Abstract
Leg length discrepancy (LLD) is an underrecognized and prevalent condition among the U.S. population, with effects varying depending on the cause and size of the discrepancy. LLD occurs when the paired lower extremities are unequal in length and can be etiologically classified as functional or structural. Length differences are typically less than 10 mm and asymptomatic or easily compensated for by the patient through self-lengthening or shortening of the lower extremities. Literature review of the etiology, diagnostic modalities, clinical complications, and treatment option for patients with LLD. LLD can be assessed directly through tape measurements or indirectly through palpation of bony landmarks. Imaging modalities, specifically radiography, are more precise and help identify coexistent deformity. Once LLD has been diagnosed, evaluation for potential adverse complications is necessary. Discrepancies greater than 20 mm can alter biomechanics and loading patterns with resultant functional limitations and musculoskeletal disorders, such as functional scoliosis. Functional scoliosis is nonprogressive and involves a structurally normal spine with an apparent lateral curvature, which regresses fully or partially when the LLD is corrected. Long-standing LLD and functional scoliosis often result in permanent degenerative changes in the facet joints and intervertebral discs of the spine. Further understanding of the contribution of LLD in the development of scoliosis and degenerative spine disease will allow for more effective preventative treatment strategies and hasten return to function.
Keywords: Leg length discrepancy, Functional scoliosis, Degenerative joint disease, Disc degeneration, Radiography
LEG LENGTH DISCREPANCY
Leg length discrepancy (LLD) is a common orthopedic condition among the pediatric and adult population, with a prevalence rate of 90% of the general population and 40% among athletes.1,2) LLD occurs when the paired lower extremities are unequal in length. Length differences are typically less than 10 mm and asymptomatic or easily compensated for by the patient through self-lengthening or shortening of the lower extremities.3) Some children are born with leg discrepancies, while other causes of LLD, such as tumor, radiation, infection, or injury, are acquired. Common symptoms include trouble walking (limping or toe-walking), pain in the back, hip, knee, or ankle, posture abnormalities (tilting shoulder), and a hyperextended knee on the shorter leg and flexed on the longer leg. Larger LLDs present with more complex symptoms and require extensive treatment.3)
The effects of LLD vary from patient to patient depending on the cause and size of the discrepancy. Classification of leg length inequality was initially conducted by Reid and Smith,4) in which a discrepancy 0–30 mm was considered mild, 30–60 mm considered moderate, and 60 mm or more was considered severe. However, current research supports discrepancies greater than 10 mm as clinically significant.5) Such discrepancies can alter biomechanics and result in functional limitations, such as gait, posture, and balance problems, as well as musculoskeletal disorders, such as lower back pain, scoliosis, and degenerative spinal changes. Our study reviews the etiology, diagnostic modalities, clinical complications, and treatment options for patients with LLD.
ETIOLOGY OF LLD
LLD can be classified etiologically as structural or functional (Table 1). Structural or anatomical LLD refers to physical shortening or lengthening of a unilateral lower extremity. Such osseous change occurs between the ilium and the foot. Causes that shorten the limb are more common than causes that lengthen it. Sources of structural LLD include congenital and acquired conditions.6) Congenital LLD due to limb shortening consists of a group of rare diseases, such as fibular hemimelia and proximal focal femoral deficiency. Acquired LLD involves trauma, infection, neoplasms, radiation, or other idiopathic causes, such as Blount disease or Legg-Perthes disease. Trauma involving fractures along the physis (Salter-Harris fractures) can disrupt growth rate and result in LLD. Infectious causes of LLD include osteomyelitis that extends to the physis, while inflammatory causes include juvenile idiopathic arthritis (JIA).7,8,9) Untreated JIA interferes with bone growth around the affected joint. This may lead to accelerated bone growth, with a resultant longer limb, or rapid premature closure of the epiphyseal growth plate, with a resultant shorter limb. Simon et al.8) demonstrates accelerated bone growth in children in whom JIA developed before nine years of age. Premature growth plate closure occurs in adolescents with JIA. Premature closure and growth arrest is aggravated by treatment of JIA with corticosteroids.9) Other causes of limb lengthening include rare diseases of congenital hemi-hypertrophy, such as Beckwith-Wiedemann Syndrome or idiopathic nonsyndromic hemihypertrophy, as well as Klippel-Trenaunay-Weber syndrome.
Table 1. Structural (Anatomical) and Functional Causes of Leg Length Discrepancy.
| Type of LLD | Definition | Subcategory | Possible cause | |
|---|---|---|---|---|
| Structural (anatomical) | Physical shortening or lengthening of the tibia or femur | Congenital | Limb shortening | |
| • Fibular hemimelia, tibial hemimelia | ||||
| • Proximal focal femoral deficiency | ||||
| • Skeletal dysplasias Limb lengthening | ||||
| • Congenital hemihypertrophy (idiopathic non-syndromic hemihypertrophy) | ||||
| • Dysmorphic syndromes (Beckwith-Wiedemann syndrome; proteus syndrome, Klippel-Trenaunay-Weber syndrome) | ||||
| • Gigantism with neurofibromatosis | ||||
| Acquired | Shortening | |||
| • Trauma (Salter-Harris fractures, slipped capital femoral epiphysis, iatrogenic) | ||||
| • Infection (osteomyelitis, septic arthritis) | ||||
| • Osteonecrosis following developmental dysplasia of the hip | ||||
| • Inflammation (juvenile idiopathic arthritis) | ||||
| • Neoplasms | ||||
| • Radiation | ||||
| • Idiopathic (Blount disease or Legg-Perthes disease) | ||||
| • Neurologic disorders (cerebral palsy, polio, peripheral nerve injury) Lengthening | ||||
| • Neoplasms | ||||
| • Osteomyelitis stimulating growth plate | ||||
| • Chronic hyperemia | ||||
| Functional | Apparent asymmetry of the lower extremity, without shortening or lengthening of the osseous components of the lower limb | Pelvic obliquity due to | ||
| • Adaptive soft-tissue shortening | ||||
| • Joint or muscle contractures | ||||
| • Ligamentous laxity | ||||
| • Axial malalignment | ||||
| • Developmental dysplasia of the hip | ||||
LLD: leg length discrepancy.
Functional LLD refers to apparent asymmetry of the lower extremity, without physical shortening or lengthening of the osseous components of the lower limb.6) Functional LLD can occur anywhere from the superior aspect of the ilium to the inferior aspect of the foot. Functional LLD typically results from pelvic obliquity related to adaptive soft-tissue shortening, joint or muscle contractures, ligamentous laxity, or axial malalignment.6) As the pelvis rotates, the legs are pulled into apparent different lengths. In contrast, true limb shortening or lengthening (structural LLD) is a primary disorder with shortening or lengthening of one extremity.
DIAGNOSIS OF LLD
Proper physical examination and imaging modalities for measuring and assessing LLD are crucial for diagnosis, classification, and treatment. Tape measurements are widely used as a direct method to determine LLD. Tape measurements can differentiate functional LLD from structural LLD. Functional LLD can be measured from the umbilicus to the medial malleolus, with the lower limbs in line with the trunk. Structural LLD can be measured from the anterior superior iliac spine to the medial malleolus or the anterior inferior iliac spine to the lateral malleolus.6) Tape measurements allow precision up to 5 mm and are comparable to gold standard computed tomography (CT) measurements.10) However, potential sources of error can contribute to incorrect length measurement and must be considered carefully. Sources of error include differences in girth, difficulty identifying bony prominences, the presence of concomitant deformity, and the mobility of the skin above the bony prominence.5) To avoid such error, physicians should always use the mean of at least 2–3 measurements. In addition, physicians should measure the leg circumference to assess for muscle atrophy or muscle hyperplasia.
Various indirect methods of assessing LLD exist, including palpation of bony landmarks. The most accurate and precise indirect method involves the use of increasingly thick blocks placed under the patient's shorter leg.11) The patient stands with his/her feet 10 cm apart and knees extended. The clinician places his/her hands on a bilateral anatomic structure, such as the posterior superior iliac spines, anterior superior iliac spines, or the left or right iliac crests. The clinician then places 0.5 cm blocks continuously underneath the patient's foot until equal leg length is achieved. As such, reliability is highly dependent on the clinician's skill. Other confounding factors include incorrect feet positioning, joint contractures, obesity, scoliosis, or pelvic asymmetry.
While direct and indirect clinical evaluation of LLD is inexpensive and noninvasive, imaging modalities are more precise, help identify coexistent deformity, and aid in guiding treatment. The current gold standard involves the use of radiography. Three types of radiographic methods have been utilized to determine LLD: teleroentgenography, orthoroentgenography, and scanography.6)
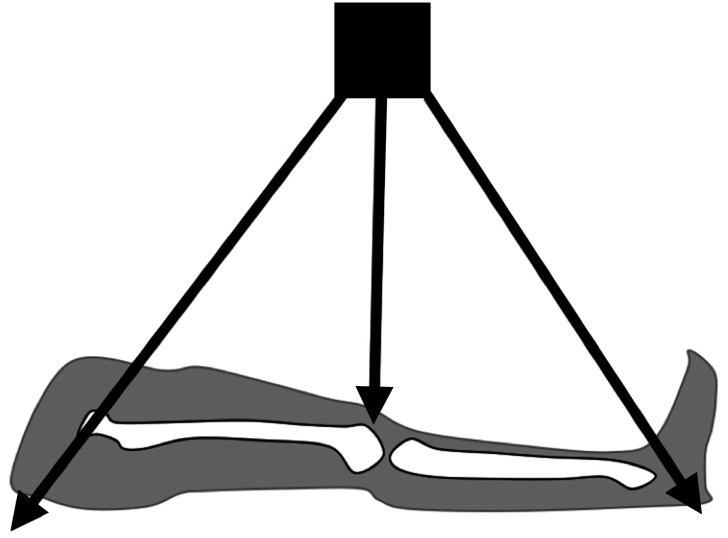
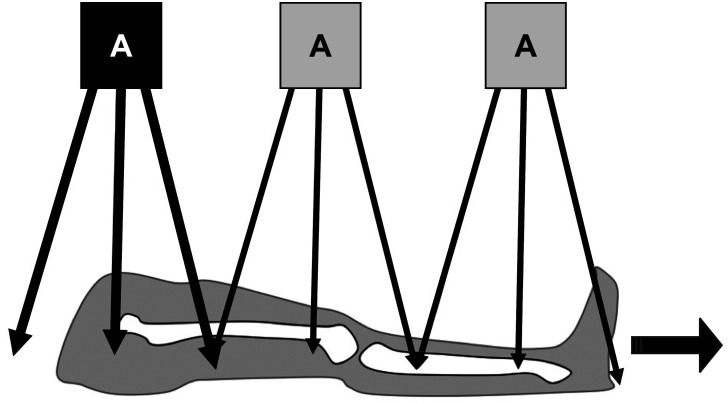
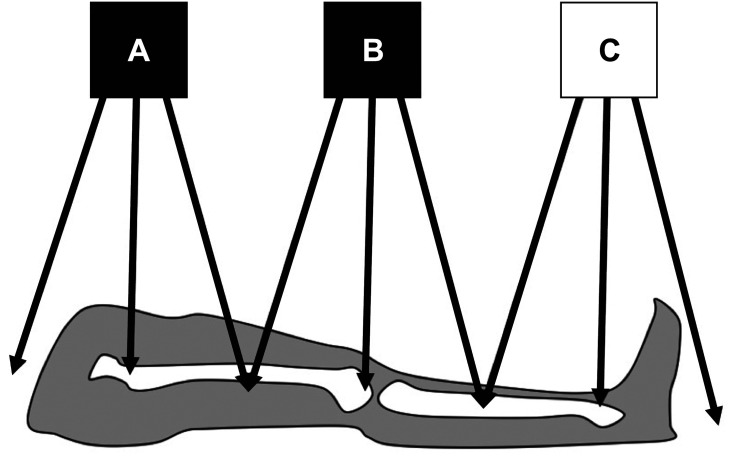
A teleroentgenogram is a conventional radiograph of a standing patient that minimizes radiation exposure by capturing the entire lower extremity in one radiation exposure (Fig. 1). Teleroentgenography offers multiple advantages, including the ability to demonstrate axial deformities, frontal plane deformities, and LLD on a single film.5) However, teleroentgenography is subject to magnification error and is not practical for adults due to the size of the films required and beam distortion. The technique is also limited in patients with a hip or knee contracture.12) Similarly, orthoroentgenography is limited in patients with fixed joint contractures. However, orthoroentgenography and scanography attempt to minimize magnification error risk by creating three distinct exposures at the hip, knee, and ankle. Orthoroentgenography utilizes a single imaging receptor that remains stationary, while the table and X-ray tube move to the unexposed region (Fig. 2). In contrast, scanography utilizes three separate imaging receptors (Fig. 3). Error can occur if there is movement between exposures. 6) Scanography is a commonly used radiographic imaging modality due to its high accuracy and reliability for measuring LLD. However, a standing anterior-posterior radiograph (standing teleroentgenogram) is the imaging modality of choice when evaluating patients with LLD and potential associated angular deformities.13) Standing teleroentgenography offers comparable cost and reduced radiation exposure to scanography.13) Similarly, CT should be utilized in evaluation of LLD in patients with hip or knee flexion contractures.14) As CT has not shown increased accuracy in detection of LLD except in such cases, the increased cost of CT would only be justified if the contracture has been identified or radiation exposure must be minimized.14) Radiation exposure may be further reduced with the use of the Palpation Meter (PALM). PALM was developed as a pelvic leveling tool, measuring pelvic crest height differences, to assess for LLD in the upright position. PALM is a reliable tool that combines palpation with the precision of a caliper and an inclinometer to indirectly assess LLD. PALM has the potential to serve as an alternative to radiography for diagnosing LLD.15)
Fig. 1. Mechanism of obtaining teleroentgenogram, utilizing a single imaging receptor and a single exposure.
Fig. 2. Mechanism of obtaining an orthoroentgenogram, using a single imaging receptor that remains stationary while the table and X-ray tube move to the unexposed section.
Fig. 3. Mechanism of obtaining a scanogram, utilizing three separate imaging receptors to capture three exposure centers (hip, knee, and ankle).
COMPLICATIONS OF LLD
Once LLD has been diagnosed, evaluation for potential adverse complications is performed. Both structural and functional LLDs place a tremendous amount of uneven stress on the lower extremities. LLDs alter the normal biomechanics and result in functional limitations, including posture and gait abnormalities5,16,17,18,19,20,21,22,23) Khamis and Carmeli16) found that LLDs greater than 10 mm produce substantial alterations in gait, with greater LLDs having greater impact. Such gait alterations result in compensatory strategies in both the shorter and longer limbs.16,17) Common compensatory mechanisms of the shorter leg include supination or plantar flexion of the foot, along with extension of the hip and knee. Common compensatory mechanisms of the longer leg include foot pronation and flexion of the hip and knee.5) Such changes in kinematics contribute to pelvic tilt in the coronal and sagittal planes.
Azizan et al.18) focused on the effect of LLD on postural stability during the swing phase of the gait cycle. The results demonstrated greater loading forces on the shorter limb during gait and increased postural instability with increased LLD height.18) These findings may be explained by a greater step-down distance of the shorter limb in transition to the stance phase. These results were consistent with previous studies.19,20) Uneven loading on the shorter limb results in alterations at the lower limb joints, pelvis, and spine.21) Eek et al.22) demonstrated alterations in postural stability in a cohort of children with spastic hemiplegia due to increased flexion in the shorter limb. Walsh et al.23) showed worsening postural changes due to pelvic obliquity, the most common compensatory mechanism. Pelvic obliquity can progress to functional scoliosis and lower back pain. In line with such findings, Zeitoune et al.24) utilized a three-dimensional motion analysis system to measure pelvic, hip, knee, and ankle gait kinematics to predict anatomical LLD. LLD was predicted with mild accuracy based on hip and knee gait kinematics, demonstrating a potential screening tool to identify patients with LLD and reduce radiation from diagnostic imaging.
In addition to posture and gait abnormalities, LLDs are associated with several musculoskeletal disorders, including scoliosis and resultant degenerative spinal changes. However, the degree of LLD required to cause such disorders remains a topic of debate. LLD has been shown to cause pelvic obliquity in the frontal plane. In order to maintain shoulder balance and compensate for the pelvic obliquity, Cummings et al.25) noted that a lumbar scoliosis occurs with convexity directed towards the shorter limb. This scoliosis is nonprogressive and involves a structurally normal spine with an apparent lateral curvature. It is termed functional scoliosis. It is evident while standing, but reduced when sitting, supine, or prone.6) The greater the degree of LLD, the more apparent the functional scoliosis may be.
Specht and De Boer26) reviewed the radiographs of 106 patients with LLDs greater than 3 mm. LLDs greater than 6 mm (53% of the cases) were associated with at least one abnormal spinal adaptation. Spinal adaptations included scoliosis, hypolordosis, and hyperlordosis.26) Similarly, Giles and Taylor27) studied the vertebral differences in 100 patients, aged 19–61 years, with lower back pain and LLDs greater than 9 mm or LLD 0–3 mm. A high prevalence of functional scoliosis with the convexity of the curve toward the shorter leg was found among individuals with an LLD greater than 9 mm. In addition, structural changes in vertebral morphology on the side of the shorter leg were observed. Such structural changes included increased L5 vertebral height and inferior end-plate asymmetry of the apical vertebra. Traction osteophytes were also observed in patients over 40 years old, suggestive of degenerative changes in the spine in response to long-standing functional scoliosis. Giles and Taylor27) thus concluded that superimposed functional scoliosis was likely to accelerate the rate of disc degeneration.
Reduced intervertebral disc height is associated with increased load of the zygapophyseal joints. Biomechanical studies have demonstrated that zygapophyseal joints carry 12%–16% of the total load during compression and bending. This load can increase up to 70% when the intervertebral disc height is reduced.28) LLDs of greater than 10 mm have been associated with asymmetrical apical and lumbar zygapophyseal joint cartilage and subchondral bone changes in patients with functional scoliosis.29) Such changes in hyaline articular cartilage may appear differently from age-related changes, as the cartilage often develops areas of disintegration and erosion. This can occur even early in life. In addition, degenerative joint disease results in diffuse degradation and repair, rather than generalized thinning as seen in aging.30,31) Similarly, Murray et al.29) demonstrated increased prevalence of degenerative joint disease among patients with LLD compared to corresponding male and female cohorts without LLD. Correlation between LLD and degenerative joint disease was more pronounced at the L5–S1 motion segment, as compared to the L4–L5 spinal level in both men and women.31)
Another complication of LLD involves reduced health-related quality of life (HrQoL) in children and parents. Quitmann et al.32) analyzed parents of short-statured children to validate the Quality of Life in Short Stature Youth (QoLISSY) questionnaire. Quitmann et al.32) found the QoLISSY questionnaire to possess a three-domain core HrQoL structure. The questionnaire demonstrated test-retest reliability, validity, and good criterion. The questionnaire was easy to administer and relevant internationally.32) Similarly, Vitale et al.33) utilized the Child Health Questionnaire to assess the parental perspective. While differences in HrQoL became more apparent with increasing LLD, no definitive cutoff point was observed. Vitale et al.33) thus questioned the cut-off point of 20 mm for surgical equalization. Such findings are consistent with Gordon and Davis,1) in which discrepancies of 5 mm led to pathologic consequences. Furthermore, the influence of LLD on HrQoL should be assessed in the elderly population. Iversen et al.34) studied the effect of LLD on patient satisfaction and function six years following total hip replacement. Approximately 32% of patients reported perceived LLD following primary surgery. Patients with LLD were twice as likely to report dissatisfaction with surgery and worsening functional status, as well as the need for assistive devices or external support.34)
TREATMENT OF LLD
Equalization of limb length is difficult to achieve. Optimal treatment depends on various factors including patient age, general health, LLD measurements, and severity of symptoms. LLDs less than 20 mm are often asymptomatic and represent a normal variant. Internal shoe lifts with a thickness corresponding to the discrepancy are added for such individuals, typically ranging from 5 mm to 15 mm. External heel lifts are often more comfortable for patients with LLD 15–20 mm.35) Nonsurgical intervention also includes physical therapy, such as stretching the muscles of the lower extremity. Physical therapy is only used for functional scoliosis, as the apparent shortening or lengthening is due to pelvic obliquity from adaptive soft-tissue shortening, joint or muscle contracture, or ligamentous laxity.
Surgical correction is recommended for LLD greater than 20 mm.1,2,33) LLD between 20 mm and 50 mm are often corrected with shortening of the longer limb. This can occur by growth arrest in children or adolescents, or by limb shortening with bone resection in adults. Growth arrest (epiphysiodesis) can be accomplished through a minimally invasive procedure called percutaneous epiphysiodesis using transphyseal screws (PETS). PETS utilizes two screws inserted on the medial and lateral aspects of the physis to promote temporary bone growth arrest. The screws are removed when the leg lengths equalize or the skeleton reaches maturity.5) When skeletal maturity is already reached, limb shortening can be considered. Tibial shortening is associated with a greater risk of complications as compared to the femur. Such complications include muscle bunching or weakness, delayed union, delayed circulatory return, compartment syndrome, and infection. Another option for epiphysiodesis includes guided growth using tension-band plates.36,37) However, tension band plates for LLD correction has been associated with inferior correction, as well as higher complication and revision rates as compared to definitive percutaneous epiphysiodesis.38)
LLD ranging from 60 mm to 150 mm require external fixator lengthening of the shorter limb. External fixators can be classified as circular or mono-lateral. Due to high complication rates related to external fixators, several techniques have been developed that allow for early removal of the frame.5) Such techniques include external fixation over an intramedullary nail and external fixation over the plate. To reduce the risk of external fixation complications, the PRECICE intramedullary expanding nail system was developed.5) The PRECICE system utilizes a telescopic rod with magnetic expansion control to allow for an extremely accurate and controlled lengthening rate. While intramedullary nail techniques reduce the risk of complications, they may result in nerve injuries, nail fractures, and bone nonunions.5)
LLD greater than 150 mm require combined treatment of lengthening the shorter limb and shortening the longer limb. If total equalization is not achieved postoperatively, an internal or external lift can be utilized. LLD greater than 200 mm is often unsuccessful and thus external prostheses are used.39)
TREATMENT OF FUNCTIONAL SCOLIOSIS
Functional scoliosis is thought to regress fully or partially when its cause, the LLD, is removed. Gibson et al.40) demonstrated near complete resolution of functional scoliosis after LLD correction in patients with an LLD 15–55 mm secondary to femoral shaft fracture. No degenerative changes were noted in these patients, as all patients were less than 31 years old and had acquired, not congenital, LLD due to trauma.37) In contrast, Papaioannou et al.39) studied 23 patients who had notable LLD ranging from 12 mm to 52 mm since childhood. All patients demonstrated compensatory lumbar scoliosis that was nearly corrected when the pelvis was leveled using blocks placed under the shorter limb.39) Degenerative changes were seen on radiographs, suggesting possible permanent structural changes in the spine from long-duration functional scoliosis and LLD.
Similarly, Radcliff et al.41) demonstrates LLD in 87% of patients with degenerative scoliosis. Distinct patterns of LLD were observed corresponding to degenerative scoliotic curve morphology. In patients with single degenerative scoliotic curves, LLD appeared to counteract the scoliotic curve and result in overall decrease in truncal shift. Radcliff et al.41) suggests that single degenerative curves are rarer and likely develop as a compensatory mechanism for LLD. In contrast, double degenerative curves likely develop from primary lumbar degenerative pathology, independent of LLD.41) Consequently, correction of degenerative scoliosis with fusion may upset the compensatory mechanisms involving pelvic parameters and lumbar deformity. Further studies are needed to assess whether LLD is an independent risk factor for adjacent segment breakdown or development of SIJ pathology following lumbar fusion.
CONCLUSION
LLD is an underrecognized and prevalent condition among the U.S. population. LLD may be present from birth due to a congenital disorder or may be acquired through illness, infection, or trauma. The effects of LLD vary on an individual basis depending on the cause and size of the discrepancy. Most cases of LLD are less than 10 mm and are asymptomatic or normal variant. Current research supports discrepancies greater than 20 mm to be clinically significant and require treatment. Such discrepancies can alter biomechanics and result in functional limitations, such as gait, posture, and balance problems. The asymmetrical loading patterns of LLD can also result in various musculoskeletal disorders, such as functional scoliosis and degenerative disease of the spine. Functional scoliosis is nonprogressive and involves a structurally normal spine with an apparent lateral curvature. Long-standing LLD and functional scoliosis can result in permanent degenerative changes in the facet joints and intervertebral discs of the spine, as well as structural curvature. Functional scoliosis regresses fully or partially when the LLD is fixed and the scoliosis is flexible. Further understanding of the contribution of LLD in the development of scoliosis and degenerative disease will allow for more effective preventative treatment strategies and hasten return to function.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Gordon JE, Davis LE. Leg length discrepancy: the natural history (and what do we really know) J Pediatr Orthop. 2019;39(6 Suppl 1):S10–S13. doi: 10.1097/BPO.0000000000001396. [DOI] [PubMed] [Google Scholar]
- 2.Krawiec CJ, Denegar CR, Hertel J, Salvaterra GF, Buckley WE. Static innominate asymmetry and leg length discrepancy in asymptomatic collegiate athletes. Man Ther. 2003;8(4):207–213. doi: 10.1016/s1356-689x(03)00012-2. [DOI] [PubMed] [Google Scholar]
- 3.D'Amico JC. Keys to recognizing and treating limb length discrepancy. Podiatry Today. 2014;27(5):66–75. [Google Scholar]
- 4.Reid D, Smith B. Leg length discrepancy assessment: accuracy and precision in five clinical methods of evaluation. Physiother Can. 1984;36:177–182. doi: 10.2519/jospt.1984.5.5.230. [DOI] [PubMed] [Google Scholar]
- 5.Queiros AF, Costa FG. Leg length discrepancy: a brief review. Port J Orthop Traumatol. 2018 [Google Scholar]
- 6.Brady RJ, Dean JB, Skinner TM, Gross MT. Limb length inequality: clinical implications for assessment and intervention. J Orthop Sports Phys Ther. 2003;33(5):221–234. doi: 10.2519/jospt.2003.33.5.221. [DOI] [PubMed] [Google Scholar]
- 7.Bedoya MA, Chauvin NA, Jaramillo D, Davidson R, Horn BD, Ho-Fung V. Common patterns of congenital lower extremity shortening: diagnosis, classification, and follow-up. Radiographics. 2015;35(4):1191–1207. doi: 10.1148/rg.2015140196. [DOI] [PubMed] [Google Scholar]
- 8.Simon S, Whiffen J, Shapiro F. Leg-length discrepancies in monoarticular and pauciarticular juvenile rheumatoid arthritis. J Bone Joint Surg Am. 1981;63(2):209–215. [PubMed] [Google Scholar]
- 9.Packham JC, Hall MA. Long-term follow-up of 246 adults with juvenile idiopathic arthritis: functional outcome. Rheumatology (Oxford) 2002;41(12):1428–1435. doi: 10.1093/rheumatology/41.12.1428. [DOI] [PubMed] [Google Scholar]
- 10.Jamaluddin S, Sulaiman AR, Imran MK, Juhara H, Ezane MA, Nordin S. Reliability and accuracy of the tape measurement method with a nearest reading of 5 mm in the assessment of leg length discrepancy. Singapore Med J. 2011;52(9):681–684. [PubMed] [Google Scholar]
- 11.Woerman AL, Binder-Macleod SA. Leg length discrepancy assessment: accuracv and precision in five clinical methods of evaluation. J Orthop Sports Phys Ther. 1984;5(5):230–239. doi: 10.2519/jospt.1984.5.5.230. [DOI] [PubMed] [Google Scholar]
- 12.Mannello DM. Leg length inequality. J Manipulative Physiol Ther. 1992;15(9):576–590. [PubMed] [Google Scholar]
- 13.Sabharwal S, Zhao C, McKeon J, Melaghari T, Blacksin M, Wenekor C. Reliability analysis for radiographic measurement of limb length discrepancy: full-length standing anteroposterior radiograph versus scanogram. J Pediatr Orthop. 2007;27(1):46–50. doi: 10.1097/01.bpo.0000242444.26929.9f. [DOI] [PubMed] [Google Scholar]
- 14.Stanitski DF. Limb-length inequality: assessment and treatment options. J Am Acad Orthop Surg. 1999;7(3):143–153. doi: 10.5435/00124635-199905000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Petrone MR, Guinn J, Reddin A, Sutlive TG, Flynn TW, Garber MP. The accuracy of the Palpation Meter (PALM) for measuring pelvic crest height difference and leg length discrepancy. J Orthop Sports Phys Ther. 2003;33(6):319–325. doi: 10.2519/jospt.2003.33.6.319. [DOI] [PubMed] [Google Scholar]
- 16.Khamis S, Carmeli E. Relationship and significance of gait deviations associated with limb length discrepancy: a systematic review. Gait Posture. 2017;57:115–123. doi: 10.1016/j.gaitpost.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 17.Taillard W, Morscher E. Beinlängenunterschiede. Basel: Karger; 1965. pp. 90–134. [Google Scholar]
- 18.Azizan NA, Basaruddin KS, Salleh AF, Sulaiman AR, Safar MJ, Rusli WM. Leg length discrepancy: dynamic balance response during gait. J Healthc Eng. 2018;2018:7815451. doi: 10.1155/2018/7815451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonnet CT, Cherraf S, Szaffarczyk S, Rougier PR. The contribution of body weight distribution and center of pressure location in the control of mediolateral stance. J Biomech. 2014;47(7):1603–1608. doi: 10.1016/j.jbiomech.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Swaminathan V, Cartwright-Terry M, Moorehead JD, Bowey A, Scott SJ. The effect of leg length discrepancy upon load distribution in the static phase (standing) Gait Posture. 2014;40(4):561–563. doi: 10.1016/j.gaitpost.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 21.White SC, Gilchrist LA, Wilk BE. Asymmetric limb loading with true or simulated leg-length differences. Clin Orthop Relat Res. 2004;(421):287–292. doi: 10.1097/01.blo.0000119460.33630.6d. [DOI] [PubMed] [Google Scholar]
- 22.Eek MN, Zugner R, Stefansdottir I, Tranberg R. Kinematic gait pattern in children with cerebral palsy and leg length discrepancy: effects of an extra sole. Gait Posture. 2017;55:150–156. doi: 10.1016/j.gaitpost.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 23.Walsh M, Connolly P, Jenkinson A, O'Brien T. Leg length discrepancy: an experimental study of compensatory changes in three dimensions using gait analysis. Gait Posture. 2000;12(2):156–161. doi: 10.1016/s0966-6362(00)00067-9. [DOI] [PubMed] [Google Scholar]
- 24.Zeitoune G, Nadal J, Batista LA, Metsavaht L, Moraes AP, Leporace G. Prediction of mild anatomical leg length discrepancy based on gait kinematics and linear regression model. Gait Posture. 2019;67:117–121. doi: 10.1016/j.gaitpost.2018.09.027. [DOI] [PubMed] [Google Scholar]
- 25.Cummings G, Scholz JP, Barnes K. The effect of imposed leg length difference on pelvic bone symmetry. Spine (Phila Pa 1976) 1993;18(3):368–373. doi: 10.1097/00007632-199303000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Specht DL, De Boer KF. Anatomical leg length inequality, scoliosis and lordotic curve in unselected clinic patients. J Manipulative Physiol Ther. 1991;14(6):368–375. [PubMed] [Google Scholar]
- 27.Giles LG, Taylor JR. Lumbar spine structural changes associated with leg length inequality. Spine (Phila Pa 1976) 1982;7(2):159–162. doi: 10.1097/00007632-198203000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Adams MA, Hutton WC. The effect of posture on the role of the apophyseal joints in resisting intervertebral compressive forces. J Bone Joint Surg Br. 1980;62(3):358–362. doi: 10.1302/0301-620X.62B3.6447702. [DOI] [PubMed] [Google Scholar]
- 29.Murray KJ, Molyneux T, Le Grande MR, Castro Mendez A, Fuss FK, Azari MF. Association of mild leg length discrepancy and degenerative changes in the hip joint and lumbar spine. J Manipulative Physiol Ther. 2017;40(5):320–329. doi: 10.1016/j.jmpt.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Giles LG, Singer KP. Clinical anatomy and management of low back pain. Oxford: Butterworth-Heinemann; 1997. p. 411. [Google Scholar]
- 31.Giles LG, Taylor JR. The effect of postural scoliosis on lumbar apophyseal joints. Scand J Rheumatol. 1984;13(3):209–220. doi: 10.3109/03009748409100389. [DOI] [PubMed] [Google Scholar]
- 32.Quitmann J, Rohenkohl A, Bullinger M, et al. Parental perception of health-related quality of life in children and adolescents with short stature: literature review and introduction of the parent-reported QoLISSY instrument. Pediatr Endocrinol Rev. 2013;11(2):147–160. [PubMed] [Google Scholar]
- 33.Vitale MA, Choe JC, Sesko AM, et al. The effect of limb length discrepancy on health-related quality of life: is the ‘2 cm rule’ appropriate? J Pediatr Orthop B. 2006;15(1):1–5. doi: 10.1097/01202412-200601000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Iversen MD, Chudasama N, Losina E, Katz JN. Influence of self-reported limb length discrepancy on function and satisfaction 6 years after total hip replacement. J Geriatr Phys Ther. 2011;34(3):148–152. doi: 10.1519/JPT.0b013e31820e16dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raczkowski JW, Daniszewska B, Zolynski K. Functional scoliosis caused by leg length discrepancy. Arch Med Sci. 2010;6(3):393–398. doi: 10.5114/aoms.2010.14262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kenwright J, Albinana J. Problems encountered in leg shortening. J Bone Joint Surg Br. 1991;73(4):671–675. doi: 10.1302/0301-620X.73B4.2071658. [DOI] [PubMed] [Google Scholar]
- 37.Broughton NS, Olney BW, Menelaus MB. Tibial shortening for leg length discrepancy. J Bone Joint Surg Br. 1989;71(2):242–245. doi: 10.1302/0301-620X.71B2.2925743. [DOI] [PubMed] [Google Scholar]
- 38.Borbas P, Agten CA, Rosskopf AB, Hingsammer A, Eid K, Ramseier LE. Guided growth with tension band plate or definitive epiphysiodesis for treatment of limb length discrepancy? J Orthop Surg Res. 2019;14(1):99. doi: 10.1186/s13018-019-1139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papaioannou T, Stokes I, Kenwright J. Scoliosis associated with limb-length inequality. J Bone Joint Surg Am. 1982;64(1):59–62. [PubMed] [Google Scholar]
- 40.Gibson PH, Papaioannou T, Kenwright J. The influence on the spine of leg-length discrepancy after femoral fracture. J Bone Joint Surg Br. 1983;65(5):584–587. doi: 10.1302/0301-620X.65B5.6643562. [DOI] [PubMed] [Google Scholar]
- 41.Radcliff KE, Orozco F, Molby N, et al. Is pelvic obliquity related to degenerative scoliosis. Orthop Surg. 2013;5(3):171–176. doi: 10.1111/os.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]