Abstract
Nepal suffers from high burden of antimicrobial resistance (AMR) due to inappropriate use of antibiotics. The main objective of this study was to explore knowledge, attitude and practices of antibiotics uses among patients, healthcare workers, laboratories, drug sellers and farmers in eight districts of Nepal. A cross-sectional survey was conducted between April and July 2017. A total of 516 individuals participated in a face-to-face interview that included clinicians, private drug dispensers, patients, laboratories, public health centers/hospitals and, livestock and poultry farmers. Out of 516 respondents, 62.8% (324/516) were patients, 16.9% (87/516) were clinicians, 6.4% (33/516) were private drug dispensers. A significant proportion of patients (42.9%; 139/324) thought that fever could be treated with antibiotics. Majority (79%; 256/324) of the patients purchased antibiotics over the counter. The knowledge of antibiotics used among patients increased proportionately with the level of education: literate only [AOR = 1.4 (95% Cl = 0.6–4.4)], versus secondary education (8–10 grade) [AOR = 1.8 (95% Cl = 1.0–3.4)]. Adult patients were more aware of antibiotic resistance. Use of antibiotics over the counter was found high in this study. Knowledge, attitude and practice related to antibiotic among respondents showed significant gaps and need an urgent effort to mitigate such practice.
Subject terms: Antimicrobials, Policy and public health in microbiology, Microbiology
Introduction
Over the last 50 years, antibiotics have been widely used in human and animal as prophylaxis, therapeutics, and growth promoters1. Nonetheless, widespread and inappropriate use of antibiotics has led to the emergence of antibiotic resistance—a condition in which pathogenic bacteria develop resistance to the specific antibiotic prescribed against it2,3. Emergence of drug resistance became even more prominent since the end of twentieth century when the declining efficacy of antibiotics were increasingly reported worldwide4,5. Increasing AMR constrains therapeutic options, prolongs hospital stays and mortalities, lowers quality of life, and adds to the economic burden6.
Antimicrobial resistance is a growing global health threat with multifaced phenomenon7. Infection with drug resistant microbes increases the morbidity, mortality, length of hospitalization and treatment cost of patients7,8. Such scenario will continue to affect low- and middle- income countries as they suffer from high overuse and misuse of antibiotics9,10. A multitude of factors contribute to the development of antimicrobial resistance. Increasing demands for food from animals and environment for a growing population has further added pressure in the eco-system. Also, increasing food and environmental links has facilitated the rapid transfer of drug-resistant pathogens11. In response to these challenges, a holistic ‘One Health’ approach has been advocated in recent years that aims to include the health of human, animal and the environment12. One health approach can be utilized to enhance risk analysis on emergence, spread and control strategies of AMR at the human-animal-local environment interfaces13.
A number of previous studies conducted in low- and lower middle- income countries (LMICs) have documented a poor level of knowledge, attitude and practice (KAP) on antibiotics prescription with remarkable over-the-counter (OTC) use which can ultimately lead to increase in AMR10. LMICs are adding more burdens to the ever-increasing AMR in comparison to the developed countries14,15. In LMICs, less than 40% of the patients attending public and private healthcare centers receive treatment according to the WHO guidelines16. Worryingly, healthcare professionals do not follow standard antimicrobial guidelines such as WHO guidelines and often prescribe broad spectrum antibiotics without laboratory evidence17.
Common to most LMICs, Nepal experiences an extremely huge burden of infectious diseases such as respiratory tract infections, enteric fever (typhoid, paratyphoid fever), urinary tract infections and other bacterial infections9. Researchers have reported high burden of drug resistant/multidrug resistant bacteria in Nepal18–23. Discrepancy between prescription and OTC use of antibiotic is found to contribute to the rise of AMR in Nepal10. Nonetheless, much less is explored around how behavior related to the use of antimicrobials in various sectors have contributed to the rising trend of AMR in Nepal9,10.
AMR surveillance in Nepal was commenced in 1999 by National Public health Laboratory (NPHL) with technical support from Bangladesh24. However, the AMR surveillance among animal pathogens started only in 2011 by joint efforts of NPHL and other veterinary laboratories24. Researchers have investigated different components of antimicrobial use and AMR in Nepal such as use of antibiotic in primary health care25, awareness of antibiotic use and its resistance in the general population26,27 and self-medication (with antibiotics) among medical students28. Nonetheless, these efforts have not been successful in documenting true burden of AMR in the country as these reports did not encompass all the sectors that used antimicrobials in various proportions. Traditionally, much of the focus has been invested in exploring the use of antimicrobials in humans, and use of antimicrobials among food animals is persistently neglected. To address these gaps in effective surveillance program, a Fleming fund has initiated a multi-disciplinary one health approach to tackle antimicrobial resistance in Nepal29,30. To the best of our knowledge, there are no KAP surveys on prescription and use of antibiotics through a comprehensive one health approach in Nepal. The main objective of this study was to explore knowledge and practices of antibiotics prescription and uses in various sectors utilizing a one health approach in eight districts of Nepal.
Materials and methods
Study settings
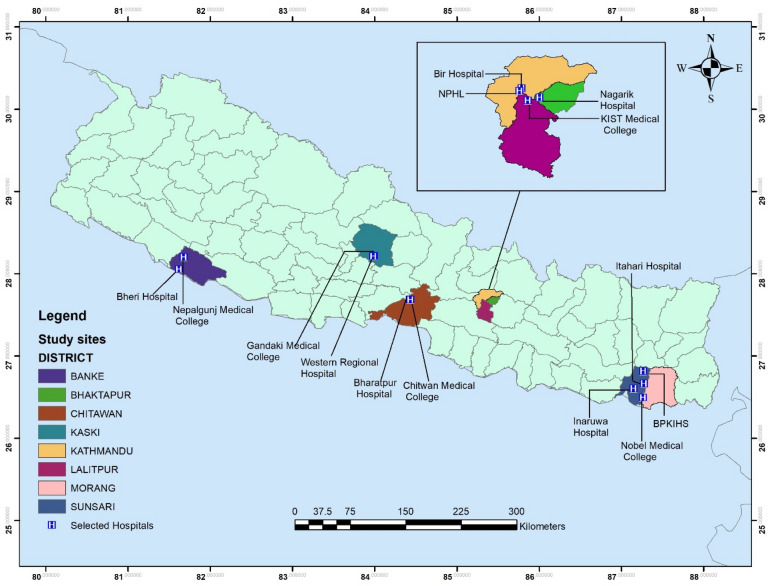
This study was conducted in selected eight districts of Nepal. Nepal is a landlocked mountainous country that shares 1800 km porous border with India in the east, west and south while it shares northern border with China31,32. It covers an area of 147,516 km2 between 2622′–3027′N and longitudes 8004′–8812′E. With varying population densities, the population of the country was 26.4 million in 201133. Currently, Nepal is divided into seven provinces with 6 metropolitan cities, 11 sub-metropolitan cities, 276 municipalities, and 460 rural municipalities. The health system of Nepal is divided into three parts: Federal, Provincial and Local level health facility. In addition, private hospitals, private diagnostic centers, nursing homes, polyclinics and private drug dispensaries serve a large population in all seven provinces of Nepal. This study was conducted in tertiary hospitals and primary health care centers, private drug shops around the hospitals, laboratories (private/public), and food producing farmers and two rural primary health centers (PHC) from the selected eight districts of Nepal (Fig. 1). There are, a total of 4,863 public health care facilities in Nepal. The distribution of public health care facilities based on the provinces are: province 1 (n = 816), province 2 (n = 822), Bagmati province (province-3) (n = 934), Gandaki province (province 4) (n = 635), Lumbini province (province-5) (n = 741), Karnali province (province-6) (n = 404) and Sudurpashchim province (province-7) (n = 511) different health care facilities. In our study, 17 different health care facilities in seven different provinces were selected to represent the entire nation.
Figure 1.
Study sites of selected eight districts of Nepal. The map was created using ArcGIS desktop version 10.8. The shapefile of the administrative districts and location for Nepal was obtained from the Government of Nepal, Ministry of Land Management, Survey Department website and were publicly available for unrestricted use (http://www.dos.gov.np/nepal-map)".
Study population
Knowledge, Attitude and Practice (KAP) surveys were conducted among five groups (clinicians, patients, private drug sellers, livestock and poultry farmers and diagnostic laboratories) of key informants. A total of 550 participants were approached for the survey out of which (93.8%; 516/550) responses were received. 516 respondents in this study included clinicians and health workers (n = 87), private drug dispensers (n = 33), patients (n = 324), laboratory head/or representative of selected laboratories (n = 23), government hospital/ primary health centre (n = 17) and livestock farmers (n = 32).
Study design
This was a descriptive cross-sectional survey using a previously validated and reliable WHO questionnaire between May 2017 and October 2017.
Participant recruitment
This study was led by a research assistant in each province and supervised by co-investigator/principal investigator. At first a national and sub-national list of hospitals, pharmacies, and farms were obtained. Using a proportional sampling method, a proportional sample size for each category was calculated. Based on the sample size required for each category (hospitals/health facilities, pharmacies and farms) respondents were chosen using a simple random sampling. At the hospitals, patients were first requested and explained about the study. Broadly, patients who were willing to participate regardless of their illnesses were included in the study. They were also introduced about the procedure, risks and benefits of the study. All patients who agreed to participate in the study were included. The procedure was repeated for other categorical respondents. A written informed consent or thumb print from each participant or their guardian in case of children below 16 years was obtained. Written informed consents were obtained from health care workers, private drug sellers, Laboratory personnel, hospital/PHC pharmacist and food producing farmers from the list prior to the interview.
Questionnaire development and pre-testing
Questionnaires were used following the WHO guidelines, publications and consultation with experts. Four different questionnaires for medicine prescribers, drug dispensers, patients and farmers were developed. Developed questionnaires were pre-tested during the orientation of the research assistants. Knowledge survey among HCWs consisted of three sections: firstly, on causes of emergence of antibiotic resistance, secondly on their existing know-how on treatment and development of drug resistance, and lastly on the mechanisms of antibiotic resistance. Pre-testing was conducted in hospitals and few sites within Kathmandu valley which were precluded from the data for this study.
Methods of data collection
Six research assistants having a qualification of Masters of Science (M.Sc.) in Microbiology were recruited for this study. A week-long workshop was organized in Kathmandu to discuss about the study, protocol and pre-testing of the questionnaire. They were proportionately deployed in the study sites for data collection, enumeration and data entry. Principal and co-investigators provided the complete guidance during the entire study.
This study involved data collection at different levels—individual patients, healthcare providers, pharmacists, farms, and healthcare facilities. KAP on antimicrobial resistance were explored using standardized and pre-tested questionnaires adapted to each type of respondents34. Interviews were conducted with patients visiting health centers. All the participations were voluntary and were not provided any forms of incentives. The survey was conducted in collaboration with National Public Health Laboratory (NPHL) and the hospitals, data on AMR in different settings were collected from available reports which were tracked through laboratory registers/Laboratory Management Information System (LMIS) and were analyzed to assess AMR patterns. Assessment of recording, reporting and LMIS systems on antimicrobial resistance and review of the registers were also conducted in the laboratories. Information on morbidity and mortality related to AMR, through review of medical-records data were examined.
Data management and analysis
All the questionnaires were screened for completeness, and errors; and were processed for data analysis. First, all the data were entered into Epi-data version 3.1 and were imported into SPSS software version 24.0 (IBM; Chicago, USA). Descriptive and univariate analysis was carried out using Chi-squared tests/Fisher’s exact test and are presented in supplementary files. Statistically significant variables based on the univariate analysis and research question were selected and were analyzed using multivariate logistic regression models. Various independent variables were controlled and evaluated to calculate adjusted odds ratio predicting the outcome variables that included knowledge of antibiotic and antibiotic resistance. A difference with p-value less than 0.05 was considered as statistically significant.
Ethics approval and consent to participate
This study received ethical approval from Ethical review board (ERB) of Nepal Health Research Council (NHRC), (Reg No. 68/2017). Written informed consent was obtained from each participant for their voluntary participation in the study. This study was conducted in accordance with the Declaration of Helsinki.
Results
Demographic characteristics of respondents
Of the 87 health care workers, 75.9% (66/87) were male and 24.1% (21/87) were female. Majority of health care workers were medical doctors (90.2%;79/87). More than two-thirds of the health care workers belonged to age group 25–50 years (63.2%; 55/87), followed by age group (0–25) years (23%; 20/87) and > 50 years (13.8; 12/87). Of 324 patients interviewed, 72.8% (236/324 were male and 27.2% (88/324) were female. Half of the patients were from 26–50 years age group (50%; 162/324), followed by 0–25 years (32.7%;106/324) and > 50 years (17.3%; 56/324). Of 32 livestock farmers, 87.5% (28/32) were male and 12.5% (4/32) were female (Table 1). Their distribution in selected eight districts is summarized (Supplementary Table S1).
Table 1.
Demographics characteristics of respondents.
| Characteristics | Number (%) |
|---|---|
| Health care workers (n = 87) | |
| Gender | |
| Male | 66 (75.9) |
| Female | 21 (24.1) |
| Age group (in years) | |
| 0–25 | 20 (23) |
| 26–50 | 55 (63.2) |
| > 50 | 12 (13.8) |
| Profession | |
| Medical doctor | 79 (90.2) |
| Paramedics | 8 (9.8) |
| Qualifications | |
| MBBS only | 42 (53.2) |
| MD General Practitioner | 7 (8.9) |
| Internal Medicine | 10 (12.7) |
| Pediatrics | 2 (2.5) |
| Gynecology | 3 (3.8) |
| Surgery | 7 (8.9) |
| Others | 8 (10.1) |
| Working medical institutions | |
| Public/ Government Hospital | 37 (42.5) |
| Primary health care center | 2 (2.3) |
| Private hospital of 100 beds or more | 48 (55.2) |
| Private Drug Seller (n = 33) | |
| Gender | |
| Male | 14 (42.4) |
| Female | 19 (57.6) |
| Age group (in years) | |
| 0–25 | 6 (18.2) |
| 26–50 | 20 (60.6) |
| > 50 | 7 (21.2) |
| Education | |
| Pharmacy degree (D. Pharma, B. Pharma) | 19 (57.6) |
| Health related other degree | 10 (30.3) |
| Non-Health related degree | 4 (12.1) |
| Hospital Patients (n = 324) | |
| Gender | |
| Male | 236 (72.8) |
| Female | 88 (27.2) |
| Age group (in years) | |
| 0–25 | 106 (32.7) |
| 26–50 | 162 (50) |
| > 50 | 56 (17.3) |
| Type of patients | |
| Inpatients | 64 (19.8) |
| Out patients | 260 (80.2) |
| Education of the patients | |
| Illiterate | 40 (12.3) |
| Literate | 36 (11.1) |
| Primary education (Class 1 -7) | 36 (11.1) |
| Secondary Education (8–10) | 97 (29.9) |
| Highest Secondary (10 + 2) | 65 (20.1) |
| Bachelor and above | 50 (15.4) |
| Hospital /Primary Health Center, Chief / Representative (n = 17) | |
| Gender | |
| Male | 12 (70.6) |
| Female | 5 (29.4) |
| Age group (in years) | |
| 0–25 | 1 (5.9) |
| 26–50 | 14 (82.4) |
| > 50 | 2 (11.7) |
| Type of Health center | |
| Primary health center | 12 (70.5) |
| Hospital | 5 (29.5) |
| Livestock Farmers (n = 32) | |
| Gender | |
| Male | 28 (87.5) |
| Female | 4 (12.5) |
| Age group (in years) | |
| 0–25 | 4 (12.5) |
| 26–50 | 20 (62.5) |
| > 50 | 8 (25) |
Knowledge
More than eighty percentage (range 80–96%) of the HCWs thought the role of various underlying factors (listed in questionnaire) on the emergence of resistance. Almost 40% of them were aware and had encountered different drug-resistant bacteria including extended spectrum beta-lactamase producing Escherichia coli, penicillin-resistant Streptococcus pneumoniae (PRSP), multi-drug resistant Pseudomonas aeruginosa, and Methicillin-resistant Staphylococcus aureus (MRSA). Of the listed mechanism of development of drug resistance in questionnaire, majority (69–91%) of the HCWs had sound knowledge (Supplementary Table S2).
Hospital patients were first asked about possibility of antibiotics in curing some bacterial and viral diseases. Nonetheless, in this study, we did not ask patients what they understood by the term ‘antibiotics.’ Out of 324 patients interviewed, 42.9% (139/324) of them thought that fever can be treated with antibiotics; more than one third (35.2%; 114/324) believed that antibiotics were used for treatment of cold and flu; 17.3% (56/324) believed that antibiotics can be used for treatment for sore throat, and 22.2% (72/ 324) believed that antibiotics cure headache and skin or wound infection. This study also assessed about their knowledge on terminologies related to resistance such as AMR, superbugs, drug-resistance, and drug-resistant bacteria. Of 324 patients, majority (84%; 272/324) of the respondents had never heard anything about antibiotic resistance and related terms and only 16% (52/324) of the patients had heard about different terms for antibiotics. Of the total (324) patients, 5.6% (18/324) heard about AMR and its related terms from media (newspaper, TV, radio); 3.7% (12/324) heard from family members and friend; 3.1% (10/324) heard from doctor or nurse; and a few (1.2%; 4/324) heard from pharmacists. Final section of the questionnaire explored on the perception on the drug resistance. Out of 324 patients interviewed, 75% of the respondents were not aware on anything related to antibiotic resistance issues (Supplementary Table S3).
Of 33 private drug sellers interviewed, majority (87.9%; 29/33) of them had heard about antibiotic resistance. However, a few (18%; 6/33) of them thought that antibiotics could treat all sorts of diseases listed in the questionnaire. Of 33 respondents, more than half (51.5%; 17/33) responded that antibiotics were used for treatment of bacterial diseases and oral thrush. Surprisingly, 23.3% (9/33) and 12.1% of them responded that antibiotics can be used to treat viral diseases and general weakness (Supplementary Table S4). Among livestock farmers, a few (12.5%; 4/32) of them had heard about antibiotic resistance. They had heard it mainly from the doctors or nurses (50%; 2/4), and pharmacists (50%; 2/4) (Supplementary Table S5).
Attitude
Majority of HCWs (76/87) believed that antibiotics are overused all over the country; and majority (82/87) of them were in support of a more controlled policy for antibiotic use. Almost two-thirds of them (63.3%; 55/87) thought that AMR has already become significant problem in their hospitals. Out of 87 participants, majority (92%; 80/87) believed that prescribing broad spectrum antibiotics can aggravate the problem of resistance (Supplementary Table S6).
Most of the participants (92.6%; 300/324) believed that antibiotics should be used only when they are prescribed by a medical practitioner; exactly equal proportion of them also stressed on the fact that HCWs should prescribe antibiotics only when it is needed. Out of 324 patients, most (98.1%; 318/324) were well-informed on the hand hygiene practice. Nearly two-thirds of the participants (65.7%; 213/324) agreed that farmers should use antibiotics as minimum as possible. When they were asked on immunization, majority (96.6%;313/324) of the participants were certain that parents should ensure up to date vaccinations to their children. When it came to antibiotic resistance, they were unaware about this issue as 78.7% (255/340) of the respondents were either unaware or rejected the problem of AMR as one of the global crises. Nearly half of the respondents (161/324; 49.7%) were unsure about whether the AMR crisis has hit them individually. More than half (58.6%;190/324) were unsure on whether correct use of antibiotics could avoid AMR crisis at individual level. Another important finding was that 71.3% (231/324) of the individuals had no idea about their role in combating AMR crisis (Supplementary Table S7).
Practice
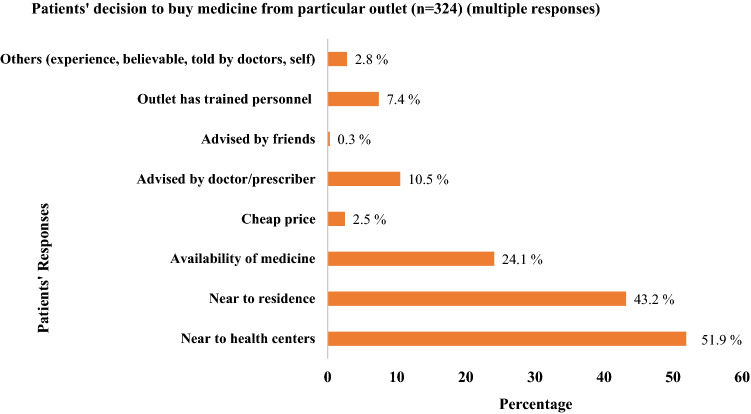
Out of 87 HCWs, majority (71.3%; 62/87) reported that they prescribed antibiotics at least once a day, and only a few (13.8%; 12/87) prescribed at least once a week (Supplementary Table S8). Out of 324 patients interviewed, majority (79%; 256/324) of the respondents were purchasing antibiotics for themselves while 61.7% (200/324) respondents were currently taking antibiotics. Findings regarding the use of antibiotics among patients revealed that a few (18%; 58/324) of them used antibiotics without prescription (self-medicated); and few others (14.8%; 48/324) complained that they were not advised by HCWs on how to use the antibiotics. Almost half (46.3%; 150/324) admitted that they had the habit of discontinuing antibiotics (did not complete the prescribed dose) after they felt better (Supplementary Table S9). On patient’s response (multiple choice) to buy medicine from particular outlet, 51.9% (168/324) bought medicines from near the health centers and 43.2% (140/324) bought from near the residence (Fig. 2).
Figure 2.
Patient’s responses to buy medicine from particular outlet.
Multivariate analysis
Multivariate analysis of people's knowledge on antibiotics showed that gender, age, person buying drug were not significant predictors. As the education level increased, the knowledge of patients on antibiotics also increased. Education level of patients was associated with the knowledge of antibiotics: literate only [ AOR = 1.4 (95% CI = 0.6–4.4)], primary education (1–7 grade [AOR = 1.0 (95% CI = 0.4–2.3)], secondary education (8–10 grade) [AOR = 1.8 (95% CI = 1.1–3.4)], Higher secondary (11–12 grade) [AOR = 1.6 (95% CI (0.8–3.1)] and bachelor and above [AOR = 2.0 (95% CI = 0.9–4.6)] (Table 2).
Table 2.
Multivariate analysis of predictors of knowledge on antibiotics.
| Variables | Regression coefficient | p-value | Odds ratio | 95% CI for odds ratio | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Gender | |||||
| Male | Ref | – | 1 | – | – |
| Female | 0.093 | 0.723 | 1.097 | 0.657 | 1.833 |
| Age category (years) | |||||
| < or 20 | Ref | ||||
| 21–40 | 0.108 | 0.715 | 1.115 | 0.622 | 1.997 |
| 41–60 | 0.175 | 0.612 | 1.191 | 0.606 | 2.344 |
| > 60 | 0.438 | 0.294 | 1.550 | 0.683 | 3.514 |
| Education level | |||||
| Illiterate | Ref | 1 | |||
| Literate only | 0.392 | 0.359 | 1.481 | 0.640 | 3.427 |
| Primary education (1–7 grade) | 0.073 | 0.856 | 1.075 | 0.491 | 2.357 |
| Secondary education (8–10 grade) | 0.627 | 0.042 | 1.872 | 1.024 | 3.422 |
| Higher secondary (11–12 grade) | 0.489 | 0.147 | 1.631 | 0.842 | 3.157 |
| Bachelor and above | 0.734 | 0.074 | 2.083 | 0.932 | 4.655 |
| Person buying the drug in the family | |||||
| Self | Ref | 1 | |||
| Other | −0.047 | 0.868 | 0.954 | 0.548 | 1.661 |
Related to knowledge about antibiotic resistance among patients, adults (41–60 years) were apparently more aware of antibiotic resistance. Knowledge of AMR on age group (21–40) years [AOR = 0.6 (95% CI = 0.3- 1.1)], age groups (41–60) years [AOR = 0.7 (95% CI = 0.3–1.5)].and > 60 years [AOR = 0.2 (95% CI = 0.07–0.73)]. Knowledge of antibiotic, person purchasing the drug were not associated with knowledge of antibiotic resistance; however, age of the patient was found as a predictor of knowledge of antibiotic resistance (Table 3).
Table 3.
Multivariate analysis of predictors of Knowledge related to antibiotic resistance.
| Variables | Regression coefficient | p-value | Odds ratio | 95% CI for odds ratio | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Gender | |||||
| Male | Ref | 1 | |||
| Female | –0.401 | 0.186 | 0.670 | 0.370 | 1.213 |
| Age category (years) | 0.057 | ||||
| < or 20 | Ref | 1 | |||
| 21–40 | –0.496 | 0.126 | 0.609 | 0.323 | 1.150 |
| 41–60 | –0.324 | 0.399 | 0.723 | 0.341 | 1.535 |
| > 60 | –1.441 | 0.012 | 0.237 | 0.077 | 0.730 |
| Education level | 0.000 | ||||
| Illiterate | Ref | 1 | |||
| Literate only | –1.947 | 0.004 | 0.143 | 0.038 | 0.533 |
| Primary education (1–7 grade) | –1.171 | 0.022 | 0.310 | 0.114 | 0.846 |
| Secondary education (8–10 grade) | –0.736 | 0.036 | 0.479 | 0.240 | 0.953 |
| Higher secondary (11–12 grade) | –0.296 | 0.421 | 0.744 | 0.362 | 1.529 |
| Bachelor and above | 1.017 | 0.019 | 2.766 | 1.180 | 6.484 |
| Person buying the drug in the family | |||||
| Self | Ref | 1 | |||
| Other | 0.234 | 0.449 | 1.263 | 0.690 | 2.312 |
| Knowledge of antibiotic | |||||
| No | Ref | 1 | |||
| Yes | 0.082 | 0.758 | 1.085 | 0.645 | 1.827 |
The existing condition of LMIS on AMR in hospital/laboratories
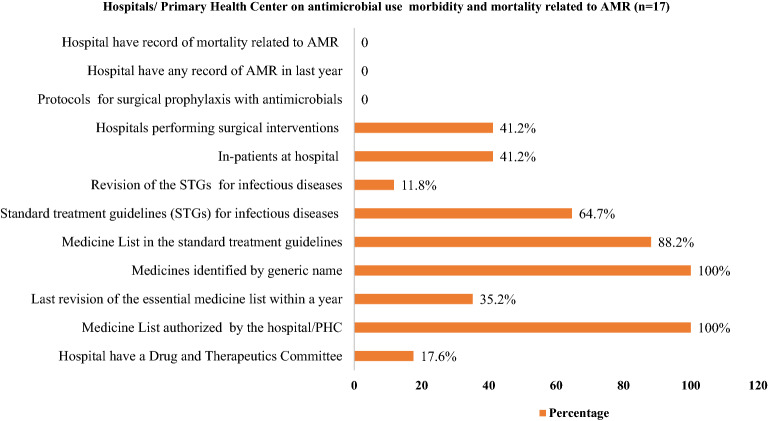
A total of 17 Hospitals/Primary health center were selected to gather information on antimicrobial use, morbidity and mortality related to AMR. Out of 17, all hospitals/primary health center had medicine by generic name and had an essential formulary list of medicines. Out of 17, only 35.2% had last revision of the essential medicine list with in one year and 17.6% had drugs and therapeutics committee. None of the hospital/PHC had protocols or norms for surgical prophylaxis, record of AMR in previous years and AMR related mortality (Fig. 3).
Figure 3.
Hospitals provisions on antimicrobial use and morbidity and mortality related to AMR from Hospital/ PHC Record.
A total of twenty-three laboratories were assessed for performance, reporting and recording of AMR. More than half (13/23) of the laboratories were not purchasing ready-to-use culture media, thus were preparing those media manually as per routine requirements. In addition to this, 78.3% (18/23) of the laboratories did not have an automated blood culture system and relied on conventional procedures. Majority (69.5%; 16/23) of the laboratories did not have trends of performing biochemical assay for the confirmation of pathogenic organisms. Twenty laboratories were reliant on convention disk diffusion method (Kirby-Bauer method) for antimicrobial susceptibility testing (AST); only two laboratories adopted minimum inhibitory concentration (MIC) determination method for AST. However, majority (69.5%; 16/23) of the laboratories were following CLSI guidelines for interpretation of the result while all (100%; 23/23) of the labs did have AST recording system of the strains by clearly indicating them as resistant, intermediate or sensitive (Supplementary Table S10).
Discussion
Rational use of antimicrobials is dependent on its main stakeholders that include patients, clinicians, veterinary professionals, drug retailers and farmers. It is essential to explore how these stakeholders interact, practice and use the antibiotics. A number of factors such as easy access to over the counter (OTC) use of drugs, lack of clear policy guiding empirical treatment regimens, poor literacy level among a major proportion of the population in the country, pressure among healthcare professionals for prescriptions, lack of proper diagnostic facilities, and other socioeconomic constrains were found to augment the irrational use of antimicrobial drugs35.
Participants Knowledge on Antibiotics
Although this study did not explore what patients understood by the term ‘antibiotics’, majority of patients are not aware of what constitute such type of medicine, their scientific rationale and uses despite that ‘antibiotics’ were familiar term among the patients35. More than two-fifth of the patients still believed that fever was cured by antibiotics and echoed with previous studies from Nepal27 and Indonesia36. Around one-third of the respondents believed that viral diseases such as cold and flu, measles and HIV/AIDS could be treated with antibiotics. The widespread beliefs that antibiotics can treat a wide range of illnesses (ranging from non-specific body aches to the life-threatening viral diseases) may have multiple consequences including undermining the medical professional’s role in prescription and guidance37. In addition, beliefs that antibiotics could treat all diseases may have promoted buying of over-the-counter medications (self-medications), and also may have exerted pressure on HCWs to provide/prescribe antibiotics9,10.
In the context of basic knowledge on antibiotics, our study showed that the news media were reported to be the major source of information about the antibiotics in generating mass awareness38. A report published by the WHO also underscores the role of televisions, radios, internets, and press in spreading knowledge of antibiotics among general population37. Heightened awareness among participants regarding hand hygiene (98.1%) and immunization (96.6%) was observed in our study. The improved knowledge and practices may have been contributed by the mass media and vaccine campaign that served the population at the household over the last two decades39 and are reflected in previous studies from Nepal40–45.
Knowledge on AMR
Overall, majority (84%) of the respondents had never heard about AMR while more than three-fourth of the total participants had no any idea on AMR related issues. This finding is similar (88.6%) to the previous study conducted in Nepal46. The poor knowledge on antibiotics may have impact on their attitude towards the use of antibiotics. Unlike their responses on knowledge questions, majority (93%) of the people stressed the need for the use of antibiotics by health professionals’ prescription only. When it came to the use of antibiotics, eighteen percentages of them were purchasing antibiotics over-the-counter (OTC) and were often driven by the distance, cost and perceived benefits. This finding is similar (24%) to one conducted in Bhutan47. Nonetheless, these responses suffer from social desirability bias (as patients very well knew that medicines ought to be taken with the prescription), and we believe OTC is way more prevalent than the reported percentage in this study. Even so, this trend highlights the urgent need for strict rules and regulations to discourage the OTC use of antibiotics10. Nearly half of the respondents (46.3%) in this study explained that they would not comply to the complete dose of medicines. Several previous studies have also concluded that the self-medication remains as one of the commonest practices in Nepal48. Most of the patients do not adhere to the treatment therapies as prescribed by the clinicians49 and thus do not follow a complete course of antibiotic therapy. Patients stop taking medicines once the disease symptoms begin to fade away and they stockpile the remaining drugs for future use. They often recommend such drugs to their family members, relatives and peers, which augments the rapid spread of the resistance50,51.
High prevalence of OTC medication practices could also be attributed to the low number of doctors to patients (1:1724) ratio in Nepal, which can inevitably oblige patients to rely on range and types of health workers available thus shaping the treatment seeking behavior33,52. Doctors are disproportionately distributed in Nepal. For instance, the Kathmandu valley has one doctor for 850 individuals but in rural zones the number is one doctor for each 150,000 individuals53. Moreover, Nepal is predominated by high proportion of rural and inaccessible areas where tertiary care centres are heavily constrained54,55. Therefore, the majority of the rural people rely on health assistants, quacks (unqualified persons pretending to have medical skills), and poorly trained drug dispensers for their treatment decision(s)56. Nonetheless, drug shops are a popular choice for OTC medication in both rural and urban regions and are affected by accessibility and functionality of the formal health services35. Despite that this study was conducted in a relatively urban areas, patients represented rural regions too. Follow-ups in patients from rural region were heavily constrained by their poor socio-economic conditions57.
Factors influencing on KAP
Prescriber’s decisions may be affected by various factors such as lack of adequate professional knowledge on rational use of drugs, lucrative intensions often guided by themselves and/or pharmaceutical companies’ offer and/or hospital administration’s pressure, fear of losing clients (patients), patients’ self-demand, prescribing practices even for non-bacterial infections, and inappropriate doses and route of administration of the drugs46. Similarly, patients’ decisions on the use of antibiotics might be influenced by self-medication, sharing drug among family members, relatives and peers, ignorance of proper dose during medication, and lack of trust on medical professionals58. More importantly, poor infection control and augmented use of antibiotics in clinics, livestock and poultry farming, agriculture and aquaculture have aggravated the onset and rapid transfer of AMR globally58.
Education level of the respondents affected the knowledge and attitude related to antibiotics and AMR. Previous studies from Nepal27, India59, Bhutan47, Saudi Arabia60, Hong Kong61, Malaysia62, South Korea63, Oman64, Greece65, and Lithuania66 have echoed with our findings on the role of education. With higher education, respondents perhaps had good knowledge about proper use of antibiotics and adversities such as antibiotic resistance. As majority of them belonged to the age group of 25–50 years, their education level may have role rather than their age in affecting the KAP on antibiotics.
Healthcare workers, especially physicians are an integral stakeholder of antibiotics prescription and the AMR. Driven by the demand, and high prevalence of infectious diseases, health care workers are also one of the main facilitators of irrational prescriber of antibiotics10,49. In our study, more than eighty percentage (80–96%) of the HCWs had the knowledge on antibiotics, and AMR, and mechanisms leading to the emergence of resistance. This finding was slightly higher than a previous finding in Nepal67, India68, and was similar to other previous studies conducted in Egypt69, Zambia70 and Pakistan71. Improving knowledge and practice related to antibiotics might be attributable to the improved education and mass awareness carried out by media, activists, government, and NGO(s)/INGOs. In addition to this, improved status of surveillance, enforcement of law and orders could be other factors to be considered.
The majority of HCWs agreed that there is an overuse of antibiotics all over the country and almost two-third (63.3%) of them believed that AMR has already become a challenge in their own hospital(s). Similar findings were reported in USA in which 88% and 72% of the physicians agreed that AMR was a challenge in general and in their hospital72. In Egypt, 94% and 80% of the clinicians believed the AMR as a problem in their country and in their hospital69. Similar finding was reported in Nepal in which 87% of the physicians believed that antibiotics were overused throughout the country67. A previous study conducted in Nepal has reported that almost half the doctors (50.2%) used to prescribe antibiotics more than once a day67.
Previous studies have well documented that some of the healthcare professionals unnecessarily prescribed broad-spectrum antibiotics73. Minor health problems such as colds, coughs and diarrhea can be conservatively managed (without antibiotics). Nonetheless, clinicians prescribe antibiotics to these non-specific illnesses50. In addition to this, incorrect dose(s) and misleading guidance are also frequently observed in practices50. Similarly, pharmaceutical companies visit clinicians with lucrative offers which adds pressure on antibiotics prescriptions74.
Unwarranted demands from the patients’ side for any specific drug is another leading factor for irrational prescriptions. Such practices are predominant in the low-and middle-income countries (LMICs)71. Above all, poor infection control practices in the LMICs are often neglected but significant factor in the rapid spread of drug-resistant pathogens48.
Policy implications
Findings from this study bear policy implications, particularly related to high rate of antibiotic prescription, OTC use of drugs, physicians’ neglect on AST guided therapy, and lack of knowledge on regulating policies for use of antimicrobials48. Common to resource constrained settings, Nepal suffers from scarcity of laboratory infrastructure, particularly in rural regions, which can inevitably lead to high empirical treatment10. Government should aim to establish laboratories with well-resourced with equipment and trained lab personnel48.
Nepal’s unique geographical landscape, population and urban–rural distribution of health services further affects the treatment seeking behavior including the (over-)use of antibiotics75,76. Aligning with these reports, HCWs also suggested for the culturally and geographically tailored guidelines for antimicrobial use in addition to the national guideline68. In addition, Nepal also suffers from disproportionate physician to patients’ ratio and should be properly addressed77. For example, by establishing the health care structure in rural regions and incentivizing the health care workers to serve the rural regions in addition to other developmental works77,78. At the same time, patients in remote regions of Nepal with simple infectious conditions should not be restricted to utilize antibiotics where qualified health care workers including doctors and tertiary care centers can be days away9. In addition, counselling on rationale use of antibiotics, and its adversities should be essential elements of prescription by health care workers which can be a sustainable method to increase the public awareness10.
Patients’ pressure also plays crucial role in the prescription and use of antibiotics which may be well reflected in the primary health centers where treatment options are limited and drugs are easily available OTC10. In such geographically remote regions, there is a good relationship between healthcare providers and community members, which creates pressure in prescription and sell of the antibiotics asked by patients rather than the appropriate ones10,79–81. Although policy guidelines demand for use of antibiotics based on the identification of causative agents, in practice, the empirical and over the counter use of antibiotics are high9,10.
As the positive roles of mass awareness and media are reported from several studies, promotion and incorporation of effective media/programs and routine trainings to HCWs are highly recommended. The importance of education and mass awareness in promoting the rational use of antibiotics are also supported by several previous studies in Nepal82,83 in India83 and in Europe84.
Antibiotic stewardships aligning with the ‘One Health’ approach need to be considered in future efforts in Nepal85. Lack of protocols for surgical prophylaxis, and record of AMR and associated mortality in this study implies need for investment in protocol development and robust surveillance in all the health care institutions. National and sub-national AMR surveillance agencies, hospitals, organizations and all stakeholders working in the sector of human health, livestock and agriculture can utilize the findings of this study to forge future strategies against AMR in Nepal. Collaborative efforts between ministry of health, ministry of agriculture & livestock development, WHO and their stakeholders are essential to implement ‘one health approach’. Government of Nepal (GoN) together with the leadership of WHO can forge ‘one health approach’ in national action plan on AMR.
Strengths and limitations
Although this study explored knowledge, attitude and practice related to antibiotics uses among patients, healthcare workers, and farmers; using quantitative methods alone constrained us to understand the deeper reasons behind the responses including extent and nature on how they affect the use of antibiotics in Nepal. Future studies utilizing qualitative methods are critical to disentangle how these factors interact in various sectors to facilitate the use of antibiotics. For example, future ethnographic studies can explore why and how patients choose distance to the health center (against other factors) as an influence in buying antibiotics over the counter. This study was conducted in a select of districts/hospitals and inevitably constraints us to generalize the findings for the entire country. Although respondents in all categories were attempted to be balanced, number of pharmacists, and farmers were limited due to their proportion. In future, studies exploring farmers and pharmacists in greater proportion can help understand the true burden of antibiotic use in these sectors. Only 12.5% of farmers in our study had heard of AMR. This suggests need for more engagement with the animal and livestock sector in the future. Furthermore, this study did not include some of the underlying factors such as the extent of patients’ pressure in prescription, physician’s knowledge on microbiology laboratory reports, commercial interests in selling the antibiotics, pharmaceutical companies’ competing offers to clinicians. This survey may have incurred recall and social desirability bias. Data collected through prescription audit, observation and in-depth interviews were precluded in the analysis due to inconsistencies in their collection and inadequacy of the data which affected data saturation. Triangulation of various methods could have enriched our findings. Nonetheless, this study serves as a reference to the context and situation of AMR knowledge, attitudes and practices in Nepal.
Conclusion
Although the level of knowledge, attitude and practice related to prescriptions and use of antibiotics apparently seems improved compared to previous studies, the prescription and overuse of antibiotics are unregulated in the study areas. Antibiotic stewardship programs in Nepal can at first address these gaps through health education, awareness and mass campaigns to mitigate the inappropriate use of antibiotics. Importantly, reformation in policy related to use of antibiotics should consider the multiple stakeholders that shape demand, supply, and compliance, including a ‘One Health approach’ to ensure animal health considerations are also taken into account.
Supplementary Information
Acknowledgements
We thank all of the staffs of entire health centers under study. We are grateful to all the patients, farmers, drug-sellers and laboratory personnel for their involvement in the study. Lastly, we would like to express our sincere gratitude to Dr. Reuben Samuel and Dr. Sophie Goyet (WHO-Country Office for Nepal) for their technical support.
Abbreviations
- AMR
Antimicrobial resistance
- ANM
Auxiliary Nurse Midwife
- AST
Antibiotic Susceptibility Test
- CLSI
Clinical Laboratory Standard Institute
- EML
Essential Medicine List
- ESBL
Extended Spectrum Beta Lactamase
- HA
Health Assistant
- HP
Health Post
- KAP
Knowledge, Attitude and Practice
- LMIS
Logistic Management Information System
- MRSA
Methicillin Resistant Staphylococcus aureus
- NPHL
National Public Health Laboratory
- PHC
Primary Health Care Center
- SPSS
Statistical Package for Social Sciences
- WHO
World Health Organization
Author contributions
Conceived and designed the study: K.R.R., M.R.B., P.G. Performed the study/Data Collection: B.D., S.K., K.G., N.A., U.T.S., P.G., S.D., D.R.S. Analyzed the data: K.R.R., M.R.B., B.D., B.G. Map creation: K.R.R., B.A., D.R.S. Wrote the original draft: K.R.R., B.D. Reviewed edited and finalized the draft: K.R.R., B.A., P.G.
Funding
The research presented was supported by WHO- country office for Nepal. We declare that this study is free from any sort of interests of the funding source. We are responsible for the entire contents and thus do not necessarily represent the views of the funding agency.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Komal Raj Rijal and Megha Raj Banjara.
Contributor Information
Komal Raj Rijal, Email: komal.rijal@cdmi.tu.edu.np.
Prakash Ghimire, Email: prakash.ghimire@cdmi.tu.edu.np.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-90812-4.
References
- 1.Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014;5:229–241. doi: 10.1177/2042098614554919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO). Fact Sheet Antibiotic Resistance. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance. Accessed 31 Aug 2020 (2020).
- 3.Mazzon D. Ethical use of antibiotics in the era of multiresistance: a common good for the individual or the society? Recenti. Prog. Med. 2016;107:71–74. doi: 10.1701/2152.23268. [DOI] [PubMed] [Google Scholar]
- 4.Gaynes R. The Discovery of Penicillin—New Insights After More Than 75 Years of Clinical Use. Emerg. Infect. Dis. 2017;23(5):849–853. doi: 10.3201/eid2305.161556. [DOI] [Google Scholar]
- 5.Aminov RI. A brief history of the antibiotic era: lessons learned and challenges for the future. Front. Microbiol. 2010;1:134. doi: 10.3389/fmicb.2010.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gajdacs, M. & Albericio, F. Antibiotic Resistance: From the Bench to Patients. Antibiotics (Basel)8, 1. 10.3390/antibiotics8030129 (2019). [DOI] [PMC free article] [PubMed]
- 7.Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog. Glob. Health. 2015;109:309–318. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandra S, Barter DM, Laxminarayan R. Economic burden of antibiotic resistance: how much do we really know? Clin. Microbiol. Infect. 2014;20:973–980. doi: 10.1111/1469-0691.12798. [DOI] [PubMed] [Google Scholar]
- 9.Pokharel S, Raut S, Adhikari B. Tackling antimicrobial resistance in low-income and middle-income countries. BMJ Glob. Health. 2019;4:e002104. doi: 10.1136/bmjgh-2019-002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pokharel S, Adhikari B. Antimicrobial resistance and over the counter use of drugs in Nepal. J. Glob. Health. 2020;10:010360. doi: 10.7189/jogh.10.010360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manyi-Loh C, Mamphweli S, Meyer E, Okoh A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules. 2018;23:1. doi: 10.3390/molecules23040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Destoumieux-Garzon D, et al. The one health concept: 10 years old and a long road ahead. Front. Vet. Sci. 2018;5:14. doi: 10.3389/fvets.2018.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEwen SA, Collignon PJ. Antimicrobial resistance: a one health perspective. Microbiol. Spectr. 2018;6:1. doi: 10.1128/microbiolspec.ARBA-0009-2017. [DOI] [PubMed] [Google Scholar]
- 14.Hogerzeil HV, et al. Field tests for rational drug use in twelve developing countries. Lancet. 1993;342:1408–1410. doi: 10.1016/0140-6736(93)92760-q. [DOI] [PubMed] [Google Scholar]
- 15.Gebeyehu E, Bantie L, Azage M. Inappropriate Use of Antibiotics and Its Associated Factors among Urban and Rural Communities of Bahir Dar City Administration, Northwest Ethiopia. . PLoS ONE. 2015;10:e0138179. doi: 10.1371/journal.pone.0138179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holloway, K. & van Dijk, L. WHO: The World Medicines Situation 2011. Rational use of medicines, 3rd edition (Geneva, 2011).
- 17.Chem ED, Anong DN, Akoachere JKT. Prescribing patterns and associated factors of antibiotic prescription in primary health care facilities of Kumbo East and Kumbo West Health Districts, North West Cameroon. . PLoS ONE. 2018;13:e0193353. doi: 10.1371/journal.pone.0193353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurung, S. et al. Detection of OXA-48 Gene in Carbapenem-Resistant Escherichia coli and Klebsiella pneumoniae from Urine Samples. Infect. Drug Resist 13, 2311–2321. 10.2147/IDR.S259967 (2020). [DOI] [PMC free article] [PubMed]
- 19.Raut S, et al. Trend and Characteristics of Acinetobacter baumannii Infections in Patients Attending Universal College of Medical Sciences, Bhairahawa, Western Nepal: A Longitudinal Study of 2018. Infect. Drug Resist. 2020;13:1631–1641. doi: 10.2147/IDR.S257851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kayastha K, et al. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella species in pediatric patients visiting International Friendship Children's Hospital, Kathmandu, Nepal. . Infect. Dis. (Auckl) 2020;13:1178633720909798. doi: 10.1177/1178633720909798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rijal, K. R. et al. Methicillin resistance Staphylococcus aureus in patients visiting Western Regional Hospital, Pokhara. J. Inst. Med.30(1), 21–25 (2008).
- 22.Guragin N, et al. Extended spectrum β-lactamase producing Gram Negative bacterial isolates from urine of patients visiting Everest Hospital, Kathmandu, Nepal. . TUJM. 2019;6(1):26–31. doi: 10.3126/tujm.v6i0.26575. [DOI] [Google Scholar]
- 23.Muktan B, et al. Plasmid mediated colistin resistant mcr-1 and co-existence of OXA-48 among Escherichia coli from clinical and poultry isolates: first report from Nepal. Gut. Pathog. 2020;12(44):2020. doi: 10.1186/s13099-020-00382-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Resource mobilization for antimicrobial resistance (AMR): Getting AMR into plans and budgets of government and development partners: Nepal country report. 2018: 1–34. https://www.who.int/antimicrobial-resistance/national-action-plans/Nepal-AMR-integration-Report-WHO-Sept-2018.pdf.
- 25.Shrestha, S., Yadav, R. S. & Deo, S. K. Burgeoning Irrational Antibiotics use in Primary Health Care in Nepal. J. Nepal Health Res. Counc.16, 473–475 (2019). [PubMed]
- 26.Deo SK, Rijal S, Kunwar SD, Dahal A, Gupta S. Knowledge of Use of Antibiotic, its Resistance and Consequences among Students in Private Schools. JNMA J. Nepal Med. Assoc. 2018;56:740–744. doi: 10.31729/jnma.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nepal A, Hendrie D, Robinson S, Selvey LA. Knowledge, attitudes and practices relating to antibiotic use among community members of the Rupandehi District in Nepal. BMC Public Health. 2019;19:1558. doi: 10.1186/s12889-019-7924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nepal G, Bhatta S. Self-medication with Antibiotics in WHO Southeast Asian Region: A Systematic Review. Cureus. 2018;10:e2428. doi: 10.7759/cureus.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Department of Health Services, Ministry of Health. National Antimicrobial Resistance Containment Action Plan Nepal 2016. 1–24. https://www.flemingfund.org/wp-content/uploads/3d9bf4b7ab190c600921a99cf1803059.pdf.
- 30.FHI 360. FHI 360 Nepal – Consultancy Opportunities for The Fleming Fund Country Grant for Nepal – Xplore International. 2018. https://ixplore.info/fhi-360-nepal-consultancy-opportunities-3/.
- 31.Rijal KR, et al. Epidemiology of Plasmodium vivax Malaria Infection in Nepal. Am. J. Trop. Med. Hyg. 2018;99:680–687. doi: 10.4269/ajtmh.18-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adhikari, B., Ozaki, A., Marahatta, S. B., Rijal, K. R. & Mishra, S. R. Earthquake rebuilding and response to COVID-19 in Nepal, a country nestled in multiple crises. J. Global. Health 10 (2), 020367. 10.7189/jogh.10.020367 (2020). [DOI] [PMC free article] [PubMed]
- 33.Central Bureau of Statistics . Statistical Yearbook of Nepal. Central Bureau of Statistics; 1997. [Google Scholar]
- 34.World Health Organization (WHO). WHO Global Strategy for Containment of Antimicrobial Resistance. Available online: http://www.who.int/csr/resources/publications/drugresist/en/EGlobal_Strat.pdf. Accessed 14 Aug 2020.
- 35.Adhikari, B. et al. Why do people purchase antibiotics over-the-counter? A qualitative study with patients, clinicians and dispensers in central, eastern and western Nepal. BMJ Glob. Health6, e005829. 10.1136/bmjgh-2021-005829 (2021). [DOI] [PMC free article] [PubMed]
- 36.Widayati A, Suryawati S, de Crespigny C, Hiller JE. Knowledge and beliefs about antibiotics among people in Yogyakarta City Indonesia: a cross sectional population-based survey. Antimicrob. Resist. Infect. Control. 2012;1:38. doi: 10.1186/2047-2994-1-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization . Worldwide country situation analysis: response to antimicrobial resistance. WHO; 2015. [Google Scholar]
- 38.Shah P, et al. Knowledge, Attitude, and Practice Associated with Antibiotic Use among University Students: A Survey in Nepal. Int. J. Environ. Res. Public Health. 2019;16:1. doi: 10.3390/ijerph16203996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greenwood B. The contribution of vaccination to global health: past, present and future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369:20130433. doi: 10.1098/rstb.2013.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adhikari, A. K., Acharya, H. N., Ahmad, T., Shrestha S. Innovative sanitation social movement: experiences from Nepal. Loughborough University. Conference contribution. https://hdl.handle.net/2134/31419 (2017).
- 41.Cunningham K, et al. Suaahara in Nepal: An at-scale, multi-sectoral nutrition program influences knowledge and practices while enhancing equity. Matern. Child Nutr. 2017;13:1. doi: 10.1111/mcn.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adhikari D, Khatri RB, Paudel YR, Poudyal AK. Factors Associated with Underweight among Under-Five Children in Eastern Nepal: Community-Based Cross-sectional Study. Front. Public Health. 2017;5:350. doi: 10.3389/fpubh.2017.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Routray P, Torondel B, Clasen T, Schmidt WP. Women's role in sanitation decision making in rural coastal Odisha, India. . PLoS ONE. 2017;12:e0178042. doi: 10.1371/journal.pone.0178042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Briceno B, Coville A, Gertler P, Martinez S. Are there synergies from combining hygiene and sanitation promotion campaigns: Evidence from a large-scale cluster-randomized trial in rural Tanzania. PLoS ONE. 2017;12:e0186228. doi: 10.1371/journal.pone.0186228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Czerniewska A, Muangi WC, Aunger R, Massa K, Curtis V. Theory-driven formative research to inform the design of a national sanitation campaign in Tanzania. PLoS ONE. 2019;14:e0221445. doi: 10.1371/journal.pone.0221445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shrestha, R. Knowledge, Attitude and Practice on Antibiotics Use and its Resistance Among Medical Students in A Tertiary Care Hospital. J. Nepal Med. Assoc.57, 74–79 (2019). [DOI] [PMC free article] [PubMed]
- 47.Tshokey T, Adhikari D, Tshering T, Wangmo S, Wangdi K. Assessing the Knowledge, Attitudes, and Practices on Antibiotics Among the General Public Attending the Outpatient Pharmacy Units of Hospitals in Bhutan: A Cross-Sectional Survey. Asia Pac. J. Public Health. 2017;29:580–588. doi: 10.1177/1010539517734682. [DOI] [PubMed] [Google Scholar]
- 48.Acharya KP, Wilson RT. Antimicrobial Resistance in Nepal. . Front. Med. (Lausanne) 2019;6:105. doi: 10.3389/fmed.2019.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Basnyat B, Pokharel P, Dixit S, Giri S. Antibiotic Use, Its Resistance in Nepal and Recommendations for Action: A Situation Analysis. J. Nepal Health Res. Counc. 2015;13:102–111. [PubMed] [Google Scholar]
- 50.Karki, K. B. et al. Quality of Drugs and Drug Use Patterns at Different Level of Health Care Settings in Nepal, 2016 (Nepal Health Research Council, Kathmandu, 2017). [DOI] [PubMed]
- 51.Engberg, J., Neimann, J., Nielsen, E. M., Aerestrup, F. M. & Fussing, V. Quinolone-resistant Campylobacter infections: risk factors and clinical consequences. Emerg. Infect. Dis.10, 1056–1063. 10.3201/eid1006.030669 (2004). [DOI] [PMC free article] [PubMed]
- 52.Adhikari B, et al. Treatment-seeking behaviour for febrile illnesses and its implications for malaria control and elimination in Savannakhet Province, Lao PDR (Laos): a mixed method study. BMC Health Serv. Res. 2019;19:252. doi: 10.1186/s12913-019-4070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shankar P. Brain drain and practice locations of Nepalese medical students. Janaki Med. Coll. J. Med. Sci. 2018;5(2):1–4. doi: 10.3126/jmcjms.v5i2.19010. [DOI] [Google Scholar]
- 54.Adhikari B, et al. Earthquakes, Fuel Crisis, Power Outages, and Health Care in Nepal: Implications for the Future. Disaster Med. Public. Health Prep. 2017;11:625–632. doi: 10.1017/dmp.2016.195. [DOI] [PubMed] [Google Scholar]
- 55.Marahatta SB, et al. Barriers in the access, diagnosis and treatment completion for tuberculosis patients in central and western Nepal: a qualitative study among patients, community members and health care workers. PLoS ONE. 2020;15:e0227293. doi: 10.1371/journal.pone.0227293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghimire U, et al. Health system's readiness to provide cardiovascular, diabetes and chronic respiratory disease related services in Nepal: analysis using 2015 health facility survey. BMC Public Health. 2020;20:1163. doi: 10.1186/s12889-020-09279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walson JL, Marshall B, Pokhrel BM, Kafle KK, Levy SB. Carriage of antibiotic-resistant fecal bacteria in Nepal reflects proximity to Kathmandu. J. Infect. Dis. 2001;184:1163–1169. doi: 10.1086/323647. [DOI] [PubMed] [Google Scholar]
- 58.Grigoryan L, et al. Is self-medication with antibiotics in Europe driven by prescribed use? J. Antimicrob. Chemother. 2007;59:152–156. doi: 10.1093/jac/dkl457. [DOI] [PubMed] [Google Scholar]
- 59.Agarwal S, Yewale VN, Dharmapalan D. Antibiotics Use and Misuse in Children: A Knowledge, Attitude and Practice Survey of Parents in India. J. Clin. Diagn. Res. 2015;9:21–24. doi: 10.7860/JCDR/2015/14933.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yezli S, et al. Knowledge, attitude and practice (KAP) survey regarding antibiotic use among pilgrims attending the 2015 Hajj mass gathering. Travel Med. Infect. Dis. 2019;28:52–58. doi: 10.1016/j.tmaid.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 61.You JH, et al. Public knowledge, attitudes and behavior on antibiotic use: a telephone survey in Hong Kong. Infection. 2008;36:153–157. doi: 10.1007/s15010-007-7214-5. [DOI] [PubMed] [Google Scholar]
- 62.Ling Oh, A. , et al. Public knowledge and attitudes towards antibiotic usage: a cross-sectional study among the general public in the state of Penang, Malaysia. J. Infect. Dev. Ctries. 2011;5:338–347. doi: 10.3855/jidc.1502. [DOI] [PubMed] [Google Scholar]
- 63.Kim SS, Moon S, Kim EJ. Public knowledge and attitudes regarding antibiotic use in South Korea. J. Korean Acad. Nurs. 2011;41:742–749. doi: 10.4040/jkan.2011.41.6.742. [DOI] [PubMed] [Google Scholar]
- 64.Jose J, Jimmy B, Alsabahi AG, Al Sabei GA. A study assessing public knowledge, belief and behavior of antibiotic use in an omani population. Oman Med. J. 2013;28:324–330. doi: 10.5001/omj.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mitsi G, Jelastopulu E, Basiaris H, Skoutelis A, Gogos C. Patterns of antibiotic use among adults and parents in the community: a questionnaire-based survey in a Greek urban population. Int. J. Antimicrob. Agents. 2005;25:439–443. doi: 10.1016/j.ijantimicag.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 66.Pavyde E, et al. Public Knowledge, Beliefs and Behavior on Antibiotic Use and Self-Medication in Lithuania. Int. J. Environ. Res. Public Health. 2015;12:7002–7016. doi: 10.3390/ijerph120607002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sarraf, D. P., Rai, D. & Rauniar, G. P. Attitude and Practices on Antibiotic use and resistance among doctors in B.P. Koirala Institute Of Health Sciences. J. Drug Deliv. Ther.8(4), 170–175. 10.22270/jddt.v8i4.1753 (2018).
- 68.Nair M, et al. Knowledge, attitudes, and practices related to antibiotic use in Paschim Bardhaman District: A survey of healthcare providers in West Bengal, India. . PLoS ONE. 2019;14(5):e0217818. doi: 10.1371/journal.pone.0217818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abdel Wahed WY, Ahmed EI, Hassan SK, et al. Physicians’ knowledge, attitudes, and practice concerning antimicrobial resistance & prescribing: a survey in Fayoum Governorate, Egypt. . J. Public Health (Berl.) 2020;28:429–436. doi: 10.1007/s10389-019-01027-x. [DOI] [Google Scholar]
- 70.Kalungia AC, et al. Antimicrobial stewardship knowledge and perception among physicians and pharmacists at leading tertiary teaching hospitals in Zambia: implications for future policy and practice. J. Chemother. 2019;31:378–387. doi: 10.1080/1120009X.2019.1622293. [DOI] [PubMed] [Google Scholar]
- 71.Faizullah MRN, Umar MI, Anwar M, Sarfraz M. A cross-sectional study on knowledge, attitude and practices of medical doctors towards antibiotic prescribing patterns and resistance in Khyber Pakhtun Khawah, Pakistan. . J. Appl.Pharm. Sci. 2017;7(12):38–46. doi: 10.7324/JAPS.2017.71205. [DOI] [Google Scholar]
- 72.Srinivasan A, et al. A survey of knowledge, attitudes, and beliefs of house staff physicians from various specialties concerning antimicrobial use and resistance. Arch. Intern. Med. 2004;164:1451–1456. doi: 10.1001/archinte.164.13.1451. [DOI] [PubMed] [Google Scholar]
- 73.Acharya, K.P., Karki, S., Shrestha, K., Kaphle, K. One health approach in Nepal: Scope, opportunities and challenges. One Health12(8), 100101, 10.1016/j.onehlt.2019.100101 (2019). [DOI] [PMC free article] [PubMed]
- 74.Nirmal, B. K. Sectoral response to AMR containment: animal health. In Paper presented at One Health and Antibiotic Awareness Workshop. Available online at: https://apua.org/nepal%20. Accessed 7 Sept 2020 (2017).
- 75.Subedi, MS. Healer choice in medically pluralistic cultural settings: an overview of Nepali medical pluralism, Occasional. Papers in Sociol. Anthropol.8, 128–158. 10.3126/opsa.v8i0.1125 (2003).
- 76.Wasti SP, Simkhada P, Randall J, Freeman JV, van Teijlingen E. Factors influencing adherence to antiretroviral treatment in Nepal: a mixed-methods study. PLoS ONE. 2012;7:e35547. doi: 10.1371/journal.pone.0035547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adhikari B, Mishra SR. Urgent need for reform in Nepal's medical education. Lancet. 2016;388:2739–2740. doi: 10.1016/S0140-6736(16)32423-0. [DOI] [PubMed] [Google Scholar]
- 78.Ghimire U, Shrestha N, Adhikari B, et al. Health system’s readiness to provide cardiovascular, diabetes and chronic respiratory disease related services in Nepal: analysis using 2015 health facility survey. BMC Public Health. 2020;20:1163. doi: 10.1186/s12889-020-09279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu C, Liu C, Wang D, Zhang X. Knowledge, attitudes and intentions to prescribe antibiotics: a structural equation modeling study of primary care institutions in Hubei, China. Int. J. Environ. Res. Public Health. 2019;16:1. doi: 10.3390/ijerph16132385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barker AK, Brown K, Ahsan M, Sengupta S, Safdar N. Social determinants of antibiotic misuse: a qualitative study of community members in Haryana, India. . BMC Public Health. 2017;17:333. doi: 10.1186/s12889-017-4261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nepal A, Hendrie D, Selvey LA, Robinson S. Factors influencing the inappropriate use of antibiotics in the Rupandehi district of Nepal. Int. J. Health Plann. Manag. 2020 doi: 10.1002/hpm.3061. [DOI] [PubMed] [Google Scholar]
- 82.World Health Organization. The role of education in the Rational Use of Medicines (No. Technical Publication No. 45). WHO Regional Office for South-East Asia. Acessed Aug 2020 (2006).
- 83.Jha, N., Shrestha, S., Shankar, P. R. & Bhandary, S. Knowledge, attitude and practice about antibiotic use, self-medication and antibiotic resistance among final year medical students and interns at a medical college in Lalitpur, Nepal. J. Chitwan Med. Coll.10(33), 69–73 (2020).
- 84.Machowska A, Stalsby Lundborg C. Drivers of Irrational Use of Antibiotics in Europe. Int. J. Environ. Res. Public Health. 2018;16:1. doi: 10.3390/ijerph16010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pokharel S, Shrestha P, Adhikari B. Antimicrobial use in food animals and human health: time to implement ‘One Health’ approach. Antimicrob. Resist. Infect. Control. 2020;9(181):2020. doi: 10.1186/s13756-020-00847-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.