Abstract
A biosurfactant producing strain was isolated and the rhamnolipid type biosurfactant was extracted for soil washing of a synthetically and naturally hydrocarbon-contaminated soil. Following the primary screening, Pseudomonas aeruginosa strain R4 was selected and the effect of the carbon and nitrogen source and the salinity on biosurfactant production was studied. Of the best results were observed for glucose as a carbon source, NH4Cl as a nitrogen source and salinity of 1.4%. The produced biosurfactant was a glycolipid type biosurfactant and reduced the surface tension to 32.5 mN/m with a critical micelle concentration (CMC) of 50 mg/L and production yield of 90 mg/L. Using produced biosurfactant, a pyrene desorption rate of 82% was observed in selected conditions for initial pyrene concentration of 200 mg/L.
Keywords: Pyrene, Soil pollution, Biosurfactant, Soil washing, Pseudomonas aeruginosa strain R4
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are persistent compounds which usually originate from urban and rural non-point sources through sewage, road run-off and atmospheric dissemination and pursuant deposition of particulate air pollution. Soil and river fouling near industrial sites such as creosote manufacture facilities can be contaminated with PAHs. In addition to creosote, oil spills, coal mining dust and the other fossil fuel sources have led to PAHs accumulation into the environmental resources. These compounds are ranked as one of the most hazardous soil pollutants, due to hydrophobic nature and a strong attraction to the soil texture, [1–3]. Pyrene (C16H10) is a polycyclic aromatic hydrocarbon (PAH) containing four fused benzene rings that has low biodegradability and high persistence in environment. These compounds are considered as priority pollutants by US Environmental Protection Agency (USEPA) [4]. The presence of PAHs in soil poses serious health threatening human population, because their persistency, toxicity, mutagenicity and carcinogenicity [5]. There are several biological, physical and chemical methods proposed to remediate soils contaminated with petroleum hydrocarbons [6]. Among remediation techniques, bioremediation which relies, principally, on living organisms, especially fungi, bacteria and plants, is an effective technique, due to less toxicity for the environment, but it often takes time and is not cost-effective on handling great volumes of polluted materials. However, some methods, such as using soil washing to remediate contaminated soils without causing chemical harms to the soil, may markedly raise the biodegradation rate ([7–9]). Soil washing is a technique of concentrating contaminants through separation which have received many attentions, due to the high-performance and economic aspects for remediation of contaminated soils [10]. PAHs are the highly hydrophobic materials with low water solubility. Surfactants, at low concentrations, are soluble in water and in enhanced concentrations, they form micelle in solution. Critical micelle concentration (CMC) is the concentration at which micelle begins to form. Since formation of micelles, solid polycyclic aromatic hydrocarbons can divide into the interior of the micelle, which enhance hydrocarbons solubility. Surfactants can alter the interactions between the cells and the substrate which interplay with microbial cell surface that can have both stimulatory and inhibitory effects on the biodegradation hydrocarbon uptake for a special microorganism [11–13]. Biosurfactants are a group of amphiphilic biomolecules which have hydrophobic and hydrophilic moieties and are produced by variety of microorganisms. Biosurfactants have lower toxicity, higher biodegradability, better environmental compatibility and stability towards temperature and pH conditions, in comparison with chemical surfactants. These advantages put the biosurfactants ahead of chemical surfactants [14]. Hence, the main objective of this current study was isolation and characterization of biosurfactant-producing bacteria for application of extracted biosurfactant in soil washing of hydrocarbon-contaminated soil.
Materials and methods
Materials
All chemicals used in the current study were in of analytical grade and purchased from Merck, sigma-Aldrich, USA including: Pyrene (purity >98%), agar, nutrient agar, nutrient broth, n-hexane, yeast extract, acetone (C3H6O), chloroform (CHCl3, 99.5%), (NH4)(NO3), CH3OH, H2SO4, C2H5OH, NH4Cl, (NH4)2SO4, (NH4)(NO3).
Soil sampling and preparation
The soil samples were prepared from oil fields of south-west of Iran. The soil samples were collected from the upper layer 5–20 cm by soil cores, air-dried, and then passed through a 2 mm sieve. Then, the sieved samples were sterilized after drying in an autoclave (121 °C and 15 psi during 15 min), washed three times with acetone. Finally, to remove acetone, the samples were washed with autoclaved distilled water, and stored in plastic containers at 4 °C. Since the washed soil still contained pyrene, a specific amount of pyrene was dissolved in n-Hexane and added to the soil to achieve a final pyrene concentration of 200 mg/kg soil in both synthetic fresh and aged samples [15]. Physical and chemical characteristics of soil were analyzed using X-ray diffraction (XRD) and X-ray fluorescence (XRF) (XRF, PW1410, Holland).). pH was measured using a digital pH-meters (Cyberscaneutech-instruments 5500). The pyrene and its degradation metabolites were also determined by GC-MS (Model: Agilent 7890, USA) with HP-5MS capillary column (30 m × 0.25 mm × 0.25 μm film thickness, 5% Phenyl – 95% Methyl Siloxane phase).
Isolation and purification of biosurfactant producing strain
To isolate the surfactant producing bacterial strains, 1 g of soil polluted by petroleum hydrocarbons was transferred to 250-mL Erlenmeyer flask containing 100 mL phosphate mineral salt (PMS) medium, the suspension was shaken vigorously for 20 min, and allowed to settle. Then, an aliquot of 5 mL supernatant was transferred into a 250-mL flask containing 95 mL sterilized PMS. The phosphate mineral salt (PMS) medium used in this study consisted of: 6.3 g L−1 K2HPO4, 1.8 g L−1 KH2PO4, 0.5 g L−1 yeast extract, 0.1 g L−1 MgSO4.7H2O, 0.1 g L−1 CaCl2.H2O, 0.1 g L−1 FeSO4.7H2O, 0.1 g L−1 MnSO4.H2O, and 1 mL/L of trace elements solution. The trace elements solution consisted of 0.03 g L−1 H3BO3, 0.01 g L−1 ZnSO4.7H2O, 0.02 g L−1 COCl2.6H2O, 0.006 g L−1 NaMoO4, 0.001 g L−1 CuSO4.2H2O. The pH of all culture media was adjusted by using hydrochloric acid (HCl) and sodium hydroxide (NaOH) solution at 7 ± 0.3. Pyrene, glucose (200 mg/L), crude oil and olive oil (2% V/V) were used as the sources of carbon and energy for bacterial growth and each one were transferred to a separate culture medium. Glucose and olive oil were selected according to literature for their enhancing effect on biosurfactant production. The flasks containing enriched media were incubated at 31 °C for 7 days at 180 rpm. The bacterial growth was monitored by measuring the absorbance at 600 nm. After 7 days of incubation, 5 mL of supernatant were transferred into flask of fresh phosphate mineral salt (PMS) medium. This procedure was repeated six times. At the end of this step, several pure strains were isolated for each carbon source and again transferred to liquid culture media for evaluation and screening of biosurfactant producing bacteria under sterile conditions [16–18].
Screening of biosurfactant producing strain
After purification and selection of bacterial strains for each carbon source, nine bacterial isolates (Table 1), were used for screening of biosurfactant production. 5 mL of pure strains were transferred to liquid culture medium as inoculums with OD 600 nm = 1 and to screen biosurfactant-producing strain, oil displacement and surface tension tests were carried out [19].
Table 1.
The number of pure bacterial strains for each carbon source
| Carbon source | Strains Number | Strain code |
|---|---|---|
| Pyrene | 2 | R1 |
| R2 | ||
| Glucose | 3 | R3 |
| R4 | ||
| R5 | ||
| olive oil | 2 | R6 |
| R7 | ||
| Crude oil | 2 | R8 |
Oil displacement test
Bacterial cells were harvested by centrifugation (5000 rpm for 15 min). Then, 40 mL of distilled water was added into a plate and 15 μL of crude oil was added to the surface. Afterwards, 15 μL culture solution was added to the oil surface. Spreading medium on the surface of oil is a criterion for the presence of surface active agents in the medium. The radius of dispersion and the hole created by the liquid medium were measured for the presence or absence of biosurfactant. The distilled water and sodium dodecyl sulphate (SDS) were used as negative and positive witness tests [6, 20].
Extraction of biosurfactant
The liquid medium was centrifuged (10,000 rpm for 15 min) to remove bacterial cells. Then, pH was adjusted to 2 by using HCl (2 N) to achieve the complete precipitation of biosurfactant. The mixture was kept at 4 °C for 24 h. The mixture was again centrifuged for 15 min at 10000 rpm. The resulting precipitates were extracted by chloroform: methanol (2:1 v/v) solvent system. The ratio of water to solvent was 3 to 2. Solvent evaporation in vacuum conditions resulted in the production of pure biosurfactant [21].
Biosurfactant characterization
Emulsification index (E24) test
E24 test was used to evaluate the emulsification activity. Briefly, 2 mL n-Hexadecane and 2 mL strains grown inside the test tubes were added to the mineral medium, shaken for 2 min and left to stand for 24 h. Then, the emulsification index (E24) was evaluated as the percentage of the height of the emulsified layer (mm) divided by the total height of the liquid column (mm) at each sample for each carbon source at each sample, according to Eq. (1).
| 1 |
where, E24 is the emulsification activity after 24 h, HEL is the height of the emulsified layer, and HS is the height of the total liquid column [22].
Critical micelle concentration (CMC) measurement
The efficiency of biosurfactant can be determined by critical micelle concentration (CMC), that displays the minimum concentration required for stability of surface tension. The surface tension of the biosurfactant reaches the lowest amount at CMC, and the concentrations exceeding CMC, have no reduction effect on surface tension by biosurfactant. Initially, serially diluted concentrations of the biosurfactant solution were prepared and their surface tension was measured and the CMC was calculated by drawing the surface tension values against the concentration of the solution biosurfactant. In this study, sodium dodecyl sulphate (SDS) was used to compare chemical surfactant with biological surfactant [23].
Chemical composition
The chemical structure of extracted biosurfactant was identified by liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis (Model: Q-trap 3200, USA).
Factors affecting on biosurfactant production efficiency
Effect of different nitrogen sources (ammonium chloride, urea and ammonium sulfate) and salinity levels (0–5%) on surface tension of the biosurfactants produced by Pesudomonas areuginosa strain R4 was evaluated. This effect was determined through measurement of the solution surface tension. The surface tension of the solution was measured using a Model 10 Tensiometer (Khushboo Sci. Co., Mumbai) employing the Du-Nouy ring method [23].
Pyrene desorption assay
The performance of biosurfactant produced on the pyrene desorption in contaminated soil was determined and the effects of operational parameters including, CMC (1,2,3 and 5), reaction time (6 to 72 h) and stirring speed (100 to 200 rpm) were investigated, according to one factor at the time. The pyrene desorption efficiency was calculated using Eq. (2)
| 2 |
where, qt is pyrene desorption efficiency in time t (mg/kg), C0 is the pyrene concentration in the aqueous phase at the beginning of the reaction (mg/L) and Ct is the the pyrene concentration in the aqueous phase at different reaction times (mg/L).
Results and discussion
Soil characterization
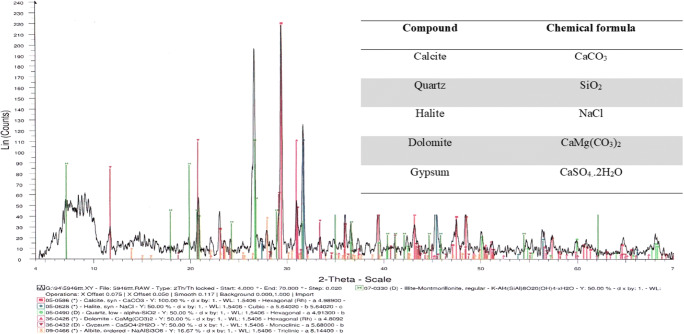
Sampling was made of points with old pollution to petroleum hydrocarbons. Therefore, in these areas, there is the probability of finding microorganisms that are potentially biosurfactant producing strains. Physical and chemical characteristics of soil are shown in Table 2. According to the obtained results, soil was sandy clay loam with 21.6% clay, 10.03% silt and 68.91% sand. The dominant elements were SiO2, CaO, Cl and over 20% organic matter. The pH value of the soil sample was about 7 and in the neutral range. In addition the crystalline structure is presented in Fig. 1.
Table 2.
Physical and chemical characteristics of the studied soil obtained by X-ray diffraction and X-ray fluorescence analysis
| Parameter | Amount (%) |
|---|---|
| Clay | 21.06 |
| Silt | 10.03 |
| Sand | 68.91 |
| Na | 4.4 |
| TiO2 | 0.28 |
| MgO | 4.8 |
| Cl | 7.5 |
| Fe2O3 | 3.3 |
| Al2O3 | 4.5 |
| K2O | 1.5 |
| L.O.I | 22.6 |
| SiO2 | 26.5 |
| CaO | 24.3 |
| La and Lu | 1> |
| pH | 7 |
| EC (μS/cm) | 46,000 |
| Soil type | Sandy clay loam |
Fig. 1.
X-ray diffraction analysis of the studied soil sample
Biosurfactant producing strain
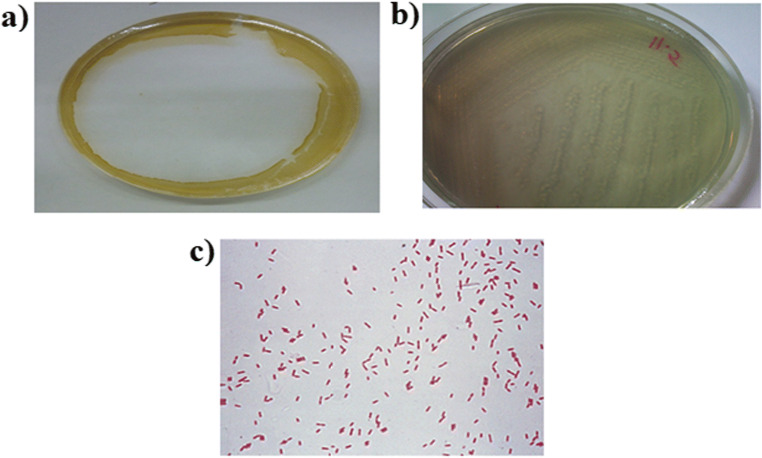
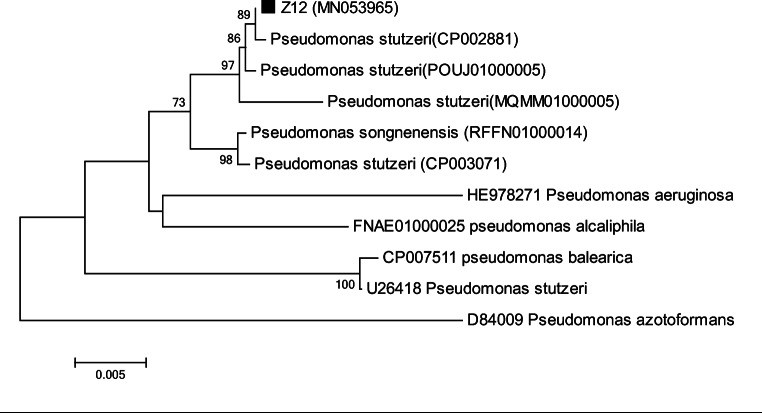
The choice of suitable substrate for the optimal growth of microorganisms is an important factor, determining the destiny of biosurfactant production by microorganisms [24, 25]. Therefore, different carbon sources namely pyrene, glucose, olive oil, and crude oil were used to evaluate the suitable carbon source for maximum production of biosurfactant. Based on the method described in section 2.4, bacterial strains were screened according to Table 3. Different studies showed that the measurement of surface tension of culture medium indicates the production of biosurfactant and an appropriate feature of the microbial strains examined in petroleum hydrocarbon decomposition [15]. On the other hand, the oil displacement method is a criterion for the presence of surface active agents in media [26]. Therefore, to determine the of biosurfactant producing strain and primary screening, oil displacement test and surface tension were performed and the corresponding results are shown in Table 3. Results showed that among nine different strains, strain R4 (source of carbon: glucose) with capability to create a cavity with a diameter of 4.4 cm and reduction of solution surface tension to 32.5 mN/m was selected (Fig.2a and b) for further experiments. Microscopic, biochemical, and genetic analyses showed that strain R4 was classified as gram-negative and rod-shape bacteria called Pseudomonas areuginosa (Fig. 2c). The sequence data was submitted to the NCBI Gen Bank database under the accession number MN053965. Phylogen of tree of Pesudomonas areuginosa strain R4 is presented in Fig. 3.
Table 3.
Isolation and purification of biosurfactant producing strain
| Row | Strain Code | Cavity diameter (cm) | surface tension (mN/m) | carbon source |
|---|---|---|---|---|
| 1 | R1 | 1.5 | 53.5 | pyrene |
| 2 | R2 | 1.9 | 51.2 | pyrene |
| 3 | R3 | 2.3 | 47.8 | Glucose |
| 4 | R4 | 4.3 | 32.5 | Glucose |
| 5 | R5 | 3.1 | 39.3 | Glucose |
| 6 | R6 | 3.6 | 38.47 | olive oil |
| 7 | R7 | 1.2 | 56.6 | olive oil |
| 8 | R8 | 1.4 | 48.3 | Crude oil |
| 9 | R9 | 1.8 | 50.7 | Crude oil |
Fig. 2.
a Results of oil spreading test, b Pure bacterial strain R4 and c the picture of gram stain Pseudomonas aeruginosa R4 strain
Fig. 3.
Phylogenetic tree of the strain Pseudomonas areuginosa strain R4 based on 16S rDNA sequences
Characterization of biosurfactant
CMC
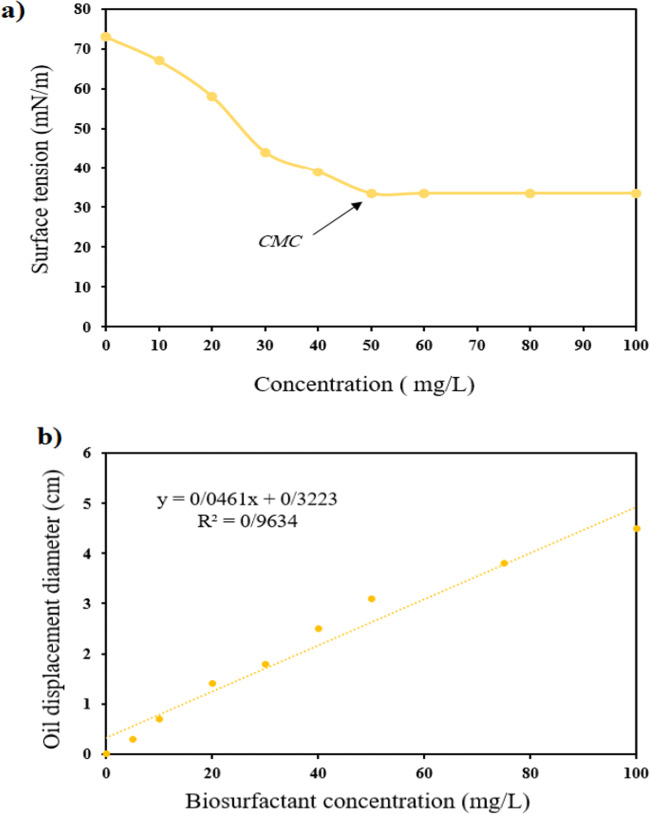
CMC is an important parameter which shows the surface activity of a biosurfactant and the solubility in aqueous phase, affecting the primary characteristics of the biosurfactant [27]. In current work, CMC of extracted biosurfactant was evaluated by measuring the surface tension at varying dilutions (10- to100 mg/L) of the samples. According to Fig. 4a, CMC of extracted biosurfactant was about 50 mg/L, which reduced surface tension to 32.5 mN/ m. Also, according to Fig. 4b, the efficiency of produced biosurfactant was 91 mg/L. Ayed et al. [28] reported that the biosurfactant produced by the Bacillus strain An6 was able to reduce culture-broth surface tension to below 30 mN/m and the CMC concentration of about 100 mg/L, which is higher than the values obtained in the present study, and indicates the potential of production of biosurfactants over chemical alternatives [28]. Yin et al. reported that the CMC concentration of the biosurfactant produced by Pseudomonas aeruginosa S6 equivalent was 50 mg/L, which is similar to the results obtained in this study. Different carbon and nitrogen sources and in general, bacterial type and cultivation conditions may be the cause of these differences [29].
Fig. 4.
a CMC value of biosurfactant produced and b oil spreading test for evaluation of biosurfactant yield
Chemical compositions
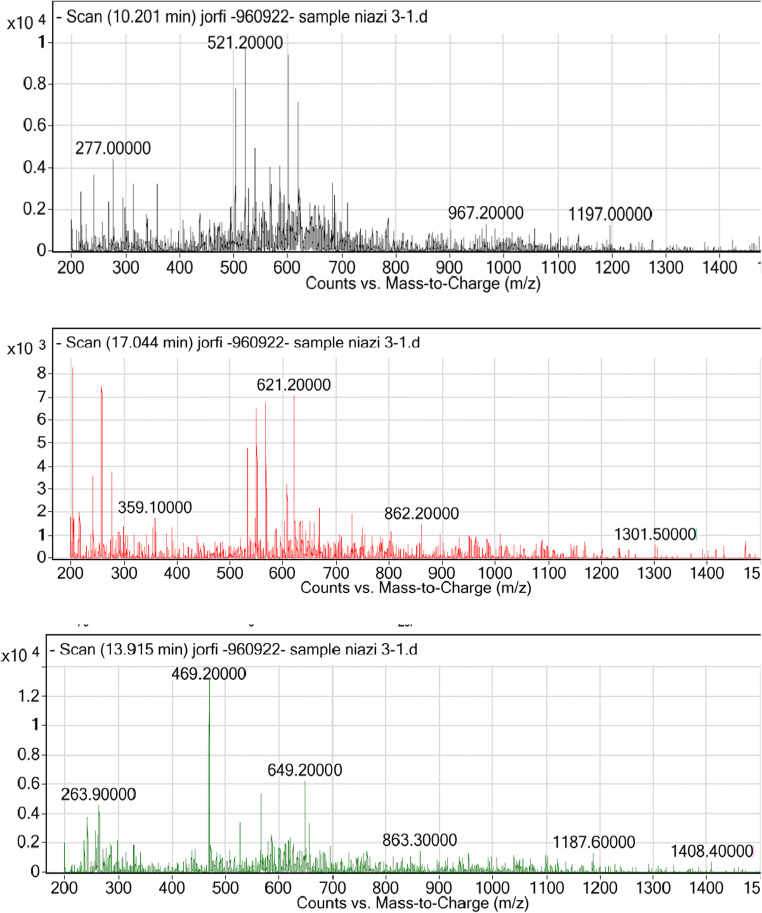
According to the chromatograms in Fig. 5 and Table 4, the biosurfactant by Pseudomonas aeruginosa strain R4 consists of three components, which is a case of a rhamnose molecule attached to fatty acids and two other rhamnose molecules bound to fatty acids containing from 8 to 10 carbons. LC MS/MS analysis revealed three main components at m/z equivalent to 496.2, 503.3 and 621.2, which represented the RhaC8C10, RhaC10C10 and RhaRhaC8C10 molecules, respectively. According to the obtained results, the produced biosurfactant was of the rhamnolipid.
Fig. 5.
Liquid chromatography tandem mass spectrometry (LC–MS/MS) spectra of the biosurfactant produced by Pesudomonas areuginosa R4
Table 4.
Chemical structure of rhamnolipid produced Pesudomonas areuginosa R4
| Biosurfactant structure | Retention time (min) | Molecular ion peak (m/z) | (%) |
|---|---|---|---|
| RhaC8C10 | 10.201 | 469.2 | 13,414 |
| RhaRhaC8C10 | 17.044 | 621.2 | 7097 |
| RhaC10C10 | 10.221 | 503.3 | 7815 |
Emulsification index (E24)
The emulsification index of rhamnolipid produced by Pesudomonas areuginosa R4 for various hydrocarbons was presented in Table 5. According to the obtained results, the highest emulsifier attributed to oil diesel and equals to 56% and the lowest was 40% for n-Hexane.
Table 5.
The emulsification index (E24) produced biosurfactant for various hydrocarbons
| Hydrocarbon | Emulsification Index E(24) % |
|---|---|
| Oil diesel | 56 |
| Crude oil | 44 |
| Hexane | 47 |
| Crude oil | 52 |
| n-Hexane | 40 |
Effect of culture conditions on biosurfactant production
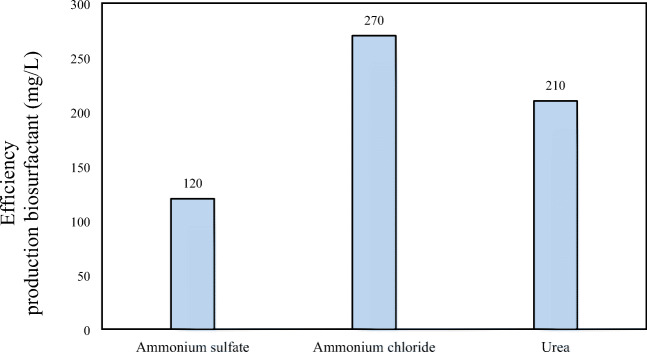
Effect of nitrogen source
The nitrogen source is an important factor that affects the quality and production rate of biosurfactant, according to the origin of microorganisms [30]. The effect of nitrogen sources including ammonium chloride, urea and ammonium sulfate on the biosurfactant production efficiency was investigated. Results showed that addition of nitrogen has a great effect on cell growth and reduced the surface tension. As can be seen in Fig.6, ammonium chloride had the best results with a yield of 90 mg/l. The nitrogen source in the environment is very important for metabolism and bacterial functions. In metabolism and especially cellular synthesis, in the absence of ammonia nitrogen, double energy was used to convert nitrogen to ammonia. In a study by Abouseoud et al. [31] for production of biosurfactant by Pseudomonas fluorescens strain, olive oil and ammonium nitrate were used, as the best carbon and nitrogen sources, respectively in which the similar to the the current research were obtained [31]. Bagheri et al. used glucose as a source of carbon and ammonium sulfate as a source of nitrogen in rhamnolipid produced by Pseudomonas aeruginosa [20]. In other studies for the production of biosurfactant by different bacterial strains including Aeromonas, Bacillus subtilis and Nocardia, the sources of nitrogen were ammonium chloride, ammonium sulfate and niobium extract as optimal conditions. As mentioned, different sources of carbon and nitrogen for producing biosurfactants and especially biosurfactant rhamnolipid have been introduced from different bacterial species (especially pseudomonads) as optimal sources of carbon and nitrogen [6, 11, 30]. The main cause of these differences is the different bacterial origin and changing environmental conditions in each study which leads to the selection of different sources as an optimized source; therefore, the optimum conditions should be determined in each study and region.
Fig. 6.
Effect of nitrogen source on the biosurfactant produced by Pesudomonas areuginosa R4
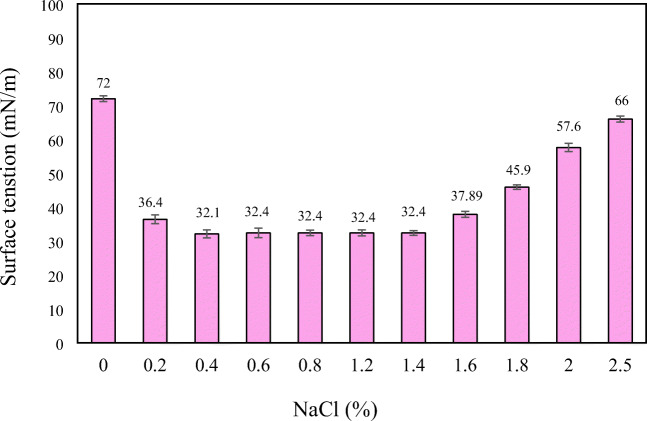
Effect of salinity
Since the isolated bacteria was a halotolerant strain and many marine and petroleum-contaminated soils have a saline environment, the effect of salinity on the performance of Pseudomonas aeruginosa in the production of biosurfactant was studied by measuring the surface tension of media. According to the results presented in Fig. 7, the reduction of surface tension in media containing Pseudomonas aeruginosa was maintained over 1.4% concentration in the best quality (32.1 mN/m) and in higher salt concentrations, the surface tension of solution gradually increased. So, salinity in the range of 0.4–1.4% (in terms of NaCl) did not show a negative effect on the performance of Pseudomonas aeruginosa in production of biosurfactant. Halophiles bacteria have two mechanisms to overcome the salinity of the environment: first, high concentrations of a balancing agent such as K+ are found to be equivalent to the surrounding media within the bacterial cell and prevent cell plasmolysis. The bacteria also contain proteins with low levels of non-polar amino acids that help tolerating salinity. In various studies on the production of biosurfactant, various salinity values have been introduced as optimal conditions. Kiran et al. [6] reported the highest yield of biosurfactant in salinity level of 2% and claimed that the biosurfactant production rate was stable to 5% salinity [6]. In another study, Abouseoud et al. [31] did not show a significant effect on the biosurfactants production rate with salinity up to 20% [31]. We believe that the kind and origin of bacteria and the interactions between the various factors affecting the production of biosurfactant resulted in higher yields in salinity levels of 0.4–1.4% in the current study.
Fig. 7.
Effect of salinity (NaCl%) on the biosurfactant produced by Pesudomonas areuginosa R4
Pyrene desorption
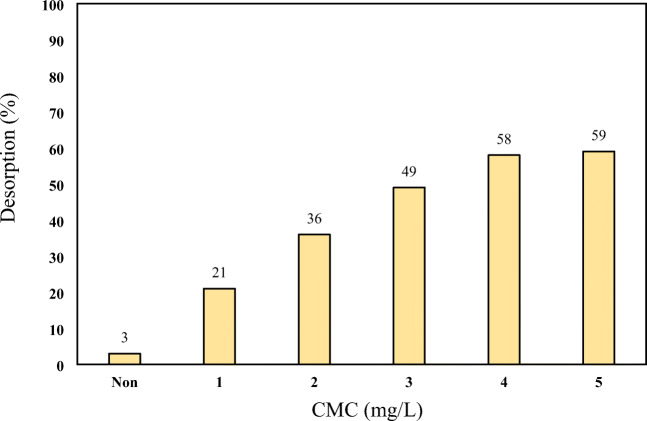
Effect of CMC
To select the proper concentration of biosurfactant, the effect of biosurfactant quantity (CMC) in the range of 0–5 mg/L was investigated at neutral pH and 24 h contact time. The results obtained in Fig.9 showed that by increasing the CMC concentration, the pyrene desorption efficiency increased and the highest desorption rates were 58 and 59% in CMC concentrations of 4 and 5 mg/L, respectively. The advantage of the biosurfactant-containing samples would be their ability to dissolve hydrophobic pyrene and the availability was attributed to bacteria. Water molecules can form hydrogen bonds, which is why the intermolecular forces and surface tension of water are very strong. Given that the molecule is polar water and the surfactants have a polar and a non-polar one, the polar heads of the surfactant molecules are adhered to water molecule and their non-polar tail is placed adjacent to and away from water molecules. Consequently, as the surfactant molecules are placed between water molecules, as shown in Fig. 8, the hydrogen bonding of the water molecules breaks down and consequently, the surface tension of the water significantly decreases. This leads to the dissolution of hydrophobic contaminants in the fluid. It is natural that the higher the biosurfactant is, the more powerful this study is, and the current study also confirms this theory.
Fig. 9.
Effect of contact time on the pyrene desorption from soil (pH = 7, CMC concentration = 4 mg/L, pyrene concentration = 200 mg/kg and stirring speed = 100 rpm)
Fig. 8.
Effect of CMC concentration on the pyrene desorption from soil (pH = 7, contact time 24 h, pyrene concentration = 200 mg/kg and stirring speed = 100 rpm)
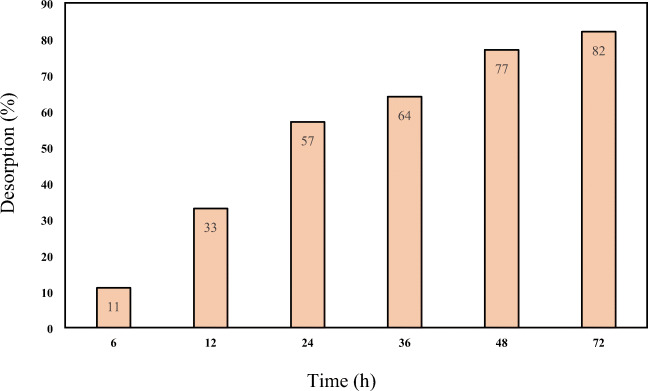
Effect of contact time
The effect of the reaction time on the percolation of the soil at the CMC level 4 mg/L and at the reaction time for zero to 72 h was presented in Fig. 9. According to the obtained results, the desorption efficiencies of 57, 64, 72 and 82% at contact times 24, 36, 48 and 72 h were obtained, respectively. Results indicated that the desorption rate represented an increasing trend along with contact time. At higher contact time the pyrene removal was further increased, due to the greater dissolution of the petroleum compounds in the biosurfactant. But, to determine the optimum time for washing, less energy is required. Hence, the reaction time of 48 h was considered as an optimum time.
Effect of stirring speed
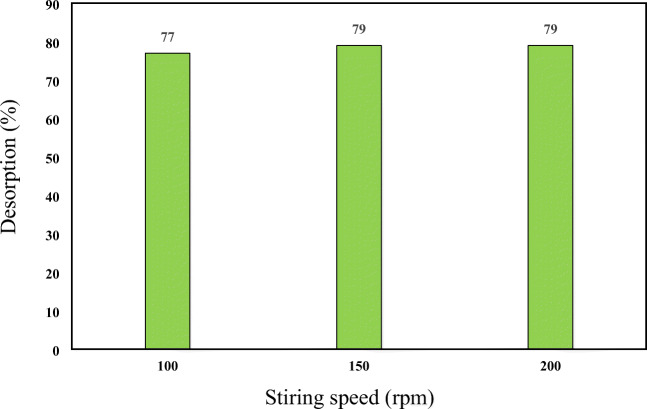
With increasing the washing speed, the pollutant removal rate increases, due to increasing soil particles collision which results in crushing of soil particles and separation of its hydrocarbon compounds which can be easily reacted with biosurfactant. On the other hand, increasing the speed of washing creates a bulky foam and reduces the collision of the biosurfactant molecules with petroleum components which in turn leads to lower removal rate. Therefore, the effect of the stirring speed from 100 to 200 rpm at reaction time 48 h and the CMC value of 4 was investigated. The results are presented in Fig. 10 which showed that the stirring speed had no effect on pyrene desorption.
Fig. 10.
Effect of stirring speed on the pyrene desorption from soil (pH = 7, CMC concentration = 4 mg/L, pyrene concentration = 200 mg/kg and contact time = 48 h)
Desorption of total petroleum hydrocarbons (TPH) from naturally contaminated soil samples
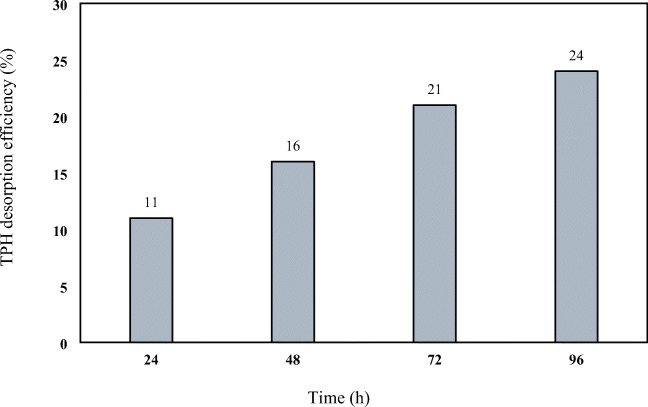
To investigate the performance of rhamnolipid biosurfactant produced by Pseudomonas aeruginosa in total petroleum hydrocarbons (TPH) removal, in optimal conditions obtained from previous steps on a sample with artificial contamination, were used From one sample the soil is not washed. As shown in Fig. 11, TPH desorption rates at reaction times 24, 48, 72 and 96 h were 11, 16, 21 and 24%, respectively.
Fig. 11.
TPH desorption efficiency using biosurfactant produced by Pseudomonas aeruginosa R4
Conclusions
In this study, nine strains of pyrene-degrading bacteria were isolated. With primary screening, a Pseudomonas aeruginosar4 strain was isolated and purified in the laboratory. Pseudomonas aeruginosa strain was able to produce a rhamnolipid biosurfactant. The optimum operational conditions of biosurfactant production were included the carbon source of Glucose, nitrogen source of ammonium chloride and salinity of 1.4%. The surface tension of the solution containing biosurfactant decreased to 32.5 mN/m. The CMC concentration of the produced biosurfactant was obtained to be 50 mg/L. The yield of biosurfactant production was 91 mg/L in the optimum conditions. Also, the pyrene desorption efficiencies were 58 and 59% in CMC 4 and 5, respectively. A rhamnolipid can be used for removal of total petroleum hydrocarbons (TPH) from contaminated soil. According to data obtained from this study, it can be concluded that biological remediation in optimum conditions can be an efficient, viable and competitive alternative for PAHs-contaminated soils.
Acknowledgments
This paper was issued from the thesis of Forud Niyazi under grant number ETRC-9526. Special thanks to Ahvaz Jundishapur University of Medical Sciences for their supports in conducting the current research.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mehdi Ahmadi, Email: ahmadi241@gmail.com.
Forud Niazi, Email: forudniazi@yahoo.com.
Neematollah Jaafarzadeh, Email: n.jaafarzade@yahoo.com.
Shokouh Ghafari, Email: shokouh_gh@ymail.com.
Sahand Jorfi, Email: sahand369@yahoo.com.
References
- 1.Abdel-Shafy HI, Mansour MS. A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt J Pet. 2016;25(1):107–123. [Google Scholar]
- 2.Bento FM, Camargo FA, Okeke BC, Frankenberger WT. Comparative bioremediation of soils contaminated with diesel oil by natural attenuation, biostimulation and bioaugmentation. Bioresour Technol. 2005;96:1049–1055. doi: 10.1016/j.biortech.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Ravindra K, Sokhi R, Van Grieken R. Atmospheric polycyclic aromatic hydrocarbons: source attribution, emission factors and regulation. Atmos Environ. 2008;42:2895–2921. doi: 10.1016/j.atmosenv.2007.12.010. [DOI] [Google Scholar]
- 4.Freire C, Abril A, Fernández M, Ramos R, Estarlich M, Manrique A, et al. Urinary 1-hydroxypyrene and PAH exposure in 4-year-old Spanish children. Sci Total Environ. 2009;407:1562–1569. doi: 10.1016/j.scitotenv.2008.10.068. [DOI] [PubMed] [Google Scholar]
- 5.Guo C, Zhou H, Wong Y, Tam N. Isolation of PAH-degrading bacteria from mangrove sediments and their biodegradation potential. Mar Pollut Bull. 2005;51:1054–1061. doi: 10.1016/j.marpolbul.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Kiran GS, Thomas TA, Selvin J. Production of a new glycolipid biosurfactant from marine Nocardiopsis lucentensis MSA04 in solid-state cultivation. Colloids Surf. B. 2010;78:8–16. doi: 10.1016/j.colsurfb.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 7.U.S. EPA . T. t. f. s. c. a. s. r. O., and of solid waste and emergency response. 10 2001. [Google Scholar]
- 8.Urum K, Grigson S, Pekdemir T, McMenamy S. A comparison of the efficiency of different surfactants for removal of crude oil from contaminated soils. Chemosphere. 2006;62:1403–1410. doi: 10.1016/j.chemosphere.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Urum K, Pekdemir T, Gopur M. Optimum conditions for washing of crude oil-contaminated soil with biosurfactant solutions. Process Saf Environ. 2003;81:203–209. doi: 10.1205/095758203765639906. [DOI] [Google Scholar]
- 10.Urum K, Pekdemir T, Çopur M. Surfactants treatment of crude oil contaminated soils. J Colloid Interf Sci. 2004;276:456–464. doi: 10.1016/j.jcis.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 11.Ilori M, Amobi C, Odocha A. Factors affecting biosurfactant production by oil degrading Aeromonas spp. isolated from a tropical environment. Chemosphere. 2005;61:985–992. doi: 10.1016/j.chemosphere.2005.03.066. [DOI] [PubMed] [Google Scholar]
- 12.Mehdi H, Giti E. Investigation of alkane biodegradation using the microtiter plate method and correlation between biofilm formation, biosurfactant production and crude oil biodegradation. Int Biodeterior Biodegrad. 2008;62:170–178. doi: 10.1016/j.ibiod.2008.01.004. [DOI] [Google Scholar]
- 13.Saeki H, Sasaki M, Komatsu K, Miura A, Matsuda H. Oil spill remediation by using the remediation agent JE1058BS that contains a biosurfactant produced by Gordonia sp. strain JE-1058. Bioresour Technol. 2009;100:572–577. doi: 10.1016/j.biortech.2008.06.046. [DOI] [PubMed] [Google Scholar]
- 14.Varjani SJ, Upasani VN. Critical review on biosurfactant analysis, purification and characterization using rhamnolipid as a model biosurfactant. Bioresour Technol. 2017;232:389–397. doi: 10.1016/j.biortech.2017.02.047. [DOI] [PubMed] [Google Scholar]
- 15.Jorfi S, Rezaee A, Moheb-ali G-a, alah Jaafarzadeh N. Pyrene removal from contaminated soils by modified Fenton oxidation using iron nano particles. J Environ Health Sci. 2013;11:17. doi: 10.1186/2052-336X-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaskin S, Bentham R. Comparison of enrichment methods for the isolation of pyrene-degrading bacteria. Int Biodeterior Biodegradation. 2005;56:80–85. doi: 10.1016/j.ibiod.2005.04.004. [DOI] [Google Scholar]
- 17.Jorfi S, Rezaee A, Mobeh-Ali G-A, Jaafarzadeh NA. Application of biosurfactants produced by Pseudomonas aeruginosa SP4 for bioremediation of soils contaminated by pyrene. Soil Sediment Contam. 2013;22:890–911. doi: 10.1080/15320383.2013.770439. [DOI] [Google Scholar]
- 18.Nayak AS, Vijaykumar M, Karegoudar T. Characterization of biosurfactant produced by Pseudoxanthomonas sp. PNK-04 and its application in bioremediation. Int Biodeterior Biodegradation. 2009;63:73–79. doi: 10.1016/j.ibiod.2008.07.003. [DOI] [Google Scholar]
- 19.Adebajo S, Akintokun A, Bolaji S. Biosurfactants producing bacteria from oil-polluted soil in Abeokuta, Ogun state. IFE J Sci. 2018;20:287–297. doi: 10.4314/ijs.v20i2.9. [DOI] [Google Scholar]
- 20.Lotfabad TB, Shourian M, Roostaazad R, Najafabadi AR, Adelzadeh MR, Noghabi KA. An efficient biosurfactant-producing bacterium Pseudomonas aeruginosa MR01, isolated from oil excavation areas in south of Iran. Colloids Surf. B. 2009;69:183–193. doi: 10.1016/j.colsurfb.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 21.Darvishi P, Ayatollahi S, Mowla D, Niazi A. Biosurfactant production under extreme environmental conditions by an efficient microbial consortium, ERCPPI-2. Colloids Surf B. 2011;84:292–300. doi: 10.1016/j.colsurfb.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Talaie AR, Talaie MR, Haghighifar NJ. Optimizing biodegradation of floating diesel fuel contaminated wastewater using the Taguchy method. J Water Wastewater. 2009;20:57–68. [Google Scholar]
- 23.Assadi T, Bargahi A, Nabipour I, Mohebbi GH, Kholdebarin B, Mohajerani S, et al. Determination of fatty acid composition of halophyte plant (Suaeda vermiculata) collected from the shorelines of Persian gulf region (Bushehr Province) ISMJ. 2014;17:638–646. [Google Scholar]
- 24.Adamczak M, odzimierz Bednarski W. Influence of medium composition and aeration on the synthesis of biosurfactants produced by Candida antarctica. Biotechnol Lett. 2000;22:313–316. doi: 10.1023/A:1005634802997. [DOI] [Google Scholar]
- 25.Robert M, Mercade M, Bosch M, Parra J, Espuny M, Manresa M, et al. Effect of the carbon source on biosurfactant production byPseudomonas aeruginosa 44T1. Biotechnol Lett. 1989;11:871–874. doi: 10.1007/BF01026843. [DOI] [Google Scholar]
- 26.Bezza FA, Chirwa EMN. Biosurfactant from Paenibacillus dendritiformis and its application in assisting polycyclic aromatic hydrocarbon (PAH) and motor oil sludge removal from contaminated soil and sand media. Process Saf Environ. 2015;98:354–364. doi: 10.1016/j.psep.2015.09.004. [DOI] [Google Scholar]
- 27.Sousa M, Melo V, Rodrigues S, Sant’ana H, Gonçalves L. Screening of biosurfactant-producing Bacillus strains using glycerol from the biodiesel synthesis as main carbon source. Bioprocess Biosyst Eng. 2012;35:897–906. doi: 10.1007/s00449-011-0674-0. [DOI] [PubMed] [Google Scholar]
- 28.Ayed HB, Jemil N, Maalej H, Bayoudh A, Hmidet N, Nasri M. Enhancement of solubilization and biodegradation of diesel oil by biosurfactant from Bacillus amyloliquefaciens An6. Int Biodeterior Biodegradation. 2015;99:8–14. doi: 10.1016/j.ibiod.2014.12.009. [DOI] [Google Scholar]
- 29.Yin H, Qiang J, Jia Y, Ye J, Peng H, Qin H, et al. Characteristics of biosurfactant produced by Pseudomonas aeruginosa S6 isolated from oil-containing wastewater. Process Biochem. 2009;44:302–308. doi: 10.1016/j.procbio.2008.11.003. [DOI] [Google Scholar]
- 30.Joshi S, Bharucha C, Desai AJ. Production of biosurfactant and antifungal compound by fermented food isolate Bacillus subtilis 20B. Bioresour Technol. 2008;99:4603–4608. doi: 10.1016/j.biortech.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 31.Abouseoud M, Maachi R, Amrane A, Boudergua S, Nabi A. Evaluation of different carbon and nitrogen sources in production of biosurfactant by Pseudomonas fluorescens. Desalination. 2008;223:143–151. doi: 10.1016/j.desal.2007.01.198. [DOI] [Google Scholar]