Abstract
Background: Older people often receive multiple medications for chronic conditions, which often result in polypharmacy (concomitant use of 5‒9 medicines) and hyperpolypharmacy (concomitant use of ≥10 medicines). A limited number of studies have been performed to evaluate the prevalence of polypharmacy, hyperpolypharmacy, and potentially inappropriate medication (PIM) use in older people of developing countries. The present study aimed to investigate regional variations in the prevalence of polypharmacy, hyperpolypharmacy, and PIM use in older people (60 + years) in India.
Methods: Studies were identified using Medline/PubMed, Scopus, and Google Scholar databases published from inception (2002) to September 31, 2020. Out of the total 1890 articles, 27 were included in the study.
Results: Overall, the pooled prevalence of polypharmacy was 49% (95% confidence interval: 42–56; p < 0.01), hyperpolypharmacy was 31% (21–40; p < 0.01), and PIM use was 28% (24–32; p < 0.01) among older Indian adults. Polypharmacy was more prevalent in North-east India (65%, 50–79), whereas hyperpolypharmacy was prevalent in south India (33%, 17–48). Region-wize estimates for the pooled prevalence of PIM use in India were as follows: 23% (21–25) in East, 33% in West (24–42), 17.8% in North (11–23), and 32% (26–38) in South India. The prevalence of PIM use in adults aged ≥70°years was 35% (28–42), in those taking more medications (≥5.5/day) was 27% (22–31), and in adults using a high number of PIMs (≥3) was 29% (22–36). Subgroup analysis showed that cross-sectional studies had a higher pooled prevalence of polypharmacy 55% (44–65) than cohorts 45% (37–54). Hyperpolypharmacy in inpatient care settings was 37% (26–47), whereas PIM use was higher in private hospitals 31% (24–38) than government hospitals 25% (19–31).
Conclusion: Polypharmacy and hyperpolypharmacy are widely prevalent in India. About 28% of older Indian adults are affected by PIM use. Thus, appropriate steps are needed to promote rational geriatric prescribing in India.
Systematic Review Registration: https://clinicaltrials.gov, identifier [CRD42019141037].
Keywords: polypharmacy (source: MeSH, NML); India; potentially inappropriate medication (PIM); prevalence; older (diseased) population; hyperpolypharmacy
Introduction
There were 703 million people aged 65°years or over in the world in 2019. The number of the older people is projected to double to 1.5 billion by 2050, with a more prominent increase in developing countries (He and Kinsella, 2020). According to the United Nations Population Fund’s (UNFPA) 2021 flagship State of World population report, there are nearly 93 million (6.8%) older people (aged 65°years or above) in India, and the number is projected to exceed 227 million in 2050 (United Nations Population Fund).
In general, older people often receive multiple medications for chronic conditions, which often result in polypharmacy (concomitant use of 5‒9 medicines) and hyperpolypharmacy (concomitant use of ≥10 medicines) (Masnoon et al., 2017). Research shows that older adults in India frequently use multiple medications. There are wide regional variations in the prevalence ranging from 5.8% in West Bengal (west region) and 93.1% in Uttaranchal (North India) (Sharma et al., 2019). Although medications are essential to improve a patient’s health status and quality of life, suboptimal prescribing and the use of multiple drugs may have adverse outcomes (Pravodelov, 2020; O’Mahony, 2020; Bala et al., 2019). Moreover, polypharmacy and hyperpolypharmacy are strongly linked to a broad range of negative health outcomes and are considered proxy indicators of potentially inappropriate medication (PIM) use (Guillot et al., 2020).
The term PIM is defined as medications that have adverse effects and, when used by older adults, may outweigh the clinical advantages of the drug, such as mental and functional decline, adverse drug events, drug interactions, unplanned hospitalization, morbidity, and mortality (Thomas and Thomas, 2019; Xing et al., 2019; de Oliveira et al., 2020; Weeda et al., 2020). Higher-income countries have taken several steps to improve rational prescribing in older adults and have developed evidence-based explicit tools to screen and prevent PIM use in older patients. Explicit tools comprise lists of drugs or drug classes (developed from literature reviews, expert opinion, and consensus techniques) that, when prescribed or underprescribed, can cause harm in older people. Beers criteria and the Screening Tool of Older Persons’ prescription (STOPP) and the Screening Tool to Alert to Right Treatment (START) are the most commonly referenced tools (Topinková et al., 2008; Hill-Taylor et al., 2013; American Geriatrics Society 2015 Beers Criteria Update Expert Panel, 2015; Thomas and Thomas, 2019; Weeda et al., 2020).
Several systematic reviews and meta-analyses on the prevalence of polypharmacy and PIM use in the older population, using data from developed countries (Kaufmann et al., 2014; Muhlack et al., 2017; Liew et al., 2019; Thomas and Thomas, 2019; Xing et al., 2019; Davies et al., 2020; de Oliveira et al., 2020; Liew et al., 2020; Mohamed et al., 2020; Weeda et al., 2020), indicated a rising trend of inappropriate medication use in the current healthcare system. However, differences in the population, healthcare settings, and medication use process may limit the generalizability of these findings in developing countries, including India. Given the rapidly increasing older population, increasing burden of chronic diseases, and wide variations in polypharmacy use across India, the prevalence of PIM use in the Indian older population is pertinent. We hypothesized that the prevalence of polypharmacy, hyperpolypharmacy, and PIM use in India would be higher than in the western countries, and their distribution may vary across different states in India. Thus, this study aimed to perform a systematic review and meta-analysis to assess the overall prevalence and regional variations (north, east, west, and south: NEWS) of polypharmacy, hyperpolypharmacy, and PIM use in older people in India.
Methods
The study was performed according to the MOOSE (Meta-analysis of Observational Studies in Epidemiology) guidelines (Stroup et al., 2000). The research protocol is registered on PROSPERO, 2019 (CRD42019141037).
Search Strategy
We comprehensively searched Medline/PubMed, Scopus, Google Scholar, and bibliographic databases from inception (2002) to September 31, 2020. The search process was initiated in april 2019 and updated until September 31, 2020. We used combinations of Medical Subject Headings (MeSH) and free text words to identify the relevant studies related to the exposure (e.g., polypharmacy, hyperpolypharmacy, potentially inappropriate prescribing (PIP), PIMs, and to search terms related to outcomes (e.g., prevalence, estimates, percentage, burden). Complete details about the search terms used in various databases have been listed in Supplementary Table S1.
Selection Criteria and Data Extraction
The studies met the following criteria; observational (cross-sectional, case-cohort, or cohort) on the older population (aged 60 and older), conducted in India, and reported prevalence of polypharmacy, hyperpolypharmacy, and PIM use, using any explicit criteria to assess the appropriateness of drugs prescribed. The following articles were excluded; duplicate studies, abstracts, letters, editorials, conference proceedings, review articles, meta-analyses, non-population-based studies, and interventional studies.
Selection of Studies
Three reviewers (ASB, RS and KVS) independently screened the titles and abstracts of the initially identified studies to determine whether each study met the predefined eligibility criteria. Full-text articles were retrieved for selected titles. References of the retrieved articles were also screened to identify the additional eligible articles. Any disagreements regarding selection were resolved through discussion, consensus, or consultation with other team authors (MC, MR, and SPS).
Data Extraction
Full texts of included studies were read, and three reviewers (RS, MC, and KVS) extracted the relevant data from the selected studies. The extracted data included author details, year of publication, geographic origin, study design and settings, patient sampling, participant characteristics (e.g., age range, mean age, sex, comorbidities, and number of prescribed medications), measurements (explicit criteria), and information on outcomes (type of medication use, number of patients exposed to PIM, number of PIMs identified and percentage of the older population on polypharmacy and hyperpolypharmacy). Prevalence estimates of PIM use were stratified to provide specific estimates of the subsets (mean age, gender, study duration, and the average number of medications).
Quality Assessment
The methodological quality of the included studies was evaluated using the Newcastle Ottawa Scale (NOS) for cross-sectional and cohort studies (Luchini et al., 2017). The NOS assesses the representativeness of the sample, sample size, response rate, ascertainment of exposure, control of confounding variables, assessment of preventability, and appropriate statistical analysis. The NOS scores range from 0 (lowest grade) to 9 (highest grade). Studies scoring seven or above were considered high quality, and those with scores below seven were of low quality.
Statistical Analysis
The estimates of polypharmacy, hyperpolypharmacy, and PIM use were expressed as proportions (%) with corresponding 95% confidence intervals (CI). The pooled prevalence estimates of outcome variables were calculated using regional population size weights. The magnitude of heterogeneity between the studies was assessed using the I 2 statistic (% residual variation due to heterogeneity), and Tau2 (method of moments estimate of between-study variance) was used for each of the pooled estimates. I 2 values range between 0 and 100%, and is considered low for I 2 <25%, modest for 25–50%, and large for >50% (Higgins et al., 2003). As differences between the studies were very high (95–99% inconsistency), a random effect DerSimonian-Laird model was used in all analyses (Higgins et al., 2003). In case of substantial heterogeneity, the source of heterogeneity was investigated using stratified analyses and meta-regression analysis, based on the study-level characteristics, such as year of publication, study duration, mean age, women-to-men ratio, the mean number of drugs, number of PIM use, and quality of studies based on NOS scale. The interaction between the subgroups of each factor was assessed using Cochran's Q test, degree of freedom (df), and p-value resulting from Cochran’s Q test. A p-value of <0.10 was considered statistically significant for Cochran’s Q test (Huedo-Medina et al., 2006). The risk of publication bias was inspected by using the symmetry of funnel plots, and Egger’s and Begg’s tests were also used. Statistical analyses were performed using STATA software, version 16 MP (StataCorp, College Station, TX).
Results
Study Selection
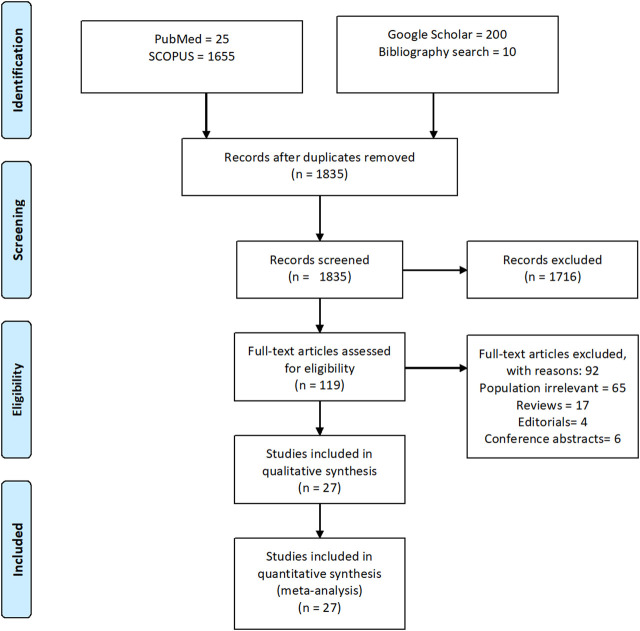
A total of 1890 references were initially identified through electronic databases. After removing 165 duplicates, a total of 1835 titles and abstracts were screened to determine if they met the inclusion criteria, as described in the methodology section. Full-text assessment of 119 potentially relevant articles resulted in 27 eligible studies (Bhatt et al., 2019; Chandrasekhar et al., 2019; Motallebzadeh et al., 2019; Pradhan and Panda, 2018; Benjamin et al., 2018; Devarapalli et al., 2017; Kumar et al., 2017; Pradhan et al., 2017; Narvekar et al., 2017; Borah et al., 2017; Rakesh et al., 2017; Anjum et al., 2017; Swathi and Bhavika, 2016; Salwe et al., 2016; Chowta et al., 2016; Kashyap et al., 2015; Danisha et al., 2015; Umar et al., 2015; Undela et al., 2014; Dhikav et al., 2014; Karandikar et al., 2013; Momin et al., 2013; Nagendra et al., 2012; Shah et al., 2011; Mandavi et al., 2011; Zaveri et al., 2010; Harugeri et al., 2010), as shown in Figure 1. The list of articles that are excluded (n = 92) due to various reasons is presented in Supplementary Table S2.
FIGURE 1.
PRISMA diagram of the literature selection in this systematic literature review and meta-analysis.
Characteristics of Included Studies
All the studies included in the present study were published between 2010 and 2019. Sample size varied on regional basis from 90 to 1,510, making a total of 11,649 patients. All the studies included both women and men (Bhatt et al., 2019; Chandrasekhar et al., 2019; Motallebzadeh et al., 2019; Pradhan and Panda, 2018; Benjamin et al., 2018; Devarapalli et al., 2017; Kumar et al., 2017; Pradhan et al., 2017; Narvekar et al., 2017; Borah et al., 2017; Rakesh et al., 2017; Anjum et al., 2017; Swathi and Bhavika, 2016; Salwe et al., 2016; Chowta et al., 2016; Kashyap et al., 2015; Danisha et al., 2015; Umar et al., 2015; Undela et al., 2014; Dhikav et al., 2014; Karandikar et al., 2013; Momin et al., 2013; Nagendra et al., 2012; Shah et al., 2011; Mandavi et al., 2011; Zaveri et al., 2010; Harugeri et al., 2010); however, seven studies included more women than men(Dhikav et al., 2014; Umar et al., 2015; Salwe et al., 2016; Kumar et al., 2017; Narvekar et al., 2017; Bhatt et al., 2019; Chandrasekhar et al., 2019). Among the studies, nineteen were cohort design (Chandrasekhar et al., 2019; Motallebzadeh et al., 2019; Pradhan and Panda, 2018; Benjamin et al., 2018; Devarapalli et al., 2017; Pradhan et al., 2017; Narvekar et al., 2017; Anjum et al., 2017; Salwe et al., 2016; Chowta et al., 2016; Kashyap et al., 2015; Danisha et al., 2015; Umar et al., 2015; Undela et al., 2014; Dhikav et al., 2014; Karandikar et al., 2013; Nagendra et al., 2012; Shah et al., 2011; Harugeri et al., 2010), and eight were cross-sectional studies (Zaveri et al., 2010; Mandavi et al., 2011; Momin et al., 2013; Swathi and Bhavika, 2016; Borah et al., 2017; Kumar et al., 2017; Rakesh et al., 2017; Bhatt et al., 2019). The majority of the studies were conducted in South India (Bhatt et al., 2019; Chandrasekhar et al., 2019; Motallebzadeh et al., 2019; Benjamin et al., 2018; Rakesh et al., 2017; Anjum et al., 2017; Swathi and Bhavika, 2016; Salwe et al., 2016; Chowta et al., 2016; Danisha et al., 2015; Umar et al., 2015; Nagendra et al., 2012; Harugeri et al., 2010), six in North India (Mandavi et al., 2011; Karandikar et al., 2013; Dhikav et al., 2014; Undela et al., 2014; Kashyap et al., 2015; Kumar et al., 2017), three in Eastern states (Devarapalli et al., 2017; Pradhan et al., 2017; Pradhan and Panda, 2018), four in Western region (Zaveri et al., 2010; Shah et al., 2011; Momin et al., 2013; Narvekar et al., 2017) and only one study in North-east India (Borah et al., 2017). Twenty-one studies provided data on the prevalence of polypharmacy (Bhatt et al., 2019; Chandrasekhar et al., 2019; Motallebzadeh et al., 2019; Pradhan and Panda, 2018; Benjamin et al., 2018; Devarapalli et al., 2017; Pradhan et al., 2017; Borah et al., 2017; Rakesh et al., 2017; Anjum et al., 2017; Swathi & Bhavika, 2016; Salwe et al., 2016; Chowta et al., 2016; Kashyap et al., 2015; Umar et al., 2015; Undela et al., 2014; Karandikar et al., 2013; Momin et al., 2013; Nagendra et al., 2012; Mandavi et al., 2011; Harugeri et al., 2010), fourteen studies reported estimates of hyperpolypharmacy (Bhatt et al., 2019; Chandrasekhar et al., 2019; Benjamin et al., 2018; Devarapalli et al., 2017; Anjum et al., 2017; Salwe et al., 2016; Chowta et al., 2016; Kashyap et al., 2015; Umar et al., 2015; Undela et al., 2014; Karandikar et al., 2013; Momin et al., 2013; Nagendra et al., 2012; Harugeri et al., 2010), whereas all the twenty-seven studies reported PIM use in the older population (Bhatt et al., 2019; Chandrasekhar et al., 2019; Motallebzadeh et al., 2019; Pradhan and Panda, 2018; Benjamin et al., 2018; Devarapalli et al., 2017; Kumar et al., 2017; Pradhan et al., 2017; Narvekar et al., 2017; Borah et al., 2017; Rakesh et al., 2017; Anjum et al., 2017; Swathi and Bhavika, 2016; Salwe et al., 2016; Chowta et al., 2016; Kashyap et al., 2015; Danisha et al., 2015; Umar et al., 2015; Undela et al., 2014; Dhikav et al., 2014; Karandikar et al., 2013; Momin et al., 2013; Nagendra et al., 2012; Shah et al., 2011; Mandavi et al., 2011; Zaveri et al., 2010; Harugeri et al., 2010). Most of the studies used 2012 Beers criteria (Bhatt et al., 2019; Motallebzadeh et al., 2019; Pradhan and Panda, 2018; Devarapalli et al., 2017; Kumar et al., 2017; Pradhan et al., 2017; Narvekar et al., 2017; Borah et al., 2017; Anjum et al., 2017; Swathi and Bhavika, 2016; Salwe et al., 2016; Kashyap et al., 2015; Danisha et al., 2015; Umar et al., 2015; Dhikav et al., 2014), only one study used 2015 STOPP/START criteria (Chandrasekhar et al., 2019), while the rest of the studies used a different version of the Beers criteria in combination with other PIM criteria (Shah et al., 2011; Nagendra et al., 2012; Karandikar et al., 2013; Chowta et al., 2016; Rakesh et al., 2017; Benjamin et al., 2018). The characteristics of the included studies are summarized in Table 1.
TABLE 1.
Characteristics of included studies.
| Author, year | Study characteristics | Explicit criteria | Prevalence (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| States | Design | Period | Setting | Sample size | Age, years (Mean/median) | Explicit criteria | Polypharmacy a | Hyperpolypharmacy b | PIM use | |
| Bhatt et al. (2019) | Kerala | Cross-sectional | 6 | Outpatient | 400 | 73.6 ± 6.7 | Beer's criteria | 45.8 | 13.5 | 34 |
| Chandrasekhar et al. (2019) | Kerala | Cohort | 12 | Inpatient | 210 | Phase 1: 72.59 ± 6.37 | STOPP/START criteria | 60 | 35.7 | Overall: 41.9, phase 1: 43.5, phase 2: 40.2 |
| Phase 2: (71.99 ± 6.30 | ||||||||||
| Motallebzadeh et al., (2019) | Karnataka | Cohort | 6 | Inpatient | 480 | Unspecified | Beers criteria | 36.4 | Unspecified | 11.6 |
| Benjamin et al. (2018) | Karnataka | Cohort | 7 | Inpatient | 350 | 92 (68) | Beers criteria, STOPP criteria | 37.1 | 58.6 | 2012 Beers: 27.7, STOPP: 24.6 |
| Pradhan et al. (2018) | Odisha | Cross-sectional | 3 | Outpatient | 425 | 72.5 ± 7.6 | Beers criteria | 75.1 | Unspecified | 23.8 |
| Devarapalli et al. (2017) | Andhra Pradesh | Cohort | Unspecified | Inpatient | 135 | 66.9 ± 0.2 | Beers criteria | 38.5 | 35.5 | 25.9 |
| Kumar et al., (2017) | Jammu & kashmir | Cohort | 6 | Inpatient | 203 | Unspecified | Beers criteria | Unspecified | Unspecified | 3.7 |
| Pradhan et al. (2017) | Odisha | Cross-sectional | 4 | Outpatient | 800 | 75.8 ± 6.9 | Beers criteria | 41.5 | Unspecified | 21.8 |
| Narvekar et al. (2017) | Goa | Cohort | 5 | Inpatient | 150 | 68.88 (range: 60–87) | Beers criteria | Unspecified | Unspecified | 44 |
| Borah et al. (2017) | Assam | Cross-sectional | 6 | Both | 150 | Unspecified | Beers criteria | 72 | Unspecified | 28.7 |
| Rakesh et al. (2017) | Karnataka | Cross-sectional | 16 | Outpatient | 426 | 71.6 ± 6.4 | MAI, beers criteria, STOPP criteria, and START criteria | 66.2 | Unspecified | 19.9 |
| Anjum et al. (2017) | Tamil nadu | Cohort | 6 | Inpatient | 90 | Unspecified | Beers criteria | 40 | 50 | 51.1 |
| Burla et al. (2016) | Telangana | Cohort | 3 | Outpatient | 287 | Unspecified | Beers criteria | 68.3 | Unspecified | 20.2 |
| Salwe et al. (2016) | Puducherry | Cross-sectional | 3 | Inpatient | 100 | 71.64 ± 6.51 | Beers criteria | 53 | 27 | 48 |
| Chowta et al. (2016) | Karnataka | Cross-sectional | 12 | Outpatient | 120 | 71.56 ± 6.61 | Medication appropriateness index, STOPP/START, Beer’s criteria | 42.5 | 2.5 | 32.5 |
| Kashyapa et al. (2015) | Chandigarh | Cohort | Unspecified | Inpatient | 1,510 | 67.2 ± 0.2 | Beers criteria | 39 | 38.7 | 21 |
| Pattani et al. (2015) | Kerala | Cohort | 12 | Inpatient | 200 | 72.2 ± 8.04 | Beers criteria | Unspecified | Unspecified | 53 |
| Umar et al. (2015) | Karnataka | Cohort | 6 | Inpatient | 203 | 70 ± 2.4 | Beers criteria | 57.1 | 7.9 | 37.4 |
| Undela et al. (2013) | Chandigarh | Cohort | 9 | Inpatient | 1,215 | 68 ± 7.0 | Beers criteria 2003 and beers criteria 2012 | 46 | 40 | 2003 Beers: 11 |
| 2012 Beers: 16 | ||||||||||
| Dhikav et al. (2014) | New Delhi | Cohort | 12 | Outpatient | 143 | 70.1 ± 10.1 | Beers criteria | Unspecified | Unspecified | 41.9 |
| Karandikar et al. (2013) | Maharashtra | Cross-sectional | 8 | Both | 600 | Unspecified | Beers criteria and STOPP/START criteria | 41 | 15 | STOPP: 11.9 Beers: 7.3 |
| Momin et al. (2013) | Gujarat | Cohort | 12 | Inpatient | 210 | 69.34 ± 5.26 | Beers criteria 2003 and 2012 | 50.9 | 34.7 | 2003 Beers: 40 2012 Beers: 28.57 |
| Vishwas et al. (2012) | Karnataka | Cohort | 9 | Inpatient | 540 | 66 (range: 60–95) | Beers criteria and STOPP | 50.2 | 44.4 | 24.6 |
| Shah et al. (2011) | Gujarat | Cohort | 27 | Both | 400 | Unspecified | Beers criteria and Phadke’s criteria | Unspecified | Unspecified | 27.2 |
| Mandavi et al. (2011) | Chandigarh | Cohort | 5 | Outpatient | 1,081 | 68.2 ± 0.20 | Beers criteria | 58 | Unspecified | 10.8 |
| Zaveri et al. (2010) | Gujarat | Cohort | 4 | Outpatient | 407 | Unspecified | Beers criteria | Unspecified | Unspecified | 23.6 |
| Harugeri et al. (2010) | Karnataka | Cohort | 18 | Inpatient | 814 | 66 years (range: 60–95) | Beers criteria | 36.6 | 53.7 | 23.5 |
using >5 drugs;
using ≥10 drugs, PIM: potentially inappropriate medication; STOPP: screening tool of older persons’ prescriptions; START: screening tool to alert to right treatment.
Quality of Included Studies
The quality assessment of included studies was assessed using NOS for the cross-sectional and cohort studies. The highest quality score was 9, and the lowest was 4. The average score of the NOS scale was 7.4, indicating high quality. In the risk of bias assessment, four studies (14.8%) were of lower quality, with a NOS score of <7. Based on NOS criteria, three studies were of lower quality based on criteria 1 (representativeness of the exposure group or sample representation), seven studies based on criteria 2 (selection of non-exposure group or sample selection), and three studies based on criteria 3 (not report the definition of the exposure); only four of eight cross-sectional studies performed appropriate statistical tests (criteria 7). Detailed results on the NOS quality assessment are presented in Supplementary Table S3.
Prevalence of Polypharmacy
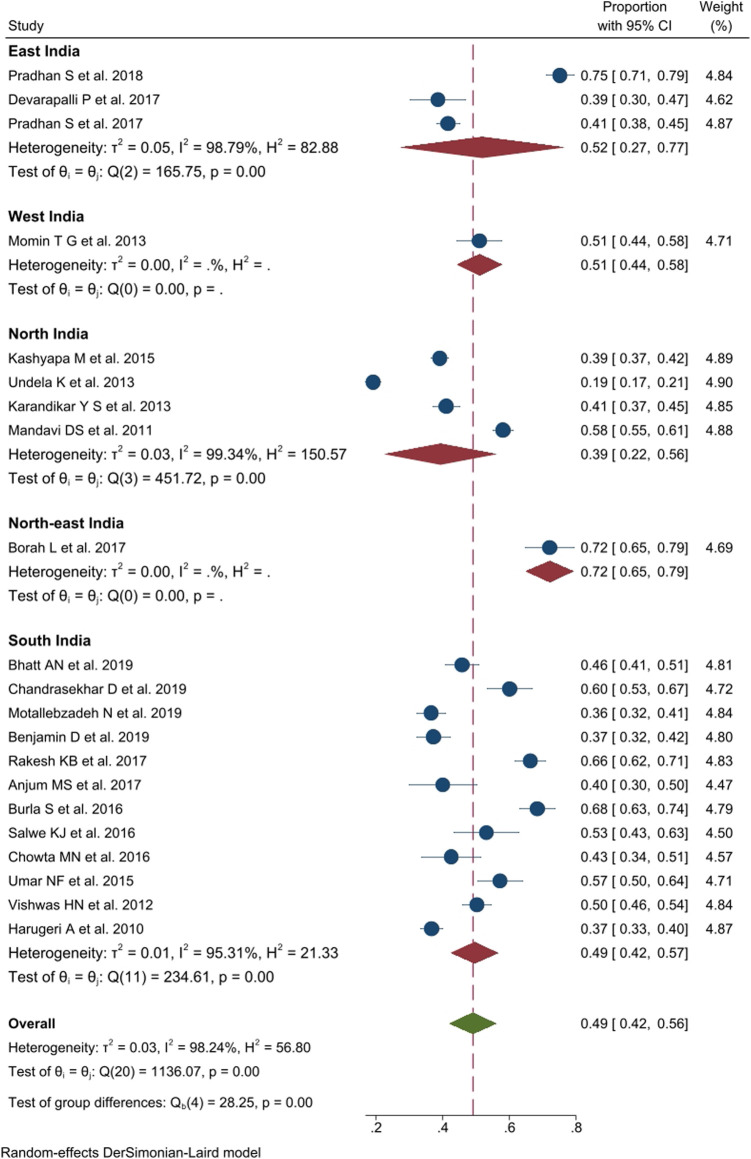
Out of 27 publications, twenty-one studies, comprising 9,391 participants, reported a prevalence of polypharmacy among older adults. The pooled prevalence of polypharmacy in India, after weighing the regional population size, was 49% (n = 10,146, 95% CI: 42–56; I 2 = 98.2%, p < 0.01, τ2 0.03). Region-wize data showed significant differences in the prevalence of polypharmacy between different regions of India (Q = 5.47, df = 4; p < 0.01) ranging from 39% (95% CI: 22–56; I 2 = 99.3%, p < 0.01) in Northern states to 52% (95% CI: 27–77, I 2 = 98.8%, p < 0.01) in East India. Studies from West India (51%, 95% CI: 44–58), and North-east India reported higher prevalence of polypharmacy (72%, 95% CI: 65–79). Moreover, the majority of studies were conducted in South India (Harugeri et al., 2010; Nagendra et al., 2012; Kashyap et al., 2015; Umar et al., 2015; Chowta et al., 2016; Salwe et al., 2016; Swathi and Bhavika, 2016; Devarapalli et al., 2017; Narvekar et al., 2017; Pradhan and Panda, 2018; Bhatt et al., 2019; Chandrasekhar et al., 2019; Motallebzadeh et al., 2019), where the prevalence of polypharmacy was 49% (95% CI: 42–57; I 2 = 95.3%, p < 0.01). The data on the prevalence of polypharmacy in other regions is summarized in Figure 2.
FIGURE 2.
Prevalence of polypharmacy use (5-9 medications) in older people across various geographic regions in India.
Hyperpolypharmacy
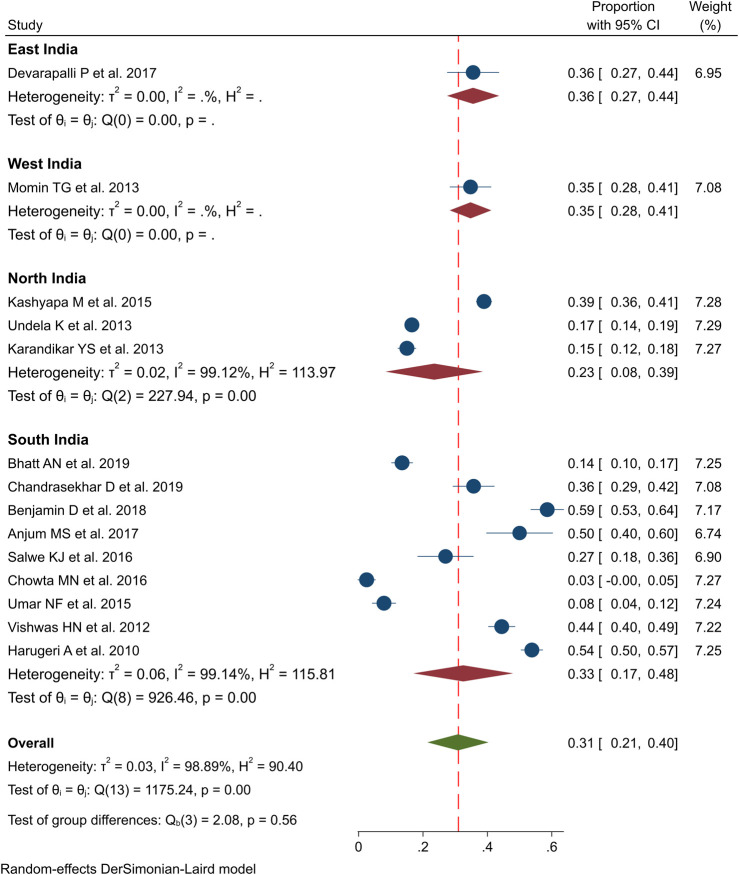
Fourteen studies investigated the prevalence of hyperpolypharmacy among the older population in India (Bhatt et al., 2019; Chandrasekhar et al., 2019; Benjamin et al., 2018; Devarapalli et al., 2017; Anjum et al., 2017; Salwe et al., 2016; Chowta et al., 2016; Kashyap et al., 2015; Umar et al., 2015; Undela et al., 2014; Karandikar et al., 2013; Momin et al., 2013; Nagendra et al., 2012; Harugeri et al., 2010). The pooled estimate of hyperpolypharmacy was 31% in India (n = 6,497, 95% CI: 21–40; I 2 = 98.9%; p < 0.01; τ2 0.0321). Region-wize data on the prevalence of hyperpolypharmacy among older adults showed considerable variations with 36% prevalence was seen in East India (95% CI: 27–44), 35% in West India (95% CI: 28–41), 23% in North India (95% CI: 8–39) and 33% (95% CI: 17–48) in South India, as shown in Figure 3. However, these differences between the regions were not statistically significant (Q = 2.08, df = 3; p = 0.560).
FIGURE 3.
Prevalence of hyperpolypharmacy (≥10 drugs) use in older people across various geographic regions in India.
PIM Use
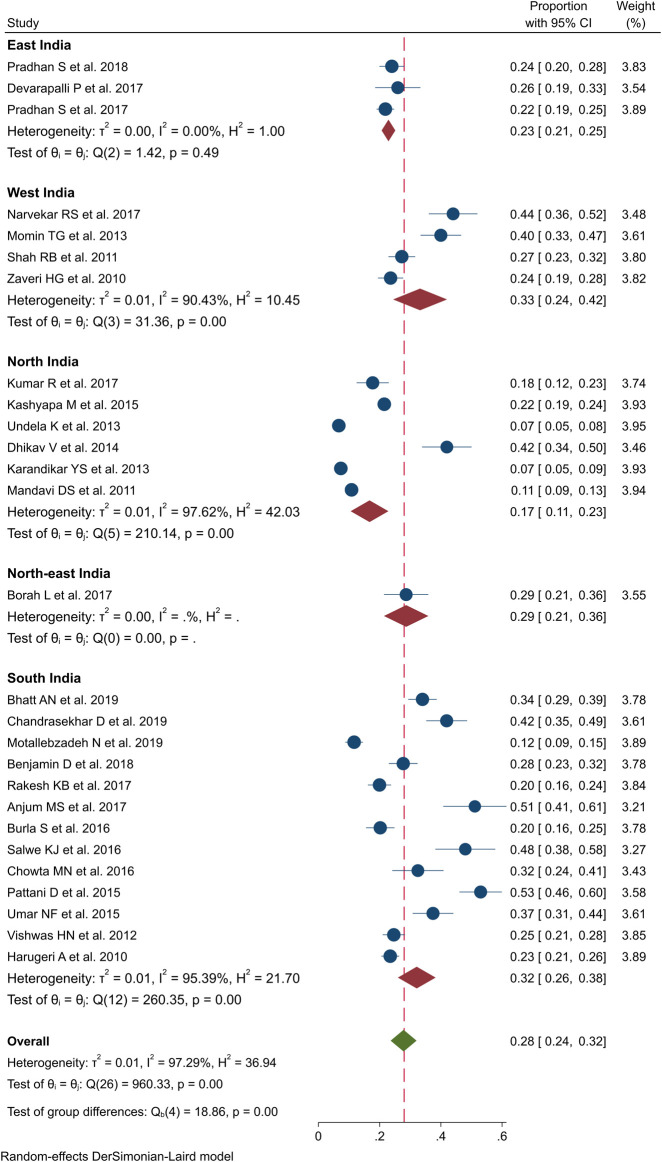
All the 27 studies provided PIM estimates among the older population in India (Bhatt et al., 2019; Chandrasekhar et al., 2019; Motallebzadeh et al., 2019; Pradhan and Panda, 2018; Benjamin et al., 2018; Devarapalli et al., 2017; Kumar et al., 2017; Pradhan et al., 2017; Narvekar et al., 2017; Borah et al., 2017; Rakesh et al., 2017; Anjum et al., 2017; Swathi and Bhavika, 2016; Salwe et al., 2016; Chowta et al., 2016; Kashyap et al., 2015; Danisha et al., 2015; Umar et al., 2015; Undela et al., 2014; Dhikav et al., 2014; Karandikar et al., 2013; Momin et al., 2013; Nagendra et al., 2012; Shah et al., 2011; Mandavi et al., 2011; Zaveri et al., 2010; Harugeri et al., 2010). The pooled prevalence of PIM was found to be 28% by using random-effect model (n = 11,649, 95% CI: 24–32; I 2 = 97.3; p < 0.01; τ2 0.0117), which indicated substantial heterogeneity, as shown in Figure 4. Comparison of PIM proportions in India showed significant differences among the four regions (NEWS) (Q = 18.8, df = 4; p < 0.01). West India and South India demonstrated a relatively higher pooled prevalence of 33% (95% CI: 24–42, p < 0.01) and 32% (95% CI: 26–38, p < 0.01), respectively, while North India and East India had a lower pooled prevalence of 17 and 23%, respectively. The variations in the pooled prevalence of PIM use are further illustrated in the forest plot in Figure 4.
FIGURE 4.
Prevalence of potential inappropriate medication (PIM) use in older people across various geographic regions in India.
Stratified Analysis
A stratified meta-analysis of the prevalence of polypharmacy, hyperpolypharmacy, and PIM use in India is summarized in Table 2. We stratified the studies by various baseline characteristics and interrogated the source of heterogeneity and differences between the groups. Significant heterogeneity was detected among all the subgroups; for instance, studies performed for less than 6°months of duration had a higher pooled prevalence of polypharmacy (59%), compared to those conducted for 6–12°months (46%) and >1 year (47%). A significant heterogeneity between the groups was observed (Q = 26.4, df = 3; p < 0.01). Regarding PIM use, studies conducted before 2013 had a lower pooled prevalence (22%) than those conducted between 2013 and 2016 (31%); however, the pooled prevalence slightly decrease for studies conducted after 2017. Grouping the studies by various subgroups did not reduce heterogeneity, and no significant difference was observed between the groups (duration of the study, mean age, percentage of females, the mean number of medications prescribed, and the number of PIM identified). However, significant differences in the heterogeneity were observed between low-quality and high-quality studies (Q = 5.30, df = 1; p = 0.021).
TABLE 2.
Stratified meta-analysis of the prevalence of polypharmacy, hyperpolypharmacy, and potential inappropriate medication (PIM) use in India.
| Characteristics | Number of studies | Pooled prevalence in percentage (95% CI) | p For interaction a | I 2 (%) | Z | Heterogeneity between groups | ||
|---|---|---|---|---|---|---|---|---|
| 1. Polypharmacy | Q | df | p | |||||
| Year of publication | 0.001 | 0.43 | 2 | 0.807 | ||||
| ≤2012 | 3 | 48 (42–56) | - | 7.08 | ||||
| 2013–2016 | 8 | 46 (34–58) | 98.4 | 7.57 | ||||
| ≥2017 | 10 | 51 (41–61) | 97.3 | 10.1 | ||||
| Study duration | 0.001 | 26.43 | 3 | <0.001 | ||||
| <6°months | 5 | 59 (47–72) | 97.6 | 9.28 | ||||
| 6–12°months | 12 | 46 (36–55) | 97.7 | 9.51 | ||||
| >1°year | 2 | 47 (44–50) | - | 34.6 | ||||
| Mean age | 0.001 | 0.41 | 2 | 0.81 | ||||
| <70 | 8 | 46 (33–59) | 99.1 | 6.90 | ||||
| ≥70 | 8 | 50 (42–58) | 93.9 | 12.36 | ||||
| NA | 5 | 52 (37–66) | 97.1 | 6.96 | ||||
| Percentage of female | 0.001 | 0.04 | 1 | 0.84 | ||||
| <50% | 15 | 49 (41–58) | 98.6 | 11.0 | ||||
| ≥50% | 6 | 48 (38–58) | 93.1 | 9.37 | ||||
| Average number of drugs | 0.001 | 14.55 | 2 | 0.001 | ||||
| <5.5 | 2 | 61 (57–65) | - | 30.2 | ||||
| ≥5.5 | 15 | 49 (41–58) | 98.6 | 11.07 | ||||
| NA | 4 | 45 (36–54) | 88.8 | 9.78 | ||||
| Number of PIM use | 0.001 | 0.37 | 1 | 0.54 | ||||
| <3 | 12 | 47 (41–53) | 95.3 | 14.7 | ||||
| ≥3 | 9 | 52 (37–66) | 99.0 | 7.2 | ||||
| Quality of studies b | 0.001 | 0.66 | 1 | 0.42 | ||||
| High (≥7) | 18 | 48 (40–54) | 98.4 | 12.36 | ||||
| Low (<7) | 3 | 56 (38–74) | - | 6.01 | ||||
| 2. Hyperpolypharmacy | ||||||||
| Year of publication | 0.001 | 29.81 | 2 | 0.001 | ||||
| ≤2012 | 2 | 50 (47–53) | - | 37.0 | ||||
| 2013–2016 | 7 | 20 (10–31) | 98.7 | 3.76 | ||||
| ≥2017 | 5 | 39 (19–58) | 98.2 | 3.79 | ||||
| Study duration | 0.001 | 72.9 | 3 | 0.001 | ||||
| <6°months | 1 | 27 (19–36) | - | 6.08 | ||||
| 6–12°months | 10 | 28 (18–37) | 98.6 | 5.46 | ||||
| >1°year | 1 | 54 (50–57) | - | 30.79 | ||||
| Mean age | 0.001 | 8.83 | 2 | 0.012 | ||||
| <70 | 6 | 37 (24–51) | 98.8 | 5.55 | ||||
| ≥70 | 6 | 24 (8–40) | 98.8 | 2.99 | ||||
| Na | 2 | 17 (15–20) | - | 12.45 | ||||
| Percentage of female | 0.001 | 0.82 | 1 | 0.365 | ||||
| <50% | 10 | 33 (22–44) | 98.8 | 5.99 | ||||
| ≥50% | 4 | 25 (9–40) | 97.9 | 3.07 | ||||
| Average number of drugs | 0.001 | 0.001 | ||||||
| <5.5 | 1 | 3 (1–7) | - | 1.75 | ||||
| ≥5.5 | 10 | 36 (26–46) | 98.6 | 6.82 | ||||
| Na | 1 | 23 (8–37) | - | 3.06 | ||||
| Number of PIM use | 0.001 | 0.70 | 1 | 0.403 | ||||
| <3 | 9 | 34 (21–47) | 98.85 | 5.12 | ||||
| ≥3 | 5 | 25 (9–41) | 98.12 | 3.08 | ||||
| Quality of studies b | 0.001 | 31.2 | 1 | 0.001 | ||||
| High (≥7) | 12 | 34 (24–43) | 98.7 | 6.76 | ||||
| Low (<7) | 2 | 5 (2–5) | - | 3.53 | ||||
| 3. PIM use | ||||||||
| Year of publication | 0.449 | 3.33 | 2 | 0.189 | ||||
| ≤2012 | 5 | 22 (15–29) | 93.9 | 5.87 | ||||
| 2013–2016 | 10 | 31 (24–39) | 97.3 | 8.17 | ||||
| ≥2017 | 12 | 28 (23–34) | 96.3 | 10.04 | ||||
| Study duration | 0.930 | 7.46 | 3 | 0.059 | ||||
| <6°months | 7 | 27 (19–34) | 96.2 | 7.0 | ||||
| 6–12°months | 15 | 31 (24–37) | 97.0 | 9.21 | ||||
| >1°year | 3 | 23 (20–27) | - | 12.41 | ||||
| Mean age | 0.072 | 5.76 | 2 | 0.056 | ||||
| <70 | 9 | 24 (19–30) | 96.7 | 8.64 | ||||
| ≥70 | 9 | 35 (28–42) | 93.9 | 9.66 | ||||
| Na | 9 | 25 (19–32) | 94.5 | 7.68 | ||||
| Percentage of female | 0.179 | 1.57 | 1 | 0.210 | ||||
| <50% | 20 | 26 (22–30) | 96.0 | 12.92 | ||||
| ≥50% | 7 | 34 (22–46) | 96.3 | 5.74 | ||||
| Average number of drugs | 0.548 | 4.08 | 2 | 0.130 | ||||
| <5.5 | 4 | 23 (18–27) | 70.5 | 9.54 | ||||
| ≥5.5 | 17 | 27 (22–31) | 96.1 | 12.11 | ||||
| Na | 6 | 35 (22–48) | 97.5 | 5.31 | ||||
| Number of PIM use | 0.782 | 0.15 | 1 | 0.702 | ||||
| <3 | 17 | 27 (23–32) | 95.7 | 11.57 | ||||
| ≥3 | 10 | 29 (22–36) | 96.8 | 8.24 | ||||
| Quality of studies b | 0.112 | 5.30 | 1 | 0.021 | ||||
| High (≥7) | 23 | 27 (23–30) | 96.2 | 13.50 | ||||
| Low (<7) | 4 | 37 (29–46) | 75.8 | 8.68 | ||||
p-value from meta-regression analyses,
New-Castle Ottawa scale score, PIM: potential inappropriate medication.
Subgroup Analysis
Subgroup analysis by geographic region, study design, type of hospital, and study settings did not influence the prevalence estimates of polypharmacy, hyperpolypharmacy, and PIM use, as shown in Table 3. However, the prevalence of hyperpolypharmacy in outpatient settings (8%) and cross-sectional studies (14%) was low. The prevalence of PIM use varied between inpatient (31%) and outpatient settings (25%); however, lower prevalence of PIM use was reported in government hospitals (25%).
TABLE 3.
Subgroup analysis for the potential variables between studies of prevalence of polypharmacy, hyperpolypharmacy and potential inappropriate medication (PIM) use in older population in India.
| Subgroups | No of studies | Prevalence (95%CI) | Test for heterogeneity | Between subgroup differences | |||||
|---|---|---|---|---|---|---|---|---|---|
| Tau 2 | p | I 2 | Q | df | p | ||||
| Polypharmacy | |||||||||
| Geographical region | South India | 12 | 49% (42–57%) | 0.0148 | <0.01 | 99% | 28.25 | 4 | <0.001 |
| East India | 3 | 52% (27–77%) | 0.0472 | <0.01 | 99% | ||||
| North India | 4 | 39% (22–56%) | 0.0298 | <0/01 | 99% | ||||
| West India | 1 | 51% (44–58%) | - | - | - | ||||
| North east India | 1 | 72% (65–79%) | - | - | - | ||||
| Study design | Cross-sectional | 8 | 55% (44–65%) | 0.0234 | <0.01 | 97% | 1.70 | 1 | 0.191 |
| Cohort | 13 | 45% (37–54%) | 0.0242 | <0.01 | 98% | ||||
| Hospital | Government | 8 | 53% (38–67%) | 0.0428 | <0.01 | 99% | 0.65 | 1 | 0.418 |
| Private | 13 | 46% (41–52%) | 0.0102 | <0.01 | 93% | ||||
| Setting | Inpatient | 12 | 43% (35–51%) | 0.0168 | <0.01 | 97% | 5.11 | 2 | 0.077 |
| Outpatient | 7 | 57% (47–67%) | 0.0169 | <0.01 | 97% | ||||
| Both in-and-outpatient | 2 | 56% (26–87%) | 0.0472 | <0.01 | 98% | ||||
| Hyper polypharmacy | |||||||||
| Geographical region | South India | 9 | 33% (17–48%) | 0.0551 | <0.01 | 99% | 2.08 | 3 | 0.555 |
| East India | 1 | 36% (27–44%) | - | - | - | ||||
| North India | 3 | 23% (8–39%) | 0.0176 | <0.01 | 99% | ||||
| West India | 1 | 35% (28–41%) | - | - | - | ||||
| Study design | Cross-sectional | 4 | 14% (6–22%) | 0.0059 | <0.01 | 95% | 11.65 | 1 | <0.001 |
| Cohort | 10 | 38% (26–49%) | 0.0313 | <0.01 | 99% | ||||
| Hospital | Government | 3 | 30% (13–47%) | 0.0218 | <0.01 | 99% | 0.01 | 1 | 0.916 |
| Private | 11 | 31% (19–44%) | 0.0444 | <0.01 | 99% | ||||
| Setting | Inpatient | 11 | 37% (26–47%) | 0.0305 | <0.01 | 99% | 17.44 | 2 | <0.001 |
| Outpatient | 2 | 8% (0–19%) | 0.0058 | <0.01 | 96% | ||||
| Both in-and-outpatient | 1 | 15% (12–18%) | - | - | - | ||||
| PIM use | |||||||||
| Geographical region | South India | 13 | 32% (26–38%) | 0.0118 | <0.01 | 95% | 18.86 | 4 | 0.001 |
| East India | 3 | 23% (21–25%) | 0 | 0.49 | 0% | ||||
| North India | 6 | 17% (11–23%) | 0.0055 | <0.01 | 98% | ||||
| West India | 4 | 33% (24–42%) | 0.0071 | <0.01 | 90% | ||||
| North east India | 1 | 29% (21–36%) | - | - | - | ||||
| Study design | Cross-sectional | 8 | 27% (18–35%) | 0.0127 | <0.01 | 97% | 0.16 | 1 | 0.693 |
| Cohort | 19 | 28% (23–34%) | 0.0127 | <0.01 | 98% | ||||
| Hospital | Government | 13 | 25% (19–30%) | 0.0095 | <0.01 | 97% | 1.82 | 1 | 0.176 |
| Private | 14 | 31% (24–38%) | 0.0166 | <0.01 | 97% | ||||
| Setting | Inpatient | 15 | 31% (24–38%) | 0.0169 | <0.01 | 98% | 2.47 | 2 | 0.290 |
| Outpatient | 9 | 25% (19–31%) | 0.0075 | <0.01 | 95% | ||||
| Both in-and-outpatient | 3 | 21% (5–37%) | 0.0190 | <0.01 | 98% | ||||
Publication Bias Assessment
The Egger’s and Begg’s tests indicated statistically significant publication bias for the polypharmacy estimates (Egger test: p = 0.034) and PIM use (Egger test: p = 0.027 & Begg’s test: p = 0.001). Visual examination of the funnel plots showed asymmetry and suggested publication bias, as shown in Supplementary Figure S1.
Discussion
Overuse and misuse of medications in the older population are among the major concerns in India (Porter and Grills, 2016). The growing culture of irrational and unnecessary prescribing of medications in the older population may increase the risk of adverse outcomes. Multiple studies demonstrated that poor prescribing practices (Chaturvedi et al., 2012), inappropriate medication selection (Boralkar et al., 2011; Castelino et al., 2011), and frequent misuse of drugs to earn profits (Roy et al., 2007; Kotwani et al., 2010) are some of the factors that result in polypharmacy, hyperpolypharmacy, and PIM use in India. In particular, older people with multiple comorbidities are exposed to polypharmacy, and suboptimal prescribing may increase their likelihood of receiving PIMs (Fabbietti et al., 2018).
We assessed the prevalence of polypharmacy, hyperpolypharmacy, and PIM use among the older population through a comprehensive systematic review and reported regional differences in prevalence across four regions in India. Data from 27 studies (11,649 participants) reported a higher prevalence of polypharmacy (49%), hyperpolypharmacy (31%), and PIM use (28%) among the older population in India. Region-specific estimates showed that polypharmacy is widely prevalent in Northern India (72%), hyperpolypharmacy in the eastern and western parts of India (36%), and PIM use (33%) in Western states. Furthermore, polypharmacy is more frequently observed in outpatient settings (57%) and hyperpolypharmacy in inpatient settings (37%). Stratified analysis showed variations in PIM exposure across subsets, and governmental hospitals showed a lower prevalence of PIM use than private hospitals (25 vs. 27%). Considerable variations in polypharmacy and hyperpolypharmacy are seen among cross-sectional studies in comparison to cohort studies.
The regional differences in the prevalence of polypharmacy, hyperpolypharmacy, and PIM use among the older population noted in our study could be due to the inclusion of a limited number of studies, smaller sample size, and differences in socioeconomic conditions, risk factors, and quality of healthcare services across the four regions of India. Two-thirds of the studies were conducted in South India, where the level of awareness of geriatric care and polypharmacy prevalence was very high compared to other regions. The Sharma et al. study demonstrated the differences in the prevalence of polypharmacy in the Indian states and reported that Uttaranchal (93.1%) from North India and Southern states, such as Telangana (82.8%) and Karnataka (84.6%), had the highest prevalence of polypharmacy compared to Northeast -West Bengal (5.8%), Tripura (East India) (6.8%), Madhya Pradesh (central India) (8.3%) and Goa (West India) (13.8%) (Pravodelov, 2020). While the underlying reasons for the increasing prevalence of polypharmacy are still unknown, our findings highlight the need to develop strategies to reduce polypharmacy in clinical practice and motivate physicians to adopt more judicious prescribing to reduce the number of medications among the older population in India.
Polypharmacy and hyperpolypharmacy are proxy indicators for PIM use in older populations, leading to adverse clinical outcomes. The findings of the current study revealed an increasing incidence of hyperpolypharmacy. The pooled estimates showed a much higher prevalence of hyperpolypharmacy (31%) in India than the developed countries like the United States of America (1%) (Assari et al., 2019), New Zealand (2.1%) (Nishtala and Salahudeen, 2015), Australia (8%) (Wylie et al., 2020), Sweden (18%) (Hovstadius et al., 2010), and Finland (28%) (Jyrkkä et al., 2009). Several review articles suggest that the application of clinical guidelines in the older population may contribute to hyperpolypharmacy (Hilmer and Gazarian, 2008; Scott and Guyatt, 2010; Kojima et al., 2020). However, it is widely recognized that the evidence-based guidelines are derived from clinical trials that generally exclude older patients with comorbidity (Sheridan and Julian, 2016; Guthrie and Boyd, 2018). This research provides vital information to alert clinicians and researchers about the dire need to reduce the medication burden in older people.
Our findings on the pooled prevalence of PIM use showed that 28% of older patients in India are affected by PIM; a similar trend was observed over the years in high-income countries (33.3%) (Liew et al., 2019). This study did not identify potential variations across different regions ranging from 33 to 36%, except North India (23%). In a recent meta-analysis of studies conducted among older patients in primary care settings, the pooled prevalence of PIM use was 33.3% in high-income countries, varying from 24.8% in North America to 59.2% in Australasians (Liew et al., 2019). In the same study, the prevalence of PIM use in middle-income countries was 23.2%. With the increase in the older population, our pooled results suggest a need for multi-pronged approaches to address PIM use in India. Some approaches include medication reviews by clinical pharmacists and the implementation of a computerized clinical decision support system. Moreover, there is a need to plan broader interprofessional interventions to motivate clinicians to reduce polypharmacy and improve the optimal use of medications in older people. The World Health Organization suggested monitoring and rectifying potential PIM prescribing regularly and prioritizing medication safety at the national level to reduce PIM use in the older population (World Health Organization, 2017).
Findings from this study demonstrate the prevalence of polypharmacy, hyperpolypharmacy, and PIM use in Indian states and highlight the urgency to address inappropriate medication use in the older population. Therefore, future studies with a multi-pronged approach should be conducted, focusing on comprehensive geriatric medication reviews by clinical pharmacists, computerized clinical decision support systems, and prioritizing rational geriatric prescribing at the national level. Moreover, multifaceted randomized controlled trials are needed to evaluate the effects of the intervention on clinically relevant outcomes such as hospitalization, medication costs, and health-related quality of life.
Strengths and Limitations
This is the first systematic review and meta-analyses to consolidate the quantitative evidence on the wide-ranging impact of polypharmacy, hyperpolypharmacy, PIM use in various states of India. We also thoroughly assessed the risk of bias in each of the 27 observational studies. We further conducted meta-analyses stratified according to the suspected potential source of heterogeneity between the studies and subgroups.
The study findings are subject to some limitations. First, factors like geographic areas, cultures, and practices vary widely across the states in India, which may influence the results. Second, higher heterogeneity in the outcomes may be due to differences in sample size (ranging from a few hundred to a few thousand). Low power and precision may produce higher Cochran Q (heterogeneity x2 test statistics) and I 2. More studies were conducted in South India than in any other region. Third, publication bias was present in the selected studies and has been known to affect heterogeneity. We performed a more stratified subgroup analysis to explore the source of heterogeneity and differences within the subsets.
Conclusion
The prevalence of polypharmacy and hyperpolypharmacy among older Indian adults is relatively high. Almost a quarter of the older people are affected by PIM use in India. Significant regional differences exist in the prevalence of polypharmacy, hyperpolypharmacy, and PIM use. These findings highlight the need for urgent steps to promote rational geriatric prescribing and prioritize pharmacist-led comprehensive medication reviews to reduce medication-related problems among older people in India.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
Conception and design: ASB, RS, KV. Acquisition of data: all authors. Analysis and interpretation of data: ASB, KV, MC, RS, DKB. Drafting the article: ASB, MC, MR. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: ASB. Statistical analysis: ASB, KV, MC, RS. Administrative/technical/material support: DKB. Study supervision: DF.
Funding
Research works have been funded by SVV260551 grant of the Faculty of Pharmacy in Hradec Králové, Charles University, Czech Republic (supervisor: Assoc. Prof. D. Fialová). Research works of D. Fialová has been financially supported also by the project InoMed NO.CZ.02.1.01/0.0/0.0/18_069/0010046 “Pre-application research into innovative medicines and medical technologies (InoMed)” co-funded by the European Union; the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant No. 764632; PROGRESS Q42 program at the Faculty of Pharmacy, Charles University, Czech Republic (KSKF-2 scientific group) and I-CARE4OLD Horizon 2020 project ID: 965341.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.685518/full#supplementary-material
References
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel (2015). American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J. Am. Geriatr. Soc. 63, 2227–2246. 10.1111/jgs.13702 [DOI] [PubMed] [Google Scholar]
- Anjum S. M., Jacob C., Benchamin A., Rodrigues P. A. (2017). A Prospective Study on Geriatric Prescribing Pattern and Medication Adherence in a Tertiary Care Hospital. Asian J. Pharm. Clin. Res. 10 (12), 220–225. 10.22159/ajpcr.2017.v10i12.20460 [DOI] [Google Scholar]
- Assari S., Helmi H., Bazargan M. (2019). Polypharmacy in African American Adults: A National Epidemiological Study. Pharmacy 7 (2), 33. 10.3390/pharmacy7020033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bala S. S., Chen T. F., Nishtala P. S. (2019). Reducing Potentially Inappropriate Medications in Older Adults: A Way Forward. Can. J. Aging 38 (4), 419–433. 10.1017/S0714980819000084 [DOI] [PubMed] [Google Scholar]
- Benjamin D., Thankachan M., Saranya R. (2018). Beers Criteria versus Screening Tool of Older Persons' Potentially Inappropriate Prescriptions in Evaluation of Drug-Prescribing Practice in an Indian Hospital. Asian J. Gerentol Geriatr. 13 (1), 13–18. 10.1111/jgs.12891 [DOI] [Google Scholar]
- Bhatt A. N., Paul S. S., Krishnamoorthy S., Baby B. T., Mathew A., Nair B. R. (2019). Potentially Inappropriate Medications Prescribed for Older Persons: A Study from Two Teaching Hospitals in Southern India. J. Fam. Community Med 26 (3), 187–192. 10.4103/jfcm.JFCM_81_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borah L., Devi D., Debnath P. K., Deka D. (2017). A Study of Drug Utilization Pattern of the Geriatric Patients in the Department of Geriatric Medicine in a Tertiary Care Hospital in Assam, India. Asian J. Pharm. Clin. Res. 10, 122–126. 10.22159/ajpcr.2017.v10i2.14762 [DOI] [Google Scholar]
- Boralkar A., Bobhate P., Khairnar A. (2011). Prospective Cross-Sectional Study in Evaluation of Prescribing Pattern of Doctors for Oncology Treatment. Res. J. Pharm. Technol. 4 (4), 634–637. [Google Scholar]
- Castelino R. L., Sathvik B. S., Parthasarathi G., Gurudev K. C., Shetty M. S., Narahari M. G. (2011). Prevalence of Medication-Related Problems Among Patients with Renal Compromise in an Indian Hospital. J. Clin. Pharm. Ther. 36 (4), 481–487. 10.1111/j.1365-2710.2011.01266.x [DOI] [PubMed] [Google Scholar]
- Chandrasekhar D., Samjas M., pattani D. (2019). Evaluation of Potentially Inappropriate Medications Among Hospitalized Geriatric Patients in Tertiary Care Referral Hospital Using STOPP/START Criteria. Clin. Epidemiol. Glob. Health 7 (3), 268–273. 10.1016/j.cegh.2018.10.008 [DOI] [Google Scholar]
- Chaturvedi V. P., Mathur A. G., Anand A. C. (2012). Rational Drug Use - as Common as Common Sense? Med. J. Armed Forces India 68 (3), 206–208. 10.1016/j.mjafi.2012.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowta M. N., Adhikari P. M., Raj S., Laxman M., Kariappa A., George J., et al. (2016). Evaluation of Appropriateness of Prescription and Polypharmacy in the Geriatric Population: A Cross-Sectional Study at a Comprehensive Geriatric Clinic in a Tertiary Care Hospital. Int. J. Pharm. Pharm. Sci. 8 (3), 119–123. [Google Scholar]
- Danisha P., Dilip C., Mohan P. L., Shinu C., Parambil J. C., Sajid M. (2015). Identification and Evaluation of Potentially Inappropriate Medications (PIMs) in Hospitalized Geriatric Patients Using Beers Criteria. J. Basic Clin. Physiol. Pharmacol. 26 (4), 403–410. 10.1515/jbcpp-2014-0054 [DOI] [PubMed] [Google Scholar]
- Davies L. E., Spiers G., Kingston A., Todd A., Adamson J., Hanratty B. (2020). Adverse Outcomes of Polypharmacy in Older People: Systematic Review of Reviews. J. Am. Med. Directors Assoc. 21 (2), 181–187. 10.1016/j.jamda.2019.10.022 [DOI] [PubMed] [Google Scholar]
- de Oliveira L. M., Diel J. D. A. C., Nunes A., da Silva Dal Pizzol T. (2020). Prevalence of Drug Interactions in Hospitalised Elderly Patients: a Systematic Review. Eur. J. Hosp. Pharm. 28, 4–9. 10.1136/ejhpharm-2019-002111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarapalli P., Soni S., T.K.N.V R. K., Swaraj G., A.M.S S. B. (2017). Assessment of Inappropriate Medication Use in Elderly Inpatients of a Tertiary Care Hospital in South-Eastern India Using the Modified Updated Beers Criteria 2003. Drugs Ther. Perspect. 33 (11), 543–549. 10.1007/s40267-017-0442-6 [DOI] [Google Scholar]
- Dhikav V. I., Sethi M. A., Singhal A. K., Anand K. S. (2014). Polypharmacy and Use of Potentially Inappropriate Medications in Patients with Dementia and Mild Cognitive Impairment. Asian J. Pharma Clin. Res. 7 (2), 218–220. [Google Scholar]
- Fabbietti P., Ruggiero C., Sganga F., Fusco S., Mammarella F., Barbini N., et al. (2018). Effects of Hyperpolypharmacy and Potentially Inappropriate Medications (PIMs) on Functional Decline in Older Patients Discharged from Acute Care Hospitals. Arch. Gerontol. Geriatr. 77, 158–162. 10.1016/j.archger.2018.05.007 [DOI] [PubMed] [Google Scholar]
- Guillot J., Maumus-Robert S., Bezin J. (2020). Polypharmacy: a General Review of Definitions, Descriptions and Determinants. Therapies 75 (5), 407–416. 10.1016/j.therap.2019.10.001 [DOI] [PubMed] [Google Scholar]
- Guthrie B., Boyd C. M. (2018). Clinical Guidelines in the Context of Aging and Multimorbidity. Public Pol. Aging Rep. 28 (4), 143–149. 10.1093/ppar/pry038 [DOI] [Google Scholar]
- Harugeri A., Joseph J., Parthasarathi G., Ramesh M., Guido S. (2010). Potentially Inappropriate Medication Use in Elderly Patients: a Study of Prevalence and Predictors in Two Teaching Hospitals. J. Postgrad. Med. 56 (3), 186–191. 10.4103/0022-3859.68642 [DOI] [PubMed] [Google Scholar]
- He W., Kinsella K. (2020). Global Aging in the New Millennium. Cult. Context Aging Worldwide Perspect., 27. [Google Scholar]
- Higgins J. P. T., Thompson S. G., Deeks J. J., Atlman D. G. (2003). Measuring Inconsistency in Meta-Analyses. BMJ 327 (7414), 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Taylor B., Sketris I., Hayden J., Byrne S., O'Sullivan D., Christie R. (2013). Application of the STOPP/START Criteria: a Systematic Review of the Prevalence of Potentially Inappropriate Prescribing in Older Adults, and Evidence of Clinical, Humanistic and Economic Impact. J. Clin. Pharm. Ther. 38, 360–372. 10.1111/jcpt.12059 [DOI] [PubMed] [Google Scholar]
- Hilmer S. N., Gazarian M. (2008). Clinical Pharmacology in Special Populations: the Extremes of Age. Expert Rev. Clin. Pharmacol. 1 (4), 467–469. 10.1586/17512433.1.4.467 [DOI] [PubMed] [Google Scholar]
- Hovstadius B., Hovstadius K., Åstrand B., Petersson G. (2010). Increasing Polypharmacy – an Individual-Based Study of the Swedish Population 2005–2008. BMC Clin. Pharmacol. 10, 16. 10.1186/1472-6904-10-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huedo-Medina T. B., Sánchez-Meca J., Marín-Martínez F., Botella J. (2006). Assessing Heterogeneity in Meta-Analysis: Q Statistic or I² Index? Psychol. Methods 11 (2), 193–206. 10.1037/1082-989x.11.2.193 [DOI] [PubMed] [Google Scholar]
- Jyrkkä J., Enlund H., Korhonen M. J., Sulkava R., Hartikainen S. (2009). Patterns of Drug Use and Factors Associated with Polypharmacy and Excessive Polypharmacy in Elderly Persons. Drugs & Aging 26 (6), 493–503. 10.2165/00002512-200926060-00006 [DOI] [PubMed] [Google Scholar]
- Karandikar Y. S., Chaudhari S. R., Dalal N. P., Sharma M., Pandit V. A. (2013). Inappropriate Prescribing in the Elderly: a Comparison of Two Validated Screening Tools. J. Clin. Gerontol. Geriatr. 4 (4), 109–114. 10.1016/j.jcgg.2013.04.004 [DOI] [Google Scholar]
- Kashyap M., D’Cruz S., Sachdev A., Tiwari P. (2015). Evidence-based Information Leads to Reduction in Inappropriate Drug Prescribing: Results from Indian Older Inpatients. Jrs 27 (4), 209–217. 10.3233/jrs-150665 [DOI] [PubMed] [Google Scholar]
- Kaufmann C. P., Tremp R., Hersberger K. E., Lampert M. L. (2014). Inappropriate Prescribing: a Systematic Overview of Published Assessment Tools. Eur. J. Clin. Pharmacol. 70, 1–11. 10.1007/s00228-013-1575-8 [DOI] [PubMed] [Google Scholar]
- Kojima T., Mizokami F., Akishita M. (2020). Geriatric Management of Older Patients with Multimorbidity. Geriatr. Gerontol. Int. 20, 1105–1111. 10.1111/ggi.14065 [DOI] [PubMed] [Google Scholar]
- Kotwani A., Wattal C., Katewa S., Joshi P. C., Holloway K. (2010). Factors Influencing Primary Care Physicians to Prescribe Antibiotics in Delhi India. Fam. Pract. 27 (6), 684–690. 10.1093/fampra/cmq059 [DOI] [PubMed] [Google Scholar]
- Kumar R., Bhat N. K., Kumar D., Gupta S. (2017). Drug Prescribing Pattern in Elderly Patients Admitted in Medicine Department of a Tertiary Care Teaching Hospital in North India-A Prescription Evaluation Study. JK Sci. 19 (4), 233–237. [Google Scholar]
- Liew T. M., Lee C. S., Goh Shawn K. L., Chang Z. Y. (2019). Potentially Inappropriate Prescribing Among Older Persons: a Meta-Analysis of Observational Studies. Ann. Fam. Med. 17 (3), 257–266. 10.1370/afm.2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew T. M., Lee C. S., Goh S. K. L., Chang Z. Y. (2020). The Prevalence and Impact of Potentially Inappropriate Prescribing Among Older Persons in Primary Care Settings: Multilevel Meta-Analysis. Age Ageing 49 (4), 570–579. 10.1093/ageing/afaa057 [DOI] [PubMed] [Google Scholar]
- Luchini C., Stubbs B., Solmi M., Veronese N. (2017). Assessing the Quality of Studies in Meta-Analyses: Advantages and Limitations of the Newcastle Ottawa Scale. Wjma 5, 80–84. 10.13105/wjma.v5.i4.80 [DOI] [Google Scholar]
- Mandavi D. S., D'Cruz S., Sachdev A., Tiwari P. (2011). Prevalence and Risk Factors for Inappropriate Drug Use Among Older People: Results from an Indian Public Outpatient Setting. J. Pharm. Health Serv. Res 2 (1), 29–34. 10.1111/j.1759-8893.2010.00028.x [DOI] [Google Scholar]
- Masnoon N., Shakib S., Kalisch-Ellett L., Caughey G. E. (2017). What Is Polypharmacy? A Systematic Review of Definitions. BMC Geriatr. 17 (1), 230. 10.1186/s12877-017-0621-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M. R., Ramsdale E., Loh K. P., Arastu A., Xu H., Obrecht S., et al. (2020). Associations of Polypharmacy and Inappropriate Medications with Adverse Outcomes in Older Adults with Cancer: A Systematic Review and Meta‐Analysis. Oncol. 25 (1), e94. 10.1634/theoncologist.2019-0406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momin T. G., Pandya R. N., Rana D. A., Patel V. J. (2013). Use of Potentially Inappropriate Medications in Hospitalized Elderly at a Teaching Hospital: a Comparison between Beers 2003 and 2012 Criteria. Indian J. Pharmacol. 45 (6), 603–607. 10.4103/0253-7613.121372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motallebzadeh N., Jayaprakash G., Mohammadi E. (2019). Evaluation of Rationality of Geriatric Patients' Prescription Based on Beers Criteria in A Tertiary Care Hospital in India. Open Access Maced J. Med. Sci. 7 (6), 987–991. 10.3889/oamjms.2019.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlack D. C., Hoppe L. K., Weberpals J., Brenner H., Schöttker B. (2017). The Association of Potentially Inappropriate Medication at Older Age with Cardiovascular Events and Overall Mortality: a Systematic Review and Meta-Analysis of Cohort Studies. J. Am. Med. Directors Assoc. 18 (3), 211–220. 10.1016/j.jamda.2016.11.025 [DOI] [PubMed] [Google Scholar]
- Nagendra H. V., Harugeri A., Parthasarathi G., Ramesh M. (2012). Potentially Inappropriate Medication Use in Indian Elderly: Comparison of Beers' Criteria and Screening Tool of Older Persons' Potentially Inappropriate Prescriptions. Geriatr. Gerontol Int 12 (3), 506–514. 10.1111/j.1447-0594.2011.00806.x [DOI] [PubMed] [Google Scholar]
- Narvekar R. S., Bhandare N. N., Gouveia J. J., Bhandare P. N. (2017). Utilization Pattern of Potentially Inappropriate Medications in Geriatric Patients in a Tertiary Care Hospital: a Retrospective Observational Study. J. Clin. Diagn. Res. 11 (4), FC04–FC08. 10.7860/JCDR/2017/21080.9731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishtala P. S., Salahudeen M. S. (2015). Temporal Trends in Polypharmacy and Hyperpolypharmacy in Older New Zealanders over a 9-year Period: 2005-2013. Gerontology 61 (3), 195–202. 10.1159/000368191 [DOI] [PubMed] [Google Scholar]
- O'Mahony D. (2020). STOPP/START Criteria for Potentially Inappropriate Medications/potential Prescribing Omissions in Older People: Origin and Progress. Expert Rev. Clin. Pharmacol. 13 (1), 15–22. 10.1080/17512433.2020.1697676 [DOI] [PubMed] [Google Scholar]
- Porter G., Grills N. (2016). Medication Misuse in India: a Major Public Health Issue in India. J. Public Health 38 (2), 1–8. 10.1093/pubmed/fdv072 [DOI] [PubMed] [Google Scholar]
- Pradhan S., Panda A. (2018). Effect of Potentially Inappropriate Medication on Treatment Adherence in Elderly with Chronic Illness. Biomed. Pharmacol. J. 11 (2), 935–943. 10.13005/bpj/1451 [DOI] [Google Scholar]
- Pradhan S., Panda A., Panigrahy S. R. (2017). Determinants of Potentially Inappropriate Medication in Elderly: A Hospital Based Cross Sectional Study. J. Clin. Gerontol. Geriatr. 8 (3), 93–97. 10.24816/jcgg.2017.v8i3.05 [DOI] [Google Scholar]
- Pravodelov V. (2020). Thoughtful Prescribing and Deprescribing. Med. Clin. North America 104 (5), 751–765. 10.1016/j.mcna.2020.06.001 [DOI] [PubMed] [Google Scholar]
- Rakesh K. B., Chowta M. N., Shenoy A. K., Shastry R., Pai S. B. (2017). Evaluation of Polypharmacy and Appropriateness of Prescription in Geriatric Patients: A Cross-Sectional Study at a Tertiary Care Hospital. Indian J. Pharmacol. 49 (1), 16–20. 10.4103/0253-7613.201036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy N., Madhiwalla N., Pai S. A. (2007). Drug Promotional Practices in Mumbai: a Qualitative Study. Indian J. Med. Ethics 4 (2), 57–61. 10.20529/IJME.2007.020 [DOI] [PubMed] [Google Scholar]
- Salwe K. J., Kalyansundaram D., Bahurupi Y. (2016). A Study on Polypharmacy and Potential Drug-Drug Interactions Among Elderly Patients Admitted in Department of Medicine of a Tertiary Care Hospital in Puducherry. J. Clin. Diagn. Res. 10 (2), FC06–10. 10.7860/JCDR/2016/16284.7273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott I. A., Guyatt G. H. (2010). Cautionary Tales in the Interpretation of Clinical Studies Involving Older Persons. Arch. Intern. Med. 170 (7), 587–595. 10.1001/archinternmed.2010.18 [DOI] [PubMed] [Google Scholar]
- Shah R., Desai S., Gajjar B. (2011). Evaluation of the Appropriateness of Prescribing in Geriatric Patients Using Beers Criteria and Phadke′s Criteria and Comparison Thereof. J. Pharmacol. Pharmacother. 2 (4), 248. 10.4103/0976-500x.85948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P., Gupta N., Chauhan H. (2019). Prevalence of Polypharmacy: Comparing the Status of Indian States. Indian J. Community Fam. Med. 5 (1), 4–9. 10.4103/ijcfm.ijcfm_10_19 [DOI] [Google Scholar]
- Sheridan D. J., Julian D. G. (2016). Achievements and Limitations of Evidence-Based Medicine. J. Am. Coll. Cardiol. 68 (2), 204–213. 10.1016/j.jacc.2016.03.600 [DOI] [PubMed] [Google Scholar]
- Stroup D. F., Berlin J. A., Morton S. C., Olkin I., Willliamson G. D., Rennie D., et al. (2000). Meta-analysis of Observational Studies in EpidemiologyA Proposal for Reporting. JAMA 283 (15), 2008–2012. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- Swathi B., Bhavika D. (2016). Pattern of Medication Use Among Elderly Patients Attending Medicine Department in a Tertiary Care Hospital in India. Asian J. Pharma Clin. Res. 9 (9), 266–269. [Google Scholar]
- Thomas R. E., Thomas B. C. (2019). A Systematic Review of Studies of the STOPP/START 2015 and American Geriatric Society Beers 2015 Criteria in Patients ≥ 65 Years. Cas 12 (2), 121–154. 10.2174/1874609812666190516093742 [DOI] [PubMed] [Google Scholar]
- Topinková E., Mádlová P., Fialová D., Klán J. (2008). [New Evidence-Based Criteria for Evaluating the Appropriateness of Drug Regimen in Seniors. Criteria STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert Doctors to Right Treatment)]. Vnitr Lek 54, 1161–1169. [PubMed] [Google Scholar]
- Umar N. F., Joel J. J., Sharma R., Shastry C. S., Adepu R.(2015). Significant Role of Clinical Pharmacists in the Assessment of Inappropriate Medications Prescribed to the Elderly Patients in a University Teaching Hospital. Asian J. Pharm. Clin. Res. 8 (1), 109–112. [Google Scholar]
- Undela K., Bansal D., D'Cruz S., Sachdev A., Tiwari P. (2014). Prevalence and Determinants of Use of Potentially Inappropriate Medications in Elderly Inpatients: a Prospective Study in a Tertiary Healthcare Setting. Geriatr. Gerontol. Int. 14 (2), 251–258. 10.1111/ggi.12081 [DOI] [PubMed] [Google Scholar]
- United Nations Population Fund. State of World Population 2021. Available at: http://https://www.unfpa.org/publications/state-world-population-2021.html (Accessed April 19, 2021).
- Weeda E. R., AlDoughaim M., Criddle S. (2020). Association between Potentially Inappropriate Medications and Hospital Encounters Among Older Adults: A Meta-Analysis. Drugs Aging 37, 529–537. 10.1007/s40266-020-00770-1 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2017). Medication without Harm: Global Patient Safety Challenge on Medication Safety. Geneva, Switzerland: World Health Organization. [Google Scholar]
- Wylie C. E., Daniels B., Brett J., Pearson S. A., Buckley N. A. (2020). A National Study on Prescribed Medicine Use in Australia on a Typical Day. Pharmacoepidemiol. Drug Saf. 29 (9), 1046–1053. 10.1002/pds.5093 [DOI] [PubMed] [Google Scholar]
- Xing X. X., Zhu C., Liang H. Y., Wang K., Chu Y. Q., Zhao L. B., et al. (2019). Associations between Potentially Inappropriate Medications and Adverse Health Outcomes in the Elderly: a Systematic Review and Meta-Analysis. Ann. Pharmacother. 53 (10), 1005–1019. 10.1177/1060028019853069 [DOI] [PubMed] [Google Scholar]
- Zaveri H. G., Mansuri S. M., Patel V. J. (2010). Use of Potentially Inappropriate Medicines in Elderly: A Prospective Study in Medicine Outpatient Department of a Tertiary Care Teaching Hospital. Indian J. Pharmacol. 42 (2), 95. 10.4103/0253-7613.64499 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.