Abstract
The presence of anti-human leucocyte antigen (HLA) antibodies in the potential solid organ transplant recipient’s blood is one of the main barriers to access to a transplantation. The HLA sensitization is associated with longer waitlist time, antibody mediated rejection and transplant lost leading to increased recipient’s morbidity and mortality. However, solid organ transplantation across the HLA immunological barriers have been reported in recipients who were highly sensitized to HLA using desensitization protocols. These desensitization regimens are focused on the reduction of circulating HLA antibodies. Despite those strategies improve rates of transplantation, it remains several limitations including persistent high rejection rate and worse long-term outcomes when compare with non-sensitized recipient population. Currently, interest is growing in the development of new desensitization approaches which, beyond targeting antibodies, would be based on the modulation of alloimmune pathways. Plasma cells appears as an interesting target given their critical role in antibody production. In the last decade, CD38-targeting immunotherapies, such as daratumumab, have been recognized as a key component in the treatment of myeloma by inducing an important plasma cell depletion. This review focuses on an emerging concept based on targeting CD38 to desensitize in the field of transplantation.
Keywords: anti-CD38, daratumumab, HLA desensitization, DSA, solid organ transplantation
Introduction
HLA Sensitization and Antibody-Mediated Rejection
Solid organ transplantation (SOT) has become the best therapeutic option for end-stage organ disease but faces two major issues: the limited transplant supply and the poor long-term transplants outcome which have not improved over the past 30 years (1–3). This observation is related to the occurrence of antibody-mediated rejection (ABMR) which remains the death-censored leading cause of transplant loss across all solid organ transplants (3, 4). ABMR is defined on the association of histologic lesions (microvascular inflammation), histologic evidence of alloantibodies–endothelium interaction (c4d staining) and circulating donor-specific antibodies mostly directed against human leucocyte antigens (HLA) (3–10). Following blood transfusion, pregnancy or previous graft failure, candidates for organ transplantation can become sensitized against HLA and produce circulating anti-HLA antibodies (11, 12). In particular, pending on their properties donor-specific anti-HLA antibodies (DSA), are responsible for ABMR leading to allograft dysfunction and graft loss (13–18). Currently, immunomotoring of the transplant candidate’s is routinely performed in order to stratify the immunological risk by determining the presence and specificity of anti-HLA antibodies and potential DSA (11, 16). The highly sensitized patients have longer waitlist times with significant adverse effect on both quality and quantity of life (1, 2). Several strategies are applied to limit the time on the waiting list of highly immunized patients such as prioritization in transplant’s access, promotion of transplantation from living-donor allografts, development of kidney paired donation and desensitization.
Desensitization and Solid Organ Transplantation’s Outcome
Current desensitization strategies have been developed in kidney transplantation and extended to other solid organ transplantation (17–21). The goal of desensitization regimens in presensitized transplant candidates is twofold including the reduction of anti-HLA level to allow transplantation and the improvement of transplantation outcome through the prevention of ABMR (22). A stepwise approach is commonly used to desensitize including, (i) either high-dose intravenous immunoglobulin (IVIG) or low dose IVIG in association with plasmapheresis to remove antibodies and, (ii) anti-CD20 targeting agent, such as rituximab, to prevent rebound antibodies development by B cell depletion (23–27). Regarding the kidney transplantation field, despite the desensitizing effect, the subsequent transplantation is associated with higher rate of rejection and higher rate of hospital readmission after transplantation (28–30). However, long term outcomes for patient and graft survival have been reported to be similar to that of non-sensitized patients (31). Furthermore, the benefit of desensitization compared to remaining on the transplant waiting list has been evaluated only in few large studies and their results remain controversial (32, 33). Montgomery et al. and Orandi et al. reported a survival benefit at five years after kidney transplantation in 211 and 1025 desensitized patients respectively compared to patients remaining on the waiting list (34, 35). Interestingly, in a study performed on 213 desensitized recipients of living donor transplants, Manook et al. showed that desensitization was not associated with a survival benefit compared to matched sensitized control patients who were waitlisted (36). On the other hand, keeping patients a long time on dialysis represent a considerable financial burden while decreasing the quality and length of life for affected patients (32, 33).Thus, it appear as necessary to develop novel therapeutic approaches in order to prevent ABMR and improve long-term survival of transplanted organs in highly immunized recipient.
Desensitization Regimens Targeting Plasma Cells
The available therapeutic tools to manage the humoral response appears modestly successful in the context of SOT and alloimmunity. Indeed, antibody rebound due to plasma cells (PC), which do not express CD20, limit the efficacy of the most commonly used strategy combining IGIV, plasmapheresis and B cell depletion by anti-CD20 depleting agent. Targeting PC with new pharmacological tool from autoimmunity and cancer research could allow a better management of the humoral response in desensitization protocols (37). In the germinal center, after the enhancement of alloantigen responses by T follicular helper (Tfh), activated B cells develop into memory-B cells, progress to plasmablasts and ultimately to antibody-producing PC (38, 39). These PC are the long-lived mediators of lasting humoral immunity and persist in medullary niche where they can secrete high-affinity complement-activating DSAs (38, 40). Several emerging strategies aim to deplete PCs compartment in order to prevent ABMR (37, 41). First, Interleukin 6 (IL-6) is a cytokine promoting Tfh and enhancing the progression of B cells to high-affinity antibodies producing PC (42). Tocilizumab, a first-in-class humanized monoclonal antibody (mAb) with specificity for IL-6R, reduce inflammation within the allograft during ABMR in heart and kidney transplantation (43) and induce circulating DSA reduction (44). Clazakizumab is a humanized IgG1 mAb with specificity for IL6 which can also induce circulating DSA reduction (45). Both Tocilizumab and Clazakizumab are pharmacological agents with major interest in the development of desensitization strategies targeting PC (37, 46). On another hand, proteasome inhibitors represent one of the most promising solution to deplete PC in the setting of desensitization, targeting more selectively PCs population. Bortezomib and carfilzomib have been evaluated in desensitization trials, lacking control group, leading to controversial results (47, 48). Both induce significant PCs depletion whereas DSA level did not significantly decrease or rebound occurred rapidly. In fact, targeting PC may lead to rapid germinal center activation by deleting the negative feedback usually provided by PC and rebound humoral immunity and compensation (49). Therefore, dual targeting approach (combining PCs depletion with proteasome inhibitors and costimulation blockade) may silence the germinal center and prevent humoral compensation. This strategy has been recently evaluated using carfilzomib and belatacept as desensitization in highly sensitized non-human primate model with a reduction of bone marrow PC, DSA levels reduction, and prolongation of allograft survival. Most animals experienced ABMR with humoral-response rebound, suggesting desensitization must be maintained after transplantation using ongoing suppression of the B cell response (50, 51). An emerging therapy to induce DSA reduction and to prevent rebound DSA development is the use of antiplasma cell therapies such as anti-CD38, anti-CD19 or bispecific anti-CD3/anti-BCMA (B cell maturation antigen). In this review, we propose to focus on anti-CD38 as a desensitization regimen in SOT.
CD38-Targeting Strategies
CD38 and CD38-Targeting Antibodies
The protein CD38 is a type II transmembrane glycoprotein known as a multifunctional molecule. CD38 play dual roles as receptors and ectoenzymes (52). The CD38/CD31 interactions are crucial to leukocyte adhesion and transmigration through the endothelium (53). CD38 is also an enzyme that catalyzes several reactions leading to the regulation of cytoplasmic calcium fluxes and a wide range of others physiological functions such as cellular metabolism (52). CD38, found throughout the immune system especially natural killer and PC, is highly expressed in multiple myeloma cells (54). Altogether, this has triggered the development of several CD38 antibodies to treat multiple myeloma (54–56). Daratumumab (DARZALEX®, Janssen), fully human IgG1-kappa, was the first CD38 antibody that was recognized as an emerging therapy against myeloma in the last decade (57). Daratumumab have multiple effects including Fc-dependent immune-effector mechanisms and direct effects. The Fc-dependent immune-effector mechanisms include antibody-dependent cellular cytotoxicity, antibody-dependent cellular phagocytosis, and complement-dependent cytotoxicity (54, 55). Direct effects include induction of apoptosis, as well as inhibition of CD38 ectoenzyme function, which may lead to disruption of the PCs niche. Those Fc-dependent effects and direct effects are associated with deep and sustained CD38+ cells depletion, mostly PC and NK cells (54, 55, 58). The ability of daratumumab to efficacy deplete PCs compartment permit to use it as an new agent in therapeutic armamentarium for multiple myeloma (56). Large clinical trials have demonstrated significant improvements in the outcome of patients with relapsed multiple myeloma with use of daratumumab and it has been recently approved in front-line regimens (56–60). Isatuximab (SARCLISA®, Sanofi) is a chimeric IgG1-kappa which has stronger direct effects than daratumumab but lower ability to induce Fc-dependent immune-effector mechanisms, while it remains unknown whether these functional differences observed between different CD38 antibodies affect their therapeutic utility (55, 61). Many other strategies targeting CD38 are under development and a selection is listed in Table 1 . The CD38-targeting antibodies generally represent a safe treatment. Indeed, the most reported toxicity is infusion related reactions which remain successfully controlled by premedication and infusion rate management with low frequency of recurrence during subsequent injections (62). A higher rate of viral infections in patients treated with daratumumab has been reported in some studies leading to a recommended administration of valaciclovir during the administration of anti-CD38 antibodies (62).
Table 1.
Selection of therapeutical regimens targeting CD38.
| Anti-CD38 strategies | Nature and mechanism | Statut | NCT number |
|---|---|---|---|
|
Daratumumab
Janssen |
Fully human IgG1-kappa anti-CD38 mAb | Approved | X |
|
Isatuximab
Sanofi |
Chimeric IgG1-kappa anti-CD38 mAb | Approved | X |
|
Felzartamab - MOR202
MorphoSys AG |
Fully human IgG1-lambda anti-CD38 mAb | Ongoing in auto-immune field |
NCT04733040 NCT04145440 |
|
Mezagitamab - TAK-079
Takeda |
Fully human IgG1-lambda anti-CD38 mAb | Ongoing in hemato-oncology | NCT03439280 |
|
CID-103
CASI Pharmaceuticals |
Fully human IgG1 anti-CD38 mAb | Ongoing in hemato-oncology | NCT04758767 |
|
ISB 1342
Glennmark Phamaceuticals |
CD3xCD38 bispecific antibody to redirect cytotoxic potential of T cells to CD38+ cells | Ongoing in hemato-oncology | NCT03309111 |
|
TAK-169
Takeda |
Antibody drugs conjugates: anti-CD38 Ab fragment combined to a Shiga-like toxin (payload: ribosome inactivation) | Ongoing in hemato-oncology | NCT04017130 |
|
TAK-573
Takeda |
Antibody drugs conjugates: humanized IgG4 anti-CD38 mAb combined to interferon α (payload: anti-proliferative effects) | Ongoing in hemato-oncology | NCT03215030 |
|
²¹¹At-OKT10-B10
Fred Hutchinson Cancer Research Center |
Antibody drugs conjugates: anti-CD38 mAb combined to radioactive Astatine ²¹¹At (payload: radiation) | Ongoing in hemato-oncology |
NCT04579523 NCT04466475 |
|
STI-6129
Sorrento Therapeutics |
Antibody drugs conjugates: anti-CD38 mAb combined to Duostatin5 (payload: tubulin inhibition) | Ongoing in hemato-oncology | NCT04316442 |
|
KP1237
Kleo Pharmaceuticals |
Endogenous-antibodies recruiting molecule targeting CD38 in order to enhance antibody-dependent destruction mechanism | Ongoing in hemato-oncology | NCT04634435 |
|
Anti-CD38 CAR-T Cells
Sorrento Therapeutics |
Imunne cell therapy based on autologous T cells modified into anti-C38 CAR-T cells | Ongoing in hemato-oncology | NCT03464916 |
Immunomodulatory Effects of CD38-Targeting Antibodies
CD38-targeting antibodies have immunomodulatory effects such as improving the host-anti-tumor immune response (63). Krejcik et al. showed that daratumumab monotherapy against myeloma was associated with both CD4+ and CD8+ T cell expansion (64). This increase in T-helper cells and cytotoxic T-cell was associated with functional modification including elevated antiviral and alloreactive functional responses, and significantly greater increases in T-cell clonality as measured by T-cell receptor sequencing (63, 64). These modifications are associated with depletion of CD38+ immunosuppressive cells including regulatory T cells, regulatory B cells, and myeloid-derived suppressor cells. It is well known that such regulatory cells inhibit the host-anti-tumor immune response in the context of several malignancies including multiple myeloma (65–67). Altogether, this immunomodulatory activity of CD38 antibodies may be essential to their therapeutic efficacy. Indeed, it has been highlighted in clinical trials showing that expansion of effector T-cells and eradication of immune suppressors cells by daratumumab used against refractory and newly diagnosed multiple myeloma was correlated to a marked improvement in response and progression-free survival (57, 59, 63, 67). It might be hypothesized that these immunomodulatory abilities have important implication for sustained control of the tumor and further deepening of response (63). As a result of these pleiotropic immune modulation, CD38 antibodies also enhance anti-tumor activity of others anti-cancer drugs with several studies highlighting that CD38-targeting antibodies have strong synergistic activity, such as combination to lenalidomide as well as to PD1/PD-L1 inhibitors (56, 68). Besides effect on immune cells, CD38 antibodies may also modulate immunometabolic pathway. Indeed, CD38-targeting agent’s exposure could lead to lower adenosine level in tumoral microenvironment, which is known as immunosuppressive metabolite (69, 70). All these properties enhancing the anti-tumoral response are of major interest in the field of oncology while it could be problematic in immunosuppressive strategies such as autoimmune diseases treatment or desensitization and SOT’s context.
CD38 Antibodies in Solid Organ Transplantation
CD38 Antibodies in Non Tumoral Context
In the last decade, several strategies to handle with autoimmune or alloimmune pathologic situations include CD38 antibodies (71–73). Indeed, long-lived plasma cells, which produce pathogenic antibodies, are unresponsive to standard immunosuppression. Besides PC depletion and immunomodulatory effect, CD38 expression on PCs from patients with autoimmune condition (74) and reduction of auto-antibodies in patients exposed to daratumumab (75) support the evaluation of daratumumab in patients with autoantibody-dependent disorders and, in extension, to alloimmune situation such as SOT. Available evidence about CD38 antibodies efficacy in these situations are mostly cases reports of daratumumab use against immune cytopenia. Daratumumab were used to treat warm autoimmune hemolytic anemia post-hematopoietic stem cell transplant (76), refractory cold agglutinin disease (77), Evans syndrome (78) and pure red cell aplasia (79) with improvement in the majority of cases. Regarding other autoimmune disease, the administration of daratumumab in two patients with refractory lupus was recently described exhibiting clinical responses associated with significant depletion of long-lived plasma cells and modulation of effector T-cell responses (80). As regard as autoimmune encephalitis, targeting CD38 was achieved with daratumumab in one case of life-threatening anti-NMDA receptor encephalitis and in one case of refractory anti-CASPR2 encephalitis with improvements of neurological sequelae (81, 82). In the last case, severe septicemia leading to patient death highlight an unmet need of rigorous clinical investigation to determine the efficacy and tolerance of CD38-targeting agent in autoimmune disease.
CD38 Antibodies and ABMR Treatment
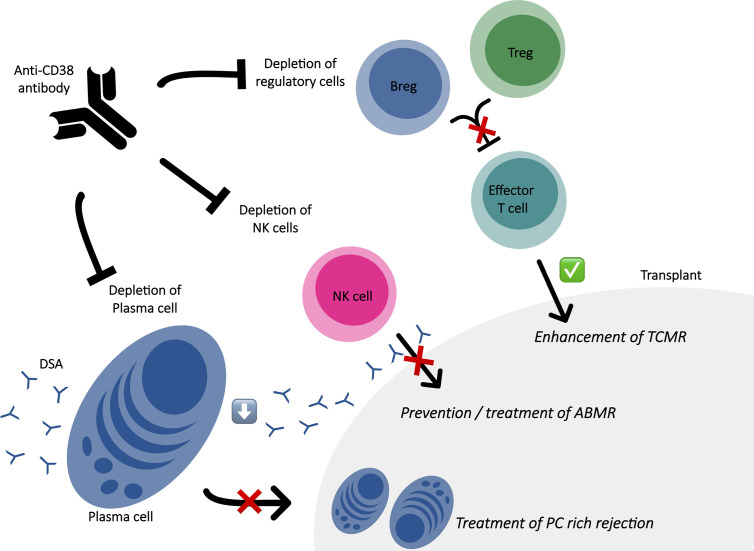
In antibody-mediated non-neoplastic diseases, alloimmune situation such as SOT represent a field where targeting CD38 is promising as shown in Figure 1 . As alloantibody-producing PC express CD38 at a higher level than other CD38+ hematopoietic cells and CD38 antibodies induce a profound depletion of CD38+ PC, CD38 appears as a rational target to handle with harmful alloantibodies such as DSA (83, 84). Currently, only few studies have been published regarding the use of CD38 antibodies for desensitization in patients awaiting transplantation or for treatment of ABMR as shown in Table 2 . Concerning treatment of ABMR, the first report was in a patient with refractory early active ABMR caused by anti-A isohemagglutinins after kidney transplantation from his ABO-incompatible sister (85). Based on the efficacy of daratumumab in the treatment of pure red cell aplasia following ABO-incompatible hematopoietic stem cell (79) and non-response of several therapies; daratumumab were tested as a rescue solution leading to a significant decrease of the pathogenic isohemagglutinins and resolution of tissue damage in the kidney biopsy. Kwun and colleagues also published a case report of daratumumab as a therapeutic strategy for refractory heart and kidney rejection in a patient who received heart and kidney transplants due to systemic lupus (72). Both transplant biopsy showed T cell–mediated rejection, ABMR and diffuse PC infiltration associated to the presence of several DSA. To face refractory cardiogenic shock and acute kidney failure dependent to dialysis, a compassionate use of daratumumab lead to the resolution of both allograft function, improvement in acute kidney lesions with decreased PCs infiltrate and dramatic decline for the majority of DSA. A recurrent acute PC-rich rejection on kidney biopsy and significant ascension of DSA were successfully managed with daratumumab. Recently, two others cases were reported: one refractory ABMR after a heart transplant successfully treated with daratumumab and one chronic active ABMR in a kidney allograft recipient diagnosed with myeloma exposed to daratumumab (73, 86). In the last one, the exhaustive immuno-monitoring showed that the main mode of action seems to be based on PC depletion, with profound PCs reduction in the bone marrow and peripheral blood and the abrogation of in vitro alloantibody production by PC enriched from bone marrow aspirates, leading to significant reduction in DSA levels (73). Another observation is that daratumumab led to depletion of NK cells infiltrating the allograft and circulating NK cells, which is major interest knowing the potential role of NK cells in microvasculature inflammation through engagement of their Fc gamma receptor IIIA with endothelium-bound DSA (87). Interestingly, while follow up biopsy showed resolution of humoral activity, it was observed tubulointerstitial inflammation which prompted steroid treatment. The author highlighted that the molecular signature of this infiltrate was not similar to signature of T-cell mediated rejection leading to question the trigger of this infiltrate not associated with graft dysfunction. Indeed, daratumumab may trigger T-cell alloresponse, even if circulating regulatory T cells were not reduced in the patient’s blood which is not necessarily correlated to the modification of immune cell populations at a tissue level. Moreover, the authors recently reported long term data of this case without evidence of ABMR rebound after daratumumab discontinuation (88). Although it is difficult to decipher the role of a rescue with daratumumab added to a complex antirejection therapy, a drug that specifically deplete PC with a favorable safety profile could represent a step forward in the field.
Figure 1.
Immune effects of anti-CD38 antibody in the context of solid organ transplantation. ABMR, antibody mediated rejection; Breg, regulatory B cell; DSA, donor specific antibodies; PC, plasma cell; TCMR, T cell mediated rejection; Treg, regulatory T cell.
Table 2.
CD38 antibody use in solid organ transplantation.
| ABMR Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|
| Réf. | Transplant | Sensitization | IS strategy | Immune event | Treatment | AntiCD38 use | Evolution | Observation |
| (72) | Heart + Kidney | Immunized: Preformed DSA |
- Induction: ATG -Maintenance: + Tacrolimus + MMF + Steroid |
-Delay post-Tx: 17 months -Clinical findings: Cardiogenic shock and acute kidney injury requiring dialysis -Anti-HLA: de novo DSA and one preformed DSA -Histology: TCMR and ABMR with PC-predominant infiltration in both transplants |
Steroid pulses + ATG + Plasmapheresis + IVIG + Rituximab + Eculizumab |
Daratumumab: - 16 mg/kg - 8 weekly infusions |
-Clinical: Heart allograft function returned to baseline + no more need of dialysis -Anti-HLA: Dramatic decline of MFI for majority of DSA at 3 months -Histology: Significant improvement in acute lesions and the PC infiltrate significantly decreased |
-20 weeks after: recurrent acute PC-rich rejection on kidney biopsy -Significant reascension of the MFI of two class 2 DSAs -New series of Daratumumab infusions with kidney allograft function improvement |
| (73) | Kidney | Immunized: Preformed DSA |
- Induction: ? -Maintenance: + Tacrolimus + MMF + Steroid |
-Delay post-Tx: 13 years -Clinical findings: Progressive graft dysfunction and proteinuria in the context of newly diagnosed myeloma -Anti-HLA: 1 DSA -Histology: chronic active ABMR |
None other treatment | Daratumumab: - 16 mg/kg - 8 weekly infusions + 8 fortnightly infusions + 1 monthly infusion thereafter for 9 months |
-Clinical: Stabilization of renal function and proteinuria -Anti-HLA: DSA levels became undetectable after 14 weeks -Histology: Abrogation of microvascular inflammation with a decrease of intragraft NK cells densities |
-3 months after: subclinical borderline rejection - High-grade tubulitis and mild interstitial infiltrates which were dominated by T-cells -Improvement with high-dose intravenous steroid. |
| (85) | Kidney | Immunized: ABOi (Anti-A) |
- Induction: + Basiliximab + Rituximab -Maintenance: + Tacrolimus + MMF + Steroid |
-Delay post-Tx: 30 days -Clinical findings: acute kidney failure -Antibodies: rise in Anti-A titers -Histology: ABMR |
Steroid pulses + ATG + Immunoadsorption + Eculizumab |
Daratumumab: - 16 mg/kg - 6 weekly infusions |
-Clinical: Recovering of kidney function at baseline -Anti-A: Reduction in Anti-A titers leading to discontinuation of immunoadsorption -Histology: No lesion |
|
| (86) | Heart | Immunized: History ABMR Preformed DSA |
- Induction: ? -Maintenance: + Tacrolimus + MMF + Steroid |
-Delay post-Tx: 13 years -Clinical findings: congestive heart failure -Anti-HLA: increase of DSA titers -Histology: ABMR |
Steroid pulses + Immunoadsorption |
Daratumumab: - 16 mg/kg - 8 weekly infusions + 8 fortnightly infusions + 1 monthly infusion thereafter for 9 months |
-Clinical: Renal and cardiac improvement in 4 weeks -Anti-HLA: DSA titers are only slightly reduced -Histology: No lesions |
|
| (72) | Preclinical: NHP |
Kidney | Daratumumab: -16 mg/kg -4 weekly infusions (8 weeks before Tx) |
Plerixafor (anti‐CXCR4): -0.24 mg/kg -same frequency |
Significant reduction of DSA levels and prolonged graft survival | None | Induction: anti-CD4 + anti-CD8 Maintenance: Tacrolimus + MMF + Steroid |
-Delayed ABMR -DSA rebound -TCMR -Reduction of Breg and Treg -Emergence of activated T cells after kidney transplantation in the desensitization group |
| (72) | Clinical | Heart | Daratumumab: -16 mg/kg -8 weekly infusions |
Plasmapheresis + high-dose IVIG + Rituximab |
Significant and persistent reduction of DSA levels and heart transplant access at 6 months | None | NA | Died from surgical complication |
ABMR, antibody mediated rejection; ATG, anti-human thymocytes globulins; DSA, donor specific antibodies; IVIG, intravenous immunoglobulins; MMF, mycophenolate mofetil; NHP, nonhuman primate; PC, plasma cells; Ref., reference; TCMR, T cell mediated rejection; Tx, transplantation.
CD38 Antibodies and Desensitization
The ability of CD38 to desensitize has been evaluated in both preclinical and clinical contexts and published in the same study (72). The preclinical study was based on the use of daratumumab in a non-human primate model which has the most biological similarity to humans for solid organ transplant biology (41, 89). The authors paired donors and recipients for maximal HLA mismatching and practiced, for allosensitization, two serial skin grafts before transplantation with a kidney from paired skin graft donor (72). Daratumumab and plerixafor (anti‐CXCR4), known to induce mobilization of PC from bone marrow to peripheral blood, were given as desensitization therapy with an initiation 8-12 weeks after sensitization and 8 weeks before kidney transplantation. Animals received for induction anti-CD4 and anti-CD8 antibodies and for maintenance immunosuppression tacrolimus, mycophenolate mofetil and a methylprednisolone taper. This desensitization regimen reduced significantly preformed DSA, with more than 50% reduction compared with the pretreatment time point, and prolonged graft survival with a depletion of PC without altering the germinal center response since the Tfh population was not eliminated (72). However, desensitized monkeys showed delayed ABMR associated to DSA rebound and T cell–mediated rejection perhaps due to immune deviation. Indeed, the authors observed a reduction of regulatory B and T cells after desensitization with rapid emergence of activated T cells after kidney transplantation. This observation could be related to immunomodulatory effects of daratumumab but CXCR4 inhibition, due to plerixafor, is also known to limit regulatory compartment and to promote effector cells with a potential role in these cell‐mediated rejection (90). Thus, in transplant recipients following desensitization with daratumumab, it would be interesting to elaborate new strategies than current immunosuppressive regimens in order to manage these DSA rebounds and the risk of T cell–mediated rejection. Concerning the clinical setting, the authors used daratumumab in a heart transplant candidate remaining highly sensitized after multiple courses of plasmapheresis, high-dose IVIG, and rituximab. It was observed a significant and persistent decrease of allosensitization allowing a heart transplantation six months after daratumumab infusion (72). Currently, based on these promising results, daratumumab are under investigation for desensitization in patients awaiting solid-organ transplantation in two clinical trial, one ruled by the nephrology department of Henri Mondor Hospital (Créteil, France) and another one directed by Stanford University [ClinicalTrials.gov, NCT04204980 and NCT04088903 (91, 92)]. Regarding the trial in kidney transplantation, sensitized patients with calculated panel reactive antibodies (cPRA) > 95% awaiting on the French National kidney allograft waiting-list for at least three years are eligible for the study and are randomly assigned to one of the two steps: (step 1) dose-escalation with 4 mg/kg of daratumumab weekly for four weeks, then with 8 mg/kg weekly for four weeks and then 16 mg/kg weekly for four weeks; (step 2) expansion cohort with eight weekly doses of 16 mg/kg. The primary outcomes are defined as: adverse events, intra-patient variation of cPRA and anti-HLA levels. Several other outcomes are also of interest such as percentage of patients engrafted, and intra-patient variation of ABO antibody titers (91).
Conclusion
Therapeutic improvement is required for both prevention and treatment of humoral alloresponse in solid organ transplantation. CD38 antibodies are a promising solution to profoundly deplete high affinity anti-HLA producing plasma cells. Preclinical and clinical experimental results suggests that daratumumab is a potentially therapeutic strategy to reduce DSA production and prevent and/or treat antibody-mediated rejection. However, CD38-targeting agent induce immune deviation which could be deleterious for solid organ transplants enhancing cellular-mediated rejection. Clinical studies are now needed to clarify the indications and efficacy of these promising therapeutic strategies.
Author Contributions
NJ, MM, and PG designed the review, collected and interpreted data from literature, and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Optn: Organ Procurement and Transplantation Network. OPTN. Available at: https://optn.transplant.hrsa.gov/. [Google Scholar]
- 2. Agence De La Biomédecine . Available at: https://www.agence-biomedecine.fr/.
- 3. Loupy A, Lefaucheur C. Antibody-Mediated Rejection of Solid-Organ Allografts. N Engl J Med (2018) 379:2579–82. 10.1056/NEJMra1802677 [DOI] [PubMed] [Google Scholar]
- 4. Valenzuela NM, Reed EF. Antibody-Mediated Rejection Across Solid Organ Transplants: Manifestations, Mechanisms, and Therapies. J Clin Invest (2017) 127(7):2492–504. 10.1172/JCI90597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bruneval P, Angelini A, Miller D, Potena L, Loupy A, Zeevi A, et al. The XIIIth Banff Conference on Allograft Pathology: The Banff 2015 Heart Meeting Report: Improving Antibody-Mediated Rejection Diagnostics: Strengths, Unmet Needs, and Future Directions. Am J Transplant (2017) 17(1):42–53. 10.1111/ajt.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schinstock CA, Askar M, Bagnasco SM, Batal I, Bow L, Budde K, et al. A 2020 Banff Antibody-Mediatedinjury Working Group Examination of International Practices for Diagnosing Antibody-Mediated Rejection in Kidney Transplantation - a Cohort Study. Transpl Int Off J Eur Soc Organ Transpl (2021) 34(3):488–98. 10.1111/tri.13813 [DOI] [PubMed] [Google Scholar]
- 7. Roux A, Levine DJ, Zeevi A, Hachem R, Halloran K, Halloran PF, et al. Banff Lung Report: Current Knowledge and Future Research Perspectives for Diagnosis and Treatment of Pulmonary Antibody-Mediated Rejection (AMR). Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg (2019) 19(1):21–31. 10.1111/ajt.14990 [DOI] [PubMed] [Google Scholar]
- 8. Drachenberg CB, Torrealba JR, Nankivell BJ, Rangel EB, Bajema IM, Kim DU, et al. Guidelines for the Diagnosis of Antibody-Mediated Rejection in Pancreas Allografts-Updated Banff Grading Schema. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg (2011) 11(9):1792–802. 10.1111/j.1600-6143.2011.03670.x [DOI] [PubMed] [Google Scholar]
- 9. Demetris AJ, Bellamy C, Hübscher SG, O’Leary J, Randhawa PS, Feng S, et al. 2016 Comprehensive Update of the Banff Working Group on Liver Allograft Pathology: Introduction of Antibody-Mediated Rejection. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg (2016) 16(10):2816–35. 10.1111/ajt.13909 [DOI] [PubMed] [Google Scholar]
- 10. Loupy A, Haas M, Roufosse C, Naesens M, Adam B, Afrouzian M, et al. The Banff 2019 Kidney Meeting Report (I): Updates on and Clarification of Criteria for T Cell- and Antibody-Mediated Rejection. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg (2020) 20(9):2318–31. 10.1111/ajt.15898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang R. Donor-Specific Antibodies in Kidney Transplant Recipients. Clin J Am Soc Nephrol CJASN (2018) 13(1):182–92. 10.2215/CJN.00700117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Butler CL, Valenzuela NM, Thomas KA, Reed EF. Not All Antibodies Are Created Equal: Factors That Influence Antibody Mediated Rejection. J Immunol Res (2017) 2017:7903471. 10.1155/2017/7903471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lefaucheur C, Viglietti D, Mangiola M, Loupy A, Zeevi A. From Humoral Theory to Performant Risk Stratification in Kidney Transplantation. J Immunol Res (2017) 2017:5201098. 10.1155/2017/5201098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lefaucheur C, Viglietti D, Bentlejewski C, Duong van Huyen J-P, Vernerey D, Aubert O, et al. IgG Donor-Specific Anti-Human HLA Antibody Subclasses and Kidney Allograft Antibody-Mediated Injury. J Am Soc Nephrol JASN (2016) 27(1):293–304. 10.1681/ASN.2014111120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen J-P, Mooney N, et al. Complement-Binding anti-HLA Antibodies and Kidney-Allograft Survival. N Engl J Med (2013) 369(13):1215–26. 10.1056/NEJMoa1302506 [DOI] [PubMed] [Google Scholar]
- 16. Montgomery RA, Tatapudi VS, Leffell MS, Zachary AA. HLA in Transplantation. Nat Rev Nephrol (2018) 14(9):558–70. 10.1038/s41581-018-0039-x [DOI] [PubMed] [Google Scholar]
- 17. Tinckam KJ, Keshavjee S, Chaparro C, Barth D, Azad S, Binnie M, et al. Survival in Sensitized Lung Transplant Recipients With Perioperative Desensitization. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg (2015) 15(2):417–26. 10.1111/ajt.13076 [DOI] [PubMed] [Google Scholar]
- 18. Plazak ME, Gale SE, Reed BN, Hammad S, Ton V-K, Kaczorowski DJ, et al. Clinical Outcomes of Perioperative Desensitization in Heart Transplant Recipients. Transplant Direct (2021) 7(2):e658. 10.1097/TXD.0000000000001111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bourassa-Blanchette S, Patel V, Knoll GA, Hutton B, Fergusson N, Bennett A, et al. Clinical Outcomes of Polyvalent Immunoglobulin Use in Solid Organ Transplant Recipients: A Systematic Review and Meta-Analysis - Part II: Non-Kidney Transplant. Clin Transpl (2019) 33(7):e13625. 10.1111/ctr.13625 [DOI] [PubMed] [Google Scholar]
- 20. Shah KS, Patel J. Desensitization in Heart Transplant Recipients: Who, When, and How. Clin Transpl (2019) 33(8):e13639. 10.1111/ctr.13639 [DOI] [PubMed] [Google Scholar]
- 21. Matsumoto CS, Rosen-Bronson S. Donor-Specific Antibody and Sensitized Patients in Intestinal Transplantation. Curr Opin Organ Transpl (2021) 26(2):245–9. 10.1097/MOT.0000000000000853 [DOI] [PubMed] [Google Scholar]
- 22. Schinstock CA, Smith BH, Montgomery RA, Jordan SC, Bentall AJ, Mai M, et al. Managing Highly Sensitized Renal Transplant Candidates in the Era of Kidney Paired Donation and the New Kidney Allocation System: Is There Still a Role for Desensitization? Clin Transplant (2019) 33(12):e13751. 10.1111/ctr.13751 [DOI] [PubMed] [Google Scholar]
- 23. Vo AA, Choi J, Cisneros K, Reinsmoen N, Haas M, Ge S, et al. Benefits of Rituximab Combined With Intravenous Immunoglobulin for Desensitization in Kidney Transplant Recipients. Transplantation (2014) 98(3):312–9. 10.1097/TP.0000000000000064 [DOI] [PubMed] [Google Scholar]
- 24. Vo AA, Peng A, Toyoda M, Kahwaji J, Cao K, Lai C-H, et al. Use of Intravenous Immune Globulin and Rituximab for Desensitization of Highly HLA-Sensitized Patients Awaiting Kidney Transplantation. Transplantation (2010) 89(9):1095–102. 10.1097/TP.0b013e3181d21e7f [DOI] [PubMed] [Google Scholar]
- 25. Vo AA, Lukovsky M, Toyoda M, Wang J, Reinsmoen NL, Lai C-H, et al. Rituximab and Intravenous Immune Globulin for Desensitization During Renal Transplantation. N Engl J Med (2008) 359(3):242–51. 10.1056/NEJMoa0707894 [DOI] [PubMed] [Google Scholar]
- 26. Loupy A, Suberbielle-Boissel C, Zuber J, Anglicheau D, Timsit M-O, Martinez F, et al. Combined Posttransplant Prophylactic IVIg/anti-CD 20/Plasmapheresis in Kidney Recipients With Preformed Donor-Specific Antibodies: A Pilot Study. Transplantation (2010) 89(11):1403–10. 10.1097/TP.0b013e3181da1cc3 [DOI] [PubMed] [Google Scholar]
- 27. Jordan SC, Toyoda M, Kahwaji J, Vo AA. Clinical Aspects of Intravenous Immunoglobulin Use in Solid Organ Transplant Recipients. Am J Transplant (2011) 11(2):196–202. 10.1111/j.1600-6143.2010.03400.x [DOI] [PubMed] [Google Scholar]
- 28. Amrouche L, Aubert O, Suberbielle C, Rabant M, Van Huyen J-PD, Martinez F, et al. Long-Term Outcomes of Kidney Transplantation in Patients With High Levels of Preformed Dsa: The Necker High-Risk Transplant Program. Transplantation (2017) 101(10):2440–8. 10.1097/TP.0000000000001650 [DOI] [PubMed] [Google Scholar]
- 29. Orandi BJ, Luo X, King EA, Garonzik-Wang JM, Bae S, Montgomery RA, et al. Hospital Readmissions Following HLA-incompatible Live Donor Kidney Transplantation: A Multi-Center Study. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg (2018) 18(3):650–8. 10.1111/ajt.14472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Motter JD, Jackson KR, Long JJ, Waldram MM, Orandi BJ, Montgomery RA, et al. Delayed Graft Function and Acute Rejection Following HLA-incompatible Living Donor Kidney Transplantation. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg (2021) 21(4):1612–21. 10.1111/ajt.16471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kahwaji J, Jordan SC, Najjar R, Wongsaroj P, Choi J, Peng A, et al. Six-Year Outcomes in Broadly HLA-sensitized Living Donor Transplant Recipients Desensitized With Intravenous Immunoglobulin and Rituximab. Transpl Int Off J Eur Soc Organ Transpl (2016) 29(12):1276–85. 10.1111/tri.12832 [DOI] [PubMed] [Google Scholar]
- 32. Süsal C, Opelz G. Transplantation: Desensitization and Survival in Kidney Transplant Recipients. Nat Rev Nephrol (2017) 13(4):196–8. 10.1038/nrneph.2017.24 [DOI] [PubMed] [Google Scholar]
- 33. Heidt S, Claas FHJ. Transplantation in Highly Sensitized Patients: Challenges and Recommendations. Expert Rev Clin Immunol (2018) 14(8):673–9. 10.1080/1744666X.2018.1498335 [DOI] [PubMed] [Google Scholar]
- 34. Montgomery RA, Lonze BE, King KE, Kraus ES, Kucirka LM, Locke JE, et al. Desensitization in HLA-incompatible Kidney Recipients and Survival. N Engl J Med (2011) 365(4):318–26. 10.1056/NEJMoa1012376 [DOI] [PubMed] [Google Scholar]
- 35. Orandi BJ, Luo X, Massie AB, Garonzik-Wang JM, Lonze BE, Ahmed R, et al. Survival Benefit With Kidney Transplants From HLA-Incompatible Live Donors. N Engl J Med (2016) 374(10):940–50. 10.1056/NEJMoa1508380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Manook M, Koeser L, Ahmed Z, Robb M, Johnson R, Shaw O, et al. Post-Listing Survival for Highly Sensitised Patients on the UK Kidney Transplant Waiting List: A Matched Cohort Analysis. Lancet Lond Engl (2017) 18 389(10070):727–34. 10.1016/S0140-6736(16)31595-1 [DOI] [PubMed] [Google Scholar]
- 37. Jordan SC, Ammerman N, Choi J, Huang E, Peng A, Sethi S, et al. The Role of Novel Therapeutic Approaches for Prevention of Allosensitization and Antibody-Mediated Rejection. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg (2020) 20 Suppl 4:42–56. 10.1111/ajt.15913 [DOI] [PubMed] [Google Scholar]
- 38. Leibler C, Thiolat A, Elsner RA, El Karoui K, Samson C, Grimbert P. Costimulatory Blockade Molecules And B-Cell-Mediated Immune Response: Current Knowledge and Perspectives. Kidney Int (2019) 95(4):774–86. 10.1016/j.kint.2018.10.028 [DOI] [PubMed] [Google Scholar]
- 39. Tangye SG, Ma CS, Brink R, Deenick EK. The Good, the Bad and the Ugly - TFH Cells in Human Health and Disease. Nat Rev Immunol (2013) 13(6):412–26. 10.1038/nri3447 [DOI] [PubMed] [Google Scholar]
- 40. Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM. The Generation of Antibody-Secreting Plasma Cells. Nat Rev Immunol (2015) 15(3):160–71. 10.1038/nri3795 [DOI] [PubMed] [Google Scholar]
- 41. Kwun J, Knechtle S. Experimental Modeling of Desensitization: What Have We Learned About Preventing AMR? Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg (2020) 20 Suppl 4:2–11. 10.1111/ajt.15873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brynjolfsson SF, Persson Berg L, Olsen Ekerhult T, Rimkute I, Wick M-J, Mårtensson I-L, et al. Long-Lived Plasma Cells in Mice and Men. Front Immunol (2018) 9:2673. 10.3389/fimmu.2018.02673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jordan SC, Ammerman N, Choi J, Kumar S, Huang E, Toyoda M, et al. Interleukin-6: An Important Mediator of Allograft Injury. Transplantation (2020) 104(12):2497–506. 10.1097/TP.0000000000003249 [DOI] [PubMed] [Google Scholar]
- 44. Choi J, Aubert O, Vo A, Loupy A, Haas M, Puliyanda D, et al. Assessment of Tocilizumab (Anti-Interleukin-6 Receptor Monoclonal) as a Potential Treatment for Chronic Antibody-Mediated Rejection and Transplant Glomerulopathy in HLA-Sensitized Renal Allograft Recipients. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg (2017) 17(9):2381–9. 10.1111/ajt.14228 [DOI] [PubMed] [Google Scholar]
- 45. Jordan SC, Ammerman N, Toyoda M, Huang E, Nast CC, Peng A, et al. Clazakizumab as an Agent to Reduce Donor Specific Hla Antibodies and Improve Outcomes in Patients With Chronic & Active Antibody-Mediated Rejection Post-Kidney Transplantation. ATC Abstracts. Available at: https://atcmeetingabstracts.com/abstract/clazakizumab-as-an-agent-to-reduce-donor-specific-hla-antibodies-and-improve-outcomes-in-patients-with-chronic-active-antibody-mediated-rejection-post-kidney-transplantation/. [Google Scholar]
- 46. Jordan SC. A Phase I/II Trial to Evaluate the Safety and Tolerability of Clazakizumab (Anti-Il-6 Monoclonal) to Eliminate Donor Specific Hla Antibodies (Dsas) and Improve Transplant Rates in Highly-HLA Sensitized Patients Awaiting Renal Transplant. clinicaltrials.gov; California: Cedars-Sinai Medical Center; (2020). Available at: https://clinicaltrials.gov/ct2/show/NCT03380962. Report No.: NCT03380962. [Google Scholar]
- 47. Woodle ES, Shields AR, Ejaz NS, Sadaka B, Girnita A, Walsh RC, et al. Prospective Iterative Trial of Proteasome Inhibitor-Based Desensitization. Am J Transplant (2015) 15(1):101–18. 10.1111/ajt.13050 [DOI] [PubMed] [Google Scholar]
- 48. Tremblay S, Driscoll JJ, Rike-Shields A, Hildeman DA, Alloway RR, Girnita AL, et al. A Prospective, Iterative, Adaptive Trial of Carfilzomib-Based Desensitization. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg (2020) 20(2):411–21. 10.1111/ajt.15613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kwun J, Burghuber C, Manook M, Iwakoshi N, Gibby A, Hong JJ, et al. Humoral Compensation After Bortezomib Treatment of Allosensitized Recipients. J Am Soc Nephrol JASN (2017) 28(7):1991–6. 10.1681/ASN.2016070727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ezekian B, Schroder PM, Mulvihill MS, Barbas A, Collins B, Freischlag K, et al. Pretransplant Desensitization With Costimulation Blockade and Proteasome Inhibitor Reduces DSA and Delays Antibody-Mediated Rejection in Highly Sensitized Nonhuman Primate Kidney Transplant Recipients. J Am Soc Nephrol JASN (2019) 30(12):2399–411. 10.1681/ASN.2019030304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schroder PM, Schmitz R, Fitch ZW, Ezekian B, Yoon J, Choi AY, et al. Preoperative Carfilzomib and Lulizumab Based Desensitization Prolongs Graft Survival in a Sensitized non-Human Primate Model. Kidney Int (2021) 99(1):161–72. 10.1016/j.kint.2020.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Quarona V, Zaccarello G, Chillemi A, Brunetti E, Singh VK, Ferrero E, et al. CD38 and CD157: A Long Journey From Activation Markers to Multifunctional Molecules. Cytometry B Clin Cytom (2013) 84B(4):207–17. 10.1002/cyto.b.21092 [DOI] [PubMed] [Google Scholar]
- 53. Dianzani U, Malavasi F. Lymphocyte Adhesion to Endothelium. Crit Rev Immunol (1995) 15(2):167–200. 10.1615/CritRevImmunol.v15.i2.40 [DOI] [PubMed] [Google Scholar]
- 54. van de Donk NWCJ, Usmani SZ. CD38 Antibodies in Multiple Myeloma: Mechanisms of Action and Modes of Resistance. Front Immunol (2018) 9:2134. 10.3389/fimmu.2018.02134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van de Donk NWCJ, Richardson PG, Malavasi F. CD38 Antibodies in Multiple Myeloma: Back to the Future. Blood (2018) 131(1):13–29. 10.1182/blood-2017-06-740944 [DOI] [PubMed] [Google Scholar]
- 56. van de Donk NWCJ, Pawlyn C, Yong KL. Multiple Myeloma. Lancet Lond Engl (2021) 397(10272):410–27. 10.1016/S0140-6736(21)00135-5 [DOI] [PubMed] [Google Scholar]
- 57. Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, et al. Targeting CD38 With Daratumumab Monotherapy in Multiple Myeloma. N Engl J Med (2015) 373(13):1207–19. 10.1056/NEJMoa1506348 [DOI] [PubMed] [Google Scholar]
- 58. Casneuf T, Xu XS, Adams HC, Axel AE, Chiu C, Khan I, et al. Effects of Daratumumab on Natural Killer Cells and Impact on Clinical Outcomes in Relapsed or Refractory Multiple Myeloma. Blood Adv (2017) 1(23):2105–14. 10.1182/bloodadvances.2017006866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med (2016) 375(14):1319–31. 10.1056/NEJMoa1607751 [DOI] [PubMed] [Google Scholar]
- 60. Touzeau C, Moreau P. Daratumumab for the Treatment of Multiple Myeloma. Expert Opin Biol Ther (2017) 17(7):887–93. 10.1080/14712598.2017.1322578 [DOI] [PubMed] [Google Scholar]
- 61. Lammerts van Bueren J, Jakobs D, Kaldenhoven N, Roza M, Hiddingh S, Meesters J, et al. Direct in Vitro Comparison of Daratumumab With Surrogate Analogs of CD38 Antibodies MOR03087, SAR650984 and Ab79. Blood (2014) 124(21):3474–4. 10.1182/blood.V124.21.3474.3474 [DOI] [Google Scholar]
- 62. Radocha J, van de Donk NWCJ, Weisel K. Monoclonal Antibodies and Antibody Drug Conjugates in Multiple Myeloma. Cancers (2021) 13(7):1571. 10.3390/cancers13071571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. van de Donk NWCJ. Immunomodulatory Effects of CD38-targeting Antibodies. Immunol Lett (2018) 199:16–22. 10.1016/j.imlet.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 64. Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, et al. Daratumumab Depletes CD38+ Immune Regulatory Cells, Promotes T-cell Expansion, and Skews T-cell Repertoire in Multiple Myeloma. Blood (2016) 128(3):384–94. 10.1182/blood-2015-12-687749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang L, Tai Y-T, Ho M, Xing L, Chauhan D, Gang A, et al. Regulatory B Cell-Myeloma Cell Interaction Confers Immunosuppression and Promotes Their Survival in the Bone Marrow Milieu. Blood Cancer J (2017) 7(3):e547. 10.1038/bcj.2017.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dwivedi S, Rendón-Huerta EP, Ortiz-Navarrete V, Montaño LF. CD38 and Regulation of the Immune Response Cells in Cancer. J Oncol (2021) 2021:6630295. 10.1155/2021/6630295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Adams HC, Stevenaert F, Krejcik J, Van der Borght K, Smets T, Bald J, et al. High-Parameter Mass Cytometry Evaluation of Relapsed/Refractory Multiple Myeloma Patients Treated With Daratumumab Demonstrates Immune Modulation as a Novel Mechanism of Action. Cytom Part J Int Soc Anal Cytol (2019) 95(3):279–89. 10.1002/cyto.a.23693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen L, Diao L, Yang Y, Yi X, Rodriguez BL, Li Y, et al. Cd38-Mediated Immunosuppression as a Mechanism of Tumor Cell Escape From PD-1/PD-L1 Blockade. Cancer Discovery (2018) 8(9):1156–75. 10.1158/2159-8290.CD-17-1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vijayan D, Young A, Teng MWL, Smyth MJ. Targeting Immunosuppressive Adenosine in Cancer. Nat Rev Cancer (2017) 17(12):709–24. 10.1038/nrc.2017.86 [DOI] [PubMed] [Google Scholar]
- 70. Chatterjee S, Daenthanasanmak A, Chakraborty P, Wyatt MW, Dhar P, Selvam SP, et al. Cd38-Nad+Axis Regulates Immunotherapeutic Anti-Tumor T Cell Response. Cell Metab (2018) 27(1):85–100.e8. 10.1016/j.cmet.2017.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zaninoni A, Giannotta JA, Gallì A, Artuso R, Bianchi P, Malcovati L, et al. The Immunomodulatory Effect and Clinical Efficacy of Daratumumab in a Patient With Cold Agglutinin Disease. Front Immunol (2021) 12:649441. 10.3389/fimmu.2021.649441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kwun J, Matignon M, Manook M, Guendouz S, Audard V, Kheav D, et al. Daratumumab in Sensitized Kidney Transplantation: Potentials and Limitations of Experimental and Clinical Use. J Am Soc Nephrol JASN (2019) 30(7):1206–19. 10.1681/ASN.2018121254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Doberer K, Kläger J, Gualdoni GA, Mayer KA, Eskandary F, Farkash EA, et al. Cd38 Antibody Daratumumab for the Treatment of Chronic Active Antibody-mediated Kidney Allograft Rejection. Transplantation (2021) 105(2):451–7. 10.1097/TP.0000000000003247 [DOI] [PubMed] [Google Scholar]
- 74. Cole S, Walsh A, Yin X, Wechalekar MD, Smith MD, Proudman SM, et al. Integrative Analysis Reveals CD38 as a Therapeutic Target for Plasma Cell-Rich Pre-Disease and Established Rheumatoid Arthritis and Systemic Lupus Erythematosus. Arthritis Res Ther (2018) 20(1):85. 10.1186/s13075-018-1578-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Frerichs KA, Verkleij CPM, Bosman PWC, Zweegman S, Otten H, van de Donk NWCJ. CD38-Targeted Therapy With Daratumumab Reduces Autoantibody Levels in Multiple Myeloma Patients. J Transl Autoimmun (2019) 2:100022. 10.1016/j.jtauto.2019.100022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tolbert VP, Goldsby R, Huang J, Shimano K, Melton A, Willert J, et al. Daratumumab Is Effective in the Treatment of Refractory Post-Transplant Autoimmune Hemolytic Anemia: A Pediatric Case Report. Blood (2016) 128(22):4819–9. 10.1182/blood.V128.22.4819.4819 [DOI] [Google Scholar]
- 77. Tomkins O, Berentsen S, Arulogun S, Sekhar M, D’Sa S. Daratumumab for Disabling Cold Agglutinin Disease Refractory to B-cell Directed Therapy. Am J Hematol (2020) 95(10):E293–5. 10.1002/ajh.25932 [DOI] [PubMed] [Google Scholar]
- 78. Blennerhassett R, Sudini L, Gottlieb D, Bhattacharyya A. Post-Allogeneic Transplant Evans Syndrome Successfully Treated With Daratumumab. Br J Haematol (2019) 187(2):e48–51. 10.1111/bjh.16171 [DOI] [PubMed] [Google Scholar]
- 79. Chapuy CI, Kaufman RM, Alyea EP, Connors JM. Daratumumab for Delayed Red-Cell Engraftment After Allogeneic Transplantation. N Engl J Med (2018) 08 379(19):1846–50. 10.1056/NEJMoa1807438 [DOI] [PubMed] [Google Scholar]
- 80. Ostendorf L, Burns M, Durek P, Heinz GA, Heinrich F, Garantziotis P, et al. Targeting CD38 With Daratumumab in Refractory Systemic Lupus Erythematosus. N Engl J Med (2020) 383(12):1149–55. 10.1056/NEJMoa2023325 [DOI] [PubMed] [Google Scholar]
- 81. Ratuszny D, Skripuletz T, Wegner F, Groß M, Falk C, Jacobs R, et al. Case Report: Daratumumab in a Patient With Severe Refractory Anti-NMDA Receptor Encephalitis. Front Neurol (2020) 11:602102. 10.3389/fneur.2020.602102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Scheibe F, Ostendorf L, Reincke SM, Prüss H, von Brünneck A-C, Köhnlein M, et al. Daratumumab Treatment for Therapy-Refractory anti-CASPR2 Encephalitis. J Neurol (2020) 267(2):317–23. 10.1007/s00415-019-09585-6 [DOI] [PubMed] [Google Scholar]
- 83. Martin TG, Corzo K, Chiron M, Velde HV de, Abbadessa G, Campana F, et al. Therapeutic Opportunities With Pharmacological Inhibition of CD38 With Isatuximab. Cells (2019) 8(12):1522. 10.3390/cells8121522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Flores-Montero J, de Tute R, Paiva B, Perez JJ, Böttcher S, Wind H, et al. Immunophenotype of Normal vs. Myeloma Plasma Cells: Toward Antibody Panel Specifications for MRD Detection in Multiple Myeloma. Cytometry B Clin Cytom (2016) 90(1):61–72. 10.1002/cyto.b.21265 [DOI] [PubMed] [Google Scholar]
- 85. Spica D, Junker T, Dickenmann M, Schaub S, Steiger J, Rüfli T, et al. Daratumumab for Treatment of Antibody-Mediated Rejection After ABO-Incompatible Kidney Transplantation. Case Rep Nephrol Dial (2019) 9(3):149–57. 10.1159/000503951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Aguilera Agudo C, Gómez Bueno M, Krsnik Castello I. Daratumumab for Antibody-Mediated Rejection in Heart Transplant-a Novel Therapy: Successful Treatment of Antibody-mediated Rejection. Transplantation (2021) 105(3):e30–1. 10.1097/TP.0000000000003505 [DOI] [PubMed] [Google Scholar]
- 87. Parkes MD, Halloran PF, Hidalgo LG. Evidence for CD16a-Mediated Nk Cell Stimulation in Antibody-Mediated Kidney Transplant Rejection. Transplantation (2017) 101(4):e102–11. 10.1097/TP.0000000000001586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mayer KA, Doberer K, Eskandary F, Halloran PF, Böhmig GA. New Concepts in Chronic Antibody-Mediated Kidney Allograft Rejection: Prevention and Treatment. Curr Opin Organ Transplant (2021) 26(1):97–105. 10.1097/MOT.0000000000000832 [DOI] [PubMed] [Google Scholar]
- 89. Kirk AD. Crossing the Bridge: Large Animal Models in Translational Transplantation Research. Immunol Rev (2003) 196:176–96. 10.1046/j.1600-065X.2003.00081.x [DOI] [PubMed] [Google Scholar]
- 90. Mollica Poeta V, Massara M, Capucetti A, Bonecchi R. Chemokines and Chemokine Receptors: New Targets for Cancer Immunotherapy. Front Immunol (2019) 10:379. 10.3389/fimmu.2019.00379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Matignon M, Joher N. Assistance Publique - Hôpitaux De Paris. Desensitization in Kidney Allograft Recipients Before Transplantation Using Daratumumab. clinicaltrials.gov; France: Créteil; (2020). Available at: https://clinicaltrials.gov/ct2/show/NCT04204980. Report No.: NCT04204980. [Google Scholar]
- 92. Witteles R. A Phase 1 Study of Daratumumab for Reduction of Circulating Antibodies in Patients With High Allosensitization Awaiting Heart Transplantation. clinicaltrials.gov; (2020). Available at: https://clinicaltrials.gov/ct2/show/NCT04088903. Report No.: NCT04088903. [Google Scholar]