Abstract
The purpose of this pilot study was to evaluate and determine the concentration of prostaglandin GF2α (PGF2α) and isoprostane 8‐iso‐PGF2α in plasma and intestine of specific pathogen-free (SPF) Leghorn chickens challenged with Eimeria maxima, with or without dietary supplementation of curcumin using solid‐phase microextraction and ultra‐performance liquid chromatography/tandem mass spectrometry. Eighty 1-day-old male SPF chickens were randomly allocated to one of four groups with four replicates (n = 5 chickens/replicate). Groups consisted of: (1) Control (no challenge), (2) Curcumin (no challenge), (3) Eimeria maxima (challenge), and (4) Eimeria maxima (challenge) + curcumin. At day 28 of age, all chickens in the challenge groups were orally gavaged with 40,000 sporulated E. maxima oocysts. No significant differences (P > 0.05) were observed in the groups regardless of the treatment or challenge with E. maxima. Enteric levels of both isoprostane 8‐iso‐PGF2α and PGF2α at 7 days and 9 days post-challenge were significantly increased (P < 0.01) compared to the non-challenge control chickens. Interestingly, the enteric levels of both isoprostane 8‐iso‐PGF2α and PGF2α at 7 days post-challenge were significantly reduced in chickens fed curcumin, compared to control chickens challenge with E. maxima. At 9 days post-challenge, only levels of isoprostane 8‐iso‐PGF2α in the enteric samples were significantly reduced in chickens challenged with E. maxima supplemented with curcumin, compared with E. maxima challenge chickens. No differences of isoprostane 8‐iso‐PGF2α or PGF2α were observed in plasma at both days of evaluation. Similarly, no significant differences were observed between the challenge control or chickens challenge with E. maxima and supplemented with curcumin at both times of evaluation. The results of this pilot study suggests that the antioxidant anti-inflammatory properties of curcumin reduced the oxidative damage and subsequent intestinal mucosal over-production of lipid oxidation products. Further studies to confirm and extend these results in broiler chickens are required.
Subject terms: Biological techniques, Immunology, Microbiology, Gastroenterology
Introduction
Coccidiosis is a parasitic enteric disease of animals caused by coccidian protozoa from the Apicomplexa phylum. In a recent study, the global cost of coccidiosis in broiler chickens was estimated at ~ £10.36 billion1. In commercial poultry, coccidiosis has been controlled effectively with anticoccidial products, however, the extensive use of anticoccidial drugs has led to development of resistance against all these drugs2. To reduce the occurrence of resistance, the rotation of various anticoccidial drugs in single and shuttle programs is used3. Unfortunately, this has not solved the anticoccidial resistance problem. Live anticoccidial vaccines have been incorporated into rotation programs, resulting in an increased incidence of anticoccidial drug-sensitive Eimeria spp. field isolates, which may improve the efficacy of anticoccidial drugs4. Nevertheless, possible upcoming bans restricting the use of anticoccidials as feed additives, consumer concerns on residues, and increasing regulations have prompted the quest for alternative coccidiosis control strategies5,6. Although management and biosecurity measures could halt the introduction of Eimeria spp. to a farm, in practice, they do not suffice to prevent coccidiosis outbreaks.
Several phytogenics have been evaluated as feed additives in the poultry industry to protect feed from degradation and deterioration during storage, as well as for nutritional purposes7. However, it has been reported that these additives play an essential role in the prevention of several diseases in poultry due to their antioxidant, anti-inflammatory, antibacterial, antiviral, antifungal, and immunomodulatory properties8–10. Hence, in recent years, our laboratory has been evaluating curcumin as a feed additive to control Salmonella Enteritidis and necrotic enteritis in broiler chickens11–13. Curcumin is a bright yellow chemical and the principal curcuminoid of turmeric (Curcuma longa), a member of the ginger family (Zingiberaceae). For centuries, curcumin has been used as spice and food-coloring agent. In the poultry industry, curcumin has been used as anticoccidial, anti-inflammatory, immunomodulatory, antimicrobial, antioxidant and to promote growth performance14–16. Diets supplemented with of 1% of curcumin reduced intestinal lesion scores, oocyst per gram excretion (OPG) and improved weight gains during E. maxima infections and this anticoccidial activity was suggested to result from its antioxidant properties17. Other studies have been shown that curcumin inhibits induction of nitric oxide synthase in macrophages stimulated with endotoxin, as well as serum nitrogen dioxide and nitrate in E. maxima-infected chickens fed curcumin18,19.
Eimeria spp. have a remarkable and complex life cycle, including sexual and asexual reproduction with intracellular and extracellular phases20–22. Hence, during the disease, the gut-associated lymphoid tissues respond with a series of innate and acquired immune reactions against the parasite23,24. Several investigators have extensively studied and documented the immunopathology of cellular responses involving the secretion of pro-inflammatory cytokines to Eimeria infections in chickens25–30. However, little is known about the role of prostaglandins (PG) and isoprostanes (F2-Ips) as part of the innate response during clinical coccidiosis. Prostaglandins are a group of lipid compounds from the eicosanoid family implicated in inflammation, allergy, fever, and other immune responses that are generated from arachidonic acid by the action of cyclooxygenases (COXs) isoenzymes. Conversely, F2-Ips are PG-like complexes formed from free radical catalyzed oxidation of arachidonic acid, without the action of COXs. The measurement of F2-Ips, especially 8-epi-PGF2α, is recognized as a consistent biomarker of lipid peroxidation and is currently used as a sensitive index of oxidative stress in vivo.
The purpose of this pilot study was to evaluate and determine the concentration of prostaglandin GF2α (PGF2α) and isoprostane 8‐iso‐PGF2α in plasma and intestine of specific pathogen-free (SPF) Leghorn chickens challenged with Eimeria maxima, with or without dietary supplementation of curcumin, using solid‐phase microextraction and ultra‐performance liquid chromatography/tandem mass spectrometry.
Results
The evaluation of body weight and body weight gain (in grams) of specific pathogen-free Leghorn chickens without or with Eimeria maxima challenge (7 days post-challenge) are summarized in Table 1. In the present study, challenge with 40,000 sporulated oocysts of E. maxima did not affect the body weight or body weight gain of SPF Leghorn chickens. No significant differences (P > 0.05) were observed in the groups regardless of the treatment or challenge with E. maxima (Table 1).
Table 1.
Evaluation of body weight and body weight gain (in grams) of specific pathogen-free Leghorn chickens with or without Eimeria maxima challenge (7 days post-challenge).
| Group | Body weight 21 days | Body weight 28 days | Body weight gain | Daily body weight gain |
|---|---|---|---|---|
| Control non-challenge | 258.25 ± 9.28 | 275.55 ± 8.53 | 17.30 ± 2.11 | 2.47 ± 0.30 |
| Curcumin non-challenge | 254.95 ± 8.22 | 273.80 ± 8.14 | 18.85 ± 1.63 | 2.69 ± 0.23 |
| E. maxima challenge | 248.95 ± 6.91 | 266.30 ± 6.32 | 17.35 ± 2.07 | 2.48 ± 032 |
| E. maxima + curcumin | 251.40 ± 7.79 | 267.90 ± 8.51 | 16.50 ± 2.30 | 2.36 ± 0.29 |
Data expressed as mean ± standard error. P > 0.05.
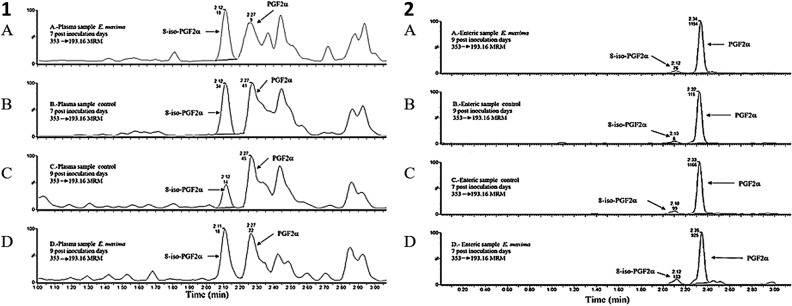
Table 2 presents the results of the evaluation of isoprostane 8‐iso‐PGF2α and PGF2α from jejunum and plasma in SPF chickens challenged with E. maxima at 7- and 9-days post-challenge. Enteric levels of both isoprostane 8‐iso‐PGF2α and PGF2α at 7 days and 9 days post-challenge were significantly increased (P < 0.01) compared to the non-challenge control chickens (Table 2; Fig. 1). Interestingly, the enteric levels of both isoprostane 8‐iso‐PGF2α and PGF2α at 7 days post-challenge were significantly reduced in chickens fed with curcumin compared to control chickens challenge with E. maxima. At 9 days post-challenge, only levels of isoprostane 8‐iso‐PGF2α in the enteric samples were significantly reduced in chickens challenged with E. maxima supplemented with curcumin, as compared with E. maxima challenge chickens. No differences of isoprostane 8‐iso‐PGF2α or PGF2α were observed in the plasma at both days of evaluation (Table 2; Fig. 1).
Table 2.
Evaluation of isoprostane 8‐iso‐PGF2α and prostaglandin GF2α from enteric (jejunum) and plasma of specific pathogen-free Leghorn chickens at 7- and 9-days post-challenge.
| Group | Prostaglandin GF2α | Isoprostane 8-iso-PGF2α | ||
|---|---|---|---|---|
| Enteric (pg/g) | Plasma (pg/mL) | Enteric (pg/g) | Plasma (pg/mL) | |
| 7 days post-challenge | ||||
| Control non-challenge | 6934.47 ± 572.87b | 107.92 ± 11.64 | 760.10 ± 75.56b | 97.17 ± 8.96 |
| Curcumin non-challenge | 5843.27 ± 631.55b | 117.29 ± 22.34 | 582.97 ± 70.68b | 95.58 ± 15.27 |
| E. maxima challenge | 12,076.52 ± 770.55a | 151.50 ± 17.87 | 1272.80 ± 81.97a | 101.99 ± 17.87 |
| E. maxima + curcumin | 8,088.87 ± 698.27b | 118.54 ± 9.89 | 864.93 ± 55.21b | 97.97 ± 17.16 |
| 9 days post-challenge | ||||
| Control non-challenge | 8984.66 ± 603.25b | 162.05 ± 15.73 | 669.16 ± 81.47b | 110.55 ± 9.22 |
| Curcumin non-challenge | 7606.78 ± 721.99b | 121.19 ± 14.62 | 602.21 ± 79.93b | 106.39 ± 19.53 |
| E. maxima challenge | 14,191.48 ± 750.61a | 124.38 ± 11.82 | 1363.84 ± 89.12a | 105.33 ± 8.83 |
| E. maxima + curcumin | 10,884.00 ± 740.07a,b | 135.66 ± 22.59 | 834.82 ± 125.43b | 104.83 ± 15.92 |
Data expressed as mean ± standard error.
a,bDifferent superscripts within columns and days indicate a significant difference at P < 0.01.
Figure 1.
Chromatograms of 8-iso-PGF2α and PGF2α. (1) Obtained from plasma samples: A—sample of E. maxima 7 days post-inoculation chickens, B—sample of control of 7 days post-inoculation chickens, C—sample of control of 9 days post-inoculation chickens, and D—sample of E. maxima 9 days post-inoculation chickens. (2) Obtained from enteric (jejunum) samples: A—sample of E. maxima 9 days post-inoculation chickens, B—sample of control of 9 days post-inoculation chickens, C—sample of control of 7 days post-inoculation chickens, and D—sample of E. maxima 7 days post-inoculation chickens.
The results of the evaluation of E. maxima oocyst per gram in the feces of specific pathogen-free Leghorn chickens at 7- and 9-days post-challenge are summarized on Table 3. No significant differences were observed between the challenge control or chickens challenged with E. maxima and supplemented with curcumin at both times of evaluation (Table 3).
Table 3.
Eimeria maxima oocyst per gram in the feces of specific pathogen-free Leghorn chickens at 7- and 9-days post-challenge.
| Group | 7 days post-challenge | 9 days post-challenge |
|---|---|---|
| Non-challenge control | 0 (0)b | 0 (0)b |
| Non-challenge Curcumin | 0 (0)b | 0 (0)b |
| E. maxima challenge | 24,240 (20,200)a | 2750 (1700)a |
| E. maxima + Curcumin | 22,593 (28,962.5)a | 3023 (1650)a |
Each value represents the mean (median).
a,bValues within groups columns with different superscripts differ significantly at P < 0.05.
Discussion
Coccidiosis remains one of the most critical diseases in the poultry industry. Due to international regulations and consumer pressures, there is a need to develop alternatives for antibiotic growth promoters in animal and poultry feed. Phytogenics seem to be candidates of interest as alternatives to antibiotic growth promoters because they have been shown to control of Eimeria infections due to the association of coccidial infection with lipid peroxidation of the intestinal mucosa31. Other studies have confirmed the benefits of phytogenics in reducing gastrointestinal infections and increasing performance32–34. Moreover, several studies have confirmed the reduction E. maxima infection severity in broiler chickens due to curcumin’s antioxidant properties17–19.
In addition to the critical job of absorbing water and nutrients, enterocytes also play an essential role in the mucosal immune response, maintaining tolerance to beneficial microbiota, and identifying luminal pathogens. The invasion of Eimeria spp. in intestinal epithelial cells is a complex process that includes several events, beginning with the excystation of sporozoites after oral ingestion of the oocysts35,36. As intracellular parasites, attachment and invasion of the sporozoites to the host cell is recognized by Toll-like receptors 4 and 15, involved in pathogen recognition and activation of the mucosal inflammasome IL-1/IL-18 axis, which is responsible for recruiting and activating heterophils, natural killer cells, mast cells, macrophages, and increased production of transcription factor NF-κB37–40. Nevertheless, sporozoites have evolved a unique molecular system fueling motility and invasion of epithelial cells through gliding motility, allowing them to rapidly invade host cells and form an intracellular parasitophorous vacuole that protects them from the intracellular hostile environment41–44. Within this vacuole, these Apicomplexa parasites gain precious time to continue with their multifaceted life cycle. Each phase of the sexual, asexual, intracellular, or extracellular stages of this prehistoric and remarkable parasite are associated with severe local inflammation, autophagy, apoptosis, cellular death, hemorrhages, and necrosis in the intestinal mucosa42–47. Hence, coccidia infections are characterized by excessive tissue damage caused by the parasite infection and chronic inflammation of the host immune response elicited against the invaders. In chickens, macrophages are the primary sources of nitric oxide, superoxide, and hydrogen peroxide as essential mediators of both innate and acquired immunity, thus increasing during coccidia infections48–52. In the present study, chickens challenged with E. maxima presented with a significant increase (P < 0.01) in enteric PGF2α at 7- and 9-days post-challenge when compared with non-challenged chickens. However, the serum levels of PGF2α remained similar in both groups. Interestingly, chickens challenged with E. maxima and supplemented with curcumin showed a significant reduction of PGF2α levels at 7 days post challenge when compared with E. maxima control chickens. PGs are produced from arachidonic acid release from phospholipids in the cellular membrane by cyclooxygenases (COXs). They are fundamental in generating inflammatory responses against pathogens53,54. While they have a rapid response during the acute phases of the inflammatory response, there is crosstalk with cytokines to synergistically activate NF-κB factor and induce gene expression of pro-inflammatory cytokines and more COXs, mediating positive feedback loops and consequently, chronic inflammation55,56.
Since the cellular components that suffer immediate damage are the lipids and proteins of the cell membrane and mitochondrial membrane by lipid peroxidation, the whole-cell physiology is then compromised. One of the cellular mechanisms to revert oxidative stress is the production of several heat shock proteins that repair damage proteins and regulate apoptosis57–59. A noteworthy result observed in this study was the significant increase in isoprostane 8‐iso‐PGF2α in the jejunum of chickens challenged with E. maxima at 7- and 9-days post-challenge compared to the non-challenge control chickens. Furthermore, chickens in the group supplemented with curcumin showed a significant reduction in isoprostane 8‐iso‐PGF2α in the jejunum of chickens challenged with E. maxima at both days of evaluation post-challenge compared to the E. maxima challenge control chickens. Excessive generation of reactive oxygen species has been implicated in a variety of pathological events. However, lipid peroxidation is the primary marker of oxidative stress in many pathological conditions, so isoprostanes are reliable evaluation biomarkers evaluate60,61. In contrast, F2-isoprostanes (8-Iso-PGF2α) have harmful and potent bioactivities, including vasoconstriction, platelet aggregation, and cardiac hypertrophy62–65. As far as we know, this is the first report of detection of 8-Iso-PGF2α following a challenge of E. maxima in the jejunum, as well as demonstrating the protective antioxidant properties of curcumin reducing the enteric levels of 8-Iso-PGF2α, despite plasma levels of 8-Iso-PGF2α remaining similar in all groups, regardless of the challenge with E. maxima. It is known that in humans, the plasma half-life of 8-Iso-PGF2α is one minute at the distribution stage and the removal stage half-life is four minutes66. Hence, the half-life in chicken plasma may also be short, which may be why we were not able to detect it. However, pharmacokinetic and metabolic studies evaluating earlier points as well as daily oocyst count are required to confirm and extend these results.
In summary, in the present study, SPF Leghorn chickens challenged with E. maxima showed an inflammatory response associated with a significant increase at 7 days and 9 days post challenge in enteric PGF2α. These changes were related to a significant increase of enteric 8-Iso-PGF2α and oocyst excretion at both days of evaluation, suggesting that the active disease phase was accompanied by inflammation and oxidative stress within the intestinal layer. Nevertheless, dietary supplementation of curcumin reduced the levels of PGF2α and 8-Iso-PGF2α at 7 days post challenge, and 8-Iso-PGF2α at 9 days post challenge compared with E. maxima challenged control chickens. Since polyunsaturated fatty acids and cholesterol are the principal targets of oxidative stress, lipid peroxidation end products, such as 8-Iso-PGF2α, are also a part of the pathogenesis of inflammation-related changes caused by E. maxima, confirming the role of 8-Iso-PGF2α as a sensitive biomarker of oxidative stress in chickens. The results of this pilot study suggest that the antioxidant and anti-inflammatory properties of curcumin are able to reduce oxidative damage and subsequently intestinal mucosal over-production of lipid oxidation products. Further studies to confirm and extend these results in broiler chickens are required.
Methods
Challenge strain
Eimeria maxima M6 oocysts were provided by Dr. John. R. Barta, University of Guelph, Canada. The methods for detecting and recovering oocysts from challenged chickens, oocyst sporulation, and the preparation of infective doses were conducted as described previously67,68.
Starter diet
A control basal non-supplemented diet and a basal diet supplemented with 2% curcumin were used in this experiment (Table 4). Starter feed used in this experiment was formulated to approximate the nutritional requirements for Leghorn chickens as recommended by the National Research Council69 and adjusted to Hy-Line Management Guide, W36 Commercial Layers recommendations70. No antibiotics, coccidiostats, or enzymes were added to the feed.
Table 4.
Ingredient composition (kg) and nutrient content of feed supplied to the experimental SPF chickens.
| Ingredients | Pre-starter (0–3 weeks) |
|---|---|
| Yellow corn 7.1% | 622.62 |
| Soybean meal 46.5% | 323 |
| Limestone 38% Ca | 18 |
| Phosphate 21/27% | 12 |
| Vegetable oil | 10 |
| NaCl (refined salt) | 4 |
| Vitamin premixa | 1.4 |
| Mineral premixb | 1.1 |
| DL-Methionine 99%c | 3.700 |
| Liquid l-lysine 50%d | 3.500 |
| l-Threoninee | 0.640 |
| 6-Phytasef | 0.040 |
| Nutrients70 | |
| Weight | 1.0 |
| Dry matter (%) | 88.300 |
| Crude protein (%) | 20.000 |
| Metabolizable energy (Mcal kg−1) | 3.087 |
| Choline (mg kg−1) | 2.000 |
| Arginine (%) | 1.210 |
| Linoleic acid (%) | 1.200 |
| Total lysine (%) | 1.150 |
| Total calcium (%) | 1.050 |
| Methionine + cystine (%) | 0.830 |
| Valine (%) | 0.830 |
| Threonine total (%) | 0.820 |
| Isoleucine (%) | 0.790 |
| Methionine total (%) | 0.510 |
| Phosphorus available (%) | 0.480 |
| Phosphorus digestible (%) | 0.440 |
| Total tryptophan (%) | 0.210 |
| Total chlorine (%) | 0.180 |
| Total sodium (%) | 0.180 |
aVitamin premix supplied per kg of diet: Retinol, 6 mg; cholecalciferol, 150 µg; dl-α-tocopherol, 67.5 mg; menadione, 9 mg; thiamine, 3 mg; riboflavin, 12 mg; pantothenic acid, 18 mg; niacin, 60 mg; pyridoxine, 5 mg; folic acid, 2 mg; biotin, 0.3 mg; cyanocobalamin, 0.4 mg.
bMineral premix supplied per kg of diet: Mn, 120 mg; Zn, 100 mg; Fe, 120 mg; copper, 10 to 15 mg; iodine, 0.7 mg; selenium, 0.2 mg; and cobalt, 0.2 mg.
cMetAMINO® (Evonik, Essen, Germany).
dLiquid l-lysine 50% (ADM, Chicago, IL, USA).
eThreAMINO® (Evonik, Essen, Germany).
fAxtra PHY TPT 10,000® (Dupont Industrial Biosciences, Marlborough, UK).
Animal source and experimental design
Eighty one-day-old male specific pathogens-free (SPF) Leghorn chickens (ALPES® Tehuacan, Puebla, Mexico) were randomly allocated to one of four groups with four replicates per group (n = 5 chickens/replicate). Chickens were placed in battery cages with a controlled age-appropriate environment at the diagnostic laboratory of the Avian Medicine Department of the Faculty of Veterinary Medicine and Zootechnics (FMVZ) at the National Autonomous University of Mexico (UNAM). Groups consisted of: (1) Control (no challenge), (2) Curcumin (no challenge), (3) Eimeria maxima (challenge), and (4) Eimeria maxima (challenge) + curcumin. Chickens were provided with ad libitum access to water. At day 28 of age, all chickens in the challenge groups were orally gavaged with 40,000 sporulated E. maxima oocysts in a volume of 1 mL of sterile phosphate-buffered saline solution (PBS). The dose used in the present study did not cause clinical coccidiosis in SPF Leghorn chickens. The dose was selected based on a previous trial conducted to determine a challenge dose causing sub-clinical coccidiosis as described previously13. Negative control chickens were sham inoculated with 1 mL of PBS. Seven days after challenge, all chickens were bled, and half of them were euthanized to collect the second half of the jejunum to determine plasma and enteric concentrations of isoprostane 8‐iso‐PGF2α and PGF2α. At 9 days post-challenge, remaining chickens from all groups were bled and jejunum was collected to perform the evaluations. Oocysts per gram (OPG) of feces were evaluated at 7- and 9-days post-challenge.
The standards for 8‐iso‐PGF2α and 8‐iso‐PGF2α‐d4
The standards for 8‐iso‐PGF2α and 8‐iso‐PGF2α‐d4 (internal standard) were purchased from Cayman Chemicals (Ann Arbor, MI), while the standard for PGF2α was obtained from Sigma-Aldrich (St Louis, MO). Acetonitrile and methanol (HPLC grade) were purchased from JT Baker. Milli‐Q water (Millipore system) was used throughout the experiments. Formic acid (FA: 95%, reactive grade) and isopropanol (LC/MS grade) were purchased from Sigma-Aldrich (St Louis, MO). Ammonium hydroxide (NH4OH, reactive grade, 29.60%) and potassium hydroxide (KOH) were purchased from JT Baker. For solid‐phase microextraction (micro‐SPE), 96‐well Oasis® MAX μElution cartridges containing a water‐wettable reversed‐phase strong ammonium exchange mixed‐mode polymer, which is selective for acids and stable in organic eluents, were used. A Positive Pressure‐96 processor purchased from Waters was also used. Figure 1 shows the chromatograms of standards.
Procedure for the extraction of 8-iso-PGF2α and PGF2α in chicken plasma
Extraction of 8-iso-PGF2α and PGF2α were determined as previously described71. An aliquot of 500 µL chicken plasma was transferred to 2 mL vials, followed by the addition of 100 µL of 4 ng/mL 8‐iso‐PGF2α‐d4 as an internal standard and 500 µL of hydrolysis solution (KOH, 15%) to release 8-iso-PGF2α-esterified. The vials were mixed and incubated in an ultrasonic bath for 30 min at 40 °C. Subsequently, the vials were cooled to room temperature and 225 µL of 6 M formic acid (FA) was added, mixed, and centrifuged at 15,000 rpm for 10 min at 4 °C. Solid‐phase microextraction using a 96‐well Oasis® MAX μElution plate conditioned with 500 μL of methanol and 500 μL of 20 mM FA was used. Finally, the cartridges were loaded with 350 μL of plasma and washed with 350 μL of 2% NH4OH. Samples were then eluted with 50 μL of a mixture of 5% FA in acetonitrile and isopropanol (40:60) and diluted with 150 μL of Milli‐Q water. Samples were analyzed (30 μL) using ultra‐performance liquid chromatography/tandem mass spectrometry (UPLC/MS/MS).
Procedure for the extraction of 8-iso-PGF2α and PGF2α in chicken intestine
For the extraction of 8-iso‐PGF2α and PGF2α, 0.1 g of homogenized second half of the jejunum (Meckel’s diverticulum to cecal tonsils) were transferred to 2 mL vials, followed by the addition of 100 µL of 4 ng/mL 8‐iso‐PGF2α‐d4 as the internal standard and 1.5 mL of chloroform: methanol (80:20) mixture. The vials were mixed 30 s by vortex and 15 min in an ultrasonic bath. Samples were then centrifuged at 15,000 rpm for 20 min. The supernatant was evaporated and 500 µL of methanol and 500 µL of hydrolysis solution (KOH 15%) were added, mixed, and incubated in an ultrasonic bath for 30 min at 40 °C. Subsequently, the vials were cooled to room temperature and 225 µL of 6 M formic acid (FA) and 50 µL of 88% FA were added, mixed, and centrifuged at 15,000 rpm for 10 min at 4 °C. Solid‐phase microextraction and analysis of samples were performed in the same way as for the determination of 8-iso-PGF2α and PGF2α in chicken plasma using a 96‐well Oasis® MAX μElution plate conditioned with 500 μL of methanol and 500 μL of 20 mM FA. Finally, the cartridges were loaded with 350 μL of jejunum sample and washed with 350 μL of 2% NH4OH. Samples were then eluted with 50 μL of a mixture of 5% FA in acetonitrile and isopropanol (40:60) and diluted with 150 μL of Milli‐Q water. The sample (30 μL) was injected into a UPLC-MS/MS system for analysis, under the chromatographic and mass spectrometric conditions described previously by Rodriguez Patiño et al.71.
Ethics
This study was carried out in accordance with the guidelines for the management of chickens as recommended by the Internal Committee for Care and Use of Experimental Animals (CICUAE, from its abbreviation in Spanish) of the National Autonomous University of Mexico (UNAM), Ethical approval code CICUAE: C20_06, and the study is in compliance with the ARRIVE guidelines where animals are involved.
Quantification of oocysts
The quantification of OPG from feces was performed at 7- and 9-days post-challenge by using the McMaster technique as previously described67.
Data and statistical analysis
PGF2α and 8-iso-PGF2α data are presented as means with standard deviation (S.D.). The number of samples per variable group was 20, implying a normal distribution (Shapiro–Wilk test), and the homoscedasticity was verified (Levene's test). Accordingly, the parametric test of analysis of variance (ANOVA) was performed, and the differences between the means were evaluated using Tukey’s honestly significant difference (HSD) test, and the P value was established with an alpha level of P < 0.01. OPG data are presented as means with median. The number of samples per variable group was 20; however, the hypotheses of normal distribution (Shapiro–Wilk test) and homoscedasticity (Levene’s test) were not confirmed. Consequently, non-parametric tests of non-parametric tests of the two-tailed Kruskal–Wallis was applied and subsequently the Mann–Whitney’s U test to compare between pairs of groups was applied with an alpha level P < 0.0572.
Acknowledgements
The research obtained funding from the PAPIIT IT201620 project of DGAPA‐UNAM, Universidad Nacional Autónoma de México. The authors thank CONACyT for the doctoral grant number 494367. The research was supported in part by funds provided by USDA-NIFA Sustainable Agriculture Systems, Grant No. 2019-69012-29905. Title of Project: Empowering U.S. Broiler Production for Transformation and Sustainability USDA-NIFA (Sustainable Agriculture Systems): No. 2019-69012-29905.
Author contributions
Conceptualization: V.M.P.-G., R.L.-A., and G.T.-I. Investigation and Methodology: V.M.P.-G., G.R.P., M.A.C.R., D.H.-P., B.S.-C., and X.H.-V. Formal analysis and Software: F.A.-H., C.N.V., I.C.-H. Supervision: V.M.P.-G., and G.T.-I. Validation and Visualization: D.H.-P. and B.S.-C. Writing-original draft: V.M.P.-G. and G.T.-I. Writing-review and editing: X.H.-V. and G.T.-I. All the authors reviewed, edited, and approved the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Blake DP, et al. Re-calculating the cost of coccidiosis in chickens. Vet. Res. 2020;51:115. doi: 10.1186/s13567-020-00837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman HD. Anticoccidial drugs and their effects upon the development of immunity to Eimeria infections in poultry. Avian Pathol. 1999;28:521–535. doi: 10.1080/03079459994317. [DOI] [PubMed] [Google Scholar]
- 3.Chapman H. Biochemical, genetic and applied aspects of drug resistance in Eimeria parasites of the fowl. Avian Pathol. 1997;26:221–244. doi: 10.1080/03079459708419208. [DOI] [PubMed] [Google Scholar]
- 4.Shivaramaiah C, Barta JR, Hernandez-Velasco X, Téllez G, Hargis BM. Coccidiosis: recent advancements in the immunobiology of Eimeria species, preventive measures, and the importance of vaccination as a control tool against these Apicomplexan parasites. Vet. Med. 2014;28:23–24. doi: 10.2147/VMRR.S57839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalloul RA, Lillehoj HS. Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert Rev. Vaccines. 2006;5:143–163. doi: 10.1586/14760584.5.1.143. [DOI] [PubMed] [Google Scholar]
- 6.Tewari AK, Maharana BR. Control of poultry coccidiosis: Changing trends. J. Parasit. Dis. 2011;35:10–17. doi: 10.1007/s12639-011-0034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luna A, et al. Thymol as natural antioxidant additive for poultry feed: Oxidative stability improvement. Poult. Sci. 2017;96:3214–3220. doi: 10.3382/ps/pex158. [DOI] [PubMed] [Google Scholar]
- 8.Hashemi SR, Davoodi H. Phytogenics as new class of feed additive in poultry industry. J. Anim. Vet. Adv. 2010;9:2295–2304. doi: 10.3923/javaa.2010.2295.2304. [DOI] [Google Scholar]
- 9.Bhattacharyya A, Shukla PK, Shukla M. Effect of Poly herbal phytobiotic on the growth, immunocompetence, development of digestive organs and carcass characteristics of commercial broilers. J. Anim. Res. 2015;5:347–351. doi: 10.5958/2277-940X.2015.00060.1. [DOI] [Google Scholar]
- 10.Saadat Shad H, Mazhari M, Esmaeilipour O, Khosravinia H. Effects of thymol and carvacrol on productive performance, antioxidant enzyme activity and certain blood metabolites in heat stressed broilers. Iran. J. Appl. Anim. Sci. 2016;6:195–202. [Google Scholar]
- 11.Hernandez-Patlan D, et al. Evaluation of a solid dispersion of curcumin with polyvinylpyrrolidone and boric acid against Salmonella Enteritidis infection and intestinal permeability in broiler chickens: A pilot study. Front. Microbiol. 2018;9:1289. doi: 10.3389/fmicb.2018.01289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernandez-Patlan D, et al. Evaluation of the dietary supplementation of a formulation containing ascorbic acid and a solid dispersion of curcumin with boric acid against Salmonella Enteritidis and necrotic enteritis in broiler chickens. Animals. 2019;9:184. doi: 10.3390/ani9040184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez-Patlan D, et al. Evaluation of ascorbic acid or curcumin formulated in a solid dispersion on Salmonella Enteritidis infection and intestinal integrity in broiler chickens. Pathogens. 2019;8:229. doi: 10.3390/pathogens8040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gowda NKS, Ledoux D, Rottinghaus G, Bermudez A, Chen Y. Efficacy of turmeric (Curcuma longa), containing a known level of curcumin, and a hydrated sodium calcium aluminosilicate to ameliorate the adverse effects of aflatoxin in broiler chicks. Poult. Sci. 2008;87:1125–1130. doi: 10.3382/ps.2007-00313. [DOI] [PubMed] [Google Scholar]
- 15.Khan RU, et al. The use of turmeric (Curcuma longa) in poultry feed. Worlds Poult. Sci. J. 2012;68:97–103. doi: 10.1017/S0043933912000104. [DOI] [Google Scholar]
- 16.Guil-Guerrero JL, Ramos L, Paredes JCZ, Carlosama-Yépez M, Moreno C, Ruales P. Effects of turmeric rhizome powder and curcumin in poultry production. A review. J. Anim. Feed Sci. 2017;26:293–302. doi: 10.22358/jafs/78511/2017. [DOI] [Google Scholar]
- 17.Allen PC, Danforth HD, Augustine PC. Dietary modulation of avian coccidiosis. Int. J. Parasitol. 1998;7:1131–1140. doi: 10.1016/s0020-7519(98)00029-0. [DOI] [PubMed] [Google Scholar]
- 18.Brouet I, Ohshima H. Curcumin, an anti-tumor promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthase in activated macrophages. Biochem. Biophys Res Commun. 1995;206:533–540. doi: 10.1006/bbrc.1995.1076. [DOI] [PubMed] [Google Scholar]
- 19.Malik P, Mukherjee TK. Structure-function elucidation of antioxidative and prooxidative activities of the polyphenolic compound curcumin. Chinese J. Biol. 2014;2014:1–8. doi: 10.1155/2014/396708. [DOI] [Google Scholar]
- 20.Lal K, et al. Proteomic comparison of four Eimeria tenella life-cycle stages: Unsporulated oocyst, sporulated oocyst, sporozoite and second-generation merozoite. Proteomics. 2009;9:4566–4576. doi: 10.1002/pmic.200900305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lillehoj H, Kim C, Keeler C, Zhang S. Immunogenomic approaches to study host immunity to enteric pathogens. Poult. Sci. 2007;86:1491–1500. doi: 10.1093/ps/86.7.1491. [DOI] [PubMed] [Google Scholar]
- 22.Chapman H. Milestones in avian coccidiosis research: A review. Poult. Sci. 2014;93:501–511. doi: 10.3382/ps.2013-03634. [DOI] [PubMed] [Google Scholar]
- 23.Allen PC, Fetterer R. Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry. Clin. Microbiol. Rev. 2002;15:58–65. doi: 10.1128/cmr.15.1.58-65.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yun C, Lillehoj H, Lillehoj E. Intestinal immune responses to coccidiosis. Dev. Comp. Immunol. 2000;24:303–324. doi: 10.1016/s0145-305x(99)00080-4. [DOI] [PubMed] [Google Scholar]
- 25.Kim WH, Chaudhari AA, Lillehoj HS. Involvement of T cell immunity in avian coccidiosis. Front. Immunol. 2019;10:2732. doi: 10.3389/fimmu.2019.02732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong YH, Lillehoj HS, Lee SH, Dalloul RA, Lillehoj EP. Analysis of chicken cytokine and chemokine gene expression following Eimeria acervulina and Eimeria tenella infections. Vet. Immunol. Immunopathol. 2006;114:209–223. doi: 10.1016/j.vetimm.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Hong YH, Lillehoj HS, Lillehoj HP, Lee SH. Changes in immune-related gene expression and intestinal lymphocyte subpopulations following Eimeria maxima infection of chickens. Vet. Immunol. Immunopathol. 2006;114:259–272. doi: 10.1016/j.vetimm.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Lillehoj H, Min W, Dalloul R. Recent progress on the cytokine regulation of intestinal immune responses to Eimeria. Poult. Sci. 2004;83:611–623. doi: 10.1093/ps/83.4.611. [DOI] [PubMed] [Google Scholar]
- 29.Lillehoj HS, Trout JM. Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clin. Microbiol. Rev. 1996;9:349–360. doi: 10.1128/CMR.9.3.349-360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park SS, et al. Immunopathology and cytokine responses in broiler chickens coinfected with Eimeria maxima and Clostridium perfringens with the use of an animal model of necrotic enteritis. Avian Dis. 2008;52:14–22. doi: 10.1637/7997-041707-Reg. [DOI] [PubMed] [Google Scholar]
- 31.Naidoo V, McGaw LJ, Bisschop SPR, Duncan N, Eloff JN. The value of plant extracts with antioxidant activity in attenuating coccidiosis in broiler chickens. Vet. Parasitol. 2008;153:214–219. doi: 10.1016/j.vetpar.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Wunderlich F, Al-Quraishy S, Steinbrenner H, Sies H, Dkhil MA. Towards identifying novel anti-Eimeria agents: Trace elements, vitamins, and plant-based natural products. Parasitol. Res. 2014;113:3547–3556. doi: 10.1007/s00436-014-4101-8. [DOI] [PubMed] [Google Scholar]
- 33.Snow Setzer M, Sharifi-Rad J, Setzer WN. The search for herbal antibiotics: An in-silico investigation of antibacterial phytochemicals. Antibiotics. 2016;5:30. doi: 10.3390/antibiotics5030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X, et al. The genome of medicinal plant macleaya cordata provides new insights into benzylisoquinoline alkaloids metabolism. Mol. Plant. 2017;10:975–989. doi: 10.1016/j.molp.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Tomley FM, Clarke LE, Kawazoe U, Dijkema R, Kok JJ. Sequence of the gene encoding an immunodominant microneme protein of Eimeria tenella. Mol. Biochem. Parasitol. 1991;49:277–288. doi: 10.1016/0166-6851(91)90071-d. [DOI] [PubMed] [Google Scholar]
- 36.Tomley FM, Billington KJ, Bumstead JM, Clark JD, Monaghan P. EtMIC4: a microneme protein from Eimeria tenella that contains tandem arrays of epidermal growth factor-like repeats and thrombospondin type-I repeats. Int. J. Parasitol. 2001;31:1303–1310. doi: 10.1016/s0020-7519(01)00255-7. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Z, et al. Upregulation of chicken TLR4, TLR15 and MyD88 in heterophils and monocyte-derived macrophages stimulated with Eimeria tenella in vitro. Exp. Parasitol. 2013;133:427–433. doi: 10.1016/j.exppara.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Crane JK, Mongiardo KM. Pro-inflammatory effects of uric acid in the gastrointestinal tract. Immunol. Invest. 2014;43:255–266. doi: 10.3109/08820139.2013.864667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sellin ME, Müller AA, Hardt W-D. Consequences of epithelial inflammasome activation by bacterial pathogens. J. Mol. Biol. 2018;430:193–206. doi: 10.1016/j.jmb.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 40.Hornung V, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang L, et al. Identification and characterization of Eimeria tenella apical membrane antigen-1 (AMA1) PLoS ONE. 2012;7:e41115. doi: 10.1371/journal.pone.0041115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soldati D, Foth BJ, Cowman AF. Molecular and functional aspects of parasite invasion. Trends Parasitol. 2004;20:567–574. doi: 10.1016/j.pt.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 43.Dowse T, Soldati D. Host cell invasion by the apicomplexans: the significance of microneme protein proteolysis. Curr. Opin. Microbiol. 2004;7:388–396. doi: 10.1016/j.mib.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 44.Russell D. Host cell invasion by Apicomplexa: An expression of the parasite’s contractile system? Parasitology. 1983;87:199–209. doi: 10.1017/s0031182000052562. [DOI] [PubMed] [Google Scholar]
- 45.Duszenko M, et al. Autophagy in protists. Autophagy. 2011;7:127–158. doi: 10.4161/auto.7.2.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nouwen LV, Everts B. Pathogens MenTORing macrophages and dendritic cells: Manipulation of mTOR and cellular metabolism to promote immune escape. Cells. 2020;9:161. doi: 10.3390/cells9010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Escalante AA, Ayala FJ. Evolutionary origin of Plasmodium and other Apicomplexa based on rRNA genes. Proc. Natl. Acad. Sci. USA. 1995;92:5793–5797. doi: 10.1073/pnas.92.13.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lillehoj HS, Li G. Nitric oxide production by macrophages stimulated with coccidia sporozoites, lipopolysaccharide, or interferon-α, and its dynamic changes in SC and TK strains of chickens infected with Eimeria tenella. Avian Dis. 2004;48:244–253. doi: 10.1637/7054. [DOI] [PubMed] [Google Scholar]
- 49.Lauridsen C. From oxidative stress to inflammation: Redox balance and immune system. Poult. Sci. 2019;98:4240–4246. doi: 10.3382/ps/pey407. [DOI] [PubMed] [Google Scholar]
- 50.Gebicki JM. Oxidative stress, free radicals, and protein peroxides. Arch. Biochem. Biophys. 2016;595:33–39. doi: 10.1016/j.abb.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 51.Cadenas E, Packer L, Traber MG. Antioxidants, oxidants, and redox impacts on cell function: A tribute to Helmut Sies. Arch. Biochem. Biophys. 2016;595:94–99. doi: 10.1016/j.abb.2015.11.01. [DOI] [PubMed] [Google Scholar]
- 52.Yodoi J, Tian H, Masutani H, Nakamura H. Thiol redox barrier; local and systemic surveillance against stress and inflammatory diseases. Arch. Biochem. Biophys. 2016;595:88–93. doi: 10.1016/j.abb.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 53.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler. Thromb. Vas. Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yao C, Narumiya S. Prostaglandin-cytokine crosstalk in chronic inflammation. Br. J. Pharmacol. 2019;176:337–354. doi: 10.1111/bph.14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao G, et al. Pivotal role of reactive oxygen species in differential regulation of lipopolysaccharide-induced prostaglandins production in macrophages. Mol. Pharmacol. 2013;83:167–178. doi: 10.1124/mol.112.080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang D, DuBois RN. Role of prostanoids in gastrointestinal cancer. J. Clin. Invest. 2018;128:2732–2742. doi: 10.1172/JCI97953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeng T, et al. Effects of heat stress on antioxidant defense system, inflammatory injury, and heat shock proteins of Muscovy and Pekin ducks: Evidence for differential thermal sensitivities. Cell Stress Chaperones. 2014;19:895–901. doi: 10.1007/s12192-014-0514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu J, Tang S, Song E, Yin B, Bao E. Inhibition of heat shock protein 70 intensifies heat-stressed damage and apoptosis of chicken primary myocardial cells in vitro. Mol. Med. Rep. 2017;15:2881–2889. doi: 10.3892/mmr.2017.6337. [DOI] [PubMed] [Google Scholar]
- 59.Çenesiz S. The role of oxidant and antioxidant parameters in the infectious diseases: A systematic literature review. Kafkas Üniv. Vet. Fak. Derg. 2020;26:849–858. doi: 10.9775/kvfd.2020.24618. [DOI] [Google Scholar]
- 60.Milne GL, Dai Q, Roberts LJ., II The isoprostanes: 25 years later. Biochim. Biophys. Acta. 2015;1851:433–445. doi: 10.1016/j.bbalip.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Czerska M, Zieliński M, Gromadzińska J. Isoprostanes: A novel major group of oxidative stress markers. Int. J. Occup. Med. Environ. Health. 2016;29:179–190. doi: 10.13075/ijomeh.1896.00596. [DOI] [PubMed] [Google Scholar]
- 62.Wen S-H, et al. Role of 15–F2t-isoprostane in intestinal injury induced by intestinal ischemia/reperfusion in rats. Free Radic. Res. 2014;48:907–918. doi: 10.3109/10715762.2014.926010. [DOI] [PubMed] [Google Scholar]
- 63.Crankshaw D, Rangachari P. Isoprostanes: More than just mere markers. Mol. Cell. Biochem. 2003;253:125–130. doi: 10.1023/a:1026052123843. [DOI] [PubMed] [Google Scholar]
- 64.Kaviarasan S, Muniandy S, Qvist R, Ismail IS. F2-isoprostanes as novel biomarkers for type 2 diabetes: A review. J. Clin. Biochem. Nutr. 2009;45:1–8. doi: 10.3164/jcbn.08-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Janicka M, Kot-Wasik A, Kot J, Namie’snik J. Isoprostanes-biomarkers of lipid peroxidation: Their utility in evaluating oxidative stress and analysis. Int. J. Mol. Sci. 2010;11:4631–4659. doi: 10.3390/ijms11114631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Basu S. Metabolism of 8-iso-prostaglandin F2α. FEBS Lett. 1998;428:32–36. doi: 10.1016/s0014-5793(98)00481-5. [DOI] [PubMed] [Google Scholar]
- 67.Long PL, Millard BJ, Joyner LP, Norton CC. A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet. Lat. 1976;6:201–217. [PubMed] [Google Scholar]
- 68.Haug A, Williams R, Larsen S. Counting coccidial oocysts in chicken faeces: a comparative study of a standard McMaster technique and a new rapid method. Vet. Parasitol. 2006;136:233–242. doi: 10.1016/j.vetpar.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 69.National Research Council. Nutrient Requirements of Poultry. http://www.nap.edu/catalog/2114.html (1994).
- 70.Hy-Line International. Management Guide, W36 Commercial Layers.https://www.hyline.com/varieties/w-36 (2020).
- 71.Rodriguez Patiño G, et al. Development of a method for the determination of 8-iso-PGF2α in sheep and goat plasma using solid-phase microextraction and ultra-performance liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2018;32:1675–1682. doi: 10.1002/rcm.8224. [DOI] [PubMed] [Google Scholar]
- 72.SAS Institute Inc. SAS/STAT® 9.2 User´s Guide. 3530–3541. (SAS Institute Inc, 2009).