Abstract
Background: Beyond non-genetic risk factors, familial hypercholesterolemia (FH) plays a major role in the development of CHD. FH is a genetic disorder characterized by heritable and severely elevated levels of low-density lipoprotein (LDL) cholesterol, which can lead to premature cardiovascular disease, particularly familial coronary heart disease (FH-CHD).
Method: To explore genes indicating a risk of familial (premature) coronary heart disease (FH-CHD) development in FH, 30 Thai male volunteers were enrolled: 7 healthy controls (N), 6 patients with hypercholesterolemia (H), 4 with FH, 10 with CHD, and 3 with FH-CHD. Transcriptome data were investigated using next-generation sequencing analysis in whole blood (n = 3). Genes that were significantly expressed in both FH and FH-CHD, but not in N, H, and CHD groups, were selected and functionally analyzed.
Results: The findings revealed that 55 intersecting genes were differentially expressed between FH and FH-CHD groups. Ten of the 55 genes (MAPK14, TRPM2, STARD8, PDLIM5, BCL3, BLOC1S5, GBA, RBMS1, CD14, and CD36 were selected for validation. These 10 genes play potential roles in chronic inflammation and are involved in pathways related to pathogenesis of CHD. Using quantitative real-time PCR, we evaluated the mRNA expression of the selected genes in all 30 volunteers. TRPM2, PDLIM5, BCL3 were significantly upregulated and GBA was significantly downregulated in both FH and FH-CHD compared with the N, H, and CHD groups.
Conclusion: our preliminary investigation reveals that the TRPM2, PDLIM5, BCL3, and GBA genes may have potential for further development as predictive markers for FH-CHD.
Keywords: familial hyperlipidemia, premature coronary heart disease, biomarker, transcriptome, predictive genes
Introduction
Atherogenesis and the complication of coronary heart disease (CHD) involve a long preclinical process and are poorly understood. Diverse risk factors have been for CHD, including behavioral, dietary, and lifestyle factors such as smoking, fatty dietary intake, physical activity level, infection (exogenous exposure), change of endogenous blood compositions such as lipid and lipoprotein, inflammation and coagulation factors, intermediary metabolites, and oxidant markers of stress, obesity, blood pressure, and diabetes mellitus (Anand et al., 2008; Weber and Noels, 2011). Beyond these non-genetic risk factors, familial hypercholesterolemia (FH) has a major role in development of CHD (Scheuner, 2003). FH is a genetic disorder characterized by heritable and severely increased levels of low-density lipoprotein (LDL) cholesterol, which can lead to premature cardiovascular disease, particularly familial coronary heart disease (FH-CHD) (Anand et al., 2008; Weber and Noels, 2011). Several previous investigations have reported that in FH, expression of at least four genes in sterol and lipoprotein pathways, including LDL receptors, apolipoprotein (apo) B, proprotein convertase subtilisin/kexin9, and the autosomal recessive hypercholesterolemia adaptor protein, are disordered in cholesterol metabolisms, e.g., in sterol and lipoprotein pathways (Defesche, 2001; Marais, 2004). Moreover, other roles of these receptors and related receptors and other genes associated with pathophysiology of disease are of interest in evaluating the risk of developing FH-CHD. In this study, we attempted to identify alternative biomarkers for risk of FH-CHD. We conducted a cross-sectional study using Thai male volunteers, including healthy controls (N) and patients with hypercholesterolemia (H), FH, CHD, and FH-CHD. Transcriptome data were analyzed by next-generation sequencing analysis in whole blood (n = 3/group) and validated by quantitative real-time PCR (qPCR). We selected genes that were significantly expressed in patients with FH and FH-CHD but not in the N, H, and CHD groups to create an intersecting gene profile and then functionally analyzed selected genes. We identified 55 intersecting genes between FH and FH-CHD groups. Similar to our previous study (Maneerat et al., 2017), the expressions of intersecting genes that shared between FH and FH-CHD groups are potential for further development as predictive markers for FH-CHD in FH patients. In this study, we selected that 10 of 55 genes, MAPK14, TRPM2, STARD8, PDLIM5, BCL3, BLOC1S5, GBA, RBMS1, CD14, and CD36 showed significant co-expression and potentially play roles in chronic inflammation and pathways related to pathogenesis of CHD. The selected genes were further validated in 30 volunteer samples using qPCR. The result revealed that TRMS2, PDLIM5, BCL3, CD14, and GBA genes were the most strongly associated with FH-CHD development and show potential for further application as inflammatory markers to predict the risk of FH-CHD development in Thai patients with FH.
Materials and Methods
Subjects
Thirty volunteers, males born to Thai parents, were enrolled in this study. Healthy volunteers who had no infections and underlying diseases or cardiovascular disease (CVD) risk factors were recruited as controls (N group; n = 7). Twenty-three patients were diagnosed, classified, treated and selected under supervision of a specialist (KP) at Pramongkutklao Hospital. They were classified into four groups based on their clinical manifestations according to the American College of Cardiology/American Heart Association criteria (2013) (Stone et al., 2014), and included 6 patients with high cholesterol levels [total cholesterol (TC), LDL, and high-density lipoprotein (HDL)], but with no evidence of vital organ dysfunction (H group) (Stone et al., 2014); 10 patients diagnosed with CHD (Stone et al., 2014) who were about to undergo coronary bypass grafting (CHD group); 3 patients with FH-CHD and 4 FH patients who were related to the 3 FH-CHD.
The workflow followed in the present study is illustrated in Figure 1. The study was performed at the Faculty of Tropical Medicine, Mahidol University. Approval for the study was considered from the Ethics Committees of the Faculty of Tropical Medicine, Mahidol University (MUTM2017-025-02), and Pramongkutklao Hospital (Q031b/59). Before enrollment, all participants were informed of the study aims, and filled an informed consent form.
FIGURE 1.
Experimental design and study population.
Blood Sample Collection and Methods
Heparinized blood samples (5 mL) were obtained once from healthy controls and all patients before hyperlipidemia treatment or coronary bypass grafting. Sera from 1 mL of clotted blood were collected for lipid measurement.
Packed blood cells were resuspended in 5 mL of Dulbecco’s-PBS (Wisent Inc., Quebec, Canada). Approximately 1 mL of blood suspension was immediately used to extract total RNA using QIAamp RNA Blood Mini kit (Qiagen Inc., Germantown, MD, United States). RNA samples were kept at −70°C to investigate expression of genes profiled by next-generation sequencing (NGS) and further validated by qPCR.
Lipid profiles, TC, triglycerides (TG), LDL cholesterol (LDL-c), and HDL cholesterol (HDL-c), were analyzed enzymatically using kits (Randox Laboratories Ltd., Crumlin, United Kingdom) and a biochemistry analyzer (Architect CI 16200, Abbott Laboratories, Abbott Park, IL, United States).
cDNA Library Construction and Sequencing by NGS Technique
All total RNA samples were examined for amount and quality before analysis. Integrity of total RNA was assessed using Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, United States). Approximately 500 ng of the total RNA from each sample was used to create individually indexed strand-specific RNA-seq libraries using TruSeq stranded mRNA library preparation kit (Illumina Inc., San Diego, CA, United States). Briefly, poly-A-containing mRNA molecules was captured using magnetic oligo (dT) beads, purified, and directed to cDNA synthesis. AMPure XP beads (Beckman Coulter Genomic, Atlanta, GA, United States) were used to separate the cDNA from reaction mix. Indexing adapters were ligated to the cDNA, and all cDNA libraries were checked for quality using an Agilent 2100 Bioanalyzer (Agilent Technologies) and quantified with DeNovix fluorometer (DeNovix Inc., Wilmington, DE, United States). The indexed sequencing libraries were pooled in equimolar quantities and subjected to cluster generation and paired-end 2 × 75 nucleotide read sequencing on an Illumina NextSeq 500 sequencer. The sequencing process was carried out at Omics Sciences and Bioinformatics Center (Bangkok, Thailand).
Differential Expression Analyses of RNA-Seq Data and Statistical Methods
Bioinformatics analyses comprised an initial quality check of the raw data files using FASTQC software (Bioinformatics Group, Babraham Institute, Cambridge, United Kingdom). Adapter and low-quality reads were removed using Trimmomatic1 (Bolger et al., 2014). The filtered reads were aligned to a human reference genome using HISAT2 aligner software (Center for Computational Biology, Johns Hopkins University, Baltimore, MD, United States). StringTie (Center for Computational Biology, Johns Hopkins University) was used to assemble transcripts from RNA-seq reads that were aligned to the genome, reconstructing all isoforms expressed from each gene as well as estimates of the relative abundance of those isoforms. Subsequently, differential isoform expression among five groups was performed using edgeR program (Robinson et al., 2010) via Cuffdiff 2.0 (Trapnell et al., 2013) (University of Maryland Center for Bioinformatics and Computational Biology)2. Fold change ≥ 1, p-value ≤ 0.05, and false discovery rate (FDR) ≤ 0.05 was interpreted statistically significant. In addition, the gffcompare utility (Pertea and Pertea, 2020) (Center for Computational Biology, Johns Hopkins University, Baltimore, MD, United States)3. StringTie (Center for Computational Biology, Johns Hopkins University) was used to discover a novel transcript. Gene Ontology (GO) and pathway enrichment analyses was conducted using a web-based bioinformatics tool DAVID (omicX, Seine Innopolis, Le-Petit-Quevilly, France).
Validation of Possible Marker Genes for Risk of FH-CHD by RT-qPCR
Fifty-five intersecting genes, significantly expressed in both FH and FH-CHD but not in N, H, and CHD groups, were selected for further validation (Figure 2). qPCR of the mRNA expression of 10 selected genes was performed in all volunteer samples. These genes were MAPK14, TRPM2, STARD8, PDLIM5, BCL3, BLOC1S5, GBA, RBMS1, CD14, and CD36, as described in Table 1 (in bold). Table 2 lists the primers designed to amplify these genes and their expected fragment lengths. qPCR was conducted in triplicate (Susomboon et al., 2006). Each 10-μL PCR reaction contained 5 μL of iTaq universal SYBR Green supermix (BioRad Laboratories Inc., Hercules, CA, United States) mixed with 100 ng of cDNA and 10 μM of each set of forward and reverse primers (Table 2). Amplification was run in a Bio-Rad CFX96 Real-time system (BioRad Laboratories Inc.). The qPCR conditions were 95°C for 3 min, followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 60°C for 30 s, and melting curve analysis at 65°C for 5 min. β-Actin (ACTB) (primers: forward: 5′-TCACCCACACTGTGCCCATCTACGA-3′ and reverse: 5′-CAGCGGAACCGCTCATTGCCAATGG-3′) was used to normalize the relative expression of each gene (Heid et al., 1996; Kotepui et al., 2012) and the relative expression level was calculated using the 2–ΔΔCt method.
FIGURE 2.
Transcriptome analysis by next-generation sequencing in four patient groups, hyperlipidemia (H), familial hyperlipidemia (FH), coronary heart disease (CHD), and familial CHD (FH-CHD) compared with healthy volunteers (N). Total RNA was extracted from blood (n = 3). Differentially expressed genes were performed using edgeR program via Cuffdiff 2.0 (fold change ≥ 1), p-value ≤ 0.05 and false discovery rate (FDR) ≤ 0.05. (A) Venn diagram illustrates all 1,080 differentially expressed genes, and (B) number of up- and downregulated genes expressed in the four patient groups compared with N. [(C), upper panels] MA plots for differential expression analysis in H, CHD, FH, or FH-CHD versus N samples. The y- and x-axes represent the log fold change (FC) of gene expression and log number of genes, respectively. The red and black points in the plot indicate significant and not significant differentially expressed genes, respectively. [(C), lower panels] Volcano plots of differentially expressed transcripts in H, CHD, FH, or FH-CHD versus N samples. The y- and x-axes represent the FDR value and log FC of gene expression, respectively. The filter threshold is FDR < 0.0001. The red and black points in the plot represent up- and downregulated transcripts, respectively.
TABLE 1.
Fifty-five intersecting genes expressed in patients in the FH and FH-CHD groups but not in the N, H, and CHD groups, including 37 significantly up-regulated and 18 down-regulated genes.
| Transcript ID (Ensembl_Gene_ID) | Gene ID | DE (FH vs. N)a |
DE (FH-CHD vs. N)b |
||||
| logFC | p-value | FDR | logFC | p-value | FDR | ||
| Upregulated genes | |||||||
| ENST00000521724.5 | CTNNA1 | 13.4 | <0.001 | < 0.001 | 13.7 | <0.001 | <0.001 |
| ENST00000427641.2 | NCBP2 | 12.7 | <0.001 | 0.018 | 13.0 | <0.001 | <0.001 |
| ENST00000370793.5 | USP33 | 12.6 | <0.001 | 0.026 | 10.1 | <0.001 | 0.04 |
| ENST00000467471.5 | PPM1M | 12.1 | <0.001 | 0.025 | 10.9 | <0.001 | 0.039 |
| ENST00000424201.6 | CACNA2D2 | 12.0 | <0.001 | 0.003 | 8.5 | <0.001 | 0.049 |
| ENST00000433797.5 | KDM6A | 11.8 | <0.001 | 0.035 | 10.6 | <0.001 | 0.041 |
| ENST00000372384.6 | TSC22D3 | 11.7 | <0.001 | 0.03 | 10.5 | <0.001 | 0.034 |
| ENST00000468133.5 | MAPK14 | 11.7 | <0.001 | 0.031 | 11.5 | <0.001 | 0.035 |
| ENST00000397708.1 | MCM3AP | 11.7 | <0.001 | 0.0049 | 9.8 | <0.001 | 0.041 |
| ENST00000633130.1 | GPI | 11.6 | <0.001 | 0.03 | 12.0 | <0.001 | 0.03 |
| ENST00000498430.5 | TRPM2 | 11.5 | <0.001 | 0.003 | 10.9 | <0.001 | 0.04 |
| ENST00000498133.5 | BCL2L13 | 11.4 | <0.001 | 0.035 | 12.3 | <0.001 | 0.013 |
| ENST00000374597.3 | STARD8 | 11.2 | <0.001 | 0.033 | 11.3 | <0.001 | 0.028 |
| ENST00000528516.5 | LTBP3 | 11.2 | <0.001 | 0.033 | 10.5 | <0.001 | 0.04 |
| ENST00000340645.9 | GOLGB1 | 11.2 | <0.001 | 0.033 | 13.1 | <0.001 | <0.001 |
| ENST00000495421.1 | DPH2 | 11.1 | <0.001 | 0.033 | 11.1 | <0.001 | 0.038 |
| ENST00000245960.9 | CDC25B | 11.0 | <0.001 | 0.018 | 11.9 | <0.001 | 0.024 |
| ENST00000627233.2 | ARHGAP27 | 10.9 | <0.001 | 0.035 | 11.1 | <0.001 | 0.038 |
| ENST00000531427.5 | CUL5 | 10.8 | <0.001 | 0.035 | 10.5 | <0.001 | 0.04 |
| ENST00000401743.6 | CD14 | 10.7 | <0.001 | 0.003 | 12.5 | <0.001 | 0.029 |
| ENST00000580168.5 | HELZ | 10.6 | <0.001 | 0.017 | 9.7 | <0.001 | 0.042 |
| ENST00000357364.8 | IKZF1 | 10.6 | <0.001 | 0.035 | 11.3 | <0.001 | 0.003 |
| ENST00000359741.9 | SLC39A14 | 10.4 | <0.001 | 0.021 | 9.6 | <0.001 | 0.045 |
| ENST00000487620.1 | ZNF3 | 10.3 | <0.001 | 0.035 | 10.7 | <0.001 | 0.04 |
| ENST00000538969.5 | CD36 | 10.2 | <0.001 | 0.039 | 10.1 | <0.001 | 0.048 |
| ENST00000380176.7 | PDLIM5 | 10.1 | <0.001 | 0.037 | 10.1 | <0.001 | 0.015 |
| ENST00000221232.9 | CNOT3 | 10.1 | <0.001 | 0.036 | 10.2 | <0.001 | 0.04 |
| ENST00000486484.5 | MBOAT2 | 9.8 | <0.001 | 0.038 | 10.5 | <0.001 | 0.04 |
| ENST00000436439.6 | HMGCL | 9.8 | <0.001 | 0.038 | 10.0 | <0.001 | 0.04 |
| ENST00000543133.5 | BCL2L13 | 9.6 | <0.001 | 0.045 | 10.4 | <0.001 | 0.004 |
| ENST00000402312.7 | WDR25 | 9.5 | <0.001 | 0.047 | 9.8 | <0.001 | 0.005 |
| ENST00000575898.5 | ZNF232 | 8.8 | <0.001 | 0.04 | 8.8 | <0.001 | 0.025 |
| ENST00000464356.6 | MEF2D | 8.5 | <0.001 | 0.027 | 9.6 | <0.001 | 0.003 |
| ENST00000513565.6 | CEP120 | 8.4 | <0.001 | 0.013 | 7.7 | <0.001 | 0.033 |
| ENST00000397053.6 | UPF2 | 8.3 | <0.001 | 0.021 | 7.1 | <0.001 | 0.043 |
| ENST00000487394.1 | BCL3 | 7.6 | <0.001 | 0.033 | 8.0 | <0.001 | 0.04 |
| ENST00000354958.6 | PLEC | 5.6 | <0.001 | 0.041 | 7.1 | <0.001 | 0.021 |
| Down-regulated genes | |||||||
| ENST00000470843.5 | RPL5 | −8.0 | <0.001 | 0.021 | −9.5 | <0.001 | 0.004 |
| ENST00000439657.5 | LENG8 | −9.2 | <0.001 | 0.017 | −9.1 | <0.001 | 0.018 |
| ENST00000543936.5 | BLOC1S5 | −9.8 | <0.001 | 0.004 | −9.8 | <0.001 | 0.004 |
| ENST00000574897.5 | NPLOC4 | −9.9 | <0.001 | 0.038 | −9.8 | <0.001 | 0.041 |
| ENST00000463567.5 | ZNF767P | −9.9 | <0.001 | 0.014 | −9.9 | <0.001 | 0.015 |
| ENST00000514886.1 | PRMT9 | −10.1 | <0.001 | 0.036 | −10.0 | <0.001 | 0.040 |
| ENST00000562631.5 | ADGRG1 | −10.3 | <0.001 | 0.024 | −8.4 | <0.001 | 0.040 |
| ENST00000523864.5 | STARD8 | −10.3 | <0.001 | 0.036 | −10.2 | <0.001 | 0.040 |
| ENST00000264244.7 | TIMMDC1 | −10.7 | <0.001 | <0.001 | −10.7 | <0.001 | 0.039 |
| ENST00000551043.5 | CNOT2 | −10.8 | <0.001 | 0.037 | −10.7 | <0.001 | 0.041 |
| ENST00000242848.8 | ZC3H13 | −10.8 | <0.001 | 0.02 | −8.9 | <0.001 | 0.038 |
| ENST00000621749.4 | RPS9 | −10.9 | <0.001 | 0.034 | −10.9 | <0.001 | 0.038 |
| ENST00000368373.7 | GBA | −11.1 | <0.001 | 0.035 | −11.0 | <0.001 | 0.040 |
| ENST00000332704.5 | TBL3 | −11.6 | <0.001 | 0.03 | −11.6 | <0.001 | 0.034 |
| ENST00000368060.7 | MED23 | −12.1 | <0.001 | 0.027 | −12.0 | <0.001 | 0.031 |
| ENST00000474820.5 | RBMS1 | −12.1 | <0.001 | 0.026 | −10.2 | <0.001 | 0.040 |
| ENST00000546079.5 | CLPTM1 | −12.9 | <0.001 | 0.015 | −12.9 | <0.001 | 0.016 |
| ENST00000376554.8 | STK24 | −13.0 | <0.001 | 0.02 | −13.0 | <0.001 | 0.021 |
Ten of the 55 intersecting genes (in bold) were selected for further validation by quantitative real-time PCR. DE, significant differential gene expression (fold change) comparing between familial hyperlipidemia patient (FH) versus normal (N) groups (a), and familial coronary heart disease (FH-CHD) versus normal (N) groups (b). Statistical analysis was considered using edgeR program via Cuffdiff 2.0 (fold change ≥ 1), p-value ≤ 0.05 and false discovery rate (FDR) ≤ 0.05 was significant.
TABLE 2.
Primers for gene amplification in quantitative real-time PCR.
| Genes | Primer sequence (5′→ 3′) | Product size (bp) | Ta (°C) | References |
| MAPK14 | F: GAGCGTTACCAGAACCTGTCTC | 161 | 60.0 | Khalaf et al. (2013), Maimaiti et al. (2016), Zhou et al. (2020) |
| R: AGTAACCGCAGTTCTCTGTAGGT | ||||
| TRPM2 | F: ATTGTGAAGCGGATGATGAAGGA | 158 | 57.0 | Lin et al. (2018) |
| R: ATGGTGAGGTAGGAGTGGTAGAC | ||||
| STARD8 | F: GCAGCTTTTTGAAGGAGGCTGAT | 127 | 60.6 | * |
| R: TGGGGCCAACAGATCAGAGG | ||||
| PDLIM5 | F: CTCGCTCTTTCCGAATCCTTGC | 126 | 60.0 | * |
| R: AAGCTACCGAGGAAGCCAACTG | ||||
| BCL3 | F: GAACACCGAGTGCCAAGAAACC | 121 | 57.0 | * |
| R: GCTAAGGCTGTTGTTTTCCACGG | ||||
| BLOC1S5 | F: CCAAATGTAGAGACACAATGCGG | 106 | 57.0 | * |
| R: TCCTGTTCCCTCTGTTGGAGTC | ||||
| GBA | F: TGCTGCTCTCAACATCCTTGCC | 135 | 57.0 | Ivankovic et al. (2016) |
| R: TAGGTGCGGATGGAGAAGTCAC | ||||
| RBMS1 | F: GCATCTCCTGTATCTGCCTACC | 170 | 60.0 | * |
| R: GGCTGTAGTGACATGGTGTGCT | ||||
| CD14 | F: CTGGAACAGGTGCCTAAAGGAC | 120 | 60.0 | Hidaka et al. (2013) |
| R: GTCCAGTGTCAGGTTATCCACC | ||||
| CD36 | F: CAGGTCAACCTATTGGTCAAGCC | 119 | 60.0 | Soriano-Romani et al. (2015a, b) |
| R: GCCTTCTCATCACCAATGGTCC | ||||
| ACTB | F: TCACCCACACTGTGCCCATCTACGA | 295 | 60.0 | Susomboon et al. (2006), Stone et al. (2014) |
| R: CAGCGGAACCGCTCATTGCCAATGG |
*The primer sequences were designed according to ORIGENE (http://www.origene.com). Ta, annealing temperature.
Results and Discussion
Characteristics of Healthy Controls and Patients
General descriptions and clinical characteristics of the four patient groups and controls are presented as medians and ranges (Table 3).
TABLE 3.
Characteristics of the participants.
| Group | N | FH | FH-CHD | H | CHD |
| Variables | (n = 7) | (n = 4) | (n = 3) | (n = 6) | (n = 10) |
| Age (years) | 27 | 47.5 | 45 | 52.5 | 51.5 |
| (19–36) | (20–56) | (45–79) | (26–65) | (41–61) | |
| TC (mg/dL) | 182 | 227.5 | 176 | 194.5 | 164.5 |
| (165–187) | (171–276) | (171–232) | (148–233) | (131–233) | |
| TG (mg/dL) | 130 | 119.5 | 177 | 218.5 | 172.5 |
| (82–168) | (102–256) | (125–271) | (134–289) | (73–368) | |
| HDL (mg/dL) | 48 | 51.5 | 46 | 49 | 45 |
| (41–66) | (35–78) | (42–48) | (37–78) | (40–65) | |
| LDL (mg/dL) | 105 | 152 | 100 | 102.5 | 89 |
| (98–111) | (89–175) | (80–149) | (43–153) | (15–126) | |
| WBC (103/uL) | 7.8 | 7.5 | 7.3 | 6.1 | 5.7 |
| (3.8–12) | (5.4–8.8) | (5.4–8.5) | (5.2–9.4) | (4.9–8.6) | |
| RBC (103/uL) | 4.87 | 5.24 | 5.99 | 4.8 | 4.775 |
| (4.58–7.35) | (4.46–6.31) | (4.19–6.38) | (3.6–7.85) | (4.46–12.5) | |
| Hb (g/dL) | 14.3 | 14.4 | 13.4 | 14.35 | 14.1 |
| (10.9–18.2) | (13.9–15) | (13.3–16.2) | (11.2–14.5) | (12.5–16.2) | |
| HCT (%) | 41.9 | 42.85 | 42 | 42.25 | 41.5 |
| (32–53.7) | (39.5–43.8) | (38.3–49.7) | (32.9–44.3) | (38–46.5) | |
| Lymphocyte (%) | 35.4 | 38.3 | 28.8 | 33.2 | 35.4 |
| (25–50.3) | (25–47) | (27–34.5) | (21.4–42.2) | (22.3–40.7) | |
| Monocyte (%) | 5.2 | 4.85 | 7.2 | 6.05 | 6.65 |
| (4.6–6.9) | (4.0–6.0) | (4–8.7) | (4.5–8.8) | (5.3–9.0) |
All patients and controls were male. Data are shown as median (ranges). N, normal controls; H and CHD, patients with hyperlipidemia and coronary heart disease; respectively. FH and FH-CHD, familial hyperlipidemia and familial coronary heart disease patients, respectively. TC, total cholesterol; TG, triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein; WBC, white blood cells; RBC, red blood cells; Hb, hemoglobin; HCT, hematocrit.
Preselection of Regulatory Sequences
Transcriptome sequences of healthy volunteers (N) and patients in the H, FH, CHD, and FH-CHD were analyzed. We found approximately 500 differentially expressed genes in the patient groups compared with the healthy controls, as illustrated in a Venn diagram (Figure 2A). The number of significantly up- and down-regulated genes in the four patient groups compared with the N group are displayed in bar graphs (Figure 2B) and volcano plots (Figure 2C).
Intersecting and Selected Genes
To pick up sequence group to identify predictive markers for FH-CHD risk in the FH group, we focused on the 55 genes that intersected between FH and FH-CHD (white area in Figure 2A). The intersecting gene profiles include 37 up- and 18 down-regulated genes (Table 1). Gene Ontology and pathway enrichment analyses were conducted and the results are shown in Figure 3. The GO terms were in three categories: biological process, cellular component, and molecular functions (Figure 3A). The number of up- and down-regulated genes in each category is shown in Figure 3B.
FIGURE 3.
Gene Ontology (GO) enrichment analysis of differential expression of 55 intersecting genes in the familial hyperlipidemia (FH) and familial coronary heart disease (FH-CHD) versus N groups. (A) The ordinate represents the next level GO term of the three categories including biological process (upper panel), cellular component (middle panel), and molecular functions (lower panel). The abscissa represents the gene number under the term. (B) Bar graphs show number of upregulated (gray bar) and downregulated (white bar) genes in each category.
To investigate the importance of the intersecting genes, we conducted functional enrichment analyses of genes differentially expressed at the mRNA level analyzed by Metascape pathway4. We observed functional correlations between the top 11 clusters in the 55 intersecting genes between FH and FH-CHD; the genes were mainly involved in lipid metabolism, cellular process, oxidative stress, and inflammation (Figure 4A). Focusing on 10 differentially expressed genes, 5 clusters are displayed as the same network enrichment analysis of the 55 intersecting genes (Figure 4B). In addition, the expected protein–protein interaction networks in the 55 intersecting genes and the 10 selected genes analyzed by web-based bioinformatics tool are shown in Figures 5A,B, respectively.
FIGURE 4.
Functional enrichment analyses of differentially expressed genes at the mRNA level analyzed by Metascape pathway. (A) Association between the top 11 clusters and (B) 5 clusters of enriched terms displayed as a network enrichment analysis of 55 intersected and of 10 selected differentially expressed mRNAs, respectively. Nodes of the same color belong to the same cluster. Terms with a similarity score > 0.3 are linked by an edge. The network was visualized with Cytoscape with force-directed layout and edge bundled for clarity. The analysis was conducted using the web-based bioinformatics tool DAVID (omicX, Seine Innopolis, Le-Petit-Quevilly, France).
FIGURE 5.
The protein–protein interaction network of (A) the 55 intersecting genes between familial hyperlipidemia (FH) and familial coronary heart disease (FH-CHD) groups, and (B) in 10 genes selected from the 55 intersecting genes using web-based bioinformatics tool: functional protein association networks analysis.
Verification of 10 Selected Genes Using RT-qPCR
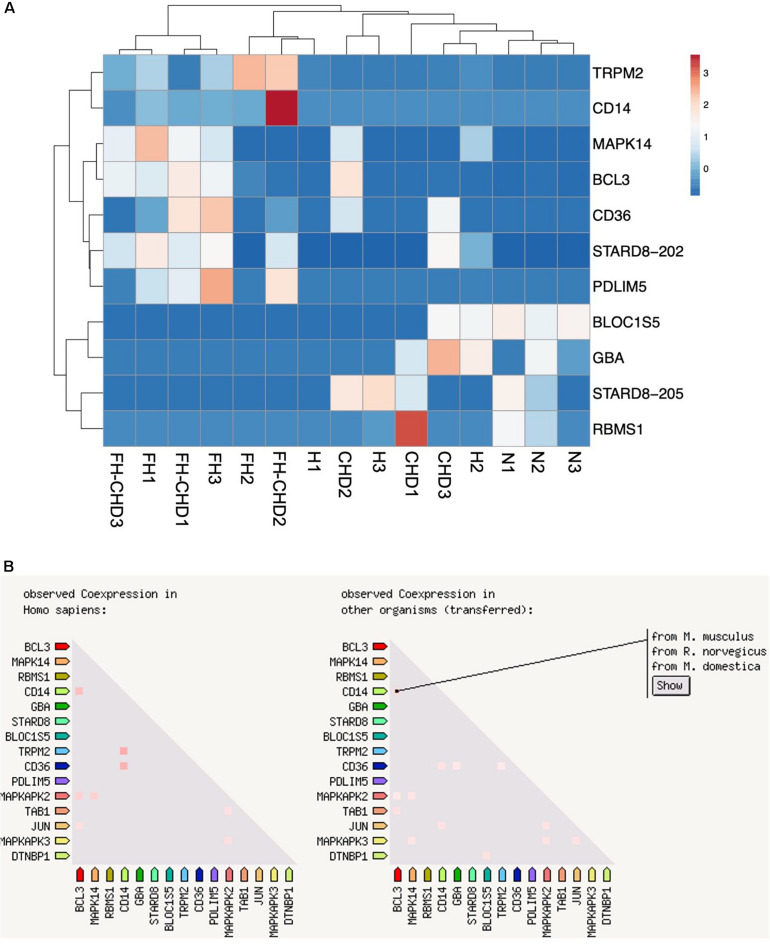
Based on focusing regulatory and immune response roles of target genes, we selected 10 genes potentially involved in atherogenesis, chronic inflammation, or lipid metabolism. These genes include MAPK14, TRPM2, STARD8, PDLIM5, BCL3, BLOC1S5, GBA, RBMS1, CD14, and CD36. Table 4 summarizes the selected genes and their cardiovascular syndrome (CVS)-related functions. In Figure 6, we compared the expression of these genes among groups (Figure 6A) and detected high co-expression between CD14 and BCL3, between TRMP2 and CD14, and between CD14 and CD36 (Figure 6B), which may indicate a possible further extension of our indicative synergistic markers for risk of FH-CHD. The qPCR results of relative mRNA expression (mean two-fold changes) of the 10 genes in all groups using total RNA extracted from blood of volunteers are shown in Figure 7. The mRNA expression of these genes was compared among the groups. To identify ideal biomarkers, we selected only genes that were significantly up- or down-regulated in patients with FH and FH-CHD compared with healthy individuals (not differently expressed between FH and FH-CHD). The results reveal that TRPM2, PDLIM5, BCL3, and CD14 were up-regulated and GBA was down-regulated in both FH and FH-CHD compared with the N, H and CHD groups. These five target genes have potential as markers for risk of FH-CHD in FH.
TABLE 4.
Description and potential functions of 10 genes selected from the 55 intersecting genes expressed in both FH and FH-CHD but not in N, H, and CHD groups.
| Gene ID | Description | Function |
| MAPK14 | Mitogen-activated protein kinase 14; p38 MAPK | Its activation promotes cardiomyocyte hypertrophy (Zechner et al., 1997; Liang and Molkentin, 2003), promotes myocyte apoptosis (Sharov et al., 2003) via downstream targets STAT1, CHOP, FAK, SMAD, cytochrome c, NF-κB, PTEN, and p53 (Fiordaliso et al., 2001; Ghosh et al., 2009), and regulate cardiomyocyte cytokinesis and promote cell cycle exit (Engel et al., 2006). |
| TRPM2 | Transient receptor potential cation channel subfamily M member 2 | The encoded protein is activated by oxidative stress and confers susceptibility to cell death (Maglott et al., 2011). Ca2+ entry via TRMP2 is necessary for proper cardiac function through modulation of mitochondrial oxidative signals, especially after I/R and reducing reactive oxygen species levels. TRPM2 is also protective of doxorubicin cardiomyopathy (Miller et al., 2014; Hoffman et al., 2015). |
| STARD8 | StAR related lipid transfer domain containing 8; Rho-GTPase-activating-protein domain (RhoGAP) | This gene encodes a member of a subfamily of Rho GTPase activating proteins that contain a steroidogenic acute regulatory protein related lipid transfer domain. The encoded protein localizes to focal adhesions and may be involved in regulating cell morphology. START proteins are involved in several different biological processes: lipid transfer between cellular compartments; lipid metabolism, which involves START proteins that contain thioesterase catalytic activities; and signal transduction, which involves the RhoGAP START proteins (Alpy and Tomasetto, 2005). |
| PDLIM5 | PDZ and LIM domain 5 | PDLIM5 encodes several splice variants, whose expression is tissue specific and temporally regulated (Zheng et al., 2010). Alternative splicing plays an important role in heart development and in the development of cardiopathies (Weeland et al., 2015). Polymorphisms in PDLIM3 (rs4861669, rs4862543) and PDLIM5 (rs1056772) were significantly associated with idiopathic dilated cardiomyopathy (IDCM) in Chinese Han patients (Wang et al., 2019) |
| BCL3 | BCL3 transcription coactivator | BCL3 gene expression is induced via NF-κB and play role in cell proliferation regulation (Ohno et al., 1990; Wulczyn et al., 1992). Down regulated expression of BCL3 gene affect their transcription regulatory networks, which subsequently alter a number of biological processes in human ischemia cardiomyopathy (Herrer et al., 2015). |
| BLOC1S5 | Biogenesis of lysosomal organelles complex 1 subunit 5 | Component of the BLOC-1 complex, a complex that is required for normal biogenesis of lysosome-related organelles (LRO), such as platelet dense granules. Dense granules are important in platelet aggregation and play role in thrombus formation (Ambrosio et al., 2012). |
| GBA | Glucosylceramidase beta | Glucosylceramidase that catalyzes the hydrolysis of glucosylceramide/GlcCer into free ceramide and glucose within the lysosomal compartment. Thereby, GBA plays a central role in the degradation of complex lipids and the turnover of cellular membranes (Rijnboutt et al., 1991). Under specific conditions, GBA may alternatively catalyze the reverse reaction, transferring glucose from cholesteryl-beta-D-glucoside to ceramide, finally, may also hydrolyze cholesteryl-beta-D-glucoside to produce D-glucose and cholesterol (Akiyama et al., 2013; Marques et al., 2016). Alterations in the level of glucosylceramide are noted in cells and tissues in response to cardiovascular disease, diabetes, skin disorders and cancer. Reducing synthesis of glycosphingolipids with pathological effects could be a new approach for preventing cardiac hypertrophy. Overall, upregulation of glucosylceramide offers cellular protection and primes certain cells for proliferation (Messner and Cabot, 2010). |
| RBMS1 | RNA binding motif single stranded interacting protein 1 | This gene encodes a member of a small family of proteins, which bind single stranded DNA/RNA. These proteins are characterized by the presence of two sets of ribonucleoprotein consensus sequence (RNP-CS) that contain conserved motifs, RNP1 and RNP2, originally described in RNA binding proteins, and required for DNA binding. These proteins have been implicated in such diverse functions as DNA replication, gene transcription, cell cycle progression and apoptosis (Maglott et al., 2011). RBMS1 interact with polymerase (DNA directed), alpha 1 (Niki et al., 2000). |
| CD14 | Cluster of differentiation 14 molecule | CD14 is expressed mainly by macrophages in innate immunity (Simmons et al., 1989). CD14 acts as co-receptor for Toll like receptor (TLR) 4 and MD-Z in response to lipopolysaccharide (LPS) (Kitchens et al., 2000). Increase in proinflammatory monocyte (CD14+CD16+) is an independent risk factor for CAD and plaque process (Schlitt et al., 2004). Monocyte (CD14++CD16+) and neutrophil may be involved in small to large vessel vasculitis (Corbera-Bellalta et al., 2016). Soluble CD14 from peripheral blood monocytes could be biological markers for screening and monitoring inflammatory disease activity in patients (Scherberich and Nockher, 1999). |
| CD36 | Cluster of differentiation 36 molecule; fatty acid translocase | The CD36 encoded protein is the fourth major glycoprotein of the platelet surface and serves as a receptor for thrombospondin in platelets and various cell lines. This protein may have important functions as a cell adhesion molecule. It binds to collagen (Tandon et al., 1989), thrombospondin (Silverstein et al., 1992), anionic phospholipids and oxidized LDL (Podrez et al., 2002). CD36 is a key mediator of phagocytic oxLDL (oxidized low-density lipoprotein) uptake. CD36 was significantly reduced with plaque enriched long non-coding RNA in atherosclerotic macrophage regulation (PELATON) knockdown (Hung et al., 2020). CD36 (fatty acid translocase) as a key target gene for this miRNA and showed that the induced expression of CD36 is responsible for increased fatty acid uptake, thereby causing lipotoxicity in the heart (Li et al., 2019). The heat shock protein/glucocorticoid receptor (HSP/GR) complex-mediated CD36 axis was involved in the regulation of plaque formation in atherosclerosis development in mice (Silverstein et al., 1992). High-fat food-induced metabolic disorders promote lipoproteins accumulation, oxidative stresses, and active inflammation in macrophage of the vascular wall and accordingly result in the formation of atherosclerotic plaques by affecting the expression of GC, GR, HSP, CD36, and ABCA1 (cholesterol efflux regulatory protein) (Fu et al., 2019). CD36, a scavenger receptor, was at higher levels in the serum of patients with acute coronary syndrome or chronic coronary heart disease than in normal subjects (Bai et al., 2019). CD36 mediates foam cell formation and promotes inflammatory response and oxidative stress (Park, 2014; Xu et al., 2018) and promote atherosclerosis (Bai et al., 2019). |
FIGURE 6.
Ten selected genes from 55 intersecting genes between familial hyperlipidemia (FH) and familial coronary heart disease (FH-CHD) groups. (A) Heat maps of the 10 differentially expressed transcripts in the patient groups versus the normal control group (n = 3). (B) Co-expression of 10 selected genes. Note the high co-expression between CD14 and BCL3, between TRMP2 and CD14, and between CD14 and CD36 (left panel) in Homo sapiens and in other organisms (right panel).
FIGURE 7.
Quantitative reverse transcription PCR analysis of mRNA expression of (A) MAPK14, (B) TRPM2, (C) STARD8, (D) PDLIM5, (E) BCL3, (F) BLOC1S5, (G) GBA, (H) RBMS1, (I) CD14, and (J) CD36 genes, showing altered expression in patient groups vs. controls. mRNA expression (2.0-fold change) relative to ACTB mRNA in RNA samples obtained from the patient groups; familial hyperlipidemia (FH; n = 4), familial coronary heart disease (FH-CHD; n = 3), hyperlipidemia (H; n = 6), and coronary heart disease (CHD; n = 10) compared with the healthy (N) group (n = 7). Bracketed p-values indicate significant differences between groups.
The present study had some limitations; (1) We used whole blood to prepare transcriptome data. Therefore, our data lacks cardiac genes (reviewed in Saucerman et al., 2019) that play role in cardiovascular system but not mainly express in blood cells. In our opinion, atherosclerosis and its complication, coronary heart disease and stroke are consequence of chronic inflammation of vascular wall (Hansson, 2005). Blood contains inflammatory cells, which play an important role in atherogenesis. Therefore, blood is an accessible source particularly fitting surrogate for atherosclerotic tissue (Moore et al., 2005; Kang et al., 2006; Sharp et al., 2007). In consistence, Perisic et al., 2016 demonstrated similar gene expression profiles in carotid atherosclerosis compared between PBMC and vascular tissue sources (Perisic et al., 2016). These approaches contribute that our study using blood to investigate the transcriptome data is feasible; (2) we focused only on male volunteers to control the influence of the sex-hormone factor. Estrogens, in particular, are primary examples of female sex steroids. Previous studies in animal models, e.g., with rabbits, mice, and monkeys, have reported that estrogen has protective effects in CVD. Earlier evidence has also indicated that estrogen protects women against CHD pre-menopause (reviewed in Arnal et al., 2007). Estrogen ameliorates lipidaemia by up-regulating LDL-receptor production. In addition, estrogen attenuates inflammation in the atherosclerotic plaque. It is exerted via the sex hormone receptors on various inflammatory cells in the plaques, including reduced LDL oxidation, EC activation and the adhesion of neutrophils and monocytes to the endothelial lining, and impedes nitric oxide activity (Boese et al., 2017). We expect that our preliminary findings will help further studies to find appropriate biomarkers to predict CHD in both male and female Thai hyperlipidemia patients; (3) we illustrate our findings with low statistical power due to the small genetic sample size and single-center analysis. Further studies with a larger sample size and multi-center analysis in genotypic and phenotypic expressions of the 10 potential genes will be needed to clarify our current findings; (4) We conducted this study using a single ethnic background cohort. Ethnicity is a source of health inequalities. In particular, ethnic inheritance influences the occurrence rates of different cardiovascular disorders (Scarborough et al., 2010). In this study, we performed a small preliminary study only in Thai ethnicities to elucidate appropriate biomarkers to predict FH-CHD. We expect that further studies in larger sample sizes and multi-center Thai male and female populations will help to confirm the feasibility of using the FH-CHD biomarker in Thai ethnicities. Moreover, further meta-analysis studies using publicly available transcriptomic data from different ethnic populations to compare and share appropriate CHD biomarker is expected to be more reliable and advantageous; (5) Several current studies into the genetic basis of coronary heart disease have intensively reported the discovery and aggregation of genetic variants from multiple genes from lipid and other pathologic pathways (Yao et al., 2015; Glicksberg et al., 2019; Cohain et al., 2021). Based on our current NGS data, we are continuing to investigate the variant genes observed only in FH and FH-CHD patient groups, to identify potential predictive markers.
Taken together, based on previous knowledge and our findings, we suggest that among 10 potential markers of FH-CHD risk, TRMS2, PDLIM5, BCL3, CD14, and GBA genes were the most strongly correlated with development of FH-CHD. These show potential for further application as inflammatory markers for the risk of FH-CHD development in Thai patients with FH.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI BioProject, accession no: PRJNA663423.
Ethics Statement
The studies involving human participants were reviewed and approved by The Ethics Committees of the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand and Pramongkutklao Hospital, Bangkok, Thailand. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
KP chose healthy control, patients with hyperlipidemia, familial hyperlipidemia, coronary heart disease (CHD), familial CHD, and performed their coronary bypass grafting. YM and WD were responsible for laboratory work including blood collection and RNA extraction. YM and WD analyzed the NGS data. SB designed primers and conducted the qRT-PCR assays and analyses. WD worked on data analysis and statistical calculations. YM conceived the study and prepared the manuscript. All authors discussed the results, and read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the volunteers and patients who donated their blood and the staff at the Hospital for Tropical Diseases, Faculty of Tropical Medicine, Mahidol University taking consent from patients and filling out the questionnaire voluntarily. We appreciate Edanz Group Ltd. (www.edanzediting.com) and Mr. Paul Adams, who provided editorial assistance. We also thank Louise Adam, ELS(D), from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Funding. This research project is supported by Mahidol University and the Faculty of Tropical Medicine, Mahidol University.
References
- Akiyama H., Kobayashi S., Hirabayashi Y., Murakami-Murofushi K. (2013). Cholesterol glucosylation is catalyzed by transglucosylation reaction of beta-glucosidase 1. Biochem. Biophys. Res. Commun. 441 838–843. 10.1016/j.bbrc.2013.10.145 [DOI] [PubMed] [Google Scholar]
- Alpy F., Tomasetto C. (2005). Give lipids a START: the StAR-related lipid transfer (START) domain in mammals. J. Cell Sci. 118(Pt. 13), 2791–2801. 10.1242/jcs.02485 [DOI] [PubMed] [Google Scholar]
- Ambrosio A. L., Boyle J. A., Di Pietro S. M. (2012). Mechanism of platelet dense granule biogenesis: study of cargo transport and function of Rab32 and Rab38 in a model system. Blood 120 4072–4081. 10.1182/blood-2012-04-420745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand S. S., Islam S., Rosengren A., Franzosi M. G., Steyn K., Yusufali A. H., et al. (2008). Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur. Heart J. 29 932–940. 10.1093/eurheartj/ehn018 [DOI] [PubMed] [Google Scholar]
- Arnal J. F., Douin-Echinard V., Tremollieres F., Terrisse A. D., Sie P., Payrastre B., et al. (2007). Understanding the controversy about hormonal replacement therapy: insights from estrogen effects on experimental and clinical atherosclerosis. Arch. Mal. Coeur Vaiss. 100 554–562. [PubMed] [Google Scholar]
- Bai H. L., Lu Z. F., Zhao J. J., Ma X., Li X. H., Xu H., et al. (2019). Microarray profiling analysis and validation of novel long noncoding RNAs and mRNAs as potential biomarkers and their functions in atherosclerosis. Physiol. Genomics 51 644–656. 10.1152/physiolgenomics.00077.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boese A. C., Kim S. C., Yin K. J., Lee J. P., Hamblin M. H. (2017). Sex differences in vascular physiology and pathophysiology: estrogen and androgen signaling in health and disease. Am. J. Physiol. Heart Circ. Physiol. 313 H524–H545. 10.1152/ajpheart.00217.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A. M., Lohse M., Usadel B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30 2114–2120. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohain A. T., Barrington W. T., Jordan D. M., Beckmann N. D., Argmann C. A., Houten S. M., et al. (2021). An integrative multiomic network model links lipid metabolism to glucose regulation in coronary artery disease. Nat. Commun. 12:547. 10.1038/s41467-020-20750-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbera-Bellalta M., Planas-Rigol E., Lozano E., Terrades-Garcia N., Alba M. A., Prieto-Gonzalez S., et al. (2016). Blocking interferon gamma reduces expression of chemokines CXCL9, CXCL10 and CXCL11 and decreases macrophage infiltration in ex vivo cultured arteries from patients with giant cell arteritis. Ann. Rheum. Dis. 75 1177–1186. 10.1136/annrheumdis-2015-208371 [DOI] [PubMed] [Google Scholar]
- Defesche J. (2001). World health organisation report on familial hypercholesterolemia. Atherosclerosis 154:242. 10.1016/s0021-9150(00)00646-8 [DOI] [PubMed] [Google Scholar]
- Engel F. B., Hsieh P. C., Lee R. T., Keating M. T. (2006). FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc. Natl. Acad. Sci. U.S.A. 103 15546–15551. 10.1073/pnas.0607382103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiordaliso F., Leri A., Cesselli D., Limana F., Safai B., Nadal-Ginard B., et al. (2001). Hyperglycemia activates p53 and p53-regulated genes leading to myocyte cell death. Diabetes 50 2363–2375. 10.2337/diabetes.50.10.2363 [DOI] [PubMed] [Google Scholar]
- Fu W., Chen M., Ou L., Li T., Chang X., Huang R., et al. (2019). Xiaoyaosan prevents atherosclerotic vulnerable plaque formation through heat shock protein/glucocorticoid receptor axis-mediated mechanism. Am. J Transl. Res. 11 5531–5545. [PMC free article] [PubMed] [Google Scholar]
- Ghosh J., Das J., Manna P., Sil P. C. (2009). Taurine prevents arsenic-induced cardiac oxidative stress and apoptotic damage: role of NF-kappa B, p38 and JNK MAPK pathway. Toxicol. Appl. Pharmacol. 240 73–87. 10.1016/j.taap.2009.07.008 [DOI] [PubMed] [Google Scholar]
- Glicksberg B. S., Amadori L., Akers N. K., Sukhavasi K., Franzen O., Li L., et al. (2019). Integrative analysis of loss-of-function variants in clinical and genomic data reveals novel genes associated with cardiovascular traits. BMC Med. Genomics 12(Suppl. 6):108. 10.1186/s12920-019-0542-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K. (2005). Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352 1685–1695. 10.1056/NEJMra043430 [DOI] [PubMed] [Google Scholar]
- Heid C. A., Stevens J., Livak K. J., Williams P. M. (1996). Real time quantitative PCR. Genome Res. 6 986–994. 10.1101/gr.6.10.986 [DOI] [PubMed] [Google Scholar]
- Herrer I., Rosello-Lleti E., Ortega A., Tarazon E., Molina-Navarro M. M., Trivino J. C., et al. (2015). Gene expression network analysis reveals new transcriptional regulators as novel factors in human ischemic cardiomyopathy. BMC Med. Genomics 8:14. 10.1186/s12920-015-0088-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka M., Wakabayashi I., Takeda Y., Fukuzawa K. (2013). Vitamin D(3) derivatives increase soluble CD14 release through ERK1/2 activation and decrease IL-8 production in intestinal epithelial cells. Eur. J. Pharmacol. 721 305–312. 10.1016/j.ejphar.2013.09.014 [DOI] [PubMed] [Google Scholar]
- Hoffman N. E., Miller B. A., Wang J., Elrod J. W., Rajan S., Gao E., et al. (2015). Ca(2)(+) entry via Trpm2 is essential for cardiac myocyte bioenergetics maintenance. Am. J. Physiol. Heart Circ. Physiol. 308 H637–H650. 10.1152/ajpheart.00720.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung J., Scanlon J. P., Mahmoud A. D., Rodor J., Ballantyne M., Fontaine M. A. C., et al. (2020). Novel plaque enriched long noncoding RNA in atherosclerotic macrophage regulation (PELATON). Arterioscler. Thromb. Vasc. Biol. 40 697–713. 10.1161/ATVBAHA.119.313430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivankovic D., Chau K. Y., Schapira A. H., Gegg M. E. (2016). Mitochondrial and lysosomal biogenesis are activated following PINK1/parkin-mediated mitophagy. J. Neurochem. 136 388–402. 10.1111/jnc.13412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J. G., Patino W. D., Matoba S., Hwang P. M. (2006). Genomic analysis of circulating cells: a window into atherosclerosis. Trends Cardiovasc. Med. 16 163–168. 10.1016/j.tcm.2006.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalaf H., Demirel I., Bengtsson T. (2013). Suppression of inflammatory gene expression in T cells by Porphyromonas gingivalis is mediated by targeting MAPK signaling. Cell Mol. Immunol. 10 413–422. 10.1038/cmi.2013.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchens R. L., Thompson P. A., O’Keefe G. E., Munford R. S. (2000). Plasma constituents regulate LPS binding to, and release from, the monocyte cell surface. J. Endotoxin Res. 6 477–482. 10.1179/096805100101532450 [DOI] [PubMed] [Google Scholar]
- Kotepui M., Thawornkuno C., Chavalitshewinkoon-Petmitr P., Punyarit P., Petmitr S. (2012). Quantitative real-time RT-PCR of ITGA7, SVEP1, TNS1, LPHN3, SEMA3G, KLB and MMP13 mRNA expression in breast cancer. Asian Pac. J. Cancer Prev. 13 5879–5882. 10.7314/apjcp.2012.13.11.5879 [DOI] [PubMed] [Google Scholar]
- Li H., Fan J., Zhao Y., Zhang X., Dai B., Zhan J., et al. (2019). Nuclear miR-320 Mediates diabetes-induced cardiac dysfunction by activating transcription of fatty acid metabolic genes to cause lipotoxicity in the heart. Circ. Res. 125 1106–1120. 10.1161/CIRCRESAHA.119.314898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Q., Molkentin J. D. (2003). Redefining the roles of p38 and JNK signaling in cardiac hypertrophy: dichotomy between cultured myocytes and animal models. J. Mol. Cell Cardiol. 35 1385–1394. 10.1016/j.yjmcc.2003.10.001 [DOI] [PubMed] [Google Scholar]
- Lin R., Wang Y., Chen Q., Liu Z., Xiao S., Wang B., et al. (2018). TRPM2 promotes the proliferation and invasion of pancreatic ductal adenocarcinoma. Mol. Med. Rep. 17 7537–7544. 10.3892/mmr.2018.8816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglott D., Ostell J., Pruitt K. D., Tatusova T. (2011). Entrez gene: gene-centered information at NCBI. Nucleic Acids Res. 39 D52–D57. 10.1093/nar/gkq1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimaiti A., Maimaiti A., Yang Y., Ma Y. (2016). MiR-106b exhibits an anti-angiogenic function by inhibiting STAT3 expression in endothelial cells. Lipids Health Dis. 15:51. 10.1186/s12944-016-0216-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneerat Y., Prasongsukarn K., Benjathummarak S., Dechkhajorn W. (2017). PPBP and DEFA1/DEFA3 genes in hyperlipidaemia as feasible synergistic inflammatory biomarkers for coronary heart disease. Lipids Health Dis. 16:80. 10.1186/s12944-017-0471-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais A. D. (2004). Familial hypercholesterolaemia. Clin. Biochem. Rev. 25 49–68. [PMC free article] [PubMed] [Google Scholar]
- Marques A. R., Mirzaian M., Akiyama H., Wisse P., Ferraz M. J., Gaspar P., et al. (2016). Glucosylated cholesterol in mammalian cells and tissues: formation and degradation by multiple cellular beta-glucosidases. J. Lipid Res. 57 451–463. 10.1194/jlr.M064923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner M. C., Cabot M. C. (2010). Glucosylceramide in humans. Adv. Exp. Med. Biol. 688 156–164. 10.1007/978-1-4419-6741-1_11 [DOI] [PubMed] [Google Scholar]
- Miller B. A., Hoffman N. E., Merali S., Zhang X. Q., Wang J., Rajan S., et al. (2014). TRPM2 channels protect against cardiac ischemia-reperfusion injury: role of mitochondria. J. Biol. Chem. 289 7615–7629. 10.1074/jbc.M113.533851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. F., Li H., Jeffries N., Wright V., Cooper R. A., Jr., Elkahloun A., et al. (2005). Using peripheral blood mononuclear cells to determine a gene expression profile of acute ischemic stroke: a pilot investigation. Circulation 111 212–221. 10.1161/01.CIR.0000152105.79665.C6 [DOI] [PubMed] [Google Scholar]
- Niki T., Galli I., Ariga H., Iguchi-Ariga S. M. (2000). MSSP, a protein binding to an origin of replication in the c-myc gene, interacts with a catalytic subunit of DNA polymerase alpha and stimulates its polymerase activity. FEBS Lett. 475 209–212. 10.1016/s0014-5793(00)01679-3 [DOI] [PubMed] [Google Scholar]
- Ohno H., Takimoto G., McKeithan T. W. (1990). The candidate proto-oncogene bcl-3 is related to genes implicated in cell lineage determination and cell cycle control. Cell 60 991–997. 10.1016/0092-8674(90)90347-h [DOI] [PubMed] [Google Scholar]
- Park Y. M. (2014). CD36, a scavenger receptor implicated in atherosclerosis. Exp. Mol. Med. 46:e99. 10.1038/emm.2014.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perisic L., Aldi S., Sun Y., Folkersen L., Razuvaev A., Roy J., et al. (2016). Gene expression signatures, pathways and networks in carotid atherosclerosis. J. Intern. Med. 279 293–308. 10.1111/joim.12448 [DOI] [PubMed] [Google Scholar]
- Pertea G., Pertea M. (2020). GFF Utilities: GffRead and GffCompare. F1000Research 9:304. 10.12688/f1000research.23297.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podrez E. A., Poliakov E., Shen Z., Zhang R., Deng Y., Sun M., et al. (2002). A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J Biol. Chem. 277 38517–38523. 10.1074/jbc.M205924200 [DOI] [PubMed] [Google Scholar]
- Rijnboutt S., Aerts H. M., Geuze H. J., Tager J. M., Strous G. J. (1991). Mannose 6-phosphate-independent membrane association of cathepsin D, glucocerebrosidase, and sphingolipid-activating protein in HepG2 cells. J. Biol. Chem. 266 4862–4868. 10.1016/s0021-9258(19)67728-8 [DOI] [PubMed] [Google Scholar]
- Robinson M. D., McCarthy D. J., Smyth G. K. (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26 139–140. 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucerman J. J., Tan P. M., Buchholz K. S., McCulloch A. D., Omens J. H. (2019). Mechanical regulation of gene expression in cardiac myocytes and fibroblasts. Nat. Rev. Cardiol. 16 361–378. 10.1038/s41569-019-0155-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough P., Rayner M., van Dis I., Norum K. (2010). Meta-analysis of effect of saturated fat intake on cardiovascular disease: overadjustment obscures true associations. Am. J. Clin. Nutr. 92 458–459. 10.3945/ajcn.2010.29504 [DOI] [PubMed] [Google Scholar]
- Scherberich J. E., Nockher W. A. (1999). CD14++ monocytes, CD14+/CD16+ subset and soluble CD14 as biological markers of inflammatory systemic diseases and monitoring immunosuppressive therapy. Clin. Chem. Lab. Med. 37 209–213. 10.1515/CCLM.1999.039 [DOI] [PubMed] [Google Scholar]
- Scheuner M. T. (2003). Genetic evaluation for coronary artery disease. Genet Med. 5 269–285. 10.1097/01.GIM.0000079364.98247.26 [DOI] [PubMed] [Google Scholar]
- Schlitt A., Heine G. H., Blankenberg S., Espinola-Klein C., Dopheide J. F., Bickel C., et al. (2004). CD14+CD16+ monocytes in coronary artery disease and their relationship to serum TNF-alpha levels. Thromb. Haemost. 92 419–424. 10.1160/TH04-02-0095 [DOI] [PubMed] [Google Scholar]
- Sharov V. G., Todor A., Suzuki G., Morita H., Tanhehco E. J., Sabbah H. N. (2003). Hypoxia, angiotensin-II, and norepinephrine mediated apoptosis is stimulus specific in canine failed cardiomyocytes: a role for p38 MAPK, Fas-L and cyclin D1. Eur. J. Heart Fail 5 121–129. 10.1016/s1388-9842(02)00254-4 [DOI] [PubMed] [Google Scholar]
- Sharp F. R., Xu H., Lit L., Walker W., Pinter J., Apperson M., et al. (2007). Genomic profiles of stroke in blood. Stroke 38(Suppl. 2), 691–693. 10.1161/01.STR.0000247940.27518.38 [DOI] [PubMed] [Google Scholar]
- Silverstein R. L., Baird M., Lo S. K., Yesner L. M. (1992). Sense and antisense cDNA transfection of CD36 (glycoprotein IV) in melanoma cells. role of CD36 as a thrombospondin receptor. J. Biol. Chem. 267 16607–16612. 10.1016/s0021-9258(18)42046-7 [DOI] [PubMed] [Google Scholar]
- Simmons D. L., Tan S., Tenen D. G., Nicholson-Weller A., Seed B. (1989). Monocyte antigen CD14 is a phospholipid anchored membrane protein. Blood 73 284–289. 10.1182/blood.v73.1.284.bloodjournal731284 [DOI] [PubMed] [Google Scholar]
- Soriano-Romani L., Contreras-Ruiz L., Garcia-Posadas L., Lopez-Garcia A., Masli S., Diebold Y. (2015a). Inflammatory cytokine-mediated regulation of thrombospondin-1 and CD36 in conjunctival cells. J. Ocul. Pharmacol. Ther. 31 419–428. 10.1089/jop.2015.0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano-Romani L., Garcia-Posadas L., Lopez-Garcia A., Paraoan L., Diebold Y. (2015b). Thrombospondin-1 induces differential response in human corneal and conjunctival epithelial cells lines under in vitro inflammatory and apoptotic conditions. Exp. Eye Res. 134 1–14. 10.1016/j.exer.2015.03.004 [DOI] [PubMed] [Google Scholar]
- Stone N. J., Robinson J. G., Lichtenstein A. H., Bairey Merz C. N., Blum C. B., Eckel R. H., et al. (2014). 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J. Am. Coll. Cardiol. 63(25 Pt. B), 2889–2934. 10.1016/j.jacc.2013.11.002 [DOI] [PubMed] [Google Scholar]
- Susomboon P., Maneerat Y., Dekumyoy P., Kalambaheti T., Iwagami M., Komaki-Yasuda K., et al. (2006). Down-regulation of tight junction mRNAs in human endothelial cells co-cultured with Plasmodium falciparum-infected erythrocytes. Parasitol. Int. 55 107–112. 10.1016/j.parint.2005.11.054 [DOI] [PubMed] [Google Scholar]
- Tandon N. N., Kralisz U., Jamieson G. A. (1989). Identification of glycoprotein IV (CD36) as a primary receptor for platelet-collagen adhesion. J. Biol. Chem. 264 7576–7583. 10.1016/s0021-9258(18)83273-2 [DOI] [PubMed] [Google Scholar]
- Trapnell C., Hendrickson D. G., Sauvageau M., Goff L., Rinn J. L., Pachter L. (2013). Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 31 46–53. 10.1038/nbt.2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Fang J., Lv J., Pan Z., Yin X., Cheng H., et al. (2019). Novel polymorphisms in PDLIM3 and PDLIM5 gene encoding Z-line proteins increase risk of idiopathic dilated cardiomyopathy. J. Cell Mol. Med. 23 7054–7062. 10.1111/jcmm.14607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeland C. J., van den Hoogenhof M. M., Beqqali A., Creemers E. E. (2015). Insights into alternative splicing of sarcomeric genes in the heart. J. Mol. Cell Cardiol. 81 107–113. 10.1016/j.yjmcc.2015.02.008 [DOI] [PubMed] [Google Scholar]
- Weber C., Noels H. (2011). Atherosclerosis: current pathogenesis and therapeutic options. Nat. Med. 17 1410–1422. 10.1038/nm.2538 [DOI] [PubMed] [Google Scholar]
- Wulczyn F. G., Naumann M., Scheidereit C. (1992). Candidate proto-oncogene bcl-3 encodes a subunit-specific inhibitor of transcription factor NF-kappa B. Nature 358 597–599. 10.1038/358597a0 [DOI] [PubMed] [Google Scholar]
- Xu C., Zhang C., Ji J., Wang C., Yang J., Geng B., et al. (2018). CD36 deficiency attenuates immune-mediated hepatitis in mice by modulating the proapoptotic effects of CXC chemokine ligand 10. Hepatology 67 1943–1955. 10.1002/hep.29716 [DOI] [PubMed] [Google Scholar]
- Yao C., Chen B. H., Joehanes R., Otlu B., Zhang X., Liu C., et al. (2015). Integromic analysis of genetic variation and gene expression identifies networks for cardiovascular disease phenotypes. Circulation 131 536–549. 10.1161/CIRCULATIONAHA.114.010696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner D., Thuerauf D. J., Hanford D. S., McDonough P. M., Glembotski C. C. (1997). A role for the p38 mitogen-activated protein kinase pathway in myocardial cell growth, sarcomeric organization, and cardiac-specific gene expression. J. Cell Biol. 139 115–127. 10.1083/jcb.139.1.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M., Cheng H., Banerjee I., Chen J. (2010). ALP/Enigma PDZ-LIM domain proteins in the heart. J. Mol. Cell Biol. 2 96–102. 10.1093/jmcb/mjp038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Liang Y., Zhang X., Liao L., Yang Y., Ouyang W., et al. (2020). SHARPIN Promotes Melanoma Progression via Rap1 Signaling Pathway. J. Invest. Dermatol. 140 395–403.e6. 10.1016/j.jid.2019.07.696 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI BioProject, accession no: PRJNA663423.