Abstract
The efficient proliferation of Newcastle disease virus (NDV) depends on its inhibition of host cell innate immunity. V protein acts as a nonstructural protein which plays a significant role in virus replication, whereas its function remains to be further explored. In this study, Musashi RNA binding protein 1 (MSI1) was selected and its interaction with V protein was further verified by Co-immunoprecipitation (Co-IP) and Immuno-colocalization test. Through the transfection of pCMV-HA-MSI1 in DF-1 cells, the overexpression of MSI1 reduced virus particles in the cell supernatant but not reduced mRNA and virus protein in cells pellet, which suggests that MSI1may act as a new antiviral molecule by inhibiting viral release. Cell early apoptosis was detected by flow cytometry (FCM), the result shows that overexpression of MSI1 inhibit cell apoptosis, implying MSI1 Inhibit virus release may through this way. Taken together, MSI1 and NDV V protein has a detectable interaction, and may block apoptosis to inhibit the release of NDV. However, this is the first report about the interaction between MSI1 and V protein of NDV that can inhibit the NDV replicated.
Key words: Newcastle disease virus; V protein, Musashi1; replication; interaction
INTRODUCTION
Since the first report of Newcastle disease virus (NDV) in 1926 (Ganar et al., 2014), it acts as a serious infectious disease that affects the poultry industry across the world. According to the latest virus taxonomy, the virus has been classified into the order family Paramyxoviridae, subfamily Avulavirinae, and genus Orthoavulavirus (Butt et al., 2019). NDV genome transcript six fundamental genes in the order of 3′-NP-P-M-F-HN-L-5′ (Wang et al., 2019). Like other Paramyxovirus, NDV encodes V protein through RNA editing (Galinski et al., 1992). The NDV Clone-30 mutants that have lost its V protein were unable to propagate in SPF chicken eggs (Mebatsion et al., 2001), which indicates that the V protein plays a direct role in virus replication. Studies have shown that expression of the V protein prevents the activation of the Alpha Interferon response, by its C-terminal domain (Park et al., 2003a). Overexpression of V protein promotes NDV replication by anti-innate immune responses (Jang et al., 2010). But, in interferon-deficient cells, the deletion of the C-terminal domain of V protein decreased the replication level of rNDV (Park et al., 2003b), which indicates that the function of V protein is not fully understood.
To further explore the function of V protein, several host proteins that interacted with V protein were discovered by the yeast two-hybrid technology (Y2H) in our lab. MSI1, one of the molecules that have been screened, belongs to an evolutionarily conserved family of RNA-binding proteins was first identified in 1994 in Drosophila by Makoto Nakamura (Nakamura et al., 1994). MSI1 has two RNA-recognition motifs, in the N-terminal region that mediate the binding of MSI1 to target RNAs (Kudinov et al., 2017). According to previous studies, MSI1 may control hundreds of targets, regulating apoptosis, differentiation, proliferation, and cell cycle (Bish and Vogel, 2014). Indicating that MSI1 can regulate cell fate through multiple ways (Chen et al., 2016). However, the antiviral function of MSI1 has yet to be studied in relation to NDV.
V protein is indispensable for the efficient replication of NDV, whereas its function remains to be further explored. The present study revealed a molecule MSI1 which has a detectable interaction with V protein. We investigated the function of MSI1 in the progression of NDV infection. In this context, we delve into the mechanism of MSI1 to further interpret the function of NDV V protein.
MATERIALS AND METHODS
Plasmid Construction
The primers for MSI1 were designed according to the published Gallus MSI1 gene in NCBI (XM_015275405.2). The ORF of MSI1 was amplified by PCR from DF-1 cell and was cloned into the pCMV-HA expression vector. The constructed plasmid was finally identified using double restriction enzyme digestion, and MSI1 overexpression was confirmed by western blotting. The V gene, with a Flag tag, cloned into the pCAGEN (maintained in our lab).
Cell Culture and Plasmid Transfection
DF-1, HEK 293T and BHK21 cells were maintained in our lab. All cell lines were cultured in DMEM (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (SERA-PRO, USA) in a 5% CO2 condition in a 37°C incubator.
DF-1 cells were plated with 5 × 105 cells per well in a 12-well plate and cultivated for 24 h. The cells were transfected with pCMV-HA-MSI1 or pCAGEN-Flag-V using TurboFect (Thermo, USA) according to the manufacturer's instructions.
Viruses and Viral Infection
NDV F48E9 (genotype IX) was maintained in our lab. It is a kind of neurotropic immediate virulent strain. DF-1 cells in 12-well culture plate were incubation with F48E9 (0.01 MOI, 300 µL) for 1 h. The incubation process was performed at 37°C with 5% CO2. In this study, viral titers were measured by plaque assay through BHK21 cells.
Co-IP Assay
293T cells were cultured in 60 mm dishes grown to a confluence of 80%, each plasmid was transfected with 3 μg for 48 h. Scrap the cells from dishes. Centrifugally collect cell pellets after washing cells twice with cold PBS. The cell pellet was lysed by the NP40 buffer for 30 min on ice. Centrifuge at 13000 rpm for 10 min, collecting supernatant subassembly 20 µL as input. Resuspend magnetic beads by vortex 30 s and transfer 50 μL beads to a tube. Place the tube on the magnet to remove the supernatant. Add antibody diluted in 200 μL premade PBST into the processed magnetic beads and rotation for 30 min at room temperature. Place the tube on the magnet and remove the supernatant. Incubate antigen and bead-Ab complex for 45 min at room temperature. Wash the bead-Ab-Ag complex with PBST gently three times. Remove the supernatant, add 20 μL of Elution Buffer and 10 μL protein loading buffer heat at 95℃ for 5 min, 10 μL sample was used for Western blotting assay.
Western Blot Analysis
DF-1 cells on a 12-well plate were washed with PBS and lysed by RIPA buffer (contained PMSF) for 30 min at 4°C. Proteins were boiled for 10 min with a 5×protein loading buffer. The equal volume of each protein sample was separated by 12% or 15% SDS-PAGE, then, transferred onto PVDF sheets (Millipore, Billerica, MA, USA). After blocking for 2 h at room temperature with 10% skim milk, the PVDF membranes containing target protein were incubated with diluted primary antibodies for one night at 4°C, then incubated with diluted second antibodies for 1 h at room temperature. After washing by TBST, the membranes were observed by the imaging system.
Quantitative Real Time PCR (Q-PCR)
The mRNA levels of NDV M genes were detected by Q-PCR. The total RNAs were extracted by RNAiso (TaKaRa, Dalian, China) from DF-1 cells. Then, cDNA was obtained via Star Script II First-strand cDNA synthesis kit II (Genstar, China). Real-time PCR was used to detect cDNA samples according to the protocol.
Indirect Immunofluorescence Assay (IFA)
DF-1 cells were grown to 70% confluence on confocal dishes (NEST, Wuxi, China), cells were co-transfection with pCAGEN-Flag-V and pCMV-HA-MSI1. After 24 h, the treated cell samples were observed under a confocal microscope.
RESULTS
V. Protein Interacts With MSI1 in DF-1 Cells
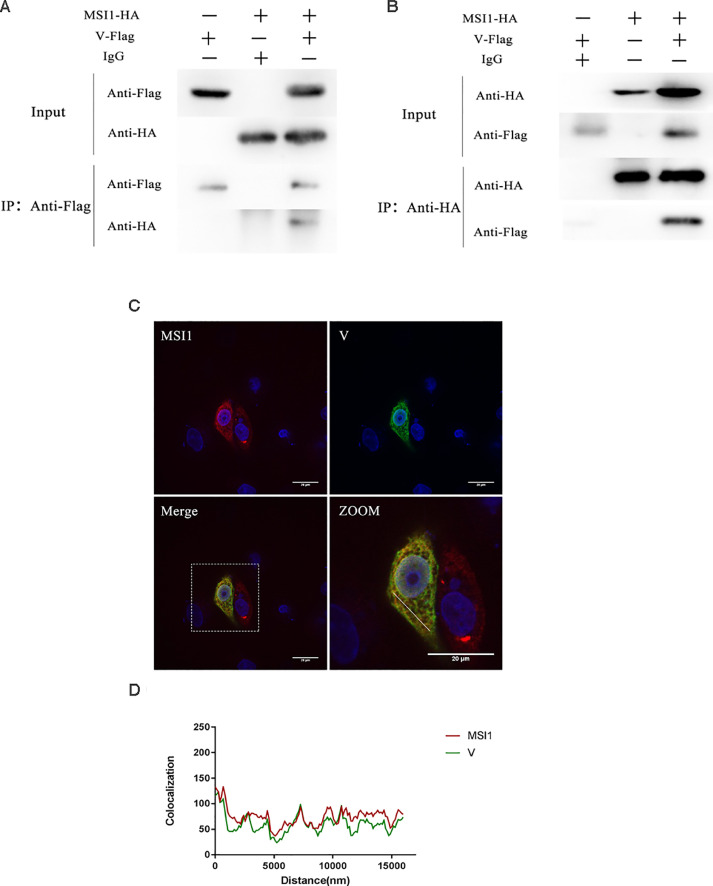
Our previous work screened hundreds of molecules that interact with NDV V protein including MSI1 through the yeast two-hybrid system. In this work, a Co-IP assay confirmed the interaction of MSI1 with V in 293T cells (Figure 1A), magnetic beads were treated with Flag antibody, and HA antibodies were used to detect MSI1 in eluent. The results show the interaction between V and MSI1. The interaction was further confirmed by a reverse Co-IP assay by using HA antibody treated magnetic and Flag antibody to detect V in eluent (Figure 1B). The localization of MSI1 and V in DF-1 cells was observed using immunofluorescence and confocal microscopy. As is shown above, the V protein (RFP) and MSI1 (GFP) colocalized in the cytoplasmic region (Figures 1C and 1D).
Figure 1.
V protein interacts with MSI1. (A, B) Co-Immunoprecipitation analysis of MSI1 and V in 293T cells. MSI1 and V were co-transfected or separate transfected into 293T cells. Cells pellet was collected after 36 h post-transfection. (C) Confocal analysis of MSI1 and V in DF-1 cells. MSI1 and V protein was transfected into DF-1 cells and assessed by immunofluorescence staining. V protein was detected with goat antimouse monoclonal antibody and visualized with Alexa Fluor 488 (green). MSI1 was detected with goat antirabbit monoclonal antibody and visualized with Alexa Fluor 594 (red). Yellow indicates colocalization of the V protein and MSI1 in the merged image. (D) Histograms represent the fluorescence intensity for MSI1 and V protein in determined area (white continuous line) demonstrating the correlation between two signals. In MSI1 and V co-expression cells, MSI1 is colocalized with V.
Infected With F48E9 or Transfection With V Protein in DF-1 Cells Promote MSI1 Expression
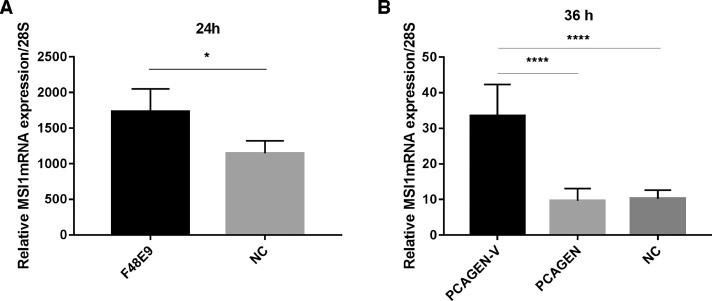
After identifying the interaction between MSI1 and V protein, we further explored their relationship. The expression of endogenous MSI1 at the mRNA levels was detected 24 h postinfection. The results show that infected with F48E9 promotes MSI1 expression (Figure 2A). Then, DF-1 cells which were transfected with V protein, compared to the control group, have prominently higher MSI1 mRNA level (Figure 2B).
Figure 2.
Infected with F48E9 or transfection with V protein promote MSI1 expression. (A) DF-1 cells were infected with F48E9 for 24 h. The RNA was extracted according to the method previously described. The mRNA levels of endogenous MSI1 were detected using Q-PCR. (B) DF-1 cells were transfected with V protein for 36 h. The RNA was extracted and endogenous MSI1 mRNA was detected using Q-PCR.
MSI1 Inhibits NDV Release in DF-1 Cells
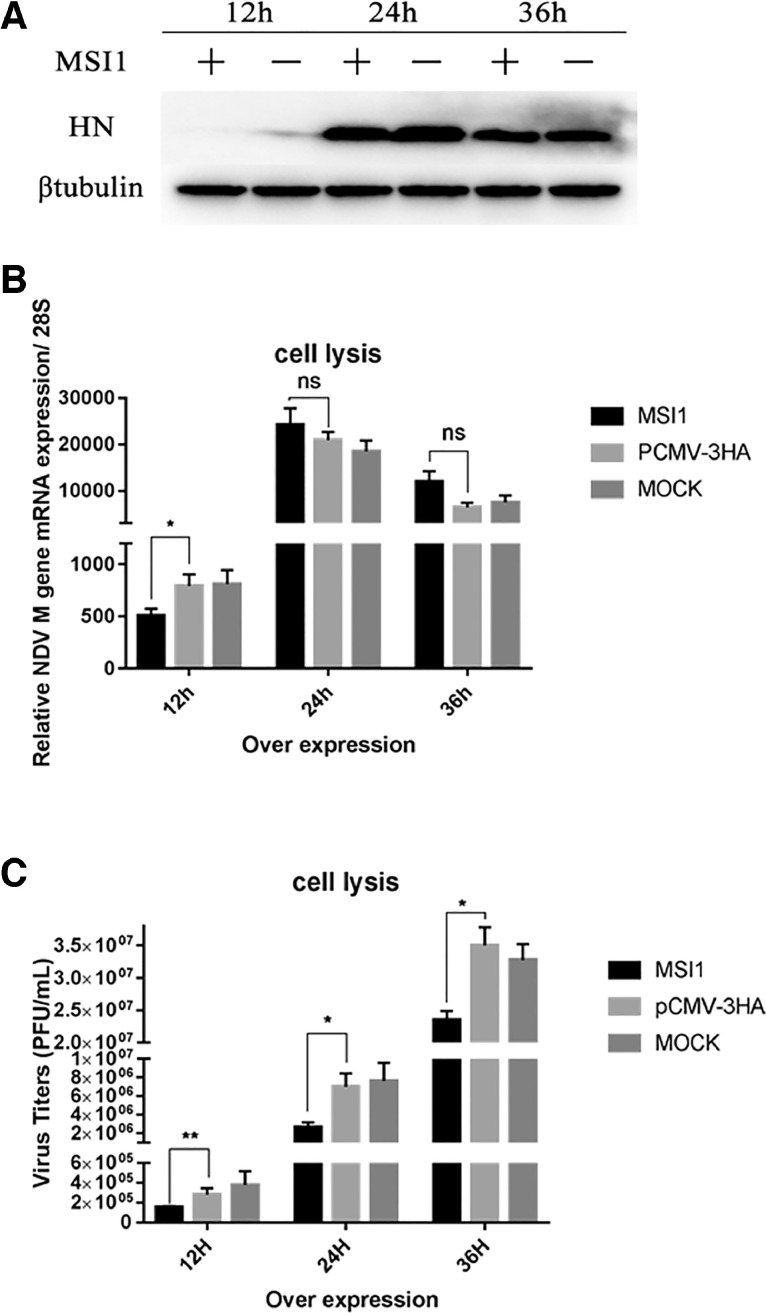
Previous studies have shown that V protein promotes the proliferation of NDV through a variety of functions. Next, we measured the effect of MSI1 on NDV replication. DF-1 cells were transfected with pCMV-HA-MSI1 and pCMV-HA (control) for 24 h and then infected with F48E9 (0.01 MOI), collect the samples at 12 h, 24 h and, 36 h postinfection. Western blot (Figure 3A) and Q-PCR (Figure 3B) results show that there is significant inhibition in 12 h postinfection, the inhibition weakened in the 24 h and 36 h groups. However, plaque assay shows the titer of virus decreased in the supernatant at three time points (Figure 3C). Indicating that overexpression of MSI1 inhibits NDV release in DF-1 cells.
Figure 3.
MSI1 inhibits NDV release. (A) Western blot was used to detect the protein of the virus. DF-1 cells were transfected with pCMV-HA-MSI1 and control plasmids for 24 h and infected with F48E9 (0.01 MOI). The cell pellet was collected after 12 h, 24 h, and 36 h postinfection. (B) DF-1 cells were transfected with pCMV-HA- MSI1 and control plasmids for 24 h and infected with F48E9 (0.01 MOI). The RNA was extracted after 12 h, 24 h and 36 h postinfection. Then mRNA level of NDV was detected using Q-PCR. (C) The viral titer of F48E9 was detected using the viral plaque assay.
Overexpression of MSI1 Inhibited Apoptosis in DF-1 Cells
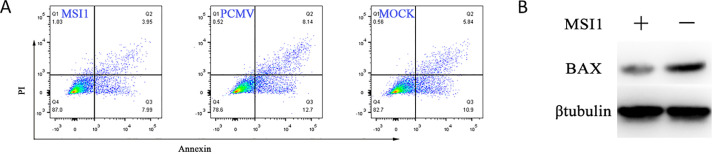
MSI1 acts as a tumor factor affecting apoptosis. Flow cytometry analysis of early apoptosis showed that early apoptotic cells were decreased in MSI1 transfected cells compared with control group 36 h post-transfection (Figure 4A). Western blot results showed that BCL2 associated X protein decreased in DF-1 cells (Figure 4B) which were transfected with pCMV-HA-MSI1, compared with the control group. In conclusion, overexpression of MSI1 inhibits cell apoptosis.
Figure 4.
MSI1 Inhibit apoptosis in DF-1 cells. (A) Detection of apoptosis by Annexin V/PI staining and flow cytometry. DF-1 cells were transfected with pCMV-HA-MSI1, control plasmid or mock transfected for 48 h. Stained with annexin V and PI, and analyzed with flow cytometry. (B) Protein expression level for BAX in MSI1 overexpressed cells 36 h post-transfection detected by western blot.
DISCUSSION
In our previous studies, we employed a yeast two-hybrid system to select the host proteins which interact with NDV V protein. The result shows that V proteins have a wide range of interactions with intracellular molecules, such as TXNL1 (Wang et al., 2018), cacyBP (Chu et al., 2018). Many of these molecules are associated with tumorigenesis, which suggests that V protein may participate in cell cycle regulation. As one of the molecules which interact with V protein, MSI1 belongs to a family of RBPs that possess various functions, including apoptosis, differentiation, proliferation and cell cycle. In cancers, MSI1 has been identified as a factor supporting proliferation and apoptosis (Kudinov et al., 2017).
Further, this interaction was confirmed by Co-IP assays. The results show that the interaction between MSI1 and V protein could be detected whether MSI1 or V protein was used as bait protein, which gave us convincing proof, suggesting that the V protein may exert its function through MSI1 while the molecular details of this interaction need further investigation. Next, Endogenous MSI1 mRNA was detected after NDV infection or V protein over-expression. Results show that after infection with F48E9 for 24 h, the expression of MSI1 increased with a small range in DF-1 cells while over-expression of V protein 36 h post-transfection prominently promoted MSI1 expression. This may be because the expression of V protein after virus infection is lower than that after transfection.
Current studies are devoted to making MSI1 a cancer treatment target and there have been no reports on the antiviral effect of MSI1. However, our study showed that overexpression of MSI1 inhibited NDV replication in DF-1 cells. Then we investigated the role of MSI1 in virus replication. Notably, previous studies have indicated that MSI1 transduction also promoted OP survival, significantly reduced the cleaved of caspase-3, protective against OP cell death in vitro (Dobson et al., 2008). Ether extrinsic or intrinsic pathways of apoptosis are both ultimately initiated by the cleavage of caspase-3/7 (De et al., 2018). Consistent with the results, DF-1 cells which were transfected with pCMV-HA-MSI1 the BAX expression is reduced and the proportion of early apoptotic cells decreased compared to the control group. How does MSI1 regulate apoptosis still needs to be further identified. NDV replicates and aggregates in host cells, eventually destroying the cells and resulting in the mass release of virions. The inhibition of apoptosis in cells which were infected probably results in the prevention of virus release. Observe in microscope, cells which transfected with pCMV-HA-MSI1 shows higher viability after infection with F48E9 compared with cells transfected with vector (Supplemental Figure 1). Consequently, we used the plaque assay to detect the cell supernatant. The result shows a significant decrease of virus titer, at 12 h, 24 h, and 36 h, compared with control cells. Indicating that, MSI1 can be an anti-NDV molecule. Suggesting that MSI1 may be an anti-NDV target, could contribute to innovative vaccines to control NDV in poultry flocks. However, in cells pellet, mRNA and protein level of virus have no significant differences between the experimental group and the control group. We speculate that the overexpression of MSI1 prevented the release of virus, thus inhibiting their replication.
CONCLUSION
In summary, MSI1 was identified as a novel antiviral molecule, and it has a detectable interaction with NDV V protein. It is speculated that MSI1 can be a potential target for V protein to regulate apoptosis, which gives new information about the interactive relationships between viral genes and host proteins. Future studies need to verify the regulatory relationship between V protein and MSI1. The findings of this study may help us better understand the molecular mechanisms by which NDV replicates in host cells.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundations of China (NO. 31572538).
DISCLOSURES
All authors declare that there is no conflict of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi: https://doi.org/10.1016/j.psj.2021.101105.
Appendix. Supplementary materials
Figure S1 DF-1 cells which transfected with MSI1 shows higher viability. DF-1cells were cultured in 12-well plate reached 80%, transfected with pCMV-HA-MSI1 or pCMV-HA, incubated with F48E9 24 h post-transfection, the cells were observed under a microscope at 12h and 24h after infection.
REFERENCES
- Bish R., Vogel C. RNA binding protein-mediated post-transcriptional gene regulation in medulloblastoma. Mol. Cells. 2014;37:357–364. doi: 10.14348/molcells.2014.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt S.L., Moura V., Susta L., Miller P.J., Hutcheson J.M., Cardenas-Garcia S., Brown C.C., West F.D., Afonso C.L., Stanton J.B. Tropism of Newcastle disease virus strains for chicken neurons, astrocytes, oligodendrocytes, and microglia. BMC Vet. Res. 2019;15:317. doi: 10.1186/s12917-019-2053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.Y., Lin L.T., Wang M.L., Lee S.H., Tsai M.L., Tsai C.C., Liu W.H., Chen T.C., Yang Y.P., Lee Y.Y., Chang Y.L., Huang P.I., Chen Y.W., Lo W.L., Chiou S.H., Chen M.T. Musashi-1 regulates AKT-derived IL-6 autocrinal/paracrinal malignancy and chemoresistance in glioblastoma. Oncotarget. 2016;7:42485–42501. doi: 10.18632/oncotarget.9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z., Wang C., Tang Q., Shi X., Gao X., Ma J., Lu K., Han Q., Jia Y., Wang X., Adam F., Liu H., Xiao S., Wang X., Yang Z. Newcastle disease virus V protein inhibits cell apoptosis and promotes viral replication by targeting cacyBP/SIP. Front. Cell. Infect. Microbiol. 2018;8:304. doi: 10.3389/fcimb.2018.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De P., Carlson J.H., Leyland-Jones B., Williams C., Dey N. Triple fluorescence staining to evaluate mechanism-based apoptosis following chemotherapeutic and targeted anti-cancer drugs in live tumor cells. Sci. Rep. 2018;8:13192. doi: 10.1038/s41598-018-31575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson N.R., Zhou Y.X., Flint N.C., Armstrong R.C. Musashi1 RNA-binding protein regulates oligodendrocyte lineage cell differentiation and survival. Glia. 2008;56:318–330. doi: 10.1002/glia.20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinski M.S., Troy R.M., Banerjee A.K. Rna editing in the phosphoprotein gene of the human parainfluenza virus type 3. Virology. 1992;186:543–550. doi: 10.1016/0042-6822(92)90020-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganar K., Das M., Sinha S., Kumar S. Newcastle disease virus: current status and our understanding. Virus Res. 2014;184:71–81. doi: 10.1016/j.virusres.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J., Hong S.H., Choi D., Choi K.S., Kang S., Kim I.H. Overexpression of Newcastle disease virus (NDV) v protein enhances NDV production kinetics in chicken embryo fibroblasts. Appl. Microbiol. Biotechnol. 2010;85:1509–1520. doi: 10.1007/s00253-009-2189-z. [DOI] [PubMed] [Google Scholar]
- Kudinov A.E., Karanicolas J., Golemis E.A., Boumber Y. Musashi RNA-binding proteins as cancer drivers and novel therapeutic targets. Clin. Cancer Res. 2017;23:2143–2153. doi: 10.1158/1078-0432.CCR-16-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebatsion T., Verstegen S., De Vaan L.T.C., Romer-Oberdorfer A., Schrier C.C. A recombinant Newcastle disease virus with low-level v protein expression is immunogenic and lacks pathogenicity for chicken embryos. J. Virol. 2001;75:420–428. doi: 10.1128/JVI.75.1.420-428.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Okano H., Blendy J.A., Montell C. Musashi, a neural RNA-binding protein required for Drosophila adult external sensory organ development. Neuron. 1994;13:67–81. doi: 10.1016/0896-6273(94)90460-x. [DOI] [PubMed] [Google Scholar]
- Park M.S., Garcia-Sastre A., Cros J.F., Basler C.F., Palese P. Newcastle disease virus v protein is a determinant of host range restriction. J. Virol. 2003;77:9522–9532. doi: 10.1128/JVI.77.17.9522-9532.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M.S., Shaw M.L., Muñoz-Jordan J., Cros J.F., Nakaya T., Bouvier N., Palese P., García-Sastre A., Basler C.F. Newcastle disease virus (NDV)-based assay demonstrates interferon-antagonist activity for the NDV V protein and the Nipah virus V, W, and C proteins. J. Virol. 2003;77:1501–1511. doi: 10.1128/JVI.77.2.1501-1511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Chu Z., Liu W., Pang Y., Gao X., Tang Q., Ma J., Lu K., Adam F., Dang R., Xiao S., Wang X., Yang Z. Newcastle disease virus V protein inhibits apoptosis in DF-1 cells by downregulating TXNL1. Vet. Res. 2018;49:102. doi: 10.1186/s13567-018-0599-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Jia Y., Ren J., Liu H., Adam F.A., Wang X., Yang Z. Insights into the chicken bursa of fabricius response to Newcastle disease virus at 48 and 72 hours post-infection through RNA-seq. Vet. Microbiol. 2019;236:108389. doi: 10.1016/j.vetmic.2019.108389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 DF-1 cells which transfected with MSI1 shows higher viability. DF-1cells were cultured in 12-well plate reached 80%, transfected with pCMV-HA-MSI1 or pCMV-HA, incubated with F48E9 24 h post-transfection, the cells were observed under a microscope at 12h and 24h after infection.