Abstract
Objective:
To evaluate darunavir and cobicistat pharmacokinetics during pregnancy compared to postpartum and in infant washout samples after delivery.
Design:
Nonrandomized, open-label, parallel-group, multi-center phase-IV prospective study of darunavir and cobicistat pharmacokinetics in pregnant women with HIV and their children in the U.S.
Methods:
Intensive steady-state 24 hour pharmacokinetic profiles were performed after administration of 800 mg of darunavir and 150 mg of cobicistat orally in fixed dose combination once-daily during the second trimester, third trimester, and postpartum. Infant washout samples were collected after birth. Darunavir and cobicistat were measured in plasma by validated HPLC-UV and LC-MS/MS assays, respectively. A two-tailed Wilcoxon signed-rank test (α = 0.10) was employed for paired within-participant comparisons.
Results:
A total of 29 pregnant women receiving darunavir and cobicistat once-daily enrolled in the study. Compared to paired postpartum data, darunavir AUC0–24 was 53% lower in the second trimester (n=12, P=0.0024, Geometric mean of ratio (GMR)=0.47, 90% CI 0.33 – 0.68) and 56% lower in the third trimester (n=18, p<0.0001, GMR=0.44, 90% CI 0.36 – 0.54), while cobicistat AUC0–24 was 50% lower in the second trimester (n=12, P=0.0024, GMR=0.50, 90% CI 0.36 – 0.69) and 56% lower in the third trimester (n=18, p<0.0001, GMR=0.44, 90% CI 0.35 – 0.55). Placental transfer of darunavir and cobicistat was limited.
Conclusions:
Standard darunavir/cobicistat dosing during pregnancy results in significantly lower exposure during pregnancy which may increase the risk of virologic failure and perinatal transmission.
Keywords: Darunavir, cobicistat, HIV, pharmacokinetics, perinatal transmission, pregnancy, protease inhibitor, HIV infection
Introduction
Antiretroviral treatment is recommended for pregnant women living with HIV for both primary treatment of maternal HIV infection and for prevention of perinatal HIV transmission. Use of potent antiretroviral regimens by pregnant women living with HIV has reduced the rate of perinatal HIV transmission to 1% or less in the United States and Europe.[1] While safe and effective antiretroviral treatment options and dosing regimens have increased for pregnant women with HIV, pharmacokinetic and safety data on newer agents during pregnancy remain limited.
Physiological changes during pregnancy may alter pharmacokinetics and affect exposure to antiretrovirals.[2, 3] For example, the activity of the cytochrome P450 3A (CYP3A) enzyme system is increased during pregnancy leading to reduced exposure to drugs which are CYP3A substrates.[4–9] Subtherapeutic antiretroviral exposure increases the risk of virologic failure and consequent perinatal transmission of HIV, while increased drug exposure may subject both mother and child to drug-related toxicities.[10]
Darunavir is an HIV-1 protease inhibitor (PI) with virologic potency, durability, and a high genetic barrier to resistance.[11] The US Department of Health and Human Services Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV recommend a PI-based regimen with darunavir in certain clinical situations, including in patients who must begin antiretroviral treatment urgently before resistance tests are available and in patients who are at risk for intermittent therapy due to poor adherence.[12] Darunavir is metabolized primarily by CYP3A and must be boosted by coadministration with one of the licensed CYP3A4 inhibitors ritonavir or cobicistat. Darunavir boosted with ritonavir has been well-characterized in pregnancy; however, the pharmacokinetics of darunavir boosted with cobicistat have only been studied in a small number of pregnant women.[13] The primary objective of this study was to evaluate the pharmacokinetics and safety of darunavir and cobicistat in a larger cohort of pregnant women with HIV-1 infection receiving a fixed dose combination of darunavir with cobicistat.
Methods
Study population and design
IMPAACT P1026s “Pharmacokinetic Properties of Antiretroviral and Related Drugs during Pregnancy and Postpartum” (ClinicalTrials.gov NCT00042289), was a non-randomized, open-label, parallel-group, multi-center study. The study included an arm for pregnant women with HIV receiving darunavir 800 mg and cobicistat 150 mg (Prezcobix®, Jassen Pharmaceuticals, Inc.) prescribed for clinical care. Participants had to be between 20 and 38 weeks gestation, be stable on their antiretroviral regimen for two weeks, and intend to continue the same regimen through 6 – 12 weeks postpartum. Maternal exclusion criteria were multiple gestation, toxicity necessitating a medication change during the study, and the use of specific medications known to interact with darunavir or cobicistat.
Each study site received local institutional review board approval. All participants gave informed consent prior to participation. Pharmacokinetic sampling was performed during the third trimester (30–38 weeks gestation), and postpartum, as well as the second trimester (20–26 weeks gestation) for participants enrolling before 26 weeks gestation. Samples collected during pregnancy were assayed in real-time with results reported to each study participant and her clinician.
Infant enrollment occurred immediately after maternal enrollment with maternal consent, with eligibility confirmed at birth. Infant inclusion criteria were birth weight >1,000g, singleton delivery and maternal enrollment in P1026s. Infant exclusion criteria were a severe congenital malformation or medical condition that would interfere with study participation as deemed by site clinicians and use of specific medications known to interfere with darunavir disposition.
Clinical and laboratory monitoring
Each study visit included monitoring of HIV-1 RNA, CD4+ lymphocyte cell count, and hematology and serum biochemistry tests. All infants received physical examinations after birth and laboratory evaluations if clinically indicated. Adverse events were reported at each study visit and management was determined by each participant’s clinician. The NIAID/DAIDS Table for Grading the Severity of Adult and Pediatric Adverse Events Version 2.0 (2014) was used to grade adverse event severity.
Sample collection
Intensive 24-hour pharmacokinetic evaluations were performed during the second trimester (20–26 weeks gestation), third trimester (30–38 weeks gestation) and postpartum (6–12 weeks following delivery). Requirements prior to pharmacokinetic sampling were self-reported darunavir and cobicistat adherence for two weeks and consistent dosing times for the last three doses. On sampling days the pre-dose sample was drawn and study medications were administered under observation. Post-dose samples were drawn at 1, 2, 4, 6, 8, 12 and 24 hours. At delivery, cord blood and maternal plasma samples were collected when possible. Four plasma samples were collected from study infants at 2–10 hours, 18–28 hours, 36–72 hours, and 5–9 days after birth.
Darunavir and cobicistat plasma concentration measurements
Quantitative determination of darunavir and cobicistat in human plasma was accomplished by the use of protein precipitation and high pressure liquid chromatography with UV detection and high-performance liquid chromatography with tandem mass spectrometry detection (LC-MS/MS), respectively.
Darunavir was precipitated from 200 μL of plasma with 240 μL of 100% acetonitrile (MeCN). A total of 50 μL of supernatant was injected directly onto a C-18 reversed phase HPLC column (Ace 5, 4.6 × 150 mm). Darunavir was separated isocratically using a mobile phase consisting of 51% buffer (10mM potassium phosphate buffer, pH 3.0 – 3.1) and 49% ACN at a flow rate of 0.5mL/min. UV detection was at 202nm. Mean recovery of drug from plasma was 99.08%. The method was linear over the range of 0.092 – 23.58 μg/mL. Quantitation was by external calibration standards used to generate a curve using a least-squares linear regression algorithm to plot the peak area versus concentration with 1/response weighting.
Cobicistat was precipitated from 10 μL of plasma with 300 μL of 100% acetonitrile (MeCN) plus the internal standard [2H8]-Cobicistat (D8-Cobicistat) (300ng/mL). A total of 5 μL of supernatant was injected directly onto a C-18 reversed phase HPLC column (MacMod Ace-5, 2.1 × 150 mm). Cobicistat was eluted using an gradient mobile phase consisting of 90% 0.1% formic acid in water and 10% 0.1% formic acid in MeCN to 5% 0.1% formic acid in water and 95% 0.1% formic acid in MeCN at a flow rate of 0.6mL/min to 0.8mL/min. MS/MS detection was made in positive electrospray ionization mode, with MRM monitoring of transitions (776.5→606.2) and (784.5→614.5) for cobcistat and D8-cobcistat, respectively. Mean recovery efficiency of drug from plasma was 100.8%. The method had a dynamic range of 4.9–2,500 ng/mL. Calibration standards are used to generate a curve using a linear regression algorithm to plot the peak area ratio of cobcistat/D8-cobicistat versus concentration with 1/x weighting, over the full dynamic range of analyte concentrations. Concentrations of incurred and quality control samples are calculated with the same regression analysis.
Pharmacokinetic analyses
Darunavir and cobicistat maximum, minimum, and last plasma concentrations (Cmax, Cmin, C24) along with corresponding time points (Tmax, Tmin) were observed directly. Steady-state area under the plasma concentration versus time curve over the 24-hour dosing interval (AUC0–24) was estimated with the trapezoidal rule. The terminal elimination half-life (t1⁄2) was calculated as 0.693/λz, where λz is the elimination rate constant derived from the terminal slope of the log concentration versus time curve. For participants with pre-dose concentrations below the assay quantitation limit, single-dose AUC from time 0 to infinity was estimated as AUC0–24 plus C24 divided by λz. Apparent oral clearance (CL/F) was calculated as dose divided by AUC0–24. Concentrations that were below the limit of quantitation of the assay were set at half the lower limit of quantitation to calculate summary statistics. The minimum exposure target for darunavir (70.4 μg*hr/mL) was defined as a 30% reduction from the typical AUC0–24 in non-pregnant adult patients with HIV (100.6 μg*hr/mL) which was taken from published pharmacokinetic parameters.[14]
Statistical analyses
The target sample size was 25 women with evaluable third trimester pharmacokinetic data, with at least 12 who had evaluable second trimester data. Descriptive statistics were calculated for pharmacokinetic parameters during each study period. Pharmacokinetic parameters during the second trimester versus postpartum and during the third trimester versus postpartum were compared at the within-participant level using the Wilcoxon signed-rank test, with a two-sided P-value ≤0.10 considered statistically significant. Within-participant geometric mean ratios (GMR) and 90% confidence intervals (CI) for pharmacokinetic parameters in the pregnant versus non-pregnant conditions were calculated for darunavir and cobicistat to estimate the range of percentage changes between the two conditions that would be consistent with the observed data and to assess clinical importance in order to inform dosing recommendations. Participants with no postpartum data or with non-evaluable postpartum data were excluded from matched comparisons
Results
Participant Characteristics
Twenty-nine pregnant women taking darunavir and cobicistat once-daily enrolled in the study. Evaluable pharmacokinetic data were available for 16, 26, and 19 women in the second trimester, third trimester, and postpartum, respectively. All darunavir and cobicistat plasma concentrations in one woman in the postpartum period were below or near the limit of quantitation of both the darunavir and cobicistat assays, consistent with lack of absorption of both drugs. Postpartum pharmacokinetic data from this participant were deemed non-evaluable and excluded from both postpartum summary statistics and from matched comparisons. Two participants missed scheduled third trimester visits. Overall, evaluable paired pregnancy and postpartum data were available for 12 of 16 women who had second trimester visits and for 18 of 26 women who had third trimester visits. Maternal and infant clinical characteristics are summarized in Table 1.
Table 1.
Clinical Characteristics.
| N (%) and/or Median (Range) | |
|---|---|
| Maternal Demographics (n = 29) | |
| Age at Delivery (years) | 27.4 (17.2 – 43.2) |
| Weight at Delivery (kg) | 94.3 (72.5 – 124.2) |
| Race/Ethnicity – Black Non-Hispanic; Hispanic (Regardless of Race) | 18 (62%); 11 (38%) |
| Concomitant ARVs 2T PK visit: FTC; TAF; 3TC; ZDV; TDF; DTG; LPV; RTV | 10 (62.5%); 8 (50%); 6 (37.5%); 6 (37.5%); 2 (12.5%); 1 (6.25%); 1 (6.25%); 1 (6.25%) |
| Concomitant ARVs 3T PK visit: FTC; ZDV; TAF; 3TC; TDF; DTG; LPV; RTV | 15 (57.7%); 12 (46.2%); 11 (42.3%); 9 (34.6%); 4 (15.4%); 3 (11.5%); 1 (3.8%); 1 (3.8%) |
| Country: United States | 29 (100%) |
| 2T: HIV-1 RNA ≤ 50 copies/mL; HIV-1 RNA copies/mL | 11/16 (68.8%); 20 (20, 684) |
| 2T: CD4 (cells/mm3) | 506.5 (237 – 1596) |
| 3T: HIV-1 RNA ≤ 50 copies/mL; HIV-1 RNA copies/mL | 21/25 (84.0%); 20 (20, 124) |
| 3T: CD4 (cells/mm3) | 473 (153 – 1581) |
| Delivery: HIV-1 RNA ≤ 50 copies/mL; HIV-1 RNA copies/mL | 25/29 (86.2%); 20 (20, 429) |
| Delivery: CD4 (cells/mm3) | 517 (153 –1822) |
| PP: HIV-1 RNA ≤ 50 copies/mL; HIV-1 RNA copies/mL | 15/19 (78.9%); 24 (20, 822) |
| PP: CD4 (cells/mm3) | 569 (322 – 1807) |
| Infant Demographics (n=28)* | |
| Gestational Age (weeks) | 38.0 (35.9 – 40.9) |
| Birth Weight (grams) | 2912.5 (2400–3800) |
| HIV Status: Uninfected; No testing data available | 26 (93%); 2 (7%) |
2T, second trimester; 3T, third trimester; PP, postpartum; PK, pharmacokinetic; FTC, emtricitabine; TAF, tenofovir alafenamide; 3TC, lamivudine; ZDV, zidovudine; TDF, tenofovir disoproxil fumarate; DTG, dolutegravir; LPV, lopinavir; RTV, ritonavir
One mother went off study before delivery
Darunavir Pharmacokinetics
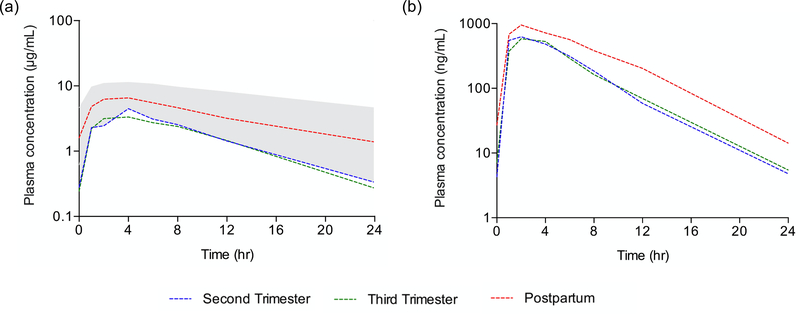
The median (IQR) darunavir AUC0–24 in the second trimester, third trimester, and postpartum periods were 50.00 μg*hr/mL (27.03 – 58.90), 42.05 μg*hr/mL (26.83 – 50.50) and 95.55 μg*hr/mL (67.20 – 118.95), respectively (Figure 1, Table 2). Compared to paired postpartum data, darunavir AUC0–24 was 53% lower in the second trimester (n=12, P=0.0024, GMR=0.47, 90% CI 0.33 – 0.68) and 56% lower in the third trimester (n=18, p<0.0001, GMR=0.44, 90% CI 0.36 – 0.54). The frequency of participants meeting the darunavir AUC0–24 target (70.4 μg*hr/mL) was 3/16 (19%) in the second trimester, 4/26 (15%) in the third trimester, and 14/20 (70%) postpartum.
Figure 1.
Median antepartum and postpartum plasma darunavir (a) and cobicistat (b) concentration versus time profiles at steady state following once-daily dosing of 800/150 mg darunavir/cobicistat. The shaded area displays the 10th to 90th percentile concentrations of darunavir in non-pregnant adults.
Table 2.
Maternal Darunavir Pharmacokinetic Parameters, Median (IQR)
| Parameter | Second Trimester n = 16 | Third Trimester n = 26 | Postpartum n = 19 | Non-pregnant Adults with HIV1 | GMR2 (90% CI) 2T/PP, n=12 | GMR2 (90% CI) 3T/PP, n=18 |
|---|---|---|---|---|---|---|
| AUC0–24 (μg*hr/mL) | 50.00 (27.032, 58.90) | 42.05 (26.83, 50.50) | 95.55 (67.20, 118.95) | 100.15 (±32.04) | 0.47 (0.33, 0.68)* | 0.44 (0.36, 0.54) * |
| C0 (μg/mL) | 0.28 (0.045, 0.60) | 0.25 (0.045, 1.13) | 1.62 (0.045, 3.44) | 2.04 (±1.26) | 0.29 (0.07, 1.16)* | 0.32 (0.10, 1.05) * |
| Cmax (μg/mL) | 4.59 (2.38, 6.12) | 3.67 (3.29, 4.65) | 7.04 (5.70, 10.75) | - | 0.56 (0.41, 0.76)* | 0.54 (0.46, 0.63) * |
| Tmax (hr) | 3 (2, 4) | 2 (2, 4) | 2 (2, 4) | - | - | - |
| C24 (μg/mL) | 0.33 (0.045, 0.47) | 0.27 (0.045, 0.63) | 1.43 (0.73, 1.86) | - | 0.15 (0.08, 0.30)* | 0.21 (0.12, 0.36) * |
| Cmin (μg/mL) | 0.33 (0.045, 0.47) | 0.25 (0.045, 0.45) | 1.34 (0.56, 1.86) | - | 0.15 (0.08, 0.30)* | 0.21 (0.11, 0.38) * |
| CL/F (L/hr) | 16.00 (13.64, 29.74) | 19.04 (15.84, 29.82) | 8.37 (6.73, 11.91) | - | 2.12 (1.47, 3.05)* | 2.28 (1.87, 2.77) * |
| T1/2 (hr) | 4.80 (3.91, 6.07) | 5.18 (3.76, 6.61) | 8.11 (6.19, 10.55) | - | 0.55 (0.44, 0.67)* | 0.62 (0.50, 0.76) * |
p<0.10 compared to postpartum
Historical data from Prezcobix® (darunavir and cobicistat) package insert, represented as mean (±S.D.)
Paired comparisons
GMR, geometric mean ratio; 2T, second trimester, 3T, third trimester; PP, postpartum
Darunavir C24 was 85% lower in the second trimester and 79% lower in the third trimester compared to paired postpartum data (p=0.001 and p=0.004, respectively). The median (IQR) darunavir C24 was 0.33 μg/mL (0.045 – 0.47) in the second trimester, 0.27 μg/mL (0.045 – 0.63) in the third trimester, and 1.43 μg/mL (0.72 – 1.86) postpartum. A total of 6/16 (38%), 8/26 (31%), and 1/20 (5%) mothers had 24-hour darunavir trough concentrations below the limit of quantitation of the assay (0.09 μg/mL) in the second trimester, third trimester, and postpartum, respectively, suggesting trough concentrations in these women may have fell below the average protein binding-adjusted EC50 for wild-type virus (0.055 μg/mL[15]).
A total of 20 maternal plasma samples at delivery and 19 cord blood samples were available. Of these, 6 maternal plasma samples and 15 cord blood samples were below the lower limit of quantitation of the assay for darunavir (0.09 μg /mL). The median (IQR) concentration of darunavir in maternal plasma at delivery was 0.61 μg/mL (0.045 – 1.78, n=20). The highest darunavir concentration observed in cord blood was 0.30 μg/mL. A total of 13 sets of paired samples had quantifiable darunavir concentrations in maternal plasma at delivery along with a cord blood sample (including cord blood samples below the limit of quantitation). Of these paired samples, the median (IQR) ratio of cord blood to maternal plasma was 0.07 (0.03 – 0.15).
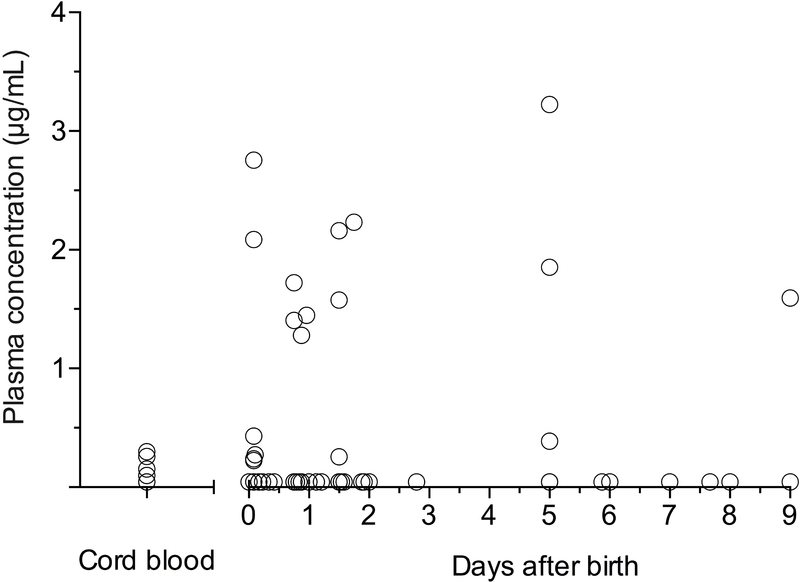
A total of 85 washout samples were obtained from 26 infants after birth. Infant darunavir plasma concentrations post-delivery are displayed in Figure 2. In 17 infants all samples were below the quantitation limit for darunavir. In the remaining 9 infants with detectable darunavir concentrations, the median (IQR) maximum observed plasma concentration was 0.43 μg/mL (0.27 – 2.23).
Figure 2.
Scatter plot of darunavir plasma concentrations in cord blood and in infants after birth. Concentrations below the limit of quantitation (BLQ; 0.09 μg/mL) are displayed as 1/2 the lower limit of quantitation (0.045 μg/mL). Darunavir was BLQ in 15 of 19 available cord blood samples. A total of 85 washout samples were obtained from 26 infants over the first 9 days of life. In 17 infants all samples were BLQ for darunavir.
Cobicistat Pharmacokinetics
The median (IQR) cobicistat AUC0–24 in the second trimester, third trimester, and postpartum periods was 4.46 μg*hr/mL (3.21 – 5.69), 3.91 μg*hr/mL (3.15 – 6.24), and 8.52 μg*hr/mL (6.28 – 11.39), respectively. Compared to paired postpartum data, cobicistat AUC0–24 was 50% lower in the second trimester (n=12, P=0.0024, GMR=0.50, 90% CI 0.36 – 0.69) and 56% lower in the third trimester (n=18, p<0.0001, GMR=0.44, 90% CI 0.35 – 0.55) (Figure 1, Table 3). Cobicistat Cmax was 37% lower in the second trimester and 42% lower in the third trimester compared to paired postpartum data (p=0.0034 and p<0.0001, respectively). Pre-dose cobicistat concentrations below the quantitation limit were observed in 7/16 (44%), 10/26 (38%) and 8/20 (40%) women in the second trimester, third trimester, and postpartum, respectively.
Table 3.
Maternal Cobicistat Pharmacokinetic Parameters, Median (IQR)
| Parameter | Second Trimester n = 16 | Third Trimester n = 26 | Postpartum n = 19 | Non-pregnant Adults with HIV1 | GMR2 (90% CI) 2T/PP, n=12 | GMR2 (90% CI) 3T/PP, n=18 |
|---|---|---|---|---|---|---|
| AUC0–24 (μg*hr/mL) | 4.46 (3.21, 5.69) | 3.91 (3.15, 6.24) | 8.52 (6.28, 11.39) | 7.6 (± 3.7) | 0.50 (0.36, 0.69)* | 0.44 (0.35, 0.55)* |
| C0 (ng/mL) | 4.1 (2.5, 10.8) | 7.2 (2.5, 26.3) | 28.5 (2.5, 171.0) | - | 0.28 (0.07, 1.10) | 0.31 (0.09, 1.07)* |
| Cmax (ng/mL) | 713.5 (525.0, 1050.5) | 662.5 (497.0, 969.0) | 1190.0 (838.0, 1300.0) | 990 (± 300) | 0.63 (0.51, 0.78)* | 0.58 (0.48, 0.70)* |
| Tmax (hr) | 2 (1, 3) | 2 (1, 4) | 2 (1, 4) | - | - | - |
| C24 (ng/mL) | 4.4 (2.45, 8.9) | 5.5 (2.45, 8.8) | 16.5 (7.2, 50.1) | 0.03 (±0.1) | 0.40 (0.23, 0.69)* | 0.26 (0.16, 0.43)* |
| Cmin (ng/mL) | 3.3 (2.45, 7.7) | 5.5 (2.45, 8.8) | 12.0 (7.2, 30.9) | - | 0.32 (0.20, 0.53)* | 0.29 (0.16, 0.52)* |
| CL/F (L/hr) | 33.67 (26.39, 46.68) | 38.38 (24.05, 47.60) | 17.61 (13.17, 23.88) | - | 2.01 (1.45, 2.77)* | 2.27 (1.82, 2.83)* |
| T1/2 (hr) | 2.87 (2.28, 3.43) | 2.81 (2.27, 3.31) | 3.43 (2.99, 4.53) | - | 0.86 (0.70, 1.06)* | 0.75 (0.65, 0.86)* |
p<0.10 compared to postpartum
Historical data from TYBOST® (cobicistat) package insert, represented as mean (±S.D.)
Paired comparisons
GMR, geometric mean ratio; 2T, second trimester, 3T, third trimester; PP, postpartum
A total of 20 maternal plasma samples at delivery and 19 cord blood samples were available. Of these, 6 maternal plasma samples and 13 cord blood samples were below the lower limit of quantitation of the assay for cobicistat (4.9 ng/mL). The median (IQR) concentration of cobicistat in maternal plasma at delivery was 27.1 ng/mL (2.45 – 112.3, n=20). The highest cobicistat concentration observed in cord blood was 60.6 ng/mL. A total of 14 sets of paired samples had quantifiable cobicistat concentrations in maternal plasma at delivery along with a cord blood sample (including cord blood samples below the limit of quantitation). Of these paired samples, the median (IQR) ratio of cord blood to maternal plasma was 0.08 (0.05 – 0.12). Cobicistat was not quantifiable in any neonatal washout plasma samples after birth.
Maternal and Infant Outcomes
Nine mothers had adverse events grade 3 or higher. All were considered unrelated to study drugs except for preterm labor in two women, which were classified as possibly treatment related. Six infants had adverse events grade 3 or higher. All were considered unrelated to study drugs except for a Grade 2 perimembranous ventricular septal defect in one infant which was classified as possibly treatment related by the study team. Six infants had clinical abnormalities observed at birth, including perimembranous ventricular septal defect and a patent foramen ovale, sacral dimple, congenital anemia from ABO incompatibility, bilateral undescended testes and inguinal hernias, ankyloglossia (tongue tie), and slate gray nevi (Mongolian blue spots).
The percentage of women with suppression of HIV replication (viral suppression, defined in this study as HIV-1 RNA < 50 copies/mL) at the second trimester, third trimester, delivery, and postpartum was 68.8%, 84.0%, 86.2%, and 78.9%, respectively. Of the 28 infants included in this study, the most definitive HIV status was uninfected for 26 infants (93%) while 2 infants (7%) had no testing data available (Table 1).
Discussion
In pregnant women with HIV receiving darunavir in combination with cobicistat, darunavir exposure was significantly lower during pregnancy compared to postpartum. Compared to paired postpartum data, darunavir AUC0–24 was 53% lower in the second trimester and 56% lower in the third trimester, while C24 was 85% lower in the second trimester and 79% lower in the third trimester. In this study, the minimum AUC target for darunavir (70.4 μg*hr/mL) was defined as a 30% reduction from the typical AUC in non-pregnant adult patients with HIV (100.6 μg*hr/mL).[14] Fewer participants met this minimum threshold during pregnancy (19% in second trimester; 15% in third trimester) compared to postpartum (70%).
The result of this study are consistent with a prior report that described decreased darunavir and cobicistat exposure in seven pregnant women with HIV receiving darunavir/cobicistat 800/150 mg once daily plus a background ARV regimen.[13] In these seven women, darunavir AUC0–24 was 56% and 50% lower in the second and third trimesters of pregnancy relative to postpartum, respectively, while darunavir 24-hour trough concentrations were 92% and 89% lower. Cobicistat AUC0–24 was 63% and 49% lower in the second and third trimesters of pregnancy relative to postpartum, respectively.[13] In November 2019 the U.S. Food and Drug Administration revised drug labeling for cobicistat products with a recommendation that cobicistat should not be used during pregnancy to boost atazanavir, darunavir, and elvitegravir due to substantially lower exposures of these antiretrovirals with cobicistat boosting in pregnant women.[13, 16–18] The results of the present study – from a larger cohort of pregnant women – are consistent with these prior results and support the recommendation that cobicistat at the standard adult dose is an inadequate pharmacokinetic booster during pregnancy for antiretroviral drugs that are primarily eliminated by CYP3A-mediated hepatic metabolism.
Prior studies have shown only a weak correlation between darunavir exposure and virologic response.[19] In clinical trials, a shallow exposure-response relationship has been observed, whereby virologic response in the first quartile of AUC0–24 or Ctrough is comparable to that in the last quartile.[20] Inhibitory quotients – representing the ratio of drug exposure to viral susceptibility – are more predictive of virologic response for PIs compared to pharmacokinetic or resistance data alone.[21] For example, the ratio of darunavir Ctrough to the 50% inhibitory concentration (IC50) of darunavir, estimated through phenotypic resistance assays, was strongly related to the virologic response. In the current study, 44% and 38% of mothers in the second trimester and third trimester had 24-hour darunavir trough concentrations below the limit of quantitation of the assay (0.09 μg/mL), respectively, suggesting trough concentrations in these women fell below 0.055 μg/mL. Phentoypic assays to determine individual darunavir IC50 values was were not performed in this study.
Physiologic changes in pregnancy contribute to the observed altered pharmacokinetics of darunavir and cobicistat during pregnancy. Increases in blood volume, total body water and body mass can have a dilutional effect on drug concentrations and plasma proteins. Higher production of several hormones, such as progesterone, induce metabolic enzymes, including CYP3A. Changes in gastrointestinal function, including gastric pH and hepatic plasma flow, can affect drug absorption, distribution, metabolism, and excretion. The metabolism of both darunavir and cobicistat is primarily by CYP3A which is localized in the liver, intestine, uterus, placenta, and elsewhere.[22, 23] Hormones such as placental growth hormone, estrogen, cortisol and progesterone induce up to a two-fold increase in CYP3A activity.[9] Increases in CYP3A metabolism may contribute to decreased exposure to both drugs during pregnancy. Cobicistat is used as a pharmacokinetic enhancer to inhibit CYP3A-mediated metabolism of darunavir, increasing darunavir systemic exposure and facilitating once daily dosing. In this study, cobicistat AUC0–24 was 50% lower during the second trimester and 56% lower during the third trimester relative to paired postpartum data. This reduction in cobicistat exposure during pregnancy likely plays a major role in the reductions in darunavir exposure, although other pregnancy related changes (decreased absorption, increased volume of distribution, and/or increased metabolism) may also contribute.
Darunavir is highly protein-bound in plasma (95%), primarily to alpha 1-acid glycoprotein.[24] The concentration of alpha 1-acid glycoprotein is decreased in pregnancy.[25, 26] In addition, drug binding to alpha 1-acid glycoprotein may be displaced by increased hormone binding to this protein during pregnancy. Although the unbound darunavir concentration is responsible for pharmacologic activity, unbound drug concentrations were not measured in this study. While lower darunavir exposure was observed during pregnancy, the therapeutic unbound free fraction during pregnancy is unknown. Unbound darunavir concentrations during pregnancy were measured in the smaller group of pregnant women (n=7) previously described.[13] In this cohort, darunavir protein binding in pregnancy was reduced but these changes had a minor impact as unbound darunavir concentrations also showed large reductions during pregnancy, with total darunavir AUC0–24 56% and 50% lower in the second and third trimesters of pregnancy relative to postpartum, respectively, and unbound darunavir AUC0–24 45% and 40% lower.[13]
This study assessed in utero transfer of darunavir and cobicistat and the washout kinetics of these drugs transferred in utero across the placenta in infants born to mothers receiving darunavir and cobicistat during pregnancy. Darunavir concentrations were quantifiable (≥0.09 μg /mL) in 14 of 20 plasma samples at delivery and 4 of 19 cord blood samples. Cobicistat concentration were quantifiable (≥4.9 ng/mL) in 14 of 20 plasma samples at delivery and 6 of 19 cord blood samples. For darunavir, the median ratio of cord blood to maternal plasma was 0.15 (from 4 available paired sets of cord blood and maternal plasma at delivery with quantifiable darunavir concentrations in both matrices). For cobicistat, the median ratio of cord blood to maternal plasma was 0.05 (from 5 available paired sets of cord blood and maternal plasma at delivery with quantifiable cobicistat concentrations in both matrices). These data suggest that the placental transfer of both drugs is limited. The majority of infants (17/26; 65%) did not have any quantifiable darunavir washout plasma concentrations during the first 9 days of life. In the remaining 9 infants with detectable darunavir concentrations, the median (IQR) maximum observed plasma concentration was 0.43 μg/mL (0.27 – 2.23). Cobicistat was not quantifiable in any neonatal washout samples. Due to the low placental transfer of these drugs, infant washout elimination of these drugs could not be assessed.
A limitation of this study is the opportunistic approach of only enrolling women successfully receiving darunavir and cobicistat as part of clinical care may result in a population that is more likely to respond to the regimen without treatment-limiting adverse outcomes. This selection bias may overestimate positive outcomes and underestimate adverse outcomes. Finally, infant washout analysis included wide sampling windows with sparse time points.
In conclusion, standard darunavir/cobicistat dosing during pregnancy results in significantly lower exposure which may increase the risk of virologic failure and perinatal transmission, and this drug combination should be avoided during pregnancy.
Acknowledgements
We thank the study participants and their families. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH).
Site Acknowledgements
3801 Texas Children’s Hospital CRS (Shelley Buscher, BA, RN, CNM; Chivon McMullen-Jackson, RN, BSN; Mariam Pontifes, CCRP); 4001 Lurie Children’s Hospital of Chicago (Donna McGregor, APN; Patricia Garcia, MD; Sarah Sutton, MD); 4201 University of Miami Pediatric Perinatal HIV/AIDS CRS (Grace A. Alvarez, FMD, MPH, CCRP; Charles D. Mitchell, MD; Adriana Drada, FMD, CCRP); 5048 University of Southern California School of Medicine– Los Angeles County NICHD CRS (Françoise Kamer, MD; LaShonda Spencer, MD; James Homans, MD); 5112 David Geffen School of Medicine at UCLA CRS (Jaime G. Deville, MD; Michele F. Carter, RN; Carla Janzen, MD); 5114 Bronx-Lebanon Hospital Center CRS (Martha Cavallo, PNP; Marvin Alvarado, MD; Murli Purswani, MD); 6601 University of Puerto Rico Pediatric HIV/AIDS Research Program CRS (Carmen D. Zorrilla, MD; Juana Rivera MD; Jessica Ibarra, MD)
Support: Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH.
References
- 1.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission [September 30, 2019]; Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf.
- 2.Costantine MM. Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol 2014; 5:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39(7):512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mirochnick M, Capparelli E. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet 2004; 43(15):1071–1087. [DOI] [PubMed] [Google Scholar]
- 5.Stek A, Best BM, Wang J, Capparelli EV, Burchett SK, Kreitchmann R, et al. Pharmacokinetics of Once Versus Twice Daily Darunavir in Pregnant HIV-Infected Women. J Acquir Immune Defic Syndr 2015; 70(1):33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stek AM, Mirochnick M, Capparelli E, Best BM, Hu C, Burchett SK, et al. Reduced lopinavir exposure during pregnancy. AIDS 2006; 20(15):1931–1939. [DOI] [PubMed] [Google Scholar]
- 7.Acosta EP, Bardeguez A, Zorrilla CD, Van Dyke R, Hughes MD, Huang S, et al. Pharmacokinetics of saquinavir plus low-dose ritonavir in human immunodeficiency virus-infected pregnant women. Antimicrob Agents Chemother 2004; 48(2):430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tracy TS, Venkataramanan R, Glover DD, Caritis SN, National Institute for Child H, Human Development Network of Maternal-Fetal-Medicine U. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A Activity) during pregnancy. Am J Obstet Gynecol 2005; 192(2):633–639. [DOI] [PubMed] [Google Scholar]
- 9.Papageorgiou I, Grepper S, Unadkat JD. Induction of hepatic CYP3A enzymes by pregnancy-related hormones: studies in human hepatocytes and hepatic cell lines. Drug Metab Dispos 2013; 41(2):281–290. [DOI] [PubMed] [Google Scholar]
- 10.Rakhmanina NY, van den Anker JN, Soldin SJ. Safety and pharmacokinetics of antiretroviral therapy during pregnancy. Ther Drug Monit 2004; 26(2):110–115. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Liu Z, Brunzelle JS, Kovari IA, Dewdney TG, Reiter SJ, et al. The higher barrier of darunavir and tipranavir resistance for HIV-1 protease. Biochem Biophys Res Commun 2011; 412(4):737–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.HHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf.
- 13.Crauwels HM, Osiyemi O, Zorrilla C, Bicer C, Brown K. Reduced exposure to darunavir and cobicistat in HIV-1-infected pregnant women receiving a darunavir/cobicistat-based regimen. HIV Med 2019; 20(5):337–343. [DOI] [PubMed] [Google Scholar]
- 14.Tashima K, Crofoot G, Tomaka FL, Kakuda TN, Brochot A, Van de Casteele T, et al. Cobicistat-boosted darunavir in HIV-1-infected adults: week 48 results of a Phase IIIb, open-label single-arm trial. AIDS Res Ther 2014; 11:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curran A, Gutirerrez M, Deig E, Mateo G, Lopez RM, Imaz A, et al. Efficacy, safety and pharmacokinetics of 900/100 mg of darunavir/ritonavir once daily in treatment-experienced patients. J Antimicrob Chemother 2010; 65(10):2195–2203. [DOI] [PubMed] [Google Scholar]
- 16.Boyd SD, Sampson MR, Viswanathan P, Struble KA, Arya V, Sherwat AI. Cobicistat-containing antiretroviral regimens are not recommended during pregnancy: viewpoint. AIDS 2019; 33(6):1089–1093. [DOI] [PubMed] [Google Scholar]
- 17.Momper JD, Best BM, Wang J, Capparelli EV, Stek A, Barr E, et al. Elvitegravir/cobicistat pharmacokinetics in pregnant and postpartum women with HIV. AIDS 2018; 32(17):2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Momper JD, Stek A, Wang J, Shapiro DE, Smith E, Chakhtoura N, Capparelli EV, Mirochnick M, Best BM. Pharmacokinetics of atazanavir boosted with cobicistat during pregnancy and postpartum. 20th International Workshop on Clinical Pharmacology of HIV, Hepatitis and Other Antiviral Drugs. Noordwijk, Netherlands. May 16, 2019. [Google Scholar]
- 19.Molto J, Santos JR, Perez-Alvarez N, Cedeno S, Miranda C, Khoo S, et al. Darunavir inhibitory quotient predicts the 48-week virological response to darunavir-based salvage therapy in human immunodeficiency virus-infected protease inhibitor-experienced patients. Antimicrob Agents Chemother 2008; 52(11):3928–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Food and Drug Administration. Office of Clinical Pharmacology Review Prezista® (NDA: 202895). Available at: https://www.fda.gov/files/drugs/published/202895--Darunavir-Clinpharm-BPCA.pdf.
- 21.Mikula JM, Hsiao CB, Sawyer JR, Ma Q, Morse GD. Comparative Effectiveness of Darunavir 1,200 mg Daily and Approved Dosing Strategies for Protease Inhibitor-Experienced Patients. AIDS Res Treat 2013; 2013:687176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hakkola J, Raunio H, Purkunen R, Pelkonen O, Saarikoski S, Cresteil T, et al. Detection of cytochrome P450 gene expression in human placenta in first trimester of pregnancy. Biochem Pharmacol 1996; 52(2):379–383. [DOI] [PubMed] [Google Scholar]
- 23.Sarkar MA, Vadlamuri V, Ghosh S, Glover DD. Expression and cyclic variability of CYP3A4 and CYP3A7 isoforms in human endometrium and cervix during the menstrual cycle. Drug Metab Dispos 2003; 31(1):1–6. [DOI] [PubMed] [Google Scholar]
- 24.Prezcobix (darunavir and cobicistat) [package insert].Titusville, NJ: Janssen Pharmaceutical Companies; 2015. [Google Scholar]
- 25.Honda M, Omori Y, Minei S, Oshiyama T, Shimizu M, Sanaka M, et al. Quantitative analysis of serum alpha 1-acid glycoprotein levels in normal and diabetic pregnancy. Diabetes Res Clin Pract 1990; 10(2):147–152. [DOI] [PubMed] [Google Scholar]
- 26.Notarianni LJ. Plasma protein binding of drugs in pregnancy and in neonates. Clin Pharmacokinet 1990; 18(1):20–36. [DOI] [PubMed] [Google Scholar]