Abstract
Sand fleas infestation, Tunga penetrans, remains a neglected tropical disease of public-health concern in many countries. Tungiasis can lead to destruction of the feet causing serious discomfort and deformities. This study aim was to determine the intensity rate and clinical morbidities of tungiasis among the people living in Igbokoda, Ondo State, Nigeria. A community-based cross-sectional survey was conducted among households in three villages in Igbokoda. Hands and feet of selected household members were examined, while fleas on different floor types of houses were sampled by soil collection and extraction by tullgren funnel method. Sand fleas were equally sampled from legs of infested individuals by hand picking. Intensity was determined in terms of number of lesions and fleas in different floor-types. Tungiasis-associated morbidities was assessed using acute and chronic lesions severity scores. The study revealed that moderate infestation was observed in 173 infested individuals and severe infestation in 16 infested individuals. A total of 5293 lesions was observed out of which 3098 were viable. The flea burdens on sampled floors and legs were 127 and 146 fleas respectively occurring mostly in unpaved veranda and rooms (65 and 62 fleas respectively). Tungiasis lesions observed in males were more intense compared to females. Flea lesions and burdens with respect to age stratification and location were significant P < 0.0001. Difficulty in walking, pain upon pressure and deformation of the feet were the most common tungiasis-associated morbidity recorded. Also, tungiasis-associated morbidities, and flea number sampled on legs and on floor of environment were reportedly intense. Therefore, scaling up an appropriate and affordable intervention approach targeted at the fleas would serve as a relief to the scourge of tungiasis in Igbokoda community.
Keywords: Clinical morbidity, Igbokoda, Intensity, Tunga penetrans, Tungiasis
1. Introduction
The sand flea genera Tunga is an important cause of tungiasis, a neglected ecto-parasite disease worldwide (Pampiglione et al., 2009). The sand fleas according to Heukelbach et al. (2002) and Muehlen et al. (2003) has a very high penetration preference for the human feet, particularly the periungual skin of the toes compared to other ectopic sites such as hands, arm, and elbow causing discomfort and most times interfering with blood circulation to the affected part. Penetrations on cutaneous surfaces may vary widely due to close contact with flea infested soils or floor-type conditions that promote their existence in houses. The pathological processes of tungiasis are debilitating and severe re-occurring morbidity including penetration, implantation, infestation and destruction of body parts. The main clinical manifestations of tungiasis include erythema, hyper-erythema, lymphoedema, abscesses, ulceration, hypertrophy causing desquamation, painful fissures, frailty, loss of toe and toe nails, deformation of toes and toe nails, and Hyperkeratosis (Heukelbach et al., 2001; Wilcke et al., 2002; Feldmeier et al., 2003; Joseph et al., 2006; Ugbomoiko et al., 2007; Kehr et al., 2007).
Tungiasis remains one of the significant neglected disease in endemic settings where environmental conditions are favourable. Despite the notable studies reported over decades, the status of the disease has remained neglected with about 80% of populations living in endemic countries being at risk of infestation. Haiti suffers the highest burden for Tunga penetrans. Tungiasis burdens as a result of prevalence was highest 74.6% in Haiti followed by 15.7% to 54.8% Latin American, Caribbean, Trinidad and Tobago, Brazil, and sub-Saharan African countries, and Nigeria (Ade-Serrano and Ejezie, 1981; Chadee, 1994; Heukelbach et al., 2001; Wilcke et al., 2002; De Carvalho et al., 2003; Ehrenberg and Ault, 2005; Heukelbach, 2005; Joseph et al., 2006). In Cameroon, tungiasis burdens was highly prevalent and accounts for 50% of the total infestation in school children (Njeumi et al., 2002). Unlike other diseases, tungiasis has a unique reproductive stage that can cause re-infestation in weeks or months after the initial infestation. Although, the infestation is host-limiting and non-contagious. The embedded female fleas have the potentials of dispersing eggs that infests the soil and cause a continuous cycle of human infection. The habit of removing embedded fleas using inappropriate sharp instruments due to discomfort results in manipulated lesions which are characterized as severe mutilated lesions (Feldmeier and Keysers, 2013). Pathogenic bacteria can gain entry into severely manipulated lesions or tiny holes made by flea penetrations, leading to superinfection in non-vaccinated individuals and causing inflammation to increase, tetanus and gangrene, intense pain and difficulty in walking. (Heukelbach et al., 2004; Feldmeier et al., 2004; Feldmeier et al., 2006; Ugbomoiko et al., 2007; Feldmeier et al., 2009). Fischer et al. (2002) and Heukelbach et al. (2004) have isolated varieties of aerobic and anaerobic bacteria from individuals with embedded sand fleas.
It is well known that the female sand flea infest ostracized populations in urban squatter settlements, mostly indigent, unhygienic and remote villages in the hinterland, at the coast and traditional fishing communities along the littoral regions (Wilcke et al., 2002; Muehlen et al., 2003; De Carvalho et al., 2003; Feldmeier et al., 2003; Heukelbach, 2005; Pilger et al., 2008; Linardi et al., 2010; Feldmeier and Keysers, 2013). Tourist who travel to endemic areas are equally prone to infestation. In endemic areas, constant re-infestation may occur and infested individuals often harbour dozens, sometimes hundreds of embedded parasites which they acquired intra- and peri-domiciliary (Heukelbach et al., 2004; Feldmeier et al., 2006; Pilger et al., 2008; Linardi et al., 2010). Besides human tungiasis, the sand flea also infest a wide range of mammals such as dogs, cats, pigs, goats and rodents (Rattus rattus) which may act as important reservoir host for the transmission of sand fleas (Heukelbach et al., 2001; Heukelbach et al., 2003; Heukelbach et al., 2004; Ugbomoiko et al., 2008; Mutebi et al., 2016; Mutebi et al., 2017). The epidemiology of tungiasis in Nigeria is scanty, with little or no published case studies except the prevalence studies on pre-primary, post-primary student and residents by Ugbomoiko et al. (2017) and Ade-Serrano and Ejezie (1981) in Lagos State and Osun State, and Arene (1984) in Choba, River State.
Mechanical destruction of affected bodily parts causing serious pain, discomfort, and associated morbidity have been reported where children of school age drop out of school since they are unable to walk properly and adults in turn faced with inability to carry out their day to day activities normally (Ugbomoiko et al., 2008). Ngunjiri and Keiyoro (2011) have observed that infested persons in Kenya are labelled as socially unwelcomed and therefore ostracized. There have been no proper attention and documentation of the intensity, severity and clinical morbidity in Nigeria. More so, no record on National control intervention measures are available for the disease and epidemiological data on the prevalence and control intervention of this disease are still very scanty. Therefore, the need to measure the intensity rate and clinical morbidities associated in the area in order to bring to lamplight the empirical evidence of tungiasis infestation as an issue that can draw the attention of concerned government agencies for intervention.
2. Materials & methods
2.1. Study site
The study was conducted in Igbokoda, Ilaje Local Government Area (LGA) of Ondo State, Nigeria. Three villages namely Zion, Laranda and Kofawe was selected out of six villages in Igbokoda community as representative sample locations considering nearness to the community health facility, available resources and geographic spread of sampling. According to observations by the local government and community health officials, community individuals have frequent complaints about the infestation. Although with no published intensity records. Igbokoda is located 704 km north of the equator and 532 km east of the prime meridian with a latitude of 6o 21′09.6″ N and longitude of 4o 48′14.6″. Igbokoda is bounded in the North by Okititpupa, in the south by the Atlantic Ocean, in the East by Ese-Odo Local Government and Delta State and in the West by Ogun State. It has a coastline of about 80 km which runs in a northwest to southeast direction and a shoreline covering about 180 km thereby making Ondo State the state with the longest coastline in Nigeria. There are several features that makes the three selected villages similar. This include sandy soil surrounding, mixture of thatched and block buildings with paved and unpaved floors, free range domestic animals and majority of the inhabitants walk about barefooted. As fishing communities, the selected villages are surrounded by rivers and lagoons.
2.2. Determination of sample size
The sample size was determined using the formula below for this study, with the main aim of estimating prevalence (reported in detail in Anyaele and Enwemiwe (2021):
where,
n = required sample size.
Z = standard normal value corresponding to 95% confidence level set at 1.96.
P = prevalence of tungiasis in Erekiti Lagos State, Nigeria = 45.2% (Ugbomoiko et al., 2007).
e = level of error tolerance 5%
Adjusting the sample size for 10% non-response
where,
nf = adjusted sample size due to attrition.
nf = non-response rate 10%
2.3. Study design
A cross-sectional study based on home visits with voluntary participation was conducted between March and July 2017 which coincided with the end of dry season and part of raining season. The study evaluated the intensity rate of tungiasis and fleas, and clinical morbidities in individuals in Igbokoda. A total of 705 participants made up of 239, 255 and 211 individuals from Zion, Laranda and Kofawe respectively was recruited for this study after a written informed consent was obtained. The participants in each household was visited and the body parts examined thoroughly for the presence of lesions and embedded sand fleas to determine the intensity of infestation. Lesions on the hands and feet was examined and recorded. Houses with at least one flea had its household examined for tungiasis infestation.
Similarly, 90 household which comprise of 15 thatched and 15 concrete houses were randomly selected each from the three locations using the National Population Census House Number list. The floors of these selected household was characterized into paved rooms and veranda, unpaved rooms and veranda, paved room and unpaved veranda, and unpaved rooms and paved verandas. The number of floors sampled for Zion, Laranda and Kofawe were 119, 129, and 114 floors respectively. The intensity of fleas in the environment and on legs of participants were determined. Fleas on the legs of infested individuals were sampled by hand picking. This was carried out by wetting the finger before picking the fleas from the leg and stored in EDTA bottle containing 10% formaldehyde. Fleas in the environment were sampled by soil collection and extraction by tullgren funnel method, a method modified from Mc Gavin (2007). Houses were sampled by using a clean white cloth laid half-way the paved and unpaved floors. The white cloth was observed carefully for the presence of fleas while recording the fleas for both sampled rooms and verandas. Also, sand in the house was swept and gathered into containers and preserved with 10% formaldehyde to be examined in the laboratory for flea count. During house sweeping, sand on floor were beaten at every two stroke of sweep before the sand was gathered and transported to the laboratory.
2.4. Determination of intensity of tungiasis
The intensity of infestation on infested individuals and the environment was measured in terms of number of viable lesions, type of lesions and the number of fleas. All the participants were examined for macroscopic lesions on bodily parts and the number of lesions was recorded. The number of viable lesions on infested individuals were characterized as mild, moderate and severe infestations for the presence of 5 viable lesions and below, 6 to 30 viable lesions, and above 30 viable lesions respectively according to Muehlen et al. (2003). Also the numbers of viable lesions localized on feet and hands was determined. The type of lesions were differentiated into manipulated, viable and dead lesions. Manipulated lesions are lesions treated with sharp object including needle, razor blade, and thorns while stage I-III lesions represent viable lesions with the presence of fleas in the process of penetrating, early or later stages of development respectively and stage IV represented the dead lesions or fleas been sloughed off from the epidermis. Lesions were categorized into stages as described in “Fortaleza classification” by Eisele et al. (2003). All lesions including manipulated, viable and dead lesions were counted and documented with the use of modified clinical form chart adopted from Feldmeier et al. (2013). The numbers of fleas sampled from legs of infested individuals and on the floors were characterized as low, medium and high for the presence of 10 fleas and below, 11 to 30 fleas, and above 30 fleas respectively.
2.5. Determination of morbidity
Morbidity was assessed semi-quantitatively using severity score for acute tungiasis (SSAT) and a severity score for chronic tungiasis (SSCT) adopted from Kehr et al. (2007). SSAT score of infested individuals was determined using the following sign and symptoms: oedema, erythema, pain upon pressure or spontaneously generated pain, itching, sleeping disturbance due to itching, difficulty in walking, abscess, fissure and ulceration and suppuration as indication of bacteria superinfection. Depending on the acute symptoms and number of affected sites, the acute symptoms are assigned number that range from 0 to 24. The SSCT score of infested individuals was determined using the following sign and symptoms: loss of toe nails, hyperkeratosis, deformation of toe nails, deformation of toes and hypertrophy of nail rim. Depending on the chronic symptoms and the number of affected sites, the chronic symptoms are assigned numbers that range from 0 to 33. The attributed points assigned range from 0 to 4 for both acute and chronic symptoms.
The topographic sites recognized in this scoring scheme are the toes, heels soles and fingers and the total affected sites that are scored is 24.
2.6. Ethical considerations
The study involved human subjects and thus was conducted according to the “Nigeria National Code for Health Research Ethics (NNCHRE)”. The ethical review committee of the College of Medicine, University of Ibadan (reference no.: UI/EC/17/0404) approved the proposal for the study. The participation of individuals was optional and voluntary after written informed consent was obtained from the household heads on behalf of their children. Since the most understood local language is Yoruba, the consent form was translated into that for better understanding. The generated data was then used only for the purpose explained by the principal investigator to ensure confidentiality. The subjects found with Tunga penetrans infestation were not left unattended to but instead referred to the community health facilities for hygienic extractions while designing an affordable intervention from the available resources in the community. Participants agreed that photographs of affected parts be used for this study as long as their faces were not shown.
2.7. Data analysis
Data was inputted into Excel database (Microsoft office, 2013) and rechecked for completeness. Data was analyzed using XL Statistical software version 2020 for descriptive statistics. Descriptive statistics used include mean, standard error, range and percentages. Bar chart and pie chart was used to show relationships. One and two-way ANOVA test was used to determine the differences of significance. Tukey's test was used to separate means using single factor ANOVA set at α = 0.05.
3. Result
3.1. Prevalence of tungiasis
The prevalence of tungiasis in Igbokoda, Nigeria and the sampled villages has been reported elsewhere (Anyaele and Enwemiwe, 2021) and is summarized in Table 1, Table 2. Of which the 705 participants examined for the study had 252 infested, this repreesemted 35.8% of the prevalence. Children in the age of 10–14 years had the highest prevalence. This was closely followed by children within the ages of 5–9 years. The older aged individuals (40–59 years) suffered low infestation. With regards to the locations, 105, 74 and 73 individuals representing 44%, 29% and 35% prevalence were infested out of the 239, 255 and 211 individuals examined in Zion, Laranda and Kofawe respectively. Infestation prevalence was highest in Zion compared to the other locations (Table 2). Similarly, within the age groups in the locations, children within the ages of 10–14 years in Zion and Kofawe, and those within the ages of 5–9 years in Laranda had the highest prevalence compared to the other age groups. It was observed that individuals in the older age group (40–59 years) had low infestation prevalence in the three locations. The differences within age groups were not significant in Zion (P > 0.05) except for the age of 20–39 years against 10–14 age group (Table 2). The differences in prevalence observed within the age groups in Laranda and Kofawe were highly significant (P < 0.05).
Table 1.
Prevalence of tungiasis in Igbokoda, Nigeria.
| Age group (years) | Both sexes (n = 705) |
||
|---|---|---|---|
| Examined | Infested (%) | (95% CI) | |
| 0–4 | 75 | 28 (37.3) | 26.4–48.3 |
| 5–9 | 175 | 88 (50.3) | 42.9–57.7 |
| 10–14 | 166 | 85 (51.2) | 43.6–58.8 |
| 15–19 | 73 | 15 (20.6) | 11.3–29.9 |
| 20–39 | 73 | 14 (19.2) | 10.2–28.2 |
| 40–59 | 61 | 6 (9.8) | 2.3–17.3 |
| 60 & above | 82 | 16 (18.2) | 9.9–26.6 |
| Total | 705 | 252 (35.8) | 32.3–39.3 |
Source: Anyaele and Enwemiwe, 2021.
Table 2.
Prevalence of tungiasis in the sampled villages, Igbokoda, Nigeria.
| Age group (years) | Zion |
Laranda |
Kofawe |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Examined | Infested (Mean %) | 95% CI | Examined | Infested (Mean %) | 95% CI | Examined | Infested (Mean %) | 95% CI | |
| 0–4 | 18 | 8 (44.4)b | 21.5–67.4 | 28 | 7 (25.0)c | 9.0–41.0 | 29 | 13 (44.8)b | 26.7–62.9 |
| 5–9 | 58 | 34 (58.6)a | 45.9–71.3 | 69 | 33 (47.8)b | 36.0–59.6 | 48 | 21 (43.8)b | 29.8–57.8 |
| 10–14 | 63 | 38 (60.3)a | 48.2–72.4 | 61 | 25 (36.2)c | 24.1–48.3 | 42 | 22 (52.4)a | 37.3–67.5 |
| 15–19 | 28 | 8 (28.6)c | 11.9–45.3 | 24 | 3 (12.5)d | −0.7–25.73 | 21 | 4 (19.1)d | 2.3–35.9 |
| 20–39 | 26 | 5 (19.2)d | 4.1–34.3 | 24 | 2 (8.3)e | −2.7–19.3 | 23 | 7 (30.4)c | 11.6–49.2 |
| 40–59 | 21 | 4 (19.1)d | 2.3–35.9 | 18 | 0 (0.0)f | 0.0–0.0 | 22 | 2 (9.1)e | −2.9–21.1 |
| ≥ 60 | 25 | 8 (32.0)c | 13.7–50.3 | 31 | 4 (12.9)e | 1.1–24.7 | 26 | 4 (15.4)d | 1.5–29.3 |
| Total | 239 | 105 (43.9) | 37.6–50.2 | 255 | 74 (29.0) | 23.4–34.6 | 211 | 73 (34.6) | 28.2–41.0 |
Mean percentages of the same superscript letter do not differ significantly (P < 0.05) within age group using Turkey test. (Source: Anyaele and Enwemiwe, 2021).
3.2. Number, localization and classification of lesions
A total of 5293 lesions was recorded in 252 individuals with 3098 (58.5%) representing viable lesions, 1780 (33.6%) manipulated lesions and 415 dead lesions (7.8%). The infestation burdens represented as number of viable lesions in infested individuals is presented in Table 3. Of the 252 infested individuals, majority had moderate infestation while the proportion of infested individuals with mild and severe infestations were respectively low (Table 3). Considering infestation burdens within the age group, severe infestations was recorded more in children of 10–14 age group (8.2%) which is followed by children in the 5–9 age group (6.8%). These two age groups also have the highest moderate infestations while the least number of infestations was recorded in the 40–59 age group (Table 3). With regards to location, moderate infestation was the highest and represented 70.5%, 66.2% and 68.5% for Zion, Laranda and Kofawe respectively. While severe infestation was the least recorded and represented 10.5%, 4.1% and 2.7% for Zion, Laranda and Kofawe respectively (F (Location) = 8.47, P = 0.00017; F (Infestation burden) = 11.97, P = 0.00013).
Table 3.
Infestation burden in individuals with viable lesions in Igbokoda, Ondo State, Nigeria (n = 252).
| Age group (years) | No. infested | Parasite burden: no. (%) |
||
|---|---|---|---|---|
| Mild | Moderate | Severe | ||
| 0–4 | 28 | 11 (39.3) | 17 (60.7) | 0 (0.0) |
| 5–9 | 88 | 18 (20.5) | 64 (72.7) | 6 (6.8) |
| 10–14 | 85 | 18 (21.2) | 60 (70.6) | 7 (8.2) |
| 15–19 | 15 | 4 (26.7) | 10 (66.7) | 1 (6.7) |
| 20–39 | 14 | 6 (42.9) | 8 (57.1) | 0 (0.0) |
| 40–59 | 6 | 2 (33.3) | 3 (50.0) | 1 (16.7) |
| ≥60 | 16 | 4 (25.0) | 11 (68.8) | 1 (6.3) |
| Total | 252 | 63 (25.0) | 173 (68.7) | 16 (6.4) |
Note: 1–5 viable lesions = mild infestation; 6–30 viable lesions = moderate infestation; >30 viable lesions = severe infestation. Analysis of variance (α = 0.05): F (Age-group) = 2.67, P = 0.00271; F(Infestation burden) = 17.21, P < 0.0001.
The localization of viable lesions on different topographic sites affected is presented in Table 4. The highest viable lesions (1276) were recorded in 10–14 years age group individuals while the lowest in the 40–59 years age group individuals (106). Although, the number of viable lesions recorded in the 5–9 age group were also high (Table 4). Considering the topographic sites affected, the highest viable lesions were recorded on toes (96.0% of the infested individuals) followed by sole (75.8% of infested individuals) and the lowest on hands (5.2% of the infested individuals). Considering the localization of lesions within the age group, individuals in the 60 years of age and above had the highest sole and heel infestation and this was respectively followed by 40–59 age group (Table 4). Also, the 40–59 age group recorded the highest toes infestations and this was followed by the 5–9 age group. Hand infestation was highest in the 5–9 age group and followed by the 0–4 and 10–14 age group (Table 4). Tungiasis infestation on sole (64.3%), toes (85.7%), and heel (10.7%) was recorded low in the 20–39 age group for sole and toes, 0–4 age group for heel and no infestation recorded on the hand in four age groups (Table 4).
Table 4.
Localization of viable lesions on individuals with tungiasis in Igbokoda, Ondo State, Nigeria (n = 252).
| Age group (years) | No. of viable lesions | Topographic site affected |
|||
|---|---|---|---|---|---|
| Sole (%) | Toes (%) | Heel (%) | Hands (%) | ||
| 0–4 | 199 | 21 (75.0) | 27 (96.4) | 3 (10.7) | 1 (3.6) |
| 5–9 | 1078 | 64 (72.7) | 87 (98.9) | 18 (20.5) | 9 (10.2) |
| 10–14 | 1278 | 69 (81.2) | 82 (96.5) | 22 (25.9) | 3 (3.5) |
| 15–19 | 142 | 8 (53.3) | 14 (93.3) | 4 (26.7) | 0 (0.0) |
| 20–39 | 114 | 9 (64.3) | 12 (85.7) | 4 (28.6) | 0 (0.0) |
| 40–59 | 106 | 5 (83.3) | 6 (100.0) | 2 (33.3) | 0 (0.0) |
| ≥60 | 181 | 15 (93.8) | 14 (87.5) | 6 (37.5) | 0 (0.0) |
| Total | 3098 | 191 (75.8) | 242 (96.0) | 59 (23.4) | 13 (5.2) |
Analysis of variance (α = 0.05): F(Age-group) = 5.57, P = 0.0021; F(Localization) = 7.03, P = 0.0025.
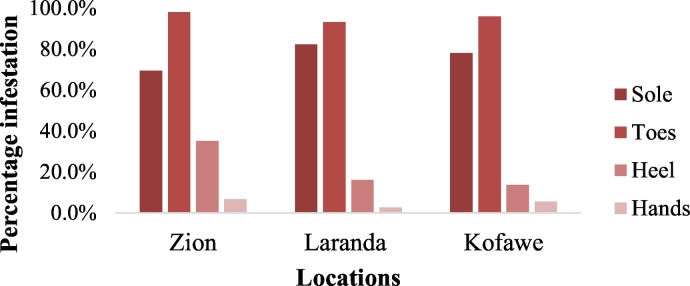
According to the 105, 74, and 73 infested individuals in the various sampled locations, Zion had the highest toes, heel and hand infestation while sole infestation was highest in Laranda (Fig. 1). On the other hand, hand and sole infestation was recorded low in Laranda (2.7% and 93.2%) while sole and heel infestation was recorded low in Zion and Kofawe (69.5% and 13.7% respectively).
Fig. 1.
Localization of viable lesions on individuals with tungiasis in Igbokoda, Ondo State, Nigeria. Analysis of variance (α = 0.05): F(Location) = 0.36, P = 0.712; F(Localization) = 58.73, P < 0.0001.
Furthermore, the type of lesions in individuals with tungiasis is presented in Table 5. In total, viable lesions were highly recorded compared to dead and manipulated lesions. Considering the age group, the highest viable, manipulated lesions and dead lesions were recorded in the 40–59, 5–9 and 40–59 age groups respectively while the lowest viable, manipulated lesions and dead lesions were recorded in 20–39, 20–39 and 0–4 age group respectively (Table 5). On a general note, the highest lesions (2700: 51.0%) was recorded in Zion out of the 5293 lesions recorded in this study, while the lowest lesions (1194: 22.6%) was recorded in Laranda. Although, the (1399: 26.4%) lesions recorded in Kofawe was also high. The highest viable lesions was recorded in Zion and the lowest in Kofawe while within the manipulated lesions, the highest was recorded in Kofawe and lowest in Zion (Fig. 2). Similarly, the highest dead lesions were recorded in Kofawe and the lowest in Laranda.
Table 5.
Type of lesions in individuals with tungiasis from Igbokoda, Ondo State, Nigeria.
| Age group (years) | Igbokoda (n = 252) |
|||
|---|---|---|---|---|
| No. infested | Mean of lesions (range) |
|||
| Viable lesions | Manipulated lesions | Dead lesions | ||
| 0–4 | 28 | 7.1 ± 1.5 (1–15) | 5.1 ± 0.9 (1–18) | 0.7 ± 0.01 (0–3) |
| 5–9 | 88 | 12.3 ± 2.1 (1–55) | 8.1 ± 1.3 (0–18) | 1.5 ± 0.1 (0−20) |
| 10–14 | 85 | 15.0 ± 2.7 (3–127) | 7.0 ± 1.1 (0–76) | 2.1 ± 0.3 (0–14) |
| 15–19 | 15 | 9.5 ± 1.3 (2−31) | 6.1 ± 1.5 (0–51) | 1.7 ± 0.03 (0–14) |
| 20–39 | 14 | 8.1 ± 1.1 (1–25) | 4.3 ± 0.9 (0–37) | 1.1 ± 0.03 (0–3) |
| 40–59 | 6 | 17.7 ± 3.1 (3–73) | 5.3 ± 0.4 (0−32) | 5.2 ± 0.4 (0–25) |
| ≥ 60 | 16 | 11.3 ± 2.5 (2–63) | 7.7 ± 1.5 (0–54) | 0.9 ± 0.01 (0–4) |
| Total | 252 | 12.3 (1–127) | 7.1 (0–76) | 1.7 (0–25) |
Note: viable lesions are lesions in stage I-III; manipulated lesions are extracted lesions and dead lesions are stage IV. Values in parenthesis are minimum and maximum values of lesions. Analysis of variance (α = 0.05): F (Age-group) = 2.48, P = 0.02366; F (Infestation burden) = 15.18, P < 0.0001.
Fig. 2.
Type of tungiasis lesions stratified with the sampled locations in Igbokoda, Ondo State, Nigeria. Legend: 1–5 viable lesions = mild infestation; 6–30 viable lesions = moderate infestation; >30 viable lesions = severe infestation. Analysis of variance (α = 0.05): F(Location) = 1.91, P = 0.9917; F(Lesion types) = 63.71, P < 0.0001.
3.3. Infestation burden and classification with sex
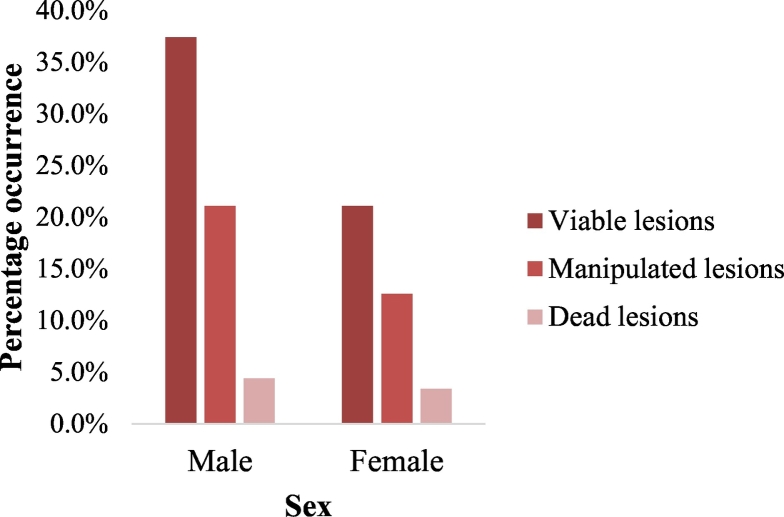
Of the 3098 viable lesions recorded in 252 infested individuals, 1980 (63.9%) represented viable lesions in males and 1118 (36.1%) represent viable lesions in females. Viable lesions were significantly different between males and females P = 0.02. The type of tungiasis lesions stratified with sex is shown in Fig. 3. Of the 5293 lesions recorded in this study, males had the highest lesions 3327 (62.9%) than females 1966 (37.1%) lesions. Furthermore, viable, manipulated and dead lesions were preponderant in males than in females.
Fig. 3.
Classification of tungiasis lesions according to sex in Igbokoda, Ondo State, Nigeria. Legend: 1–5 viable lesions = mild infestation; 6–30 viable lesions = moderate infestation; >30 viable lesions = severe infestation. Analysis of variance (α = 0.05): F(Sex) = 3.76, P = 0.0168; F(Lesion types) = 76.61, P < 0.0001.
3.4. Classification and flea burdens with environment
The type of lesions in infested individuals with floor-type in Igbokoda is presented in Table 6. Of the 252 flea infested individuals recorded in this study, unpaved veranda and room recorded the highest infestations in 135 individuals while infestations was reportedly low in paved veranda and unpaved room; this representing 20 infested individuals. Individuals that lived in unpaved veranda and room had the highest viable, manipulated and dead lesions (Table 6). However, dead and viable lesions was also recorded high in the paved rooms and veranda (mean of 1.9) and in paved veranda and unpaved room (mean of 12.3) respectively. On the other hand, infested individuals that lived in paved rooms and unpaved veranda had the lowest viable and dead lesions while paved veranda and unpaved rooms recorded the lowest manipulated lesions (Table 6). The highest range of viable, manipulated and dead lesions was recorded in household with unpaved veranda and room and the lowest range in paved veranda and unpaved rooms.
Table 6.
Type of tungiasis lesions on infested individuals stratified with floor-types from Igbokoda, Ondo State, Nigeria.
| Floor-type | Igbokoda n = 252 |
|||
|---|---|---|---|---|
| No. infested | Mean of lesions (range) |
|||
| Viable lesions | Manipulated lesions | Dead lesions | ||
| Paved room and unpaved veranda | 54 | 9.6 ± 1.4 (2–75) | 6.4 ± 1.1 (0–54) | 1.0 ± 0.2 (0–5) |
| Paved veranda and unpaved room | 20 | 12.3 ± 2.1 (1−23) | 5.9 ± 1.3 (0–18) | 1.3 ± 0.3 (0–4) |
| Paved room and paved veranda | 43 | 10.1 ± 2.0 (1–52) | 6.3 ± 1.5 (0–54) | 1.9 ± 0.4 (0–17) |
| Unpaved veranda and room | 135 | 14.1 ± 2.5 (1–127) | 7.7 ± 1.1 (0–76) | 1.9 ± 0.4 (0–25) |
| Total | 252 | 12.3 (1–127) | 7.1 (0–76) | 1.7 (0–25) |
Note: viable lesions are lesions in stage I-III; manipulated lesions are extracted lesions and dead lesions are stage IV. Values in parenthesis are minimum and maximum values of lesions. Analysis of variance (α = 0.05): F (Floor-types) = 2.41, P = 0.076; F (Lesion types) = 20.58, P < 0.0001.
Flea burdens represented as number of fleas on the sampled floors in Igbokoda is presented in Table 7. Eighty-one out of the 362 sampled floors had fleas. The total number of fleas in the various floor-types were 127 and this representing a high flea intensity for Igbokoda. The highest intensity of flea was recorded in the unpaved veranda and room (51.2%) and this is followed by paved rooms and unpaved veranda. The lowest intensity of flea was recorded in paved veranda and unpaved rooms (1.6%) and this is followed by paved room and veranda (Table 7).
Table 7.
Flea burdens on the floor of sampled houses in Igbokoda, Ondo State, Nigeria.
| Floor-type | No. examined | Total flea (%) | Status of intensity |
|---|---|---|---|
| Paved room and unpaved veranda | 141 | 53 (41.7b) | +++ |
| Paved veranda and unpaved room | 8 | 2 (1.6d) | + |
| Paved room and veranda | 130 | 7 (5.5c) | + |
| Unpaved veranda and room | 83 | 65 (51.2a) | +++ |
| Total | 362 | 127 | +++ |
Note: High intensity of fleas = +++, medium intensity of fleas = ++ and low intensity of fleas = +. Mean percentages of the same superscript letter do not differ significantly (P < 0.05) using Turkey test.
More so, flea burdens on the legs of infested individuals in Igbokoda is presented in Table 8. Of the 252 legs examined, 59 legs had fleas which represent a total of 146 fleas recorded. The total flea on legs of infested individuals is high for Igbokoda. The highest flea intensity was recorded in unpaved veranda and room (42.5%) and this is followed by the paved rooms and unpaved veranda while the lowest flea intensity was recorded in paved room and unpaved verandas (5.5%) and this is followed by paved room and veranda (Table 8).
Table 8.
Flea burdens on legs of tungiasis infested individuals living in the various floor-types from Igbokoda, Ondo State, Nigeria.
| Floor-type | No. of flea positive legs | Total flea (%) | Status of intensity |
|---|---|---|---|
| Paved room and unpaved veranda | 23 | 54 (37.0b) | +++ |
| Paved veranda and unpaved room | 3 | 8 (5.5d) | + |
| Paved room and veranda | 10 | 22 (15.1c) | ++ |
| Unpaved veranda and room | 23 | 62 (42.5a) | +++ |
| Total | 59 | 146 | +++ |
Note: High intensity of fleas = +++, medium intensity of fleas = ++, low intensity of fleas = + and total number of legs examined = 252. Mean percentages of the same superscript letter do not differ significantly (P < 0.05) using Turkey test.
3.5. Tungiasis-associated morbidity in Igbokoda
The acute severity score of tungiasis in infested individuals in Igbokoda is presented in Table 9. A total of 904 affected sites were recorded for acute tungiasis from the 252 infested individuals. The acute tungiasis represented 504, 228 and 172 affected sites for Zion, Laranda and Kofawe respectively. Difficulty in walking was the highest recorded acute symptom, this was followed by pain upon pressure and the lowest acute sign was itching. Plate 1 A to E shows individuals presenting some acute signs of tungiasis predominant in the study.
Table 9.
Acute clinical signs associated with tungiasis infested individuals in Igbokoda, Nigeria.
| Symptoms | Affected sites | Mean sites affected ± SE | % occurrence |
|---|---|---|---|
| Erythema and oedema | 115.5 | 23.1 ± 3.55 | 12.8 |
| Pustulation | 290 | 58 ± 11.72 | 32.1 |
| Spontaneous pain | 300 | 60 ± 13.13 | 33.2 |
| Pain upon pressure | 593 | 118.6 ± 15.73 | 65.6 |
| Fissure | 214 | 42.8 ± 8.90 | 23.7 |
| Ulcer | 229 | 45.8 ± 9.12 | 25.3 |
| Itching | 149 | 29.8 ± 2.98 | 16.5 |
| Sleeping disturbance | 175.5 | 35.1 ± 3.88 | 19.4 |
| Suppuration | 394 | 78.8 ± 15.62 | 43.6 |
| Lesion in cluster | 261 | 52.2 ± 10.71 | 28.9 |
| Pain on walking | 462 | 92.4 ± 18.09 | 51.1 |
| Difficulty in walking | 650 | 130 ± 27.45 | 71.9 |
Analysis of variance (α = 0.05): F (Acute signs) = 18.02, P < 0.05.
Plate 1.
Acute symptoms of tungiasis lesions recorded in infested individuals from Igbokoda, Ondo State, Nigeria. A. Individual presenting cluster of lesions on the heel. B. Lesions in cluster C. Severely infested individual presenting multiple symptoms. D. Severely infested leg presenting all symptoms. E. Infested individual presenting multiple acute symptoms.
The chronic severity score of tungiasis in infested individuals in Igbokoda is presented in Table 10. A total of 702 affected sites were recorded for chronic tungiasis from the 252 infested individuals. The chronic tungiasis represented 367, 203 and 132 affected sites for Zion, Laranda and Kofawe respectively. Deformation of toes was the most occurring chronic symptom and hypertrophy of nail rim was the lowest recorded. Plate 2 A to D shows individuals presenting some chronic signs of tungiasis predominant in the study.
Table 10.
Chronic clinical signs associated with tungiasis infested individuals in Igbokoda, Nigeria.
| Symptoms | Affected sites | Mean sites affected ± SE | % occurrence |
|---|---|---|---|
| Hyperkeratosis | 198 | 39.6 ± 4.01 | 28.2 |
| Hypertrophy of nail rim | 141 | 28.2 ± 2.95 | 20.1 |
| Deformation of toe nail | 159 | 31.8 ± 3.76 | 22.7 |
| Loss of toe nail | 251 | 50.2 ± 8.97 | 35.8 |
| Deformation of toes | 434 | 86.8 ± 12.27 | 61.8 |
Analysis of variance (α = 0.05): F(Chronic signs) = 11.35, P < 0.05.
Plate 2.
Chronic symptoms of tungiasis lesions recorded in infested individuals from Igbokoda, Ondo State, Nigeria. A. Infested individual presenting hyperkeratosis. B. Individual presenting hypertrophy of nail rim. C. Individual presenting loss of toe nails. D. Infested individual presenting deformation of toe nails.
4. Discussion
This study recorded two hundred and fifty-two infested individuals. According to the observation made by Anyaele and Enwemiwe (2021), children aged 10 to 14 years had the highest prevalence which was closely followed by children within the ages of 5 to 9 years and a low prevalence in the older aged individuals (40–59 years). It is probable that infestation prevalence influenced the intensity recorded in this study, majority of which had moderate infestation and a low proportion of mild and severe infestations. Comparing intensity of infestation within age group, severe infestations was common among children of the 10–14 and 5–9 age group. Reasons for this being that children within these age groups are often active playing feet unprotected on sands and at best putting on sandals and slippers as earlier observed by Ugbomoiko et al. (2007). Infestation prevalence was highest in Zion (44%) compared to the other two fish villages and was equally higher in children within the ages of 10 to 14 years in Zion and Kofawe, and those within the ages of 5 to 9 years in Laranda compared to the other age groups (Anyaele and Enwemiwe, 2021). Adults in the 40–59 age group on the other hand were the individuals with the lowest proportion of infestations. This may be due to the fact that individuals in this age group have knowledge of the infestation and at best put on cover shoes while out for work. Since moderate infestations were commonly observed in all locations, it is indicative that all sites frequently observed infestations by sand fleas. However, the severity of infestation especially common in Zion is a function of majority of the houses not having a paved floor thus supporting the development of fleas. The high occurrence of moderate infestation in this study correlates the fact that viable lesions were highest compared to other lesions and that frequent infestations occurred in Igbokoda, Nigeria. Also, the preponderance of viable lesions in males corresponds with the number of viable lesions recorded. The mean viable lesions recorded in this study is higher than those reported in an urban slum in Brazil and in Trinidad with a mean number of 7.8 and of 8.0 lesions respectively (Chadee, 1994; Wilcke et al., 2002). Viable, manipulated and dead lesions were highest in males compared to females. This being that houses were infected, maybe due to the lack of shoes inside the house, associated that men usually do less work inside the house, turns these individuals with less movements than females and maybe predisposing them to flea infestation. More so, that the individuals spent time in unpaved floors and removing foot covers during fishing and farming, may equally predispose them more to the infestation too.
The mean of viable lesions observed was highest in the unpaved veranda and rooms compared to the other floor-types. This is most likely due to that flea thrives more in dry loosed sand and since the sand in the surrounding is connected to those in the house, the likelihood of fleas jumping into house with no barrier is possible. Comparing the mean of viable lesion recorded in the unpaved veranda and rooms to the paved rooms and unpaved verandas with low mean viable lesion, it can be deduced that fleas do not thrive in the paved floors in room. This can be best explained by where individuals spend most time whether in rooms with paved floors or verandas which are unpaved. Considering the flea burdens in the floor and on infested individuals living in houses with different floor-types, infestation intensity was observed to be high in house with unpaved veranda and room, and also in houses with paved rooms and unpaved verandas. On the contrary, moderate and low intensity recorded in houses with paved rooms and veranda, and also rooms with paved veranda and unpaved rooms can be linked to the fact that pavement of floors served as barriers to fleas development.
Tungiasis-related morbidities are dependent on the number of lesions present on the topographic localized body parts. A total of 5293 lesions recorded in 252 individuals is considerably high and the severe infestation recorded in Zion showed that individuals were more predisposed than in other locations. The high viable lesions localized on toes and sole of individuals are capable of inflicting difficulty in walking which could resultantly cause poverty. Poverty driven by this infestation is linked to the fact that severely infested and even some moderately infested individuals are often discouraged from carrying out their day-to-day activities. Since individuals were faced with difficulty in walking, persistent itching, sleeping difficulty and other related symptoms, farming and fishing smoothly could be discouraged. The high flea lesions localized on feet supports the findings of Mazigo et al. (2012) which observed flea lesions on the feet other than the hands and ectopic sites such as elbows, neck, buttocks and the genital region. The high number of viable flea lesions recorded in children in the 10–14 and 5–9 age group indicating that children in this age group are often mobile walking foot unprotected through the central paths of the villages which are sandy, laying on mat positioned under shady trees to rest and playing around sandy beach of coastlines. Furthermore, children that play on loosed sand at best put on sandals or slippers, which do not in actual fact act as a barrier to penetrating jigger fleas. This explains convincingly the reason children are predisposed more to infestation thus making these age groups severely infested.
It was previously suggested that increased keratinization of the skin in adolescence and growing adult individuals may increasingly hinder the penetration of jigger fleas (Ade-Serrano and Ejezie, 1981; Chadee, 1994). However, this justification seems questionable as this study frequently observed fleas lesions on heavily keratinized parts of the sole, toes and heels of older aged individuals compared to younger age individuals. Penetration by sand fleas could occur regardless of age. The observation of flea lesions on sole, toes, and heel in the elderly age group is in accordance with an unpublished observation made by Heukelbach which pointed that increased keratinization of skin in 60 years and above individuals fails to justify the major increase in prevalence and infestation burden in this study. Heukelbach also observed that the thickness of the stratum corneum is increased at this age. Similarly, it is probable that dermal ossification observed with the treatment of hands and feet treated with topical ointment of naphthalene mixture were more effective than keratinization (Enwemiwe et al., 2020). The percentage of manipulated lesions observed in the locations were high. This indicates that in locations where infestation are high, individuals with embedded sand fleas are often faced with the feelings of extracting the embedded fleas in lesion. The observation reported by Wilcke et al. (2002) that adult individuals become experienced in better extracting embedded fleas is not in accordance with this study because manipulated lesions were recorded high in the 5–9 age group. On the other hand, infestation by fleas on the hands were common in the 5–9 age group where the possibility of playing with sand in environment is high.
The majority of tungiasis lesions recorded in this study were topographically localized on the sole, toes, heel than on the hands. The resulting preponderance of tungiasis lesion on the feet is linked to the observation of Heukelbach et al. (2002) which ascribed this to the presence of resilin in legs of fleas that enables them jump to a desirable heights and penetrating the host skin in close contact with the infested soil. This study did not encountered lesions at ectopic sites and as such do not supports Heukelbach et al. (2002) observation that embedded fleas at ectopic sites including elbows, thighs and gluteal region is predominant in children reaching up to one quarter of the lesions. Tungiasis infestation on the ectopic sites are uncommon except in cases where children on bare bodied sits, lay or play on sandy floors or surroundings. However, the high occurrence of tungiasis lesions at ectopic sites have been explained by Muehlen et al. (2003) indicating that the high possibility of lesions occurring at the ectopic sites in children is due to the fact that their bodies may frequently comes in contact with infested soil than older aged individuals. Muehlen et al. (2003) related high tungiasis prevalence to high infestation burden in communities. Their report corroborates with this present study where prevalence in Zion is 44%, and moderate and severe infestations was equally higher compared to Laranda and Kofawe where prevalence of infestation was lower relating to intensity (Anyaele and Enwemiwe, 2021).
Difficulty in walking and pain upon pressure was the acute morbidity while deformation of toes was the most occurring chronic morbidity highly observed in infested individuals in Igbokoda, Nigeria. Difficulty in walking as well as a deformation of toes have the potentials of stigmatizing infested individuals since tungiasis played major role in psychoanalysis of societal significance. Tungiasis with this stigmatization cannot remain a mere infestation (Heukelbach, 2006; Mazigo et al., 2012). Ulcerations and fissures was observed to be a secured significant entry for pathogenic agent including Clostridium tetani which have the potentials of causing acute complications and sometimes death in non-immune individuals (Greco et al., 2001; Feldmeier et al., 2003). Tungiasis-associated morbidities have apparently been reported by various survey from other endemic areas (Njeumi et al., 2002; Heukelbach, 2006; Joseph et al., 2006; Ugbomoiko et al., 2007). Ulceration and fissure were not common among infested individuals in this study. The occurrence of acute and chronic symptoms including pain upon pressure, difficulty in walking, deformation of toes among others suggests that infested individuals were severally re-infested without intervention.
5. Conclusion
This study revealed that intensity of infestation and clinical morbidities was high in Igbokoda community occurring severely in children compared to adults. Fleas sampled on legs and on floors of the environment was also reportedly high. The high flea intensity in unpaved rooms and verandas was the major cause of infestation and re-infestations in this study. The high infestation intensities on toes, sole, heel other than hands, and on the floors, and tungiasis-morbidities including difficulty in walking, pain upon pressure and deformation of toes observed is capable of impacting on the socio-economic standards of individuals in Igbokoda community.
Funding
Not applicable.
Ethical approval
The study was conducted according to “Nigeria National Code for Health Research Ethics (NNCHRE)”. The ethical review committee of the College of Medicine, University of Ibadan (reference no.: UI/EC/17/0404) approved the written protocol for the study.
Consent to participate
Participation was sort by written document. A written consent for participants transcribed to their language for better understanding.
Consent to publication
Authors declare that the article be published.
Availability of data and material
All analyzed data are available in the body of the article.
Declaration of Competing Interest
Authors declare that there is no competing interest.
Acknowledgements
We are grateful to the participants who voluntarily consented to this study.
References
- Ade-Serrano M.A., Ejezie G.C. Prevalence of tungiasis in Oto-Ijanikin village, Badagry, Lagos state, Nigeria. Ann. Trop. Med. Parasitol. 1981;75:471–472. doi: 10.1080/00034983.1981.11687469. [DOI] [PubMed] [Google Scholar]
- Anyaele O.O., Enwemiwe V.N. African Zoology; 2021. Prevalence of Tungiasis in Rural Poor Neighbourhood in Igbokoda, Ondo State, Nigeria; pp. 1–7. [DOI] [Google Scholar]
- Arene F.O. The prevalence of sand flea (Tunga penetrans) among primary and post-primary school pupils in Choba area of the Niger Delta. Public Health. 1984;98:282–283. doi: 10.1016/s0033-3506(84)80004-9. [DOI] [PubMed] [Google Scholar]
- Chadee D.D. Distribution patterns of Tunga penetrans within a community in Trinidad, West Indies. J. Trop. Med. Hyg. 1994;97:167–170. [PubMed] [Google Scholar]
- De Carvalho R.W., De Almeida A.B., Barbosa-Silva S.C., Amorim M., Ribeiro P.C. The patterns of Tungiasis in Araruama township, state of Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz. 2003;98(1):31–36. doi: 10.1590/s0074-02762003000100005. [DOI] [PubMed] [Google Scholar]
- Ehrenberg J.P., Ault S.K. Neglected diseases of neglected populations: thinking to reshape the determinants of health in Latin America and the Caribbean. BMC Public Health. 2005;5:119. doi: 10.1186/1471-2458-5-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enwemiwe V.N., Ojianwuna C.C., Anyaele O.O. Assessing the potentials of two local topical ointments as affordable treatment against tungiasis infestation: a self-experimentation in Igbokoda, Nigeria. Parasite Epidemiol. Control. 2020;11 doi: 10.1016/j.parepi.2020.e00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeier H., Keysers A. Tungiasis–a Janus-faced parasitic skin disease. Travel Med. Infect. Dis. 2013;11(6):357–365. doi: 10.1016/j.tmaid.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Feldmeier H., Eisele M., Saboia-Moura R.C., Heukelbach J. Severe tungiasis in underprivileged communities: case series from Brazil. Emerg. Infect. Dis. 2003;9:949–955. doi: 10.3201/eid0908.030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeier H., Kehr J.D., Poggensee G. High exposure to Tunga penetrans (Linnaeus, 1785) correlates with intensity of infestation. Mem. Inst. Oswaldo Cruz. 2006;101:65–69. doi: 10.1590/s0074-02762006000100012. [DOI] [PubMed] [Google Scholar]
- Feldmeier H., Sentongo E., Krantz I. Tungiasis (sand flea disease): a parasitic disease with particular challenges for public health. Eur. J. Clin. Microbiol. Infect. Dis. 2013;32(1):19–26. doi: 10.1007/s10096-012-1725-4. [DOI] [PubMed] [Google Scholar]
- Greco J.B., Sacramento E., Tavares-Neto J. Chronic ulcers and myasis as ports of entry for Clostridium tetani. Braz. J. Infect. Dis. 2001;5(6):319–323. doi: 10.1590/s1413-86702001000600005. [DOI] [PubMed] [Google Scholar]
- Heukelbach J. Tungiasis. Revista Instantaneous Medecine de Tropica Sa˜o Paulo. 2005;47:307–313. doi: 10.1590/s0036-46652005000600001. [DOI] [PubMed] [Google Scholar]
- Heukelbach J. Revision on tungiasis: treatment options and prevention. Expert Rev. Anti-Infect. Ther. 2006;4:151–157. doi: 10.1586/14787210.4.1.151. [DOI] [PubMed] [Google Scholar]
- Heukelbach J., Araujo S.D., Hesse G., Feldmeier H. Tungiasis: a neglected health problem of poor communities. Tropical Med. Int. Health. 2001;6:267–272. doi: 10.1046/j.1365-3156.2001.00716.x. [DOI] [PubMed] [Google Scholar]
- Heukelbach J., Wilcke T., Eisele M., Feldmeier H. Ectopic localization of Tungiasis. Am. J. Trop. Med. Hyg. 2002;67(2):214–216. doi: 10.4269/ajtmh.2002.67.214. [DOI] [PubMed] [Google Scholar]
- Heukelbach J., Eisele M., Jackson A., Feldmeier H. Topical treatment of tungiasis: a randomized, controlled trial. Ann. Trop. Med. Parasitol. 2003;97:743–749. doi: 10.1179/000349803225002408. [DOI] [PubMed] [Google Scholar]
- Heukelbach J., Costa A.M.L., Wilcke T., Mencke N., Feldmeier H. The animal reservoir of Tunga penetrans in severely affected communities of north-East Brazil. Med. Vet. Entomol. 2004;18(4):329–335. doi: 10.1111/j.0269-283X.2004.00532.x. [DOI] [PubMed] [Google Scholar]
- Joseph J.K., Bazile J., Mutter J., Shin S., Ruddle A. Tungiasis in rural Haiti: a community-based response. Trans. R. Soc. Trop. Med. Hyg. 2006;100:970–974. doi: 10.1016/j.trstmh.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Kehr J.D., Heukelbach J., Mehlhorn H., Feldmeier H. Morbidity assessment in sand flea disease (tungiasis) Parasitol. Res. 2007;100:413–421. doi: 10.1007/s00436-006-0348-z. [DOI] [PubMed] [Google Scholar]
- Linardi P.M., Calheiros C.M.L., Campelo-Junior E.B., Duarte E.M., Heukelbach J., Feldmeier H. Occurrence of the off-host life stages of Tunga penetrans (Siphonaptera) in various environments in Brazil. Ann. Trop. Med. Parasitol. 2010;104(4):337–345. doi: 10.1179/136485910X12743554759902. [DOI] [PubMed] [Google Scholar]
- Mazigo H.D., Bahemana E., Konje E.T., Dyegura O., Mnyone L.L., Kweka E.J., Kidenya B.R., Heukelbach J. Jigger flea infestation (tungiasis) in rural western Tanzania: high prevalence and severe morbidity. Trans. R. Soc. Trop. Med. Hyg. 2012;106(4):259–263. doi: 10.1016/j.trstmh.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Mc Gavin G.C. Royal Geographical Society; Kensington Gore, London: 2007. Expedition Field Techniques Insects and Other Terrestrial Arthropods. Geography Outdoors: The Centre Supporting Field Research, Exploration and Outdoor Learning; pp. 59–60. [Google Scholar]
- Muehlen M., Heukelbach J., Wilcke T., Winter B., Mehlhorn H. Investigations on the biology, epidemiology, pathology and control of Tunga penetrans in Brazil: II. Prevalence, parasite load and topographic distribution of lesions in the population of a traditional fishing village. Parasitol. Res. 2003;90:449–455. doi: 10.1007/s00436-003-0877-7. [DOI] [PubMed] [Google Scholar]
- Mutebi F., Krücken J., Mencke N., Feldmeier H., von Samson-Himmelstjerna G., Waiswa C. Two severe cases of Tungiasis in goat kids in Uganda. J. Insect Sci. 2016;16(1):34. doi: 10.1093/jisesa/iew016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutebi F., Krücken J., Feldmeier H., Waiswa C., Mencke N., Eneku W., von Samson-Himmelstjerna G. High intensity of Tunga penetrans infection causing severe disease among pigs in Busoga, south eastern Uganda. BMC Vet. Res. 2017;13(1):206. doi: 10.1186/s12917-017-1127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngunjiri J., Keiyoro P. Vol. 7. LAP LAMRT academic publishing GmbH & Co; KG, Germany: 2011. Soil Factors Influencing the Occurrence of Tunga penetrans in Kenya; pp. 53–54. (30) [Google Scholar]
- Njeumi F., Nsangou C., Ndjend A.G., Koga O.F., Pampiglione S. Tunga penetrans au Cameroun. Revue de Médecine Véterinaire. 2002;153(3):176–180. [Google Scholar]
- Pampiglione S., Fioravanti M.L., Gustinelli A., Onore G., Montovani B. Sand flea (Tunga spp.) infections in man and domestic animals: state of the art. Med. Vet. Entomol. 2009;23:172–186. doi: 10.1111/j.1365-2915.2009.00807.x. [DOI] [PubMed] [Google Scholar]
- Pilger D., Schwalfenberg S., Heukelbach J., Witt L., Mencke N., Khakban A., Feldmeier H. Controlling Tungiasis in an impoverished community: an intervention study. PLoS Negl. Trop. Dis. 2008;2(10) doi: 10.1371/journal.pntd.0000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugbomoiko S., Ariza L., Heukelbach J. Pigs are the most important animal reservoir for Tunga penetrans (jigger flea) in rural Nigeria. Trop. Dr. 2008;38:226–227. doi: 10.1258/td.2007.070352. [DOI] [PubMed] [Google Scholar]
- Ugbomoiko U.S., Ofoezie I.E., Heukelbach J. Tungiasis: high prevalence, parasite load, and morbidity in a rural community in Lagos state, Nigeria. Int. J. Dermatol. 2007;46:475–481. doi: 10.1111/j.1365-4632.2007.03245.x. [DOI] [PubMed] [Google Scholar]
- Ugbomoiko U.S., Ariza L., Babamale A.O., Heukelbach J. Prevalence and clinical aspects of tungiasis in south-west Nigerian schoolchildren. Trop. Dr. 2017;47(1):34–38. doi: 10.1177/0049475516657503. [DOI] [PubMed] [Google Scholar]
- Wilcke T., Heukelbach J., Cesar-Saboia M.R., Kerr-Pontes R.S., Feldmeier H. High prevalence of tungiasis in a poor neighbourhood in Fortaleza, Northeast Brazil. Acta Trop. 2002;83:255–258. doi: 10.1016/s0001-706x(02)00133-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All analyzed data are available in the body of the article.