Abstract
Purpose: To evaluate the diagnostic accuracy of PET with different radiotracers and parameters in differentiating between true glioma progression (TPR) and post treatment-related change (PTRC).
Methods: Studies on using PET to differentiate between TPR and PTRC were screened from the PubMed and Embase databases. By following the PRISMA checklist, the quality assessment of included studies was performed, the true positive and negative values (TP and TN), false positive and negative values (FP and FN), and general characteristics of all the included studies were extracted. Results of PET consistent with reference standard were defined as TP or TN. The pooled sensitivity (Sen), specificity (Spe), and hierarchical summary receiver operating characteristic curves (HSROC) were generated to evaluate the diagnostic accuracy.
Results: The 33 included studies had 1,734 patients with 1,811 lesions suspected of glioma recurrence. Fifteen studies tested the accuracy of 18F-FET PET, 12 tested 18F-FDG PET, seven tested 11C-MET PET, and three tested 18F-DOPA PET. 18F-FET PET showed a pooled Sen and Spe of 0.88 (95% CI: 0.80, 0.93) and 0.78 (0.69, 0.85), respectively. In the subgroup analysis of FET-PET, diagnostic accuracy of high-grade gliomas (HGGs) was higher than that of mixed-grade gliomas (Pinteraction = 0.04). 18F-FDG PET showed a pooled Sen and Spe of 0.78 (95% CI: 0.71, 0.83) and 0.87 (0.80, 0.92), the Spe of the HGGs group was lower than that of the low-grade gliomas group (0.82 vs. 0.90, P = 0.02). 11C-MET PET had a pooled Sen and Spe of 0.92 (95% CI: 0.83, 0.96) and 0.78 (0.69, 0.86). 18F-DOPA PET had a pooled Sen and Spe of 0.85 (95% CI: 0.80, 0.89) and 0.70 (0.60, 0.79). FET-PET combined with MRI had a pooled Sen and Spe of 0.88 (95% CI: 0.78, 0.94) and 0.76 (0.57, 0.88). Multi-parameters analysis of FET-PET had pooled Sen and Spe values of 0.88 (95% CI: 0.81, 0.92) and 0.79 (0.63, 0.89).
Conclusion: PET has a moderate diagnostic accuracy in differentiating between TPR and PTRC. The high Sen of amino acid PET and high Spe of FDG-PET suggest that the combination of commonly used FET-PET and FDG-PET may be more accurate and promising, especially for low-grade glioma.
Keywords: glioma, positron emission tomography, glioma progression, treatment outcome, meta-analysis
Introduction
Glioma is the most common primary brain tumor. It includes low-grade gliomas (LGGs) and high-grade gliomas (HGGs). The annual incidence of gliomas is approximately six cases per 100,000 individuals worldwide (1). Maximizing extent of resection (EOR) was demonstrated to be associated with improved outcomes, including progression-free survival and overall survival, by multiple retrospective analyses (2, 3). According to the NCCN guidelines, the standard treatment method for LGGs is surgical gross total resection combined with radiotherapy (RT) and adjuvant chemotherapy (CT) of temozolomide (TMZ) or PCV (procarbazine, lomustine, vincristine); for HGGs it is total resection followed by concurrent and adjuvant chemotherapy of TMZ or PCV (more than six cycles) (4–6).
Tumor progression monitoring is very important for patients with glioma after treatment. A confirmed diagnosis of tumor recurrence or progression guides surgeon and patients for the next treatment strategy. Progression and recurrence can be described as true glioma progression (TPR), while all treatment effects can (e.g., radiation necrosis and pseudoprogression) be described as post treatment-related change (PTRC). The wrong differentiation between TPR and PTRC will cause the wrong decision to be made by surgeon and patients, for example an unnecessary secondary surgery or the best surgery opportunity missed. Assessment of the glioma treatment response to differentiate between PTRC and TPR remains as much of a challenge as the treatment (7, 8).
At present, imaging follow-up was still the most common method to monitor TPR. Gadolinium-enhanced T1-weighted magnetic resonance imaging (G-T1-MRI) is often the choice for monitoring glioma patients' response to PTRC and TPR. However, this test often gets a false positive result because it only detects the disruption of the blood-brain barrier and not the tumor activity specifically. To overcome the limitations of G-T1-MRI, multimodal MRI has been proposed, including magnetic resonance spectroscopy (MRS), diffusion-weighted imaging (DWI), and perfusion-weighted imaging (PWI), among others (9, 10). Some previous meta-analyses had demonstrated the relative high diagnostic accuracy of MRS and PWI but low accuracy of DWI (11–13). However, these methods also sometimes caused false positive or false negative results because of their inability to provide metabolic information of lesions directly.
Due to the challenge to accurately determine brain tumor response by MRI both in daily practice and clinical trials, radiolabeled amino acid-positron emission tomography (PET) was proposed in the management of brain tumors by the RANO (Response Assessment in Neuro-Oncology) group (14). The radiotracers include O-(2-[18F] fluoroethyl)-L-tyrosine (18F-FET), 18F-fluorodeoxyglucose (18F-FDG), 11C-methionine (11C-MET), 3,4-dihydroxy-6-[18F]-fluoro-l-phenylalanine (18F-DOPA), and 11C-choline (11C-CHO), among others. PET of various tracers can visualize biological processes such as cell proliferation, membrane biosynthesis, glucose consumption, and uptake of amino acid analogs. Hence PET provides additional insight beyond MRI into the biology and treatment response of gliomas which may be used for non-invasive grading, differential diagnosis, delineation of tumor extent, surgical and radiotherapy treatment planning, post treatment surveillance, and prognostication (15). Many previous studies have demonstrated the usefulness of PET for the assessment of the treatment response of glioma (16, 17), yet a systematic review and meta-analysis of the diagnostic accuracy of PET using different radiotracers and parameters is lacking. Here, we conducted a systematic review and meta-analysis to assess the diagnostic accuracy of PET at differentiating TPR and PTRC.
Methods
Search Strategy
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) criteria (Supplementary Material 1). It was registered on PROSPERO with registration number CRD42020197852. PubMed and Embase were searched using the key words as follows: “glioma” or “glioblastoma” or “astrocytoma” or “oligodendroglioma” or “oligoastrocytoma”; and “Positron Emission Tomography” or “PET;” and “progression” or “recurrence” or “recurrent” or “relapse” or “pseudoprogression” or “necrosis” or “posttreatment.” The detailed search strategy is shown in Supplementary Material 2.
Selection Criteria
The inclusion criteria were as follows: English language, publication from January 1999 to December 2019, clinical diagnostic test for glioma with adequate PET data (radiotracers, imaging technique, parameters, etc.), more than 15 patients, adult patients with glioma treated with standard therapy (surgery+RT/CT), diagnostic test involving PET to differentiate between TPR and PTRC compared to a definite reference standard diagnosis (histology or clinical/imaging follow-up), and 2 × 2 tables from which true positives (TP), false positives (FP), false negatives (FN), and true negatives (TN) could be extracted. The reference standard of clinical/imaging follow-up was reasonable according to RANO criteria. For WHO grade II gliomas, the PTRC required that both the clinical and the radiological situation had to be stable/improved for at least 12 months without administration of another therapy. For WHO grade III-IV gliomas, the classification of PTRC required at least 6 months of stable or improved clinical and radiological condition, as well as no change in tumor treatment. TPR was considered present when lesions continued to increase in size on at least two subsequent MRI scans, paralleled by a deterioration in performance status, or when a patient died of glioma, which ever occurred first (18).
Exclusion criteria were the inclusion of other tumor entities besides glioma (such as brain metastases, lymphomas, or meningiomas); pediatric glioma patients; in vitro or animal studies; publication as a review, letter, comment, case report, or abstract; and treatment methods other than standard therapy recommended by NCCN guidelines (6), such as antiangiogenic bevacizumab therapy, intracavitary radiation, and tumor-treating field therapy.
Data Extraction and Quality Assessment
After duplicates were eliminated, studies were screened for eligibility based first on their title and abstract and subsequently on their full text independently by a board-certified neurosurgeon with 6 years of experience and a neuro-oncologist with 5 years of experience. Study quality was assessed independently according to the quality assessment of diagnostic accuracy studies (QUADAS-2) (19). Then these two authors reviewed in detail the abstracts, methods, results, figures, and tables. Extracted data consisted of true positives (TP), false positives (FP), true negatives (TN), and false negatives (FN); if raw numbers were not reported in the papers, we calculated them from the sensitivity (Sen) and specificity (Spe). The general characteristics extracted from each study were the total number of patients, study design, patient selection criteria, therapy method, mean or median age, sex, reference standard of diagnosis (histopathology or clinical/MRI follow-up), time point at which tumor progression was suspected, PET characteristics and time at which PET was performed, and parameters of PET with or without cut-off value. Disagreements were reassessed by the two authors together to reach a consensus.
Statistical Analysis
By using Meta-Disc statistical software version 1.4, we first evaluated the heterogeneity between each study caused by the threshold effect. The Spearman correlation coefficient between the logit of Sen and the logit of (1–Spe) was computed to assess the threshold effect (20). A strong positive correlation would suggest a threshold effect with P < 0.05.
Then, we used Stata 14.0 to perform the statistical analysis: (1) To evaluate the extent of heterogeneity in each study, we used the Q test and the inconsistency index (I2) of the diagnostic odds ratio (DOR: the ratio of LR+ and LR-). Heterogeneity was considered to be significant if P < 0.1 or I2 > 50%. In this case, the Sen and Spe of the studies were pooled using a random-effects model. Otherwise, a fixed-effects model was used (21). (2) The pooled Sen, Spe, and DOR with their 95% confidence intervals (CIs) were calculated for all studies and are presented as forest plots. If any cells of studies contained a count of zero, a value of one was added to replace zero in order to avoid any impossibilities in odds calculations for studies with a Sen or Spe of 100%. (3) The hierarchical logistic regression model was used to generate hierarchical summary receiver operating characteristic (HSROC) curves and area under the curve (AUC) to evaluate the diagnostic accuracy (22–24). (4) We assessed publication bias visually by using a scatter plot (X axis was log DOR, Y axis was ESS1/2, which is the inverse of the square root of effective sample size). A symmetric funnel shape and P > 0.01 in Deeks' asymmetry test indicated the absence of publication bias; otherwise, there was significant bias.
Subgroup analysis was performed using meta-regression if ≥3 studies could be included, and meta-regression tests were used to analyze differences between subgroups.
Results
Characteristics of Included Studies
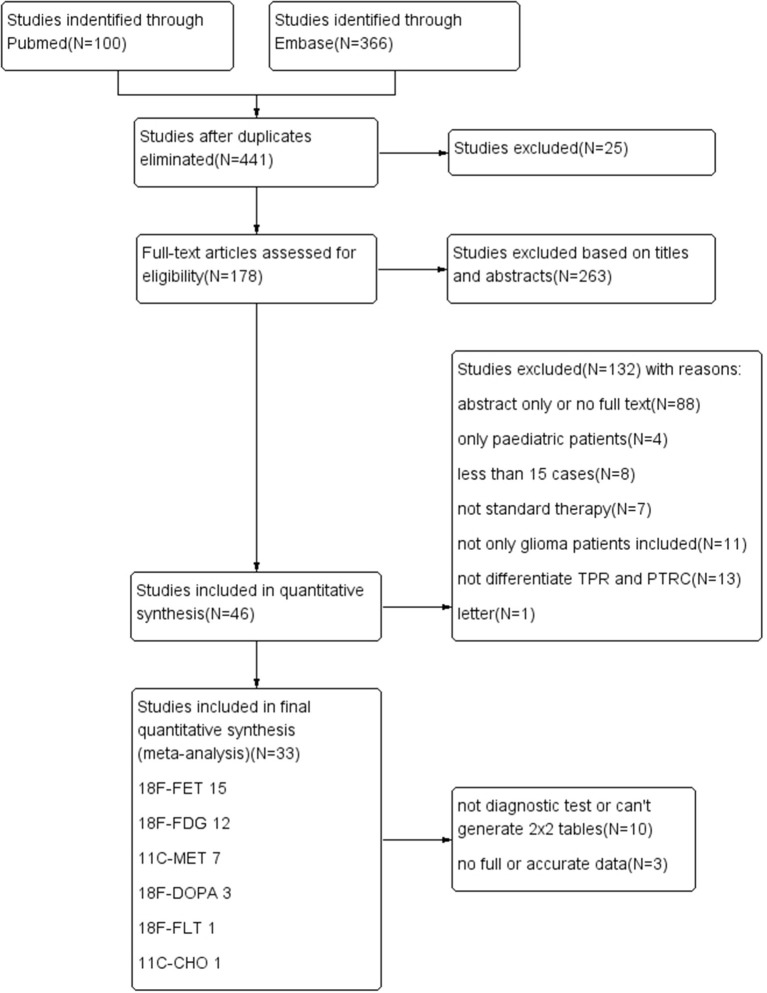
A flow diagram describing our study search process is provided in Figure 1. The results of the quality assessment are presented in Supplementary Material 3 and Figure 2. In brief, the quality of the included studies was satisfactory.
Figure 1.
Flow diagram of the study-screening process.
Figure 2.
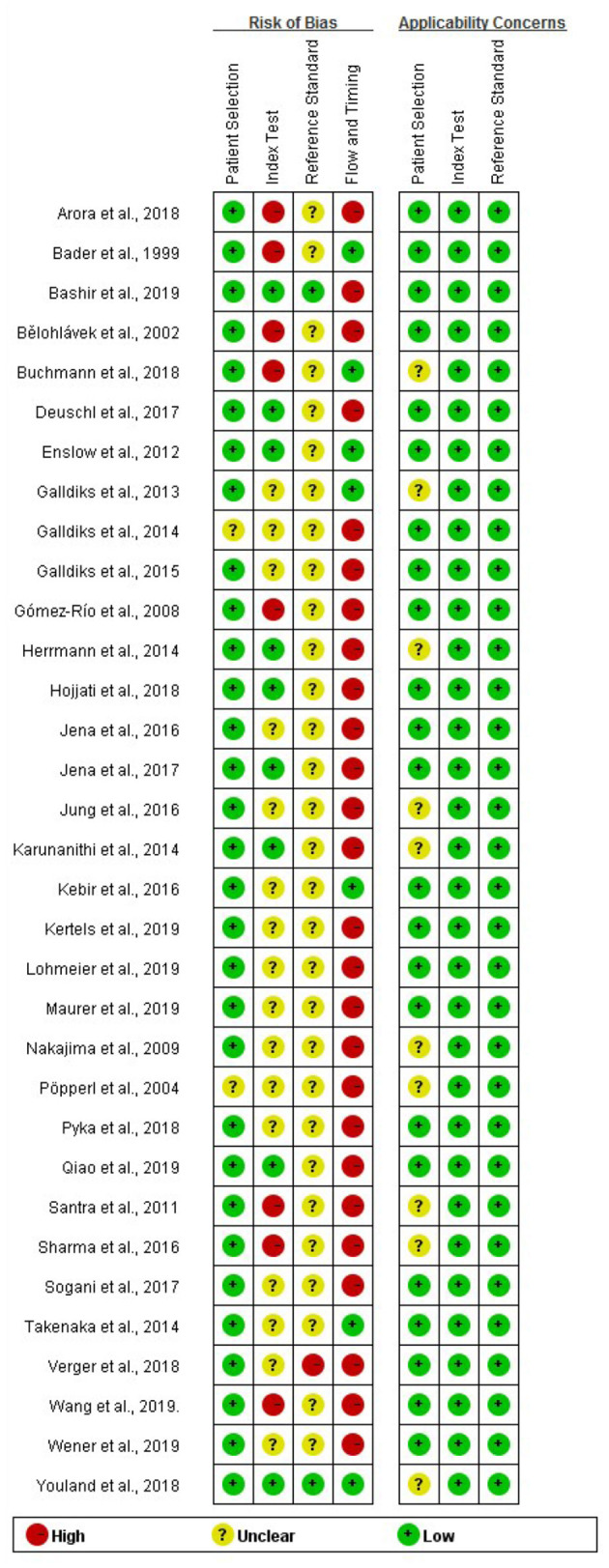
Methodological quality assessment summary of the included studies.
In total, the 33 included studies (retrospective = 21, prospective = 12) consisted of 1,734 patients and 1,811 lesions suspected of glioma recurrence. The mean ages ranged from 33.9 to 59.5 years. There were 1,110 males and 624 females. The lesions comprised 388 (21.4%) LGGs (WHO grades I and II) and 1,300 (71.8%) HGGs (WHO grades III and IV). In addition, 16 (0.9%) were other kinds of brain tumors, and 107 (5.9%) pathology results of lesions were not mentioned or determined.
Among the included studies, 15 tested the accuracy of 18F-FET PET (25–39), 12 tested 18F-FDG PET (40–51), seven tested 11C-MET PET (46, 47, 51–55), three tested 18F-DOPA PET (45, 56, 57), one tested 18F-FLT PET (44), and 1 tested 11C-CHO PET (46). Of all the included lesions, 836 (46.0%) had histopathology as the reference standard, 549 (30.2%) had clinical combined with imaging follow-up, and 81 (4.5%) only had imaging or clinical follow-up. Though the remaining 353 (19.4%) cases had either a combination or individual use of histopathology and clinical and imaging follow-up as the reference standard, the detailed data were not reported in these four studies (43, 47, 49, 51). The total incidence of TPR was 77.4% (1,394/1,801) in all patients. The incidence of TPR in HGG and LGG patients was 80.5% (577/717) and 63.1% (106/168), respectively. The incidence of PTRC was 22.6% (407/1,801) among all patients, 19.5% (140/717) in patients with HGG, and 36.9% (62/168) in patients with LGG (Supplementary Material 4).
Quantitative Synthesis
The pooled results and subgroup analysis are shown in Tables 1, 2, respectively. The HSROC curves are shown in Figure 3. The forest plots are shown in Figure 4.
Table 1.
Pooled Sen, Spe, DOR, and heterogeneity analysis results of PET tests of different radiotracers and techniques.
| Radiotracer and test technique | Quantitative parameter | Threshold range | ρ and P value | Heterogeneity of pooled Sen (upper) and Spe (lower) (P-value of Q test and I2) | Pooled Sen and its 95%CI | Pooled Spe and its 95%CI | Pooled DOR and its 95%CI | AUC of HSROC |
|---|---|---|---|---|---|---|---|---|
| 18F-FET | TBRmax (810 tests) | 1.95,3.52 | 0.068 (P = 0.816) | §P < 0.1, I2 = 85.3%||P = 0.09, I2 = 36.1% | 0.88 (0.80,0.93) | 0.78 (0.69,0.85) | 26 (12,57) | 0.86 (0.83, 0.89) |
| TBRmean (713 tests) | 1.52,2.98 | −0.677 (P = 0.022) | NA* | NA* | NA* | NA* | 0.90 (0.87, 0.92) | |
| TTP (317 tests) | 20,45 | 0.714 (P = 0.111) | §P = 0.03, I2 = 59.1%¶P = 0.1, I2 = 45.2% | 0.80 (0.68,0.88) | 0.67 (0.48,0.81) | 8 (4,16) | 0.81 (0.77, 0.84) | |
| 18F-FDG (631 tests) | NA† | NA† | 0.432 (P = 0.161) | —||P = 0.04, I2 = 46.4%¶P = 0.5, I2 = 0.0% | 0.78 (0.71,0.83) | 0.87 (0.80,0.92) | 23 (14,39) | 0.90 (0.87, 0.92) |
| 11C-MET | TBR (409 tests) | 1.43,2.51 | 0.559 (P = 0.192) | §P < 0.1, I2 = 86.3%¶P = 0.35, I2 = 10.8% | 0.92 (0.83,0.96) | 0.78 (0.69,0.86) | 39 (15,105) | 0.82 (0.78, 0.85) |
| 18F-DOPA | TBRmax (175 tests), visual (175 tests) | NA† | −0.638 (P = 0.173) | ¶P = 0.30, I2 = 18.2%¶P = 0.61, I2 = 0.0% | 0.85 (0.80,0.89) | 0.70 (0.60,0.79) | 13 (7,24) | 0.85 (0.82, 0.88) |
| FET-PET and MRI (190 tests) | NA‡ | NA‡ | 0.316 (P = 0.648) | §P = 0.06, I2 = 60.2%¶P = 0.16, I2 = 42.5% | 0.88 (0.78,0.94) | 0.76 (0.57,0.88) | 23 (9,59) | 0.90 (0.87, 0.92) |
| FET-PET static/dynamic multi-parameters analysis (354 tests) | NA‡ | NA‡ | −0.100 (P = 0.873) | ||P = 0.09, I2 = 49.5%¶P = 0.30, I2 = 18.4% | 0.88 (0.81,0.92) | 0.79 (0.63,0.89) | 26 (9,78) | 0.91 (0.88, 0.93) |
AUC, area under receiver operating curve; CI, confidence interval; DOR, diagnostic odds ratio; HSROC, hierarchical summary receiver operating characteristic; NA*, not available because of the threshold effect existed; NA†, not available because not all the studies used quantitative parameter analysis of PET; NA‡, not available because different parameters were used among studies; ρ, Spearman correlation coefficient; TBR, tumor-to-brain ratio; TTP, time-to-peak;
, significant heterogeneity;
, slight heterogeneity;
, no heterogeneity;
#, some studies only reported the value of TBR, so it was used instead of TBRmax or TBRmean to analyze the pooled effect. Bold face type indicates statistical significance.
Table 2.
Subgroup analysis between LGGs, mixed-grade gliomas, and HGGs; between static PET scan and dynamic PET scan; and between visual assessment of the PET scan and semi-quantitative parametric analysis.
| Radiotracer and parameter | Subgroup | Number of studies/PET scan tests | Pooled Sen and its 95%CI | P-value | Pooled Spe and its 95%CI | P-value | Pooled DOR and its 95%CI | AUC | |
|---|---|---|---|---|---|---|---|---|---|
| 18F-FET, TBRmax | HGGs | 6/333 | 0.92 (0.85,0.98) | 0.51 | 0.88 (0.79,0.96) | 0.90 | 75 (18,304) | 0.92 | 0.04 |
| Gliomas of mixed grades | 8/477 | 0.84 (0.75,0.93) | 0.71 (0.61,0.81) | 12 (6,23) | 0.80 | ||||
| Static scan | 5/311 | 0.94 (0.88,0.99) | 0.80 | 0.76 (0.62,0.90) | 0.09 | 47 (7,299) | 0.92 | 0.10 | |
| Dynamic scan | 9/810 | 0.82 (0.73,0.91) | 0.79 (0.71,0.88) | 15 (7,32) | 0.86 | ||||
| PET performed ≤7d after SRR | 3/108 | 0.84 (0.76,0.92) | 0.19 | 0.79 (0.56,1.00) | 0.64 | NA | NA | 0.34 | |
| PET performed >7d after SRR | 4/233 | 0.76 (0.68,0.84) | 0.82 (0.68,0.95) | NA | NA | ||||
| 18F-FDG | Visual assessment | 8/355 | 0.76 (0.63,0.85) | 0.30 | 0.90 (0.81,0.95) | <0.05 | 29 (14,60) | 0.92 | 0.17 |
| Semi-quantitative parametric analysis | 7/349 | 0.83 (0.73,0.90) | 0.82 (0.72,0.89) | 23 (10,51) | 0.85 | ||||
| HGGs | 11/214 | 0.76 (0.68,0.83) | 0.82 (0.70,0.90) | 15 (7,33) | 0.84 | ||||
| Gliomas of mixed grades | 7/417 | 0.76 (0.58,0.88) | 0.31† | 0.87 (0.80,0.92) | <0.05† | 21 (8,52) | 0.88 | 0.64† | |
| LGGs | 5/141 | 0.61 (0.34,0.83) | 0.83† | 0.90 (0.77,0.96) | 0.02† | 15 (3,88) | 0.90 | 0.10† | |
| 11C-MET, TBR | HGGs | 3/248 | 0.94 (0.87,1.00) | 0.95 | 0.82 (0.69,0.95) | 0.47 | 71 (20,251) | 0.95 | 0.58 |
| Gliomas of mixed grades | 4/384 | 0.89 (0.78,0.99) | 0.77 (0.67,0.86) | 29 (7, 117) | 0.79 | ||||
| PET performed <20 m after RT | 2/92 | 0.96 (0.91,1.00) | 0.89 | 0.71 (0.53,0.89) | 0.04 | NA | NA | 0.11 | |
| PET performed >20 m after RT | 4/110 | 091 (0.84,0.97) | 0.89 (0.79,0.99) | NA | NA | ||||
| 18F-DOPA | Visual assessment | 3/175 | 0.86 (0.80,0.92) | <0.05 | 0.72 (0.59,0.86) | 0.22 | 16 (4,62) | 0.93 | 0.8 |
| Semi-quantitative analysis of TBRmax | 3/175 | 0.84 (0.77,0.90) | 0.68 (0.54,0.83) | 14 (4,50) | 0.85 |
AUC, area under receiver operating curve; CI, confidence interval; DOR, diagnostic odds ratio; HGGs, high-grade gliomas; LGGs, low-grade gliomas; SRR, suspicious recurrence of glioma; TBR, tumor-to-brain ratio. Bold face type indicates statistical significance. *Based on meta regression.
Compared with group of HGGs.
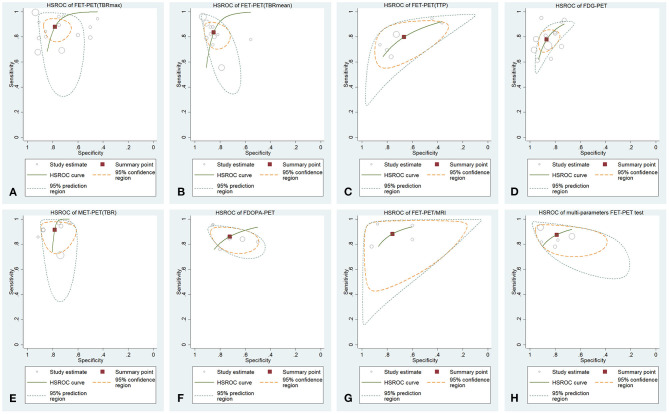
Figure 3.
HSROC curves for (A) 18F-FET PET (TBRmax), (B) 18F-FET PET (TBRmean), (C) 18F-FET PET (TTP), (D) 18F-FDG PET, (E) 11C-MET PET (TBR), (F) 18F-DOPA PET, (G) FET-PET/MRI, and (H) multi-parameters FET-PET.
Figure 4.
Forest plots for (A) 18F-FET PET (TBRmax), (B) 18F-FET PET (TTP), (C) 18F-FDG PET, (D) 11C-MET PET (TBR), and (E) 18F-DOPA PET.
18F-FET PET (TBRmax, TBRmean, TTP)
The pooled weighted values of tumor-to-brain maximum ratio (TBRmax) tests were Sen = 0.88 (95% CI: 0.80, 0.93), Spe = 0.78 (0.69, 0.85), DOR = 26 (12, 57), and AUC of HSROC curve = 0.86 (0.83, 0.89). The Sen of these studies had high heterogeneity (P < 0.1, I2 = 85.3%). Subgroup analysis of glioma histopathology showed that the HGG subgroup of the FET-PET test group benefitted from higher accuracy than the mixed-grade group (Pinteraction = 0.04). Subgroup analysis of PET scans (static and dynamic scan groups) showed no significant difference in diagnostic accuracy (Pinteraction = 0.54). Different time points of PET scans may influence the diagnostic accuracy, but subgroup analysis indicated no difference between the ≤7-day and >7-day groups after suspicious recurrence.
Thresholds of the tumor-to-brain mean ratio (TBRmean) ranged from 1.52 to 2.98. The Spearman's correlation coefficient (ρ) was −0.677 (P = 0.022), which indicated that a threshold effect was detected only in this group when quantitative synthesis was performed. Therefore, the Sen and Spe of studies could not be pooled directly. The AUC of the HSROC curve was 0.90 (0.87, 0.92), which was used to evaluate the diagnostic accuracy of the TBRmean tests of FET-PET.
The pooled weighted values of time-to-peak (TTP) tests were Sen = 0.80 (95% CI: 0.68, 0.88), Spe = 0.67 (0.48, 0.81), DOR = 8 (4, 16), and AUC = 0.81 (0.77, 0.84).
18F-FDG PET
The pooled weighted values were Sen = 0.78 (95% CI: 0.71, 0.83), Spe = 0.87 (0.80, 0.92), and DOR = 23 (14, 39). The AUC of the HSROC curve was 0.90 (0.87, 0.92). Subgroup analysis of PET visual assessment and semiquantitative analysis of one parameter (such as TBR and SUV) were performed. The difference in Spe between these two groups was statistically significant (P < 0.05). Subgroup analysis of glioma histopathology showed that the mixed-grade group and LGG group had higher pooled Spe than the HGG group according to the FDG-PET test (0.87 vs. 0.82 [P < 0.05], 0.90 vs. 0.82 [P = 0.02]). However, the pooled Sen of the HGG group was not significantly different from those of the other two groups (both P > 0.05).
11C-MET PET (TBR)
Heterogeneity was detected in pooled Sen (P < 0.1, I2 = 86.3%). The pooled Sen, Spe, and DOR were 0.92 (95% CI: 0.83, 0.96), 0.78 (0.69, 0.86), and 39 (15, 105), respectively. The AUC of the HSROC curve was 0.82 (0.78, 0.85). The subgroup of HGGs and the subgroup of mixed grades were analyzed, but no significant differences in diagnostic accuracy were detected (all P > 0.05). Subgroup analysis of time points showed a higher pooled Spe of the >20 months after radiotherapy group than the <20 months group (P = 0.04), but the diagnostic accuracy in these two groups was not different (Pinteraction = 0.11).
18F-DOPA PET (TBRmax, Visual)
The pooled values of Sen, Spe, and DOR were 0.85 (95% CI: 0.80, 0.89), 0.70 (0.60, 0.79), and 13 (7, 24), respectively. The AUC of the HSROC curve was 0.85 (0.82, 0.88). Subgroup analysis showed that there were significant differences between the visual and semiquantitative groups regarding sensitivity (P < 0.05).
Publication Bias Assessment
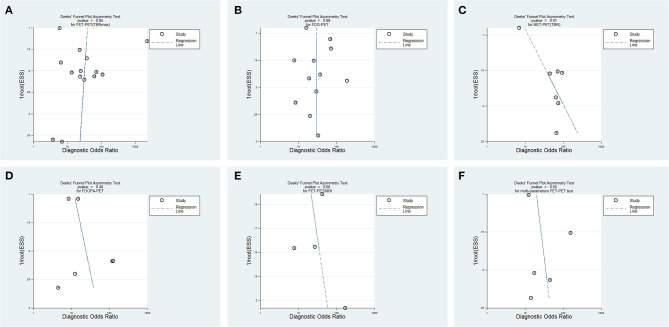
Publication bias did not exist in the FET-PET (TBRmax), FDG-PET, or 18F-DOPA-PET studies (P = 0.85, P = 0.99, P = 0.40), while bias may have existed in the MET-PET (TBR) studies (P = 0.01) (Figure 5).
Figure 5.
Funnel plots of publication bias for different PET tests. (A) 18F-FET PET (TBRmax), (B) 18F-FDG PET, (C) 11C-MET PET (TBR), (D) 18F-DOPA PET, (E) FET-PET/MRI, and (F) multi-parameters FET-PET.
MRI Combined With FET-PET and Multi-Parameter FET-PET
The pooled Sen, Spe, and DOR of the FET-PET/MRI group were 0.88 (95% CI: 0.78, 0.94), 0.76 (0.57, 0.88), and 23 (9, 59), respectively. The AUC of the HSROC curve was 0.90 (0.87, 0.92). The multi-parameter FET-PET test had pooled Sen, Spe, DOR, and AUC values of 0.88 (95% CI: 0.81, 0.92), 0.79 (0.63, 0.89), 26 (9, 78), and 0.91 (0.88, 0.93), respectively. Publication bias did not exist between these studies (P = 0.66, P = 0.65).
Discussion
To overcome the limitations of MRI, PET-CT has been suggested by RANO for the management of glioma in addition to conventional MRI at every disease stage (58). Many studies have explored the diagnostic accuracy of PET labeled by different radiotracers, which indicates its potential value. Static and dynamic PET scans can acquire different parameters to assess diagnosis. Static scans can calculate SUVmax, SUVmean, TBRmax, and TBRmean to analyze the characteristics of radiotracer uptake in lesions and unaffected brain tissue to differentiate between tumor and normal brain tissue. Dynamic scanning brings in the variable of time and can depict the time-activity curves (TACs) of radiotracer uptake by lesions, and can then identify tumors by calculating TTP or slope (the change in SUV per hour) or by visually assessing the shape of TACs (28, 30, 39). All these parameters are believed to improve the diagnostic accuracy of PET tests compared to visual assessments alone.
According to the pooled AUC of HSROC curves in this meta-analysis, the diagnostic accuracy of 18FET-PET was similar to that of 18F-FDG PET, which was higher than that of 18F-DOPA PET and 11C-MET PET.
18F-FET PET had a high diagnostic accuracy for TBR. Subgroup analysis demonstrated a higher test accuracy for HGGs than gliomas of mixed grades (Pinteraction = 0.04). Because of the higher recurrence rate of HGGs than LGGs, FET-PET will benefit HGG patients more during monitoring recurrence after surgery (Table 2). Compared with dynamic scanning, static FET-PET scanning may have similar accuracy for the differential diagnosis between TPR and PTRC (Pinteraction = 0.10).
A common PET modality for the central nervous system (53, 59), 11C-MET PET had good test accuracy when calculating TBR (pooled set: 0.92, Spe: 0.78). Although the accuracy of 11C-MET PET was not different between the HGG group and the mixed-grade group (Pinteraction = 0.58), a trend of higher diagnostic accuracy in the HGG group was detected. As seen for FET-PET, this result also indicated a better performance of MET-PET for HGG patients. 18F-DOPA is a kind of amino acid radiotracer that can be transported across the intact blood-brain barrier, but it is scarcely used or researched (60). The accuracy of 18F-DOPA PET was not superior to that of FET- or MET-PET in our analysis, which will limit the use of FDOPA-PET.
Because of the limited number of studies that reported the time when PET was performed, only the FET-PET TBRmax and MET-PET TBR could be evaluated for the influence of time point by subgroup analysis in this review. Because of the pseudoprogression caused by radiotherapy, the symptoms of patients and MRI always showed false positive results, which can be falsely considered as tumor recurrence. In our analysis, a longer time after radiotherapy showed a higher Spe of MET-PET, which indicated that the PET scan may need to be performed in the appropriate time window (perhaps a longer time after radiotherapy). Further research is needed to identify the best time point for performing PET after there is suspicious recurrence on MRI.
18F-FDG is a common radiotracer used in PET scans, but it always fails to differentiate between tumors and the brain due to the similarly high uptake rates of glucose in normal brain tissue (61). In this review, the accuracy of FDG-PET showed a similar accuracy to amino acid PET (Table 1). Compared to amino acid PET, FDG-PET had a higher Spe but a lower Sen, so we can predict that the combination of these two radiotracers would acquire better diagnostic performance. In the subgroup analysis (Table 2), the pooled Spe of visual assessment was higher than that of semiquantitative parametric analysis (P < 0.05). Compared to the mixed-grade glioma group and LGG group, the HGG group had a lower Spe (P < 0.05, P = 0.02). These results demonstrate that the high Spe of FDG-PET may be derived from the visual assessment of PET and the testing of LGG patients. Thus, visual assessment should also be an important part of PET tests in addition to parametric analysis. For HGG patients, the accuracy of amino acid PET was satisfactory, while for postoperative recurrence tests of LGG patients, amino acid PET was not good enough and should be combined with FDG-PET to acquire a higher Spe.
A confirmed diagnosis of recurrence during the follow-up of glioma patients is most important for choosing the next treatment method (secondary surgery or others). MRI is the most common and valuable method to monitor recurrence at present. From a review of some previous meta-analyses, the diagnostic accuracy of anatomical G-T1-MRI and advanced MRI is summarized in Table 3. The accuracy of G-T1-MRI and DWI was obviously not adequate for the diagnosis of glioma recurrence (10, 13). Advanced MRI showed a higher accuracy (11, 12, 62–64). Compared to advanced MRI, PET with different radiotracers did not present an obvious superiority in the differential diagnosis of glioma recurrence (Table 1). However, the high Sen of amino acid PET and high Spe of FDG-PET may suggest their combination in future applications of diagnosis.
Table 3.
Previous meta-analysis of MRI accuracy for diagnosis of glioma recurrence.
| MRI | Study | Pooled Sen and its 95%CI | Pooled Spe and its 95%CI | DOR and its 95%CI | AUC |
|---|---|---|---|---|---|
| Anatomical G-T1-MRI | (13) | 0.68 (0.51,0.81) | 0.77 (0.45,0.93) | NR | NR |
| (64) | 0.48 (0.08,0.90) | 0.85 (0.39,0.98) | NR | NR | |
| ADC of DWI | (13) | 0.71 (0.60,0.80) | 0.87 (0.77,0.93) | NR | NR |
| MRS | (63) | 0.87 (0.80,0.92) | 0.86 (0.77,0.93) | NR | 0.93 |
| (13) | 0.91 (0.79,0.97) | 0.95 (0.65,0.99) | NR | NR | |
| (64) | 0.82 (0.68,0.90) | 0.79 (0.69,0.87) | NR | NR | |
| Cho/NAA of MRS | (11) | 0.88 (0.81,0.93) | 0.86 (0.76,0.93) | 37 (12,84) | 0.92 |
| Cho/Cr of MRS | (11) | 0.83 (0.77,0.89) | 0.83 (0.74,0.90) | 24 (12,49) | 0.90 |
| DSC of PWI | (62) | 0.88 (0.82,0.93) | 0.85 (0.75,0.92) | 42 (19,94) | 0.93 |
| (12) | 0.90 (0.85,0.94) | 0.88 (0.83,0.92) | NR | NR | |
| (13) | 0.87 (0.82,0.91) | 0.86 (0.77,0.91) | NR | NR | |
| DCE of PWI | (12) | 0.89 (0.78,0.96) | 0.85 (0.77,0.91) | NR | NR |
| (13) | 0.92 (0.73,0.98) | 0.85 (0.76,0.92) | NR | NR |
ADC, apparent diffusion coefficient; Cho/Cr, choline/creatine; Cho/NAA, choline/N-acetyl-aspartate; DCE, dynamic contrast-enhanced; DSC, dynamic susceptibility contrast; DWI, diffusion-weighted imaging; G-T1-MRI, gadolinium-enhanced T1-weighted magnetic resonance; MRS, magnetic resonance spectroscopy; PWI, perfusion-weighted imaging; NR, not reported.
Some previous meta-analyses evaluated the accuracy of PET, which did not show an obviously better performance than advanced MRI. Moreover, none of them took the different parameters (TBR, TTP, etc.) of PET into consideration (63–67). Along with the evaluation of PET with different tracers, this meta-analysis concentrated on the different accuracy of parameters of PET scans and showed more accuracy in the static parameter TBRmax of FET-PET than the dynamic parameter TTP. However, most studies in this review only performed static parameter analysis, and the accuracy of dynamic parameter analysis needs more research for evaluation. Regardless of these results, as a supplement to MRI, PET with different tracers and parameters can provide additional useful information by evaluating different metabolic pathways.
Furthermore, the accuracy of FET-PET multi-parameter analysis or FET-PET/MRI in this meta-analysis was similar to that of FET-PET TBR alone (Table 1). FET-PET multi-parameter analysis and hybrid PET/MRI also were not more accurate than advanced MRI. Although hybrid PET/MRI seemed to be a promising technique for recurrent glioma diagnosis, quantitative meta-analyses are scarce, and its accuracy in our analysis was not satisfactory (7, 68). PET-CT was also commonly used to differentiate TPR and PTRC in patients with brain metastases. A meta-analysis showed that pooled Sen and Spe of FDG-PET were 0.83 (95% CI: 0.74, 0.92) and 0.88 (0.81, 0.95), respectively. The pooled Sen and Spe of amino acid PET were 0.84 (0.79, 0.90) and 0.85 (0.80, 0.91) (69). These results were similar to the results in this review, thus it demonstrated similar diagnostic accuracy of PET differentiating TPR and PTRC both in glioma and brain metastases.
To acquire high diagnostic accuracy in the management of glioma as well as high feasibility and low cost to patients, the method of radiomics analysis is applied broadly now. PET radiomics combines datasets of static and dynamic PET parameters to make full use of imaging information. Precise image segmentation and feature extraction using different classification methods make it more accuracy in diagnosis. However, many studies demonstrated that a PET radiomics model had higher accuracy than the PET single parameter analysis in differentiating TPR from PTRC (70, 71). Feature-based PET/MRI radiomics also showed higher accuracy (72). One study used FDG PET, MET PET, and structural MRI images to develop an integrated radiomics-based model which showed a very high diagnostic accuracy with an AUC of 0.99 (51). In this review we did not cover radiomics. Multi-parameter PET radiomics combined with other imaging modalities has indeed great potential in differentiating TRP and PTRC, and its diagnostic performance should be summarized and evaluated in future.
Some limitations exist in this meta-analysis. First, while 33 studies were included, only three tested 18F-DOPA PET, one tested 18F-FLT PET, and one tested 11C-CHO PET. So their credible diagnostic accuracy cannot be found in this review. The accuracy of 18F-FET, 11C-MET, and 18F-FDG PET which was synthesized in this review was credible due to the support of enough studies. Second, some studies included a small number of patients, so the correction of zero replaced by 1 in 2 × 2 tables caused a large effect on these studies.
Conclusion
This meta-analysis demonstrates that PET with different radiotracers has a moderate diagnostic accuracy in differentiating between glioma progression or recurrence and post treatment-related changes. However, their performance does not show an obvious advantage over advanced MRI. The high Sen of amino acid PET and high Spe of FDG-PET suggest that the combination of these two methods will yield a higher accuracy. The PET multi-parameter analysis and PET/MRI have a great clinical application prospect, their diagnostic performances require further research in large sample sizes in multicenter studies.
Data Availability Statement
The original contributions generated for this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
MC: conceptualization, methodology, software, formal analysis, resources, data curation, writing-original draft, and writing reviewing and editing. RZ-V: methodology, software, formal analysis, resources, data curation, and writing-reviewing and editing. JH: supervision and writing-reviewing and editing. BG: supervision and writing-reviewing and editing. XM: supervision and project administration. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thanks to Yihong Chi from the Department of Information Technology in Xian Janssen Pharmaceutical Ltd for her work and contribution to this research (including data analysis and visualization).
Glossary
Abbreviations
- 11C-CHO
11C-choline
- DCE
dynamic contrast-enhanced
- DOR
diagnostic odds ratio
- DSC
dynamic susceptibility contrast
- DWI
diffusion-weighted imaging
- FDG
18F-fluorodeoxyglucose
- 18F-FET
O-(2-[18F]fluoroethyl)-L-tyrosine
- 18F-FLT
18F-fluorothymidine
- 18F-DOPA
3,4-dihydroxy-6-[18F]-fluoro-l-phenylalanine
- HGGs
high-grade gliomas
- LGGs
low-grade gliomas
- LR+
positive likelihood ratio
- LR–
negative likelihood ratio
- 11C-MET
11C-methionine
- MRS
magnetic resonance spectroscopy
- PTRC
post treatment-related change
- PWI
perfusion-weighted imaging
- ROIs
regions of interest
- SUV
standardized uptake value
- TBR
tumor-to-brain ratio
- TBRmax/mean
tumor-to-brain maximum/mean ratio (calculated by dividing the max or mean SUV of the tumor ROIs by the max or mean SUV of healthy brain tissue)
- TMZ
temozolomide
- TPR
true progression
- TTP
time-to-peak (the time in minutes from the beginning of the dynamic acquisition to the time at which the maximum SUV of the lesion occurred).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.671867/full#supplementary-material
References
- 1.Fisher JL, Schwartzbaum JA, Wrensch M, Wiemels JL. Epidemiology of brain tumors. Neurol Clin. (2007) 25:867–90. 10.1016/j.ncl.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 2.Chaichana KL, Jusue-Torres I, Navarro-Ramirez R, Raza SM, Pascual-Gallego M, Ibrahim A, et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. (2014) 16:113–22. 10.1093/neuonc/not137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Amico RS, Englander ZK, Canoll P, Bruce JN. Extent of resection in Glioma-A review of the cutting edge. World Neurosurg. (2017) 103:538–49. 10.1016/j.wneu.2017.04.041 [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. (2005) 352:987–96. 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 5.Nabors LB, Portnow J, Ammirati M, Brem H, Brown P, Butowski N, et al. Central nervous system cancers, version 2.2014. Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. (2014) 12:1517–23. 10.6004/jnccn.2014.0151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nabors LB, Portnow J, Ammirati M, Baehring J, Brem H, Butowski N, et al. NCCN guidelines insights: central nervous system cancers, version 1.2017. J Natl Compr Canc Netw. (2017) 15:1331–45. 10.6004/jnccn.2017.0166 [DOI] [PubMed] [Google Scholar]
- 7.Dhermain FG, Hau P, Lanfermann H, Jacobs AH, van den Bent MJ. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. (2010) 9:906–20. 10.1016/S1474-4422(10)70181-2 [DOI] [PubMed] [Google Scholar]
- 8.Ahluwalia MS, Wen PY. Antiangiogenic therapy for patients with glioblastoma: current challenges in imaging and future directions. Expert Rev Anticancer Ther. (2011) 11:653–6. 10.1586/era.11.35 [DOI] [PubMed] [Google Scholar]
- 9.Bobek-Billewicz B, Stasik-Pres G, Majchrzak H, Zarudzki L. Differentiation between brain tumor recurrence and radiation injury using perfusion, diffusion-weighted imaging and MR spectroscopy. Folia Neuropathol. (2010) 48:81–92. 10.3109/15513815.2010.505628 [DOI] [PubMed] [Google Scholar]
- 10.Verma N, Cowperthwaite MC, Burnett MG, Markey MK. Differentiating tumor recurrence from treatment necrosis: a review of neuro-oncologic imaging strategies. Neuro Oncol. (2013) 15:515–34. 10.1093/neuonc/nos307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Ma L, Wang Q, Zheng X, Wu C, Xu BN. Role of magnetic resonance spectroscopy for the differentiation of recurrent glioma from radiation necrosis: a systematic review and meta-analysis. Eur J Radiol. (2014) 83:2181–9. 10.1016/j.ejrad.2014.09.018 [DOI] [PubMed] [Google Scholar]
- 12.Patel P, Baradaran H, Delgado D, Askin G, Christos P, John Tsiouris A, et al. MR perfusion-weighted imaging in the evaluation of high-grade gliomas after treatment: a systematic review and meta-analysis. Neuro Oncol. (2017) 19:118–27. 10.1093/neuonc/now148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Dijken BRJ, van Laar PJ, Holtman GA, van der Hoorn A. Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high-grade glioma, a systematic review and meta-analysis. Eur Radiol. (2017) 27:4129–44. 10.1007/s00330-017-4789-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albert NL, Weller M, Suchorska B, Galldiks N, Soffietti R, Kim MM, et al. Response assessment in neuro-oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. (2016) 18:1199–208. 10.1093/neuonc/now058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.la Fougere C, Suchorska B, Bartenstein P, Kreth FW, Tonn JC. Molecular imaging of gliomas with PET: opportunities and limitations. Neuro Oncol. (2011) 13:806–19. 10.1093/neuonc/nor054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cannon DM, Kruser TJ, Khuntia D. Principles and application of PET in brain tumors. PET Clin. (2011) 6:131–48. 10.1016/j.cpet.2011.02.004 [DOI] [PubMed] [Google Scholar]
- 17.Tsiouris S, Bougias C, Fotopoulos A. Principles and current trends in the correlative evaluation of glioma by advanced MRI techniques and PET. Hell J Nucl Med. (2019) 22:206–19. [PubMed] [Google Scholar]
- 18.Chukwueke UN, Wen PY. Use of the Response Assessment in Neuro-Oncology (RANO) criteria in clinical trials and clinical practice. CNS Oncol. (2019) 8:CNS28. 10.2217/cns-2018-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. (2003) 3:25. 10.1186/1471-2288-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. (2006) 6:31. 10.1186/1471-2288-6-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leeflang MM. Systematic reviews and meta-analyses of diagnostic test accuracy. Clin Microbiol Infect. (2014) 20:105–13. 10.1111/1469-0691.12474 [DOI] [PubMed] [Google Scholar]
- 22.Kim KW, Lee J, Choi SH, Huh J, Park SH. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-part I. General guidance and tips. Korean J Radiol. (2015) 16:1175–87. 10.3348/kjr.2015.16.6.1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J, Kim KW, Choi SH, Huh J, Park SH. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-part II. Statistical methods of meta-analysis. Korean J Radiol. (2015) 16:1188–96. 10.3348/kjr.2015.16.6.1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suh CH, Park SH. Successful publication of systematic review and meta-analysis of studies evaluating diagnostic test accuracy. Korean J Radiol. (2016) 17:5–6. 10.3348/kjr.2016.17.1.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pöpperl G, Götz C, Rachinger W, Gildehaus FJ, Tonn JC, Tatsch K. Value of O-(2-[18F]fluoroethyl)-L-tyrosine PET for the diagnosis of recurrent glioma. Eur J Nucl Med Mol Imaging. (2004) 31:1464–70. 10.1007/s00259-004-1590-1 [DOI] [PubMed] [Google Scholar]
- 26.Galldiks N, Stoffels G, Ruge MI, Rapp M, Sabel M, Reifenberger G, et al. Role of O-(2-18F-fluoroethyl)-L-tyrosine PET as a diagnostic tool for detection of malignant progression in patients with low-grade glioma. J Nucl Med. (2013) 54:2046–54. 10.2967/jnumed.113.123836 [DOI] [PubMed] [Google Scholar]
- 27.Galldiks N, Dunkl V, Stoffels G, Hutterer M, Rapp M, Sabel M, et al. Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-L-tyrosine PET. Eur J Nucl Med Mol Imaging. (2015) 42:685–95. 10.1007/s00259-014-2959-4 [DOI] [PubMed] [Google Scholar]
- 28.Galldiks N, Stoffels G, Filss C, Rapp M, Blau T, Tscherpel C, et al. The use of dynamic O-(2-18F-fluoroethyl)-l-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro Oncol. (2015) 17:1293–300. 10.1093/neuonc/nov088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jena A, Taneja S, Gambhir A, Mishra AK, D'Souza MM, Verma SM, et al. Glioma recurrence versus radiation necrosis: single-session multiparametric approach using simultaneous O-(2-18F-Fluoroethyl)-L-tyrosine PET/MRI. Clin Nucl Med. (2016) 41:e228–36. 10.1097/RLU.0000000000001152 [DOI] [PubMed] [Google Scholar]
- 30.Kebir S, Fimmers R, Galldiks N, Schafer N, Mack F, Schaub C, et al. Late pseudoprogression in glioblastoma: diagnostic value of dynamic O-(2-[18F]fluoroethyl)-L-tyrosine PET. Clin Cancer Res. (2016) 22:2190–6. 10.1158/1078-0432.CCR-15-1334 [DOI] [PubMed] [Google Scholar]
- 31.Sogani SK, Jena A, Taneja S, Gambhir A, Mishra AK, D'Souza MM, et al. Potential for differentiation of glioma recurrence from radionecrosis using integrated 18F-fluoroethyl-L-tyrosine (FET) positron emission tomography/magnetic resonance imaging: a prospective evaluation. Neurol India. (2017) 65:293–301. 10.4103/neuroindia.NI_101_16 [DOI] [PubMed] [Google Scholar]
- 32.Pyka T, Hiob D, Preibisch C, Gempt J, Wiestler B, Schlegel J, et al. Diagnosis of glioma recurrence using multiparametric dynamic 18F-fluoroethyl-tyrosine PET-MRI. Eur J Radiol. (2018) 103:32–7. 10.1016/j.ejrad.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 33.Verger A, Filss CP, Lohmann P, Stoffels G, Sabel M, Wittsack HJ, et al. Comparison of O-(2-18F-Fluoroethyl)-L-tyrosine positron emission tomography and perfusion-weighted magnetic resonance imaging in the diagnosis of patients with progressive and recurrent glioma: a hybrid positron emission tomography/magnetic resonance study. World Neurosurg. (2018) 113:e727–37. 10.1016/j.wneu.2018.02.139 [DOI] [PubMed] [Google Scholar]
- 34.Bashir A, Mathilde Jacobsen S, Molby Henriksen O, Broholm H, Urup T, Grunnet K, et al. Recurrent glioblastoma versus late posttreatment changes: diagnostic accuracy of O-(2-[18F]fluoroethyl)-L-tyrosine positron emission tomography (18F-FET PET). Neuro Oncol. (2019) 21:1595–606. 10.1093/neuonc/noz166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buchmann N, Gempt J, Ryang YM, Pyka T, Kirschke JS, Meyer B, et al. Can early postoperative O-(2-(18F)Fluoroethyl)-l-tyrosine positron emission tomography after resection of glioblastoma predict the location of later tumor recurrence? World Neurosurg. (2019) 121:e467–74. 10.1016/j.wneu.2018.09.139 [DOI] [PubMed] [Google Scholar]
- 36.Kertels O, Mihovilovic MI, Linsenmann T, Kessler AF, Tran-Gia J, Kircher M, et al. Clinical utility of different approaches for detection of late pseudoprogression in glioblastoma with O-(2-[18F]fluoroethyl)-l-tyrosine PET. Clin Nucl Med. (2019) 44:695–701. 10.1097/RLU.0000000000002652 [DOI] [PubMed] [Google Scholar]
- 37.Lohmeier J, Bohner G, Siebert E, Brenner W, Hamm B, Makowski MR. Quantitative biparametric analysis of hybrid (18)F-FET PET/MR-neuroimaging for differentiation between treatment response and recurrent glioma. Sci Rep. (2019) 9:14603. 10.1038/s41598-019-50182-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maurer GD, Brucker DP, Stoffels G, Filipski K, Filss CP, Mottaghy FM, et al. (18)F-FET PET imaging in differentiating glioma progression from treatment-related changes - a single-center experience. J Nucl Med. (2020) 61:505–11. 10.2967/jnumed.119.234757 [DOI] [PubMed] [Google Scholar]
- 39.Werner JM, Stoffels G, Lichtenstein T, Borggrefe J, Lohmann P, Ceccon G, et al. Differentiation of treatment-related changes from tumour progression: a direct comparison between dynamic FET PET and ADC values obtained from DWI MRI. Eur J Nucl Med Mol Imaging. (2019) 46:1889–901. 10.1007/s00259-019-04384-7 [DOI] [PubMed] [Google Scholar]
- 40.Bader JB, Samnick S, Moringlane JR, Feiden W, Schaefer A, Kremp S, et al. Evaluation of L-3-[123I]iodo-α-methyltyrosine SPET and [18F]fluorodeoxyglucose PET in the detection and grading of recurrences in patients pretreated for gliomas at follow-up: a comparative study with stereotactic biopsy. Eur J Nucl Med. (1999) 26:144–51. 10.1007/s002590050370 [DOI] [PubMed] [Google Scholar]
- 41.Bělohlávek O, Klener J, Vymazal J, Dbalý V, Tovaryš F. The diagnostics of recurrent gliomas using FDG-PET: Still questionable? Nucl Med Rev. (2002) 5:127–30. [PubMed] [Google Scholar]
- 42.Gómez-Río M, Rodríguez-Fernández A, Ramos-Font C, López-Ramírez E, Llamas-Elvira JM. Diagnostic accuracy of 201Thallium-SPECT and 18F-FDG-PET in the clinical assessment of glioma recurrence. Eur J Nucl Med Mol Imaging. (2008) 35:966–75. 10.1007/s00259-007-0661-5 [DOI] [PubMed] [Google Scholar]
- 43.Santra A, Kumar R, Sharma P, Bal C, Julka PK, Malhotra A. Detection of recurrence in glioma: a comparative prospective study between Tc-99m GHA SPECT and F-18 FDG PET/CT. Clin Nucl Med. (2011) 36:650–5. 10.1097/RLU.0b013e318217aee0 [DOI] [PubMed] [Google Scholar]
- 44.Enslow MS, Zollinger LV, Morton KA, Butterfield RI, Kadrmas DJ, Christian PE, et al. Comparison of 18F-fluorodeoxyglucose and 18F- fluorothymidine PET in differentiating radiation necrosis from recurrent glioma. Clin Nucl Med. (2012) 37:854–61. 10.1097/RLU.0b013e318262c76a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karunanithi S, Sharma P, Kumar A, Khangembam BC, Bandopadhyaya GP, Kumar R, et al. 18F-FDOPA PET/CT for detection of recurrence in patients with glioma: prospective comparison with 18F-FDG PET/CT. Eur J Nucl Med Mol Imaging. (2013) 40:1025–35. 10.1007/s00259-013-2384-0 [DOI] [PubMed] [Google Scholar]
- 46.Takenaka S, Asano Y, Shinoda J, Nomura Y, Yonezawa S, Miwa K, et al. Comparison of 11c-methionine, 11c-choline, and 18f-fluorodeoxyglucose-positron emission tomography for distinguishing glioma recurrence from radiation necrosis. Neurol Med Chir. (2014) 54:280–9. 10.2176/nmc.oa2013-0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma R, D'Souza M, Jaimini A, Hazari PP, Saw S, Pandey S, et al. A comparison study of 11 C-methionine and 18 F-fluorodeoxyglucose positron emission tomography-computed tomography scans in evaluation of patients with recurrent brain tumors. Indian J Nucl Med. (2016) 31:93–102. 10.4103/0972-3919.178254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jena A, Taneja S, Jha A, Damesha NK, Negi P, Jadhav GK, et al. Multiparametric Evaluation in differentiating glioma recurrence from treatment-induced necrosis using simultaneous (18)F-FDG-PET/MRI: a single-institution retrospective study. AJNR Am J Neuroradiol. (2017) 38:899–907. 10.3174/ajnr.A5124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arora G, Sharma P, Sharma A, Mishra AK, Hazari PP, Biswas A, et al. 99mTc-methionine hybrid SPECT/CT for detection of recurrent glioma: comparison with 18F-FDG PET/CT and contrast-enhanced MRI. Clin Nucl Med. (2018) 43:e132–8. 10.1097/RLU.0000000000002036 [DOI] [PubMed] [Google Scholar]
- 50.Hojjati M, Badve C, Garg V, Tatsuoka C, Rogers L, Sloan A, et al. Role of FDG-PET/MRI, FDG-PET/CT, and dynamic susceptibility contrast perfusion mri in differentiating radiation necrosis from tumor recurrence in glioblastomas. J Neuroimaging. (2018) 28:118–25. 10.1111/jon.12460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang K, Qiao Z, Zhao X, Li X, Wang X, Wu T, et al. Individualized discrimination of tumor recurrence from radiation necrosis in glioma patients using an integrated radiomics-based model. Eur J Nucl Med Mol Imaging. (2019) 47:1400–11. 10.1007/s00259-019-04604-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakajima T, Kumabe T, Kanamori M, Saito R, Tashiro M, Watanabe M, et al. Differential diagnosis between radiation necrosis and glioma progression using sequential proton magnetic resonance spectroscopy and methionine positron emission tomography. Neurol Med Chir. (2009) 49:394–401. 10.2176/nmc.49.394 [DOI] [PubMed] [Google Scholar]
- 53.Jung TY, Min JJ, Bom HS, Jung S, Kim IY, Lim SH, et al. Prognostic value of post-treatment metabolic tumor volume from 11C-methionine PET/CT in recurrent malignant glioma. Neurosurg Rev. (2017) 40:223–9. 10.1007/s10143-016-0748-1 [DOI] [PubMed] [Google Scholar]
- 54.Deuschl C, Kirchner J, Poeppel TD, Schaarschmidt B, Kebir S, El Hindy N, et al. 11C–MET PET/MRI for detection of recurrent glioma. Eur J Nucl Med Mol Imaging. (2018) 45:593–601. 10.1007/s00259-017-3916-9 [DOI] [PubMed] [Google Scholar]
- 55.Qiao Z, Zhao X, Wang K, Zhang Y, Fan D, Yu T, et al. Utility of dynamic susceptibility contrast perfusion-weighted MR imaging and11C-methionine PET/CT for differentiation of tumor recurrence from radiation injury in patients with high-grade gliomas. Am J Neuroradiol. (2019) 40:253–9. 10.3174/ajnr.A5952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herrmann K, Czernin J, Cloughesy T, Lai A, Pomykala KL, Benz MR, et al. Comparison of visual and semiquantitative analysis of 18F-FDOPA- PET/CT for recurrence detection in glioblastoma patients. Neuro Oncol. (2014) 16:603–9. 10.1093/neuonc/not166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Youland RS, Pafundi DH, Brinkmann DH, Lowe VJ, Morris JM, Kemp BJ, et al. Prospective trial evaluating the sensitivity and specificity of 3,4-dihydroxy-6-[18F]-fluoro-L-phenylalanine (18F-DOPA) PET and MRI in patients with recurrent gliomas. J Neurooncol. (2018) 137:583–91. 10.1007/s11060-018-2750-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Law I, Albert NL, Arbizu J, Boellaard R, Drzezga A, Galldiks N, et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [(18)F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. (2019) 46:540–57. 10.1007/s00259-018-4207-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yano H, Shinoda J, Iwama T. Clinical utility of positron emission tomography in patients with malignant glioma. Neurol Med Chir. (2017) 57:312–20. 10.2176/nmc.ra.2016-0312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Youland RS, Kitange GJ, Peterson TE, Pafundi DH, Ramiscal JA, Pokorny JL, et al. The role of LAT1 in (18)F-DOPA uptake in malignant gliomas. J Neurooncol. (2013) 111:11–8. 10.1007/s11060-012-0986-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Herholz K, Langen KJ, Schiepers C, Mountz JM. Brain tumors. Semin Nucl Med. (2012) 42:356–70. 10.1053/j.semnuclmed.2012.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deng SM, Zhang B, Wu YW, Zhang W, Chen YY. Detection of glioma recurrence by (1)(1)C-methionine positron emission tomography and dynamic susceptibility contrast-enhanced magnetic resonance imaging: a meta-analysis. Nucl Med Commun. (2013) 34:758–66. 10.1097/MNM.0b013e328361f598 [DOI] [PubMed] [Google Scholar]
- 63.Wang X, Hu X, Xie P, Li W, Li X, Ma L. Comparison of magnetic resonance spectroscopy and positron emission tomography in detection of tumor recurrence in posttreatment of glioma: a diagnostic meta-analysis. Asia Pac J Clin Oncol. (2015) 11:97–105. 10.1111/ajco.12202 [DOI] [PubMed] [Google Scholar]
- 64.Furuse M, Nonoguchi N, Yamada K, Shiga T, Combes JD, Ikeda N, et al. Radiological diagnosis of brain radiation necrosis after cranial irradiation for brain tumor: a systematic review. Radiat Oncol. (2019) 14:28. 10.1186/s13014-019-1228-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nihashi T, Dahabreh IJ, Terasawa T. Diagnostic accuracy of PET for recurrent glioma diagnosis: a meta-analysis. AJNR Am J Neuroradiol. (2013) 34:944–50. 10.3174/ajnr.A3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu J, Zheng J, Xu W, Weng J, Gao L, Tao L, et al. Accuracy of (18)F-FDOPA positron emission tomography and (18)F-FET positron emission tomography for differentiating radiation necrosis from brain tumor recurrence. World Neurosurg. (2018) 114:e1211–24. 10.1016/j.wneu.2018.03.179 [DOI] [PubMed] [Google Scholar]
- 67.Treglia G, Muoio B, Trevisi G, Mattoli MV, Albano D, Bertagna F, et al. Diagnostic performance and prognostic value of PET/CT with different tracers for brain tumors: a systematic review of published meta-analyses. Int J Mol Sci. (2019) 20:4669. 10.3390/ijms20194669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang Y, He MZ, Li T, Yang X. MRI combined with PET-CT of different tracers to improve the accuracy of glioma diagnosis: a systematic review and meta-analysis. Neurosurg Rev. (2019) 42:185–95. 10.1007/s10143-017-0906-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suh CH, Kim HS, Jung SC, Choi CG, Kim SJ. Comparison of MRI and PET as potential surrogate endpoints for treatment response after stereotactic radiosurgery in patients with brain metastasis. AJR Am J Roentgenol. (2018) 211:1332–41. 10.2214/AJR.18.19674 [DOI] [PubMed] [Google Scholar]
- 70.Hotta M, Minamimoto R, Miwa K. 11C-methionine-PET for differentiating recurrent brain tumor from radiation necrosis: radiomics approach with random forest classifier. Sci Rep. (2019) 9:15666. 10.1038/s41598-019-52279-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lohmann P, Elahmadawy MA, Gutsche R, Werner JM, Bauer EK, Ceccon G, et al. FET PET radiomics for differentiating pseudoprogression from early tumor progression in glioma patients post-chemoradiation. Cancers. (2020) 12:3835. 10.3390/cancers12123835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lohmann P, Meissner AK, Kocher M, Bauer EK, Werner JM, Fink GR, et al. Feature-based PET/MRI radiomics in patients with brain tumors. Neurooncol Adv. (2020) 2(Suppl. 4):iv15–21. 10.1093/noajnl/vdaa118 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions generated for this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.