Abstract
It has been known that retinal vein occlusion (RVO) is associated with chronic kidney disease, especially end-stage renal disease (ESRD). However, little is known about the effect of kidney transplantation (KT) on RVO incidence in ESRD patients. This study aimed to compare the incidence of RVO in KT recipients (n = 10,498), matched ESRD patients (n = 10,498), and healthy controls (HCs, n = 10,498), using a long-term population-based cohort. The incidence of RVO was 2.74, 5.68, and 1.02 per 1000 patient-years, for the KT group, the ESRD group, and the HCs group, respectively. Adjusted hazard ratios for RVO development compared to the HCs group, were 1.53 and 3.21, in the KT group and the ESRD group, respectively. In the KT group, multivariable regression analysis indicated that an age over 50, a Charlson Comorbidity Index score over 4, and a history of desensitization therapy were associated with an increased risk of RVO. In summary, KT recipients have a lower risk for development of RVO than ESRD patients treated with dialysis. However, the risk is still higher compared to healthy people who have normal kidney functions.
Subject terms: Retinal diseases, End-stage renal disease
Introduction
Retinal vein occlusion (RVO) is one of the most common causes of significant visual loss in developed countries1. The prevalence of RVO in hospital-based samples is variable, but prior work suggests an incidence range of 0.3–1.6% in elderly populations2. RVO risk is associated with systemic conditions, including hematologic abnormalities, cardiovascular disease, hypertension, diabetes mellitus (DM), smoking, dyslipidemia, and angina3,4.
End-stage renal disease (ESRD), the most severe form of late-stage chronic kidney disease (CKD), is associated with an enhanced mortality5,6. A steady increase in ESRD incidence has been observed globally, and subsequently kidney transplantation (KT), a highly effective replacement therapy for patients with ESRD, is also increasing in number7,8.
Recently, an association between ESRD and RVO has been reported. Comorbidities, as risk factors of RVO mentioned above, are more common in ESRD patients than in the general population9–11. This does not fully explain the increased risk of RVO in ESRD, however, common pathogenic mechanisms for ESRD, including vascular endothelial dysfunction and arteriosclerosis, might be contributing to RVO.
In order to prevent the development of RVO in ESRD patients, it is necessary to discover modifiable factors which may affect the RVO incidence. To our knowledge, the relationship between risk of RVO, and management of ESRD through KT, has not been investigated previously. In this study, we retrospectively recruited three patient groups: KT recipients, ESRD patients treated with renal replacement therapy, and healthy controls (HCs). We explored the hazard ratios (HRs) of RVO in these groups by analyzing the information found in the National Health Insurance Service (NHIS) Database of South Korea.
Results
Demographic data
Table 1 shows the baseline characteristics for the ESRD, KT, and HCs groups. The mean age of all participants at index date was 45.9 ± 10.6 years, and 59.1% of patients were male. The ESRD group and KT group exhibited a significantly higher prevalence of DM (P < 0.001), hypertension (P < 0.001), and dyslipidemia (P < 0.001), compared to the HCs group. Income status was classified into five grades. The ESRD group displayed a lower income status compared to the KT and HCs groups (P < 0.001).
Table 1.
Baseline characteristics.
| Variables | ESRD (n = 10,498) | KT (n = 10,498) | Healthy controls (n = 10,498) | P-value |
|---|---|---|---|---|
| Age, years, n (%) | 45.87 ± 10.57 | 45.87 ± 10.57 | 45.87 ± 10.57 | > 0.999 |
| 20–29 | 771 (7.3) | 771 (7.3) | 771 (7.3) | |
| 30–39 | 2186 (20.8) | 2186 (20.8) | 2186 (20.8) | |
| 40–49 | 3378 (32.2) | 3378 (32.2) | 3378 (32.2) | |
| 50–59 | 3139 (29.9) | 3139 (29.9) | 3139 (29.9) | |
| 60–69 | 974 (9.3) | 974 (9.3) | 974 (9.3) | |
| 70–79 | 48 (0.5) | 48 (0.5) | 48 (0.5) | |
| ≥ 80 | 2 (0.02) | 2 (0.02) | 2 (0.02) | |
| Gender, male, n (%) | 6200 (59.1) | 6200 (59.1) | 6200 (59.1) | > 0.999 |
| Index year, n (%) | > 0.999 | |||
| 2007–2009 | 2628 (25.03) | 2628 (25.03) | 2628 (25.03) | |
| 2010–2012 | 3643 (34.7) | 3643 (34.7) | 3643 (34.7) | |
| 2013–2015 | 4227 (40.26) | 4227 (40.26) | 4227 (40.26) | |
| Income status, n (%) | ||||
| Medical aid | 2432 (23.17) | 1496 (14.25) | 261 (2.49) | |
| Q1 | 2895 (27.58) | 2111 (20.11) | 2710 (25.81) | |
| Q2 | 2179 (20.76) | 2056 (19.58) | 2570 (24.48) | |
| Q3 | 1751 (16.68) | 2204 (20.99) | 2449 (23.33) | |
| Q4 | 1241 (11.82) | 2631 (25.06) | 2508 (23.89) | |
| DM, n (%) | 4231 (40.3) | 4231 (40.3) | 661 (6.3) | < 0.001 |
| HTN, n (%) | 9576 (91.22) | 9576 (91.22) | 1748 (16.65) | < 0.001 |
| Dyslipidemia, n (%) | 4441 (42.3) | 5904 (56.24) | 1245 (11.86) | < 0.001 |
| Dialysis modality, n (%) | ||||
| Preemptive | 0 (0) | 3383 (32.23) | 10,498 (100) | |
| Hemodialysis | 7781 (74.12) | 4663 (44.42) | 0 (0) | |
| Peritoneal dialysis | 2145 (20.43) | 1777 (16.93) | 0 (0) | |
| Mixed | 572 (5.45) | 675 (6.43) | 0 (0) | |
| Desensitization, n (%) | < 0.001 | |||
| Yes | 0 (0) | 1619 (15.42) | 0 (0) | |
| No | 10,498 (100) | 8879 (84.58) | 10,498 (100) | |
| Induction, n (%) | < 0.001 | |||
| Not use | 10,498 (100) | 563 (5.36) | 10,498 (100) | |
| ATG | 0 (0) | 884 (8.42) | 0 (0) | |
| Basiliximab | 0 (0) | 9051 (86.22) | 0 (0) | |
| Maintenance, n (%) | < 0.001 | |||
| Not use | 10,498 (100) | 253 (2.41) | 10,498 (100) | |
| Tacrolimus | 0 (0) | 8565 (81.59) | 0 (0) | |
| Cyslosporine | 0 (0) | 1680 (16) | 0 (0) | |
Expressed with the number of cases (proportion). Comparison was performed using student t-test and chi-square test.
ATG anti-thymocyte globulin, CI confidence interval, ESRD end stage renal disease, DM diabetes mellitus, HTN hypertension, Income status divided into four income classes by National health insurance premium (Q1: lowest, Q4: highest), KT kidney transplantation.
Within the ESRD group, 74.1% of patients received hemodialysis, 20.4% of patients received peritoneal dialysis, and 5.5% of patients received both types of dialysis. In the KT recipients, 32.2% of patients had not received any type of renal replacement therapy prior to KT. The average duration for renal replacement therapy was significantly longer in the ESRD group (2.9 ± 3.0 years) compared to the KT group (2.8 ± 3.2 years). In the KT recipients, 95.6% and 15.4% of patients were treated with basiliximab and/or anti-thymocyte globulin as induction therapy, and plasmapheresis and/or rituximab as desensitization therapy, respectively, prior to KT.
Incidence of RVO
The cumulative incidence of RVO in the ESRD group, the KT group, and the HCs group is shown in Table 2. During the follow-up period, the incidence rates were 5.68 for the ESRD group, 2.74 for the KT group, and 1.02 for the HCs group, per 1000 patient-years respectively. Model 3, which accounted for age, sex, DM, hypertension, dyslipidemia, CCI score, and income status, indicated that the adjusted HR (aHR) for RVO development was 3.21 times greater in the ESRD group compared to the HCs group (P < 0.001), and 1.53 times greater in the KT group compared to the HCs group (P = 0.034, Fig. 1, Table 2). The number of subjects at risk of RVO among each group, stratified by time, are provided in Supplementary Table S1. A Model 3 comparison of the ESRD group and the KT group indicated that the aHR was 0.47 times lower in the KT group compared to ESRD group (P < 0.001). Moreover, the incidence of aHR in the ischemic and non-ischemic RVO groups was 0.44 and 0.48, respectively (Table 3).
Table 2.
Incidence of RVO in ESRD group, KT group and healthy controls.
| Outcomes | Groups | RVO | Duration | Rate | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Event | Patient-years | Per 1000 patient-years | Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | |||
| RVO, total | ESRD | 290 | 51,047.6 | 5.68 | 5.54 (4.22, 7.23) | < 0.001 | 5.71 (4.35, 7.50) | < 0.001 | 3.21 (2.19, 4.70) | < 0.001 | |
| KT | 164 | 59,847.8 | 2.74 | 2.70 (2.02, 3.60) | < 0.001 | 2.72 (2.03, 3.64) | < 0.001 | 1.53 (1.02, 2.29) | 0.034 | ||
| Healthy | 63 | 62,050.0 | 1.02 | 1 (ref) | 1 (ref) | 1 (ref) | |||||
| Non-ischemic RVO | ESRD | 235 | 51,047.6 | 4.60 | 5.25 (3.90, 7.06) | < 0.001 | 5.49 (4.02, 7.27) | < 0.001 | 3.08 (2.03, 4.69) | < 0.001 | |
| KT | 135 | 59,847.8 | 2.26 | 2.59 (1.89, 3.56) | < 0.001 | 2.61 (1.91, 3.58) | < 0.001 | 1.49 (0.97, 2.30) | 0.069 | ||
| Healthy | 54 | 62,050.0 | 0.87 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |||||
| Ischemic RVO | ESRD | 55 | 51,047.6 | 1.07 | 7.27 (3.60, 14.72) | < 0.001 | 7.53 (3.72, 15.24) | < 0.001 | 3.89 (1.50, 10.06) | 0.005 | |
| KT | 29 | 59,847.8 | 0.48 | 3.33 (1.58, 7.04) | 0.002 | 3.37 (1.59, 7.11) | 0.002 | 1.75 (0.66, 4.66) | 0.263 | ||
| Healthy | 9 | 62,050.0 | 0.15 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |||||
Analysis was performed using Cox regression. Model 1: not adjusted; Model 2: adjusted with age, sex; Model 3: adjusted with age, sex, diabetes mellitus, hypertension, dyslipidemia, Charlson comorbidity index, income status.
CI confidence interval, ESRD end stage renal disease, KT kidney transplantation, RVO renal vein occlusion.
Figure 1.
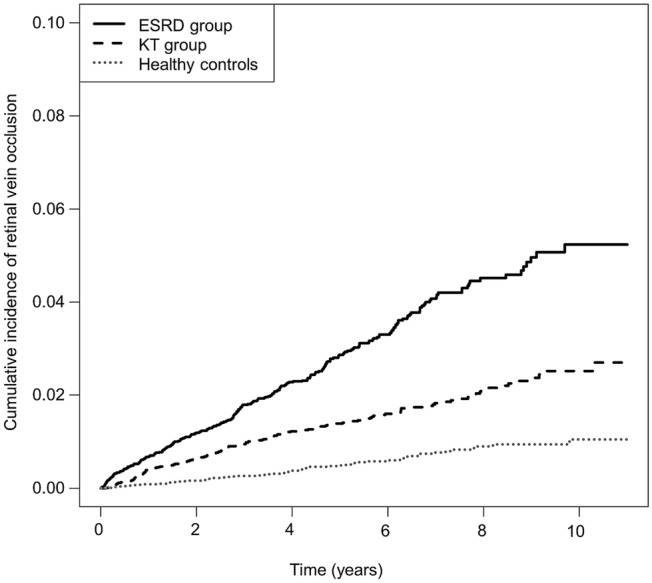
Cumulative incidence of RVO in the ESRD group, the KT group, and healthy controls. ESRD end-stage renal disease, KT kidney transplantation, RVO renal vein occlusion.
Table 3.
Hazard ratio of RVO in ESRD group and KT group.
| Outcomes | Groups | Rate | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|---|
| Per 1000 patient-years | Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | ||
| RVO, total | ESRD | 5.68 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |||
| KT | 2.74 | 0.49 (0.40, 0.59) | < 0.001 | 0.48 (0.40, 0.58) | < 0.001 | 0.47 (0.29, 0.58) | < 0.001 | |
| Non-ischemic RVO | ESRD | 4.60 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |||
| KT | 2.26 | 0.50 (0.4, 0.61) | < 0.001 | 0.49 (0.39, 0.60) | < 0.001 | 0.48 (0.39, 0.60) | < 0.001 | |
| Ischemic RVO | ESRD | 1.07 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |||
| KT | 0.48 | 0.46 (0.30, 0.72) | < 0.001 | 0.45 (0.29, 0.70) | < 0.001 | 0.44 (0.28, 0.70) | < 0.001 | |
Analysis was performed using Cox regression. Model 1: not adjusted; Model 2: adjusted with age, sex; Model 3: adjusted with age, sex, diabetes mellitus, hypertension, dyslipidemia, Charlson comorbidity index, income status.
CI confidence interval, ESRD end stage renal disease, KT kidney transplantation, RVO renal vein occlusion.
Risk factors for RVO in KT groups
In order to determine the potential risk factors for RVO in the KT group, incidence rates were assessed by univariable and multivariable logistic regression models (Table 4). Results from univariable analysis indicate that ages greater than 60 (P < 0.01), CCI scores higher than or equal to 3 (P < 0.01), and the presence of DM (P < 0.001), were significantly associated with the development of RVO. Results from the multivariable regression analysis indicated that a higher age and higher CCI scores are risk factors of RVO in the KT group (P < 0.001). Desensitization therapy is also a potential risk factor for RVO (aHR 1.64; 95% CI 1.09–2.47). The other analyzed factors including sex, income status, DM, hypertension, dyslipidemia, and duration of dialysis, showed no relationship with RVO. Medications used for maintenance and induction of immunosuppression did not have any relationship with RVO.
Table 4.
Risk factors analysis for RVO in KT group.
| Variables | Model 1 | Model 2 | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | |
| Age, years | ||||
| 20–29 | 0.20 (0.74, 0.56) | 0.48 (0.17, 1.36) | ||
| 30–39 | 0.40 (0.24, 0.65) | 0.84 (0.49, 1.43) | ||
| 40–49 | 0.53 (0.36, 0.78) | 1 (ref) | ||
| 50–59 | 1 (ref) | < 0.001 | 1.79 (1.20, 2.66) | < 0.001 |
| 60–69 | 1.69 (1.08, 2.57) | 2.98 (1.83, 4.85) | ||
| 70–79 | 4.26 (1.34, 13.56) | 7.89 (2.40, 5.99) | ||
| ≥ 80 | ||||
| Sex | ||||
| Male | 1 (Ref.) | 0.3145 | 1 (Ref.) | 0.560 |
| Female | 0.85 (0.62, 1.17) | 0.85 (0.62, 1.176) | ||
| DM | ||||
| Yes | 1.96 (1.44, 2.67) | < 0.001 | 1.2(0.86, 1.68) | 0.243 |
| No | 1 (Ref.) | 1 (Ref.) | ||
| HTN | ||||
| Yes | 1.23 (0.68, 2.21) | 0.495 | 1.05 (0.58, 1.91) | 0.846 |
| No | 1 (Ref.) | 1 (Ref.) | ||
| Dyslipidemia | ||||
| Yes | 1.20 (0.88, 1.643) | 0.255 | 1.04 (0.75, 1.43) | 0.820 |
| No | 1 (Ref.) | 1 (Ref.) | ||
| CCI score | ||||
| 0 | < 0.001 | 0.005 | ||
| 1–2 | 1 (Ref.) | 1 (Ref.) | ||
| 3–4 | 1.56 (0.69, 3.51) | 1.36 (0.60, 3.08) | ||
| ≥ 5 | 3.71 (1.73, 7.94) | 2.57 (1.17, 5.65) | ||
| Income status | ||||
| Medical aid | 1 (Ref.) | 0.7285 | 1 (Ref.) | 0.550 |
| Q1 | 1.04 (0.62, 1.72) | 0.88 (0.52, 1.48) | ||
| Q2 | 0.84 (0.49, 1.43) | 0.71 (0.41,1.22) | ||
| Q3 | 0.81 (0.48, 1.38) | 0.66 (0.38, 1.13) | ||
| Q4 | 1.06 (0.66, 1.72) | 0.75 (0.45, 1.24) | ||
| Dialysis modality | ||||
| Preemptive | 0.81 (0.56, 1.17) | 0.187 | 0.96 (0.57, 1.60) | 0.107 |
| Hemodialysis | 1 (Ref.) | 1 (Ref.) | ||
| Peritoneal dialysis | 1.29 (0.87, 1.92) | 1.52 (1.02, 2.28) | ||
| Mixed | 0.81 (0.39, 1.68) | 0.82 (0.39, 1.71) | ||
| Dialysis duration | ||||
| < 1 year | 1 (Ref.) | 0.159 | 1 (Ref.) | 0.809 |
| ≥ 1 year | 1.25 (0.92, 1.71) | 1.07 (0.67, 1.71) | ||
| Desensitization | ||||
| Yes | 1.55 (1.04, 2.30) | 0.030 | 1.64 (1.09, 2.47) | 0.018 |
| No | 1 (Ref.) | 1 (Ref.) | ||
| Induction | ||||
| Not use | 0.86 (0.33, 2.25) | 1.32 (0.48, 3.60) | ||
| ATG | 1 (Ref.) | 0.410 | 1 (Ref.) | 0.580 |
| Basiliximab | 1.30 (0.66, 2.56) | 1.43 (0.72, 2.82) | ||
| Maintenance | ||||
| Not use | 0.82 (0.26, 2.57) | 1.01 (0.31, 3.27) | ||
| Tacrolimus | 1 (Ref.) | 0.773 | 1 (Ref.) | 0.967 |
| Cyslosporine | 0.87 (0.58, 1.32) | 0.94 (0.62, 1.43) | ||
Analysis was performed using univariable and multivariable Cox regression. Model 1: univariable analysis; Model 2: multivariable Cox proportional hazards regression analysis.
ATG anti-thymocyte globulin, CCI Charlson comorbidity index, CI confidence interval, DM diabetes mellitus, HTN hypertension, Income status divided into four income classes by National health insurance premium (Q1: lowest, Q4: highest), KT kidney transplantation, RRT renal replacement therapy, RVO retinal vein occlusion.
Discussion
To our knowledge, this is the first study that has been conducted to explore the relationship between the incidence of RVO and KT in ESRD patients. We found that the risk for RVO in ESRD patients treated with dialysis was 3.21 times greater than in the HCs, while the KT group risk was only 1.53 times greater than in the HCs. In other words, KT could reduce the risk of subsequent RVO development in ESRD patients by about half. In the KT group, RVO development was associated with older age, higher CCI score, and a history of desensitization therapy.
The association between RVO and renal dysfunction has been explored in population-based studies. However, the definition of renal dysfunction differs based upon the study. In the Beaver Dam study, an increased level of serum creatinine was shown to be associated with the incidence of branched retinal vein occlusion (BRVO) in Caucasians4, but this result was not consistent with Wong’s cross-sectional study, which was conducted on 3,280 patients12. The Hisayama study revealed that CKD, which was defined as the presence of proteinuria and/or eGFR < 60 mL/min/1.73 m2, was an independent risk factor for RVO in Japanese populations13. In addition, extensive, longitudinal epidemiological studies, which were performed in Taiwan and South Korea, have also suggested a strong relationship between ESRD and RVO9,11. Similarly, our results further verify that RVO incidence is associated with ESRD.
Several mechanisms are thought to be common in the pathogenesis of RVO and ESRD. Surprisingly, the eye and kidney share many physiological features. For example, the renin–angiotensin–aldosterone hormonal cascade is found in both the kidneys as well as the eyes14. ESRD patients frequently experience retinal microvascular changes, such as retinal arteriolar narrowing, and arteriovenous nicking15. Interestingly, these retinal changes are also common in RVO patients. In addition, retinal microvascular changes are indicators of decreased renal function14, and predictors of RVO development. We believe that vascular endothelial dysfunction as a result of arteriosclerosis, which is the major mechanism of ESRD, contributes to retinal arteriolar changes. Vein compression, as facilitated by sclerotic arteriolar walls, may expedite thrombus formation and result in RVO. In addition, levels of proinflammatory cytokines are elevated in ESRD patients due to decreased renal elimination and increased induction by uremic toxins or oxidative stress16,17. The inflammation associated with these cytokines may promote the pathogenesis of RVO18,19. A hypercoagulable state, which is described in ESRD, can also contribute to the development of RVO. Known risk factors for RVO, such as elevated levels of prothrombin fragment, thrombin-antithrombin complex, and homocysteine20,21, have been described in ESRD patients22,23. Furthermore, repetitive hemodialysis increases the levels of oxidative stress, and stimulates inflammation and coagulation pathways22, which may increase the risk of RVO.
In this study, we found that in comparison to dialysis, KT can reduce the risk of RVO in ESRD patients. We also investigated the effect of dialysis duration and preemption on this result. Dialysis duration was not related to the effect of KT on RVO reduction (Table 4). Preemptive and non-preemptive transplants did not have different RVO risks (P = 0.469) and the RVO reduction effect by KT was still significant even when preemptive transplants were excluded from the KT group (aHR 0.50; 95% CI 0.41, 0.63). We hypothesized that KT diminished the harmful effects of uremic conditions, which are typically seen in ESRD patients on repetitive renal replacement therapy. Therefore, KT led to a protective effect by preventing RVO development. This result was in line with a well-established relationship between ESRD and cardiovascular disease (CVD), which shares common risk factors with RVO. Previous reports show that cardiovascular complications and mortality are reduced in KT recipients when compared to ESRD patients24–26. Interestingly, traditional risk factors (e.g., hypertension, DM, and dyslipidemia) and non-traditional risk factors (e.g., albuminuria, elevated homocysteine levels, extracellular fluid volume overload22, and uremia) are known to elevate the risk of cardiovascular mortality in ESRD27. A decreased risk of CVD in recipients has resulted from the modification of these non-traditional risk factors by KT. The common risk factors and mechanisms of pathogenesis between CVD and RVO suggest a high possibility for a protective effect of KT on RVO development in ESRD patients.
Overall, our result suggests that KT recipients had a smaller risk of RVO compared to patients with ESRD on dialysis. However, we sought to determine which KT recipients retained a higher risk of RVO development. Analysis of the KT patients indicated that older age was a significant risk factor for occurrence of RVO, as shown in previous studies11. Our results also revealed that the CCI score, which helps to predict cardiovascular risk in KT recipients, could be a relevant risk factor for occurrence of RVO28,29. In addition, immunosuppressant agents such as tacrolimus, cyclosporine, anti-thymocyte globulin and basiliximab did not affect the risk of RVO in our study. There have been some reports that cyclosporine is associated with increased hypertension and hyperlipidemia30 and tacrolimus is associated with an increased risk of new-onset diabetes after transplantation31. However, it would be possible that the risk modification for cardiovascular complication after KT as mentioned earlier ameliorated negative effect of immunosuppressant agents. Analogously, the association between the type of immunosuppression and cardiovascular death was not observed in the report investigating the effect of KT on cardiovascular death32. Interestingly, desensitization therapy before KT was a significant risk factor for the occurrence of RVO. Generally, desensitization therapies, including rituximab, plasmapheresis, or intravenous immunoglobulin, have been performed to reduce the risk of organ rejection in certain KT recipients, like ABO incompatible KT or human leukocyte antigen incompatible KT33,34. It is well known that endothelial dysfunction is induced due to up-regulation of various cytokines in sensitized KT recipients35–37. Therefore, we hypothesized that endothelial dysfunction and subsequent atherosclerosis, which are related to pathogenesis of RVO38,39, might contribute to the increased risk of RVO development in KT recipients with desensitization therapy.
Here, we present a large, population-based study to analyze KT recipients against matched ESRD patients and HCs. However, our study has some limitations. First, an ICD-10 code-dependent diagnosis can potentially lead to an inaccurate diagnosis of RVO, ESRD, and other comorbidities. Secondly, we only analyzed data from the NHIS. So detailed clinical information and aspects of the individual patients, such as vision prognosis and involvement of lesions, could not be reflected. Third, we did not distinguish subtypes of RVO, namely, central retinal vein occlusion (CRVO) and BRVO in patients. Therefore, the different pathogeneses and risk factors associated with RVO subtypes might be overlooked40. Forth, there was no information about such as ABO incompatibility or human leukocyte antigen incompatibility of kidney transplantation in our cohort data, so it was impossible to analyze the difference of RVO outcome according to immunologic incompatibility.
In conclusion, we found that KT recipients showed a significantly lower risk for RVO than ESRD patients treated with dialysis. Increasing age, high CCI scores, and desensitization therapy could be risk factors of RVO development in KT recipients. Therefore, although KT might be helpful to prevent RVO in ESRD patients, careful ophthalmic examination should be performed for older KT recipients who present with known comorbidities or a history of desensitization therapy.
Methods
This study was undertaken as a part of the RESTORE (REnal tranSplanT and OculaR disEases study) project, a retrospective, longitudinal, cohort study based on the NHIS database. The primary objective of the RESTORE project was to investigate the effect of KT on the national population-based incidence and prognosis of various eye diseases, including age-related macular degeneration, RVO, and glaucoma. The study protocol was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. E-1908-001-1050) and all methods were carried out in accordance with relevant guidelines and regulations. The need for participant consent was waived by the Institutional Review Board of Seoul National University Hospital because this study collected medical data which is managed anonymously.
Data source and collection
South Korea has a compulsory single-payer health insurance system, managed by the NHIS. All healthcare providers are required to submit medical claims with information including demographics, diagnostic codes based on the International Classification of Diseases (ICD)‐10 codes41, procedure codes, prescription records, and healthcare facilities to the NHIS for review and reimbursement. Therefore, the NHIS claims system acts as a centralized database and provides a nationwide, population-based data source.
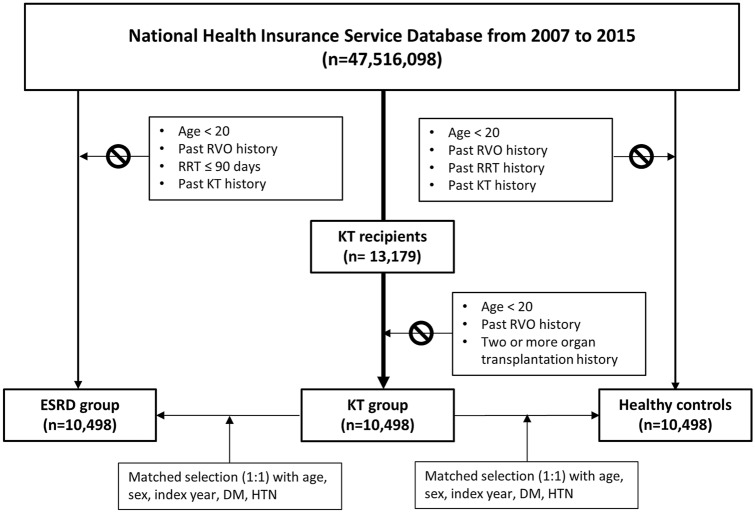
In this study, subjects were recruited from the NHIS database from the year 2007 to 2015. Using specific codes for kidney transplantation (R3280 [KT, ICD-10 code], V005 [KT related treatment, V code for Korean rare incurable diseases]) we were able to identify 13,179 KT recipients. Patients were excluded if they (1) were less than 20 years old, (2) had previously been diagnosed with RVO, and (3) had received two or more organ transplantations. After exclusions, we analyzed 10,498 KT recipients. The ESRD group consisted of patients identified by the ICD-10 codes (N18-19, Z49, Z94.0, Z99.2) and the procedural codes of renal replacement therapy (hemodialysis [O7011-7020, V001] or peritoneal dialysis [O7071-7075, V003]). Patients were required to have been diagnosed with ESRD for more than 3 months, and had not previously received a KT. By considering age, sex, inclusion year, underlying hypertension and DM, the ESRD patients were matched with the KT recipients. Individuals without history of renal replacement therapy, including KT and RVO history, were selected as HCs and were matched with KT patients by age, sex, inclusion year, and history of underlying hypertension and DM (Fig. 2).
Figure 2.
Flowchart depicting the identification of the KT group, the ESRD group, and the healthy controls. ESRD end-stage renal disease, DM diabetes mellitus, HTN hypertension, KT kidney transplantation, RRT renal replacement therapy, RVO renal vein occlusion.
Demographic characteristics including age, sex, income status, type of dialysis, duration of dialysis, time of KT, type of induction therapy, history of desensitization therapy, and the Charlson Comorbidity Index (CCI) were identified for each patient42. Preemptive KT was defined as KT performed within 3 months of dialysis or without dialysis. Hypertension was identified by a previous diagnosis of hypertension (I10-I15, I159, I150, I1522, I151, I1528) or the prescription of antihypertensive drugs more than twice within one year before KT. DM was defined by a previous DM diagnosis (E109, E119, E139, E149, E101, E111, E131, E141, E105, E115, E135, E145) or prescription of one or more oral hypoglycemic agents or insulin. Dyslipidemia was defined by a diagnosis of dyslipidemia (E78) or a prescription of lipid-lowering agents. Among the prescription lists, information regarding immunosuppressant agents such as tacrolimus, cyclosporine, and steroids, which were licensed in South Korea during the follow-up, was collected separately.
Study outcomes
The main goal was to determine the incidence of RVO in KT recipients compared to that in ESRD patients and in HCs. The study population was followed up to the date of RVO diagnosis or until December 31, 2017, whichever came first. RVO was confirmed by ICD-10 code H43.8. We subdivided the RVO patients into two subgroups: ischemic and non-ischemic. Ischemic RVO was operationally defined as those who needed scatter laser photocoagulation (S5160, S5161) because of a large non-perfusion area, or who underwent vitrectomy (S5121, S5122) within one year after RVO diagnosis. Otherwise the case was defined as non-ischemic RVO.
Statistical analysis
Descriptive characteristics are presented as mean ± SD, median (IQR), number, or frequency (%). The chi‐square test was used to determine differences in the proportion of categorical variables, and analysis of variance (ANOVA) was used to evaluate differences between the means of continuous variables. Incidence rates of RVO are expressed as events per 1000 patient-years.
The cumulative incidence of RVO was analyzed using Kaplan–Meier estimates. Log-rank test was used to compare differences among groups. HR and 95% confidence interval (CI) values were analyzed using a Cox proportional hazards analysis with multivariable-adjustment. Model 1 presented the results of an unadjusted univariable analysis, and Model 2 was adjusted for age and sex. Model 3 further adjusted Model 2 for hypertension, DM, dyslipidemia, CCI, and income status. All analyses were performed with the SAS 9.4 program (SAS Institute, Cary, NC). P values < 0.05 were considered statistically significant.
Supplementary Information
Author contributions
Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work. B.-L.O. and Y.C.K. designed the conception of this study and J.L. and H.R.C. drafted the article. J.L., H.R.C. and Y.C.K. acquired the data. J.L., H.R.C., B.-L.O., Y.C.K., and H.L. interpreted the data. S.H.P. and K.D.H. performed statistical analyses. D.K.K., K.W.J., Y.S.K., E.K.L., U.C.P., H.G.Y. and H.L. mentored all the processes of the study. B.-L.O. and Y.C.K. finally approved of the version to be published.
Funding
This study was supported by a grant from the Ministry of Health and Welfare, Republic of Korea (Grant number: HI18C1604).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jangwook Lee, Hye Rim Choe, Yong Chul Kim and Baek-Lok Oh.
Contributor Information
Yong Chul Kim, Email: imyongkim@gmail.com.
Baek-Lok Oh, Email: baeklok@snu.ac.kr.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-90765-8.
References
- 1.Zhu Z, et al. Visual impairment and major eye diseases in chronic kidney disease: The National Health and Nutrition Examination Survey, 2005–2008. Am. J. Ophthalmol. 2020;213:24–33. doi: 10.1016/j.ajo.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Cugati S, Jie JW, Rochtchina E, Mitchell P. Ten-year incidence of retinal vein occlusion in an older population: The blue mountains eye study. Arch. Ophthalmol. 2006;124:726–732. doi: 10.1001/archopht.124.5.726. [DOI] [PubMed] [Google Scholar]
- 3.Wong T, et al. Cardiovascular risk factors for retinal vein occlusion and arteriolar emboli: The atherosclerosis risk in communities & cardiovascular health studies. Ophthalmology. 2005;112:540–547. doi: 10.1016/j.ophtha.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 4.Klein R. The 15-year cumulative incidence of retinal vein occlusion. Arch. Ophthalmol. 2008;126:513. doi: 10.1001/archopht.126.4.513. [DOI] [PubMed] [Google Scholar]
- 5.Wang TJ, Wu CK, Hu CC, Keller JJ, Lin H-C. Increased risk of co-morbid eye disease in patients with chronic renal failure: A population-based study. Ophthalmic Epidemiol. 2012;19:137–143. doi: 10.3109/09286586.2012.680531. [DOI] [PubMed] [Google Scholar]
- 6.Kim KM, Oh HJ, Choi HY, Lee H, Ryu DR. Impact of chronic kidney disease on mortality: A nationwide cohort study. Kidney Res. Clin. Pract. 2019;38:382–390. doi: 10.23876/j.krcp.18.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCullough KP, Morgenstern H, Saran R, Herman WH, Robinson BM. Projecting ESRD incidence and prevalence in the United States through 2030. J. Am. Soc. Nephrol. 2019;30:127–135. doi: 10.1681/ASN.2018050531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park S, et al. Characteristics of kidney transplantation recipients over time in South Korea. Korean J. Intern. Med. 2020 doi: 10.3904/kjim.2019.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang YS, et al. Risk of retinal vein occlusion following end-stage renal disease. Medicine (Baltimore) 2016;95:e3474. doi: 10.1097/MD.0000000000003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen SN, Yang TC, Lin JT, Lian IB. End stage renal disease as a potential risk factor for retinal vein occlusion. Medicine (Baltimore) 2015;94:e1960. doi: 10.1097/MD.0000000000001960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee KS, Nam KH, Kim DW, Kang EC, Koh HJ. Risk of retinal vein occlusion in patients with end-stage renal disease: A 12-year, retrospective, nationwide cohort study in South Korea. Investig. Ophthalmol. Vis. Sci. 2018;59:39–44. doi: 10.1167/iovs.17-22638. [DOI] [PubMed] [Google Scholar]
- 12.Lim LL, et al. Prevalence and risk factors of retinal vein occlusion in an Asian population. Br. J. Ophthalmol. 2008;92:1316–1319. doi: 10.1136/bjo.2008.140640. [DOI] [PubMed] [Google Scholar]
- 13.Arakawa S, et al. Nine-year incidence and risk factors for retinal vein occlusion in a general Japanese population: The Hisayama Study. Investig. Opthalmol. Vis. Sci. 2011;52:5905. doi: 10.1167/iovs.11-7775. [DOI] [PubMed] [Google Scholar]
- 14.Wong CW, Wong TY, Cheng CY, Sabanayagam C. Kidney and eye diseases: Common risk factors, etiological mechanisms, and pathways. Kidney Int. 2014;85:1290–1302. doi: 10.1038/ki.2013.491. [DOI] [PubMed] [Google Scholar]
- 15.Yip WF, et al. Retinal microvascular abnormalities and risk of renal failure in Asian populations. PLoS One. 2015;10:e0118076. doi: 10.1371/journal.pone.0118076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimmel PL, et al. Immunologic function and survival in hemodialysis patients. Kidney Int. 1998;54:236–244. doi: 10.1046/j.1523-1755.1998.00981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stenvinkel P, et al. IL-10, IL-6, and TNF-α: Central factors in the altered cytokine network of uremia—The good, the bad, and the ugly. Kidney Int. 2005;67:1216–1233. doi: 10.1111/j.1523-1755.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- 18.Deobhakta A, Chang LK. Inflammation in retinal vein occlusion. Int. J. Inflamm. 2013;2013:1–6. doi: 10.1155/2013/438412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshimura T, et al. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS One. 2009;4:e8158. doi: 10.1371/journal.pone.0008158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rehak J, Rehak M. Branch retinal vein occlusion: Pathogenesis, visual prognosis, and treatment modalities. Curr. Eye Res. 2008;33:111–131. doi: 10.1080/02713680701851902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 22.Sarnak MJ, et al. Kidney disease as a risk factor for development of cardiovascular disease. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 23.Leskinen Y, et al. Risk factors for aortic atherosclerosis determined by transesophageal echocardiography in patients with CRF. Am. J. Kidney Dis. 2003;42:277–285. doi: 10.1016/S0272-6386(03)00674-7. [DOI] [PubMed] [Google Scholar]
- 24.van Gennip ACE, et al. Endothelial dysfunction and low-grade inflammation in the transition to renal replacement therapy. PLoS One. 2019;14:e0222547. doi: 10.1371/journal.pone.0222547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cañas L, et al. Inflammation and oxidation: Do they improve after kidney transplantation? Relationship with mortality after transplantation. Int. Urol. Nephrol. 2017;49:533–540. doi: 10.1007/s11255-016-1435-4. [DOI] [PubMed] [Google Scholar]
- 26.Cho J, et al. Coagulation profile in patients with chronic kidney disease before and after kidney transplantation: A retrospective cohort study. Clin. Transplant. 2017;31:e13051. doi: 10.1111/ctr.13051. [DOI] [PubMed] [Google Scholar]
- 27.Young JB, Neumayer H-H, Gordon RD. Pretransplant cardiovascular evaluation and posttransplant cardiovascular risk. Kidney Int. 2010;78:S1–S7. doi: 10.1038/ki.2010.209. [DOI] [PubMed] [Google Scholar]
- 28.Grosso G, et al. Predictive value of the Charlson comorbidity index in kidney transplantation. Transplant. Proc. 2012;44:1859–1863. doi: 10.1016/j.transproceed.2012.06.042. [DOI] [PubMed] [Google Scholar]
- 29.Wu C, et al. Comorbid conditions in kidney transplantation: Association with graft and patient survival. J. Am. Soc. Nephrol. 2005;16:3437–3444. doi: 10.1681/ASN.2005040439. [DOI] [PubMed] [Google Scholar]
- 30.Perrea DN, et al. Correlation between lipid abnormalities and immunosuppressive therapy in renal transplant recipients with stable renal function. Int. Urol. Nephrol. 2008;40:521–527. doi: 10.1007/s11255-007-9266-y. [DOI] [PubMed] [Google Scholar]
- 31.Vincenti F, et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am. J. Transplant. 2007;7:1506–1514. doi: 10.1111/j.1600-6143.2007.01749.x. [DOI] [PubMed] [Google Scholar]
- 32.Pilmore H, Dent H, Chang S, McDonald SP, Chadban SJ. Reduction in cardiovascular death after kidney transplantation. Transplantation. 2010;89:851–857. doi: 10.1097/TP.0b013e3181caeead. [DOI] [PubMed] [Google Scholar]
- 33.Tyden G, et al. ABO incompatible kidney transplantations without splenectomy, using antigen-specific immunoadsorption and rituximab. Am. J. Transplant. 2005;5:145–148. doi: 10.1111/j.1600-6143.2004.00653.x. [DOI] [PubMed] [Google Scholar]
- 34.Montgomery RA, et al. Desensitization in HLA-incompatible kidney recipients and survival. N. Engl. J. Med. 2011;365:318–326. doi: 10.1056/NEJMoa1012376. [DOI] [PubMed] [Google Scholar]
- 35.Valenzuela NM, Reed EF. Antibody-mediated rejection across solid organ transplants: Manifestations, mechanisms, and therapies. J. Clin. Investig. 2017;127:2492–2504. doi: 10.1172/JCI90597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bieri M, et al. Anti-HLA I antibodies induce VEGF production by endothelial cells, which increases proliferation and paracellular permeability. Int. J. Biochem. Cell Biol. 2009;41:2422–2430. doi: 10.1016/j.biocel.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 37.Reyes-Vargas E, et al. Binding of anti-HLA class I antibody to endothelial cells produce an inflammatory cytokine secretory pattern. J. Clin. Lab. Anal. 2009;23:157–160. doi: 10.1002/jcla.20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gouliopoulos N, et al. Endothelial dysfunction and impaired arterial wall properties in patients with retinal vein occlusion. Vasc. Med. (United Kingdom) 2020 doi: 10.1177/1358863X20913609. [DOI] [PubMed] [Google Scholar]
- 39.Tanano I, et al. Impaired systemic vascular endothelial function in patients with branch retinal vein occlusion. Curr. Eye Res. 2013;38:114–118. doi: 10.3109/02713683.2012.738460. [DOI] [PubMed] [Google Scholar]
- 40.Kolar P. Risk factors for central and branch retinal vein occlusion: A meta-analysis of published clinical data. J. Ophthalmol. 2014;2014:1–5. doi: 10.1155/2014/724780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quan H, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 42.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chron. Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.