Abstract
A 45-year-old man presents with 48-hour status of high temperature, cough and dyspnoea. In the context of pandemic, the patient is initially diagnosed with COVID-19 syndrome. Later, the laboratory and ultrasound study supported acute appendicitis diagnosis. Appendicectomy was performed. The histopathology study confirmed eosinophilic appendicitis and that a parasitic infection was suspected. The stool sample was positive for Strongyloides stercoralis. The diagnosis of a S.stercoralis is a rare finding in Spain. S. stercoralis simulates clinical findings of inflammatory bowel disease or eosinophilic gastroenteritis, which may lead to the wrong therapeutic choice. Since in inflammatory diseases corticosteroid treatments are considered the initial choice in many cases, in the case of S. stercoralis infection, the administration of this therapy can be fatal. In Spain, the number of diagnoses is much lower than in the past decade, although it is highly probable that the infection has been underdiagnosed due to low clinical awareness among Spanish population.
Keywords: infectious diseases, infection (gastroenterology), medical management
Background
Spain has been one of the most affected countries by the COVID‐19 outbreak. Diagnosis of rare entities in this pandemic context may be a challenge for clinicians. Strongyloidiasis is a disease caused by an infection with a soil-transmitted helminth that affects, according to largely varying rates, between 30 and 370 million people worldwide.1 Clinical, epidemiologic and therapeutic aspects are not adequately addressed to allow for effective management of the disease.2 Strongyloidiasis is acquired through contact with contaminated soil, and the infection is primarily transmitted in areas with poor sanitation, inadequate access to clean water and lack of hygiene.3
We report a case of a patient diagnosed with Strongyloides infection in SARS-CoV-2 pandemic context who develops respiratory symptoms associated with abdominal pain. This is a rare parasite diagnosis and is a challenge for clinicians in the COVID-19 pandemic context.
Case presentation
We present the case of a 45-year-old man with a history of snorting cocaine and depressive anxiety disorder who suffered a 48-hour period of high temperature, cough and dyspnoea. He reported abdominal pain and diarrhoea in the previous 10 days. Physical examination showed a 92% basal oxygen saturation, normal blood pressure and wheezing in bilateral lung auscultation.
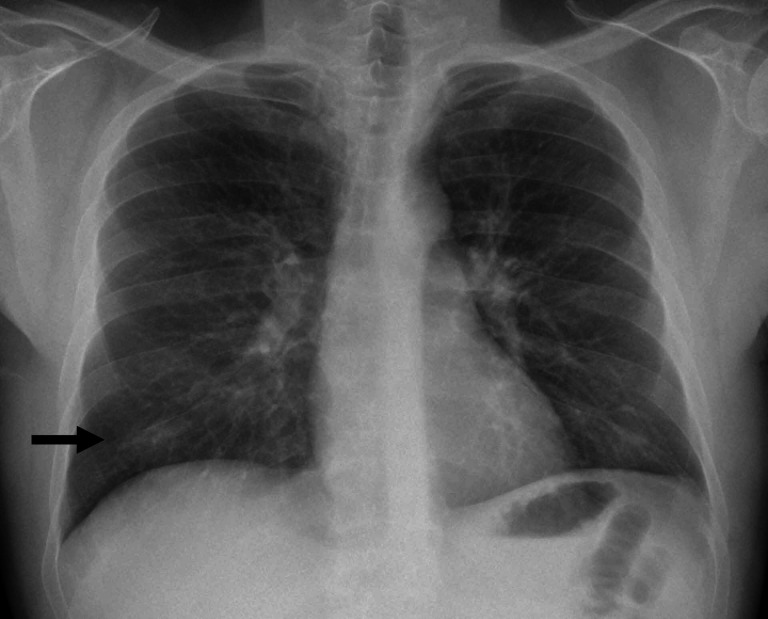
The laboratory study indicated leucocytes 10.2×109/L (reference 4–11×109 counts/L), eosinophil count 2069 counts/µL (reference 0–800 counts/µL), D-dimer 1.55 µg/mL (reference 0.1–0.5 µg/mL) and C-reactive protein 51 mg/L (reference 0–5 mg/L). On chest X-ray, we observed a mild consolidation in the right lower pulmonary lobe (figure 1). In the context of pandemic, the patient was diagnosed with COVID-19 syndrome, although SARS-CoV-2 PCR test resulted negative. The patient was treated following the initial experimental study of Spain which included azithromycin, ceftriaxone, hydroxychoroquine, Kaletra and antithrombotic prophylaxis with enoxaparin.4 The patient did not receive corticosteroids or immune therapy. Due to the early satisfactory clinical evolution, the patient was discharged within 7 days.
Figure 1.
X-ray chest examination where we observed a mild consolidation in the right lower pulmonary lobe.
Two weeks after discharge, the patient returned to hospital reporting abdominal pain located in the epigastrium and vomiting. He remained afebrile and is haemodynamically stable with 94% basal oxygen saturation. On examination, the abdomen was found to have tenderness to palpation in the right iliac fossa with a positive Blumberg’s sign. The laboratory findings showed leucocytes 19.2x109counts/L, eosinophils 13.4x109counts/Land C-reactive protein 4.8 mg/L (reference 0–5 mg/L). Chest and abdominal X-ray were normal. The ultrasound study showed a non-depressible caecal appendix of 7 mm in diameter and increased echogenicity of the adjacent fat. The patient was tested again for SARS-CoV-2 PCR, which returned a negative result.
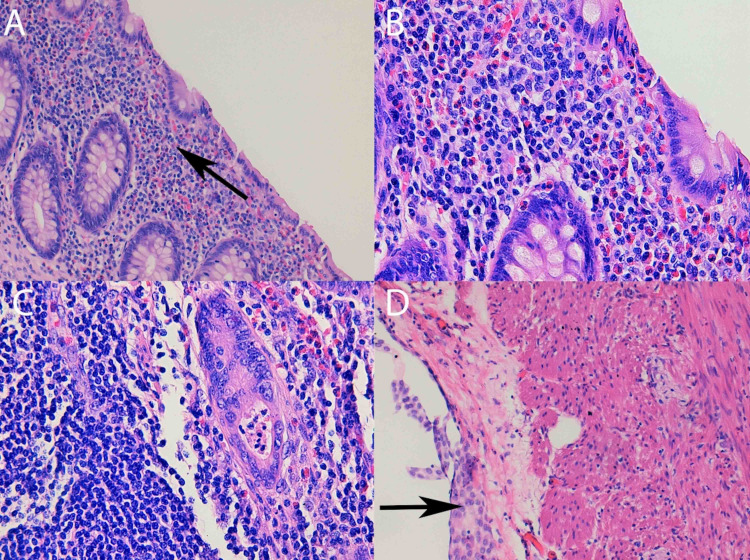
An urgent appendectomy was performed, finding mild hyperaemia of the caecal appendix. The patient developed a clinical worsening with increased leucocytosis and eosinophilia (20.8x109counts/L and 14.ox109 counts/L, respectively). On histopathological study, a significant eosinophilic polymorphonuclear cell infiltration was observed, covering the entire appendicular wall. It was counted >70 eosinophils/high magnification field (HMF) (figure 2). Test results for HIV and human T-lymphotropic virus (HTLV) were negative. Serology for SARS-CoV-2 resulted negative 1 month after the syndrome presentation.
Figure 2.
(A) At ×20 objective lens magnification, a large eosinophilic infiltrate at the mucosa level is observed. (B) At ×40 magnification, we can see an eosinophilic infiltrate that permeates the glandular epithelium. Degranulated eosinophils are observed. (C) At ×20 magnification, serosal eosinophils and reactive hyperplasia of the mesothelials are observed. (D) At ×40 magnification, a gland with proeosinophilic crypt microabscess is observed.
Differential diagnosis
The initial pneumonic presentation can be attributed to S. stercoralis, although COVID-19 cannot be ruled out after negative PCR testing, in the context of pandemic in Spain. Differential diagnosis for intestinal findings includes eosinophilic gastroenteritis and intestinal parasitosis. The patient was initially treated with analgesics, avoiding the use of corticosteroids, until the blood culture and stool examination results were received. The examination of stool sample returned positive for S. stercoralis.
Treatment
Once S. stercoralis was diagnosed, the patient initiated treatment with ivermectin (a dose of 200 µg/kg given on days 1, 2, 15 and 16).
Outcome and follow-up
The evolution of the patient after treatment was satisfactory and relapse after recovery has not been identified after 9 months of follow-up. Diagnosis of rare entities in the context of the pandemic may be a challenge for clinicians.
Discussion
Spain has been one of the most affected countries by the COVID-19 outbreak. COVID-19, which is caused by infection with SARS-CoV-2, predominantly includes pulmonary symptoms; however, <10% of cases also include gastrointestinal events, including abdominal pain, diarrhoea and vomiting.5 6
Our patient developed initially a 48-hour respiratory syndrome associated with abdominal pain. This syndrome is compatible with COVID-19 in the context of the pandemic, despite negative PCR testing or not worsening after days 7–10. Later evolution, and after presenting acute appendicitis, the stool examination and histopathology study confirmed S. stercoralis infection. Strongyloidiasis is a disease caused by an infection with a soil-transmitted helminth that affects between 30 million and 370 million people worldwide.1 Some authors consider Spain and some other southern European countries as endemic.7
The most frequent mechanism of infection is percutaneous entry of the filariform larvae. In healthy people, most of the cases are asymptomatic, although it can cause intermittent respiratory, gastrointestinal and dermatological symptoms.8 Risk factors for severe infection include immunosuppression, current malignancies, human T-cell lymphotropic virus type 1 infection and alcoholism. S. stercoralis has been associated with agricultural or mining activities.9 Our patient had a history of cocaine use and resulted negative for HIV and other viral infections, like the HTLV. S. stercoralis infection has also been associated with low socioeconomic factors.10
As in our case, S. stercoralis can simulate the symptomatology and histopathological findings of an inflammatory bowel disease or an eosinophilic gastroenteritis.11–13
This circumstance may lead to the wrong choice of treatment. Since in inflammatory diseases, corticosteroid treatment is considered the initial choice in many cases, in the S. stercoralis infection, the administration of this therapy can be fatal.14–16
A single stool sample examination has low sensitivity for S. stercoralis detection.17 For this reason, serology, which shows the highest sensitivity so far, is currently widely available and used.17 In our case, stool samples were positive for S. stercoralis. Serological tests are specific enough and useful in follow-up management. A decrease in the titres or negativisation could be observed 6–12 months after treatment.17 We did not test Strongyloides antibodies in our patient for the diagnoses, so we did not use either the study as a follow-up test. Stool samples were analysed instead and resulted negative after treatment.
The COVID-19 disease has posed a diagnostic and therapeutic challenge for clinicians worldwide. In the pandemic situation, other concurrent diseases with similar clinical symptoms to SARS-CoV-2 have been underdiagnosed. The diagnosis of a S. stercoralis in this context is a rare finding. There are still new diagnosis of strongyloidiasis in Spain every year. We could make different interpretations of our case. On one hand, our patient could have developed COVID-19 infection (despite negative PCR and serology) and acute appendicitis, in the context of chronic S. stercoralis infection. On the other hand, acute appendicitis and Strongyloides infection worsening could be the consequence of SARS-CoV-2-induced immunodysregulation.18 In relation to corticosteroids use in SARS-CoV-2 pandemic and its risk in developing parasitic complications, we know that the current recommended dexamethasone dose from the COVID-19 Treatment Panel is 6 mg per day (40 mg of prednisone) for 10 days. It is likely that the benefit of dexamethasone outweighs the risk of possible Strongyloides hyperinfection.19 A possible strategy to avoid hyperinfection syndrome in anticipation of widespread use of corticosteroids during the COVID-19 pandemic is following the 2016 Committee to Advise on Tropical Medicine and Travel (CATMAT) recommendations.20 Nevertheless, since this is a rare disease, we have not found scientific evidence to recommend screening for strongyloidiasis in patients with COVID-19 requiring treatment with corticosteroids.
Learning points.
Differential diagnosis between strongyloidiasis and other infectious diseases is a challenge for clinicians due to its low prevalence in the Western world.
Strongyloides stercoralis can simulate respiratory infection (high temperature, cough and dyspnoea) or gastrointestinal disease.
S. stercoralis can simulate the symptomatology and histopathological findings of an inflammatory bowel disease or an eosinophilic gastroenteritis.
Since in inflammatory diseases, corticosteroid treatment is considered the initial choice in many cases, in the S. stercoralis infection, the administration of this therapy can be fatal.
Footnotes
Contributors: JP-Z: Idea, writing and contributor who accepts full responsibility for the finished article. MF-A: Figures. BB-S: Writing. RL-L: Identification and treatment of the patient.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer: Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bisoffi Z, Buonfrate D, Montresor A, et al. Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis 2013;7:e2214. 10.1371/journal.pntd.0002214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Utzinger J, Becker SL, Knopp S, et al. Neglected tropical diseases: diagnosis, clinical management, treatment and control. Swiss Med Wkly 2012;142:w13727. 10.4414/smw.2012.13727 [DOI] [PubMed] [Google Scholar]
- 3.Albonico M, Becker SL, Odermatt P, et al. StrongNet: an international network to improve diagnostics and access to treatment for strongyloidiasis control. PLoS Negl Trop Dis 2016;10:e0004898. 10.1371/journal.pntd.0004898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centro de Coordinación de Alertas y Emergencias Sanitarias . Ministerio de Sanidad de España. Actualización no112. Enfermedad por el coronavirus (COVID-19), 2020. Available: https://www.mscbs.gob.es/en/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/Actualizacion_112_COVID-19.pdf
- 5.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet 2020;395:507–13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus–Infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toledo R, Muñoz-Antoli C, Esteban J-G. Strongyloidiasis with emphasis on human infections and its different clinical forms. Adv Parasitol 2015;88:165–241. [DOI] [PubMed] [Google Scholar]
- 8.Jourdan PM, Lamberton PHL, Fenwick A, et al. Soil-transmitted helminth infections. Lancet 2018;391:252–65. 10.1016/S0140-6736(17)31930-X [DOI] [PubMed] [Google Scholar]
- 9.Barroso M, Salvador F, Sánchez-Montalvá A, et al. Strongyloides stercoralis infection: a systematic review of endemic cases in Spain. PLoS Negl Trop Dis 2019;13:13. 10.1371/journal.pntd.0007230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beknazarova M, Whiley H, Ross K. Strongyloidiasis: a disease of socioeconomic disadvantage. Int J Environ Res Public Health 2016;13:13. 10.3390/ijerph13050517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minematsu H, Hokama A, Makishi T, et al. Colonoscopic findings and pathologic characteristics of Strongyloides colitis: a case series. Digestion 2011;83:210–4. 10.1159/000321812 [DOI] [PubMed] [Google Scholar]
- 12.Poveda J, El-Sharkawy F, Arosemena LR, et al. Strongyloides Colitis as a Harmful Mimicker of Inflammatory Bowel Disease. Case Rep Pathol 2017;2017:1–4. 10.1155/2017/2560719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kishimoto K, et al. Endoscopic and histopathological study on the duodenum of Strongyloides stercoralis Hyperinfection. World J Gastroenterol 2008;14:1768. 10.3748/wjg.14.1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fardet L, Généreau T, Cabane J, et al. Severe strongyloidiasis in corticosteroid-treated patients. Clin Microbiol Infect 2006;12:945–7. 10.1111/j.1469-0691.2006.01443.x [DOI] [PubMed] [Google Scholar]
- 15.Al Maslamani MA, Al Soub HA, Al Khal ALM, et al. Strongyloides stercoralis hyperinfection after corticosteroid therapy: a report of two cases. Ann Saudi Med 2009;29:397–401. 10.4103/0256-4947.55172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khadka P, Khadka P, Thapaliya J, et al. Fatal strongyloidiasis after corticosteroid therapy for presumed chronic obstructive pulmonary disease. JMM Case Rep 2018;5:5. 10.1099/jmmcr.0.005165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Requena-Méndez A, Chiodini P, Bisoffi Z, et al. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis 2013;7:e2002. 10.1371/journal.pntd.0002002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parrill A, Tsao T, Dong V, et al. SARS-CoV-2-induced immunodysregulation and the need for higher clinical suspicion for co-infection and secondary infection in COVID-19 patients. J Microbiol Immunol Infect 2021;54:105–8. 10.1016/j.jmii.2020.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stauffer WM, Alpern JD, Walker PF. COVID-19 and dexamethasone: a potential strategy to avoid Steroid-Related Strongyloides hyperinfection. JAMA 2020;324:623-624. 10.1001/jama.2020.13170 [DOI] [PubMed] [Google Scholar]
- 20.Boggild AK, Libman M, Greenaway C, et al. CATMAT statement on disseminated strongyloidiasis: prevention, assessment and management guidelines. Can Commun Dis Rep 2016;42:12–19. 10.14745/ccdr.v42i01a03 [DOI] [PMC free article] [PubMed] [Google Scholar]