work. We also thank to John D. Krenz for reviewing manuscript, and constant support. To Nicolás Cecchetto for the calibration models data and his constant support.
Author contributions
A.L., N.R.I. conceived the study design; A.L., J.E.P., E.A.S. gathered the data in the field and in laboratory; A.L. and J.C.S. conducted the analyses; J.C.S. created and prepared the maps; A.L., J.C.S., N.R.I., M.M. and B.S. wrote the manuscript. All authors reviewed and approved the final draft.
Data availability
All data needed to produce the results and discussion in the paper are present in the paper and/or the Supplementary Material.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-91058-w.
References
- 1.Møller A, Fiedler W, Berthold P. Effects of climate change on birds. Oxford University Press; 2010. [Google Scholar]
- 2.IPCC. Global Warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. (The Intergovernmental Panel on Climate Change, 2018).
- 3.Lovejoy TE, Hannah L, Wilson EO. Biodiversity and climate change. Yale University Press; 2019. [Google Scholar]
- 4.Hughes L. Biological consequences of global warming: is the signal already apparent? Trends Ecol. Evol. 2000;15:56–61. doi: 10.1016/S0169-5347(99)01764-4. [DOI] [PubMed] [Google Scholar]
- 5.Moore N. Precipitation regimes and climate change. In: Freedman B, editor. Global Environmental Change. Dordrecht: Springer; 2014. pp. 191–197. [Google Scholar]
- 6.Knapp AK, et al. Characterizing differences in precipitation regimes of extreme wet and dry years: Implications for climate change experiments. Glob. Change Biol. 2015;21:2624–2633. doi: 10.1111/gcb.12888. [DOI] [PubMed] [Google Scholar]
- 7.Tobias A, Díaz J. Heat waves, human health, and climate change. In: Freedman B, editor. Global Environmental Change. Dordrecht: Springer; 2014. pp. 447–453. [Google Scholar]
- 8.Freedman B. Global Environmental Change. Springer; 2014. [Google Scholar]
- 9.Hannah L. Climate change biology. 2. Elsevier; 2014. [Google Scholar]
- 10.Gibbons, J. W. et al. The global decline of reptiles, Déjà Vu Amphibians: Reptile species are declining on a global scale. Six significant threats to reptile populations are habitat loss and degradation, introduced invasive species, environmental pollution, disease, unsustainable use, and global climate change. BioScience50, 653–666 (2000).
- 11.Williams SE, Shoo LP, Isaac JL, Hoffmann AA, Langham G. Towards an integrated framework for assessing the vulnerability of species to climate change. PLoS ONE. 2008;6:e325. doi: 10.1371/journal.pbio.0060325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huey RB, Losos JB, Moritz C. Are Lizards Toast? Science. 2010;328:832–833. doi: 10.1126/science.1190374. [DOI] [PubMed] [Google Scholar]
- 13.Sinervo B, et al. Erosion of lizard diversity by Climate Change and altered thermal niches. Science. 2010;328:894–899. doi: 10.1126/science.1184695. [DOI] [PubMed] [Google Scholar]
- 14.Glick P, Stein BA, Edelson NA. Scanning the conservation horizon: A guide to climate change vulnerability assessment. National Wildlife Federation; 2011. [Google Scholar]
- 15.Parmesan C. Climate and species’ range. Nature. 1996;382:765–766. doi: 10.1038/382765a0. [DOI] [Google Scholar]
- 16.Parmesan C, et al. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature. 1999;399:579–583. doi: 10.1038/21181. [DOI] [Google Scholar]
- 17.Kelly AE, Goulden ML. Rapid shifts in plant distribution with recent climate change. Proc. Natl. Acad. Sci. USA. 2008;105:11823–11826. doi: 10.1073/pnas.0802891105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuckerberg B, Woods AM, Porter WF. Poleward shifts in breeding bird distributions in New York State. Glob. Change Biol. 2009;15:1866–1883. doi: 10.1111/j.1365-2486.2009.01878.x. [DOI] [Google Scholar]
- 19.Sodhi NS, Ehrlich PR. Conservation Biology for all. Oxford University Press; 2010. [Google Scholar]
- 20.Porter WP, Gates DM. Thermodynamic equilibria of animals with environment. Ecol. Monogr. 1969;39:227–244. doi: 10.2307/1948545. [DOI] [Google Scholar]
- 21.Avery RA. Field studies of body temperatures and thermoregulation. In: Gans C, Pough FH, editors. Biology of the Reptilia. New York: Academic Press; 1982. pp. 93–166. [Google Scholar]
- 22.Angilletta MJ. Thermal Adaptation: A Theoretical and Empirical Synthesis. Oxford University Press; 2009. [Google Scholar]
- 23.Huey RB. Temperature, physiology, and the ecology of reptiles. In: Gans C, Pough FH, editors. Biology of the Reptilia. New York: Academic Press; 1982. pp. 25–91. [Google Scholar]
- 24.Angilletta MJ. Estimating and comparing thermal performance curves. J. Therm. Biol. 2006;31:541–545. doi: 10.1016/j.jtherbio.2006.06.002. [DOI] [Google Scholar]
- 25.Shine R. Incubation regimes of cold-climate reptiles: the thermal consequences of nest-site choice, viviparity and maternal basking. Biol. J. Linn. Soc. 2004;83:145–155. doi: 10.1111/j.1095-8312.2004.00376.x. [DOI] [Google Scholar]
- 26.Shine R. Life-history evolution in Reptiles. Ann. Rev. Ecol. Evol. Syst. 2005;36:23–46. doi: 10.1146/annurev.ecolsys.36.102003.152631. [DOI] [Google Scholar]
- 27.Huey RB, Stevenson RD. Integrating thermal physiology and ecology of ectotherms: A discussion of approaches. Am. Zool. 1979;19:357–366. doi: 10.1093/icb/19.1.357. [DOI] [Google Scholar]
- 28.Bennett AF. The thermal dependence of lizard behaviour. Anim. Behav. 1980;28:752–762. doi: 10.1016/S0003-3472(80)80135-7. [DOI] [Google Scholar]
- 29.Christian KA, Tracy CR. The effect of the thermal environment on the ability of hatchling Galapagos land iguanas to avoid predation during dispersal. Oecologia. 1981;49:218–223. doi: 10.1007/BF00349191. [DOI] [PubMed] [Google Scholar]
- 30.Snell HL, Jennings RD, Snell HM, Harcourt S. Intrapopulation variation in predator-avoidance performance of Galápagos lava lizards: The interaction of sexual and natural selection. Evol. Ecol. 1988;2:353–369. doi: 10.1007/BF02207566. [DOI] [Google Scholar]
- 31.Robson MA, Miles DB. Locomotor performance and dominance in male Tree Lizards, Urosaurus ornatus. Funct. Ecol. 2000;14:338–344. doi: 10.1046/j.1365-2435.2000.00427.x. [DOI] [Google Scholar]
- 32.Perry G, LeVering K, Girard I, Garland T. Locomotor performance and social dominance in male Anolis cristatellus. Anim. Behav. 2004;67:37–47. doi: 10.1016/j.anbehav.2003.02.003. [DOI] [Google Scholar]
- 33.Cowles RB, Bogert CM. A preliminary study of the thermal requirements of desert reptiles. Bull. Am. Mus. Nat. Hist. 1944;83:265–296. [Google Scholar]
- 34.Bartholomew GA. Physiological control of body temperature. In: Gans C, Pough FH, editors. Biology of the Reptilia. New York: Academic Press; 1982. pp. 167–211. [Google Scholar]
- 35.Beaupre SJ. Effects of geographically variable thermal environment on bioenergetics of mottled rock rattlesnakes. Ecology. 1995;76:1655–1665. doi: 10.2307/1938166. [DOI] [Google Scholar]
- 36.Huey RB, Hertz PE, Sinervo B. Behavioral drive versus behavioral inertia in evolution: a null model approach. Am. Nat. 2003;161:357–366. doi: 10.1086/346135. [DOI] [PubMed] [Google Scholar]
- 37.Huey RB. Behavioral thermoregulation in lizards: importance of associated costs. Science. 1974;184:1001. doi: 10.1126/science.184.4140.1001. [DOI] [PubMed] [Google Scholar]
- 38.Hertz PE, Huey RB, Stevenson RD. Evaluating temperature regulation by field-active ectotherms: The fallacy of the inappropriate question. Am. Nat. 1993;142:796–818. doi: 10.1086/285573. [DOI] [PubMed] [Google Scholar]
- 39.Vitt L, Caldwell J. Herpetology: An introductory biology of amphibians and reptiles. 4. Elsevier; 2014. [Google Scholar]
- 40.Ortega Z, Mencía A, Pérez-Mellado V. Sexual differences in behavioral thermoregulation of the lizard Scelarcis perspicillata. J. Therm. Biol. 2016;61:44–49. doi: 10.1016/j.jtherbio.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Rodríguez-Serrano E, Navas CA, Bozinovic F. The comparative field body temperature among Liolaemus lizards: Testing the static and the labile hypotheses. J. Therm. Biol. 2009;34:306–309. doi: 10.1016/j.jtherbio.2009.04.002. [DOI] [Google Scholar]
- 42.Telemeco RS, Radder RS, Baird TA, Shine R. Thermal effects on reptile reproduction: Adaptation and phenotypic plasticity in a montane lizard. Biol. J. Linn. Soc. 2010;100:642–655. doi: 10.1111/j.1095-8312.2010.01439.x. [DOI] [Google Scholar]
- 43.Labra A, Pienaar J, Hansen TF. Evolution of thermal physiology in Liolaemus Lizards: Adaptation, phylogenetic inertia, and niche tracking. Am. Nat. 2009;174:204–220. doi: 10.1086/600088. [DOI] [PubMed] [Google Scholar]
- 44.Huey RB, et al. Why tropical forest lizards are vulnerable to climate warming. Proc. Biol. Sci. 2009;276:1939–1948. doi: 10.1098/rspb.2008.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deutsch CA, et al. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA. 2008;105:6668–6672. doi: 10.1073/pnas.0709472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Root TL, et al. Fingerprints of global warming on wild animals and plants. Nature. 2003;421:57–60. doi: 10.1038/nature01333. [DOI] [PubMed] [Google Scholar]
- 47.Bestion E, Teyssier A, Richard M, Clobert J, Cote J. Live fast, die young: Experimental evidence of population extinction risk due to climate change. PLoS ONE. 2015;13:e1002281. doi: 10.1371/journal.pbio.1002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang L, Yang F, Zhu W-L. Evidence for the ‘rate-of-living’ hypothesis between mammals and lizards, but not in birds, with field metabolic rate. Comp. Biochem. Physiol. Part A. 2021;253:110867. doi: 10.1016/j.cbpa.2020.110867. [DOI] [PubMed] [Google Scholar]
- 49.Dillon ME, Wang G, Huey RB. Global metabolic impacts of recent climate warming. Nature. 2010;467:704–706. doi: 10.1038/nature09407. [DOI] [PubMed] [Google Scholar]
- 50.Sinervo B, et al. Climate change, thermal niches, extinction risk and maternal-effect rescue of toad-headed lizards, Phrynocephalus, in thermal extremes of the Arabian Peninsula to the Qinghai—Tibetan Plateau. Integr. Zool. 2018;13:450–470. doi: 10.1111/1749-4877.12315. [DOI] [PubMed] [Google Scholar]
- 51.Ibarguengoytía NR, et al. Looking at the past to infer into the future: Thermal traits track environmental change in Liolaemidae. Evolution. 2021 doi: 10.1111/evo.14246. [DOI] [PubMed] [Google Scholar]
- 52.Beniston M. Climate change in mountain regions: A review of possible impacts. Clim. Change. 2003;59:5–31. doi: 10.1023/A:1024458411589. [DOI] [Google Scholar]
- 53.Thuiller W. Climate change and the ecologist. Nature. 2007;448:550–552. doi: 10.1038/448550a. [DOI] [PubMed] [Google Scholar]
- 54.Martínez Carretero E. La Puna argentina: Delimitación general y división en distritos florísticos. Bol. Soc. Argent. Bot. 1995;31:27–40. [Google Scholar]
- 55.Esquerré D, Brennan IG, Catullo RA, Torres-Pérez F, Keogh JS. How mountains shape biodiversity: The role of the Andes in biogeography, diversification, and reproductive biology in South America’s most species-rich lizard radiation (Squamata: Liolaemidae) Evolution. 2019;73:214–230. doi: 10.1111/evo.13657. [DOI] [PubMed] [Google Scholar]
- 56.Abdala CS, Laspiur A, Langstroth R. Las especies del género Liolaemus (Liolaemidae). Lista de taxones y comentarios sobre los cambios taxonómicos más recientes. Cuad. Herp. 2021;35:193–223. [Google Scholar]
- 57.Abdala CS, et al. Unravelling interspecific relationships among highland lizards: First phylogenetic hypothesis using total evidence of the Liolaemus montanus group (Iguania: Liolaemidae) Zool. J. Linn. Soc. 2020;189:349–377. doi: 10.1093/zoolinnean/zlz114. [DOI] [Google Scholar]
- 58.Cabrera MR, Monguillot JC. A new Andean species of Liolaemus of the darwinii complex (Reptilia: Iguanidae) Zootaxa. 2006;1106:35–43. doi: 10.11646/zootaxa.1106.1.3. [DOI] [Google Scholar]
- 59.Abdala CS, et al. Categorización del estado de conservación de las lagartijas y anfisbenas de la República Argentina. Cuad. Herp. 2012;26:215–248. [Google Scholar]
- 60.Avila, L. J. Liolaemus montanezi. The IUCN Red List of Threatened Species 2016: e.T56077261A56077269. 10.2305/IUCN.UK.2016-1.RLTS.T56077261A56077269.en (2016).
- 61.Barros VR, et al. Climate change in Argentina: trends, projections, impacts and adaptation. Wiley Interdiscip. Rev. Clim. Change. 2015;6:151–169. doi: 10.1002/wcc.316. [DOI] [Google Scholar]
- 62.IPCC. Climate change 2014: synthesis report. Contribution of Working Groups I, II and III to the Fifh Assessment Report of the Intergovernmental Panel on Climate Change. (IPCC, Geneva, 2014).
- 63.Bury RB. Natural history, field ecology, conservation biology and wildlife management: time to connect the dots. Herp. Con. Biol. 2006;1:56–61. [Google Scholar]
- 64.Fei T, et al. A body temperature model for lizards as estimated from the thermal environment. J. Therm. Biol. 2012;37:56–64. doi: 10.1016/j.jtherbio.2011.10.013. [DOI] [Google Scholar]
- 65.Ortega Z, et al. Disentangling the role of heat sources on microhabitat selection of two Neotropical lizard species. J. Trop. Ecol. 2019;35:149–156. doi: 10.1017/S0266467419000099. [DOI] [Google Scholar]
- 66.Bujes CS, Verrastro L. Thermal biology of Liolaemus occipitalis (Squamata, Tropiduridae) in the coastal sand dunes of Rio Grande do Sul, Brazil. Braz. J. Biol. 2006;66:945–954. doi: 10.1590/S1519-69842006000500021. [DOI] [PubMed] [Google Scholar]
- 67.Almeida-Santos P, Militão CM, Nogueira-Costa P, Menezes VA, Rocha CFD. Thermal ecology of five remaining populations of an endangered lizard (Liolaemus lutzae) in different restinga habitats in Brazil. J. Coast. Conserv. 2015;19:335–343. doi: 10.1007/s11852-015-0395-7. [DOI] [Google Scholar]
- 68.Liz AV, Santos V, Ribeiro T, Guimarães M, Verrastro L. Are lizards sensitive to anomalous seasonal temperatures? Long-term thermobiological variability in a subtropical species. PLoS ONE. 2019;14:e0226399. doi: 10.1371/journal.pone.0226399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martori R, Bignolo P, Cardinale L. Relaciones térmicas en una población de Liolaemus wiegmannii (Iguania: Tropiduridae) Rev. Esp. Herpetol. 1998;12:19–26. [Google Scholar]
- 70.Martori R, Aun L, Orlandini S. Relaciones térmicas temporales en una población de Liolaemus koslowskyi. Cuad. Herp. 2002;16:33–45. [Google Scholar]
- 71.Cánovas MG, Villavicencio HJ, Acosta JC. Liolaemus olongasta (NCN) Body temperature. Herp. Rev. 2006;37:87–88. [Google Scholar]
- 72.Villavicencio H, Acosta J, Cánovas M, Marinero J. Thermal ecology of a population of the lizard, Liolaemus pseudoanomalus in western Argentina. Amphibia-Reptilia. 2007;28:163–165. doi: 10.1163/156853807779799027. [DOI] [Google Scholar]
- 73.Ibargüengoytía NR, et al. Thermal biology of the southernmost lizards in the world: Liolaemus sarmientoi and Liolaemus magellanicus from Patagonia, Argentina. J. Therm. Biol. 2010;35:21–27. doi: 10.1016/j.jtherbio.2009.10.003. [DOI] [Google Scholar]
- 74.Castillo GN, Villavicencio HJ, Acosta JC, Marinero JA. Temperatura corporal de campo y actividad temporal de las lagartijas Liolaemus vallecurensis y Liolaemus ruibali en clima riguroso de los Andes centrales de Argentina. Multequina. 2015;24:19–31. [Google Scholar]
- 75.Laspiur A, Villavicencio HJ, Acosta JC. Liolaemus chacoensis (NCN). Body temperature. Herp. Rev. 2007;38:458–459. [Google Scholar]
- 76.Salva AG, Robles CI, Tulli MJ. Thermal biology of Liolaemus scapularis (Iguania:Liolaemidae) from argentinian northwest. J. Therm. Biol. 2021;98:102924. doi: 10.1016/j.jtherbio.2021.102924. [DOI] [PubMed] [Google Scholar]
- 77.Mesinger, F., Jovic, D., Chou, S. C., Gomes, J. L. & Bustamante, J. F. Wind forecast around the Andes using the sloping discretization of the eta coordinate. in Proceedings of the 8th International Conference on Southern Hemisphere Meteorology and Oceanography 1837–1848 (INPE, 2006).
- 78.Sannolo M, Carretero MA. Dehydration constrains thermoregulation and space use in lizards. PLoS ONE. 2019;14:e0220384. doi: 10.1371/journal.pone.0220384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nicholson KL, et al. The influence of temperatura and humidity on activity patterns of the lizards Anolis stratulus and Ameiva exsul in the British Virgin Islands. Caribb. J. Sci. 2005;41:870–873. [Google Scholar]
- 80.Adolph AS, Porter WP. Temperature, activity, and lizard life histories. Am. Nat. 1993;142:273–295. doi: 10.1086/285538. [DOI] [PubMed] [Google Scholar]
- 81.Bakken GS, Santee WR, Erskine D. Operative and standard operative temperature: Tools for thermal energetics studies. Am. Zool. 1985;25:933–943. doi: 10.1093/icb/25.4.933. [DOI] [Google Scholar]
- 82.Black IRG, Berman JM, Cadena VC, Tattersall GJ. Behavioral thermoregulation in lizards. Strategies for achieving preferred temperature. In: Bels VL, Russell AP, editors. Behavior of lizards. Evolutionary and mechanistic perspectives. Florida: CRC Press; 2019. pp. 13–46. [Google Scholar]
- 83.Pirtle EI, Tracy CR, Kearney MR. Hydroregulation. A neglected behavioral response of lizards to climate change? In: Bels VL, Russell AP, editors. Behavior of Lizards. Evolutionary and mechanistic perspectives. Florida: CRC Press; 2019. pp. 343–374. [Google Scholar]
- 84.Medina M, et al. Thermal biology of genus Liolaemus: A phylogenetic approach reveals advantages of the genus to survive climate change. J. Therm. Biol. 2012;37:579–586. doi: 10.1016/j.jtherbio.2012.06.006. [DOI] [Google Scholar]
- 85.Blouin-Demers G, Weatherhead PJ. Thermal ecology of black rat snakes (Elaphe obsoleta) in a thermally challenging environment. Ecology. 2001;82:3025–3043. doi: 10.1890/0012-9658(2001)082[3025:TEOBRS]2.0.CO;2. [DOI] [Google Scholar]
- 86.Cabezas-Cartes F, Fernández JB, Duran F, Kubisch EL. Potential benefits from global warming to the thermal biology and locomotor performance of an endangered Patagonian lizard. PeerJ. 2019;7:e7437. doi: 10.7717/peerj.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Obregón RL, Scolaro JA, Ibargüengoytía NR, Medina M. Thermal biology and locomotor performance in Phymaturus calcogaster: are Patagonian lizards vulnerable to climate change? Integr. Zool. 2021;16:53–66. doi: 10.1111/1749-4877.12481. [DOI] [PubMed] [Google Scholar]
- 88.Litmer AR, Murray CM. Critical thermal tolerance of invasion: Comparative niche breadth of two invasive lizards. J. Therm. Biol. 2019;86:102432. doi: 10.1016/j.jtherbio.2019.102432. [DOI] [PubMed] [Google Scholar]
- 89.Bels VL, Russell AP. Behavior of lizards. Evoutionary and mechanistic perspectives. Florida: CRC Press; 2019. [Google Scholar]
- 90.Panda BB, Achary VM, Mahanty S, Panda KK. Plant adaptation to abiotic and genotoxic stress: Relevance to climate change and evolution. In: Tuteja N, Gill SS, editors. Climate Change and plant abiotic stress tolerance. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2014. pp. 251–294. [Google Scholar]
- 91.Kiesling R. Flora de San Juan, República Argentina. Buenos Aires: Vázquez Mazzini; 1994. [Google Scholar]
- 92.Köeppen VP. Climatología. Con un estudio de los climas de la tierra. Pánuco, México, DF: Fondo de Cultura Económica; 1948. [Google Scholar]
- 93.Bakken GS. Measurements and application of operative and standard operative temperatures in ecology. Am. Zool. 1992;32:194–216. doi: 10.1093/icb/32.2.194. [DOI] [Google Scholar]
- 94.Cecchetto NR, Medina SM, Taussig S, Ibargüengoytía NR. The lizard abides: cold hardiness and winter refuges of Liolaemus pictusargentinus in Patagonia, Argentina. Can. J. Zool. 2019;97:773–782. doi: 10.1139/cjz-2018-0214. [DOI] [Google Scholar]
- 95.Cecchetto NR, Medina SM, Ibargüengoytía NR. Running performance with emphasis on low temperatures in a Patagonian lizard, Liolaemus lineomaculatus. Sci. Rep. 2020;10:14732. doi: 10.1038/s41598-020-71617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mahoney JJ, Hutchison VH. Photoperiod acclimation and 24-hour variations in the critical thermal maxima of a tropical and a temperate frog. Oecologia. 1969;2:143–161. doi: 10.1007/BF00379157. [DOI] [PubMed] [Google Scholar]
- 97.Christian KA, Weavers BW. Thermoregulation of monitor lizards in Australia: An evaluation of methods in thermal biology. Ecol. Monogr. 1996;66:139–157. doi: 10.2307/2963472. [DOI] [Google Scholar]
- 98.Camacho A, et al. Measuring behavioral thermal tolerance to address hot topics in ecology, evolution, and conservation. J. Therm. Biol. 2018;73:71–79. doi: 10.1016/j.jtherbio.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 99.Clusella-Trullas S, Chown SL. Lizard thermal trait variation at multiple scales: a review. J. Comp. Physiol. B. 2014;184:5–21. doi: 10.1007/s00360-013-0776-x. [DOI] [PubMed] [Google Scholar]
- 100.Sunday JM, et al. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc. Natl. Acad. Sci. USA. 2014;111:5610–5615. doi: 10.1073/pnas.1316145111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sokal RR, Rohlf FJ. Biometry: The Principles and practice of statistics in biological research. 3. Freeman W.H; 1995. [Google Scholar]
- 102.Kovach, W. Oriana ver. 4.0. Software. (Kovach Computing Services, 2001).
- 103.Fitzgerald LA, Cruz FB, Perotti G. Phenology of a lizard assemblage in the dry Chaco of Argentina. J. Herpetol. 1999;33:526–535. doi: 10.2307/1565568. [DOI] [Google Scholar]
- 104.Beasley TM, Schumacker RE. Multiple regression approach to analyzing contingency tables: Post hoc and planned comparison procedures. J. Exp. Educ. 1995;64:79–93. doi: 10.1080/00220973.1995.9943797. [DOI] [Google Scholar]
- 105.García Pérez MA, Núñez Antón V. Cellwise residual analysis in two-way contingency tables. Educ. Psychol. Meas. 2003;65:825–839. doi: 10.1177/0013164403251280. [DOI] [Google Scholar]
- 106.Peig J, Green AJ. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos. 2009;118:1883–1891. doi: 10.1111/j.1600-0706.2009.17643.x. [DOI] [Google Scholar]
- 107.Bohonak, A. J. & van Der Linde, K. RMA for JAVA Software for Reduced Major Axis regression. ver. 1.21. (2004).
- 108.Baty F, et al. A toolbox for nonlinear regression in R: The Package nlstools. J. Stat. Softw. 2015;5:1–21. [Google Scholar]
- 109.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing (2019).
- 110.Thuiller, W., Georges, D., Engler, R. & Breiner, F. biomod2: Ensemble Platform for Species Distribution Modeling. R package ver. 3.4.6. (2020).
- 111.Hijmans, R. J., Phillips, S., Leathwick, J. & Elith, J. dismo: Species Distribution Modeling. R package ver. 1.1-4. (2017).
- 112.Hijmans, R. J. raster: Geographic Data Analysis and Modeling. R package ver. 3.4-5. (2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to produce the results and discussion in the paper are present in the paper and/or the Supplementary Material.