Abstract
Background:
Image-guided bronchoscopy techniques such as virtual bronchoscopic navigation (VBN) has emerged as a means of assisting in the biopsy of peripheral pulmonary lesions. However, the role of VBN-assisted (VBNA) bronchoscopy in the diagnosing of peripheral pulmonary lesions (PPLs) has not been well established. This meta-analysis investigated the diagnostic yield of VBN-assisted versus non-VBN-assisted (NVBNA) bronchoscopy for PPLs.
Methods:
PubMed, Embase, Cochrane library, and Web of Sciences databases were searched up to and including August 2020 to identify randomized controlled trials (RCTs) evaluating the performance of VBNA compared with an NVBNA group. Results were expressed as risk ratio (RR) or mean difference (MD) with accompanying 95% confidence interval (CI).
Results:
Six RCTs with 1626 patients were included. The overall diagnostic rate was similar in the VBNA (74.17%) and NVBNA (69.51%) groups, with risk ratio of 1.07 (95% CI: 0.98–1.17). However, in the VBNA group, the total examination time was significantly shorter (MD = −3.94 min, 95% CI: −6.57 to −1.36; p = 0.003) than in the NVBNA group. VBNA had superior diagnostic yield than NVBNA for PPLs ⩽ 20 mm (RR = 1.18, 95% CI: 1.05–1.32). In addition, diagnostic yield according to nature of lesion, lesion location in the lung lobe, distance from the hilum, bronchus sign and complications were similar between VBNA and NVBNA groups.
Conclusion:
VBNA bronchoscopy did not increase overall diagnostic yield in patients with PPLs compared with NVBNA bronchoscopy. The superiority of VBNA over NVBNA was evident among patients with PPLs ⩽ 20 mm. Future multicenter RCTs are needed for further investigation.
The reviews of this paper are available via the supplemental material section.
Keywords: bronchoscopy, diagnostic yield, meta-analysis, peripheral pulmonary lesions, virtual bronchoscopic navigation
Introduction
Peripheral pulmonary lesions (PPLs) are encountered frequently in clinical practice. Traditionally, PPLs are defined pulmonary nodules located in the lung periphery and are in general hard to biopsy using conventional flexible bronchoscopy.1,2 Advances in recent sensitive imaging technologies have enabled physicians to find PPLs that previously would have remained undetected. Physicians should accurately identify and characterize lesions at high risk of malignancy before these lesions become incurable, while avoiding unnecessary procedures for benign lesions.3,4
Percutaneous needle biopsy is recommended for definitive diagnosis of peripheral lesions, with diagnostic yield ranging from 68% to 99%, but safety concerns often outweigh the benefits.4–7 Relatively high complication rates of needle biopsy are widely known,5,8 and transbronchial biopsy (TBB) has low complication rates when compared with percutaneous needle biopsy.6,9 However, TBB has a low diagnostic yield for small lesions in diameter of ⩽20 mm.2,10 Furthermore, the success of TBB is compromised due to the inability to detect lesions located beyond the subsegmental bronchus level. To overcome this problem, various bronchoscopic technologies have emerged over recent years, including virtual bronchoscopy navigation (VBN) technology. 11 VBN is an image-based novel technology that includes the spatial information derived from computed tomography (CT) images to guide a bronchoscope visually to the peripheral target lesion.
Several studies have shown improved diagnostic yield when VBN is used in conjugation with X-ray fluoroscopy,12,13 CT14,15 or endobronchial ultrasonography with guide sheath (EBUS-GS) that leverage virtual bronchoscopic technologies to further improve access to the target lesions.16–18 Recent randomized clinical trials (RCTs) of VBN- versus non-VBN-assisted techniques compared diagnostic yield and safety of peripheral pulmonary lesions. The results of RCTs have been heterogeneous in their conclusions,16–19 and it is unclear whether a VBN-assisted bronchoscopy improves diagnostic yield for PPLs.
We therefore performed a systematic review and meta-analysis of RCTs published to date to investigate the overall diagnostic yield and safety profile of VBN-assisted (VBNA) group compared with non-VBN-assisted (NVBNA) group for diagnosing PPLs. In addition, we further analyzed diagnostic yield of VBNA and NVBNA group according to lesion size, nature of lesion, lesion location, distance from the hilum, and bronchus sign.
Materials and methods
Search strategy and selection criteria
This systematic review and meta-analysis was performed in accordance with the recommendations of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidence. 20 We searched PubMed, Embase, Cochrane library, and Web of Sciences databases for the relevant papers. The last search was performed on 26 August 2020. The search combined following concepts: “Virtual bronchoscopic navigation” OR “Virtual bronchoscopy” AND “Peripheral lung lesions” OR “Peripheral pulmonary lesions”. By combing these concepts additional strategies were used to identify RCTs when possible. The reference list of retrieved studies were searched manually for relevant studies missed by electronic search.
We included all studies that meet the following criteria: (a) RCTs; (b) Patients were randomized to either VBNA or NVBNA for PPLs; and (c) reporting any of the following outcomes: total diagnostic yield, total examination time, diagnostic yield according to the lesion size, nature of lesion, lesion location in the lung lobe, distance from the hilum, bronchus sign, and complications. Exclusion criteria were non-comparatives studies, case reports, conference papers, and review papers. No restrictions were applied for study language. We performed electronic search without any time restrictions.
Data extraction and outcomes
The data were extracted by two investigators (MG and AP) independently. Disagreements were resolved with a third investigator (TW). Using a standardized data extraction form, two independent reviewers abstracted the data. The following data were extracted from eligible studies: first author, year of publication, study design, patient demographics, setting, bronchoscopy, navigation system, biopsy instruments and other auxiliaries. Total diagnostic yield, total examination time and diagnostic yield by lesion size (⩽20 mm or >20 mm), location of lesion in the lobe, distance from the hilum (central, intermediate and peripheral third), bronchus sign (presence or absence of bronchus sign), and nature of the lesion (malignant or benign), and complications were also recorded. The primary outcome was overall diagnostic yield and total examination time. Secondary outcomes included diagnostic yield according to the lesion size, lobe location of the lesion, distance from the hilum, bronchus sign, nature of the lesion, and complications. The study by Bo et al. divided subjects randomly in three groups as, per the inclusion criteria, we collected data only for the EBUS-GS and combined (EBUS-GS +VBN) groups. 17
Quality assessment
Two reviewers (MG and AP) independently evaluated studies for risk of bias using the Cochrane tool. 21 The following seven domains of each of the included studies were assessed: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), masking of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other potential sources of bias. Each domain was assigned a judgment of low risk of bias, unclear risk of bias, or high risk of bias. Any disagreements were resolved through consensus with a third reviewer. The inter-rater agreement for quality assessment between reviewers was evaluated via Cohen k coefficient.
Statistical analysis
All meta-analyses were performed using Review Manager, version 5.3. We used random-effects models for all analyses. Dichotomous outcomes were analyzed using Mantel–Haenszel (M-H) risk ratios (RR). Continuous outcomes were pooled using the inverse-variance mean difference (MD). Medians and interquartile ranges or ranges were converted to means and standard deviations (SD) according to Wan et al. 22 Heterogeneity between studies was evaluated with I2 estimation and the Cochran Q test based on Chi-squared statistics. For heterogeneity testing, Chi-squared tests with a p value <0.1 indicated heterogeneity in the results. Tests for funnel plot asymmetry were evaluated visually, but not used to assess for publication bias, as the number of studies identified was <10. 23
Results
Characteristics of included studies
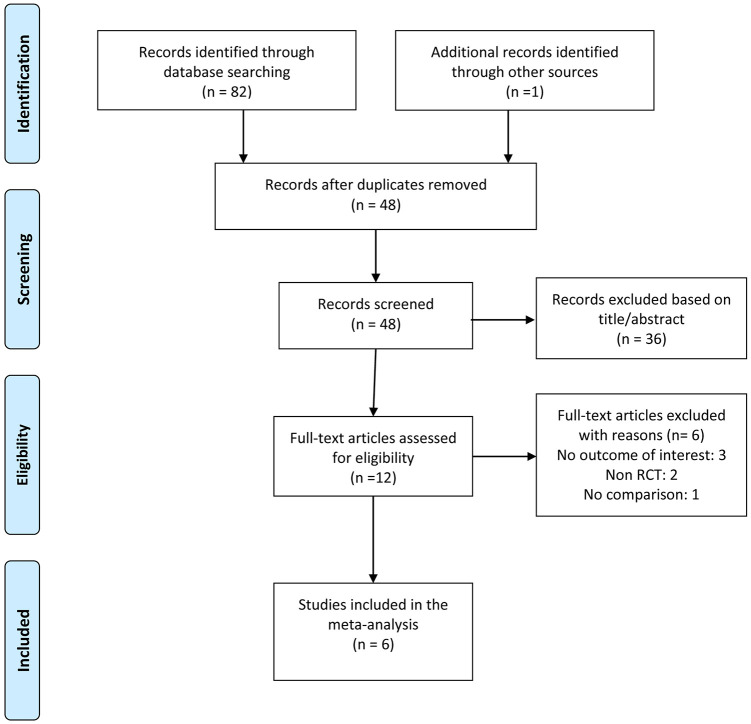
The study selection process is shown in Figure 1. The literature search identified 83 unique articles, and six RCTs fulfilling inclusion criteria were included in the meta-analysis.16–19,24,25 Table 1 shows the characteristics of the included studies. All studies were published between 2011 and 2019. All studies were conducted at tertiary care settings in China and Japan (three studies in Japan and three studies in China). Four out of the six RCTs were multi-center studies.16,17,19,24 A total of 1626 patients were included in the final analysis, with 813 patients in VBNA group and 813 patients in NVBNA group, respectively. Information on total diagnostic yield and total examination time was provided in all six studies.16–19,24,25 We did not assess the publication bias owing to the limited number of studies (<10 studies) included in each analysis.
Figure 1.
Flow chart of study selection process.
RCT, randomized controlled trial.
Table 1.
Characteristics of included studies.
| Study | Study design/location | Number of participants | Total examination time (min) a | Bronchoscope/outer diameter | Biopsy method | ||
|---|---|---|---|---|---|---|---|
| VBNA | NVBNA | VBNA | NVBNA | ||||
| Asano et al. 16 | RCT/Japan | 167 | 167 | 21.1 (8.9–45.1) | 20.8 (6.3–72.4) | XP260F, XP40/2.8 mm | Forceps, brush, lavage |
| Asano et al. 19 | RCT/Japan | 65 | 64 | 16.6 (7.6–36.5) | 18.5 (8.3–55.4) | P260F/4 mm | Forceps, brush, lavage |
| Bo et al. 17 | RCT/China | 334 | 336 | 28.34 ± 5.65 | 29.06 ± 6.40 | NA/NA | Forceps |
| Chen et al. 25 | RCT/China | 93 | 91 | 45 ± 10 | 55 ± 10 | BF-1 T260 or BF_F260/NA | Brush |
| Ishida et al. 24 | RCT/Japan | 99 | 95 | 24 (8.7–47) | 26.2 (11.6–58.6) | P260F/4 mm | Forceps, brush |
| Xu et al. 18 | RCT/China | 55 | 60 | 20.59 ± 2.12 | 21.53 ± 1.62 | Olympus BF-P260F/4 mm | Forceps |
Values are mean ± SD, or median (range).
NA, not available; NVBNA, non-virtual bronchoscopic navigation assisted; SD, standard deviation; VBNA, virtual bronchoscopic navigation assisted.
All RCTs had a low risk of random sequence generation selection bias (Figure S2, Supplemental information). There was unclear risk of allocation concealment (selection bias) in all the included RCTs, as these trials have not stated the method of allocation of subjects. The detailed methodological quality is shown in Supplemental Figures S2 and S3). The inter-rater agreement for quality assessment between reviewers was good, with the Cohen k coefficient being 0.831.
Primary outcomes
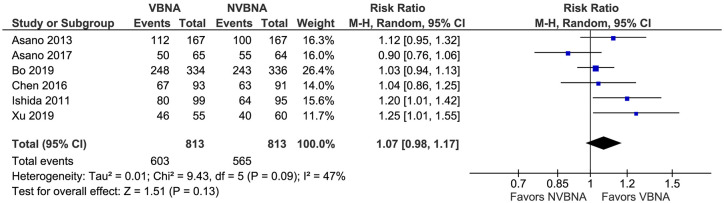
In six trials that reported overall diagnostic yield,16–19,24,25 the pooled diagnostic yield of VBN-assisted (VBNA) group was 74.17% (603/813) and non-VBN-assisted (NVBNA) group was 69.51% (565/813). There was no significant difference in the overall diagnostic yield between the VBNA group and NVBNA group (RR 1.07; 95% CI: 0.98–1.17; p = 0.13) (Figure 2). There was significant heterogeneity among the studies (I2 = 47%; p = 0.09). Two studies were an outlier in the estimate18,24 ; after excluding these two studies from the analysis, the pooled RR was 1.02 (95% CI: 0.94–1.10). After exclusion of the apparent outliers, there was no significant heterogeneity among studies (I2 = 22%; p = 0.28).
Figure 2.
Forest plot of all studies for overall diagnostic yield of VBNA versus NVBNA group for diagnosis of peripheral pulmonary lesion.
CI, confidence interval; M-H, Mantel–Haenszel; NVBNA, non-virtual bronchoscopic navigation assisted; VBNA, virtual bronchoscopic navigation assisted.
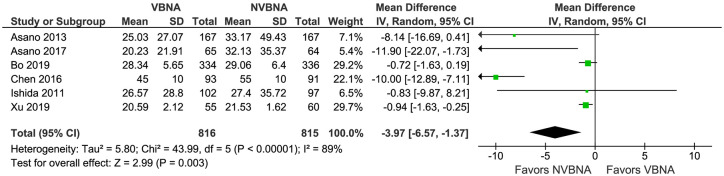
Total examination time was reported by six RCTs.16–19,24,25 VBNA significantly shortened total examination time compared with NVBNA [mean difference (MD): −3.94, 95% CI: −6.57 to −1.36; p = 0.003]; with significant heterogeneity (I2 = 89%; p < 0.00001) (Figure 3). After removing three outlier studies,18,19,25 the pooled MD was −1.79 (95% CI: −5.41 to 1.82) and there was no significant heterogeneity among studies (I2 = 30%; p = 0.24).
Figure 3.
Forest plot comparing total examination time of VBNA versus NVBNA group.
CI, confidence interval; IV, inverse variance; NVBNA, non-virtual bronchoscopic navigation assisted; VBNA, virtual bronchoscopic navigation assisted.
Secondary outcomes
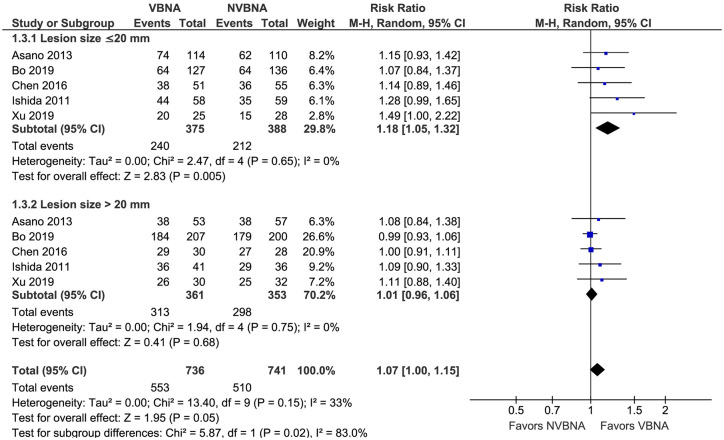
As shown in Table 2, subgroup meta-analysis was further performed to analyze diagnostic yield of VBNA and NVBNA group according to the lesion size, nature of lesion, lesion location in the lung lobe, distance from the hilum, and bronchus sign. A total of five studies were included in the pooled analysis of diagnostic yield by lesion size,16–18,24,25 and the pooled result showed significant disparities between both groups for lesion size (RR 1.07, 95% CI: 1.00–1.15), without significant heterogeneity (I2 = 33%; p = 0.15) (Figure 4). Subgroup analysis was performed to test diagnostic yield of VBNA and NVBNA bronchoscopy for diagnosing peripheral pulmonary lesions of size ⩽20 mm and lesion size >20 mm. Compared with non-virtual bronchoscopic navigation bronchoscopy, the diagnostic yield was higher in the VBNA group among the patients with a lesion size of ⩽20 mm (RR 1.18, 95% CI: 1.05–1.32) (Figure 4). When lesions >20 mm were evaluated, there was no significant difference in the pooled diagnostic yield in the VBNA group compared with the NVBNA group (RR 1.01, 95% CI: 0.96–1.06) (Figure 4).
Table 2.
Meta-analysis results of subgroup analysis.
| Variable | Number of studies | VBNA ‡ (%) | NVBNA † (%) | RR | 95% CI | Heterogeneity p value | I 2§ | Meta-analysis p value |
|---|---|---|---|---|---|---|---|---|
| Lesion size | ||||||||
| ⩽20 mm | 5 | 64 | 54.6 | 1.18 | 1.05–1.32 | 0.65 | 0 | 0.005* |
| >20 mm | 5 | 75.1 | 68.8 | 1.01 | 0.96–1.06 | 0.75 | 0 | 0.68 |
| Nature of lesion | ||||||||
| Malignant | 5 | 76.2 | 72 | 1.06 | 0.95–1.18 | 0.10 | 49 | 0.31 |
| Benign | 5 | 53.6 | 50 | 1.10 | 0.84–1.43 | 0.17 | 38 | 0.48 |
| Location of lesion | ||||||||
| Bilateral lower lobe | 5 | 74.7 | 69.7 | 1.07 | 0.91–1.26 | 0.01 | 69 | 0.39 |
| Right middle lobe | 5 | 88.2 | 82.1 | 1.04 | 0.91–1.18 | 0.82 | 0 | 0.57 |
| Bilateral lower lobe | 5 | 70 | 66.8 | 1.06 | 0.99–1.14 | 0.36 | 9 | 0.24 |
| Distance from hilum | 0 | |||||||
| Peripheral third | 3 | 70.7 | 61.7 | 1.10 | 0.89–1.36 | 0.06 | 65 | 0.39 |
| Central or intermediate third | 3 | 78.3 | 77.7 | 1.00 | 0.79–1.26 | 0.08 | 61 | 0.98 |
| Bronchus sign | ||||||||
| Present | 3 | 77.2 | 71.9 | 1.07 | 0.90–1.27 | 0.03 | 72 | 0.44 |
| Absent | 3 | 44.4 | 44.6 | 1.09 | 0.68–1.75 | 0.54 | 0 | 0.72 |
A p value <0.1 indicated heterogeneity in the results.
I2 index to quantify the degree of heterogeneity.
Diagnostic yield of VBNA.
Diagnostic yield of NVBNA.
CI, confidence interval; NVBNA, non-virtual bronchoscopic navigation assisted; RR, risk ratio; VBNA, virtual bronchoscopic navigation assisted.
Figure 4.
Forest plot comparing the diagnostic yield according to the lesion size of VBNA versus NVBNA group.
CI, confidence interval; M-H, Mantel–Haenszel; NVBNA, non-virtual bronchoscopic navigation assisted; VBNA, virtual bronchoscopic navigation assisted.
Five studies reported diagnostic yield by nature of lesion (malignant or benign lesion).16–19,25 Pooled analysis showed that there was no significant difference in the diagnosis yield of malignant lesions in VBNA group and NVBNA group (RR 1.06, 95% CI: 0.95–1.18, p = 0.31) (Supplemental Figure S4). Similarly, for benign lesions, statistical significance was not observed in the VBNA and NVBNA groups (RR 1.10, 95% CI: 0.84–1.43, p = 0.48) (Supplemental Figure S4).
Five studies reported information regarding the location of the lesion within the lobe.16–19,24 There was no significant difference in diagnostic yield between the VBNA group and NVBNA group for pulmonary lesions located in the bilateral lower lobe (RR 1.07, 95% CI 0.91–1.26, with significant heterogeneity, I2 = 51%; p = 0.01) (Supplemental Figure S5). Similarly, no significant statistical difference was observed between VBNA and NVBNA bronchoscopy for the diagnosis of peripheral pulmonary lesions located at the right middle lobe (RR 1.04, 95% CI 0.91–1.18) or bilateral lower lobe (RR 1.07, 95% CI 0.95–1.21) (Supplemental Figure S5).
Diagnostic yield according to the distance from the hilum was reported by three RCTs.16,19,24 Diagnostic yield results were similar for VBNA and NVBNA, for the lesions located in peripheral third (RR 1.10, 95% CI 0.89–1.36) and central/intermediate third of the lung field (RR 1.00, 95% CI 0.79–1.26) (Supplemental Figure S6). A total of three RCTs reported data regarding presence or absence of bronchus sign.16,19,24 Meta-analysis results showed that, in the bronchus sign-positive subgroup, VBNA bronchoscopy did not exhibit a significantly higher diagnostic rate than the NVBNA group (RR 1.07, 95% CI 0.90–1.27) (Supplemental Figure S7). Similarly, VBNA bronchoscopy was not superior to NVBNA bronchoscopy for the diagnosis of PPLs in the bronchus sign absent group (RR 1.09, 95% CI 0.68–1.75) (Supplemental Figure S7).
Complications
There were no major complications reported either in VBNA or NVBNA group in any of the included studies (Table 3). Pneumothorax and hemorrhage were complications reported by most studies.16–19,24,25 The results of our meta-analysis revealed that VBNA bronchoscopy was not associated with a higher rate of complications compared with the NVBNA bronchoscopy group (RR 0.84, 95% CI 0.42–1.67) (Supplemental Figure S2).
Table 3.
Summary of the complications from the studies included in the meta-analysis.
| Study | Complications | |
|---|---|---|
| VBNA | NVBNA | |
| Asano et al. 16 | Pneumothorax not requiring drainage (n = 1) Hemorrhage(n = 2) Transient bradycardia (n = 1) No severe adverse events |
Pneumothorax not requiring drainage (n = 1) Xylocaine intoxication (n = 1) Pneumonia (n = 1) No severe adverse events |
| Asano et al. 19 | Hyperventilation (n = 1) No severe adverse effect |
Hemorrhage (n = 2) Pneumonia (n = 1) No severe adverse effect |
| Bo et al. 17 | Pneumothorax (n = 5) Hemorrhage (n = 3) No severe adverse events |
Pneumothorax (n = 7) Hemorrhage (n = 4) No severe adverse events |
| Chen et al. 25 | No severe adverse events | No severe adverse events |
| Ishida et al. 24 | No severe or moderate adverse events | Mild pneumothorax that did not require chest drainage (n = 1) |
| Xu et al. 18 | Pneumothorax requiring intervention (n = 2) | Hemorrhage (n = 1) |
NVBNA, non-virtual bronchoscopic navigation assisted; VBNA, virtual bronchoscopic navigation assisted.
Discussion
To our knowledge, this is the first systematic review and meta-analyses of RCTs published to date to investigate the diagnostic yield and total examination time of VBNA and NVBNA bronchoscopy for diagnosing PPLs. The results of this meta-analysis demonstrate that there is no difference in total diagnostic yield between VBNA and NBVNA groups. However, VBNA significantly shortened total examination time compared with the NVBNA group. The subgroup analysis showed that the diagnostic yield was significantly higher in the VBNA group than in the NVBNA group for PPLs with lesion size ⩽20 mm, but the diagnostic yield for lesions >20 mm was not significantly different between the two groups. In addition, there were no differences between VBNA and NVBNA groups with regards to secondary outcomes, such as lobe location of the lesion, distance from the lesion to the hilum, bronchus sign, nature of the lesion (malignant or benign), and complications.
Our results are in contrast with the findings of a recent meta-analysis by Jiang et al., 26 which revealed that overall diagnostic yield of navigation bronchoscopy was statistically higher than non-navigation bronchoscopy for PPLs. The possible reasons for this contrasting finding are as follows: (1) Jiang et al. 26 pooled the results of both observational studies and RCTs in their meta-analysis, which might have overestimated the total effect, especially because observational studies were more vulnerable to selection bias. In addition, they failed to include two RCTs that compared VBNA and NVBNA for diagnosing PPLs.24,25 (2) In pooled analysis, they included one RCT that compared diagnostic yield of electromagnetic navigation bronchoscopy, 27 which might have increased the risk of bias and confounding variables that may have affected the results. We found no significant difference in the diagnostic yield between the two groups. Among the studies included in our meta-analysis, the study by Ishida et al. was the only one showing the higher diagnostic yield for the VBNA than for NVBNA bronchoscopy group. 24 The exclusion of this study from analysis resolved the issue of heterogeneity, without altering pooled results (RR 1.05; 95% CI: 0.95–1.15; I2 = 43%, p = 0.13). The choice of bronchoscopic modalities such as CT-guided biopsy and VBN and/or r-EBUS or conventional bronchoscopy varies from patient to patient. EBUS requires operation expertise and enables direct visualization of the target lesion. In addition to the VBN, study by Bo et al. used r- EBUS for diagnosis of the peripheral pulmonary lesion. 17 Of note, the diagnostic yields between the combined group (VBN + EBUS) and EBUS group were similar. The absence of benefit seen with VBN and r-EBUS may have been due to patient selection, with more difficult cases being selected for VBN and r-EBUS.
VBN can guide the bronchoscope to the more peripheral lesions in a shorter time than guided biopsy instruments. 28 The present study reported that total examination time was significantly shortened among patients with PPLs who were in the VBNA group compared with those who were in the NVBNA group. This finding is consistent with the results of individual studies included in our meta-analysis.24,25 Although VBNA demonstrated a statistically significant shortening of total examination time, this finding is far from being clinically relevant (mean difference being about 4 min); future, well-designed multi-center RCTs are needed to verify our findings. However, in terms of patients comfort, decreasing overall examination time by 4 min is significant, especially in patients undergoing the procedure under local anesthesia. In subgroup analysis, diagnostic yield of lesions ⩽20 mm was higher in the VBNA group than in the NVBNA group. This was in line with findings of previous meta-analysis. 26 At the same time, Kato et al. demonstrated that the diagnostic yield of small PPL <20 mm in diameter was significantly higher in the VBNA group than in the NVBNA group. 29 The use of VBNA might improve bronchial path selection more accurately and quickly for small lesions, in contrast to larger lesions, which could have several routes to reaching the target lesion. Additionally, a recent study that compared the diagnostic yield of VBN-guided and unguided ultrathin bronchoscopy found that the diagnostic yield was slightly higher for PPLs ⩽20 mm in the VBN-ultrathin arm, but the difference was not statistically significant (p = 0.069). 30 Diagnostic yield does not depend solely on the lesion size but is also affected by the target disease, location, and the presence or absence of an involved bronchus. 2 Our analysis suggests that VBNA bronchoscopy is a safe procedure with complication rates similar to those of NVBNA bronchoscopy – pneumothorax and bleeding being the most frequent complications. Further focused multi-center RCTs with larger sample size are needed to clarify the complications of VBNA and NVBNA bronchoscopy.
There are several caveats to this study. First, the number of RCTs include in our meta-analysis is relatively small. However, all studies exhibited moderate-to-excellent methodological quality. Second, the lack of detailed information on experience of operators, sampling methods, and equipment in included RCTs might lead to the observed heterogeneity, and further impair the robustness of our findings. Third, the high variability in diagnostic yield between individual trials included in this meta-analysis may be attributed to several factors including the expertise of the interventional pulmonologist, the presence or absence of rapid on-site evaluation (ROSE), and biopsy tools selection. This potential overestimation of diagnostic yield due to expertise bias may affect the meta-analysis results. Fourth, unlike other meta-analyses, heterogeneity may affect the results of this meta-analysis. Large multi-center RCTs comparing VBNA and NVBNA bronchoscopy, targeting subgroups of patients with PPLs are needed to generalize our findings.
In conclusion, the current systematic review and meta-analysis demonstrates that VBNA bronchoscopy does not increase the overall diagnostic yield when compared with NVBNA bronchoscopy in patients with PPLs. However, total examination time was shorter in the VBNA group than in the NVBNA group. Furthermore, subgroup analysis revealed that VBNA had a better performance than NVBNA bronchoscopy for PPLs ⩽20 mm. VBN is a form of novel guided bronchoscopy that requires no specific training, and has a low complication rate. It can shorten the positioning time and is a safe and effective promising technique for investigating pulmonary lesions. VBN improves the diagnostic yield when combined with other methods, such as EBUS or R-EBUS, and EBUS-GS-TBLB for PPLs. This technique also helps to abandon X-ray guidance, thus avoiding significant cumulative radiation dose for both patient and operator. Analysis of current several bronchoscopic technologies, including advantages and disadvantages is included in Supplemental Table S1. More RCTs that use standardized patient selection, technical approaches, outcome definitions, and statistical reporting methods are needed to elucidate the potential role of VBNA bronchoscopy for the diagnosis of PPLs.
Supplemental Material
Supplemental material, sj-pdf-1-tar-10.1177_17534666211017048 for Virtual bronchoscopic navigation versus non-virtual bronchoscopic navigation assisted bronchoscopy for the diagnosis of peripheral pulmonary lesions: a systematic review and meta-analysis by Mohan Giri, Anju Puri, Ting Wang, Guichuan Huang and Shuliang Guo in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_17534666211017048 for Virtual bronchoscopic navigation versus non-virtual bronchoscopic navigation assisted bronchoscopy for the diagnosis of peripheral pulmonary lesions: a systematic review and meta-analysis by Mohan Giri, Anju Puri, Ting Wang, Guichuan Huang and Shuliang Guo in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_17534666211017048 for Virtual bronchoscopic navigation versus non-virtual bronchoscopic navigation assisted bronchoscopy for the diagnosis of peripheral pulmonary lesions: a systematic review and meta-analysis by Mohan Giri, Anju Puri, Ting Wang, Guichuan Huang and Shuliang Guo in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-4-tar-10.1177_17534666211017048 for Virtual bronchoscopic navigation versus non-virtual bronchoscopic navigation assisted bronchoscopy for the diagnosis of peripheral pulmonary lesions: a systematic review and meta-analysis by Mohan Giri, Anju Puri, Ting Wang, Guichuan Huang and Shuliang Guo in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-5-tar-10.1177_17534666211017048 for Virtual bronchoscopic navigation versus non-virtual bronchoscopic navigation assisted bronchoscopy for the diagnosis of peripheral pulmonary lesions: a systematic review and meta-analysis by Mohan Giri, Anju Puri, Ting Wang, Guichuan Huang and Shuliang Guo in Therapeutic Advances in Respiratory Disease
Footnotes
Author contributions: Conceptualization: M.G., S.L.G, G.C.H., A.P. Data curation: M.G., A.P., T.W. Formal analysis: M.G., A.P., T.W., S.L.G., G.C.H. Investigation: M.G., A.P. Project administration: M.G., A.P., T.W. Supervision: S.L.G., M.G. Validation: S.L.G., M.G., A.P. Visualization: M.G., A.P., T.W., S.L.G. Writing: M.G., A.P., T.W., S.L.G. Writing – review and editing: M.G., A.P., T.W., S.L.G., G.C.H.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This study was supported by grants from Chongqing Science and Technology Commission project cstc2017shmsA130044.
Data availability: The data used to support the findings of this study are available from the corresponding author upon request.
ORCID iD: Mohan Giri  https://orcid.org/0000-0001-8588-5482
https://orcid.org/0000-0001-8588-5482
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Mohan Giri, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China.
Anju Puri, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China.
Ting Wang, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China.
Guichuan Huang, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China.
Shuliang Guo, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Chongqing Medical University, No 1 Youyi Road, Yuzhong, Chongqing 400016, China.
References
- 1. Ali MS, Trick W, Mba BI, et al. Radial endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions: a systematic review and meta-analysis. Respirology 2017; 22: 443–453. [DOI] [PubMed] [Google Scholar]
- 2. Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143: e142S–e165S. [DOI] [PubMed] [Google Scholar]
- 3. Callister MEJ, Baldwin DR, Akram AR, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015; 70(Suppl. 2): ii1–ii54. [DOI] [PubMed] [Google Scholar]
- 4. Wang W, Yu L, Wang Y, et al. Radial EBUS versus CT-guided needle biopsy for evaluation of solitary pulmonary nodules. Oncotarget 2018; 9: 15122–15131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Steinfort DP, Vincent J, Heinze S, et al. Comparative effectiveness of radial probe endobronchial ultrasound versus CT-guided needle biopsy for evaluation of peripheral pulmonary lesions: a randomized pragmatic trial. Respir Med 2011; 105: 1704–1711. [DOI] [PubMed] [Google Scholar]
- 6. Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012; 142: 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gupta S, Wallace MJ, Cardella JF, et al. Quality improvement guidelines for percutaneous needle biopsy. J Vasc Interv Radiol 2010; 21: 969–975. [DOI] [PubMed] [Google Scholar]
- 8. Wu CC, Maher MM, Shepard J-AO. Complications of CT-guided percutaneous needle biopsy of the chest: prevention and management. AJR Am J Roentgenol 2011; 196: W678–W682. [DOI] [PubMed] [Google Scholar]
- 9. Xu C-H, Yuan Q, Yu L-K, et al. Endobronchial ultrasound transbronchial biopsy with guide-sheath for the diagnosis of solitary pulmonary nodules. Oncotarget 2017; 8: 58272–58277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herth FJF, Ernst A, Becker HD. Endobronchial ultrasound-guided transbronchial lung biopsy in solitary pulmonary nodules and peripheral lesions. Eur Respir J 2002; 20: 972–974. [DOI] [PubMed] [Google Scholar]
- 11. Kemp SV. Navigation bronchoscopy. Respiration 2020; 99: 277–286. [DOI] [PubMed] [Google Scholar]
- 12. Tachihara M, Tamura D, Kiriu T, et al. Bronchoscopy using virtual navigation and Endobronchial Ultrasonography with a Guide Sheath (EBUS-GS) with or without fluoroscopy for peripheral pulmonary lesions. Kobe J Med Sci 2018; 63: E99–E104. [PMC free article] [PubMed] [Google Scholar]
- 13. Tachihara M, Ishida T, Kanazawa K, et al. A virtual bronchoscopic navigation system under X-ray fluoroscopy for transbronchial diagnosis of small peripheral pulmonary lesions. Lung Cancer 2007; 57: 322–327. [DOI] [PubMed] [Google Scholar]
- 14. Asano F, Matsuno Y, Shinagawa N, et al. A virtual bronchoscopic navigation system for pulmonary peripheral lesions. Chest 2006; 130: 559–566. [DOI] [PubMed] [Google Scholar]
- 15. Shinagawa N, Yamazaki K, Onodera Y, et al. Factors related to diagnostic sensitivity using an ultrathin bronchoscope under CT guidance. Chest 2007; 131: 549–553. [DOI] [PubMed] [Google Scholar]
- 16. Asano F, Shinagawa N, Ishida T, et al. Virtual bronchoscopic navigation combined with ultrathin bronchoscopy. A randomized clinical trial. Am J Respir Crit Care Med 2013; 188: 327–333. [DOI] [PubMed] [Google Scholar]
- 17. Bo L, Li C, Pan L, et al. Diagnosing a solitary pulmonary nodule using multiple bronchoscopic guided technologies: a prospective randomized study. Lung Cancer 2019; 129: 48–54. [DOI] [PubMed] [Google Scholar]
- 18. Xu C, Yuan Q, Wang Y, et al. Usefulness of virtual bronchoscopic navigation combined with endobronchial ultrasound guided transbronchial lung biopsy for solitary pulmonary nodules. Medicine (Baltimore) 2019; 98: e14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Asano F, Ishida T, Shinagawa N, et al. Virtual bronchoscopic navigation without X-ray fluoroscopy to diagnose peripheral pulmonary lesions: a randomized trial. BMC Pulm Med 2017; 17: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011; 343: d4002. [DOI] [PubMed] [Google Scholar]
- 24. Ishida T, Asano F, Yamazaki K, et al. Virtual bronchoscopic navigation combined with endobronchial ultrasound to diagnose small peripheral pulmonary lesions: a randomised trial. Thorax 2011; 66: 1072–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen ZB, Jin YP, Yu YM, et al. [A study of the diagnostic value of endobronchial ultrasound guide sheath transbronchial lung biopsy combined with virtual bronchoscopic navigation in peripheral pulmonary lesions]. Zhonghua jie he he hu xi za zhi = Zhonghua jiehe he huxi zazhi = Chinese J Tuberc Respir Dis 2016; 39: 509–513. [DOI] [PubMed] [Google Scholar]
- 26. Jiang S, Xie F, Mao X, et al. The value of navigation bronchoscopy in the diagnosis of peripheral pulmonary lesions: a meta-analysis. Thorac Cancer 2020; 11: 1191–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eberhardt R, Anantham D, Ernst A, et al. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med 2007; 176: 36–41. [DOI] [PubMed] [Google Scholar]
- 28. Asano F, Eberhardt R, Herth FJF. Virtual bronchoscopic navigation for peripheral pulmonary lesions. Respiration 2014; 88: 430–440. [DOI] [PubMed] [Google Scholar]
- 29. Kato A, Yasuo M, Tokoro Y, et al. Virtual bronchoscopic navigation as an aid to CT-guided transbronchial biopsy improves the diagnostic yield for small peripheral pulmonary lesions. Respirology 2018; 23: 1049–1054. [DOI] [PubMed] [Google Scholar]
- 30. Diez-Ferrer M, Morales A, Tebé C, et al. Ultrathin bronchoscopy with and without virtual bronchoscopic navigation: influence of segmentation on diagnostic yield. Respiration 2019; 97: 252–258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tar-10.1177_17534666211017048 for Virtual bronchoscopic navigation versus non-virtual bronchoscopic navigation assisted bronchoscopy for the diagnosis of peripheral pulmonary lesions: a systematic review and meta-analysis by Mohan Giri, Anju Puri, Ting Wang, Guichuan Huang and Shuliang Guo in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_17534666211017048 for Virtual bronchoscopic navigation versus non-virtual bronchoscopic navigation assisted bronchoscopy for the diagnosis of peripheral pulmonary lesions: a systematic review and meta-analysis by Mohan Giri, Anju Puri, Ting Wang, Guichuan Huang and Shuliang Guo in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_17534666211017048 for Virtual bronchoscopic navigation versus non-virtual bronchoscopic navigation assisted bronchoscopy for the diagnosis of peripheral pulmonary lesions: a systematic review and meta-analysis by Mohan Giri, Anju Puri, Ting Wang, Guichuan Huang and Shuliang Guo in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-4-tar-10.1177_17534666211017048 for Virtual bronchoscopic navigation versus non-virtual bronchoscopic navigation assisted bronchoscopy for the diagnosis of peripheral pulmonary lesions: a systematic review and meta-analysis by Mohan Giri, Anju Puri, Ting Wang, Guichuan Huang and Shuliang Guo in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-5-tar-10.1177_17534666211017048 for Virtual bronchoscopic navigation versus non-virtual bronchoscopic navigation assisted bronchoscopy for the diagnosis of peripheral pulmonary lesions: a systematic review and meta-analysis by Mohan Giri, Anju Puri, Ting Wang, Guichuan Huang and Shuliang Guo in Therapeutic Advances in Respiratory Disease