Abstract
Background
Hesitancy to receive COVID-19 vaccination is a major public health concern. COVID-19 vaccine willingness and the factors contributing to willingness in adults with multiple sclerosis (MS) is unknown.
We administered an online survey from 1 December 2020 to 7 January 2021 to adults with MS to estimate COVID-19 vaccine willingness among adults with MS. Bivariate analysis with chi-square testing compared categorical variables associated with vaccine willingness.
Results
Of 401 respondents, 70.1% were willing to receive an authorized COVID-19 vaccination if it was available to them, 22.7% were unsure, and 7.2% were unwilling. The most frequent concern for those unsure was vaccine safety. Vaccine willingness was associated with increased perceived personal risk of COVID-19 (χ2 = 45.4; p < 0.0001), prior influenza vaccine acceptance (χ2 = 97.6; p < 0.0001), higher educational level (χ2 = 50.2; p < 0.0001), and if respondents discussed or planned to discuss the COVID-19 vaccine with their neurologists (χ2 = 64.3; p < 0.0001).
Conclusion
While COVID-19 vaccination willingness is high among people with MS, nearly 30% were either unwilling or unsure about being vaccinated. Neurologists should be aware of patient-centered factors associated with COVID-19 vaccine willingness and address COVID-19 vaccine safety concerns in discussions with their vaccine-unsure MS patients.
Keywords: Multiple sclerosis, COVID-19, vaccine willingness, vaccine readiness, vaccine hesitancy, vaccine acceptance
Introduction
Uncontrolled spread of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is causing significant morbidity and mortality as well as substantial psychological and economic costs worldwide. 1 Case series and MS registries indicate that older people with multiple sclerosis (MS), particularly those with progressive disease and greater disability, are at increased risk of COVID-19 complications.2–4
Widespread COVID-19 vaccination will directly protect immunized individuals and likely indirectly protect the whole community by slowing virus transmission. Because it is unknown how many people with MS were included in the Pfizer-BioNTech and Moderna vaccine clinical trials, and people taking immunosuppressive agents were specifically excluded, safety of the vaccines in the MS population is unknown. However, based on a low theoretical risk and the high potential benefit of vaccination, a panel convened by the National MS Society strongly recommended that those with MS receive the COVID-19 vaccine. 5
One obstacle to widespread COVID-19 vaccination is vaccine hesitancy, defined as “delay in acceptance or refusal of vaccination despite availability”. 6 Vaccine hesitancy is a behavior influenced by a range of variables, such as knowledge, past experience, and perceived risks of vaccination. 7 Common concerns associated with vaccine hesitancy encompass a broad spectrum, including skepticism about vaccine safety, vaccine effectiveness, discomfort with vaccine policies, and those who do not perceive infection as a considerable risk. 7 Identifying the most salient concerns of those within this spectrum who may become more willing to accept the vaccine after additional counseling may be helpful. People with MS may specifically be concerned about effects of a vaccine because of the immune-mediated nature of MS and the immune-modulating to immunosuppressive nature of MS treatments. Therefore, assessing vaccine willingness and understanding sources of vaccine hesitancy in people with MS will help MS providers more effectively approach patients to encourage COVID-19 vaccination while respecting patient autonomy.
The objectives of our study are to estimate COVID-19 vaccine willingness among adults with MS and explore the factors associated with willingness to be vaccinated against COVID-19.
Materials and methods
Study design, setting, and respondents
This was a single center, observational, cross-sectional study. The study was approved by the Oregon Health & Science University (OHSU) Institutional Review Board.
We recruited respondents through a link posted on the National MS Society (NMSS) website, an e-mail sent to a list-serve for NMSS support groups in Oregon and Southwest Washington, and at the OHSU MS Center. In addition, patients of the OHSU MS Center with a diagnosis of MS seen within the last two years were sent a link to the survey through their electronic health record. Consented respondents were included in analyses if they were adults with a self-reported diagnosis of MS.
The survey
Between 1 December 2020 and 7 January 2021, respondents completed electronic surveys through REDCap, an online platform designed for electronic data capture. 8 Respondents given the link to the survey were directed to fill in responses directly into REDCap, either by themselves or with assistance.
The primary outcome for the survey was vaccine willingness, assessed by the question: “Would you be willing to get vaccinated against COVID-19 if a vaccine was available for you?” Possible responses included: “Yes, I would be willing to be vaccinated against COVID-19,” “No, I would not be willing to get vaccinated against COVID-19,” and “I am unsure if I would be willing to get vaccinated against COVID-19.” The respondent then selected up to 3 of the most important reasons influencing their vaccine willingness, including an “Other” which allowed a write-in response. The answer choices for this question were specific for COVID-19 and based on the vaccine hesitancy determinants matrix developed for the World Health Organization (Supplementary Table 1).6,9 Write-in responses were recategorized by the investigators, if appropriate.
The survey also queried demographics (sex, gender, race, ethnicity, education, household income, zip code, and health insurance); MS characteristics (subtype, duration); prior declined vaccines in adulthood; frequency of visits with their primary care provider; prior discussion or plans to discuss the COVID-19 vaccine with their neurologist; personal and/or social network history of COVID-19 infection, complications, and/or death; self-perceived risk for contracting COVID-19; home and work exposure risks; and social distancing practices. MS disease severity was captured in self-reported disability category scale correlating with the Expanded Disability Status Scale (r = 0.85). 10 Additionally, we captured and categorized disease modifying therapies (DMT) as “high efficacy” (B-cell therapies, alemtuzumab, cladribine, or natalizumab) or “low efficacy” (glatiramer acetate, interferons, sphingosine 1-phosphate receptor modulators, 11 dimethyl fumarate and other biosimilars). Finally, we inquired about the presence of medical co-morbidities that could increase the risk of COVID-19 complications, including: hypertension, pre-diabetes/diabetes, heart disease, heart failure, stroke, asthma, emphysema, kidney and liver disease, cancer, humman immunodeficiency virus (HIV), obesity, sickle cell disease, prior solid organ or bone marrow transplant, immune deficiences, cystic fibrosis, and/or thalassemia. The complete survey is included in Supplemental Materials.
Statistical methods
We reported demographics, disease characteristics, and key survey responses with descriptive statistics (mean or median and standard deviation for continuous variables and percentage for categorical variables). Respondents who completed the survey but who did not have a diagnosis of MS were excluded from statistical analysis. Our primary outcome variable, COVID-19 vaccine willingness, is a categorical variable measured at three levels (yes, no, unsure). Consequently, chi-square analyses were chosen to test associations between three levels of vaccine willingness and categorical predictor variables. We selected comparisons between outcome and predictor variables that were hypothesized to be the most important as they related to this specific population. These variables included formal education level, age, sex, presence of a high risk co-morbidity for severe COVID-19, disability, MS duration, MS subtype, current DMT use, personal concern for getting COVID-19, personal history of suspected or confirmed COVID-19, personally knowing someone who had COVID-19 or was hospitalized for COVID-19, if MS increased the respondent’s concerns about COVID-19 infection, social distancing practices, discussion or plan to discuss COVID-19 vaccine with their MS doctor; prior acceptance of the influenza vaccine, employment location, and employment status. A p-value of 0.05 or lower was considered statistically significant. No alpha adjustments were made since these analyses were exploratory. Statistical analysis was conducted using STATA16. 12
Results
Survey invitations were sent to 31 NMSS group leaders who covered 24 NMSS groups in Oregon and 1920 people were sent an invitation to participate in the survey through their electronic medical record; 477 people opened the REDCap link and 86% of these (n = 410) completed the survey. Of these 410 respondents, 9 did not report a diagnosis of MS and were therefore excluded. Overall, 401 respondents were included in the statistical analysis.
The majority of survey respondents were white (87.6%), female (76.1%), and college educated (64%; Table 1). Their median age was 51 years, and 17.8% were age 65 or older. Most respondents had relapsing MS (70.7%), and 22.2% had “none/minimal” disability, 29.5% had mild disability, and 21% had moderate disability. At the time of the survey, 110 respondents (27.8%) were not taking a DMT. The most common DMTs reported were ocrelizumab/rituximab (26.4%), dimethyl fumarate/diroximel fumarate (16.1%), and glatiramer acetate (8.3%). Overall, 157 (40.1%) were taking a low efficacy DMT, and 126 (32.1%) were taking a high efficacy DMT. The median annual income was $50,000–100,000 and nearly all respondents had health insurance (99.2%); 44.4% of respondents were working, and 38.4% were working outside the home at least 50% of the time.
Table 1.
Demographic and clinical characteristics of survey respondents (N = 401).
| Survey respondents N (%) | |
|---|---|
| Age, years, median (range), mean (sd) | 51 (18–84), 51.1 (13.5) |
| 18–49 | 176 (43.8%) |
| 50–64 | 142 (35.4%) |
| >65 | 73 (18.2%) |
| Missing | 10 (2.5%) |
| Biological sex at birth | |
| Female | 312 (77.8%) |
| Missing | 1 (0.25%) |
| Race | |
| American Indian or Alaska Native | 4 (1.0%) |
| Asian | 3 (0.7%) |
| Black or African American | 6 (1.5%) |
| Native Hawaiian or other Pacific Islander | 1 (0.2%) |
| White | 359 (89.5%) |
| More than one race | 16 (4.0%) |
| Unknown, would prefer not to say or missing | 12 (3.0%) |
| Ethnicity | |
| Hispanic or Latino | 21 (5.2%) |
| Not Hispanic/Latino | 354 (88.3%) |
| Unknown or missing | 26 (6.5%) |
| Education | |
| High school diploma or less | 25 (6.2%) |
| Some college | 111 (27.7%) |
| Bachelor’s degree | 116 (28.9%) |
| Some graduate school or more | 136 (33.9%) |
| Missing | 13 (3.2%) |
| Employment location (N = 182) | |
| Inside the home all or most of the time | 112 (61.5%) |
| Outside the home half of the time or more | 70 (38.5%) |
| Income | |
| < $25,000 | 43 (10.7%) |
| $25,000–49,999 | 61 (15.2%) |
| $50,000–99,999 | 118 (29.4%) |
| $100,000 or more | 161 (40.1%) |
| Missing | 18 (4.4%) |
| State of residence | |
| Oregon | 304 (75.8%) |
| Washington | 43 (10.7%) |
| California | 8 (2.0%) |
| Othera | 39 (9.7%) |
| Missing | 7 (1.74%) |
| Has health insurance | 396 (99.2%) |
| Multiple sclerosis subtype | |
| Relapsing remitting | 290 (72.3%) |
| Primary progressive | 30 (7.5%) |
| Secondary progressive | 52 (13.0%) |
| Not sure | 29 (7.2%) |
| Disability | |
| None/Minimal | 91 (22.7%) |
| Mild | 121(30.1%) |
| Moderate | 86 (21.4%) |
| Some support needed for walking | 60 (14.9%) |
| Walker/two-handed crutch | 29 (7.2%) |
| Wheelchair-bound | 12 (2.9%) |
| Bed-bound | 2 (0.5%) |
| DMTb | |
| No DMT | 110 (27.8%) |
| Low efficacy DMT | 157 (40.1%) |
| High efficacy DMT | 126 (32.1%) |
| Medical co-morbidities | |
| 0 | 179 (44.6%) |
| 1 or more | 222 (55.4%) |
aFour or fewer respondents each from ID, TX, OK, KS, TN, DC, NV, MI, SC, CO, UT, KY, NY, GA, IL, MT, AZ.
bDMT was categorized as “low efficacy” (glatiramer acetate, interferons, sphingosine 1-phosphate receptor modulators, dimethyl fumarate and other biosimilars) or “high efficacy” (B-cell therapies, alemtuzumab, cladribine, or natalizumab).
COVID-19 exposure, practices, and beliefs
A minority of respondents (37, 9.3%) reported a history of confirmed or suspected COVID-19 infection, and 2 (5.4%) required hospitalization (Table 2). None required admission to the intensive care unit (ICU). Almost 30% of respondents knew someone who had been hospitalized for COVID-19, and 18.5% knew someone who had passed away from COVID-19.
Table 2.
COVID-19 exposure, practices, and beliefs.
| Survey respondents N (%) | |
|---|---|
| How concerned are you that you will personally get COVID-19? (N = 400) | |
| Not at all concerned | 26 (6.5%) |
| Slightly concerned | 76 (19.0%) |
| Somewhat concerned | 128 (31.9%) |
| Moderately concerned | 128 (31.9%) |
| Extremely concerned | 50 (12.5%) |
| How concerned are you that you will require hospitalization, have severe complications, or die from COVID-19? (N = 400) | |
| Not at all concerned | 43 (10.8%) |
| Slightly concerned | 77 (19.3%) |
| Somewhat concerned | 101 (25.3%) |
| Moderately concerned | 91 (22.8%) |
| Extremely concerned | 88 (22.0%) |
| Have you ever personally tested positive or has a physician suspected you were positive for COVID-19? (N = 399) | |
| Yes | 37 (9.3%) |
| No | 362 (90.7%) |
| If you have tested positive, did you require hospitalization for COVID-19? (N = 37) | |
| Yes | 2 (5.4%) |
| No | 35 (94.6%) |
| Do you personally know anyone who has tested positive or was suspected to be positive for COVID-19? (N = 399) | |
| Yes | 280 (70.2%) |
| No | 119 (29.8%) |
| Do you personally know anyone who has been hospitalized for confirmed or suspected COVID-19? (N = 400) | |
| Yes | 118 (29.5%) |
| No | 282 (70.5%) |
| Do you personally know anyone who passed away from confirmed or suspected COVID-19? | |
| Yes | 74 (18.5%) |
| No | 327 (81.5%) |
| Which of the following best describes your current social behavior | |
| I am not going to public places and only socialize virtually with family or friends. | 175 (43.6%) |
| I am not going to public places, but I am socializing with family or friends in my, or their, home | 115 (28.7%) |
| I am only socializing in public places if I can maintain a distance of six-feet from other people. | 78 (19.5%) |
| I am continuing to socialize in public places, but less than before | 26 (6.5%) |
| I am continuing to socialize in public places | 7 (1.7%) |
| How does your MS affect your concerns about COVID-19? | |
| I’m a lot more worried about COVID-19 because of my MS | 123 (30.7%) |
| I’m a little more worried about COVID-19 because of my MS | 187 (46.6%) |
| My worries about COVID-19 are not affected by my MS | 79 (19.7%) |
| I’m a little less worried about COVID-19 because of my MS | 6 (1.5%) |
| I’m a lot less worried about COVID-19 because of my MS | 6 (1.5%) |
Respondents were somewhat (31.9%), moderately (31.9%), or extremely (12.5%) worried about personally contracting COVID-19, and 70.1% were at least somewhat concerned about having severe complications if they were to contract COVID-19. The majority of respondents were either lot more (30.7%) or a little more (46.6%) worried about COVID-19 because of their MS, whereas only 19.7% reported their worries about COVID-19 were not affected by their MS.
The largest proportion (43.6%) of respondents were not going to public places and only socializing virtually with family or friends who live outside their homes, and 28.7% reported there were not going to public places but are socializing with family/friends in either of their homes.
Eighty-nine percent of respondents reported their vaccine practices and beliefs had not changed because of COVID-19, whereas 9.0% were more likely to receive a vaccine than before COVID-19. The remaining 2.2% were less likely to receive a vaccine.
COVID-19 vaccination willingness
Most respondents (70.1%, n = 281) were willing to receive the COVID-19 vaccine if it were available to them (Table 3). The most common reasons cited were to protect themselves (77.6%) and their loved ones (58.7%) from getting COVID-19, and to decrease risk of serious illness (53.4%) (Table 3). A minority of respondents (7.2%, n = 29) were not willing to receive the vaccine, primarily due to concerns that the vaccine was developed too rapidly and that there may be potential side effects of the vaccine (both 55.2%). Over 22% of respondents (n = 91) were unsure if they would be willing to receive the vaccine. The majority of these respondents (57.1%) wanted more evidence to prove the vaccine’s safety. The unsure respondents also expressed concerns that political pressures rushed the vaccine trials (37.4%) and thought that the general population should be vaccinated before people with MS due to concerns about safety of the vaccine for people with MS (29.7%).
Table 3.
COVID-19 vaccination willingness.
| Would you be willing to get vaccinated against COVID-19? | N = 401 | Most common reasons selected | N (%) |
|---|---|---|---|
| Yes | 281 (70.1%) | To protect myself from getting COVID-19 | 218 (77.6%) |
| To protect my loved ones from getting COVID-19 | 165 (58.7%) | ||
| To decrease the chance of getting seriously ill from COVID-19 | 150 (53.4%) | ||
| No | 29 (7.2%) | I’m concerned that the vaccine was developed too rapidly | 16 (55.2%) |
| I’m concerned about potential side effects | 16 (55.2%) | ||
| I think the vaccine may have been rushed and would like more evidence to prove it is safe | 13 (44.8%) | ||
| Unsure | 91 (22.7%) | I would like it to be proven safe on a large-scale, population level to prove it is safe before I take it | 52 (57.1%) |
| I think political pressure rushed the vaccine trials and it should be tested more thoroughly to prove it is safe | 34 (37.4%) | ||
| I think that people without MS should get the vaccine before people with MS due to concerns about safety for people with MS | 27 (29.7%) |
Variables associated with willingness to receive a COVID-19 vaccine
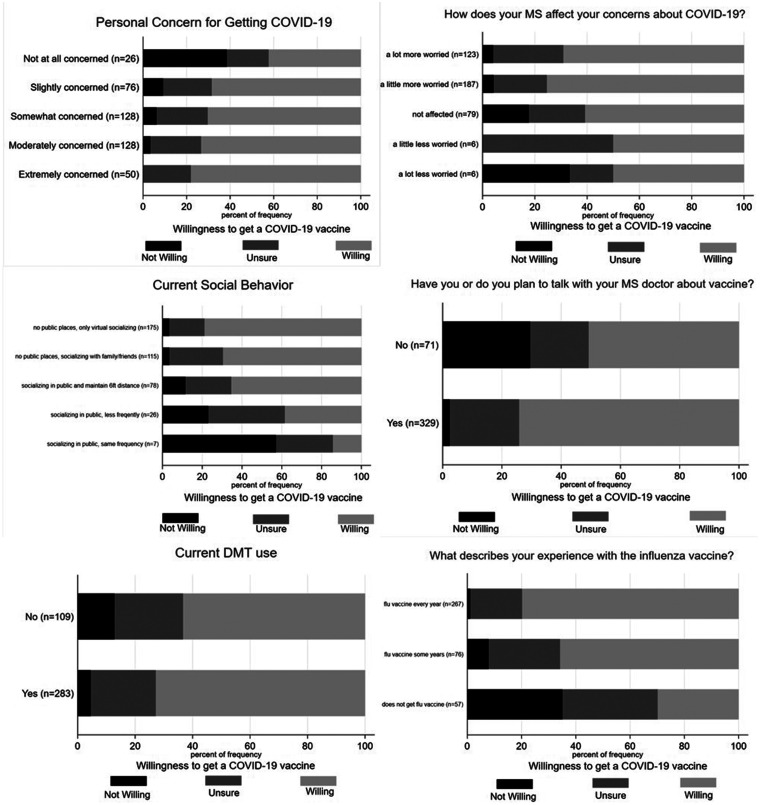
We assessed demographic, exposure, practice, and belief variables that were hypothesized to be the most important as they related to this specific population with COVID-19 vaccine willingness (three levels) using chi-square tests. COVID-19 vaccine willingness was significantly associated with the following: higher personal concern for getting COVID-19 (χ2 = 45.4, p < 0.0001); if MS increased the respondent’s concerns about COVID-19 infection (χ2 = 28, p < 0.0001); more cautious social distancing practices (χ2 = 55.6, p < 0.0001); discussion or plan to discuss COVID-19 vaccine with their MS doctor (χ2 = 64.3, p < 0.0001); prior acceptance of the influenza vaccine (χ2 = 97.6, p < 0.0001); current use of any DMT (χ2 = 8.8, p = 0.01), and higher levels of formal education (χ2 = 50.2, p < 0.0001, Table 4). See Figure 1 for graphical representation of these results. The variables that were not associated with COVID-19 vaccine willingness are summarized in Table 5. These variables included age, disability, type of MS, presence of medical co-morbidities, working outside the home ≥50% of time, personal history of COVID-19, and high efficacy vs low efficacy DMT use.
Table 4.
Variables associated with COVID-19 vaccine willingness.
| Variables associated with COVID-19 vaccine willingness | Not willing | Unsure | Willing | χ² | p |
|---|---|---|---|---|---|
| How concerned are you that you will personally get COVID-19? | 45.4 | <0.0001 | |||
| Not at all concerned | 10 (38.5%) | 5 (19.2%) | 11 (42.3%) | ||
| Slightly concerned | 7 (9.2%) | 17 (22.4%) | 52 (68.4%) | ||
| Somewhat concerned | 8 (6.3%) | 30 (23.4%) | 90 (70.3%) | ||
| Moderately concerned | 4 (3.3%) | 28 (23.3%) | 88 (73.3%) | ||
| Extremely concerned | 0 (0%) | 11 (22%) | 39 (78.0%) | ||
| How does your MS affect your concerns about COVID-19? | 28.0 | <0.0001 | |||
| I’m a lot more worried about COVID-19 because of my MS | 5 (4.1%) | 33 (26.8%) | 85 (69.1%) | ||
| I’m a little more worried about COVID-19 because of my MS | 8 (4.3%) | 38 (20.3%) | 141 (75.4%) | ||
| My worries about COVID-19 are not affected by my MS | 14 (17.7%) | 17 (21.5%) | 48 (60.8%) | ||
| I’m a little less worried about COVID-19 because of my MS | 0 (0%) | 3 (50.0%) | 3 (50.0%) | ||
| I’m a lot less worried about COVID-19 because of my MS | 2 (33.3%) | 1 (16.7%) | 3 (50.0%) | ||
| Which of the following best describes your current social behavior | 55.6 | <0.0001 | |||
| I am not going to public places and only socialize virtually with family or friends. | 6 (3.4%) | 31 (17.7%) | 138 (78.9%) | ||
| I am not going to public places, but I am socializing with family or friends in my, or their, home | 4 (3.5%) | 31 (27.0%) | 80 (69.6%) | ||
| I am only socializing in public places if I can maintain a distance of 6-feet from other people. | 9 (11.5%) | 18 (23.1%) | 51 (65.4%) | ||
| I am continuing to socialize in public places, but less than before | 6 (23.1%) | 10 (38.5%) | 10 (38.5%) | ||
| I am continuing to socialize in public places | 4 (57.1%) | 2 (28.6%) | 1 (14.3%) | ||
| Have you or do you plan to talk with your MS doctor about vaccine? | 64.3 | <0.0001 | |||
| Yes | 8 (2.4%) | 77 (23.4%) | 244 (74.2%) | ||
| No | 21 (29.6%) | 14 (19.7%) | 36 (50.7%) | ||
| Current DMT use | 8.8 | 0.01 | |||
| Yes | 13 (4.6%) | 64 (22.6%) | 206 (72.8%) | ||
| No | 14 (12.8%) | 26 (23.9%) | 69 (63.3%) | ||
| What describes your experience with the influenza vaccine? | 97.6 | <0.0001 | |||
| I get the influenza vaccine every year | 3 (1.1%) | 51 (19.1%) | 213 (79.8%) | ||
| I get the influenza vaccine some years, but not all | 6 (7.9%) | 20 (26.3%) | 50 (65.8%) | ||
| I do not get the influenza vaccine | 20 (35.1%) | 20 (35.1%) | 17 (29.8%) | ||
| Education | 50.2 | <0.0001 | |||
| High school diploma or less | 7 (28.0%) | 11 (44.0%) | 7 (28%) | ||
| Some college | 14 (12.6%) | 31 (27.9%) | 66 (59.5%) | ||
| Bachelor’s degree | 3 (2.6%) | 28 (24.1%) | 85 (73.3%) | ||
| Some graduate school or more | 3 (2.2%) | 20 (14.7%) | 113 (83.1%) |
Figure 1.
Graphical representation of six of the variables found to be significantly associated with COVID-19 vaccine willingness: higher personal concern for getting COVID-19 (χ2 = 45.4, p < 0.0001); if MS increased the respondent’s concerns about COVID-19 infection (χ2 = 28, p < 0.0001); more cautious social distancing practices (χ2 = 55.6, p < 0.0001); discussion or plan to discuss COVID-19 vaccine with their MS doctor (χ2 = 64.3, p < 0.0001), current use of any DMT (χ2 = 8.8, p = 0.01), and prior acceptance of the influenza vaccine (χ2 = 97.6, p < 0.0001).
Table 5.
Variables not associated with COVID-19 vaccine willingness.
| Variables not associated with COVID-19 vaccine willingness | Chi-square | p |
|---|---|---|
| Current DMT use (high efficacy vs low efficacy) | 9.1 | 0.06 |
| Do you personally know anyone who has been hospitalized for confirmed or suspected COVID-19? | 4.8 | 0.09 |
| Have you ever personally tested positive or has a physician suspected you were positive for COVID-19? | 3.2 | 0.20 |
| Do you personally know anyone who has tested positive or was suspected to be positive for COVID-19? | 2.7 | 0.25 |
| Disability (none/minimum, mild, moderate, some support needed for walking, walker/two-handed crutch, wheelchair-bound, bed-bound) | 6.5 | 0.887 |
| Age (>65, 50-64, 18-49) | 3.6 | 0.458 |
| Sex | 2.0 | 0.363 |
| Presence of a high risk co-morbidity for severe COVID-19 | 3.6 | 0.728 |
| Employment location (outside the home ≥50% of time) | 1.2 | 0.537 |
| MS duration | 4.6 | 0.799 |
| MS Subtype | 5.4 | 0.496 |
| Employment status (employed versus unemployed) | 0.5 | 0.786 |
To further explore the variables associated with willingness to receive a COVID-19 vaccine, we collapsed the unsure and unwilling categories together. This allowed us to use logistic regression to specifically predict willingness to get the COVID-19 vaccine and assess odds ratios and 95% confidence intervals associated with the predictor variables. Bivariate logistic regressions found COVID-19 vaccine willingness (two levels) was significantly associated with the following predictor variables: higher personal concern for getting COVID-19 χ2 = 10.9, p < 0.05) or severe COVID-19 (χ2 = 12.72, p < 0.05), more cautious social distancing practices (χ2 = 28.20, p < 0.0001); discussion or plan to discuss COVID-19 vaccine with their MS doctor (χ2 = 14.58, p < 0.001); and prior acceptance of the influenza vaccine (χ2 = 52.71, p < 0.0001). After testing several demographic variables, higher levels of formal education (χ2 = 37.22, p < 0.0001) and age (χ2 = 6.91, p < 0.01) were found to be associated with COVID-19 vaccine willingness (two levels). Multivariate logistic regressions were performed for each of the predictor variables while adjusting for education and age. See Table 6 for odds ratios and 95% confidence intervals for both univariate and adjusted logistic regression models.
Table 6.
Univariate and adjusted odds ratios for COVID-19 vaccine willingness.
| Univariate model |
Adjusted model a |
|||
|---|---|---|---|---|
| OR (95%CI) | p-value | OR (95%CI) | p-value | |
| Age: | ||||
| Age | 1.02 (1.01, 1.04) | 0.009 | – | – |
| Education: | ||||
| High School Diploma or Less | 1 (Reference) | – | – | – |
| Some College | 3.77 (1.46, 9.77) | 0.006 | – | – |
| Bachelor’s Degree | 7.05 (2.69, 18.51) | <0.001 | – | – |
| Some graduate school or more | 12.63 (4.73, 33.71) | <0.001 | – | – |
| Plan to discuss the COVID-19 vaccine with their MS provider: | ||||
| No, I do not plan to discuss it with my MS provider | 1 (Reference) | – | 1 (Reference) | – |
| Yes, I have already discussed it with my MS provider | 3.13 (1.55, 6.34) | 0.001 | 2.93 (1.32, 6.49) | 0.008 |
| Yes, I plan to discuss it with my MS provider | 2.70 (1.57, 4.64) | <0.001 | 2.57 (1.38, 4.80) | 0.003 |
| Prior acceptance of the influenza vaccine: | ||||
| Do not get the flu vaccine | 1 (Reference) | – | 1 (Reference) | – |
| Get the flu vaccine some years, but not all | 4.52 (2.16, 9.48) | <0.001 | 5.59 (2.44, 12.77) | <0.001 |
| Get the flu vaccine every year | 9.28 (4.89,17.62) | <0.001 | 8.96 (4.39, 18.31) | <0.001 |
| Level of personal concern about contracting COVID-19: | ||||
| Not at all concerned | 1 (Reference) | – | 1 (Reference) | – |
| Slightly concerned | 2.95 (1.18, 7.39) | 0.020 | 2.85 (1.05, 7.73) | 0.040 |
| Somewhat concerned | 3.23 (1.36, 7.67) | 0.008 | 2.84 (1.10, 7.33) | 0.031 |
| Moderately concerned | 3.75 (1.56, 9.01) | 0.003 | 2.94 (1.13, 7.65) | 0.027 |
| Extremely concerned | 4.83 (1.73,13.49) | 0.003 | 4.04 (1.34, 12.18) | 0.013 |
| Level of personal concern about hospitalization, severe complications and/or death if infected with COVID-19: | ||||
| Not at all concerned | 1 (Reference) | – | 1 (Reference) | – |
| Slightly concerned | 2.39 (1.11, 5.15) | 0.026 | 2.43 (1.05, 5.62) | 0.038 |
| Somewhat concerned | 3.32 (1.57, 7.00) | 0.002 | 3.27 (1.45, 7.38) | 0.004 |
| Moderately concerned | 3.04 (1.43, 6.46) | 0.004 | 2.93 (1.29, 6.63) | 0.010 |
| Extremely concerned | 3.45 (1.60, 7.45) | 0.002 | 2.99 (1.31, 6.81) | 0.009 |
| Current social behavior with those outside of the home: | ||||
| Continuing to socialize in public places with the same frequency | 1 (Reference) | – | 1 (Reference) | – |
| Socializing in public places, but less than before | 3.75 (0.39, 35.92) | 0.252 | 3.11 (0.28, 34.55) | 0.355 |
| Socializing in public places, but with ≥6 feet of distance between self and others | 11.33 (1.30, 99.04) | 0.028 | 9.47 (0.94, 95.02) | 0.056 |
| No public places, socialization in the home | 13.71 (1.59, 118.20) | 0.017 | 14.07 (1.43, 138.33) | 0.023 |
| No public places, virtual socialization only | 22.38 (2.61, 191.71) | 0.005 | 17.53 (1.80, 170.60) | 0.014 |
Note: Odds ratios represent the likelihood that the participant is willing to receive a COVID-19 vaccine, as compared to those who are unsure or unwilling to receive the vaccine.
aAdjusted model includes age and education as co-variates. Education remained significant in the adjusted model; age did not remain significant.
Discussion
We found COVID-19 vaccination willingness to be high in this sample of people with MS, but nearly 30% were either unwilling or unsure about being vaccinated. The variables associated with vaccine willingness were personal concern for contracting COVID-19, impact of their MS on their perceived COVID-19 risk, social distancing practices, discussion or plan to discuss the vaccine with their MS doctor, prior influenza vaccine acceptance, current DMT use, and education level.
Overall, the rate of COVID-19 willingness in our study sample was similar to or higher than that of the general adult population in the United States and similar to a prior study of COVID-19 vaccine willingness in people with MS.13–18 Several national surveys, with sample sizes of 316–7632, completed between April and August 2020 found that 49–69% of respondents intended to be vaccinated, 17–32% were not sure, and 11–35% did not intend to be vaccinated.13–17 The prior study of vaccine willingness among people with MS found that 66% of respondents were willing and 15.4% were unwilling to accept COVID-19 vaccination. 18 A key difference between our study and the others cited was that most of our study was conducted after the first two mRNA vaccines against COVID-19 were authorized for emergency use by the FDA. FDA authorization and imminent vaccine availability may have altered willingness to receive the vaccine.
Respondents who were unsure about receiving the COVID-19 vaccination wanted more evidence about the safety of the vaccine in large populations. Those who did not want the vaccine were primarily concerned with the speed of vaccine development and potential side effects. These reasons were similar to prior COVID-19 vaccine willingness surveys of the general public in the United States.14–16,19 In our survey, however, 29.7% of “unsure” respondents were concerned about the safety of the vaccine specifically for people with MS. Moreover, discussion or planned discussion of the COVID-19 vaccine with a neurologist was associated with vaccine willingness. This was in line with a prior study which showed that patients were more likely to accept a COVID-19 vaccination if they thought their healthcare provider would recommend it. 15
The variables associated with COVID-19 vaccine willingness in our study were similar to those reported for the general population. Past vaccine acceptance, especially for the influenza vaccine, as well as the perceived risk of acquiring COVID-19, were associated with COVID-19 vaccine willingness in our study and several others.14,15,19 In our study, however, the effect of increasing age on vaccine willingness was minor in bivariate analysis and was no longer statistically significant when other factors such as education was controlled. The presence of several other demographic characteristics, co-morbidities, and conditions that increase the risk of severe COVID-19 (e.g. disability, high-risk comorbidities, and working outside the home) were not associated with vaccine willingness. This highlights a disconnect between how objective risk factors and subjective risk perception influenced respondents’ willingness to receive a COVID-19 vaccination, which was also shown in the prior study of vaccine willingness among people with MS. 18 MS providers need to recognize this disconnect when discussing COVID-19 vaccination to provide personalized and MS-specific information in order to promote vaccination. Independent of the content, the approach to the vaccination discussion can also influence vaccine willingness. Hofstetter et al. observed that parental influenza vaccination acceptance for their children was higher when healthcare providers initiated the vaccine discussion using a presumptive approach (e.g. “today we’re going to do the flu vaccine”, 72% acceptance) as opposed to a participatory approach (e.g. “are we going to do the flu vaccine today?”, 17% acceptance). 20 While these approaches were effective in a small pediatric population, we do not know if or how these techniques would translate to adult vaccine counseling; education initiatives using personalized risk stratification incorporating these approaches could be promising directions for future research.
Our study has a number of strengths. Our study is one of the only studies so far to explore COVID-19 vaccine willingness among adults with MS and in a population with a chronic medical condition. 21 Our results support the findings reported in the prior study of COVID-19 vaccine willingness in people with MS. 18 A major difference between the two studies is the survey window, which was shorter and allowed us to explore a period of time where two COVID-19 vaccines were approved, but before COVID-19 vaccines were offered to most respondents, reducing the potential impact of a changing vaccine landscape during the study window. Our study also examined the reasons for vaccine willingness and hesitancy as well as more demographic, behavioral, and MS-related variables. Our number of respondents was similar to other published surveys of both the general public and specific populations in the US.18,19,22
Our study also has a number of limitations, primarily related to generalizability. The response rate to the survey within OHSU was low at 21.3% which may increase the risk of sampling bias. A link to the survey was distributed nationally on the NMSS website which makes it difficult to assess the overall response rate to the survey, although 86.5% of our respondents resided in Oregon and Southwest Washington. Additionally, the number of individuals not using DMT (28%) was higher than expected. Because a self-reported diagnosis of MS was required for participation, some patients may not have actually met diagnostic criteria, although all patients who were invited via a link sent to their electronic medical record had an ICD10 diagnosis code of MS. While the rates of vaccine willingness in our study were similar to national polling data, Oregon has historically had one of the highest rates of vaccine hesitancy among its general population – only 71% of 2-year-olds were up to date on their childhood vaccinations in 2019. 23 The survey was only offered online, which could have excluded socioeconomically underserved populations; this bias was likely reflected in the high education and income levels of our respondents. There was a possibility of selection bias as potential respondents distrustful of a vaccine might have been less likely to participate in this research survey regarding vaccines. Most respondents identified as White (87.6%), so we could not perform any secondary analyses related to race/ethnicity. This major limitation to our study was especially unfortunate given that COVID-19 has disproportionately affected racial and ethnic minority groups 24 and because several studies suggested that vaccine acceptance may be lower amongst Black Americans.14,15 More research on sources of vaccine hesitancy amongst racial and ethnic minority groups is imperative. Finally, the survey period of our study closed shortly before the National MS Society released their recommendations stating that the COVID-19 vaccines developed by Pfizer-BioNTech and Moderna are safe for people with MS, safe to use with MS medications, and that people with MS should therefore receive a COVID-19 vaccine. 5 The National MS Society guidance was based on data from the general population in the vaccine clinical trials and from studies of other vaccines in people with MS. 5 It is possible that these Society recommendations would address some of the concerns for people with MS and increase vaccine willingness rates.
In summary, our study in people with MS found that over 70% of respondents were willing to receive a COVID-19 vaccine. Important variables associated with vaccine willingness were personal concern for getting COVID-19, discussing or planning to discuss COVID-19 vaccine with their MS doctor, and past vaccine acceptance. Respondents who were unsure if they were willing to receive a COVID-19 vaccine had significant uncertainty about the safety of the vaccine specifically for people with MS. Patient desire for MS-specific safety data could reflect a research priority for the future. Our study highlights the importance of patient-centered factors associated with COVID-19 vaccine willingness that may be used to guide personalized discussions with vaccine-unsure MS patients.
Supplemental Material
Supplemental material, sj-pdf-1-mso-10.1177_20552173211017159 for COVID-19 vaccination willingness among people with multiple sclerosis by Xinran M Xiang Chris Hollen Qian Yang Barbara H Brumbach Rebecca I Spain Lindsey Wooliscroft in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental material, sj-pdf-2-mso-10.1177_20552173211017159 for COVID-19 vaccination willingness among people with multiple sclerosis by Xinran M Xiang Chris Hollen Qian Yang Barbara H Brumbach Rebecca I Spain Lindsey Wooliscroft in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Acknowledgements
The authors would like to thank the Oregon Chapter of National MS Society for their help with recruitment, and the survey respondents for contributing their time. The authors would like to thank Drs. Dennis Bourdette and Michelle Cameron for their contributions to editing this manuscript and providing valuable feedback. We also acknowledge the editorial assistance of the Oregon Clinical & Translational Research Institute, which is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Award Number UL1TR002369.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the OHSU Foundation. Dr Xiang would like to thank the National MS Society for their support of her fellowship. Dr Hollen would like to thank the VA MS Center of Excellence for their support of his fellowship. Dr Wooliscroft is supported by K23HD101667 from the National Institutes of Health (NIH). Dr Spain is supported by the Department of Veterans Affairs (RX002682), NMSS (R-1705-27628, CA 1073-A-4), NIH (1R44AG055388-01).
ORCID iDs: Xinran M Xiang https://orcid.org/0000-0001-6422-436X
Lindsey Wooliscroft https://orcid.org/0000-0001-5377-6185
Contributor Information
Xinran M Xiang, Department of Neurology, Oregon Health & Science University, Portland, OR, USA.
Chris Hollen, Department of Neurology, Oregon Health & Science University, Portland, OR, USA; Neurology Division, VA Portland Health Care System, Portland, OR, USA.
Qian Yang, OHSU-PSU School of Public Health, Oregon Health & Science University, Portland, OR, USA.
Barbara H Brumbach, OHSU-PSU School of Public Health, Oregon Health & Science University, Portland, OR, USA; Biostatistics and Design Program Core, Oregon Health & Science University, Portland, OR, USA.
Rebecca I Spain, Department of Neurology, Oregon Health & Science University, Portland, OR, USA; Neurology Division, VA Portland Health Care System, Portland, OR, USA.
Conflict of Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental material
Supplemental material for this article is available online.
References
- 1.World Health Organization. COVID-19 weekly epidemiological update, www.who.int/publications/m/item/weekly-epidemiological-update-19-january-2021 (accessed 24 January 2021).
- 2.Louapre C, Collongues N, Stankoff B, et al.; the Covisep investigators. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol 2020; 77: 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.North American COVID-19 MS Clinical Database. www.covims.org (accessed 24 January 2021).
- 4.Chaudhry F, Bulka H, Rathnam AS, et al. COVID-19 in multiple sclerosis patients and risk factors for severe infection. J Neurol Sci 2020; 418: 117147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.COVID-19 Vaccine Guidance for People Living with MS. www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance (accessed 18 January 2021).
- 6.MacDonald NE, Hesitancy S; SAGE Working Group on Vaccine Hesitancy. Vaccine hesitancy: definition, scope and determinants. Vaccine 2015; 33: 4161–4164. [DOI] [PubMed] [Google Scholar]
- 7.Dube E, Laberge C, Guay M, et al. Vaccine hesitancy: an overview. Hum Vaccin Immunother 2013; 9: 1763–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(SAGE) SAGoEoI. Vaccine hesitancy survey questions related to SAGE vaccine hesitancy matrix, www.who.int/immunization/programmes_systems/Survey_Questions_Hesitancy.pdf (accessed 29 April 2021).
- 10.Shinto L, Yadav V, Morris C, et al. The perceived benefit and satisfaction from conventional and complementary and alternative medicine (CAM) in people with multiple sclerosis. Complement Ther Med 2005; 13: 264–272. [DOI] [PubMed] [Google Scholar]
- 11.Weideman AM, Tapia-Maltos MA, JK, Greenwood M, et al. Meta-analysis of the Age-Dependent efficacy of multiple sclerosis treatments. Front Neurol 2017; 8: 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.StataCorp. Stata statistical software: release 16. College Station, TX: StataCorp LLC, 2019. [Google Scholar]
- 13.Coustasse A, Kimble C, Maxik K. COVID-19 and vaccine hesitancy: a challenge the United States must overcome. J Ambul Care Manage 2021; 44: 71–75. [DOI] [PubMed] [Google Scholar]
- 14.Fisher KA, Bloomstone SJ, Walder J, et al. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of U.S. adults. Ann Intern Med 2020; 173: 964–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reiter PL, Pennell ML, Katz ML. Acceptability of a COVID-19 vaccine among adults in the United States: how many people would get vaccinated? Vaccine 2020; 38: 6500–6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neergard L, Fingernut H. AP-NORC poll: half of Americans would get a COVID-19 vaccine, https://apnews.com/article/dacdc8bc428dd4df6511bfa259cfec44 (accessed 22 January 2021).
- 17.O’Keefe S. One in three Americans would not get COVID-19 vaccine, https://news.gallup.com/poll/317018/one-three-americans-not-covid-vaccine.aspx (accessed 22 January 2021).
- 18.Ehde DM, Roberts MK, Herring TE, et al. Willingness to obtain COVID-19 vaccination in adults with multiple sclerosis in the United States. Mult Scler Relat Disord 2021; 49: 102788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pogue K, Jensen JL, Stancil CK, et al. Influences on attitudes regarding potential COVID-19 vaccination in the United States. Vaccines (Basel) 2020; 8: 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofstetter AM, Robinson JD, Lepere K, et al. Clinician-parent discussions about influenza vaccination of children and their association with vaccine acceptance. Vaccine 2017; 35: 2709–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams L, Gallant AJ, Rasmussen S, et al. Towards intervention development to increase the uptake of COVID-19 vaccination among those at high risk: outlining evidence-based and theoretically informed future intervention content. Br J Health Psychol 2020; 25: 1039–1054. [DOI] [PubMed] [Google Scholar]
- 22.Lucia VC, Kelekar A, Afonso NM. COVID-19 vaccine hesitancy among medical students. J Public Health (Oxf). Epub ahead of print 2020. doi: 10.1093/pubmed/fdaa230. [DOI] [PMC free article] [PubMed]
- 23.Oregon kindergarten vaccine exemption rate increases sharply. www.oregon.gov/oha/ERD/Pages/OregonKindergartenVaccineExemptionRateIncreasesSharply.aspx (accessed 22 January 2021).
- 24.Mackey K, Ayers CK, Kondo KK, et al. Racial and ethnic disparities in COVID-19-related infections, hospitalizations, and deaths: a systematic review. Ann Intern Med. Epub ahead of print 2020. doi: 10.7326/M20-6306. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-mso-10.1177_20552173211017159 for COVID-19 vaccination willingness among people with multiple sclerosis by Xinran M Xiang Chris Hollen Qian Yang Barbara H Brumbach Rebecca I Spain Lindsey Wooliscroft in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental material, sj-pdf-2-mso-10.1177_20552173211017159 for COVID-19 vaccination willingness among people with multiple sclerosis by Xinran M Xiang Chris Hollen Qian Yang Barbara H Brumbach Rebecca I Spain Lindsey Wooliscroft in Multiple Sclerosis Journal – Experimental, Translational and Clinical