Abstract
Myrosinase enzymes play a key role in the chemical defense of plants of the order Brassicales. Upon herbivory, myrosinases hydrolyze the β-S-linked glucose moiety of glucosinolates, the characteristic secondary metabolites of brassicaceous plants, which leads to the formation of different toxic hydrolysis products. The specialist flea beetle, Phyllotreta armoraciae, is capable of accumulating high levels of glucosinolates in the body and can thus at least partially avoid plant myrosinase activity. In feeding experiments with the myrosinase-deficient Arabidopsis thaliana tgg1 × tgg2 (tgg) mutant and the corresponding Arabidopsis Col-0 wild type, we investigated the influence of plant myrosinase activity on the metabolic fate of ingested glucosinolates in adult P. armoraciae beetles. Arabidopsis myrosinases hydrolyzed a fraction of ingested glucosinolates and thereby reduced the glucosinolate sequestration rate by up to 50% in adult beetles. These results show that P. armoraciae cannot fully prevent glucosinolate hydrolysis; however, the exposure of adult beetles to glucosinolate hydrolysis products had no impact on the beetle’s energy budget under our experimental conditions. To understand how P. armoraciae can partially prevent glucosinolate hydrolysis, we analyzed the short-term fate of ingested glucosinolates and found them to be rapidly absorbed from the gut. In addition, we determined the fate of ingested Arabidopsis myrosinase enzymes in P. armoraciae. Although we detected Arabidopsis myrosinase protein in the feces, we found only traces of myrosinase activity, suggesting that P. armoraciae can inactivate plant myrosinases in the gut. Based on our findings, we propose that the ability to tolerate plant myrosinase activity and a fast glucosinolate uptake mechanism represent key adaptations of P. armoraciae to their brassicaceous host plants.
Keywords: plant-insect interaction, two-component defense, herbivore adaptation, isothiocyanate, detoxification, Phyllotreta
Introduction
Many plants deter herbivores with a chemical defense that is activated upon tissue damage (Morant et al., 2008). The chemical defense compound is usually stored as inactive glucose conjugate in the vacuole of plant cells. When plant tissue is damaged, the glucose moiety is hydrolyzed by a defensive β-glucosidase, originally localized separately from the glucose conjugate, which liberates toxic and deterrent compounds (Morant et al., 2008; Pentzold et al., 2014b). Activated chemical defenses are usually more efficient in deterring chewing herbivores that cause extensive tissue damage than in deterring insects with less invasive feeding modes, such as sap suckers (Pentzold et al., 2014b).
A number of herbivorous insects evolved resistance against this plant defense strategy (Pentzold et al., 2014b) and some highly adapted species even accumulate (sequester) plant glucosides in their bodies and deploy them for defense against predators (Kazana et al., 2007; Opitz and Müller, 2009; Beran et al., 2019). However, it is currently not well understood how chewing insects prevent the hydrolysis of ingested plant glucosides by plant β-glucosidases. A study with turnip sawfly larvae (Athalia rosae) suggests that a rapid absorption of ingested glucosides (glucosinolates) across the gut epithelium prevents their hydrolysis in the gut; this is possibly facilitated by low plant β-glucosidase activity in the anterior gut (Abdalsamee et al., 2014). A rapid uptake mechanism was also proposed to enable western corn rootworm larvae (Diabrotica virgifera virgifera), to sequester benzoxazinoid glucosides; however, there was no evidence for reduced β-glucosidase activity in the larval gut (Robert et al., 2017). Burnet moth larvae (Zygaena filipendulae) which sequester cyanogenic glucosides avoid extensive plant β-glucosidase activity by a leaf-snipping feeding mode causing only minor tissue damage. Moreover, the alkaline pH in the midgut lumen of burnet moth larvae inhibits plant β-glucosidase activity (Pentzold et al., 2014a).
Sequestering herbivores must avoid the hydrolysis of the ingested plant glucosides they sequester, but the extent to which plant β-glucosidase activity influences glucoside sequestration has rarely been assessed. The brassicaceous model plant Arabidopsis thaliana offers an ideal system to address this question. The activated defense of Arabidopsis and other plants of the order Brassicales is the glucosinolate-myrosinase system (Halkier and Gershenzon, 2006; Blažević et al., 2020). Glucosinolates are a structurally diverse group of amino acid-derived thioglucosides that are hydrolyzed by β-thioglucosidases called myrosinases. The resulting aglucone is unstable and can give rise to different hydrolysis products including isothiocyanates, nitriles and epithionitriles (Figure 1). Which hydrolysis products are formed depends on various factors such as the structure of the glucosinolate side chain, pH conditions, and the presence of so-called plant specifier proteins (Wittstock et al., 2016). Among the possible hydrolysis products, isothiocyanates are the most reactive and thus toxic for small herbivores (Jeschke et al., 2016a). The Arabidopsis tgg1 × tgg2 double knock-out mutant (tgg) is devoid of myrosinase activity in leaves (Barth and Jander, 2006) and thus can be used for comparative feeding studies with the corresponding Arabidopsis Col-0 wild type (wild type). For example, in feeding experiments performed with the cabbage stem flea beetle, Psylliodes chrysocephala, adult beetles sequestered six times more glucosinolates from the myrosinase-deficient Arabidopsis tgg mutant than from the wild type (Beran et al., 2018). In a similar feeding experiment performed with larvae of the horseradish flea beetle, Phyllotreta armoraciae, only traces of sequestered glucosinolates were detected in wild type-fed larvae, whereas comparatively high glucosinolate levels were found in tgg-fed larvae (Sporer et al., 2020). In contrast to P. armoraciae larvae, adult beetles were able to sequester glucosinolates from wild type leaves (Yang et al., 2020), which suggests that plant myrosinase activity has a stronger influence on glucosinolate sequestration in larvae compared to adults. Plant myrosinase activity influences not only sequestration, but also the feeding behavior of Phyllotreta flea beetles. In field experiments, the crucifer flea beetle, Phyllotreta cruciferae, caused less feeding damage on Brassica rapa plants selected for high myrosinase activity than on plants selected for low myrosinase activity (Siemens and Mitchell-Olds, 1996).
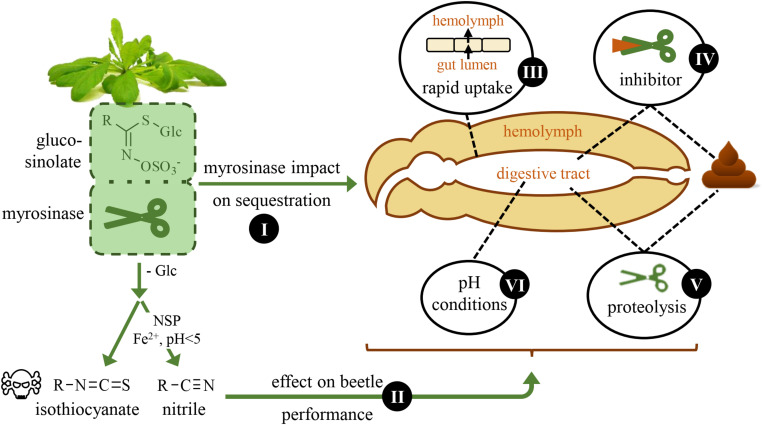
FIGURE 1.
Schematic overview of questions that are addressed in this study. The horseradish flea beetle, Phyllotreta armoraciae, sequesters glucosinolates from its brassicaceous host plants and is thus able to overcome plant myrosinase activity. Here, we investigate the impact of plant myrosinase activity on glucosinolate sequestration (I), and the effect of glucosinolate hydrolysis on beetle weight and energy reserves (II). Further, we analyze possible mechanisms that may facilitate sequestration by influencing plant myrosinase activity: a rapid uptake of ingested glucosinolates from the gut (III), the inhibition (IV) and digestion (V) of plant myrosinases, and gut pH conditions (VI).
Here, we investigated the influence of plant myrosinase activity on glucosinolate sequestration, the effect of glucosinolate hydrolysis on beetle performance and potential mechanisms that facilitate the sequestration of intact glucosinolates in the adult life stage of P. armoraciae (Figure 1). In a previous feeding study, we recovered about 35% of the total ingested glucosinolates from wild type leaves in the beetle body and feces, whereas the metabolic fate of more than 60% of the total ingested glucosinolates remained unknown (Yang et al., 2020). One possible explanation is that the unrecovered glucosinolates were hydrolyzed by plant myrosinases. To investigate this possibility, we performed a series of comparative feeding experiments with myrosinase-deficient and wild type Arabidopsis plants. In nature, P. armoraciae is closely associated with horseradish, Armoracia rusticana, a plant species that is characterized by high levels of allyl glucosinolate (Li and Kushad, 2004; Ciska et al., 2017). Therefore, we additionally investigated the influence of plant myrosinase activity on the sequestration of allyl glucosinolate by spiking the intact glucosinolate into Arabidopsis leaves. We observed a negative influence of plant myrosinase activity on glucosinolate sequestration and confirmed that a fraction of ingested glucosinolates is hydrolyzed in P. armoraciae. We thus asked whether the metabolism of glucosinolate hydrolysis products incurs a metabolic cost in P. armoraciae. Finally, we explored possible mechanisms that allow P. armoraciae to partially prevent the hydrolysis of ingested glucosinolates by plant myrosinase.
Materials and Methods
Plants and Insects
Food plants, Brassica juncea cv. “Bau Sin” and Brassica rapa cv. “Yu-Tsai-Sum” (Known-You Seed Co., Ltd., Taiwan) were cultivated in a controlled environment chamber (24°C, 55% relative humidity, 14 h light/10 h dark period). Arabidopsis thaliana plants were cultivated under short day conditions in a controlled environment chamber (21°C, 55% relative humidity, 10-h light/14-h dark period). Experiments were performed with A. thaliana Col-0 (wild type) and two mutants in the A. thaliana Col-0 background: the myrosinase deficient A. thaliana tgg1 × tgg2 (tgg) double knockout mutant (Barth and Jander, 2006), and the A. thaliana myb28 × myb29 (myb) double knockout mutant which does not produce aliphatic glucosinolates (Sønderby et al., 2007).
The laboratory rearing of Phyllotreta armoraciae was established in October 2012 with beetles collected from horseradish plants in Laasdorf, Thuringia, Germany. The beetles are relatively small with an approximate adult body length of 3 mm and weight of about 2–3 mg per beetle. Beetles were reared on potted 3–4 week old B. juncea or B. rapa plants in a controlled environment chamber (24°C, 60% relative humidity, 14 h light/10 h dark period). Adult beetles were provided with new plants every week and plants with eggs were kept separately for larval development. After 3 weeks, any remaining plant material was removed and the soil containing pupae was kept in plastic containers (9 L volume, Lock&Lock, Seoul, South Korea). Newly emerged adults were collected every 2–3 days. Unless stated otherwise, experiments were performed with newly emerged beetles that had been reared on B. juncea plants. We did not determine the sex of the beetles used in experiments unless stated otherwise.
Sequestration Experiments
To analyze whether plant myrosinase activity influences the sequestration of glucosinolates in P. armoraciae beetles, we performed sequestration experiments with the myrosinase-deficient Arabidopsis tgg mutant and the corresponding wild type Col-0.
In Experiment 1, we fed newly emerged beetles for 1 day with detached leaves of Arabidopsis wild type or tgg plants (n = 28 per genotype, two beetles per replicate). On the next day, each remaining leaf was weighed, frozen in liquid nitrogen, and stored at −20°C until freeze drying. To allow for digestion of ingested plant material, we fed the beetles one additional day on Arabidopsis myb leaves before beetles were weighed, frozen in liquid nitrogen, and stored at −20°C until extraction. The extraction and analysis of glucosinolates by high performance liquid chromatography coupled with diode array detection (HPLC-DAD) was performed as described in Beran et al. (2014). Adult P. armoraciae beetles convert ingested 4-methylsulfinylbutyl (4MSOB) glucosinolate, the major aliphatic glucosinolate in Arabidopsis wild type and tgg plants, into 4-methylthiobutyl (4MTB) glucosinolate (Yang et al., 2020). Therefore, we summed up the concentrations of 4MSOB and 4MTB glucosinolates per mg fresh weight in each beetle sample and expressed this concentration relative to the concentration of both glucosinolates per mg fresh weight in the corresponding leaf sample, which was set to 1. To establish whether beetles feed equal amounts from both Arabidopsis lines, we quantified the beetle feeding damage (2 beetles per leaf disc with 16 mm diameter) over 1 day using the software Fiji (Schindelin et al., 2012) (n = 8 per genotype).
In Experiment 2, we fed newly emerged beetles with Arabidopsis wild type, tgg, or myb leaves for 1 day (n = 5, 5 beetles per replicate). Afterwards, feces was collected in 50 μL ultrapure water containing 0.1% (v/v) formic acid, mixed with 50 μL pure methanol and stored at −20°C. Beetles were frozen in liquid nitrogen and stored at −20°C until extraction. Remaining leaves were weighed, frozen in liquid nitrogen and freeze-dried. Feces samples were homogenized for 2 min at 25 Hz in a TissueLyzerII (Qiagen, Hilden, Germany) using metal beads. Beetles were homogenized in 500 μL 50% (v/v) methanol using plastic pestles. Freeze-dried leaves were homogenized to powder as described for feces samples and extracted with 800 μL of 50% (v/v) methanol. Samples were centrifuged at 4°C for 10 min at 16,000 × g and supernatants were stored at −20°C until analysis by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS). We only quantified 4MSOB glucosinolate and 4MSOB glucosinolate-derived hydrolysis products in this experiment because currently no LC-MS/MS method for the quantification of 4MTB glucosinolate-derived hydrolysis products is available. Identification and quantification of 4MSOB glucosinolate and 4MSOB glucosinolate-derived metabolites in samples was performed on an Agilent 1200 HPLC system (Agilent, Santa Clara, CA, United States) connected to an API3200 tandem mass spectrometer (AB Sciex Germany GmbH, Darmstadt, Germany) using external standard curves prepared from authentic standards of each compound as described in Beran et al. (2018). After quantification, we subtracted the average amounts of each metabolite in bodies or feces of myb-fed control beetles (background control) from those detected in wild type- or tgg-fed beetles.
In Experiment 3, we fed newly emerged beetles for 1 day with Arabidopsis wild type or tgg leaves containing 300 nmol allyl glucosinolate that was introduced by placing the petiole into an aqueous allyl glucosinolate solution (Carl Roth, Mannheim, Germany) as described in Schramm et al. (2012). This experiment was performed with adult beetles that had been reared on B. rapa plants, which do not produce allyl glucosinolate (Beran et al., 2018). The glucosinolate profile of unfed beetles reared on B. rapa was analyzed as described in Beran et al. (2014) (n = 20, with 5 beetles per replicate) to confirm that allyl glucosinolate is not present in beetles. To facilitate the distribution of spiked allyl glucosinolate within the leaf, we placed the petiole in a reaction tube filled with water. Newly emerged beetles were fed with spiked Arabidopsis leaves for 1 day (n = 10 per genotype, 5 beetles per replicate) and allyl glucosinolate-spiked leaves without beetles were kept under the same conditions as a recovery control (n = 8–10 per genotype). Each leaf was sampled separately, frozen in liquid nitrogen, freeze-dried, and homogenized with metal beads for 2 min at 25 Hz in a TissueLyzerII (Qiagen). Beetles were weighed and frozen in liquid nitrogen. Feces was collected in 100 μL ultrapure water and mixed with 100 μL pure methanol. Leaf and beetle samples were homogenized in 1 mL and 800 μL 50% (v/v) methanol, respectively. After centrifugation for 10 min at 16,000 × g, supernatants were collected. Feces samples were homogenized as described for leaves and centrifuged at 4°C for 10 min at 16,000 × g. The supernatant was collected, the solvent evaporated using nitrogen and extracts were re-dissolved in 80 μL 50% methanol. Samples were stored at −20°C until LC-MS/MS as described in Malka et al. (2016) using a modified elution gradient. The gradient consisted of formic acid (0.2%) in water (solvent A) and acetonitrile (solvent B) and was carried out as follows: 1.5% (v/v) B (1 min), 1.5–5% (v/v) B (5 min), 5–7% (v/v) B (2 min), 7–12.6% (v/v) B (4 min), 12.6–100% (v/v) B (0.1 min), 100% (v/v) B (0.9 min), 100 to 1.5% (v/v) B (0.1 min), and 1.5% (v/v) B (3.85 min). Allyl glucosinolate was quantified using an external calibration curve. We recovered 97.8 ± 6.5 and 109.5 ± 4.4% (mean ± SD) of the spiked glucosinolate from undamaged (control) wild type and tgg leaves, respectively, showing that only small amounts of spiked allyl glucosinolate were metabolized in Arabidopsis leaves under our assay conditions. To determine how much allyl glucosinolate beetles had ingested, we subtracted the allyl glucosinolate amount detected in each fed leaf from the average allyl glucosinolate amounts recovered from corresponding unfed control leaves. The amounts of allyl glucosinolate that were recovered in beetles and feces were expressed relative to the total ingested amount, which was set to 100%.
In Experiment 4, we fed newly emerged beetles with allyl glucosinolate-spiked Arabidopsis wild type or tgg leaves (prepared as described in Experiment 3) for 1 day and simultaneously collected the headspace on Porapak-QTM volatile collection traps (25 mg; ARS, Inc., Gainsville, FL, United States) (n = 6–7 per genotype, 8 beetles per replicate). Leaves without beetles served as controls (n = 4 per genotype). The volatile collection and sample analysis by gas chromatography mass spectrometry (GC-MS) was performed as previously described in Sporer et al. (2020). Allyl isothiocyanate was quantified in headspace samples using an external calibration curve prepared from an authentic standard (Sigma-Aldrich, Steinheim, Germany). The glucosinolate amount per fed beetle was determined as described in Experiment 1.
Performance Experiment
To investigate whether the hydrolysis of ingested glucosinolates has a negative influence on beetle performance, we compared the fresh weight and energy reserves of beetles that had fed for 10 days on Arabidopsis wild type or tgg leaf discs. We separated newly emerged beetles into males and females and assigned them randomly to one of the two Arabidopsis genotypes (n = 10 females per genotype, n = 8–9 males per genotype). Each beetle was provided with a new leaf disc from an undamaged Arabidopsis plant every day for 10 consecutive days. After 10 days feeding, beetles were weighed, frozen in liquid nitrogen, and stored at −20°C until analysis of energy reserves. The contents of soluble protein, total lipids, glycogen, and soluble carbohydrates in individual beetles were determined as described in Foray et al. (2012) with minor modifications. Instead of a 96-well borosilicate microplate, we used a 96-well quartz glass microplate (Hellma Analytics, Müllheim, Germany) that was covered with MicroAmp clear adhesive film (Applied Biosystems, Waltham, MA, United States). The plate was heated using a ThermoMixer (Eppendorf, Hamburg, Germany) and for measurements we used a Tecan Infinite 200 Reader (Tecan, Crailsheim, Germany). As a control, we quantified the levels of soluble protein, amino acids, and sugars in rosette leaves of Arabidopsis wild type and tgg mutant plants (for details refer to Supplementary Material).
Distribution of Glucosinolates in P. armoraciae Shortly After Ingestion
To investigate whether ingested glucosinolates are rapidly absorbed across the gut epithelium in P. armoraciae, we allowed newly emerged beetles to feed for 1 min on wild type or tgg leaves. After 5 min, beetles were dissected into gut and remaining body (without head; n = 3, 3 beetles per replicate). Non-fed beetles were used as background control (n = 2–3, 3 beetles per replicate). Dissected guts were washed twice in phosphate-buffered saline (PBS) pH 7.4 (Bio-Rad, Munich, Germany) before sampling. Samples were homogenized in 500 μL 80% methanol containing 0.4 μM 4-hydroxybenzyl glucosinolate as internal standard using plastic pestles and stored at −20°C until extraction and analysis by LC-MS/MS as described above in Experiment 2. We quantified 4MSOB glucosinolate in each sample using an external standard curve and expressed the glucosinolate distribution in the gut and rest of the body relative to the total amount detected in both samples (set to 100%).
Myrosinase Inhibition Assays
To determine whether P. armoraciae can inhibit ingested myrosinase activity, we performed myrosinase activity assays with gut content extracts of adult beetles. The gut content of adults was collected as follows: dissected guts were washed in extraction buffer [20 mM 2-(N-morpholino)ethanesulfonic acid (MES), pH 5.2] containing protease inhibitors (cOmplete, EDTA-free, Roche, Mannheim, Germany) and cut open longitudinally to collect the gut content in 2.5 μL of extraction buffer. For each sample, gut contents from 20 beetles were pooled, frozen in liquid nitrogen and stored at −20°C until extraction (n = 4). Samples were homogenized with metal beads for 2 min at 25 Hz in a TissueLyzer II, centrifuged at 4°C for 10 min at 16,000 × g and the supernatant split into two subsamples of which one was boiled for 5 min at 99°C.
Assays (50 μL total volume) consisted of 0.1 mM ascorbic acid (Fluka, Buchs, Switzerland), 0.2 mM 4MSOB glucosinolate (substrate), 0.5 ng/μL partially purified myrosinase from Sinapis alba (Sigma-Aldrich, details of protein purification are described in the Supplementary Material) and (a) gut content extract (corresponding to four beetles), (b) boiled gut content extract, or (c) extraction buffer (control). Assays with gut extracts but without myrosinase and assays containing only substrate were used as additional controls. Assays were incubated for 15 min at 30°C, the reaction was stopped by 5 min boiling at 99°C and extracted with 100 μL 80% methanol containing 0.2 mM 4-hydroxybenzyl glucosinolate as an internal standard. Myrosinase activity was determined by quantifying the remaining 4MSOB glucosinolate amount in each assay as described in Experiment 1.
Detection of Myrosinase Enzyme and Activity in Beetle Feces
To determine whether P. armoraciae can degrade ingested plant myrosinase enzymes, we collected feces of 90 adults that had fed on Arabidopsis wild type leaves for 1 day in a total volume of 1 mL 20 mM MES buffer pH 6.5 containing protease inhibitors (cOmplete, EDTA-free). After homogenization with metal beads for 3 min at 25 Hz in a TissueLyzer II and centrifugation at 4°C for 10 min at 16,000 × g, we precipitated soluble proteins in the supernatant using trichloroacetic acid and washed the pellet with acetone. The protein pellet was dissolved in Laemmli buffer (Bio-Rad), boiled for 15 min at 95°C, and separated on a 12.5% Criterion Tris-HCl precast gel (Bio-Rad). Protein bands were stained with colloidal Coomassie G250 (Carl Roth), excised from the gel, and digested with porcine trypsin (Promega, Madison, WI, United States) as described in Shevchenko et al. (2006). Samples were re-dissolved in 30 μL 1% (v/v) formic acid and 2 μL were analyzed by nano-UPLC-MSE analysis as described in Vassão et al. (2018). Data were acquired using data-independent acquisition, referred to as enhanced MSE. MS data were collected using MassLynx v4.1 software (Waters, Milford, MA, United States).
The processing of nano-UPLC-MSE data and protein identification was performed as follows: the acquired continuum of LC-MSE data were processed using the ProteinLynx Global Server (PLGS) version 2.5.2 (Waters) to generate product ion spectra for database searching according to the ion accounting algorithm described in Li et al. (2009). Processed data were searched against a reference sequence (Refseq) database containing Arabidopsis thaliana sequences (40785 sequences, downloaded from the Identical Protein Groups database at the National Center for Biotechnology Information (NCBI)1 combined with a subdatabase containing common contaminants (O’Leary et al., 2016). Database searching was performed at a false discovery rate (FDR) of 2% with the following parameters: minimum numbers of fragments per peptide (3), peptides per protein (1), fragments per protein (7), and maximum number of missed tryptic cleavage sites (1). Searches were restricted to tryptic peptides with a fixed carbamidomethylation of cysteine residues along with variable oxidation of methionine. Proteins were classified according to the algorithm described for PAnalyzer software (Prieto et al., 2012) and divided into four groups: conclusive, indistinguishable, ambiguous, and non-conclusive. Conclusive and indistinguishable hits were considered as confident matches.
To determine whether P. armoraciae excretes active myrosinase enzyme, we analyzed myrosinase activity in feces homogenates and compared this activity with the corresponding ingested myrosinase activity in leaves. We used newly emerged P. armoraciae beetles that had been reared on B. rapa, and fed them for 1 day with myb leaves containing myrosinase activity but no 4MSOB glucosinolate (n = 6, 6 beetles per replicate). Leaves were weighed before and after feeding to determine the ingested plant fresh weight. Leaves were supplied with water during the experiment and the average proportional weight gain of intact leaves was used to correct the initial leaf weight (n = 16). Feces from each replicate were collected in 130 μL extraction buffer [20 mM MES buffer, pH 6.5, containing protease inhibitors (cOmplete, EDTA-free)]. Feces and fed leaves were frozen in liquid nitrogen and stored at −80°C until extraction. Feces samples were homogenized with metal beads at 25 Hz for 2 min in a TissueLyzer II. The corresponding frozen leaf samples were homogenized with metal beads at 25 Hz for 2 min in a pre-cooled sample holder to prevent thawing. For each replicate, we calculated the ingested fresh weight and extracted the corresponding amount of homogenized plant tissue in the same buffer volume used for feces extraction. After centrifugation at 4°C for 10 min at 16,000 × g, the supernatant was directly used for myrosinase activity assays. Assays consisted of 25 μL extraction buffer, 5 μL of an aqueous 11 mM 4MSOB glucosinolate solution, and (a) 25 μL feces homogenate or (b) 25 μL leaf extract. Assays containing only extraction buffer and substrate served as background control (n = 3). To test whether feces extracts have an inhibitory effect on plant myrosinase activity, we additionally performed assays in which we mixed 25 μL of feces homogenate with 25 μL of corresponding leaf extract and 5 μL glucosinolate substrate. Except for three assays with combined feces and leaf extracts, all assays were performed with two technical replicates. Assays were incubated for 30 min at 30°C, stopped by boiling for 5 min at 95°C, and 50 μL of 60% (v/v) methanol were added. After the activity assay, samples containing feces homogenates were centrifuged at 4°C for 10 min at 16,000 × g, the supernatant collected and final samples were stored at −20°C until LC-MS/MS analysis. 4MSOB glucosinolate was quantified as described in Experiment 2 and myrosinase activity was expressed as nmol 4MSOB glucosinolate hydrolyzed per minute and mg (ingested) plant fresh weight.
Statistical Analyses
Statistical analyses were performed in R 3.3.1 (R Core Team, 2018) or in SigmaPlot 11.0 (Systat Software). Details of statistical analyses performed for each dataset are summarized in Supplementary Table 1.
Results
Plant Myrosinase Activity Influences Glucosinolate Sequestration
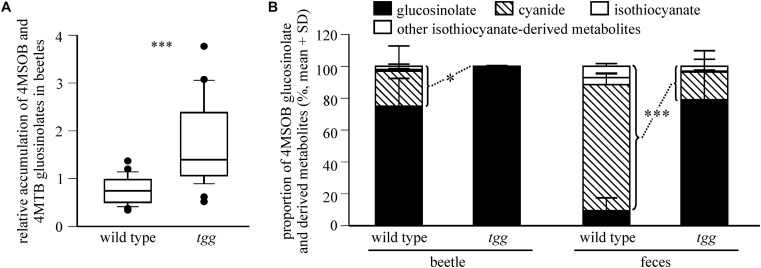
We examined the influence of plant myrosinase activity on glucosinolate sequestration in P. armoraciae by comparing the relative accumulation of 4MSOB and 4MTB glucosinolates in adults that fed on Arabidopsis leaves with (wild type) or without (tgg) myrosinase activity (Experiment 1). Tgg-fed beetles accumulated twofold higher levels of glucosinolates than wild type-fed beetles (Mann-Whitney rank sum test, U = 482.000, p < 0.001; Figure 2A), whereas beetle feeding rates and glucosinolate levels did not differ between both plant genotypes (results of statistical analyses are shown in Supplementary Table 1).
FIGURE 2.
Plant myrosinase activity influences glucosinolate sequestration in P. armoraciae. (A) Accumulation of 4-methylsulfinylbutyl (4MSOB) and 4-methylthiobutyl (4MTB) glucosinolates in P. armoraciae adults after feeding on Arabidopsis wild type and myrosinase-deficient tgg leaves (n = 28 per plant genotype). Glucosinolates in beetles and fed leaves were extracted, converted to desulfo-glucosinolates and analyzed by high performance liquid chromatography coupled to diode array detection (HPLC-DAD). The glucosinolate concentration per mg beetle fresh weight is expressed relative to the concentration per mg plant fresh weight in the corresponding fed leaf, which was set to 1. Boxplots show the median and the 25th and 75th percentile. Dots represent data points that lie outside the 10th and 90th percentiles. Data were compared by Mann-Whitney rank sum test (∗∗∗p < 0.001) (B) Relative composition of 4MSOB glucosinolate and hydrolysis products detected in bodies and feces of wild type- or tgg-fed adults (n = 5 per plant genotype). Beetles that had fed on the Arabidopsis myb mutant served as a control (n = 5). Glucosinolates and hydrolysis products were extracted with 50% methanol and analyzed by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS). Metabolites were quantified using external standard curves. The average amounts of each metabolite detected in control beetles and their feces was subtracted from the corresponding samples of wild type and tgg-fed beetles. The proportions of hydrolysis products relative to the total amount of detected glucosinolate and hydrolysis products in bodies and feces of wild type and tgg-fed beetles (set to 100%, respectively) were compared by Student’s t-tests (beetles: ∗p = 0.025, feces ∗∗∗p < 0.001). Results of statistical analyses are shown in Supplementary Table 1. 4MSOB cyanide corresponds to the nitrile hydrolysis product of 4MSOB glucosinolate. Other isothiocyanate-derived metabolites comprise 4MSOB isothiocyanate-glutathione conjugate, 4MSOB isothiocyanate-cysteinylglycine conjugate, 4MSOB isothiocyanate-cysteine conjugate, 2-(4-(methylsulfinyl)butylamino)-4,5-dihydrothiazole-carboxylic acid, 4MSOB amine, and 4MSOB acetamide.
To determine whether the lower glucosinolate accumulation in wild type-fed beetles is due to glucosinolate hydrolysis, we analyzed the levels of 4MSOB glucosinolate and known hydrolysis products in bodies and feces of wild type- and tgg-fed beetles (Experiment 2). Because the detected metabolite levels differed greatly between replicates (Supplementary Table 2), we compared the amounts of hydrolysis products relative to the total amounts of glucosinolate and hydrolysis products in each sample. We detected a significantly higher proportion of hydrolysis products in body and feces samples of wild type-fed beetles than in the corresponding samples of tgg-fed beetles (Figure 2B; beetles: Student’s t-test, t = 2.754, p = 0.025, feces: Student’s t-test, t = 7.229, p < 0.001). 4MSOB cyanide represented the dominant hydrolysis product in body and feces samples, whereas only traces of free and metabolized isothiocyanates were found (Supplementary Table 2 and Figure 2B). Overall, glucosinolate hydrolysis products accounted for 28% and 2.2% of the total detected metabolites in wild type- and tgg-fed beetles, respectively (bodies and feces). Together, our results show that a fraction of ingested 4MSOB glucosinolate was hydrolyzed by the plant myrosinase, but that glucosinolate hydrolysis also occurred independently of plant myrosinase activity.
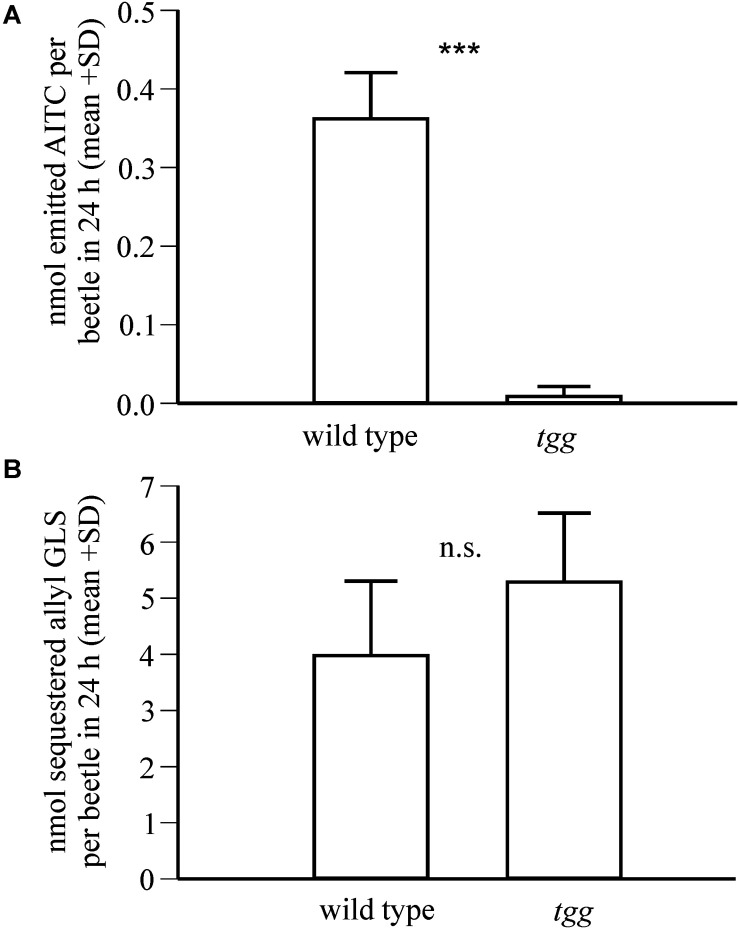
Because allyl glucosinolate represents the major glucosinolate in the natural host plant of P. armoraciae, we quantified the impact of plant myrosinase activity on the metabolic fate of ingested allyl glucosinolate (Experiment 3). This experiment was performed with beetles that were reared on B. rapa and thus do not contain allyl glucosinolate (Supplementary Table 3). Tgg-fed beetles accumulated 9% more of the total ingested allyl glucosinolate than wild type-fed beetles (Table 1), confirming a negative influence of plant myrosinase activity on sequestration. In a separate feeding experiment, we quantified the emission of the volatile allyl glucosinolate hydrolysis product allyl isothiocyanate (Experiment 4), and detected significantly higher amounts of allyl isothiocyanate in the headspace of wild type-fed beetles than in the headspace of tgg-fed beetles (Figure 3A). However, in this experiment, we found no difference regarding the amounts of sequestered allyl glucosinolate in wild type and tgg-fed beetles (Figure 3B; results of statistical analyses are summarized in Supplementary Table 1).
TABLE 1.
Recovery of ingested allyl glucosinolate in P. armoraciae.
|
Mean percentage ± SD (n = 10) |
tb | p | ||
| Wild type-fed | tgg-fed | |||
| Beetle | 43.8 ± 8.2 | 53.0 ± 4.8 | 2.924 | 0.009 |
| Feces | 0.2 ± 0.3 | 0.3 ± 0.1 | 0.655 | 0.521 |
| Unknowna | 56.0 ± 8.4 | 46.7 ± 4.8 | 2.883 | 0.010 |
aProportion of ingested allyl glucosinolate that could not be recovered. bData were arcsine square root transformed before statistical analysis by Student’s t-test.
FIGURE 3.
Impact of plant myrosinase activity on allyl glucosinolate sequestration in P. armoraciae. Adult beetles were fed with allyl glucosinolate-spiked Arabidopsis leaves with (wild type) and without (tgg) myrosinase activity for 1 day (n = 6–7 per plant genotype). (A) Headspace volatiles were collected on Porapaq-QTM adsorbent, eluted with hexane, and analyzed by gas chromatography-mass spectrometry. The amounts of emitted allyl isothiocyanate (AITC; m/z 99) were quantified using an external standard curve. Detected AITC amounts were corrected by subtracting the background emission detected in volatile collections performed without beetles, which served as controls. AITC amounts were compared by Mann-Whitney rank sum test (***p < 0.001). (B) The amounts of sequestered allyl glucosinolate in beetles were quantified after conversion to desulfo-glucosinolate using an internal standard by HPLC-DAD. The amounts of allyl glucosinolate were compared by Student’s t-test and were not significantly different (n.s., p > 0.05). Results of statistical analyses are provided in Supplementary Table 1.
Feeding on Arabidopsis Genotypes With or Without Myrosinase Activity Does Not Affect Beetle Weight and Energy Reserves
To determine whether glucosinolate hydrolysis influences beetle performance, we compared the beetle fresh weight and different energy reserves in males and females after feeding on Arabidopsis wild type and tgg leaves for 10 days after eclosion. As a control, we compared the nutritional value of wild type and tgg leaves and found no differences regarding the total levels of soluble protein, free amino acids, soluble sugars and glucosinolates between wild type and tgg plants (Supplementary Table 4). The food plant had no influence on the beetle weight or the levels of soluble protein, lipids, glycogen and soluble carbohydrates in both males and females (Supplementary Table 5).
P. armoraciae Rapidly Absorbs Glucosinolates Across the Gut
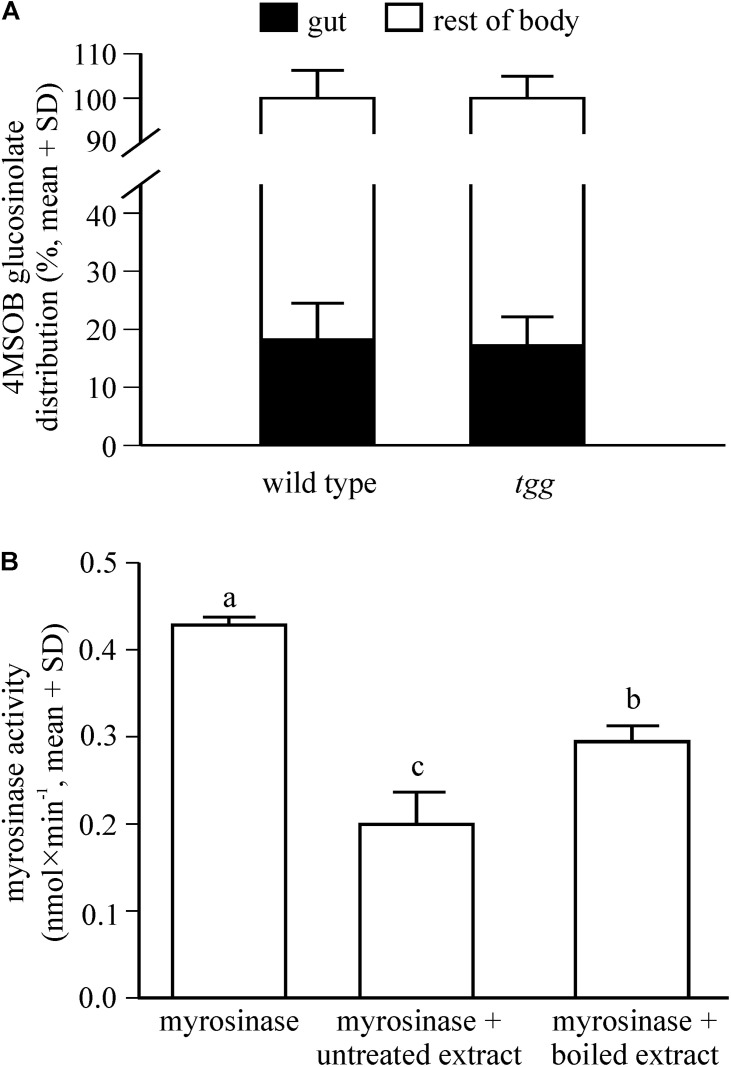
To establish whether P. armoraciae can rapidly absorb ingested glucosinolates, we analyzed the distribution of 4MSOB glucosinolate in P. armoraciae beetles shortly after feeding for 1 min on wild type and tgg leaves. We recovered more than 80% of the total detected 4MSOB glucosinolate from the body (without gut) and this glucosinolate distribution did not differ between wild type- and tgg-fed beetles (Student’s t-test, t = 0.075, p = 0.944, Figure 4A).
FIGURE 4.
Distribution of 4MSOB glucosinolate in the body of adult P. armoraciae beetles shortly after ingestion (A) and influence of beetle gut content extracts on plant myrosinase activity in in vitro enzyme assays (B). (A) Beetles were allowed to feed for 1 min on Arabidopsis leaves with (wild type) or without (tgg) myrosinase activity and were dissected into gut and rest of body 5 min later (n = 3 per plant genotype). Beetles were extracted with 80% methanol and 4MSOB glucosinolate was quantified by LC-MS/MS using an external standard curve. The distribution of 4MSOB glucosinolate in gut and rest of body is expressed relative to the total amount detected in both samples (set to 100%). (B) Enzyme assays were performed with partially purified Sinapis alba myrosinase that was incubated with 4MSOB glucosinolate as a substrate in the presence of untreated gut content extract of P. armoraciae, boiled gut content extract, or buffer (n = 4 per treatment). Myrosinase activity was determined by quantifying the 4MSOB glucosinolate substrate in each assay after conversion to desulfo-glucosinolate and analysis by HPLC-DAD. Assays without myrosinase served as background controls and activities were subtracted from the corresponding samples. Myrosinase activity was compared by One-way ANOVA (p < 0.001). Different letters indicate significant differences between groups. The result of the statistical analysis is shown in Supplementary Table 1.
Gut Content Extracts of P. armoraciae Reduce Myrosinase Activity in vitro
To determine whether glucosinolate sequestration may be facilitated by suppression of plant myrosinase activity in the gut lumen of P. armoraciae, we analyzed the influence of beetle gut content extracts on plant myrosinase activity in in vitro assays. Compared to control assays, gut content extracts significantly reduced myrosinase activity by up to 50% (ANOVA, F = 85.639, p < 0.001). Boiled gut content extracts reduced myrosinase activity significantly less than untreated extracts (Figure 4B).
P. armoraciae Excretes Inactive Myrosinase Enzyme
To investigate whether the Arabidopsis myrosinases TGG1 and TGG2 are degraded in the gut of P. armoraciae, we analyzed the fecal proteome. We detected a total of 14 peptides derived from the Arabidopsis myrosinase TGG1 in two protein bands between 55 and 70 kDa covering 34% of the TGG1 amino acid sequence (Figures 5A,B and Supplementary Table 6). The molecular weight range in which we detected TGG1 peptides corresponds approximately to the predicted (61.1 kDa) and apparent (75 kDa) molecular weight of TGG1 (Zhou et al., 2012). TGG2-specific peptides were not detected in feces with our approach.
FIGURE 5.
Detection of Arabidopsis myrosinases in the feces of P. armoraciae. (A) One dimensional SDS/PAGE gel of crude feces protein extract. Numbers indicate samples excised for proteomic analysis by nano-UPLC-MSE (only odd numbers are shown). TGG1-derived peptides were detected in bands marked with asterisks (*). (B) Amino acid sequence alignment of the Arabidopsis myrosinases TGG1 (AT5G26000.1) and TGG2 (AT5G25980.2). Identical amino acids in the TGG2-sequence are represented by a dot. Peptides highlighted with black background were detected by nano-UPLC-MSE (Supplementary Table 6). Only one detected peptide matched both TGG1 and TGG2.
Since the proteomic analysis indicates that P. armoraciae excretes intact myrosinase, we compared the levels of ingested myrosinase activity with those that were excreted. Myrosinase activity detected in feces corresponded to less than 4% of the ingested activity (Figure 6, t = 10.449, p < 0.005). We additionally spiked plant myrosinase extracts into feces homogenates, but observed similar activity as in control assays (Figure 6, t = 0.158, p = 1.000).
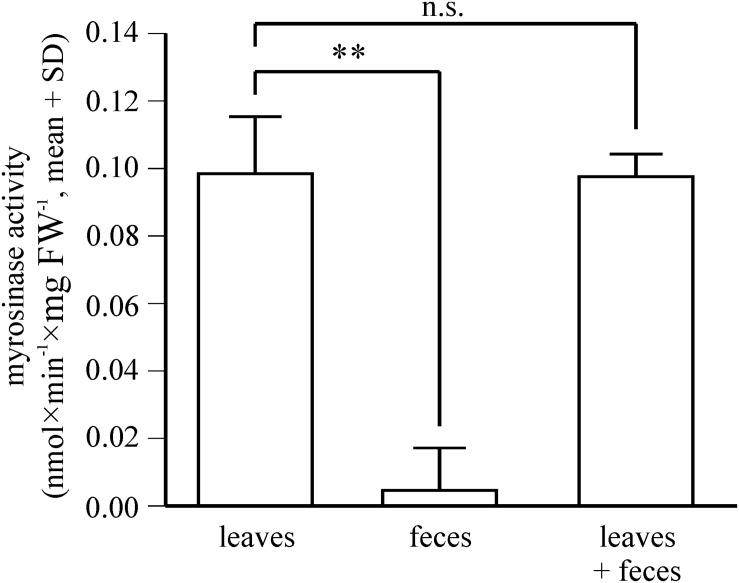
FIGURE 6.
Fate of ingested plant myrosinase activity in P. armoraciae. Levels of soluble plant myrosinase activity were analyzed in extracts prepared from intact Arabidopsis myb leaves and in the corresponding amount of digested leaf material (feces). In addition, plant myrosinase extracts were co-incubated with the corresponding feces extract (n = 5–6 per treatment). Myrosinase activity was determined by adding 4MSOB glucosinolate to each sample. After 30 min incubation, the reaction was stopped by boiling. Remaining glucosinolate substrate was quantified by LC-MS/MS. Myrosinase activity in intact leaves was compared with that in digested leaves and in the co-incubation treatment by paired Student’s t-tests, respectively (∗∗p < 0.005; n.s., p > 0.05). Results of statistical analyses are shown in Supplementary Table 1.
Discussion
The enzymatic activity of defensive plant β-glucosidases is a major barrier to the sequestration of plant glucosides by herbivorous insects (Morant et al., 2008; Pentzold et al., 2014b). Here, we investigated the impact of plant myrosinase activity on the sequestration of glucosinolates in P. armoraciae and explored possible mechanisms that enable adult beetles to suppress glucosinolate hydrolysis during feeding and digestion. We demonstrate a negative influence of plant myrosinase activity on sequestration and confirmed that a fraction of ingested glucosinolates is hydrolyzed by plant myrosinases. We found two mechanisms that can reduce the glucosinolate hydrolysis rate in the gut: the rapid absorption of ingested glucosinolates across the gut epithelium and the suppression of plant myrosinase activity in the gut lumen.
In previous quantitative feeding studies with P. chrysocephala, plant myrosinases hydrolyzed approximately 75% of the total ingested glucosinolates (Beran et al., 2018; Ahn et al., 2019), whereas only about 10% of ingested allyl glucosinolate were hydrolyzed in our quantitative study with P. armoraciae. Although P. chrysocephala and P. armoraciae belong to different genera, they have similar body sizes and feeding modes and therefore cause comparable feeding damage. Our results thus indicate that P. armoraciae adults are better adapted to overcome plant myrosinase activity than P. chrysocephala adults.
Plant myrosinases hydrolyzed a fraction of ingested glucosinolates and thus negatively influenced glucosinolate sequestration in P. armoraciae (Figures 2, 3 and Table 1). Although the sequestration rates of 4MSOB glucosinolate and allyl glucosinolate cannot be compared directly because different methods were used for quantification, our results indicate a stronger influence of plant myrosinase activity on 4MSOB glucosinolate than on allyl glucosinolate. Biochemical studies with Arabidopsis myrosinases TGG1 and TGG2 revealed similar activities of both enzymes toward these two glucosinolates (Zhou et al., 2012), making it unlikely that the substrate preferences of Arabidopsis myrosinases have affected sequestration. A similar glucosinolate-dependent effect of plant myrosinase activity on sequestration was also observed in P. armoraciae larvae (Sporer et al., 2020). Larvae sequestered allyl glucosinolate from B. juncea leaves but almost no glucosinolates from Arabidopsis wild type leaves. Although we detected similar levels of soluble myrosinase activity in crude protein extracts of Arabidopsis and B. juncea leaves in enzyme assays, we cannot rule out that different levels of myrosinase activity in Arabidopsis and B. juncea were responsible for this result. Nevertheless, our results suggest that the metabolic fate of ingested allyl glucosinolate in P. armoraciae is less affected by plant myrosinase activity than that of Arabidopsis glucosinolates. Since allyl glucosinolate represents the dominant glucosinolate in horseradish (Li and Kushad, 2004), P. armoraciae might have developed specific mechanisms to avoid the hydrolysis of the characteristic glucosinolate of its natural host plant.
Glucosinolate hydrolysis products, in particular isothiocyanates, are well-known to have a negative impact on insect growth and development by interfering with nutrition (Agrawal and Kurashige, 2003; Jeschke et al., 2016b, 2017; Sun et al., 2019). Under our experimental conditions, feeding beetles with Arabidopsis with or without myrosinase activity did not influence the weight or energy reserves of P. armoraciae adults. In addition, the food plant did not influence the developmental time, weight, and energy reserves of P. armoraciae larvae (for details refer to Supplementary Material and Supplementary Table 7). Although the exposure to glucosinolate hydrolysis products could influence other fitness parameters such as female fecundity or egg hatching rate (Sun et al., 2019), our current results indicate that P. armoraciae can tolerate glucosinolate hydrolysis.
Previous feeding experiments performed with generalist lepidopteran herbivores revealed 4MSOB isothiocyanate to be the major hydrolysis product of 4MSOB glucosinolate in Arabidopsis (Schramm et al., 2012; Jeschke et al., 2017). However, instead of the isothiocyanate, we detected mainly the nitrile hydrolysis product of 4MSOB glucosinolate in P. armoraciae bodies and feces (Figure 2B). There are several possible explanations for this unexpected result: 4MSOB isothiocyanate may not have been recovered completely with our extraction methods because it has reacted with proteins or has been metabolized by the beetle or associated gut microbes (Brown and Hampton, 2011; Jeschke et al., 2015; van den Bosch and Welte, 2017; Friedrichs et al., 2020; Shukla and Beran, 2020). Alternatively, P. armoraciae may be able to manipulate the outcome of glucosinolate hydrolysis and promote the formation of the less reactive nitrile instead of the isothiocyanate. Manipulation of glucosinolate hydrolysis occurs for example in larvae of the cabbage white butterfly, which express a so-called nitrile specifier protein in the gut (Wittstock et al., 2004). In fact, a redirection of glucosinolate hydrolysis toward less toxic nitriles could explain why plant myrosinase activity had no measurable effect on P. armoraciae performance. Further research is needed to understand the mechanism underlying the unusual composition of glucosinolate hydrolysis products in P. armoraciae.
How chewing insects that cause extensive tissue damage prevent the hydrolysis of ingested plant glucosides is currently not well understood. One proposed mechanism is a rapid absorption of plant glucosides across the gut epithelium separating substrate and enzyme (Abdalsamee et al., 2014; Pentzold et al., 2014b; van Geem et al., 2014). Although we observed a very rapid uptake of ingested glucosinolates in P. armoraciae, it remains unclear whether this indeed prevents glucosinolate hydrolysis in the gut lumen. Moreover, we had expected to find less glucosinolates in the guts of wild type-fed beetles due to the presence of myrosinase activity. Surprisingly, we recovered similar proportions of glucosinolates in the guts of tgg- and wild type-fed beetles, although we cannot rule out that glucosinolates detected in the gut were spatially separated from plant myrosinases, either in remaining intact plant tissue or in the gut epithelium.
The absorption of polar plant defense compounds such as glucosinolates across the gut is proposed to be mediated by membrane transporters (Discher et al., 2009; Petschenka and Agrawal, 2016). We recently identified glucosinolate-specific transporters belonging to the major facilitator superfamily (MFS) in P. armoraciae and found two glucosinolate transporters to be expressed in the foregut, suggesting a role in glucosinolate uptake. However, silencing the expression of these transporters did not affect the uptake of ingested glucosinolates in adults, which indicates that additional or other transporters mediate glucosinolate absorption in P. armoraciae (Yang et al., 2021).
Another explanation for the detection of glucosinolates in the gut of P. armoraciae is the inhibition of plant myrosinase activity in the gut. Gut content extracts reduced plant myrosinase activity by 30 to 50% in in vitro assays, with boiled extracts inhibiting myrosinase activity significantly less than untreated extracts (Figure 4B). These results indicate that the activity of ingested plant myrosinases is possibly reduced in the gut lumen of P. armoraciae. Interestingly, gut content extracts of the closely related flea beetle P. chrysocephala also reduced plant myrosinase activity in vitro, but, in contrast to P. armoraciae, there was no difference between the boiled and the untreated extract (refer to Supplementary Material, Supplementary Figure 1). However, the observed effects of gut content extracts on myrosinase activity may not be specific for glucosinolate-sequestering flea beetles. To investigate this further, additional controls such as protein standard solutions and gut content extracts of other insect species should be included in future experiments. In general, plant myrosinase activity can be influenced by various factors including the levels of ascorbic acid (cofactor of myrosinases), sulfate, sodium chloride and silver ions (Shikita et al., 1999; Andersson et al., 2009; Bhat and Vyas, 2019; Marcinkowska and Jelen, 2020). In addition, the gut pH can have a strong influence on the activity of ingested plant enzymes (Pentzold et al., 2014b). For example, the highly alkaline pH of the midgut lumen of burnet moth larvae drastically reduced cyanogenic β-glucosidase activity in Lotus corniculatus leaf macerates (Pentzold et al., 2014a). In contrast, the neutral pH of gut homogenates of glucosinolate-sequestering turnip sawfly larvae had only minor influence on ingested plant myrosinase activity (Abdalsamee et al., 2014). In Coleoptera, gut pH values ranging from 5 to 8 have been reported (Terra and Ferreira, 1994). For example, a comparison of the gut pH in two closely related flea beetles of the genus Longitarsus revealed species-specific pH values of 7.0 and 5.3 (Pankoke and Dobler, 2015). Gut homogenates of P. armoraciae showed an acidic pH (details are described in the Supplementary Material), which is unlikely to have a strong influence on plant myrosinase activity.
Myrosinase from S. alba and other plant defensive β-glucosidases were largely resistant to digestion in the larval gut of the generalist lepidopteran Spodoptera littoralis and thus retained most of the activity after digestion (Vassão et al., 2018). Our proteomic analysis of beetle feces also indicates that Arabidopsis myrosinase TGG1 resisted digestion in P. armoraciae, whereas TGG2 was not detected in feces (Figure 5 and Supplementary Table 5). Because TGG2 expression is restricted to phloem-associated cells (Barth and Jander, 2006), beetles have likely ingested less TGG2 than TGG1 by avoiding the leaf midrib and veins (personal observation). Despite the detection of TGG1 enzyme, we found almost no myrosinase activity in feces of P. armoraciae. We tested for the presence of myrosinase inhibitor(s) in feces homogenates but observed no suppression of spiked myrosinase activity under our assay conditions (Figure 6). We thus hypothesize that ingested TGG1 has been inactivated during gut passage in P. armoraciae. Previous studies with the turnip sawfly and the diamondback moth also indicated that plant myrosinases are not fully active in the gut (Abdalsamee et al., 2014; Sun et al., 2019). However, the underlying mechanism(s) of myrosinase inhibition in the gut of specialist herbivores including P. armoraciae remain to be determined.
P. armoraciae possesses endogenous myrosinase activity, which enables larvae to use sequestered glucosinolates for defense against predators (Sporer et al., 2020). Interestingly, we detected glucosinolate hydrolysis products in the bodies and feces of larvae and adults that had fed on the myrosinase-deficient tgg mutant (adults: Figures 2B, 3A, larvae: refer to Supplementary Material, Supplementary Table 8 and Supplementary Figure 2). These findings suggest that the beetle myrosinase also plays a role in the endogenous metabolism of sequestered glucosinolates. Similarly, volatile hydrolysis products derived from sequestered glucosinolates have also been detected in the headspace of the striped flea beetle, Phyllotreta striolata, which led to the initial discovery of a glucosinolate-myrosinase defense system in Phyllotreta flea beetles (Beran, 2011; Beran et al., 2014). To investigate the role of the beetle myrosinase in glucosinolate metabolism and defense in P. armoraciae, we currently perform experiments using beetles with suppressed myrosinase activity.
Conclusion
Defensive plant β-glucosidases represent a major target of herbivore adaptation to plants equipped with a two-component chemical defense. Our study demonstrates that the specialist herbivore P. armoraciae tolerates the hydrolysis of a fraction of ingested glucosinolates and is able to rapidly absorb glucosinolates across the gut. Moreover, we provide evidence that P. armoraciae can inactivate plant myrosinases in the gut. The ability to tolerate plant myrosinase activity could be an important prerequisite to develop mechanisms that enable the sequestration of glucosinolates.
Data Availability Statement
Source data of this study is available at the open access data repository of the Max Planck Society (Edmond) under doi: 10.17617/3.5b.
Author Contributions
TS, JK, and FB designed the experiments and wrote the manuscript. TS, JK, YH, and FB performed the experiments. TS, JK, MR, NW, and FB analyzed the data. SG-J performed the bioinformatic analyses. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the greenhouse team at the Max Planck Institute for Chemical Ecology for plant cultivation, Susanne Donnerhacke, Alexander Schilling, Fabian Seitz, and Leopold Wohlsperger for help with the rearing and experiments, Grit Kunert for help with statistical analyses, Daniel Veit and the workshop team for technical support, Caroline Müller and Helga Pankoke (University of Bielefeld) for help with the gut pH measurements, Sarah Wolf (Agroscope, Switzerland) for lending equipment for the energy budget analysis, and Felix Feistel for discussions.
Funding. This project was supported by the Max Planck Society and the International Max Planck Research School.
https://www.ncbi.nlm.nih.gov/ipg on February 28, 2020
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.645030/full#supplementary-material
References
- Abdalsamee M. K., Giampà M., Niehaus K., Müller C. (2014). Rapid incorporation of glucosinolates as a strategy used by a herbivore to prevent activation by myrosinases. Insect Biochem. Mol. Biol. 52 115–123. 10.1016/j.ibmb.2014.07.002 [DOI] [PubMed] [Google Scholar]
- Agrawal A. A., Kurashige N. S. (2003). A role for isothiocyanates in plant resistance against the specialist herbivore Pieris rapae. J. Chem. Ecol. 29 1403–1415. [DOI] [PubMed] [Google Scholar]
- Ahn S.-J., Betzin F., Gikonyo M. W., Yang Z.-L., Köllner T. G., Beran F. (2019). Identification and evolution of glucosinolate sulfatases in a specialist flea beetle. Sci. Rep. 9:15725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson D., Chakrabarty R., Bejai S., Zhang J., Rask L., Meijer J. (2009). Myrosinases from root and leaves of Arabidopsis thaliana have different catalytic properties. Phytochemistry 70 1345–1354. 10.1016/j.phytochem.2009.07.036 [DOI] [PubMed] [Google Scholar]
- Barth C., Jander G. (2006). Arabidopsis myrosinases TGG1 and TGG2 have redundant function in glucosinolate breakdown and insect defense. Plant J. 46 549–562. 10.1111/j.1365-313x.2006.02716.x [DOI] [PubMed] [Google Scholar]
- Beran F. (2011). Host Preference and Aggregation Behavior of the Striped Flea Beetle, Phyllotreta Striolata Ph.D. thesis. Berlin: Humboldt-Universität. [Google Scholar]
- Beran F., Köllner T. G., Gershenzon J., Tholl D. (2019). Chemical convergence between plants and insects: biosynthetic origins and functions of common secondary metabolites. New Phytol. 223 52–67. 10.1111/nph.15718 [DOI] [PubMed] [Google Scholar]
- Beran F., Pauchet Y., Kunert G., Reichelt M., Wielsch N., Vogel H., et al. (2014). Phyllotreta striolata flea beetles use host plant defense compounds to create their own glucosinolate-myrosinase system. Proc. Natl. Acad. Sci. U.S.A. 111 7349–7354. 10.1073/pnas.1321781111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran F., Sporer T., Paetz C., Ahn S.-J., Betzin F., Kunert G., et al. (2018). One pathway is not enough: the cabbage stem flea beetle Psylliodes chrysocephala uses multiple strategies to overcome the glucosinolate-myrosinase defense in its host plants. Front. Plant Sci. 9:1754. 10.3389/fpls.2018.01754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R., Vyas D. (2019). Myrosinase: insights on structural, catalytic, regulatory, and environmental interactions. Crit. Rev. Biotechnol. 39 508–523. 10.1080/07388551.2019.1576024 [DOI] [PubMed] [Google Scholar]
- Blažević I., Montaut S., Burčul F., Olsen C. E., Burow M., Rollin P., et al. (2020). Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 169:112100. 10.1016/j.phytochem.2019.112100 [DOI] [PubMed] [Google Scholar]
- Brown K. K., Hampton M. B. (2011). Biological targets of isothiocyanates. Biochim. Biophys. Acta 1810 888–894. 10.1016/j.bbagen.2011.06.004 [DOI] [PubMed] [Google Scholar]
- Ciska E., Horbowicz M., Rogowska M., Kosson R., Drabińska N., Honke J. (2017). Evaluation of seasonal variations in the glucosinolate content in leaves and roots of four European horseradish (Armoraica rusticana) landraces. Pol. J. Food Nutr. Sci. 67 301–308. 10.1515/pjfns-2016-0029 [DOI] [Google Scholar]
- Discher S., Burse A., Tolzin-Banasch K., Heinemann S. H., Pasteels J. M., Boland W. (2009). A versatile transport network for sequestering and excreting plant glycosides in leaf beetles provides an evolutionary flexible defense strategy. Chembiochem 10 2223–2229. 10.1002/cbic.200900226 [DOI] [PubMed] [Google Scholar]
- Foray V., Pelisson P.-F., Bel-Venner M.-C., Desouhant E., Venner S., Menu F., et al. (2012). A handbook for uncovering the complete energetic budget in insects: the van Handel’s method (1985) revisited. Physiol. Entomol. 37 295–302. 10.1111/j.1365-3032.2012.00831.x [DOI] [Google Scholar]
- Friedrichs J., Schweiger R., Geisler S., Mix A., Wittstock U., Müller C. (2020). Novel glucosinolate metabolism in larvae of the leaf beetle Phaedon cochleariae. Insect Biochem. Mol. Biol. 124:103431. 10.1016/j.ibmb.2020.103431 [DOI] [PubMed] [Google Scholar]
- Halkier B. A., Gershenzon J. (2006). Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 57 303–333. 10.1146/annurev.arplant.57.032905.105228 [DOI] [PubMed] [Google Scholar]
- Jeschke V., Gershenzon J., Vassão D. G. (2015). “Metabolism of glucosinolates and their hydrolysis products in insect herbivores,” in Recent Advances Phytochemistry: The Formation, Structure and Activity of Phytochemicals, ed. Jetter R. (Cham: Springer International Publishing; ), 163–194. 10.1007/978-3-319-20397-3_7 [DOI] [Google Scholar]
- Jeschke V., Gershenzon J., Vassão D. G. (2016a). “Chapter 8 – insect detoxification of glucosinolates and their hydrolysis products,” in Advances in Botanical Research, ed. Kopriva S. (Cambridge, MA: Academic Press; ), 199–245. 10.1016/bs.abr.2016.06.003 [DOI] [Google Scholar]
- Jeschke V., Gershenzon J., Vassão D. G. (2016b). A mode of action of glucosinolate-derived isothiocyanates: detoxification depletes glutathione and cysteine levels with ramifications on protein metabolism in Spodoptera littoralis. Insect Biochem. Mol. Biol. 71 37–48. 10.1016/j.ibmb.2016.02.002 [DOI] [PubMed] [Google Scholar]
- Jeschke V., Kearney E. E., Schramm K., Kunert G., Shekhov A., Gershenzon J., et al. (2017). How glucosinolates affect generalist lepidopteran larvae: growth, development and glucosinolate metabolism. Front. Plant Sci. 8:1995. 10.3389/fpls.2017.01995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazana E., Pope T. W., Tibbles L., Bridges M., Pickett J. A., Bones A. M., et al. (2007). The cabbage aphid: a walking mustard oil bomb. Proc. R. Soc. London Ser. B Biol. Sci. 274 2271–2277. 10.1098/rspb.2007.0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G.-Z., Vissers J. P. C., Silva J. C., Golick D., Gorenstein M. V., Geromanos S. J. (2009). Database searching and accounting of multiplexed precursor and product ion spectra from the data independent analysis of simple and complex peptide mixtures. Proteomics 9 1696–1719. 10.1002/pmic.200800564 [DOI] [PubMed] [Google Scholar]
- Li X., Kushad M. M. (2004). Correlation of glucosinolate content to myrosinase activity in horseradish (Armoracia rusticana). J. Agric. Food Chem. 52 6950–6955. 10.1021/jf0401827 [DOI] [PubMed] [Google Scholar]
- Malka O., Shekhov A., Reichelt M., Gershenzon J., Vassão D. G., Morin S. (2016). Glucosinolate desulfation by the phloem-feeding insect Bemisia tabci. J. Chem. Ecol. 42 230–235. 10.1007/s10886-016-0675-1 [DOI] [PubMed] [Google Scholar]
- Marcinkowska M., Jelen H. H. (2020). Inactivation of thioglucosidase from Sinapis alba (White Mustard) seed by metal salts. Molecules 25:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morant A. V., Jørgensen K., Jørgensen C., Paquette S. M., Sánchez-Pérez R., Møller B. L., et al. (2008). β-Glucosidases as detonators of plant chemical defense. Phytochemistry 69 1795–1813. 10.1016/j.phytochem.2008.03.006 [DOI] [PubMed] [Google Scholar]
- O’Leary N. A., Wright M. W., Brister J. R., Ciufo S., Haddad D., Mcveigh R., et al. (2016). Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 44 D733–D745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz S. E. W., Müller C. (2009). Plant chemistry and insect sequestration. Chemoecology 19 117–154. 10.1007/s00049-009-0018-6 [DOI] [Google Scholar]
- Pankoke H., Dobler S. (2015). Low rates of iridoid glycoside hydrolysis in two Longitarsus leaf beetles with different feeding specialization confer tolerance to iridoid glycoside containing host plants. Physiol. Entomol. 40 18–29. 10.1111/phen.12085 [DOI] [Google Scholar]
- Pentzold S., Zagrobelny M., Roelsgaard P. S., Møller B. L., Bak S. (2014a). The multiple strategies of an insect herbivore to overcome plant cyanogenic glucoside defence. PLoS One 9:e91337. 10.1371/journal.pone.0091337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentzold S., Zagrobelny M., Rook F., Bak S. (2014b). How insects overcome two-component plant chemical defence: plant β-glucosidases as the main target for herbivore adaptation. Biol. Rev. 89 531–551. 10.1111/brv.12066 [DOI] [PubMed] [Google Scholar]
- Petschenka G., Agrawal A. A. (2016). How herbivores coopt plant defenses: natural selection, specialization, and sequestration. Curr. Opin. Insect Sci. 14 17–24. 10.1016/j.cois.2015.12.004 [DOI] [PubMed] [Google Scholar]
- Prieto G., Aloria K., Osinalde N., Fullaondo A., Arizmendi J. M., Matthiesen R. (2012). PAnalyzer: a software tool for protein inference in shotgun proteomics. BMC Bioinformatics 13:288. 10.1186/1471-2105-13-288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2018). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: http://www.R-project.org/ [Google Scholar]
- Robert C. A. M., Zhang X., Machado R. A. R., Schirmer S., Lori M., Mateo P., et al. (2017). Sequestration and activation of plant toxins protect the western corn rootworm from enemies at multiple trophic levels. eLife 6:e29307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9 676–682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm K., Vassao D. G., Reichelt M., Gershenzon J., Wittstock U. (2012). Metabolism of glucosinolate-derived isothiocyanates to glutathione conjugates in generalist Lepidopteran herbivores. Insect Biochem. Mol. Biol. 42 174–182. 10.1016/j.ibmb.2011.12.002 [DOI] [PubMed] [Google Scholar]
- Shevchenko A., Tomas H., Havlis J., Olsen J. V., Mann M. (2006). In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 1 2856–2860. 10.1038/nprot.2006.468 [DOI] [PubMed] [Google Scholar]
- Shikita M., Fahey J. W., Golden T. R., Holtzclaw W. D., Talalay P. (1999). An unusual case of ‘uncompetitive activation’ by ascorbic acid: purification and kinetic properties of a myrosinase from Raphanus sativus seedlings. Biochem. J. 341 725–732. 10.1042/0264-6021:3410725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S. P., Beran F. (2020). Gut microbiota degrades toxic isothiocyanates in a flea beetle pest. Mol. Ecol. 29 4692–4705. 10.1111/mec.15657 [DOI] [PubMed] [Google Scholar]
- Siemens D. H., Mitchell-Olds T. (1996). Glucosinolates and herbivory by specialists (Coleoptera: Chrysomelidae, Lepidoptera: Plutellidae): consequences of concentration and induced resistance. Environ. Entomol. 25 1344–1353. 10.1093/ee/25.6.1344 [DOI] [Google Scholar]
- Sønderby I. E., Hansen B. G., Bjarnholt N., Ticconi C., Halkier B. A., Kliebenstein D. J. (2007). A systems biology approach identifies a R2R3 MYB gene subfamily with distinct and overlapping functions in regulation of aliphatic glucosinolates. PLoS One 2:e1322. 10.1371/journal.pone.0001322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporer T., Körnig J., Beran F. (2020). Ontogenetic differences in the chemical defence of flea beetles influence their predation risk. Funct. Ecol. 34 1370–1379. 10.1111/1365-2435.13548 [DOI] [Google Scholar]
- Sun R., Jiang X., Reichelt M., Gershenzon J., Pandit S. S., Vassão D. G. (2019). Tritrophic metabolism of plant chemical defenses and its effects on herbivore and predator performance. eLife 8:e51029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terra W. R., Ferreira C. (1994). Insect digestive enzymes: properties, compartmentalization and function. Comp. Biochem. Physiol. Part B Comp. Biochem. 109 1–62. 10.1016/0305-0491(94)90141-4 [DOI] [Google Scholar]
- van den Bosch T. J. M., Welte C. U. (2017). Detoxifying symbionts in agriculturally important pest insects. Microb. Biotechnol. 10 531–540. 10.1111/1751-7915.12483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Geem M., Harvey J. A., Gols R. (2014). Development of a generalist predator, Podisus maculiventris, on glucosinolate sequestering and nonsequestering prey. Naturwissenschaften 101 707–714. 10.1007/s00114-014-1207-x [DOI] [PubMed] [Google Scholar]
- Vassão D. G., Wielsch N., Gomes A. M. D. M. M., Gebauer-Jung S., Hupfer Y., Svatoš A., et al. (2018). Plant defensive β-glucosidases resist digestion and sustain activity in the gut of a lepidopteran herbivore. Front. Plant Sci. 9:1389. 10.3389/fpls.2018.01389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittstock U., Agerbirk N., Stauber E. J., Olsen C. E., Hippler M., Mitchell-Olds T., et al. (2004). Successful herbivore attack due to metabolic diversion of a plant chemical defense. Proc. Natl. Acad. Sci. U.S.A. 101 4859–4864. 10.1073/pnas.0308007101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittstock U., Kurzbach E., Herfurth A.-M., Stauber E. J. (2016). “Chapter 6 – glucosinolate breakdown,” in Advances in Botanical Research, ed. Kopriva S. (Cambridge, MA: Academic Press; ), 125–169. 10.1016/bs.abr.2016.06.006 [DOI] [Google Scholar]
- Yang Z.-L., Kunert G., Sporer T., Körnig J., Beran F. (2020). Glucosinolate abundance and composition in Brassicaceae influence sequestration in a specialist flea beetle. J. Chem. Ecol. 46 186–197. 10.1007/s10886-020-01144-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.-L., Nour-Eldin H. H., Haenniger S., Reichelt M., Crocoll C., Seitz F., et al. (2021). Sugar transporters enable a leaf beetle to accumulate plant defense compounds. bioRxiv [Preprint] 10.1101/2021.03.03.433712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Tokuhisa J. G., Bevan D. R., Esen A. (2012). Properties of β-thioglucoside hydrolases (TGG1 and TGG2) from leaves of Arabidopsis thaliana. Plant Sci. 191-192 82–92. 10.1016/j.plantsci.2012.02.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source data of this study is available at the open access data repository of the Max Planck Society (Edmond) under doi: 10.17617/3.5b.