Abstract
Objective
At present, it is not clear whether Mood Disorders (MD) and poor Health Related Quality of Life (HRQoL) in the glioma population correlate with features of the tumor, or rather with secondary symptoms associated with treatment. The aim of this study was to assess the prevalence of MD and decline in HRQoL in glioma patients, and to determine the main factors associated with these two variables.
Methods
80 patients affected by lower-grade gliomas (LGGs) and 65 affected by high-grade gliomas (HGGs) were evaluated, from admission up to 12 months after surgery, for MD, HRQoL, clinical characteristics, and cognitive functions. Independent factors associated with MD and low HRQoL were identified by using bivariate analysis.
Results
Data showed that prevalence of low HRQoL was comparable in both groups during all the time points assessed (pre, 1, 3, 6 and 12 months after surgery). In contrast at 6 months following surgery, HGGs showed a higher prevalence of MD compared to LGGs;. Bivariate analysis revealed that factors associated with MD and HRQoL in LGGs and HGGs were different over the course of the disease. In LGGs, from the pre-operative period to one year post surgery, MD and low HRQOL were associated with the occurrence of cognitive deficits and, from the third month after surgery onward, they were also associated with the effect exerted by adjuvant treatments. In HGGs, MD were associated with cognitive deficits at 3 and 6 months after surgery, along with older age (65-75 years); HRQoL, in its Physical component in particular, was associated with older age only from 6 months after surgery.
Conclusion
Factors associated with MD and low HRQoL were different in LGGs and HGGs over the course of the disease. In LGGs the effect of adjuvant treatments was prominent in determining the prevalence of both MD and poor HRQoL from the third month after surgery onward. In HGGs, MD and HRQoL were associated with age, at 3 and 6 months after surgery. In both, the occurrence of cognitive deficits was significantly associated with MD.
Keywords: mood disorders, health related quality of life, brain tumors, recovery, adjuvant treatments
Introduction
Gliomas are primary brain tumors that have a variable prognosis. Survival ranges widely, for patients with glioblastoma (15 months-2 years), other high-Grade Gliomas (HGGs) such as astrocytoma or oligodendroglioma anaplastic (3-10 years) or lower-grade gliomas (LGGs; 7-15 years) (1). Such variance depends on several factors, such as the biological behavior of the tumors, age of the patient, and treatment(s) adopted (2). Independent of prognosis, the diagnosis of brain tumor itself may create stress and significantly affects a person’s life due to the awareness of having a potentially fatal condition and to the complex treatments to be potentially/possibly adopted, along with its resulting side effects (3). Up to approximately 80% of brain tumor patients experience a variety of neurological or cognitive symptoms during the course of the disease (4–6), especially after treatments (7–9). Along with significant neurocognitive impairment, patients affected by brain tumors are also more likely to develop reactive mood disorders (MD) such as depression and/or anxiety, compared with healthy subjects or patients affected by other cancers not directly involving the central nervous system (CNS) (3, 10). MD is reported to range between 2.8% to 95% for depression, and between 13% to 60% for anxiety (11–13). Such wide variance may be associated with multiple factors, either clinical (treatment, histology, location) or functional (neurological and cognitive dysfunctions). Taken together, the alterations in physical, cognitive and psychological status, along with the side effects of the treatments can dramatically impact on the health related quality of life (HRQoL): i.e. “the subjective perception, influenced by the current health status (physical and mental) of the ability to cope with those activities important for the individual” (14). In fact, 45% of patients with a lower-grade glioma report a low global (poor) quality of life, with fewer than half of patients being able to carry out daily life activities without restriction (15).
Rather than depending on the grade of tumor, the effect on quality of life seems to be more related to the course of the tumor, whether it is stable or progressive, this supported by data suggesting that both LGG and HGG patients show a similar HRQoL when the stability of the clinical course is assured (16). However, in most cases there is a significant difference in prognosis and treatment, and patients with HGGs or LGGs may experience different treatment pathways (i.e. radiotherapy and closer follow-up for HGGs) that are unavoidably characterized by different distress profiles. Currently, tumor location (site and lobe), tumor aggressiveness, local disease control and adjuvant treatments are all considered to be factors associated with the development of mood disorders (17, 18), and are also crucial determinants of HRQoL (19).
Based on the evidence suggesting that both MD and HRQoL are associated with a short survival rate and poor medical compliance (7, 20, 21), there has been a progressive scientific effort in investigating factors affecting mood and quality of life in glioma patients.
However, despite the increasing number of studies focusing on these aspects, at present, available literature reports contradictory findings about the possible association of clinical and biological variables with the onset of MD and HRQoL, and particularly for MD, no definitive conclusions can yet be drawn (22, 23). In addition, separate data on low or high-grade gliomas are currently very limited or lacking (24).
Based on this premise, quantification of the prevalence of MD and poor HRQoL and identification of the main factors associated with these conditions in LGG and HGG patients is relevant, in order to provide an unbiased evaluation of patient’s cancer care outcomes (25). In this longitudinal study, we measured the prevalence of MD and of decline in HRQoL in a large sample of LGG and HGG patients, and we evaluated the main associated factors, focusing on demographic, clinical, anatomical, histological and functional variables, from diagnosis till 12 months after surgery. The aim was to quantify the prevalence of MD and low HRQoL and to identify, during the first year of the disease, the factors associated with the risk of developing MD or a decline in HRQoL. The identification of such predictors will help in planning specific interventions tailored to patients’ specific conditions, aimed at improving prognosis and care.
Materials and Methods
Participants and Data Collection
We prospectively enrolled 238 subjects selected from individuals consecutively admitted between January 2017 and October 2018 for a glioma resection at our Neurosurgical Oncology Unit. Inclusion criteria were: I) age ≥ 18 years; II) absence of severe comprehension deficits affecting the abilities to complete the questionnaires (comprehension abilities were tested before the administered MD and HRQoL questionnaires using standardized test (Token test) at each timepoint; III) absence of previous psychiatric symptoms or disease; IV) absence of current medications for psychiatric conditions; V) histo-molecular diagnosis of LGGs and HGGs and VI) newly diagnosed glioma with no history of treatments (surgery, chemo or radio – therapies). The LGG group included patients with a grade II and III IDH-mutated tumors; the HGG group, patients with a grade III-IV IDH-wildtype or IV IDH-mutated tumors. Tumor aggressiveness was classified upon histo-molecular profile.
The exclusion criterion was the absence of tumor progression during the assessment period, as evaluated by follow-up MRI and clinical evaluations or Tumor Board Discussion. If, at the time of the MD and HRQoL assessment (t0, t1, t2, t3 and t4), a progression of the disease was diagnosed, the patient was excluded even if he/she had performed any previous assessments. Based on the inclusion and exclusion criteria, 93 patients were excluded at the end of the study. The final sample was composed by 145 patients (see Table 1 for the clinical and demographics characteristics).
Table 1.
Frequency of the clinical and demographic characteristics of Lower (LGG) and High Grade Glioma (HGG) groups.
| LGG group (N 80) | HGG group (N. 65) | Significance (X2) | |||
|---|---|---|---|---|---|
| CLINICAL VARIABLES | % | N | % | N | |
| Histological Profile | |||||
| Astrocytoma | 30,0 | 24 | / | / | |
| Oligodendroglioma | 28,75 | 23 | / | / | |
| Gangoglioma | 17,5 | 14 | / | / | |
| Anaplastic Astrocitoma | / | / | 12,3 | 8 | |
| Anaplastic Oligodendroglioma | / | / | 18,5 | 12 | |
| Glioblastoma | / | / | 69,2 | 45 | |
| Other | 23,75 | 19 | / | / | |
| IDH | 0.001 | ||||
| Mutate | 73,8 | 59 | 33,8 | 22 | |
| Wildtype | 26,3 | 21 | 66,2 | 43 | |
| HEMISPHERIC LATERALITY | 0.335 | ||||
| Right | 53,8 | 43 | 33,8 | 22 | |
| Left | 46,3 | 37 | 66,2 | 43 | |
| LOBE AFFECTED | 0.107 | ||||
| Frontal | 51,2 | 41 | 27,7 | 18 | |
| Insular | 18,2 | 15 | 10,8 | 7 | |
| Temporal | 12,5 | 10 | 33,8 | 22 | |
| Parietal | 16,3 | 13 | 23,1 | 15 | |
| Other | 1,3 | 1 | 4,6 | 3 | |
| ADJUVANT THERAPY | 0 | ||||
| Chemotherapy | 57,5 | 46 | 98,5 | 64 | |
| Radiotherapy | 52,5 | 42 | 98,5 | 64 | |
| Gender | 0.33 | ||||
| Male | 57,5 | 46 | 64,6 | 42 | |
| Female | 42,5 | 34 | 35,4 | 23 | |
| Age | 0 | ||||
| Mean (SD) | 39,70 (11,3) | 51,2 (13,3) | |||
| Education | 0.975 | ||||
| Mean (SD) | 13,9 (3,01) | 13,7 (3,25) | |||
Clinical and demographics characteristics of Low Grade Glioma (LGG) and High Grade Glioma (HGG) groups. For each group and for each variables the percentage (%) total number (N) of subjects and X2 was reported.
For each patient, the clinical records were reviewed and the relevant data relative to tumor (type, grade, IDH-mutation, location), medications (anti-epileptic drugs -AEDs- and steroids) given before and during treatments, and adjuvant therapies (chemotherapy and radiotherapy) were recorded. For each patient we also reported the socio-demographics characteristics (age and level of education), personal or family history of psychiatric disorder and current or previous treatments with psychotropic medication. All patients gave written informed consent to the surgical and clinical procedure (IRB1299), which followed the principles outlined in the declaration of Helsinki.
Demographic and Clinical Features
One hundred and forty-five patients were enrolled. Sixty-four were HGGs and 81 LGGs. 60.7% were males; the frontal lobe (40.7%) and the left hemisphere (55.2%) were more frequently affected. 88.3% underwent a total resection and 11.7% a supra-total resection; 65.3% of patients (96.9% of HGGs and 40% of LGGs) received radiotherapy, starting 1 month after surgery, followed by adjuvant chemotherapy (Temozolamide-based standard regimen; average number of cycles: 9 (range 0-9) in HGGs and 6 (range 0-9) in LGGs. The choice of submitting a patient to adjuvant treatment was performed by Tumor Board upon histo-molecular phenotype. The majority of patients in both groups (90% of HGGs and 88% of LGGs) had seizures the year before surgery, while only 10% of HGGs and 7% of LGGs experienced (focal) seizures during the 12-months follow-up assessment. All patients were on antiepileptic drugs -AEDs - (Lacosamide and/or Levetiracetam) during the course of the disease; all patients used AEDs from the pre-operative period to 12 month after surgery, while only 50% of LGGs patients stopped treatment at 1 year. Steroids were used in all patients in the pre-operative period (desametasone, average 2 mg/day before surgery) and in the post-operative for an average of 13 days (inclusive of tapering) (average 4 mg/day); steroids were again given during the course of radiotherapy and tapered off after 10 days (average 8 mg/day). Clinical, and demographic characteristics of the participants are summarized in Table 1 .
Study Design
All patients were submitted to a preoperative (1 week before surgery - t0) and four post-operative (1 month (t1), 3 months (t2), 6 months (t3) and 12 months (t4) after surgery) extensive assessments.
At each time point, patients’ functional outcomes were assessed by neurological evaluation and standardized neuropsychological tests for five domains: language, praxis abilities, attentive, executive and memory functions ( Table 2 ). Based on the assessment, we recorded: 1) neurological deficits (motor and visual impairment) and 2) cognitive deficits. In each patient the raw score of each test was corrected for patient age and educational level to obtain a normalized score subsequently compared with the distribution of scores of an Italian control population: scores falling into the worst 5th percentile of the scores observed in the controls were defined as pathological [see (26) for an extensive description of the procedure]. A specific cognitive domain was considered to be impaired when at least one of the corresponding test scores was defined as pathological [for similar approach see (27, 28)].
Table 2.
Neuropsychological assessment.
| DOMAINS | Test |
|---|---|
| LANGUAGE | -Token Test |
| -Picture Naming test | |
| -Verbal Fluency (Phonemic and semantic) | |
| ATTENTION AND EXECUTIVE FUNCTIONS | -Attentive Matrice |
| -Trail Making Test | |
| -Stroop Test | |
| MEMORY | -Digit span backward |
| -Rey’s Auditory Verbal Learning Test | |
| -Recall Rey figure | |
| PRAXIS | -Ideomotor apraxia test |
| -Oro-facial apraxia test |
For each cognitive domain the test used is reported.
Outcome Measures
At each time point, patients were also assessed with two self-report questionnaires evaluating whether the patient was experiencing MD (anxiety and depression symptoms) or poor HRQoL.
Among the available screening tools for the evaluation of MD in cancer population the “Hospital Anxiety and Depression Scale” [HADS; (29)] was used. HADS contain fourteen-items assessing Anxiety and Depression symptoms. Patients were asked to check the sentence that best represents their feelings at the time of evaluation. Each item scored from 0 to 3 (overall score 42 points). A cut-off total score of ≥ 10 identified moderate symptoms of MD in accordance with the Italian version (30). The prevalence of mood disorders based on the HADS total cut-off scores was recorded and analyzed statistically.
HRQoL was evaluated by using the “Short Form 36 items Health survey (SF36)” (31) of the Medical Outcomes Study (MOS), a largely validated instrument for assessing HRQoL. SF36 was found reliable for assessing HRQoL in the general population and in oncological patients, including those with brain cancer (32). It consists of 36 questions measuring two different dimensions: the Physical Component Summary (PCS) assessing the role limitations due to physical problems, pain and general health, and the Mental Component Summary (MCS) assessing the role limitations caused by emotional problems, vitality, social functioning, and mental health (see 31 for scoring techniques). The PCS and MCS scores are easier to interpret statistically than the eight subscale scores, due to smaller confidence intervals, and lower floor and ceiling effects. The total scores for both components ranged from 0 to 100 and higher scores indicate better HRQoL. By following the procedure previously used to assess outcome in the oncological population3, the scores of the two components were compared with the average score recorded in the general control population (see 31 for an extensive description of the statistical procedure). Patients with a score of one standard deviation (SD: 10) below the average score of the Italian control group (PCS: 50 MCS: 49.6) were classified as patients with low HRQoL (31).
Statistical Analyses
Statistical analyses were performed using IBM SPSS Statistics Software 20. Descriptive statistics were used for the clinical and demographic features of the sample. Differences in prevalence of cognitive deficits, MD, and low HRQoL between LGG and HGG groups, and between lower grade patients treated and untreated with adjuvant therapy, were assessed for each time point (t0, t1, t2, t3 and t4) by 2x2 Chi Square tests (see Figures 1 – 3 ). In both LGG and HGG groups, the association of different functional and clinical variables with MD and HRQoL was investigated by bivariate analyses: for each time point (t0, t1, t2, t3 and t4), 2x2 Chi Square tests was performed to test association between mood disorder (MD), the Physical component of HRQoL (PCS) or Mental Components of HRQoL (MCS) and demographics, clinical, histo-molecular factors (age, sex, education level, affected lobe and hemisphere, adjuvant therapies adopted and IDH mutation) and functional factors (cognitive and neurological deficits) of each group (LGGs vs HGGs). Significant associations are shown in Table 3 and 4 . In accordance with Chi-squared Test assumptions, the associations showing expected cell frequencies smaller than five were not considered (33). Given the exploratory nature of the study, in order to avoid dramatically increase the chance of Type II errors we did not apply corrections for multiple comparisons (34, 35).
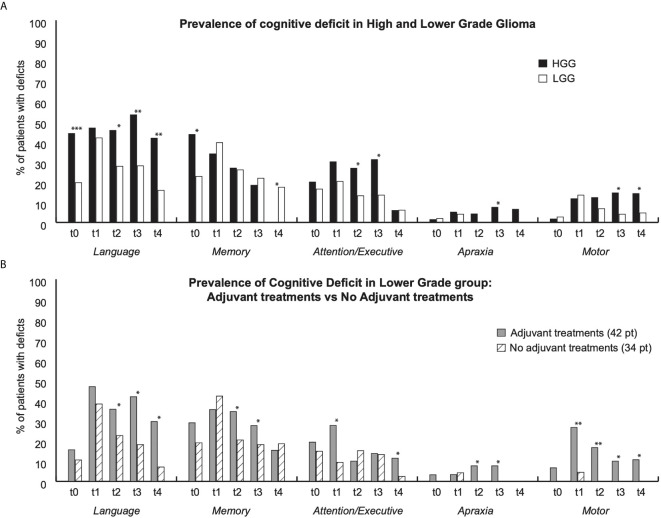
Figure 1.
Figure shows prevalence of functional deficits for each group and for each time points (t0: pre surgery; t1:1month after surgery; t2: 3 months after surgery; t3: 6 months after surgery; t4: 12 months after surgery). (A) Prevalence of deficit in High Grade glioma (HGG) (Black bars) and in lower grade (White Bars); (B) Prevalence of deficits in Lower patients who underwent adjuvant treatments (grey bars) vs patients who did not undergo adjuvant treatment (white dashed bars). * indicate p < 0,05; ** p < 0,01; *** p < 0.001.
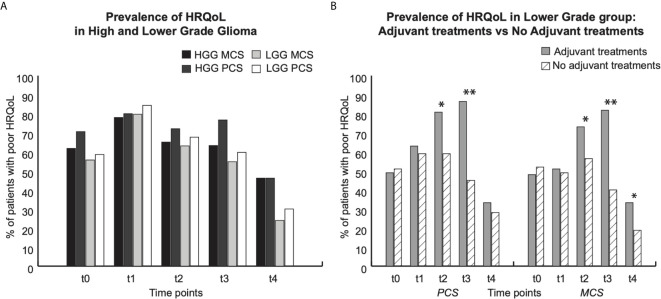
Figure 3.
Prevalence of low HRQoL (SF36 score). For each group, graphs show data at each time point: t0 (pre surgery); t1 (1 month after surgery); t2, t3 and t4 (respectively 3, 6 and 12 months after surgery). * indicate p < 0,05; ** p < 0,01. (A) Prevalence of low HRQoL in High (black and dark grey bars) and Lower (light grey and white bras) group. Dark Black and light grey bars show prevalence of Mental component score (MCS); light black and white bars show prevalence of Physical component score (PCS). (B) Prevalence of low HRQoL in lower grade group: Grey bars patients who underwent adjuvant treatments, dashed white bars: patients who did not undergo adjuvant treatments.
Table 3.
Factors associated with mood disorders in lower (LGGs) and High Grade Glioma (HGG).
| LOWER GRADE GROUP | HIGH GRADE GROUP | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor associated | Prevalence of MD % | X2(df=1) | P value | Factor associated | Prevalence of MD % | X2(df=1) | P value | ||
| t0 | Language | 4,305 | 0,038 | t0 | – | – | – | ||
| Deficit | 60 | – | – | – | |||||
| Normal | 24 | – | – | – | |||||
| t1 | Motor | 4,002 | 0,045 | t1 | – | – | – | ||
| Deficit | 88 | – | – | – | |||||
| Normal | 50 | – | – | – | |||||
| t2 | Language | 3,625 | 0,046 | t2 | Language | 4,359 | 0,037 | ||
| Deficit | 80 | Deficit | 84 | ||||||
| Normal | 44 | Normal | 54 | ||||||
| Adjuvant Treatments | 5,354 | 0,021 | Affected Hemisphere | 7,430 | 0,011 | ||||
| Yes | 70 | Left | 70 | ||||||
| No | 40 | Right | 31 | ||||||
| t3 | Language | 5,617 | 0,018 | t3 | Language | 3,758 | 0,048 | ||
| Deficit | 72 | Deficit | 90 | ||||||
| Normal | 39 | Normal | 48 | ||||||
| Adjuvant Treatments | 8.487 | 0,004 | Attentive/Executive | 4,306 | 0,046 | ||||
| Yes | 74 | Deficit | 100 | ||||||
| No | 32 | Normal | 67 | ||||||
| Age | 8,036 (df=2) | 0,038 | |||||||
| Young | 20 | ||||||||
| Adult | 33 | ||||||||
| Old | 67 | ||||||||
| t4 | Language | 5,939 | 0,015 | t4 | – | – | – | ||
| Deficit | 80 | – | – | – | |||||
| Normal | 37 | – | – | – | |||||
| Attentive/Executive | 3,968 | 0,046 | – | – | – | ||||
| Deficit | 100 | – | – | – | |||||
| Normal | 67 | – | – | – | |||||
| Adjuvant Treatments | 6,941 | 0,008 | – | – | – | ||||
| Yes | 69 | – | – | – | |||||
| No | 30 | – | – | – | |||||
For each time points (t0: pre surgery; t1:1 month after surgery; t2:3 months after surgery; t3. 6 months after surgery; t4: 1 year after surgery) and for each group (Low grade group (LGG) in the left part; and High grade glioma (HGG) on the right side) factors associated with prevalence of Mood Disorders (MD), was reported. Blank cells: no factors were statistically associated with the prevalence of MD. df, degree of freedom; X2, chi square value.
Table 4.
Factors Associated with low HRQoL in High and Lower grade glioma group.
| HIGH GRADE GROUP | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mental Component Score | Physical Component Score | |||||||
| Factors Associated | Prevalence of low MCS % | X2(df=1) | P value | Factors Associated | Prevalence of low PCS % | X2(df=2) | P value | |
| T0 | – | – | – | – | – | – | ||
| T1 | – | – | – | – | – | – | ||
| T2 | – | – | – | – | – | – | ||
| T3 | – | – | – | Age | – | 6,242 |
0,001 | |
| – | – | Young | 12 | |||||
| – | – | Adult | 21 | |||||
| Old | 78 | |||||||
| T4 | – | – | – | – | – | – | ||
| LOWER GRADE GROUP | ||||||||
| Mental Component Score | Physical Component Score | |||||||
| Factors Associated |
Prevalence of
low MCS % |
X2
(df=1) |
P value | Factors Associated |
Prevalence of
low PCS % |
X2
(df=1) |
P value | |
| T0 | – | – | – | – | – | – | ||
| T1 | – | – | – | – | – | – | ||
| T2 | – | – | – | Adjuvant Treatments | 6,232 | 0,013 | ||
| – | – | – | Yes | 74 | ||||
| – | – | – | No | 46 | ||||
| – | – | – | Language | 5,021 | 0,025 | |||
| – | – | – | Deficit | 90 | ||||
| – | – | – | Normal | 61 | ||||
| T3 | Adjuvant Treatments | 10,022 | 0,002 | Adjuvant Treatments | 10,092 | 0,001 | ||
| Yes | 80 | Yes | ||||||
| No | 37 | No | ||||||
| Language | 6,025 | 0,014 | Language | 3,869 | 0,049 | |||
| Deficit | 79 | Deficit | 90 | |||||
| Normal | 45 | Normal | 61 | |||||
| T4 | Language | 10,254 | 0,007 | Language | 7,350 | 0,014 | ||
| Deficit | 71 | Deficit | 79 | |||||
| Normal | 14 | Normal | 52 | |||||
For each time points (t0: pre surgery; t1:1 month after surgery; t2:3 months after surgery; t3. 6 months after surgery; t4: 1 year after surgery) and for each group (High grade glioma in the upper part of the table; Low grade glioma in the lower part of the table) factors associated with low Mental (left part) and Physical (right part) component of the Health Related Quality of Life (HRQoL) was reported. Blank cells: no factors were statistically associated with the prevalence of HRQoL.
df= degree of freedom; X2= chi square value.
Results
Prevalence of Cognitive Deficits
In order to better describe the sample and to collect data about the cognitive and neurological status of the patient we calculated the prevalence of deficits in HGG and LGG patients ( Figure 1A ) and in LGG patients that underwent the adjuvant treatment vs LGG patients that were not treated ( Figure 1B ).
Compared to LGG patients, HGG patients had a higher prevalence of preoperative language (44% of HGG vs 20% of LGGs) and memory (44% of HGGs vs 23% of LGGs) impairment. At the first time-point after surgery (1 month) the prevalence of cognitive deficits was similar in both groups in all the five domains assessed. Three (t2) and six months (t3) after surgery the prevalence of some deficits was higher in the HGG group with respect to LGG group, specifically: at t2, language (HGG 46% vs LGG 28%) and attentive/executive deficits (HGG 27% vs LGG 13%); at t3, language (HGG 53% vs 28%), attentive/executive deficits (HGG 31% vs 14%), apraxia (HGG 7,5% vs LGG 0%) and motor deficit (HGG 15% vs LGG 4%). One year after surgery LGG and HGG differ only for language deficits (HGG 42% vs LGG 16%), motor deficits (HGG 14% vs LGG 5%) and memory deficits (HGG 0% vs LGG 14%). Overall, data seems to suggest that HGG patients had a higher prevalence of deficits compared to LGGs at specific time points, except for memory deficits, recorded at 1 year ( Figure 1A ).
Moreover, we compared patients treated with adjuvant therapies (LGGt) with those not treated (LGGnt) in the LGG group. There was a difference between the two groups from the t1 time point onward. Specifically, at t1 (1 month after surgery and before starting the adjuvant therapies) the LGGt group had a higher prevalence of attentive/executive (28% vs 9%) and motor deficits (27% vs 5%); than LGGnt group. At t2 and t3 (3 and 6 months after surgery) the LGGt group showed a higher prevalence of language (t2 36% vs 23%; t3 42% vs 18%), memory (t2 35% vs 21%; t3 28% vs 18%); apraxia (t2 and t3 8% vs 0%) and motor deficits (t2 17% vs 0%; t3 10% vs 0%) with respect to the LGGnt group. One year after surgery, prevalence of language (30% vs 7%) and motor deficits (11% vs 0%) was higher in the LGGt group than in LGGnt group. (see Figure 1B ).
Prevalence of Mood Disorders and of Low Health Related Quality of Life
For each time point, the prevalence of pathological score in HADS and in SF36 questionnaires for (Physical (PCS) or Mental (MCS) component of HRQoL was separately presented, for both HGGs and LGGs ( Figures 2 and 3A ) and for LGG patients that underwent adjuvant treatments vs LGG patients that were not treated with adjuvant therapies ( Figures 2 and 3B ).
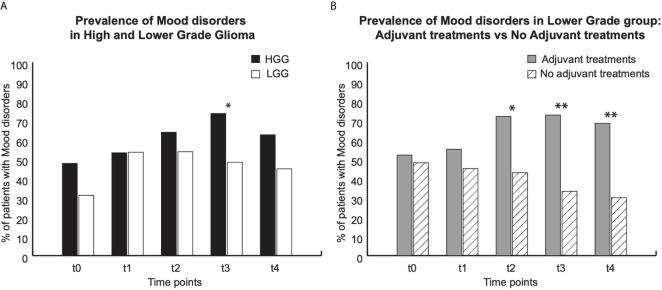
Figure 2.
Prevalence of Mood disorders (HADS score). For each group, graphs show data at each time point: t0 (pre surgery); t1 (1 month after surgery); t2, t3 and t4 (respectively 3, 6 and 12 months after surgery). (A) Prevalence of MD in High grade Glioma (HGG) (Black bars, and in lower grade glioma (white bars). (B) Prevalence of MD in lower grade group: gray bars patients underwent to adjuvant treatments, white dashed bars patients who did not undergo adjuvant treatments. * indicate p < 0,05; ** p < 0,01.
Prevalence of Mood Disorders (MD)
MD were detected in both HGG and LGG patients at each time point that was assessed ( Figure 2A ). Before surgery (t0) and at 1(t1), 3(t2) and 12(t4) months after surgery no differences were found in the prevalence of MD between LGG and HGG patients. At these time points, prevalence of MD was as follows: (t0) 31% LGG vs 48% HGG; (t1) 54% LGG vs 53% HGG; (t2) 54% LGG vs 64% HGG; (t4) 45% LGG vs 63% HGG. Only 6 months after surgery (t3) we found there to be a prevalence of MD that was higher in HGG than in the LGG group (74% HGG vs 48% LGG) ( Figure 2A ). Following 3 months after surgery the prevalence of MD was higher in those LGG patients submitted to radiotherapy and chemotherapy (t2 72% vs 43%; t3 73% vs 33%; t4 68% vs 30%), with respect to the patients in which a “wait and see” approach was adopted ( Figure 2B ).
Prevalence of Low HRQoL
Before surgery about 60% of LGGs patients and 70% of HGGs patients showed a low score in both the mental (MCS) and physical (PCS) components of the HRQoL questionnaire ( Figure 3A ). At 1 month after surgery (t1), the prevalence of low HRQoL (for both mental and physical components) was around 80% in both groups (80%MCS and 84%PCS in LGG vs 78%MCS and 80%PCS in HGG; see Figure 3A ); at 3 and 6 months after surgery, the prevalence of low scores in both components of HRQoL was around 65% (at t2) and 57% (at t3) in LGG patients (MCS t2: 63% t3 55%; PCS: t2 68%; t3 60%) and around 68% (at t2) and 70% (at t3) in HGG (MCS: t2 65%, t3 63; PCS: t2 72%; t3 77%), reaching the lowest percentage at 1 year after surgery in both groups (24%MSC and 30% PSC in LGGs vs 46% for both components in HGG) ( Figure 3A ). It is of note that there are no difference that were statistically significant, between the LGG and HGG groups.
Notably, in LGG patients, the prevalence of low HRQoL was significantly higher in those who underwent adjuvant treatments (radiotherapy/chemotherapy) in comparison to those who were just followed-up: at 3 and 6 months for the Physical and Mental components of HRQoL (PCS 81% vs 59%; MCS 73% vs 56% at t2; PCS 87% vs 45%: MCS 82% vs 40% at t3;) and also at 1 year after surgery for the Mental component only (33% vs 19%).
Variables Associated With Mood Disorders and Health Related Quality of Life
LGG Patients
Mood Disorders
At admission, MD was associated with the occurrence of language deficits while at 1 month after surgery, MD was associated with motor deficits (MD documented in 60% of patients with language deficits and in 88% of patients with motor deficits, in comparison to 24% and 50% of patients who did not experience language or motor deficits). During the subsequent course of the disease (from 3 months (t2) to 6 months (t3) after surgery), the prevalence of MD was associated with language deficits (t2:80% of patients with deficit vs 44% of patients without; t3:72% vs 39%), and with the administration of adjuvant treatments (t2: 70% of patients who underwent to adjuvant treatments vs 40% of those who were not; t3 74% vs 32%; At 12 months after surgery (t4), we found significant associations again with language deficits (80% of patients with deficit vs 37% of patients without) and adjuvant treatments (69% who underwent to adjuvant treatments vs 30% of those who were not), but also with attentive/executive deficits (100% of patients with deficit vs 67% of patients without) ( Table 3 ).
Health Related Quality of Life
At admission as well as at 1 month after surgery, low scores on the mental (MCS) and physical (PCS) components of HRQOL were not associated with any variable. On the contrary, at 3 months after surgery the low score in the Physical component of HRQoL was associated with language deficits (recorded in 90% of patients with deficits vs 61% of patients without) and undergoing adjuvant treatments (74% vs 46%); at 6 months after surgery low scores for both Mental and Physical components were associated with adjuvant treatments (PCS: 80% vs 36%; MCS: 80% vs 37%) and language deficits (PCS: 90% vs 61%; MCS: 79% vs 45%). Finally, at 12 months after surgery patients with language deficits showed a higher prevalence of low scores in both Physical and Mental components (PCS: 79% vs 52; MCS 71% vs 14%) (see Table 4 for details).
HGGs Patients
Mood Disorders
At admission, 1 month and 12 months after surgery, MD in HGGs were not associated with any variable. At 3 months after surgery, MD were associated with language deficits (recorded in 84% of patients with deficits vs 54% of patients without) and with the affected hemisphere (70% in patients with a left hemisphere tumor vs 32% in those with a right hemisphere tumor). At 6 months after surgery along with language deficits (MD recorded in 90% of patients with language deficits vs 48% of patients without), MD was also associated with attentive/executive deficits (100% vs 67%) and older age (67% in patients >65 years vs 20% in 25-35 year old patients vs 33% in 36-65 year old patients) ( Table 3 ).
Health Related Quality of Life
Both components of HRQoL (MCS and PCS) were associated with older age (78% in patients >65 years; 12% in 25-35 year old patients; 21% in 36-65 year old patients) ( Table 4 ).
Discussion
This work addresses the prevalence and time course of MD and HRQoL in LGGs and HGGs patients. It provides new insight into the secondary effect of the brain tumor on MD and HRQoL, through assessment of the clinical or functional factors that possibly contribute to their occurrence during the first year of the disease (from the pre surgical period up to 12 months after diagnosis).
The analysis was performed on a group of patients treated for a glioma who were stable for the disease during the observation time of the study, without previous or current psychiatric disorders and in a clinical condition allowing them to successfully complete the questionnaires provided. This was done to rule out the possible influence exerted by the progression of the disease and previous psychological disorders on the prevalence of MD and HQoL disorders recorded during the observation period.
The first interesting finding is the surprisingly high prevalence of poor HRQoL and MD symptoms since diagnosis, in both HGG and LGG group. Moreover, the prevalence of low HRQoL did not differ between HGG and LGGs, confirming previous observation (16) reporting that quality of life is not affected by the tumor grade per sè but by the stability of the disease over time. This hypothesis may be supported by the fact that in both groups (HGG and LGG) the prevalence of poor HRQoL recorded at 1 year after surgery was lower than at 6 months, possibly because the absence of progression allows the patient to recover from the adopted treatments. On the contrary, by the 6 month following surgery, LGG patients showed a significantly lower prevalence of MD than the high-grade patients which may suggest a potential effect of the tumor or of the different treatment approaches adopted for the management of the disease or a combined effect of the two variables. Despite the effect of treatment, it is difficult to disentangle this within the HGG group given that all patients are generally submitted to adjuvant treatments, and analyses performed within the LGG group seems to support the effect exerted by the adjuvant treatment on the prevalence of MD and HRQoL. In fact, significant differences were found in the prevalence of MD and poor HRQoL between LGGs patients who underwent adjuvant treatments at the 3 month post surgery time point and the patients that were submitted to observation only.
Bivariate analysis confirms the hypothesis that tumor aggressiveness and the adoption of therapy affect patient psychological status and the quality of life, and also highlights the effect exerted by the cognitive deficit. HGGs patients developing language deficits or affected by left hemisphere tumors experienced a higher prevalence of MD at 3 months after surgery, while at 6 months the cognitive deficits (language, attentive/executive) and older age (>65-75 years) were associated with MD. In the LGG group, the patient’s cognitive status, specifically motor, language and attentive/executive deficits, was the main factor associated with the prevalence of MD at each time point, and that influenced the course of both components of HRQoL, starting of 3 months after surgery. From the same time point, adjuvant treatments were also associated with MD and poor HRQoL.
Our findings differ from previous studies reporting no difference in mood symptoms among patients with different WHO tumor grades and no association between cognitive performance and MD (36). However, in these studies, the assessment of MD was conducted either before surgery or immediately after (7, 21, 23, 37) or at a much later stage of the follow-up, in the outpatient clinic (12, 22) in addition, by using less sensitive neuropsychological tests. As reported by Rooney and colleagues (24) it is in the intervening period, i.e. 3-6 months after surgery, the tumor grade might be expected to mediate differences in mood; it is in this period that patients can react differently to their different prognoses and to different treatment approach but also to functional recovery.
Our findings are in line with previous evidence reporting that when a detailed neuropsychological testing is used, MD is associated with cognitive impairment (38, 39). This is not surprising considering the impact that cognitive dysfunction exerts on patient’s functional performance, leading to an impairment of self-sufficiency. Regarding the lack of associations between specific single functional factors and poor HRQoL in HGG patients, we theorizes that these results may be related to the complex interplay of multiple deficits typically observed in these patients, which prevent the identification of the role of each individual factor. Moreover, HGG patients are generally older and submitted in all cases to adjuvant treatments. In itself, this may lead to a more difficult recovery and prevent patients from reaching a quality of life that is comparable to that experienced in the pre-operative phase.
In contrast to previous findings that report a relevant role of the right hemisphere in predicting the occurrence of MD (7, 12, 24) and low HRQol (40, 41), our data suggested that at 3 months after surgery, the prevalence of MD was associated with the presence of a tumor in the left hemisphere. This may be explained by the fact that the occurrence of MD was associated with that of language impairment (usually observed in patients with a tumor in the dominant hemisphere), which is associated with functional limitations and impaired the self-sufficiency.
These results point out the need to preserve the patient’s functional integrity using the best available treatments (surgical techniques and adjuvants therapies) (42–45).
The absence of significant association with clinical variables (such as histology, IDH-status, or tumor location) does not exclude that tumor-related factors play an indirect role in the etiology of MD or poor HRQoL. However, given that the prevalence of MD and HRQoL changes over time (showing a decrease especially at the long-term compared to baseline), whereas the tumor location, IDH-status, extent of resection and adopted treatments did not change, suggests that prevalence of MD and HRQoL overtime is due to additional reasons. These premises, supports a multifactorial genesis of the mood status and the subjective perception of how the disease affects an individual patients’ quality of life; it seems clear that a wide variety of factors are relevant over the course of the disease and at the individual patient level, all possibly contributing to mood status and the QoL experience.
Additional longitudinal studies are needed to better understand the patient’s psychological status and quality of life, especially for patients affected by severe cognitive deficits (for example language impairment) that make it hard to administer standardized questionnaire on mood or quality of life. The first unavoidable limitation is related to the difficulty of collecting a complete longitudinal setting of data after one year due to a high prevalence of disease progression and severe cognitive morbidity in HGG. Moreover, although the relationships between MD, HRQoL and adjuvant treatments seems to be evident, it is hard to definitively disentangle the effects of chemo/radio-therapy treatments from the role of the tumor itself, given that all the HGG patients undergo radiotherapy with concomitant adjuvant temozolomide. Further research that accounts for confounding factors is necessary in order to define specific treatment-related symptoms and their contribution to decreased HRQOL.
Other issues, such as individual differences (personality), the social context and the relationship between the HRQoL and the emotional reactions of caregivers and patients, the sense of uncertainty about treatment and life expectancy need to be also explored. The implementation of qualitative research (such as focus group studies) into research practice may allow us to obtain this crucial information also about patients affected by severe deficits or with relapse, in order to understand other psychological aspects contributing to the poor prognosis and poor HRQoL. More specifically, patients vulnerable to MD or decline in HRQoL might be identified at an earlier stage, which would allow for personalized and timely psychological interventions.
Despite these limitations, some conclusions can be drawn for HRQoL and MD in brain tumor patients. Specifically, our data show that the prevalence of MDs and low HRQoL is unexpectedly high, especially during the first months after surgery and in patients undergoing adjuvant treatment. As a matter of fact, the assessment of MD and HRQoL as primary or secondary end points must be systematically addressed in glioma patients because of their intrinsic significant prognostic value. An improvement in the patient’s well-being with in turn increase the overall survival.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: Data will be promptly available upon reasonable request to the authors. Requests to access these datasets should be directed to antonellaleonetti80@gmail.com.
Ethics Statement
The studies involving human participants were reviewed and approved by Humanitas Research Hospital Irb1299. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conceived the study: AL, GP, and LB. Selected patients, directed, and executed the surgical procedure and the intraoperative brain mapping: BL. Acquired data, analyzed data: AL, GP, MRo, FL, LB, HH, LV, MC, LG, MR, and ST. Produced figures: AL, LV, MRi, and HH. Discussed and interpreted the results: all the authors. Wrote the first draft of the paper: AL, GP, LB, and GC. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grant from “Associazione Italiana per la Ricerca sul Cancro (AIRC)” 18842 to LB.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Stupp R, Brada M, van den Bent MJ, Tonn JC, Pentheroudakis G. ESMO Guidelines Working Group. High-Grade Glioma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2014) 25 Suppl 3:iii93–101. 10.1093/annonc/mdu050 [DOI] [PubMed] [Google Scholar]
- 2. Rossi M, Gay L, Ambrogi F, Nibali MC, Sciortino T, Puglisi G, et al. Association of Supratotal Resection With Progression-Free Survival, Malignant Transformation, and Overall Survival in Lower-Grade Gliomas. Neuro Oncol (2020). 10.1093/neuonc/noaa225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goebel S, AM S, Kaup L, von H, HM M. Distress in Patients With Newly Diagnosed Brain Tumours. Psychooncology (2011) 20:623–630 8. 10.1002/pon.1958 [DOI] [PubMed] [Google Scholar]
- 4. Giovagnoli AR. Investigation of Cognitive Impairments in People With Brain Tumors. J Neurooncol (2012) 108:277–83. 10.1007/s11060-012-0815-6 [DOI] [PubMed] [Google Scholar]
- 5. Taphoorn MJB, Klein M. Cognitive Deficits in Adult Patients With Brain Tumours. Lancet Neurol (2004) 3:159–68. 10.1016/S1474-4422(04)00680-5 [DOI] [PubMed] [Google Scholar]
- 6. Mukand JA, Blackinton DD, Crincoli MG, Lee JJ, Santos BB. Incidence of Neurologic Deficits and Rehabilitation of Patients With Brain Tumors. Am J Phys Med Rehabil (2001) 8(5):346–50. 10.1097/00002060-200105000-00005 [DOI] [PubMed] [Google Scholar]
- 7. Litofsky NS, Farace E, Anderson F, Meyers CA, Huang W, Laws ER. Depression in Patients With High-Grade Glioma: Results of the Glioma Outcomes Project. Neurosurgery (2004) s54:358–67. 10.1227/01.NEU.0000103450.94724.A2 [DOI] [PubMed] [Google Scholar]
- 8. Tucha O, Smely C, Preier M, Lange KW. Cognitive Deficits Before Treatment Among Patients With Brain Tumors. Neurosurgery (2000) 47:324–333; discussion 333-334. 10.1097/00006123-200008000-00011 [DOI] [PubMed] [Google Scholar]
- 9. Klein M. Neurocognitive Functioning in Adult WHO Grade II Gliomas: Impact of Old and New Treatment Modalities. Neuro Oncol (2012) 14:17–24. 10.1093/neuonc/nos161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Janda M, Eakin EG, Bailey L, Walker D, Troy K. Supportive Care Needs of People With Brain Tumours and Their Carers. Support Care Cancer (2006) 14(11):1094–103. 10.1007/s00520-006-0074-1 [DOI] [PubMed] [Google Scholar]
- 11. Palese A, Cecconi M, Moreale R, Skrap M. Pre-Operative Stress, Anxiety, Depression and Coping Strategies Adopted by Patients Experiencing Their First or Recurrent Brain Neoplasm: An Explorative Study. Stress Heal (2012) 28:416–25. 10.1002/smi.2472 [DOI] [PubMed] [Google Scholar]
- 12. Arnold SD, Forman LM, Brigidi BD, Carter KE, Schweitzer HA, Quinn HE, et al. Evaluation and Characterization of Generalized Anxiety and Depression in Patients With Primary Brain Tumors. Neuro Oncol (2008) 10:171–81. 10.1215/15228517-2007-057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Skarstein J, Aass N, Fosså SD, Skovlund E, Dahl AA. Anxiety and Depression in Cancer Patients: Relation Between the Hospital Anxiety and Depression Scale and the European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire. J Psychosom Res (2000) 49:27–34. 10.1016/S0022-3999(00)00080-5 [DOI] [PubMed] [Google Scholar]
- 14. Romero M, Vivas-Consuelo D, Alvis-Guzman N. Is Health Related Quality of Life (HRQol) a Valid Indicator for Health Systems Evaluation? Springerplus (2013) 2(1):1–7. 10.1186/2193-1801-2-664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taphoorn MJB, Schiphorst AK, Snoek FJ, Lindeboom J, Wolbers JG, Karim ABMF, et al. Cognitive Functions and Quality of Life in Patients With Low-Grade Gliomas: The Impact of Radiotherapy. Ann Neurol (1994) 3(1):48–54. 10.1002/ana.410360111 [DOI] [PubMed] [Google Scholar]
- 16. Liu R, Page M, Solheim K, Fox S, Chang SM. Quality of Life in Adults With Brain Tumors: Current Knowledge and Future Directions. Neuro Oncol (2009) 11(3):330–9. 10.1215/15228517-2008-093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jenkins LM, Drummond KJ, Andrewes DG. Emotional and Personality Changes Following Brain Tumour Resection. J Clin Neurosci (2016) 29:128–32. 10.1016/j.jocn.2015.12.007[doi [DOI] [PubMed] [Google Scholar]
- 18. Jenkins L, Andrewes D. Social Cognition in Patients Following Surgery to the Prefrontal Cortex. Psychiatry Res Neuroimaging (2014) 224:192–203. 10.1016/j.pscychresns.2014.08.007 [DOI] [PubMed] [Google Scholar]
- 19. Jalali R, Dutta D. Factors Influencing Quality of Life in Adult Patients With Primary Brain Tumors. Neuro Oncol (2012) 14(suppl_4):iv8–16. 10.1093/neuonc/nos205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bosma I, Reijneveld JC, Douw L, Vos MJ, Postma TJ, Aaronson NK, et al. Health-Related Quality of Life of Long-Term High-Grade Glioma Survivors. Neuro Oncol (2009) 11:51–8. 10.1215/15228517-2008-049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mainio A, Tuunanen S, Hakko H, Niemelä A, Koivukangas J, Räsänen P. Decreased Quality of Life and Depression as Predictors for Shorter Survival Among Patients With Low-Grade Gliomas: A Follow-Up From 1990 to 2003. Eur Arch Psychiatry Clin Neurosci (2006) 256:516–21. 10.1007/s00406-006-0674-2 [DOI] [PubMed] [Google Scholar]
- 22. Pelletier G, Verhoef MJ, Khatri N, Hagen N. Quality of Life in Brain Tumor Patients: The Relative Contributions of Depression, Fatigue, Emotional Distress, and Existential Issues. J Neurooncol (2002) 57:41–9. 10.1023/A:1015728825642 [DOI] [PubMed] [Google Scholar]
- 23. Irle E, Peper M, Wowra B, And Kunze S. Mood Changes After Surgery for Tumors of the Cerebral-Cortex. Arch Neurol (1994) 51:164–74. 10.1001/archneur.1994.00540140070017 [DOI] [PubMed] [Google Scholar]
- 24. Rooney AG, Carson A, Grant R. Depression in Cerebral Glioma Patients: A Systematic Review of Observational Studies. J Natl Cancer Inst (2011) 103(1):61–76. 10.1093/jnci/djq458 [DOI] [PubMed] [Google Scholar]
- 25. Mauer M, Stupp R, Taphoorn MJ, Coens C, Osoba D, Marosi C, et al. The Prognostic Value of Health-Related Quality-of-Life Data in Predicting Survival in Glioblastoma Cancer Patients. Br J Cancer (2007) 97:302–7. 10.1038/sj.bjc.6603876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Capitani E, Laiacona M. Outer and Inner Tolerance Limits: Their Usefulness for the Construction of Norms and the Standardization of Neuropsychological Tests. Clin Neuropsychol (2017) 31(6-7):1219–30. 10.1080/13854046.2017.1334830 [DOI] [PubMed] [Google Scholar]
- 27. Puglisi G, Sciortino T, Rossi M, Leonetti A, Fornia L, Conti Nibali M, et al. Preserving Executive Functions in Non-Dominant Frontal Lobe Glioma Surgery: An Intraoperative Tool. J Neurosurg (2018) 28:1–7. 10.3171/2018.4.JNS18393 [DOI] [PubMed] [Google Scholar]
- 28. Capitani E, Laiacona M. Composite Neuropsychological Batteries and Demographic Correction: Standardization Based on Equivalent Scores, With a Review of Published Data. the Italian Group for the Neuropsychological Study of Ageing. J Clin Exp Neuropsychol (1997) 19:795–809. 10.1080/01688639708403761 [DOI] [PubMed] [Google Scholar]
- 29. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale (HADS). Acta Psychiatr Scand (1983) 67:361–70. 10.1016/S0016-5085(01)83173-5 [DOI] [PubMed] [Google Scholar]
- 30. Costantini M, Musso M, Viterbori P, Bonci F, Del Mastro L, Garrone O, et al. Detecting Psychological Distress in Cancer Patients: Validity of the Italian Version of the Hospital Anxiety and Depression Scale. Support Care Cancer (1999) 7(3):121–7. 10.1007/s005200050241 [DOI] [PubMed] [Google Scholar]
- 31. Apolone G, Mosconi P. The Italian SF-36 Health Survey: Translation, Validation and Norming. J Clin Epidemiol (1998) 51:1025–36. 10.1016/S0895-4356(98)00094-8 [DOI] [PubMed] [Google Scholar]
- 32. Bunevicius A. Reliability and Validity of the SF-36 Health Survey Questionnaire in Patients With Brain Tumors: A Cross-Sectional Study. Health Qual Life Outcomes (2017) 15(1):1–7. 10.1186/s12955-017-0665-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McHugh ML. The Chi-Square Test of Independence. Biochem Med (2012) 23:143–9. 10.11613/BM.2013.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Althouse AD. Adjust for Multiple Comparisons? It’s Not That Simple. Ann Thorac Surg (2016) 101(5):1644–5. 10.1016/j.athoracsur.2015.11.024 [DOI] [PubMed] [Google Scholar]
- 35. Feise RJ. Do Multiple Outcome Measures Require P-Value Adjustment? BMC Med Res Methodol (2002) 2:8. 10.1186/1471-2288-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brown PD, Ballman KV, Rummans TA, Maurer MJ, Sloan JA, Boeve BF, et al. Prospective Study of Quality of Life in Adults With Newly Diagnosed High-Grade Gliomas. J Neurooncol (2006) 76(3):283–91. 10.1007/s11060-005-7020-9 [DOI] [PubMed] [Google Scholar]
- 37. Gathinji M, McGirt MJ, Attenello FJ, Chaichana KL, Than K, Olivi A, et al. Association of Preoperative Depression and Survival After Resection of Malignant Brain Astrocytoma. Surg Neurol (2009) 71:299–303. [DOI] [PubMed] [Google Scholar]
- 38. Armstrong CL, Goldstein B, Cohen B, Jo MY, Tallent EM. Clinical Predictors of Depression in Patients With Low-Grade Brain Tumors: Consideration of a Neurologic Versus a Psychogenic Model. J Clin Psychol Med Settings (2002) 9(2):97–107. 10.1023/A:1014987925718 [DOI] [Google Scholar]
- 39. Fox S, Lyon D, Farace E. Symptom Clusters in Patients With High-Grade Glioma. J Nurs Scholarsh (2007) 39(1):61–7. 10.1111/j.1547-5069.2007.00144.x [DOI] [PubMed] [Google Scholar]
- 40. Rooney AG, McNamara S, MacKinnon M, Fraser M, Rampling R, Carson A, et al. The Frequency, Longitudinal Course, Clinical Associations, and Causes of Emotional Distress During Primary Treatment of Cerebral Glioma. Neuro Oncol (2013) 15:635–43. 10.1093/neuonc/not009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Giovagnoli AR, Silvani A, Colombo E, Boiardi A. Facets and Determinants of Quality of Life in Patients With Recurrent High Grade Glioma. J Neurol Neurosurg Psychiatry (2005) 76:562–8. 10.1136/jnnp.2004.036186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rossi M, Sciortino T, Conti Nibali M, Gay L, Viganò L, Puglisi G, et al. Clinical Pearls and Methods for Intraoperative Motor Mapping. Neurosurgery (2021) 88(3):457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Conti Nibali M, Leonetti A, Puglisi G, Rossi M, Sciortino T, Gay LG, et al. Preserving Visual Functions During Gliomas Resection: Feasibility and Efficacy of a Novel Intraoperative Task for Awake Brain Surgery. Front Oncol (2020). 10.3389/fonc.2020.01485. *co first authors [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mandonnet E, Herbet G, Duffau H. Letter: Introducing New Tasks for Intraoperative Mapping in Awake Glioma Surgery: Clearing the Line Between Patient Care and Scientific Research. Clin Neurosurg (2020) 86(2):E256–7. 10.1093/neuros/nyz447 [DOI] [PubMed] [Google Scholar]
- 45. Rossi M, Conti Nibali M, Viganò L, Puglisi G, Howells H, Gay L, et al. Resection of Tumors Within the Primary Motor Cortex Using High-Frequency Stimulation: Oncological and Functional Efficiency of This Versatile Approach Based on Clinical Conditions. J Neurosurg (2019) 133(3):642–54. 10.3171/2019.5.JNS19453 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: Data will be promptly available upon reasonable request to the authors. Requests to access these datasets should be directed to antonellaleonetti80@gmail.com.