Abstract
Purpose
There is a growing need to use green and efficient larvicidal as alternatives for conventional chemicals in vector control programs. Nanotechnology has provided a promising approach for research and development of new larvicides. Larvicidal potential of a nanoemulsion of Cinnamomum zeylanicum essential oil reports against Anopheles stephensi.
Methods
The nanoemulsion of was formulated in various ratios comprising of C. zeylanicum oil, tween 80, span 20 and water by stirrer. It was characterized by transmission electron microscopy (TEM) and dynamic light scattering (DLS). All components of C. zeylanicum essential oil were identified by GC–MS analysis. The larvicidal potential of the oil and its nanoformulation were evaluated against larvae of An. stephensi. The stability and durability of nanoemulsion was observed over a period of time.
Results
Sixty one components in the oil were identified, cinnamaldehyde (56.803%) was the main component. The LC90 and LC50 values of C. zeylanicum essential oil were calculated as 49 ppm and 37 ppm, respectively. The nanoemulsion droplets were found spherical in shape. It was able to kill 100% of larvae in up to 3 days. It was stable after dilution and increased its larvicidal activity up to 32% compared with the essential oil.
Conclusions
A novel larvicide based on nanotechnology introduced. This experiment clearly showed increasing larvicidal activity and residual effect of the nanoformulation in comparison with the bulk essential oil. It could be concluded that this nanoemulsion may be considered as safe larvicide and should be subject of more research in this field.
Graphical abstract
Keywords: Cinnamomum zeylanicum, Nanoemulsion, Larvicidal activity, Anopheles stephensi
Introduction
Vector-borne diseases (VBDs) are infection diseases caused by parasites, viruses or bacteria that are transmitted by vectors, such as human malaria which is transmitted by Anopheline mosquitoes. Malaria is still one of the major health problems worldwide. In 2019, World Health Organization reported approximately 229 million cases of malaria and approximately 409,000 deaths from malaria [1]. Vector control is the principal method for controlling many VBDs. Fighting against mosquito vectors in the larval stage is one of the important strategies endorsed by WHO. Besides, for some VBDs such as dengue fever, the only way to control the diseases is to control their vectors [2]. Epidemiological variables of malaria are associated with high genetic diversity of parasites, rapid incidence of vector insecticides and dispersal of vector species that make Anopheles vector control challenging. Anopheles stephensi is one of the major malaria vectors in the Middle East and South Asia. Recently An. stephensi has expanded its distribution to Djibouti, Ethiopia and Sudan in Africa and Sri Lanka in Asia. This is a major public health issue and WHO considers it as a significant threat to malaria control and elimination in Africa and southern Asia [3].
Synthetic chemical-based insecticides are being frequently applied to control vectors of malaria. The emergence of insecticide-resistance of An. stephensi is now becoming a key challenge in controlling the spread of this vector [4]. Insecticides can contaminate environment in different ways and pollute various sources such as soil and water. In order to prevent environmental pollution by chemical larvicides, other strategies to control vectors are being considered. Essential oils (EOs) as natural products are considered safe alternatives to synthetic pesticides as they are commonly safe for mammals and have short environmental persistence. However, low water solubility and low residual effect of EOs has provided rationale for development of efficient formulations such as nanoemulsions [5]. Nanoemulsions are mainly used in many industries today due to their special size, transparent or semi-transparent appearance, droplet size distribution, low viscosity and high stability against the phenomena of sedimentation, creaming, coalescence and clotting [6, 7]. In recent years, nanotechnology has accounted for a large share of global trade in various fields. Eliminating water pollution with the help of nanostructures is an environmentally friendly method [8]. Nanoemulsions as nanoactivators are of great importance in pharmaceutical, health, and cosmetic formulations. They are useful in controlling and disseminating the drug, releasing the active ingredients throughout the skin, targeting the drug in specific parts of the body, receiving vaccines, gene carriers, and intravenous actions, due to the precise goals in the implementation route. They are also suitable for drug delivery or DNA plasmids through the skin after dermal application. Due to the proper release of fragrances, they are also used in the formulation of alcohol-free perfumes. In the chemical industry, they are used as a polymerization reaction medium. Nanoemulsions with the ability to increase the solubility of water-insoluble pesticides are also used in the agrochemical industry [9–18]. A higher degree of delivery to the target of site, low preparation costs and lower ecological toxicity make nanoformulations as ideal formulation of EOs [5, 19].
In the field of vector control, nanotechnology providing promising larvicidal agents. So far, about 2000 plant species belonging to different families have been identified as having insecticidal properties, but essential oils and extracts have volatile components that limit their use in natural environments [20–22]. Similarly, different properties of extracts and EOs for insect control have been demonstrated in Iran [23–28]. Regrettably, the larvicidal power of extracts and EOs are generally lower than synthetic larvicides. Recently, by formulating them as nanomaterials, attempts have been made to reduce their volatility and increase their residual effects. Several studies have been conducted on extracts and EOs as an important class of natural larvicides [29–32], but there are few available articles on nanoemulsions as larvicides.
Cinnamomum zeylanicum (Family: Lauraceae), popularly known as cinnamon, is a common spice used in foods and pharmaceuticals since antiquity. EO of cinnamon bark and leaf is widely used in food flavors, cosmetics and pharmaceuticals [33], which have antibacterial, antifungal, antidiabetic and antioxidant properties [34]. The purpose of this study was to investigate the larvicides effect C. zeylanicum essential oil (CEO), prepare C. zeylanicum nanoemulsion (CNE) larvicides and evaluate the durability and stability of the larvicides properties of CNE as natural and eco-friendly product.
Materials and methods
Materials and equipment
The raw materials and equipment used in the study are shown in Table 1. CEO (100% purity) obtained from Green Plants of Life Co (Iran) with no addition of any other contents. EOs are usually degraded by oxidation, polymerization, hydrolysis and intermolecular reactions, these can be accelerated by the presence of heat, oxygen, moisture, or the presence of metals. To prevent these changes, the essential oil was stored in dark glass containers, away from sunlight at 4–8 °C.
Table 1.
Materials and equipment used in the study
| Materials & Instruments | Producer | Country |
|---|---|---|
| Cinnamomum zeylanicum Oil | Green Plants of Life | Iran |
| Tween 80 | Merck | Germany |
| Span 20 | Merck | Germany |
| Ethanol | Merck | Germany |
| DLS | K-ONE.LTD | Korea |
| GC- MS | Agilent Tecnology | US |
| TEM | Zeiss | Germany |
| Centrifuge | Eppendrof | Germany |
| Magnetic Stirrer | DOMEL | Slovenia |
Mosquito raring
Anopheles stephensi larvae were used in this study. They were reared in the insectary at 29 ± 1 °C (by electric heater) with relative humidity of 70 ± 5% (by steamer) under 12 h lightness/12 h darkness (by timer lamp). The adult and maintenance cages were 30 cm by 30 cm by 30 cm high, the floor was wooden and other sides were nylon mesh. The stock culture of adult An. stephensi was fed twice a week with artificial feeding on sheep blood. The defibrillated blood of the sheep was poured into a glass and Parafilm was placed on it and put upside down on the cage. To adjust the temperature, a container of hot water was placed on it. The egg rafts laid were then transferred to enamel larval trays. The larvae were fed with fish flakes.
Determining the larvicidal effect of CEO and CNE
Larvicidal bioassay were performed according to the WHO guideline [35]. Logarithmic concentrations were prepared from essential oil and nanoemulsion of essential oil by dissolving in ethanol. In all tests, 3rd or 4th early instar larvae were used that had been eaten the day before. During the test no food was given to the larvae. In the tests, water at room temperature, PH = 7 and chlorine-free was used. One ml of diluted essential oil or nanoemulsion essential oil was added to 249 ml of water and stirred, 25 healthy larvae were added to each container. The number of living and dead larvae was counted 24 h after the start of the assay.
Determining the durability of larvicidal CEO and CNE
In the long-time test, 1 ml of essential oil or nanoemulsion essential oil was added to 249 ml of water, then 25 live larvae were added to solution. After 24 h and observing the test results, without changing the solution, the larvae (both dead and live) were removed from the containers and 25 new live larvae were added to the containers. The larvae were exchanged for up to 8 days. The tests were performed in 16 repetitions at 4 different replicates. In each replicate 2 control groups were considered that ethanol added to those with similar mentioned manner.
Statistical analysis
Mortality rate data of An. stephensi larvae were subjected to logarithmic concentrations of CEO by probit regression analysis. Lethal concentrations of 50% and 90% (LC50 and LC90) were calculated using probit analysis [36, 37], the regression line was plotted using Excel 2007 software.
If the control group mortality was less than 5%, the data from the bioassay tests were considered correct, when the control mortality was between 5% to 20%, it was corrected using the Abbott formula [36]. If the larvae became pupae or the larvae mortality were more than 20% in the control group, the test was repeated.
Preparation of nanoemulsion
In this study, a spontaneous method was used to prepare nanoemulsion. First, surfactant (Tween 80) and co-surfactant (Span 20) were mixed thoroughly. The essential oil was then added to obtain final concentration of 90% lethal concentration and stirred for 10 min at 600 rpm. Afterwards, water was added dropwise and stirred at 600 rpm for 38 min. Different concentrations of surfactant and co-surfactant at constant essential oil concentration were prepared and stored in a dark place at room temperature for 24 h. Dispersions were visually checked for any sign of phase separation, precipitation or creaming. Dynamic light scattering (DLS), K-ONE. LTD, (Korea) was used to determine the particle size (PS) of the prepared nanoformulations. Transmission electron microscopy (TEM) was used to confirm the PS and to investigate the morphology of the particles.
Analysis of essential oil by gas chromatography–mass spectrometry (GC-MS)
Components of CEO were identified by GC–MS analysis. The GC device isolates volatile compounds, and the mass spectrometer helps identify each of the separated components based on their mass properties with high accuracy. CEO was diluted using hexane and after finding the most suitable thermal programming of the column, it was injected into the apparatus and mass spectrums and chromatograms were obtained. GC–MS was performed on HP (Agilent Technology) 7890A Network GC System connected with a 5975C VL MSD with Triple-Axis Detector. CEO was analyzed using HP-5MS Fused silica column (Length: 30 m, I.D.: 0.250 mm& Film thickness: 25 μm). The GC–MS settings were programmed as follows; initial oven temperature was held at 40 °C for 1 min, rising to 250 °C at 3 °C/min. The injector temperature was maintained at 270 °C. Detector temperature was at 230 °C. Carrier gas used was helium (He 99.999%) at a flow rate of 1 mL/min. The constituents of the essential oil were analyzed using retention indices mass spectra of the compounds and compared with the standard mass spectra available in the device library and authoritative references. The linear temperature programmed retention indices (RIs) of all the constituents were calculated from the gas chromatogram by interpolation between bracketing n-alkanes (Eq. 1) [38].
| 1 |
Where z is the number of carbon atoms in the smaller n-alkane, and tR(i), tR(z) and t are the retention times of the desired compound, the smaller n-alkane, and the larger n-alkane, respectively. In addition, the search match factor (SMF), rank number (RN) in the mass library, and five highest peaks in the mass spectra were prepared and used for identification of the components.
Results
Larvicidal bioassay of CEO
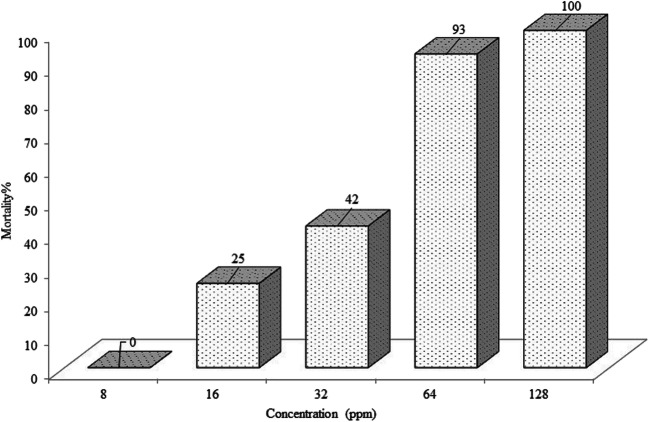
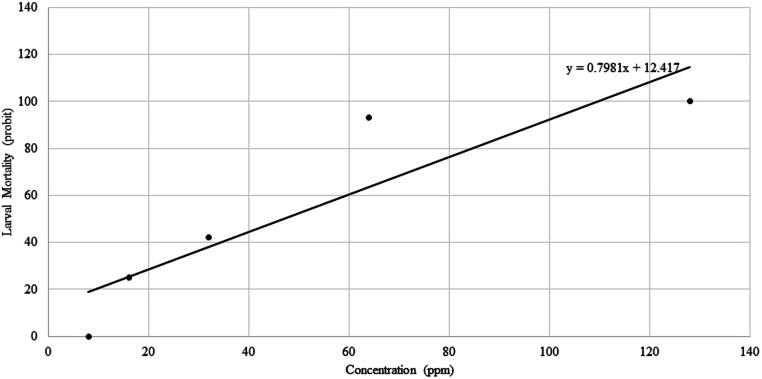
The larvicide results of 5 different concentrations of CEO are shown in Fig. 1. Sample containing 128 ppm CEO was found to be the most toxic with 100% larval mortality. There was no mortality in the control groups. In regression line a positive correlation was observed between CEO concentrations and the probit mortality (Fig. 2). The LC90 and LC50 values of CEO against An. stephensi larvae were calculated as 49 ppm and 37 ppm, respectively.
Fig. 1.
Larvicidal activity of CEO against An. stephensi
Fig. 2.
Probit regression line of An. stephensi larvae exposed to different interval concentrations of CEO
Yields and chemical constituents of CEO
The yield of CEO was 0.5% (v/w) based on fresh weight. CEO was yellowish with a distinct sharp odor. Sixty one components were determined by GC–MS analysis, with five major components of CEO including Cinnamaldehyde (56.803%), Linalool (6.310%), dl-Limonene (6.237%), trans-Caryophyllene (4.697%) and Eugenol (3.895%) as listed in Table 2.
Table 2.
Chemical composition of the CEO
| NO. | Retention time (RT) (min) | Compound | Peak area | % | Quality | Mol Weight (amu) |
|---|---|---|---|---|---|---|
| 1 | 25.606 | Cinnamaldehyde | 1,511,649,639 | 56.803 | 97 | 132.058 |
| 2 | 17.157 | Linalool | 167,945,919 | 6.311 | 97 | 154.136 |
| 3 | 13.664 | dl-Limonene | 165,991,974 | 6.237 | 99 | 136.125 |
| 4 | 31.268 | trans-Caryophyllene | 125,002,534 | 4.697 | 99 | 204.188 |
| 5 | 28.889 | Eugenol | 103,671,489 | 3.896 | 98 | 164.084 |
| 6 | 32.496 | trans-Cinnamyl acetate | 98,266,329 | 3.693 | 97 | 176.084 |
| 7 | 44.469 | Benzyl benzoate | 81,272,658 | 3.054 | 97 | 212.084 |
| 8 | 13.448 | Carvacrol | 72,963,965 | 2.742 | 97 | 134.11 |
| 9 | 26.77 | Cinnamyl alcohol | 49,436,677 | 1.858 | 98 | 134.073 |
| 10 | 14.199 | Benzenemethanol | 40,704,915 | 1.530 | 96 | 108.058 |
| 11 | 32.617 | alpha-Humulene | 19,556,247 | 0.735 | 99 | 204.188 |
| 12 | 11.228 | beta-Pinene | 18,802,634 | 0.707 | 97 | 136.125 |
| 13 | 21.267 | alpha-Terpineol | 16,495,180 | 0.620 | 91 | 154.136 |
| 14 | 9.427 | alpha-Pinene | 16,474,543 | 0.619 | 96 | 136.125 |
| 15 | 13.741 | 1,8-Cineole | 15,409,268 | 0.579 | 99 | 154.136 |
| 16 | 34.328 | Zingiberene | 11,565,807 | 0.435 | 96 | 204.188 |
| 17 | 19.473 | l-Menthone | 10,517,354 | 0.395 | 98 | 154.136 |
| 18 | 22.641 | trans-Cinnamaldehyde | 9,721,905 | 0.365 | 97 | 132.058 |
| 19 | 10.025 | Camphene | 9,579,001 | 0.360 | 98 | 136.125 |
| 20 | 37.757 | Caryophyllene oxide | 9,123,643 | 0.343 | 91 | 220.183 |
| 21 | 19.651 | Isoborneol | 7,792,915 | 0.293 | 97 | 154.136 |
| 22 | 33.819 | Benzene | 5,397,789 | 0.203 | 99 | 202.172 |
| 23 | 15.013 | gamma-Terpinene | 5,391,389 | 0.203 | 96 | 136.125 |
| 24 | 26.013 | trans-Cinnamaldehyde | 5,112,899 | 0.192 | 96 | 132.058 |
| 25 | 25.854 | trans-Cinnamaldehyde | 5,110,766 | 0.192 | 96 | 132.058 |
| 26 | 26.312 | Thymol | 4,587,550 | 0.172 | 86 | 150.104 |
| 27 | 19.008 | 3-Benzylidenecamphor | 4,545,271 | 0.171 | 98 | 152.12 |
| 28 | 34.843 | beta-Bisabolene | 4,451,083 | 0.167 | 97 | 204.188 |
| 29 | 18.614 | Dihydrolinalool | 4,391,505 | 0.165 | 86 | 156.151 |
| 30 | 35.448 | beta-Sesquiphellandrene | 4,340,898 | 0.163 | 98 | 204.188 |
| 31 | 21.585 | Isoterpinolene | 4,267,300 | 0.160 | 94 | 136.125 |
| 32 | 18.748 | trans-Limonene oxide | 3,764,993 | 0.141 | 91 | 152.12 |
| 33 | 11.947 | beta-Myrcene | 3,592,467 | 0.135 | 96 | 136.125 |
| 34 | 18.531 | Limonene oxide | 3,503,639 | 0.132 | 96 | 152.12 |
| 35 | 27.432 | 2-Propenal, 2-methyl-3-phenyl- | 3,379,580 | 0.127 | 70 | 146.073 |
| 36 | 20.421 | l-Menthol | 3,283,274 | 0.123 | 91 | 156.151 |
| 37 | 10.706 | Benzaldehyde | 3,241,273 | 0.122 | 94 | 106.042 |
| 38 | 44.291 | Cedryl acetate | 2,772,151 | 0.104 | 91 | 264.209 |
| 39 | 11.126 | Sabinene | 2,627,279 | 0.099 | 96 | 136.125 |
| 40 | 29.378 | alpha-Copaene | 2,552,346 | 0.096 | 99 | 204.188 |
| 41 | 23.684 | Kurarinone | 2,344,028 | 0.088 | 96 | 150.104 |
| 42 | 20.096 | 1-Borneol | 2,319,219 | 0.087 | 86 | 154.136 |
| 43 | 12.481 | l-Phellandrene | 2,120,629 | 0.080 | 94 | 136.125 |
| 44 | 40.327 | Benzyl ether | 1,645,951 | 0.062 | 83 | 198.104 |
| 45 | 21.451 | trans-Caryophyllene | 1,526,557 | 0.057 | 64 | 122.11 |
| 46 | 19.963 | Menthone | 1,333,607 | 0.050 | 96 | 154.136 |
| 47 | 20.599 | Terpinen-4-ol | 1,223,776 | 0.046 | 96 | 154.136 |
| 48 | 8.937 | Tricyclene | 1,001,303 | 0.038 | 96 | 136.125 |
| 49 | 31.567 | Aldrichimica acta | 1,001,002 | 0.038 | 38 | 120.094 |
| 50 | 13.041 | alpha-Terpinene | 984,871 | 0.037 | 98 | 136.125 |
| 51 | 11.851 | 5-Hepten-2-one, 6-methyl- | 918,811 | 0.035 | 95 | 126.104 |
| 52 | 30.683 | trans-Caryophyllene | 817,896 | 0.031 | 50 | 204.188 |
| 53 | 23.449 | Pulegone | 777,614 | 0.029 | 97 | 152.12 |
| 54 | 19.123 | Limonene oxide | 754,831 | 0.028 | 53 | 154.136 |
| 55 | 52.033 | alpha-Thujene | 750,049 | 0.028 | 50 | 136.125 |
| 56 | 55.793 | 1,5-Bis[dimethyl(chloromethyl)silyloxy]pentane | 691,891 | 0.026 | 37 | 316.085 |
| 57 | 28.259 | alpha-Cubebene | 612,257 | 0.023 | 86 | 204.188 |
| 58 | 42.522 | Cyclopropa | 570,689 | 0.021 | 64 | 204.151 |
| 59 | 17.991 | cis-Verbenol | 565,783 | 0.021 | 52 | 152.12 |
| 60 | 9.173 | Bicyclo | 533,073 | 0.020 | 94 | 136.125 |
| 61 | 41.937 | Nonadecane | 468,792 | 0.018 | 91 | 268.313 |
Determination of droplet size and morphology of CNE
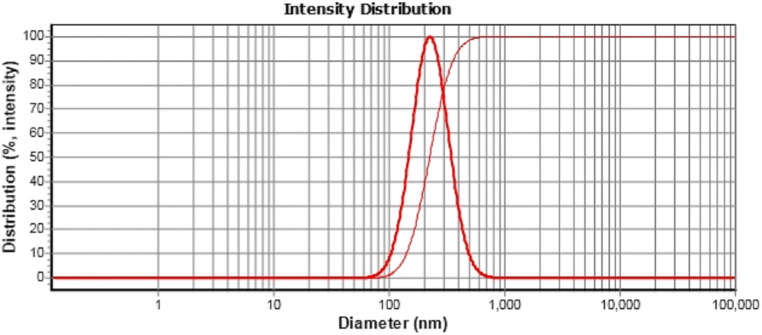
Based on previous studies, of the 10 nanoformulations made (Table 3) and determine the particle size (PS) of the prepared nanoformulations with using DLS device. The formulation with d50 of less than 200 nm, d90 of less than 400 nm and span of less than 1 was the best nanoformulation (F6 of CNE) for the next steps. Figure 3 illustrates DLS of selected samples.
Table 3.
The amount of ingredients used in making nanoemulsion of bitter C. zeylanicum essential oil and choosing the best nanoformulation based on the results of DLS (d50: The size at which 50% of the particles are smaller, d10:The size at which 10% of the particles are smaller, d90:The size at which 90% of the particles are smaller and span=d90 − d10/d50
| Formulations | C. zeylanicum essential oil | TW80 (μl) | Span (μl) |
Water (μl) |
d50 (μl) |
d10 (μl) |
d90 (μl) |
Span (μl) |
|---|---|---|---|---|---|---|---|---|
| F1 | 100 | 100 | 50 | 4750 | 9.89 | 4.12 | 30 | 2.616 |
| F2 | 100 | 150 | 50 | 4700 | 2.58 | 1.36 | 8.46 | 2.75 |
| F3 | 100 | 200 | 50 | 4650 | 24 | 5.35 | 45.5 | 1.67 |
| F4 | 100 | 250 | 50 | 4600 | 5.18 | 1.56 | 12.2 | 2.05 |
| F5 | 100 | 300 | 50 | 4550 | 6.16 | 1.97 | 20.2 | 2.95 |
| F6 | 100 | 350 | 50 | 4500 | 220 | 143 | 359 | 0.96 |
| F7 | 100 | 400 | 50 | 4450 | diphasic | diphasic | diphasic | diphasic |
| F8 | 100 | 450 | 50 | 4400 | diphasic | diphasic | diphasic | diphasic |
| F9 | 100 | 500 | 50 | 4350 | diphasic | diphasic | diphasic | diphasic |
| F10 | 100 | 550 | 50 | 4300 | diphasic | diphasic | diphasic | diphasic |
Fig. 3.
DLS results of nanoemulsion containing C. zeylanicum essential oil
F6 formulation of CNE, which was selected as the best nanoformulation based on particle size results, was prepared using transition electron microscopy (TEM) to image the morphology of their particles. The results showed that CNE was made correctly (Fig. 4).
Fig. 4.
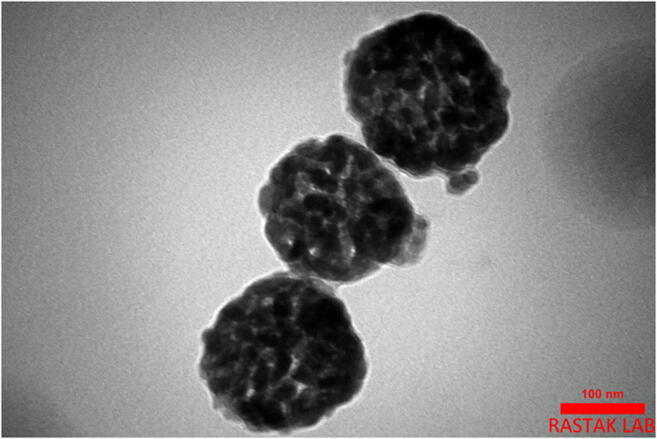
Transition electron microscopy (TEM) image of the nanoemulsion particles
Nanoemulsion physical stability
The nanoemulsion was stored at refrigerator temperature (4 °C) and at room temperature for 4 months. No phase separation or creaming was observed. No change was observed even after centrifugation.
Larvicidal effects of CNE and CEO
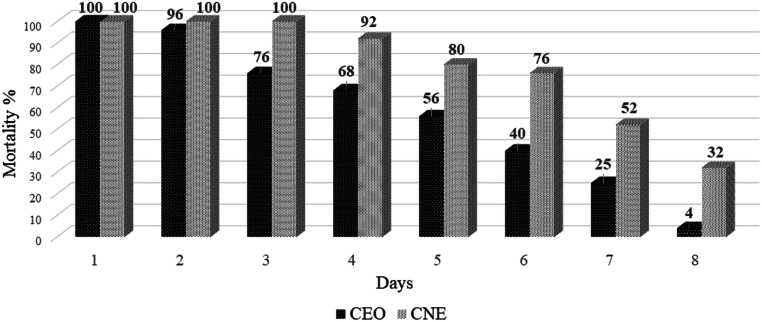
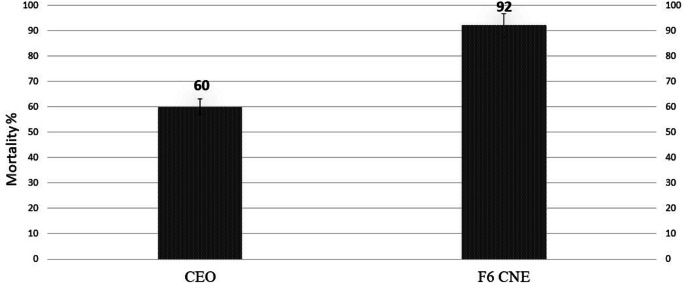
Figure 5 indicates the residual effect of CNE and CEO for 8 consecutive days. The nanoemulsion (CNE) was able to kill 100% of the larvae in the first three days of experiments. After the third day, the larval mortality decreased gradually, from 92% in fourth day, 80% in fifth day, 76% in sixth day and 52% in seventh day to finally 32% on the eighth day, respectively. The bulk of essential oil was able to control 100% of the larvae only for one day, the larval mortality decreased from 96% to 4% from the second day to the eighth day, respectively. Even at the end of the experiment the larval mortality due to CNE was eighth was 8-fold more than CEO. Although the same concentration of CEO and CNE in 24 h test were used, the larvicidal activity of CNE was stable after dilution and increased its larvicidal activity. Larvicidal effects of CEO was 60%, while it increased to 92% by CNE (Fig. 6).
Fig. 5.
Comparison of larvicidal activity of CEO vs. CNE in the long time (8 days)
Fig. 6.
Comparison of larvicidal activity of CEO vs. CNE in the short time (24 h)
Discussion
Nowadays, natural products are being considered for number of applications [39]. They provide green and efficient alternatives for vector control, having no harm to the nature. However, these substances are generally volatile and their effect is short lasting, therefore, their original forms should be formulated to be used as mosquito larvicides in the environment [28]. On the other hand, these products are not often water soluble, being usually much more soluble a second reason necessitating formulation of herbal extracts lese essential oils. This is made clear that nanotechnology can improve water solubility of these substances and increase their efficacy as larvicides [40].
EOs are complex mixtures that may contain over 300 different compounds [41]. They have a very high variability of their composition. The number of each compound of CEO in earlier studies were varied from 9 to 38 compounds [42–47], while we were able to identify 61 compounds of this essential oil, probably due to timely GC analysis used in this study.
The LC90 and LC50 values of CEO against An. stephensi were reported as 198 ppm and 55 ppm, respectively in pervious study [48] while the result of the present study is better than a result of previous report showing 49 ppm and 37 ppm, respectively. According to proposed categories of larvicidal activity of plant EOs against mosquito larvae, CEO lies in the third category as active plant [25].
To increase the stability of CEO in nature we used low temperature emulsification method for making the nanoemulsion. Nanoemulsions with a particle size of less than 20 nm have been reported to have better lactation power due to their improved penetration into the larval body [49, 50]. The larvicidal activity of the nanoemulsion is assumed due to the presence of cinnamaldehyde, which is the major component of the oil by GC–MS analysis. Although, the connections and interactions between all components of CEO need to be considered. This is the first study on nanoemulsion formulation using CEO as larvicide with span of less than 1 has been reported against An. stephensi. The results of this experiment clearly showed 32% increase in larvicidal effects of CNE in comparison with the bulk essential oil, which is probably due to droplet size distribution. CNE had a high residual effect for up to 72 h while it was reduced in bulk of essential oil after 24 h. A similar pattern of results was obtained by Osanloo et al. 2019, showing increased effect of their nanoformulation against An. stephensi from two to nine days [51]. Similarly Volpato et al. (2016) investigated the effect of essential oil and nanoemulsion of C. zeylanicum on mealworm (Alphitobius diaperinus). The nanoemulsion at concentration of 5% caused 70% mortality of the larvae after two days and had a three-fold more pronounced effect when compared to treatment with unemulsified essential oil within 3 days [52]. This is consistent with result was obtained by Balasubramani et al. (2017), their experiment showed increase in larvicidal activity of nanoformulation Vitexnegundo L. in comparison of bulk essential oil on Ae. aegypti [53]. A similar conclusion was reached by Sundararajan et al., they found that the nanoemulsion of Ocimum basilicum EO was more effective that the bulk essential oil on larvae of Cx. quinquefasciatus [54]. These findings are in agreement with the result of the present study, in which, there are worthy larvicidal and residual effects of prepared CNE against main malaria vector An. stephensi.We performed TEM as we have easier access to that instead of HRTEM. Although, it is appear that the data generated by the TEM is good enough for our study, it is recommended to use HRTEM in the future studies.
Conclusion
Our nanoemulsion larvicide based on C. zeylanicum essential oil was effective against An. stephensi. The result obtained successfully demonstrates the larvicidal activity, residual effect, and stability of C. zeylanicum nanoemulsion. The nanomodification significantly improves effectiveness of CNE as larvicide compared with bulk of the oil. EOs are rapidly volatile and low residual activity while, the nanomodification meaningfully increases the residual effect of CNE for up to 72 h. The residual efficacy of CNE shows this formulation is a good candidate for eco-friendly and safe larvicide and should be subject of more research in this field. Further research on natural products, especially essential oils to find the best candidate as larvicides is recommended. Future studies should focus on increasing residual efficacy of CNE through improvements in formulation technology. Moreover, conducting research on mode of action of such new formulations would help to better understanding on the biological effects and performance of nanoemulsion in the field of vector control. This approach may provide a solution to insecticide resistance problem of controlling vectors with no or minimal harm to environment.
Acknowledgments
This research has been supported by Tehran University of Medical Sciences & Health Services grant no. IR. TUMS. VCR. REC. 1397. 584.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. World malaria report 2020.
- 2.Beier JC, Keating J, Githure JI, Macdonald MB, Impoinvil DE, Novak RJ. Integrated vector management for malaria control. Malar J. 2008;7(S1):S4. doi: 10.1186/1475-2875-7-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organization WH. Vector alert: Anopheles stephensi invasion and spread: World Health Organization 2019.
- 4.Enayati A, Hanafi-Bojd AA, Sedaghat MM, Zaim M, Hemingway J. Evolution of insecticide resistance and its mechanisms in Anopheles stephensi in the WHO eastern Mediterranean region. Malar J. 2020;19(1):1–12. doi: 10.1186/s12936-020-03335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Echeverría J. Albuquerque RDDGd. Nanoemulsions of essential oils: New tool for control of vector-borne diseases and in vitro effects on some parasitic agents. Medicines. 2019;6(2):42. doi: 10.3390/medicines6020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutiérrez J, González C, Maestro A, Solè I, Pey C, Nolla J. Nano-emulsions: new applications and optimization of their preparation. Curr Opin Colloid Interface Sci. 2008;13(4):245–251. [Google Scholar]
- 7.Charoen R, Jangchud A, Jangchud K, Harnsilawat T, Decker EA, McClements DJ. Influence of interfacial composition on oxidative stability of oil-in-water emulsions stabilized by biopolymer emulsifiers. Food Chem. 2012;131(4):1340–1346. [Google Scholar]
- 8.Zinatloo-Ajabshir S, Baladi M, Salavati-Niasari M. Enhanced visible-light-driven photocatalytic performance for degradation of organic contaminants using PbWO4 nanostructure fabricated by a new, simple and green sonochemical approach. Ultrason Sonochem. 2021;72:105420. doi: 10.1016/j.ultsonch.2020.105420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jafari SM, He Y, Bhandari B. Production of sub-micron emulsions by ultrasound and microfluidization techniques. J Food Eng. 2007;82(4):478–488. [Google Scholar]
- 10.Solans C, Izquierdo P, Nolla J, Azemar N, Garcia-Celma MJ. Nano-emulsions. Curr Opin Colloid Interface Sci. 2005;10(3–4):102–110. [Google Scholar]
- 11.Cerqueira MÂPR, Pinheiro ACB, do Carmo CVS, Duarte CMM, da Cunha MdGC, de Oliveira Soares AAM. Nanostructured biobased systems for nutrient and bioactive compounds delivery. Nutrient Delivery. Elsevier; 2017. p. 43–85.
- 12.Mousavi-Kamazani M, Zinatloo-Ajabshir S, Ghodrati M. One-step sonochemical synthesis of Zn (OH) 2/ZnV 3 O 8 nanostructures as a potent material in electrochemical hydrogen storage. J Mater Sci Mater Electron. 2020;31(20):17332–17338. [Google Scholar]
- 13.Zinatloo-Ajabshir S, Ghasemian N, Mousavi-Kamazani M, Salavati-Niasari M. Effect of zirconia on improving NOx reduction efficiency of Nd2Zr2O7 nanostructure fabricated by a new, facile and green sonochemical approach. Ultrason Sonochem. 2021;71:105376. doi: 10.1016/j.ultsonch.2020.105376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghodrati M, Mousavi-Kamazani M, Zinatloo-Ajabshir S. Zn3V3O8 nanostructures: facile hydrothermal/solvothermal synthesis, characterization, and electrochemical hydrogen storage. Ceram Int. 2020;46(18):28894–28902. [Google Scholar]
- 15.Zinatloo AS, Taheri QN. Inverse miniemulsion method for synthesis of gelatin nanoparticles in presence of CDI/NHS as a non-toxic cross-linking system. 2014.
- 16.Zinatloo-Ajabshir S, Taheri QN. Effect of some synthetic parameters on size and polydispersity index of gelatin nanoparticles cross-linked by CDI/NHS system. J Nanostruct. 2015;5(2):137–144. [Google Scholar]
- 17.Zinatloo-Ajabshir S, Morassaei MS, Salavati-Niasari M. Simple approach for the synthesis of Dy2Sn2O7 nanostructures as a hydrogen storage material from banana juice. J Clean Prod. 2019;222:103–110. [Google Scholar]
- 18.Zinatloo-Ajabshir S, Morassaei MS, Amiri O, Salavati-Niasari M. Green synthesis of dysprosium stannate nanoparticles using Ficus carica extract as photocatalyst for the degradation of organic pollutants under visible irradiation. Ceram Int. 2020;46(5):6095–6107. [Google Scholar]
- 19.Duarte JL, Maciel De Faria Motta Oliveira AE, Pinto MC, Chorilli M. Botanical insecticide–based nanosystems for the control of Aedes (Stegomyia) aegypti larvae. Environ Sci Poll Res. 2020:1–12. [DOI] [PubMed]
- 20.Singh G, Kapoor I, Pandey S, Singh U, Singh R. Studies on essential oils: part 10; antibacterial activity of volatile oils of some spices. Phytotherapy Res: Int J Devoted Pharmacol Toxicol Eval Nat Product Derivatives. 2002;16(7):680–682. doi: 10.1002/ptr.951. [DOI] [PubMed] [Google Scholar]
- 21.Edris AE. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytotherapy Res: Int J Devoted Pharmacol Toxicol Eval Nat Product Derivatives. 2007;21(4):308–323. doi: 10.1002/ptr.2072. [DOI] [PubMed] [Google Scholar]
- 22.Piplani M, Bhagwat DP, Singhvi G, Sankaranarayanan M, Balana-Fouce R, Vats T, Chander S. Plant-based larvicidal agents: an overview from 2000 to 2018. Exp Parasitol. 2019;199:92–103. doi: 10.1016/j.exppara.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 23.Medhi SM, Reza S, Mahnaz K, Reza AM, Abbas H, Fatemeh M, Hassan V. Phytochemistry and larvicidal activity of Eucalyptus camaldulensis against malaria vector, Anopheles stephensi. Asian Pac J Trop Med. 2010;3(11):841–845. [Google Scholar]
- 24.Sedaghat M, Dehkordi AS, Abai M, Khanavi M, Mohtarami F, Abadi YS, Rafi F, Vatandoost H. Larvicidal activity of essential oils of Apiaceae plants against malaria vector, Anopheles stephensi. Iran J Arthropod Borne Dis. 2011;5(2):51–59. [PMC free article] [PubMed] [Google Scholar]
- 25.Vatandoost H, Dehkordi AS, Sadeghi S, Davari B, Karimian F, Abai M, et al. Identification of chemical constituents and larvicidal activity of Kelussia odoratissima Mozaffarian essential oil against two mosquito vectors Anopheles stephensi and Culex pipiens (Diptera: Culicidae) Exp Parasitol. 2012;132(4):470–474. doi: 10.1016/j.exppara.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Sedaghat MM, Dehkordi AS, Khanavi M, Abai MR, Mohtarami F, Vatandoost H. Chemical composition and larvicidal activity of essential oil of Cupressus arizonica EL Greene against malaria vector Anopheles stephensi Liston (Diptera: Culicidae) Pharm Res. 2011;3(2):135. doi: 10.4103/0974-8490.81962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanei-Dehkordi A, Vatandoost H, Abaei MR, Davari B, Sedaghat MM. Chemical composition and larvicidal activity of Bunium persicum essential oil against two important mosquitoes vectors. J Essential Oil Bearing Plants. 2016;19(2):349–357. [Google Scholar]
- 28.Osanloo M, Sedaghat MM, Sanei-Dehkordi A, Amani A. Plant-derived essential oils; their larvicidal properties and potential application for control of mosquito-borne diseases. Galen Med J. 2019;8:1532. doi: 10.31661/gmj.v8i0.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramachandran S, Singh SK, Larroche C, Soccol CR, Pandey A. Oil cakes and their biotechnological applications–a review. Bioresour Technol. 2007;98(10):2000–2009. doi: 10.1016/j.biortech.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Sharma A, Gupta S, Sarethy IP, Dang S, Gabrani R. Green tea extract: possible mechanism and antibacterial activity on skin pathogens. Food Chem. 2012;135(2):672–675. doi: 10.1016/j.foodchem.2012.04.143. [DOI] [PubMed] [Google Scholar]
- 31.Cheong AM, Tan KW, Tan CP, Nyam KL. Kenaf (Hibiscus cannabinus L.) seed oil-in-water Pickering nanoemulsions stabilised by mixture of sodium caseinate, tween 20 and β-cyclodextrin. Food Hydrocoll. 2016;52:934–941. [Google Scholar]
- 32.Cheong AM, Tan CP, Nyam KL. Physicochemical, oxidative and anti-oxidant stabilities of kenaf seed oil-in-water nanoemulsions under different storage temperatures. Ind Crop Prod. 2017;95:374–382. [Google Scholar]
- 33.Mallavarapu G, Rao B. Chemical constituents and uses of Cinnamomum zeylanicum Blume. Aromatic Plants From Asia Their Chem Appl Foof Therapy Har Krishan Bhalla Sons Dehradun India. 2007.
- 34.Thomas A, Mazigo HD, Manjurano A, Morona D, Kweka EJ. Evaluation of active ingredients and larvicidal activity of clove and Cinnamon essential oils against Anopheles gambiae (sensu lato) Parasit Vectors. 2017;10(1):411. doi: 10.1186/s13071-017-2355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Organization WH. Guidelines for laboratory and field testing of mosquito larvicides: World Health Organization2005.
- 36.Finney DJ. Probit analysis: Cambridge University Press; 1971.
- 37.Randhawa MA. Calculation of LD50 values from the method of miller and Tainter, 1944. J Ayub Med Coll Abbottabad. 2009;21(3):184–185. [PubMed] [Google Scholar]
- 38.Van Den Dool H, Kratz PD. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography 1963. [DOI] [PubMed]
- 39.Zinatloo-Ajabshir S, Salehi Z, Amiri O, Salavati-Niasari M. Green synthesis, characterization and investigation of the electrochemical hydrogen storage properties of Dy2Ce2O7 nanostructures with fig extract. Int J Hydrog Energy. 2019;44(36):20110–20120. [Google Scholar]
- 40.Osanloo M, Amani A, Sereshti H, Abai MR, Esmaeili F, Sedaghat MM. Preparation and optimization nanoemulsion of Tarragon (Artemisia dracunculus) essential oil as effective herbal larvicide against Anopheles stephensi. Ind Crop Prod. 2017;109:214–219. [Google Scholar]
- 41.Sell CS. The chemistry of fragrances: from perfumer to consumer: Royal Society of Chemistry; 2006.
- 42.Unlu M, Ergene E, Unlu GV, Zeytinoglu HS, Vural N. Composition, antimicrobial activity and in vitro cytotoxicity of essential oil from Cinnamomum zeylanicum Blume (Lauraceae) Food Chem Toxicol. 2010;48(11):3274–3280. doi: 10.1016/j.fct.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Dongmo PMJ, Tatsadjieu LN, Tchoumbougnang F, Sameza ML, Dongmo BN, Zollo PHA, et al. Chemical composition, antiradical and antifungal activities of essential oil of the leaves of Cinnamomum zeylanicum Blume from Cameroon. Nat Prod Commun. 2007;2(12):1934578X0700201219. [Google Scholar]
- 44.Ojagh S, Rezaei M, Razavi S, Hosseini S. Investigation of antibacterial activity cinnamon bark essential oil (Cinnamomum zeylanicum) in vitro antibacterial activity against five food spoilage bacteria. 2012.
- 45.Jayaprakasha GK. Jagan Mohan Rao L, Sakariah KK. Volatile constituents from Cinnamomum zeylanicum fruit stalks and their antioxidant activities. J Agric Food Chem. 2003;51(15):4344–4348. doi: 10.1021/jf034169i. [DOI] [PubMed] [Google Scholar]
- 46.Jayaprakasha GK, Rao LJ, Sakariah KK. Chemical composition of volatile oil from Cinnamomum zeylanicum buds. Zeitschrift für Naturforschung C. 2002;57(11–12):990–993. doi: 10.1515/znc-2002-11-1206. [DOI] [PubMed] [Google Scholar]
- 47.Uma B, Prabhakar K, Rajendran S, LAKSHMI SY. Studies on GC/MS spectroscopic analysis of some bioactive antimicrobial compounds from Cinnamomum zeylanicum. 2009. [Google Scholar]
- 48.Manimaran A, Cruz MMJJ, Muthu C, Vincent S, Ignacimuthu S. Larvicidal and knockdown effects of some essential oils against Culex quinquefasciatus Say, Aedes aegypti (L.) and Anopheles stephensi (Liston). 2012.
- 49.Anjali C, Sharma Y, Mukherjee A, Chandrasekaran N. Neem oil (Azadirachta indica) nanoemulsion—a potent larvicidal agent against Culex quinquefasciatus. Pest Manag Sci. 2012;68(2):158–163. doi: 10.1002/ps.2233. [DOI] [PubMed] [Google Scholar]
- 50.Kale NJ, Allen LV., Jr Studies on microemulsions using Brij 96 as surfactant and glycerin, ethylene glycol and propylene glycol as cosurfactants. Int J Pharm. 1989;57(2):87–93. [Google Scholar]
- 51.Osanloo M, Sedaghat M, Sereshti H, Rahmanian M, Saeedi Landi F, Amani A. Chitosan nanocapsules of tarragon essential oil with low cytotoxicity and long-lasting activity as a green nano-larvicide. J Nanostruct. 2019;9(4):723–735. [Google Scholar]
- 52.Volpato A, Baretta D, Zortéa T, Campigotto G, Galli GM, Glombowsky P, Santos RCV, Quatrin PM, Ourique AF, Baldissera MD, Stefani LM, da Silva AS. Larvicidal and insecticidal effect of Cinnamomum zeylanicum oil (pure and nanostructured) against mealworm (Alphitobius diaperinus) and its possible environmental effects. J Asia Pac Entomol. 2016;19(4):1159–1165. [Google Scholar]
- 53.Balasubramani S, Rajendhiran T, Moola AK, Diana RKB. Development of nanoemulsion from Vitex negundo L. essential oil and their efficacy of antioxidant, antimicrobial and larvicidal activities (Aedes aegypti L.) Environ Sci Pollut Res. 2017;24(17):15125–15133. doi: 10.1007/s11356-017-9118-y. [DOI] [PubMed] [Google Scholar]
- 54.Sundararajan B, Moola AK, Vivek K, Kumari BR. Formulation of nanoemulsion from leaves essential oil of Ocimum basilicum L. and its antibacterial, antioxidant and larvicidal activities (Culex quinquefasciatus) Microb Pathog. 2018;125:475–485. doi: 10.1016/j.micpath.2018.10.017. [DOI] [PubMed] [Google Scholar]