Abstract
Background:
Hemophagocytic lymphohistiocytosis (HLH) can be life-threatening if not detected and treated appropriately. The diagnosis of HLH can be confusing due to other similar febrile diseases that present with cytopenia. Natural-killer cell (NK)-cytotoxicity is an important diagnostic parameter for primary HLH; however, its role in secondary HLH in adults has not been well-elucidated.
Methods:
We prospectively enrolled 123 adult patients with febrile conditions accompanied by cytopenia or marrow hemophagocytosis. A diagnosis of HLH was based on HLH-2004 criteria and treated based on HLH-94 protocol. NK-cytotoxicity was calculated at the time of diagnosis by K562-cell direct lysis using flow-cytometry.
Results:
HLH (n = 60) was determined to be caused by Epstein–Barr virus (EBV) (n = 11), infection other than EBV (n = 16), malignancies (n = 19), and unknown (n = 14). Febrile diseases other than HLH (n = 63) were diagnosed as autoimmune disease (n = 22), malignancies (n = 21), infection (n = 12), non-malignant hematological diseases (n = 6), and unknown (n = 2). A lower NK-cytotoxicity level was observed at diagnosis in patients with HLH, compared with other causes of febrile disease (12.1% versus 26.2%, p < 0.001). However, NK-cytotoxicity had a borderline effect on diagnosis of HLH, with an area under receiver operation characteristic curve of 0.689. It also showed no significant role for the prediction of survival outcome. Multivariate analysis revealed that malignant disease and high ferritin level were related with poor survival outcome. In non-malignant disease subgroups, old age, EBV-association, and low NK-cytotoxicity were related with poor survival.
Conclusions:
Febrile disease with cytopenia was associated with decreased NK-cytotoxicity, especially in adults with HLH; however, its diagnostic role for adult HLH is still arguable. The diagnostic criteria for adult HLH should be further discussed.
Trial registration:
Clinical Research Information Service [Internet]; Osong (Chungcheongbuk-do), Korea, Centers for Disease Control and Prevention, Ministry of Health and Welfare (Republic of Korea); https://cris.nih.go.kr/cris/index.jsp; Feb, 16th 2016; KCT0001886 (KC15TISE0936);
Keywords: cytotoxicity, febrile disease, hemophagocytic lymphohistiocytosis, natural-killer cell
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is known to be caused by the impairment of granule-mediated cytotoxicity of natural-killer (NK) cells or cytotoxic T-cells, which is one of the important diagnostic criteria of HLH.1–3 This initially originated from the concept of primary or familial HLH in pediatrics. In some genetic forms of HLH, gene mutations that affect granule-mediated cytotoxicity were identified, in addition to pigment disorders and the dysregulation of cytotoxicity or cell signaling.4–7 The impaired cytotoxicity characteristically results in an overt inflammatory process that can include fever, hyperferritinemia, hypertriglyceridemia, hypofibrinogenemia, and the production of cytokines such as interferon-gamma, which has a role in macrophage activation.8,9 The increased production of interferon-gamma recruits macrophages and activates phagocytosis, which results in cytopenia in some lineages and may cause hepatosplenomegaly or neurological complications. This cascade can cause high fatalities in patients if they are not properly diagnosed and treated.
Because the HLH-2004 diagnostic criteria were based on the clinical experiences and pathologic mechanisms in pediatric familial HLH, the criteria have not yet been precisely approved in adult HLH. Decreased or absent NK cytotoxicity as a diagnostic parameter is still debated, because there is a lack of evidence that supports its diagnostic utility in adult HLH. Therefore, there are a lot of patients with other medical or surgical complications that present with features similar to HLH, which may be related to hypomorphic gene mutations important in immune regulation. 10 Such conditions can cause an imbalance between infected cells and immune effector cells, as well as transient immune dysfunction due to drugs or a low number of immune cells. 11 Decreased NK cytotoxicity can be observed in conditions other than HLH.12,13 Therefore, many febrile diseases could be initially diagnosed with HLH or the diagnosis could be underestimated and attributed to poor clinical courses of infectious conditions, rheumatology, or malignant diseases.
Overall, the diagnostic criteria for adult HLH have not yet been defined clearly; therefore, the condition and overall diagnostic parameters should be studied further in retrospective and prospective cohorts that include similar inflammatory diseases. To identify the diagnostic relevance and the significant cut-off value for NK cytotoxic function, we focused on patients that presented with fever with cytopenia or evidence of hemophagocytosis. NK cytotoxicity was calculated at the time of diagnosis and we tried to identify significant differences between the causes of febrile disease. Finally, the overall treatment response and survival outcomes were also evaluated based on the level of NK cytotoxicity in several subgroup analyses.
Materials and methods
Enrolled patients
From 2016 to 2018, a total of 123 adult patients (median age 49 years old, range 17–92 years) with febrile disease that presented to the Catholic Hematology Hospital in South Korea with cytopenia or evidence of hemophagocytosis were prospectively analyzed to identify the diagnostic role of NK cytotoxicity and survival outcomes. New patients that satisfied the following conditions were included in this study: 1) adult patients (15 years or older), 2) fever (⩾38°C) calculated by tympanic temperature, 3) patients with demonstrated hemophagocytosis in bone marrow (BM) samples, and 4) patients that presented with cytopenia with at least two lineages. The exclusion criteria were as follows: 1) patients who were treated with intensive chemotherapy within 3 months of screening, 2) patients who were already diagnosed with immune thrombocytopenic purpura, 3) patients who were already diagnosed with autoimmune hemolytic anemia or chronic anemia, and 4) patients who were previously diagnosed with aplastic anemia.
This study was approved by the Institutional Review Board of The Catholic University of Korea (No. KC15TISE0936) and was conducted in accordance with the Declaration of Helsinki. All written informed consents were provided to all patients. This study was registered in the Clinical Research Information Service (CRIS; Osong [Chungcheongbuk-do], Korea), with the Centers for Disease Control and Prevention, Ministry of Health and Welfare (Republic of Korea); KCT0001886.
Endpoints and definitions
The primary endpoint of this study was to identify the median level of NK cytotoxicity in febrile diseases and to evaluate the diagnostic value for HLH compared with other related conditions. The secondary endpoints were overall survival (OS) based on a significant level of NK cytotoxicity, or the type of disease. A diagnosis of disease concomitant with fever was defined as malignant disease, autoimmune disease, or macrophage activation syndrome (MAS), HLH, and other infectious or unspecified febrile diseases.
We placed all autoimmune disease-related cases in the MAS subgroup, although the features were very similar to HLH. Several causes were suggested in the HLH group, including malignancy-associated HLH, Epstein–Barr Virus (EBV)-associated HLH (EBV-HLH), HLH due to infections other than EBV, and HLH with other unspecified causes; these categories have also been utilized in our previous reports. 14 Therefore, if a patient has both HLH and malignant disease, we distributed the patient to HLH for current analysis. The diagnosis of HLH was based on criteria suggested in the HLH2004 protocol.3,15 However, the soluble CD25 level was not routinely checked, and was only analyzed in 49 available patients. In addition, as the role of NK cytotoxicity should have been evaluated in this study, those two values were not used for diagnosis of HLH; rather, they were diagnosed by satisfying five out of the other six suggested criteria. MAS was diagnosed based on the Ravelli 16 criteria. However, as suggested above, we allocated all patients with autoimmune disease into the ‘MAS group’ because the diagnostic criteria for adult patients are currently unclear.17,18
Clinical parameters and NK cytotoxicity assay
A fever higher than 38°C was a requirement for diagnosis in this study and cytopenia at least two lineages or BM hemophagocytosis were also required. Other parameters included in the diagnostic guidelines for HLH were reviewed, including splenomegaly, triglycerides, fibrinogen, ferritin, and cytotoxic activity of NK cells. Laboratory findings were serially measured, and the lowest level was captured for complete blood cell counts (CBC) and fibrinogen before treatment. For ferritin, the diagnostic level and the highest level within 4 weeks from initial treatment were checked and the highest level was used for triglycerides to assess clinical outcomes.
We analyzed NK cytotoxicity using a flow cytometry-based assay which was previously reported. 19 Briefly, the K562 target cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) to discriminate target cells from NK cells. The NK cells were incubated with CFSE-labeled K562 target cells at different effector-to-target ratios. Flow cytometry data, after co-culture for 4 h, were analyzed with a Fortessa flowcytometry (BD bioscience) using the FlowJo version 10.0.6 software (Tree Star, Ashland, OR). NK cytotoxicity levels were determined as the number of positive cells for both CFSE and 7-AAD/ number of total CFSE positive cells. We used peripheral blood mononuclear cell portion, rather than isolated NK-enriched samples at 32:1 of the effector-to-target ratio. As a control, we tested healthy donor’s samples used in a previous study. 19 EBV-association was evaluated by both serology and EBV deoxyribonucleic acid (DNA) reverse transcription polymerase chain reaction (RT-qPCR).
Treatment strategies
The treatment strategies for patients diagnosed with HLH were based on the HLH-94 protocol, which primarily consists of dexamethasone with an etoposide.20,21 However, the administration of the etoposide was delayed for at least 3 days after dexamethasone 15 mg, with close observation of disease improvement. If fever subsided and laboratory results, including cytopenia and ferritin, continuously improved, we did not apply the etoposide and dexamethasone treatment was tapered. However, for patients that showed progressive disease within 3 days or without appropriate improvement within 7 days, we immediately started etoposide and reduced treatment by 33–66%, according to the patients’ age, performance status, or level of cytopenia. For some patients with evidence of macrophage activation or that had experienced a severe inflammation period, we applied the same dose of dexamethasone but rapidly reduced or did not use dexamethasone in patients with evidence of sepsis. If malignancy was diagnosed, a specific chemotherapy was started, even for patients that had already been treated with dexamethasone or etoposide. We followed the standardized protocol of the Catholic Hematology Hospital for infection management. Rituximab was used for salvage therapy for patients with relapsed EBV-HLH, followed by planned allogeneic hematopoietic cell transplantation. 14
Statistical analysis
All categorical variables were calculated and compared using Fisher’s exact tests, and continuous variables were assessed by Mann–Whitney U or Kruskal–Wallis tests to determine significant differences between subgroups. A significant cut-off value was estimated using Receiver Operation Characteristic curve analysis. The OS measured the proportion of people who are alive at a specified time from the time of diagnosis. Survival curves were estimated using the Kaplan–Meier method, and log-rank analysis was used to evaluate the differences between subgroups. All statistical analyses were performed using the R software (ver. 2.15.1, R Foundation for Statistical Computing, 2012). Statistical significance was set at a p-value < 0.05.
Results
Baseline characteristics
Among the 123 enrolled patients that met the inclusion criteria, all patients had a high fever with cytopenia or evidence of hemophagocytosis. In addition, there were 68 (55.3%) patients with active hemophagocytosis in BM, and 53 (78.0%) of them were diagnosed with HLH. The other 55 patients presented with cytopenia of at least two lineages without evidence of hemophagocytosis, but seven of them were diagnosed with HLH. According to the HLH2004 diagnostic criteria, and excluding NK cytotoxicity and soluble CD25, 60 patients were finally diagnosed with HLH. A total of 63 patients were diagnosed with conditions other than HLH. The final causes were malignancies (n = 21, 33.3%), infectious diseases (n = 12, 19.0%), non-malignant hematological diseases (n = 6, 9.5%), and autoimmune diseases that were classified as MAS (n = 22, 34.9%).
The causes of HLH were determined to be cancer-association, which was observed in 19 patients (31.7%), EBV-association in 11 (18.3%), infection other than EBV in 16 (26.7%), and unknown cause in 14 (23.3%) patients. We observed that age, gender, CBC findings at diagnosis, maximal triglyceride, lowest fibrinogen level, C-reactive protein, and NK-induced interferon gamma levels were not significantly different between HLH and other than HLH. However, the median level of diagnostic and maximal ferritin, and NK cytotoxicity were significantly different between the two groups (Table 1).
Table 1.
Baseline characteristics of patients with disease presenting febrile cytopenia.
| Other than HLH (n = 63) | HLH (n = 60) | p-value | |
|---|---|---|---|
| Age, years old, median | 50 (18–92) | 49 (17–85) | |
| Age <50 years old (%) | 31 (49.2) | 30 (50.0) | 1.000 |
| Age ⩾ 50 years old (%) | 32 (50.8) | 30 (50.0) | |
| Male gender (%) | 29 (46.0) | 37 (61.7) | 0.104 |
| Splenomegaly (%) | 24 (38.1) | 50 (83.3) | <0.001* |
| CBC at diagnosis | |||
| Leukocyte (×109/l) | 2.94 (0.26–38.9) | 2.33 (0.16–18.5) | 0.425 |
| Neutrophil (×109/l) | 1.39 (0.02–25.4) | 1.04 (0.01–17.2) | 0.430 |
| Hemoglobin (g/dl) | 9.2 (5.5–15.3) | 8.8 (5.7–13.0) | 0.117 |
| Platelet (×109/l) | 65.0 (7.0–420.0) | 60.0 (7.0–322.0) | 0.232 |
| Triglyceride, diagnosis (mg/dl) | 181 (40–1929) | 160 (52–820) | 0.866 |
| Fibrinogen, diagnosis (mg/dl) | 276.0 (74.0–500.0) | 234.5 (24.0–500.0) | 0.089 |
| C-reactive protein (mg/dl) | 3.59 (0.03–29.15) | 4.21 (0.02–21.24) | 1.000 |
| Ferritin level, diagnosis (ng/ml) | 1669.0 (156.2–100000) | 5834.5 (218.3–96498.0) | <0.001* |
| Ferritin level, maximum (ng/ml) | 1898.0 (156.2–100000) | 8400.5 (218.3–100000) | <0.001* |
| BM hemophagocytosis (%) | 15 (23.8) | 53 (88.3) | <0.001* |
| K562 NK cytotoxicity (%) | 26.2 (1.6–74.3) | 12.1 (1.4–80.0) | <0.001* |
| NK-induced interferon-gamma (pg/mL) | 30.8 (0–2994) | 10.0 (1–3596) | 0.695 |
| Causes of other than HLH | - | ||
| Malignancy (%) | 21 (33.3) | - | |
| Lymphoma (%) | 12 (19.0) | - | |
| Acute leukemia (%) | 3 (4.8) | - | |
| Others (%) | 6 (9.5) | - | |
| Infection (%) | 12 (19.0) | - | |
| Hematological disease (%) | 6 (9.5) | - | |
| Unknown (%) | 2 (3.2) | - | |
| Autoimmune disease (MAS) (%) | 22 (34.9) | - | |
| Causes of HLH (%) | 0 (0.0) | 60 (100%) | - |
| Cancer-associated (%) | - | 19 (31.7%) | |
| Lymphoma (%) | - | 15 (25.0%) | |
| Acute leukemia (%) | - | 3 (5.0%) | |
| Myelodysplastic syndrome (%) | - | 1 (1.7%) | |
| EBV-associated (%) | - | 11 (18.3%) | |
| Infection other than EBV (%) | - | 16 (26.7%) | |
| Unknown (%) | - | 14 (23.3%) | |
| Treatment | 0.163 | ||
| Dexamethasone initiation (%) | 42 (66.7%) | 55 (91.7%) | |
| Cancer-specific therapy (%) | 14 (22.2%) | 14 (23.3%) | |
| HLH-94 (Dexamethasone + etoposide) (%) | 0 (0.0%) | 31 (51.7%) | |
p < 0.05.
BM, bone marrow; CBC, complete blood count; EBV, Epstein–Barr virus; HLH, hemophagocytic lymphohistiocytosis; MAS, macrophage activation syndromes; NK, natural killer cell.
NK cytotoxicity and other biomarkers for the diagnosis of HLH
We first observed the proportion of NK cells and identified that the % NK cells of the enrolled patients with febrile disease were lower than that of healthy control (15.6% versus 8.0%, p = 0.020). However, the % NK cells did not differ between the disease subgroups: malignancy versus non-malignancy (12.8% versus 7.6%, p = 0.556), malignancy HLH versus non-malignancy HLH (5.3% versus 7.2%, p = 0.500), EBV versus non-EBV (12.4% versus 6.9%, p = 0.176), and EBV-HLH versus non-EBV HLH (11.3% versus 5.8%, p = 0.116). For the % CD56dim NK cells, we also observed no significant differences between malignancy versus non-malignancy (89.2% versus 86.0%, p = 0.665), malignancy HLH versus non-malignancy HLH (69.1% versus 80.1%, p = 0.881), EBV versus non-EBV (88.0% versus 86.9%, p = 1.000), and EBV-HLH versus non-EBV HLH (81.7% versus 76.9%, p = 1.000).
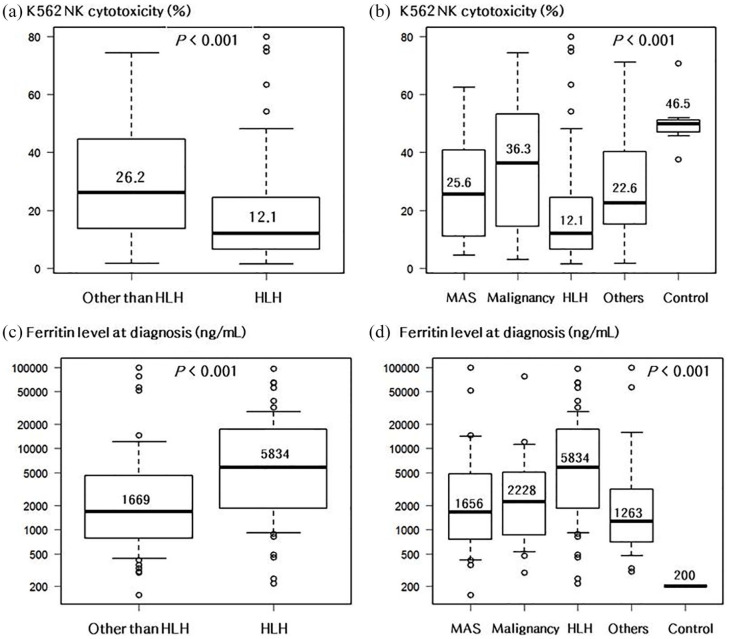
We determined that the median NK cytotoxicity level was significantly lower in HLH patients (12.1% versus 26.2%, p < 0.001) compared with patients in the group other than HLH [Figure 1(a)]. We further analyzed the median NK cytotoxicity levels according to causes that were not HLH, which revealed 25.6% in MAS, 36.3% in malignancy, and 22.6% in others. Compared with the median NK cytotoxicity levels in healthy controls (46.5%), HLH (p = 0.004), MAS (p = 0.012), and others (p = 0.051) all showed lower levels with the exception of the malignancy subgroup. However, a comparison between groups of HLH, MAS, and the others did not reveal any significant differences in the median level of NK cytotoxicity [Figure 1(b)]. We calculated a significant cut-off for diagnostically-relevant NK cytotoxicity, and the level was estimated to be lower than 22.0% of K562 lysis [area under curve (AUC) = 0.689, sensitivity 63.5%, and specificity 71.7%]. We also determined that the median ferritin level was significantly higher in HLH patients (5834 versus 1669, p < 0.001), compared with that of patients in the group other than HLH [Figure 1(c)].
Figure 1.
(a) Median level of NK cytotoxicity in patients with HLH versus other than HLH. (b) Median level of NK cytotoxicity in patients with variable febrile diseases with cytopenia. (c) Median ferritin level at diagnosis in patients with HLH versus other than HLH. (d) Median ferritin level at diagnosis in patients with variable febrile diseases with cytopenia.
HLH, hemophagocytic lymphohistiocytosis; MAS, macrophage activation syndromes; NK, natural killer.
The median ferritin levels in several causes of disease were also higher in HLH (5834, p < 0.001), MAS (1656, p = 0.003), malignancy (2228, p = 0.001), and other febrile diseases (1263, p = 0.001) compared with the levels in normal controls. In addition, a comparison between groups of HLH, MAS, and the others did not reveal any significant differences [Figure 1(d)]. We also calculated a significant cut-off for diagnostically-relevant ferritin and the level was estimated at higher than 5516 ng/ml (AUC = 0.721, sensitivity 75.0%, and specificity 64.9%), and there was a weak negative correlation between NK cytotoxicity and ferritin level (r = −0.4, p = 0.001). For soluble CD25, which was available only in 49 patients, the level was also higher in HLH patients (2551 versus 1077, p = 0.008) compared with that of patients in the other than HLH group.
Clinical outcomes according to diagnoses
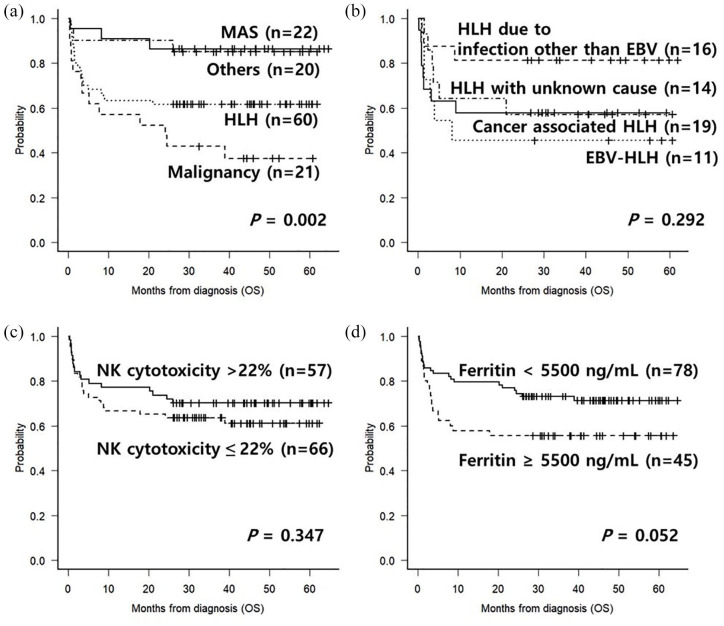
After a median follow-up duration of 45.4 months (range, 26.2–64.7) for surviving patients, the estimated 4-year OS in the MAS group was 86.4%, in the HLH group 61.7%, in the malignancy group 42.9%, and other causes that primarily included infectious diseases at 85.0% [Figure 2(a)]. In the HLH group, the 4-year OS was 81.2% in HLH with infections other than EBV, 57.9% in cancer-associated HLH, 45.5% in EBV-associated HLH, and 57.1% in HLH with unknown causes [Figure 2(b)].
Figure 2.
(a) OS according to the causes of febrile disease with cytopenia. (b) OS according to the causes of HLH. (c) OS according to NK cytotoxicity at diagnosis in all enrolled patients. (d) OS according to ferritin level at diagnosis in all enrolled patients.
HLH, hemophagocytic lymphohistiocytosis; NK, natural killer; OS, overall survival.
In all patients, a low NK cytotoxicity level [Figure 2(c)] did not significantly predict the survival outcome (61.1% versus 70.2%, p = 0.347), while the ferritin level higher than 5500 ng/ml [Figure 2(d)] showed a trend for poorer OS (55.6% versus 71.3%, p = 0.052). Multivariate analysis showed that a ferritin level higher than 5500 ng/ml [hazard ratio (HR) = 1.92, 95% confidence interval (CI) 1.04–3.52, p = 0.036] and malignancy (HR = 2.68, 95% CI 1.46–4.93, p = 0.001) were significant factors for the poor outcome (Table 2).
Table 2.
Multivariate analysis of factors affecting OS in patients with febrile disease.
| Variables | OS (entire patients) | OS (in non-malignancy) | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| % at 4 year | p-value | HR (95% CI) | p-value | % at 4 year | p-value | HR (95% CI) | p-value | |
| Age | ||||||||
| <50 years | 73.1 | 0.040 | 86.0 | 0.012 | 1 | |||
| ⩾50 years | 58.1 | 62.5 | 4.23 (1.58–11.3) | 0.004 | ||||
| Gender | ||||||||
| Male | 63.0 | 0.595 | 75.0 | 0.996 | ||||
| Female | 68.4 | 74.4 | ||||||
| EBV-association | ||||||||
| No | 67.1 | 0.326 | 78.3 | 0.075 | 1 | |||
| Yes | 55.6 | 57.1 | 5.41 (1.70–17.2) | 0.004 | ||||
| Ferritin level at diagnosis | ||||||||
| <5500 ng/ml | 71.3 | 0.052 | 1 | 82.4 | 0.033 | |||
| ⩾5500 ng/ml | 55.6 | 1.92 (1.04–3.52) | 0.036 | 62.5 | ||||
| NK cytotoxicity at diagnosis | ||||||||
| >22% | 70.2 | 0.347 | 82.1 | 0.048 | 1 | |||
| ⩽22% | 61.1 | 68.2 | 3.83 (1.29–11.4) | 0.015 | ||||
| Splenomegaly | ||||||||
| No | 70.8 | 0.336 | 77.8 | 0.519 | ||||
| Yes | 61.5 | 71.7 | ||||||
| BM hemophagocytosis | ||||||||
| No | 64.8 | 0.995 | 76.5 | 0.797 | ||||
| Yes | 66.2 | 73.5 | ||||||
| Malignancy | ||||||||
| No | 74.7 | 0.001 | 1 | - | - | - | ||
| Yes | 45.8 | 2.68 (1.46–4.93) | 0.001 | - | - | - | ||
BM, bone marrow; CI, confidence interval; EBV, Epstein-Barr virus; HR, hazard ratio; NK, natural killer cell.
Clinical outcomes according to malignant disease or not
As we believed that the survival outcomes of febrile disease due to malignancies would be influenced by the malignant disease itself, we divided patients into malignancy and non-malignancy subgroups. In the malignancy subgroup, a low NK cytotoxicity level [Figure 3(a)] did not significantly predict survival outcome (46.8% versus 44.4%, p = 0.562), similar to the result of the entire patients and no specific parameters were identified as affecting factors. However, in the non-malignancy subgroup, and NK cytotoxicity lower than 22% (68.2% versus 82.1%, p = 0.048), showed poorer 4-year OS [Figure 3(b-d)]. Final multivariate analysis showed that age older than 50 years (HR = 4.23, 95% CI 1.58–11.3, p = 0.004), EBV-association (HR = 5.41, 95% CI 1.70–17.2, p = 0.004), and NK cytotoxicity lower than 22% (HR = 3.83, 95% CI 1.29–11.4, p = 0.015) were significant factors for the poor outcome in the non-malignancy subgroup (Table 2).
Figure 3.
(a) OS according to the NK cytotoxicity in patients with malignancy. (b) OS according to the NK cytotoxicity in patients without malignancy. (c) OS according to the EBV-association in patients without malignancy. (d) OS according to the ferritin level in patients without malignancy.
EBV, Epstein–Barr virus; NK, natural killer; OS, overall survival.
Discussion
Our prospective cohort consisted of patients with febrile disease with cytopenia or evidence of BM hemophagocytosis. These clinical features are frequently observed in patients in the emergency department or intensive care unit, and are likely associated with BM suppression, severe inflammatory consumption, or hemophagocytic activity. Among them, HLH is the most life-threatening inflammatory process, if not properly diagnosed and treated,22,23 and we are frequently asked, as consultant hematologists, whether the disease status is now HLH or not from several medical departments. Therefore, a diagnosis of HLH is not only very important for early intervention, but also difficult to clearly distinguish from other diseases, such as infection, autoimmune disease, and malignant disease that shows similar signs or symptoms.24–26
The current criteria for an exact diagnosis of adult HLH are ambiguous, with some controversies. Among the diagnostic parameters, we focused on the decreased or absent NK cytotoxicity, which is mainly observed in pediatric primary HLH caused by defects in granule-mediated cytotoxicity.4–7 We tried to evaluate and determine whether the diagnostic relevance of decreased or absent NK cytotoxicity is also applicable in adult secondary HLH. We observed that the level of NK cytotoxicity was generally decreased in febrile diseases that arise from several causes, but we were not able to determine that NK cytotoxicity can be a powerful diagnostic test for diagnosis of HLH in adults. Although we calculated a significant cut-off lower than 22.0% of K562 cell lysis, the AUC was only 0.689, with a sensitivity of 63.5% and a specificity of 71.7% which is regarded as relatively-poor discriminating ability. Unlike our observations, recent reports showed normal NK cytotoxicity in adults with HLH, which was not significantly different even compared with healthy control.27–29 Therefore, unfortunately, we still cannot be sure if the decreased NK cytotoxicity is the main cause of the overt inflammatory process in HLH or a subsequent result of the most febrile diseases.
Because the ferritin level has been used as a relevant parameter for diagnosis and follow-up for response assessment, ferritin was also analyzed in a similar pattern in the current study. Previous reports have already shown that ferritin levels were diagnostically relevant for pediatric HLH, but not in adult cases, and they stated that it is not specific for diagnosing adult HLH.29–32
Our data revealed that ferritin was significantly higher in patients with HLH compared with other febrile diseases, and the diagnostically relevant level was higher than 5500 ng/ml. However, its discriminating power for diagnosis of HLH was 0.720 with a sensitivity of 75.0% and a specificity of 65.0%. This is marginal and similar to that of the NK cytotoxicity suggested priorly. Naymagon 29 and Naymagon et al. 30 revealed that the threshold ferritin levels used in diagnostic criteria for adult secondary HLH are too low to be clinically-relevant based on their analysis. We also observed that ferritin and NK cytotoxicity levels showed an inverse-relationship, but the power and significance was very weak. Therefore, based on the current results of diagnostic levels, both parameters by a certain cut-off level should be cautiously used as part of the diagnostic criteria for adult HLH. Soluble CD25 is also a relevant parameter for diagnosis of HLH, but our laboratory was set during our study period and it was not significantly analyzed in this study. We will analyze it in future evaluation rounds.
It is likely that febrile diseases that are combined with cytopenia or hemophagocytic activity are correlated with genetic susceptibility to severe inflammatory processes, even in patients without HLH. Recently, even in adult patients without a significant past medical history, hypomorphic mutations or digenetic inheritance of heterozygous mutations related to granule-mediated cytotoxicity defects have been observed, and those genetic abnormalities can also cause HLH feature.33–35 With this evidence and conditions studied in more detail, it is now difficult to distinguish boundaries between primary and secondary HLH. 36 Therefore, overt inflammation, hypercytokinemia, and macrophage-activation triggered by various antigens, at levels greater than what is expected, are based on the genetic susceptibility. This may be associated with quantitative reduction or functional decline of NK cells. Therefore, in future research, we plan to identify related gene defects or protein profiles.
In this study, we tried to identify a predictive role of NK cytotoxicity for survival outcome after disease-specific treatments including the HLH-94 protocol. Our treatment protocol for HLH patients consisted of prephase dexamethasone 10 mg/BSA/day for 3 to 7 days with close monitoring of improvement or disease progression, and the etoposide was started at any time of disease progression at reduced doses of 33 to 66%, according to the patients’ status based on previous toxic experiences and similar reports. 37 For the total survival outcomes, the effect of NK cytotoxicity level was not significant, and we hypothesized that this might be attributable to the different clinical courses for patients with malignancies, which can be difficult to analyze in combination with other diseases.38,39 Some patients with malignancies or malignancy-associated HLH die early due to rapid deterioration without proper chemotherapy at early periods. Their prognoses depend on the outcome of the specific malignant disease itself.40–42
We analyzed treatment responses in malignancy and non-malignancy subgroups separately, and we observed that an NK toxicity lower than 22% was related to poor survival outcomes in the non-malignancy subgroup.
As is the case with our previous report, 14 the current data also revealed EBV-association was still a poor prognostic factor in non-malignancy subgroup analysis. In the clinical practice, EBV-associated HLH generally shows a good response in the early treatment period, but it is hardly treated after progression with rapid multi-organ deteriorations. A few cases proceeded to allogeneic hematopoietic cell transplantation, but still showed a poor survival outcome even after transplantation. 43 In future studies we will focus on EBV-associated HLH in the context of treatment and genetic susceptibilities that present with poorer survival outcomes.
Conclusion
The current prospective study was conducted in accordance with a well-organized process and experienced support for laboratory analysis. A large proportion of patients with febrile conditions with cytopenia or hemophagocytic activity showed both decreased NK cytotoxicity and hyperferritinemia, and we observed significantly lower NK cytotoxicity and higher ferritin levels in adult patients with HLH. However, the diagnostic power and prognostic effect are not distinct, and we have to reappraise the pediatrics-inspired diagnostic criteria in adult HLH. Based on these results and other prospective studies, we hope that additional relevant diagnostic criteria for adult HLH can be introduced in the near future.
Acknowledgments
Not applicable
Footnotes
Author information: E-J.O organized the NK-cytotoxicity and immune research and wrote the manuscript; K.H.P., H.J.B, and S.J.Y mainly performed the NK-cytotoxicity and immune research; G.J.M., S-S.P., S.P., S-E.L., B-S.C., K-S.E., Y-J.K., S.L., H-J.K., C-K.M., S-G.C., J.W.L., and KJ.H. provided patients and materials and reviewed the manuscript; J-H.Y. designed and conducted the study, provided patients and materials, analyzed data, and wrote the manuscript; and all authors gave final approval of the manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF- 2019R1G1A1003013), Republic of Korea, and the authors wish to acknowledge the financial support of the Catholic Medical Center Research Foundation made in the program year of 2018 and SK plasma. The laboratory work was also supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2017R1A2B4011181), Republic of Korea.
Ethics approval and consent to participate: This study was approved by the Institutional Review Board of The Catholic University of Korea (KC15TISE0936) and was conducted in accordance with the Declaration of Helsinki. All written informed consents were provided to all patients.
Consent for publication: Written informed consent was obtained at the stage of diagnosis in all patients. This manuscript does not contain any individual’s personal data.
ORCID iDs: Jae-Ho Yoon  https://orcid.org/0000-0002-2145-9131
https://orcid.org/0000-0002-2145-9131
Yoo-Jin Kim  https://orcid.org/0000-0001-6653-2956
https://orcid.org/0000-0001-6653-2956
Jong Wook Lee  https://orcid.org/0000-0003-2949-4166
https://orcid.org/0000-0003-2949-4166
Availability of data and materials: All data generated or analyzed during this study are included in this published article. Datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Contributor Information
Eun-Jee Oh, Department of Laboratory Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Jae-Ho Yoon, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 222, Banpo-daero, Seocho-gu, Seoul, 06591, Republic of Korea.
Ki Hyun Park, Department of Biomedical Science, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Hyun Joo Bae, Department of Biomedical Science, College of Medicine, The Catholic University of Korea, Seoul, Korea.
So Jeong Yun, Department of Biomedical Science, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Gi June Min, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Sung-Soo Park, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Silvia Park, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Sung-Eun Lee, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Byung-Sik Cho, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Ki-Seong Eom, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Yoo-Jin Kim, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Seok Lee, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Hee-Je Kim, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Chang-Ki Min, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Seok-Goo Cho, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Kyungja Han, Department of Laboratory Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Jong Wook Lee, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
References
- 1. Jordan MB, Allen CE, Weitzman S, et al. How I treat hemophagocytic lymphohistiocytosis. Blood 2011; 118: 4041–4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schneider EM, Lorenz I, Muller-Rosenberger M, et al. Hemophagocytic lymphohistiocytosis is associated with deficiencies of cellular cytolysis but normal expression of transcripts relevant to killer-cell-induced apoptosis. Blood 2002; 100: 2891–2898. [DOI] [PubMed] [Google Scholar]
- 3. Henter JI, Horne A, Arico M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007; 48: 124–131. [DOI] [PubMed] [Google Scholar]
- 4. Stepp SE, Dufourcq-Lagelouse R, Le Deist F, et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science 1999; 286: 1957–1959. [PubMed] [Google Scholar]
- 5. Goransdotter Ericson K, Fadeel B, Nilsson-Ardnor S, et al. Spectrum of perforin gene mutations in familial hemophagocytic lymphohistiocytosis. Am J Hum Genet 2001; 68: 590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feldmann J, Callebaut I, Raposo G, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3). Cell 2003; 115: 461–473. [DOI] [PubMed] [Google Scholar]
- 7. Cetica V, Pende D, Griffiths GM, et al. Molecular basis of familial hemophagocytic lymphohistiocytosis. Haematologica 2010; 95: 538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jordan MB, Hildeman D, Kappler J, et al. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood 2004; 104: 735–743. [DOI] [PubMed] [Google Scholar]
- 9. Zhang L, Zhou J, Sokol L. Hereditary and acquired hemophagocytic lymphohistiocytosis. Cancer Control 2014; 21: 301–312. [DOI] [PubMed] [Google Scholar]
- 10. Fall N, Barnes M, Thornton S, et al. Gene expression profiling of peripheral blood from patients with untreated new-onset systemic juvenile idiopathic arthritis reveals molecular heterogeneity that may predict macrophage activation syndrome. Arthritis Rheum 2007; 56: 3793–3804. [DOI] [PubMed] [Google Scholar]
- 11. Usmani GN, Woda BA, Newburger PE. Advances in understanding the pathogenesis of HLH. Br J Haematol 2013; 161: 609–622. [DOI] [PubMed] [Google Scholar]
- 12. Villanueva J, Lee S, Giannini EH, et al. Natural killer cell dysfunction is a distinguishing feature of systemic onset juvenile rheumatoid arthritis and macrophage activation syndrome. Arthritis Res Ther 2005; 7: R30–R37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Epling-Burnette PK, Bai F, Painter JS, et al. Reduced natural killer (NK) function associated with high-risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood 2007; 109: 4816–4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yoon JH, Park SS, Jeon YW, et al. Treatment outcomes and prognostic factors in adult patients with secondary hemophagocytic lymphohistiocytosis not associated with malignancy. Haematologica 2019; 104: 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Henter JI, Elinder G, Ost A. Diagnostic guidelines for hemophagocytic lymphohistiocytosis. The FHL study group of the histiocyte society. Semin Oncol 1991; 18: 29–33. [PubMed] [Google Scholar]
- 16. Ravelli A, Magni-Manzoni S, Pistorio A, et al. Preliminary diagnostic guidelines for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. J Pediatr 2005; 146: 598–604. [DOI] [PubMed] [Google Scholar]
- 17. Davi S, Consolaro A, Guseinova D, et al. An international consensus survey of diagnostic criteria for macrophage activation syndrome in systemic juvenile idiopathic arthritis. J Rheumatol 2011; 38: 764–768. [DOI] [PubMed] [Google Scholar]
- 18. Schulert GS, Grom AA. Pathogenesis of macrophage activation syndrome and potential for cytokine-directed therapies. Annu Rev Med 2015; 66: 145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park KH, Park H, Kim M, et al. Evaluation of NK cell function by flowcytometric measurement and impedance based assay using real-time cell electronic sensing system. Biomed Res Int 2013; 2013: 210726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Henter JI, Arico M, Egeler RM, et al. HLH-94: a treatment protocol for hemophagocytic lymphohistiocytosis. HLH study group of the histiocyte society. Med Pediatr Oncol 1997; 28: 342–347. [DOI] [PubMed] [Google Scholar]
- 21. Trottestam H, Horne A, Arico M, et al. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood 2011; 118: 4577–4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Henter JI, Samuelsson-Horne A, Arico M, et al. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood 2002; 100: 2367–2373. [DOI] [PubMed] [Google Scholar]
- 23. Bergsten E, Horne A, Arico M, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood 2017; 130: 2728–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Janka GE. Hemophagocytic syndromes. Blood Rev 2007; 21: 245–253. [DOI] [PubMed] [Google Scholar]
- 25. Janka GE. Familial and acquired hemophagocytic lymphohistiocytosis. Eur J Pediatr 2007; 166: 95–109. [DOI] [PubMed] [Google Scholar]
- 26. Dhote R, Simon J, Papo T, et al. Reactive hemophagocytic syndrome in adult systemic disease: report of twenty-six cases and literature review. Arthritis Rheum 2003; 49: 633–639. [DOI] [PubMed] [Google Scholar]
- 27. Carvelli J, Piperoglou C, Farnarier C, et al. Functional and genetic testing in adults with HLH reveals an inflammatory profile rather than a cytotoxicity defect. Blood 2020; 136: 542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang J, Sun Y, Shi X, et al. Genotype characteristics and immunological indicator evaluation of 311 hemophagocytic lymphohistiocytosis cases in China. Orphanet J Rare Dis 2020; 15: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Naymagon L. Can we truly diagnose adult secondary hemophagocytic lymphohistiocytosis (HLH)? A critical review of current paradigms. Pathol Res Pract 2021; 218: 153321. [DOI] [PubMed] [Google Scholar]
- 30. Naymagon L, Tremblay D, Mascarenhas J. Reevaluating the role of ferritin in the diagnosis of adult secondary hemophagocytic lymphohistiocytosis. Eur J Haematol 2020; 104: 344–351. [DOI] [PubMed] [Google Scholar]
- 31. Allen CE, Yu X, Kozinetz CA, et al. Highly elevated ferritin levels and the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2008; 50: 1227–1235. [DOI] [PubMed] [Google Scholar]
- 32. Schram AM, Campigotto F, Mullally A, et al. Marked hyperferritinemia does not predict for HLH in the adult population. Blood 2015; 125: 1548–1552. [DOI] [PubMed] [Google Scholar]
- 33. Zhang K, Biroschak J, Glass DN, et al. Macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis is associated with MUNC13-4 polymorphisms. Arthritis Rheum 2008; 58: 2892–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang K, Jordan MB, Marsh RA, et al. Hypomorphic mutations in PRF1, MUNC13-4, and STXBP2 are associated with adult-onset familial HLH. Blood 2011; 118: 5794–5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang K, Chandrakasan S, Chapman H, et al. Synergistic defects of different molecules in the cytotoxic pathway lead to clinical familial hemophagocytic lymphohistiocytosis. Blood 2014; 124: 1331–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Risma K, Jordan MB. Hemophagocytic lymphohistiocytosis: updates and evolving concepts. Curr Opin Pediatr 2012; 24: 9–15. [DOI] [PubMed] [Google Scholar]
- 37. Henter JI, Chow CB, Leung CW, et al. Cytotoxic therapy for severe avian influenza A (H5N1) infection. Lancet 2006; 367: 870–873. [DOI] [PubMed] [Google Scholar]
- 38. Gurunathan A, Boucher AA, Mark M, et al. Limitations of HLH-2004 criteria in distinguishing malignancy-associated hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2018; 65: e27400. [DOI] [PubMed] [Google Scholar]
- 39. Tabata C, Tabata R. Possible prediction of underlying lymphoma by high sIL-2R/ferritin ratio in hemophagocytic syndrome. Ann Hematol 2012; 91: 63–71. [DOI] [PubMed] [Google Scholar]
- 40. Lim SH, Park S, Jang JH, et al. Clinical significance of bone marrow hemophagocytosis in adult patients with malignancy and non-malignancy-induced hemophagocytic lymphohistiocytosis. Ann Hematol 2016; 95: 325–335. [DOI] [PubMed] [Google Scholar]
- 41. Schram AM, Comstock P, Campo M, et al. Haemophagocytic lymphohistiocytosis in adults: a multicentre case series over 7 years. Br J Haematol 2016; 172: 412–419. [DOI] [PubMed] [Google Scholar]
- 42. Tamamyan GN, Kantarjian HM, Ning J, et al. Malignancy-associated hemophagocytic lymphohistiocytosis in adults: relation to hemophagocytosis, characteristics, and outcomes. Cancer 2016; 122: 2857–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Park HS, Lee JH, Lee JH, et al. Fludarabine/melphalan 100 mg/m2 conditioning therapy followed by allogeneic hematopoietic cell transplantation for adult patients with secondary hemophagocytic lymphohistiocytosis. Biol Blood Marrow Transplant 2019; 25: 1116–1121. [DOI] [PubMed] [Google Scholar]