Abstract
Purpose of Review
Coronavirus disease 2019 (COVID19) involves the heart, including pericardium. This article reviews the possible pathophysiological mechanisms in pericardial involvement in COVID19 and pericardial manifestations of COVID19. It also summarizes the patients with pericarditis secondary to COVID19 and outlines the contemporary treatment strategies in this patient population.
Recent Findings
A high degree of suspicion is required to identify the pericardial involvement in COVID19 patients. It is proposed that an underlying hyperinflammatory reaction in COVID19 leads to pericardial inflammation. Acute pericarditis with or without myocardial involvement is diagnosed on clinical presentation, serum inflammatory markers, electrocardiogram, and echocardiogram. Multimodality imaging may also have an additional diagnostic value. Patients are usually managed medically, but some patients develop a life-threatening pericardial tamponade necessitating pericardial drainage.
Summary
Pericardial involvement is an important clinical manifestation of COVID19 requiring a proper workup. Timely diagnosis and a specific management plan based on the presentation and concomitant organ involvement usually lead to a complete recovery.
Keywords: Pericardial disease, Pericardial effusion, Pericarditis, Myocarditis, Pericardial tamponade, COVID19
Introduction
Coronavirus disease 2019 (COVID19) has infected more than 80 million people worldwide and caused more than 1.5 million deaths in 2020 [1]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel coronavirus that causes COVID19. COVID19 primarily affects the lungs, and the severity of the disease ranges from mild respiratory symptoms to acute respiratory distress syndrome [2]. There is growing literature on the involvement of other organs, mainly the heart, kidneys, gastrointestinal tract, brain, and skin [3, 4]. Cardiac manifestations of COVID19 include acute heart failure, myocarditis, acute coronary syndrome, Takotsubo cardiomyopathy, arrhythmias, pericardial involvement, and cardiac arrest [5–7]. Pericardial diseases typically caused by viruses include pericarditis, pericardial effusion, and life-threatening pericardial tamponade (PT). A few cases of pericardial involvement in patients infected with other members of the coronavirus family have also been reported [8, 9, 10]. As the body of literature is expanding for COVID19, it is essential to understand some uncommon manifestations of the SARS-CoV-2 virus that require a high degree of suspicion. This review aims to identify COVID19-associated pericardial disease and briefly describe the underlying pathophysiological mechanisms. We have also summarized management strategies for COVID-19 associated pericardial diseases.
Pathophysiologic Mechanisms
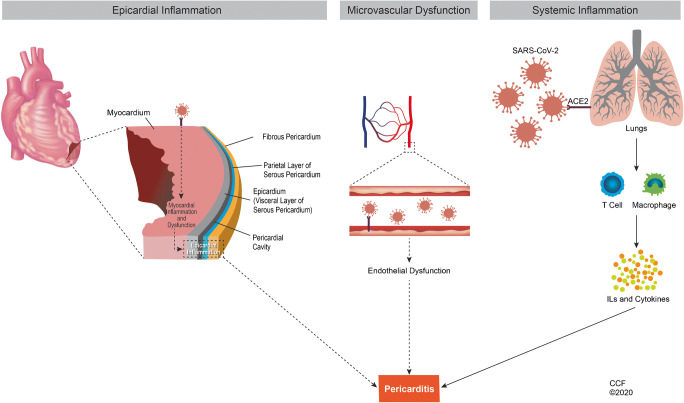
The pericardium is a double-layered protective membrane around the heart with an inner visceral (serosal) and outer parietal (fibrous) layer. The pericardial sac between these two layers usually contains around 50cc of serous fluid [11]. The pericardium is relatively avascular, and increased vascularity is the hallmark of inflammation [12]. COVID19 is primarily a disease of respiratory epithelium, and endothelial cells play a crucial role in the involvement of other organs, including the heart [13•]. The exact pathophysiological mechanism of pericardial involvement in patients with COVID-19 has not been fully elucidated. At present, there is no evidence of direct infection of the pericardium and myocardium by SARS-Cov2. However, it is proposed that systemic inflammatory reaction induced by SARS-CoV-2 leads to cardiac involvement, including pericarditis. Endothelial damage as a result of heightened inflammation may also be responsible for pericardial manifestations. Although SARS-CoV-2 has also shown cardiotropic properties, the direct damage to the cardiac structures is rare (Fig. 1).
Fig. 1.
Mechanisms of pericardial inflammation caused by SARS-CoV2. SARS-CoV2, severe acute respiratory syndrome coronavirus 2; ACE 2, angiotensin-converting enzyme; ILs, interleukins. (Reprinted with permission, Cleveland Clinic Center for Medical Arts and Photography ©2020. All rights reserved.)
COVID-19 mediated increased inflammation
The levels of various inflammatory markers and cytokines are elevated in COVID19 infection. The hyperinflammatory syndrome in COVID19 is characterized by increased interleukins (IL-1, IL-2, IL-6, IL-7), granulocyte-macrophage colony-stimulating factor, interferon-γ, inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1-α, and tumor necrosis factor (TNF)- α [14]. In a multicenter retrospective study, elevated IL6 and ferritin levels in COVID19 patients were associated with mortality [15]. This suggests hyper-inflammation and mortality associated with the systemic effects of inflammation in a subset of COVID19 patients. A cytokine storm is defined as an activation of an auto-amplifying cascade of cytokines due to unregulated host immune response to various triggers. This so-called cytokine storm is triggered by damage-associated molecular patterns and pathogen-associated molecular patterns, including infections, malignancy, autoimmune diseases, and drugs [16]. These triggers cause an imbalance of type 1 and type 2 T helper cells resulting in a hyperinflammatory response that causes a multi-organ involvement, including the heart in COVID19 patients [17]. The main mechanisms by which this heightened inflammation causes end-organ damage are mainly endothelial dysfunction, systemic cytokine circulation, and T-cell mediated immunopathology [18].
A recent study also found the activation of NLRP3 inflammasome in moderate and severe COVID19 cases [19]. Neutrophils and C-reactive protein are also significantly higher in severe cases [2, 20]. Similarly, higher levels of proinflammatory cytokines such as IL-6, IL1-B, IL-2, IL8, IL17, G-CSF, GM-CSF, IP10, MCP1, MIP1a, and TNF are seen more frequently in symptomatic COVID19 patients with severe cases as compared to the moderately ill patients [20] [21]. Higher levels of inflammatory markers and cytokines are also associated with poor prognosis in COVID19 [22, 23]. Chemokines such as CXCL10 and CCL2 are found in the bronchoalveolar lavage fluid cells of patients with SARS-CoV-2 infection [24]. Immunohistochemical endomyocardial biopsy (EMB) analysis of COVID19 patients revealed a high number of T-cells, macrophages, lymphocytes, T-memory cells, and cell adhesion molecules (CD54/ICAM-1). It also showed vascular damage and obliteration, leading to myocardial necrosis [25•]. Pericardial biopsy of a COVID19 positive patient showed reactive mesothelial cells, lymphocytes, and macrophages [26]. Postmortem histopathological analysis of the heart in patients who died of COVID19 also showed lymphocytic proliferation in the pericardium mainly composed of CD8+ lymphocytes within the visceral epicardium [27]. Cytokines such as IL1 and TNF-α are well established in the pathogenesis of pericarditis. The elevated levels of these interleukins in the activated inflammatory cascade setting predispose to pericardial inflammation [28]. The current evidence suggests that in addition to other organs, systemic inflammation may also involve the pericardium.
Cardiac tropism of SARS-Cov2
Histopathological analysis in a patient who died from COVID19 multi-inflammatory syndrome showed the infiltration of inflammatory cells in the pericardium. The inflammation was composed primarily of macrophages with a few lymphocytes and neutrophils. Viruses were identified from capillary endothelial cells and endocardial cells. Viral particles were also found in macrophages, neutrophils, and fibroblasts [29]. Tavazzi and colleagues showed SARS-CoV-2 particles in the interstitial macrophages, but not in cardiomyocytes [30]. A post-mortem analysis detected SARS-CoV-2 RNA in the heart samples of 82% of patients who died of COVID19, but no myocardial injury was found [31]. SARS-CoV-2 genome was present in 5% of patients with suspected myocarditis or unexplained heart failure. Myocardium also had inflammatory cells, increased expression of cell adhesion molecules (CD34/ICAM-1), and myocardial necrosis [25•]. The virus was also identified from the pericardial fluid of a patient with COVID19 [32]. Angiotensin-converting enzyme-2 (ACE2) receptors are considered the main portals of entry by which SARS-CoV-2 enters the organs. ACE2 is also present in the heart, endothelium, cardiomyocytes, and epicardial adipose tissue adjacent to the visceral pericardium [33–35]. The presence of the virus in the cardiac endothelial cells indicates that SARS-CoV2 can render direct damage to cardiac structures. The extent and exact pathways by which the virus potentially causes this direct damage to cardiac structures will need further exploration (Fig. 1).
COVID-19 Associated Pericardial Involvement
Pericarditis
The exact incidence of pericarditis in COVID19 patients is unknown, but the risk of pericardial involvement is higher in patients with clinical suspicion of COVID19 than in the general population [36]. Post-mortem studies have identified pericarditis in about 20% of the COVID19 cases [27, 37•]. Cardiac magnetic resonance imaging (CMR) of recently recovered COVID19 patients showed pericardial LGE in 22% of patients [38••]. In athletes recovering from COVID19 disease, 40% had pericardial late gadolinium enhancement (LGE), and 58% had pericardial effusions identified on CMR [39••]. Chest pain was positive in 29% of the healthcare workers infected with SARS-CoV2 at baseline, and they were evaluated for the presence of pericarditis and myocarditis after 10 weeks of viral infection. Only 19% of the participants had persistent chest pain, and 14% of the total participants fulfilled the pericarditis criteria at week 10. Around 37% of these healthcare workers were also diagnosed with myocarditis based on the CMR criteria [40••].
Pericarditis in COVID19 presents with chest pain and viral symptoms [41, 42]. Some pericarditis patients may not have chest pain even with an associated myocarditis [43]. Many acute pericarditis cases also had a large pericardial effusion necessitating pericardial drainage [26, 42, 44–47]. Recently, many case reports have identified acute pericarditis in COVID19 patients without myocardial involvement. A synopsis of studies reporting acute pericarditis secondary to COVID19 disease is described in Table 1.
Table 1.
Studies reporting acute pericarditis in patients with SARS-CoV-2 infection
| Study | Year/country | Age/gender | Presentation | Cardiac enzymes | ECG | TTE | CMR | Other | Management | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Amoozgar et al. [26] | 2020/USA | 56/male | Chest pain and dyspnea | NL | Atrial fibrillation | Large circumferential pericardial effusion | N/A | Cardiomegaly on CXR | Pericardial window + ibuprofen | Resolution |
| Ortiz-Martinez et al. [42] | 2020/Columbia | 25/male | Chest pain and dyspnea | NL | Sinus rhythm | Pericardial effusion | N/A | Pericardial effusion on CT | Ibuprofen and colchicine + morphine/oxycodone | Improvement |
| Asif et al. [44] | 2020/USA | 70/female | Chest pain, dyspnea, myalgia | NL | Diffuse ST elevation and PR depression | Circumferential pericardial effusion/+tamponade physiology | N/A | Cardiomegaly on CXR | Pericardiocentesis and colchicine | Resolution |
| Fox et al. [45] | 2020/USA | 43/male | Chest pain, orthopnea, dyspnea | NL | Diffuse ST elevation and PR depression | Circumferential pericardial effusion/+tamponade physiology | N/A | Cardiomegaly on CXR | Pericardiocentesis and ibuprofen + colchicine | Resolution |
| Walker et al. [46] | 2020/USA | 30/female | Chest pain, fever, cough, shortness of breath | NL | Sinus tachycardia | Moderate pericardial effusion | N/A | Pericardial effusion on CT | Pericardial window + colchicine initially and aspirin added at day 7 | Improvement |
| Blagojevic et al. [47] | 2020/Serbia | 51/male | Chest pain | NL | ST elevation and PR depression | Pericardial effusion and hyperechogenic pericardium | N/A | N/A | Aspirin, beta blocker, lopinavir/ritonavir, ceftrioxone | Resolution |
| Sauer et al. [48] | 2020/France | 51/male | Chest pain, dyspnea | Anterolateral ST-elevation and low QRS voltage | N/A | Circumferential pericardial LGE | Polyserositis on chest CT | Pericardiocentesis + colchicine | Resolution | |
| Kumar et al. [49] | 2020/Ireland | 66/male | Chest pain | NL | ST elevations in most leads and PR depression | Pericardial brightening (thickened?) | Pericardial thickening on CMR | Normal | Colchicine | Resolution |
| Dabbagh et al. [50] | 2020/USA | 57/male | Cough, shortness of breath, left shoulder pain | NL | Non-specific ST changes | Circumferential pericardial effusion/+tamponade physiology | N/A | Cardiomegaly on CXR | Pericardiocentesis and hydroxychlorquine + colchicine + glucocorticoids | Serial ECHO and troponins concerning for Takotsubo cardiomyopathy that resolved eventually |
| Tung-Chen et al. [51] | 2020/Spain | 35/female | Chest pain, cough, malaise, fever, anosmia | NL | T wave inversions | Pericardial effusion | N/A | N/A | Colchicine | Resolution |
| Karadeniz et al. [52] | 2020/Turkey | 33/male | Chest pain | N/A | Non-specific | Circumferential pericardial effusion | N/A | Pericardial effusion on chest CT | Hydroxychlorquine and indomethacin + colchicine. Anakinra added at day 5 | Resolution |
| Raymond et al. [53] | 2020/ USA | 7/female | Chest pain, cough, orthopnea | NL | Sinus tachycardia and low voltage QRS with electrical alternans | Large circumferential pericardial effusion | Enlarged cardiac silhouette with pleural effusions | N/A |
Pericardiocentesis and NSAIDS + colchicine. Pericardiectomy was done eventually |
Worsening symptoms leading to pericardiectomy and no follow-up available after surgery |
| Ashwin et al. [54] | 2020/UK | 63/female | Chest pain | NL | ST segment elevation and PR depression in inferolateral leads | Large pericardial effusion (four days later) | N/A | Pericardial thickening with no pericardial effusion on CT (baseline) | Pericardiocentesis and NSAID + colchicine | Developed pericardial effusion and then complete resolution |
ECG electrocardiogram, TTE transthoracic echocardiogram, CMR cardiac magnetic resonance imaging, CXR chest X-ray, NL normal, YO year old
Pericardial Effusion and Tamponade
Pericardial effusion is found in 5% of patients with coronavirus disease on chest computed tomography (CT) [55]. Pericardial effusion develops as a result of various insults to the myocardium or pericardium and has variable clinical presentations. Pericardial tamponade was identified in COVID19 patients with deteriorating renal and respiratory function [56, 57]. Pericardial tamponade in the setting of acute pericarditis in COVID19 patients is managed with pericardiocentesis, and occasionally a pericardial window is also required (Table 1).
A patient also developed pericardial tamponade after myocardial infarction. Interestingly, SARS-CoV-2 ribonucleic acid (RNA) was found in the pericardial fluid, but patient’s nasopharyngeal analysis was negative for the virus [32]. Pericardial analysis of another patient with COVID19-associated polyserositis also detected SARS-CoV-2 in the pericardial fluid [48]. However, other case reports evaluating pericardial effusions have not found any viral RNA in the pericardial fluid [45, 57, 58].
Myopericarditis
Acute cardiac injury is found in 7%-17% of COVID19 patients, and mortality is higher in the patients with cardiac injury [2, 59, 60]. Concomitant pericardial inflammation in patients with myocardial injury is also reported [29, 61]. COVID19-associated myopericarditis may present with or without respiratory tract symptoms [43, 62]. Chest pain is a common symptom, but some patients do not report chest pain and pose a diagnostic challenge [48, 63, 64]. Myopericarditis can also lead to cardiogenic shock and may require extracorporeal membrane oxygenation support and a left ventricular assist device for cardiac failure [65]. In a patient with myopericarditis associated with COVID19, a life-threatening cardiac tamponade was managed with pericardiocentesis [66]. Another severe case of myopericarditis was treated with intravenous immunoglobulin and methylprednisolone [67].
Diagnosis of Pericardial Involvement in COVID19
The suspicion of pericarditis must be high in patients experiencing symptoms of chest pain or raised inflammatory markers. A chest x-ray (CXR) shows an enlarged cardiac silhouette in patients with moderate to severe pericardial effusion. An electrocardiogram (ECG) may show diffuse ST-segment elevation and PR depressions, especially with concomitant myocardial involvement. However, a normal ECG does not exclude the diagnosis, and an echocardiogram may yield important information such as the presence of pericardial effusion and its hemodynamic impact [68]. Multimodality imaging, including CMR and CT scan, can show the pericardial inflammation and edema in addition to the pericardial effusion [69]. A case series of COVID19-associated pericardial diseases used a multimodality imaging-based approach to diagnose the pericardial conditions accurately [48]. Pulmonary embolism should be in the differential diagnosis of COVID19 due to an increased prothrombotic risk. These patients also develop pleuritic left-sided chest pain.
Several COVID19 patients with pericarditis had typical ECG changes associated with pericardial inflammation. Most of these patients had an enlarged cardiac silhouette on CXR, and echocardiography usually confirmed pericardial effusion (Table 1).
Management of pericardial involvement in COVID19
Anti-Inflammatory Therapies in Pericardial Diseases
The mainstay for the treatment of acute and recurrent pericarditis is NSAIDs and colchicine. Corticosteroids are used in cases of treatment failure, resistance, or contraindications to first-line therapy [68]. IL1 receptor antagonists (anakinra and rilonacept), intravenous immunoglobulins, and azathioprine are recommended in refractory recurrent pericarditis patients [70••, 71••]. Some additional drugs such as azathioprine and intravenous immunoglobulins are also used in selected refractory cases [72, 73].
Safety of Anti-Inflammatory Drugs in COVID19
The safety of NSAIDs in COVID19 patients was questioned after reports of worsening symptoms in a few patients [74]. Ibuprofen is linked to an increased expression of ACE2 receptors, but this does not establish any causative link to the severity of symptoms and warrants further investigation [75]. Observational studies on the use of aspirin in COVID19 patients have shown conflicting results. Chow et al. [76] reported decreased mortality, ICU admission, and mechanical ventilation, but Sahai et al. [77] found no change in mortality and an increased thrombotic risk in COVID19 patients taking aspirin. Current World Health Organization and Food and Drug Administration guidelines do not recommend stopping NSAIDs in symptomatic COVID19 patients [78, 79]. Recurrent pericarditis patients usually taper the anti-inflammatory drugs slowly over several months and are at risk of flare if anti-inflammatory drugs, such as NSAIDs, are stopped abruptly [80]. Colchicine is also safe to use in COVID19 patients and is currently explored as a potential treatment for COVID19. Various randomized trials have established on colchicine’s safety and efficacy in the early phases of COVID19 and show that it may also decrease complications and mortality [81•, 82, 83••]. Corticosteroids are recommended for severe or complicated COVID19 cases or concomitant disease with specific indications for steroids [84]. Moreover, anakinra (an IL1 receptor antagonist) is also used safely in COVID19 patients [70••] [85].
Patients with Established Pericarditis Developing COVID19
Patients with pericarditis (acute or recurrent) developing mild to moderate COVID19 may continue their NSAIDs and colchicine for pericardial inflammation. Recurrent pericarditis patients may also continue to use corticosteroids and anakinra. Continuous monitoring is warranted in all patients taking corticosteroids or anakinra; however, these treatments are also potential therapies for COVID-19 [86].
COVID19 Patients Developing Pericarditis
There is no concrete evidence on the management of COVID19-associated pericarditis. Physicians usually avoid the use of aspirin, but ibuprofen and colchicine are considered safe. Corticosteroids may also be used in COVID19-associated pericarditis, as dexamethasone has also been shown to improve mortality and increased ventilator-free days in COVID19 [87]. There are a few cases of successful use of anakinra in COVID19 pericarditis [52]. In our review of literature, the majority of COVID19-associated acute pericarditis cases were treated with colchicine and NSAIDs. Corticosteroids were also added in complicated cases (Table 1).
Future Directions
The current evidence shows that pericardial involvement is an important clinical manifestation of COVID19, requiring a proper workup and a specific management plan based on the presentation and concomitant organ involvement. Most pericarditis treatments (e.g., colchicine, corticosteroids, and anakinra) are safe and efficacious also for COVID-19. Further studies evaluating the incidence and pathophysiology of pericardial diseases in COVID19 patients will help understand the disease burden and refine appropriate treatment strategies.
Abbreviations
- COVID19
Coronavirus disease 2019
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus-2
- RNA
Ribonucleic acid
- PT
Pericardial tamponade
- IL
Interleukin
- TNF
Tumor necrosis factor
- ACE2
Angiotensin-converting enzyme-2
- ECG
Electrocardiogram
- CMR
Cardiac magnetic resonance
- LGE
Late gadolinium enhancement
- CT
Computed tomography
- NSAID
Non-steroidal anti-inflammatory drug
Declarations
Conflict of Interest
Muhammad M. Furqan and Beni R. Verma and declare that they have no conflict of interest.
Paul C. Cremer reports grants and personal fees from Kiniksa Pharmaceuticals, and grants from Novartis Pharmaceuticals.
Massimo Imazio has been on Advisory Board for Sobi and Kiniksa Pharmaceuticals.
Allan L. Klein receives honorarium from Sobi and Pfizer Pharmaceuticals. Also, he receives a research grant from Kiniksa Pharmaceuticals.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Pericardial Disease
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Muhammad M. Furqan, Email: furqanm@ccf.org
Beni R. Verma, Email: vermab@ccf.org
Paul C. Cremer, Email: cremerp@ccf.org
Allan L. Klein, Email: kleina@ccf.org
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.WHO Coronavirus Disease (COVID-19) Dashboard | WHO Coronavirus Disease (COVID-19) Dashboard. Available at: https://covid19.who.int/. Accessed January 11, 2021.
- 2.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA - J Am Med Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J', Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mawhirt SL, Frankel D, Diaz AM. Cutaneous manifestations in adult patients with COVID-19 and dermatologic conditions related to the COVID-19 pandemic in health care workers. Curr Allergy Asthma Rep. 2020;20:75. doi: 10.1007/s11882-020-00974-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jabri A, Kalra A, Kumar A, Alameh A, Adroja S, Bashir H, Nowacki AS, Shah R, Khubber S, Kanaa’N A, Hedrick DP, Sleik KM, Mehta N, Chung MK, Khot UN, Kapadia SR, Puri R, Reed GW. Incidence of stress cardiomyopathy during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3(7):e2014780. doi: 10.1001/jamanetworkopen.2020.14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou F, Qian Z, Wang Y, Zhao Y, Bai J. Cardiac injury and COVID-19: a systematic review and meta-analysis. CJC Open. 2020;2:386–394. doi: 10.1016/j.cjco.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malaty M, Kayes T, Amarasekera AT, Kodsi M, MacIntyre CR, Tan TC. Incidence and treatment of arrhythmias secondary to coronavirus infection in humans: a systematic review. Eur J Clin Investig. 2020;51:e13428. doi: 10.1111/eci.13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabeça TK, Bellei N. Human coronavirus NL-63 infection in a Brazilian patient suspected of H1N1 2009 influenza infection: Description of a fatal case. J Clin Virol. 2012;53(1):82–84. doi: 10.1016/j.jcv.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furqan MM, Lak HM, Mahalwar G, Abou‐Hassan O, Verma BR, Jellis CL, Klein AL. Recurrent pericarditis associated with human coronavirus (HKU1) infection in a patient with systemic lupus erythematosus (SLE). Echocardiography 2021:echo.15062. Available at: 10.1111/echo.15062. Accessed May 10, 2021. [DOI] [PubMed]
- 10.Al-Abdallat MM, Payne DC, Alqasrawi S, et al. Hospital-associated outbreak of middle east respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis. 2014;59(9):1225–1233. doi: 10.1093/cid/ciu359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holt JP. The normal pericardium. Am J Cardiol. 1970;26(5):455–465. doi: 10.1016/0002-9149(70)90702-2. [DOI] [PubMed] [Google Scholar]
- 12.Cremer PC, Kumar A, Kontzias A, Tan CD, Rodriguez ER, Imazio M, Klein AL. Complicated pericarditis: understanding risk factors and pathophysiology to inform imaging and treatment. J Am Coll Cardiol. 2016;68:2311–2328. doi: 10.1016/j.jacc.2016.07.785. [DOI] [PubMed] [Google Scholar]
- 13.Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41(32):3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76(1):16–32. doi: 10.1128/mmbr.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodrigues TS, de Sá KSG, Ishimoto AY, Becerra A, Oliveira S, Almeida L, Gonçalves AV, Perucello DB, Andrade WA, Castro R, Veras FP, Toller-Kawahisa JE, Nascimento DC, de Lima MHF, Silva CMS, Caetite DB, Martins RB, Castro IA, Pontelli MC, de Barros FC, do Amaral NB, Giannini MC, Bonjorno LP, Lopes MIF, Santana RC, Vilar FC, Auxiliadora-Martins M, Luppino-Assad R, de Almeida SCL, de Oliveira FR, Batah SS, Siyuan L, Benatti MN, Cunha TM, Alves-Filho JC, Cunha FQ, Cunha LD, Frantz FG, Kohlsdorf T, Fabro AT, Arruda E, de Oliveira RDR, Louzada-Junior P, Zamboni DS. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J Exp Med. 2021;218(3):e20201707. doi: 10.1084/jem.20201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang X, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu F, Li L, Da Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng F, Huang Y, Guo Y, Yin M, Chen X, Xiao L, Deng G. Association of inflammatory markers with the severity of COVID-19: A meta-analysis. Int J Infect Dis. 2020;96:467–474. doi: 10.1016/j.ijid.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiong Y, Liu Y, Cao L, Wang D, Guo M, Jiang A, Guo D, Hu W, Yang J, Tang Z, Wu H, Lin Y, Zhang M, Zhang Q, Shi M, Liu Y, Zhou Y, Lan K, Chen Y. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect. 2020;9(1):761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.• Escher F, Pietsch H, Aleshcheva G, et al. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Hear Fail. 2020. 10.1002/ehf2.12805This articles describes the SARS-CoV-2 genome detection in the endomyocardial biopsies in suspected myocarditis or unexplained heart failure. [DOI] [PMC free article] [PubMed]
- 26.Amoozgar B, Kaushal V, Mubashar U, Sen S, Yousaf S, Yotsuya M. Symptomatic pericardial effusion in the setting of asymptomatic COVID-19 infection: a case report. Medicine (Baltimore) 2020;99(37):e22093. doi: 10.1097/MD.0000000000022093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basso C, Leone O, Rizzo S, de Gaspari M, van der Wal AC, Aubry MC, Bois MC, Lin PT, Maleszewski JJ, Stone JR. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020;41:3827–3835. doi: 10.1093/eurheartj/ehaa664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopalco G, Rigante D, Cantarini L, Imazio M, Lopalco A, Emmi G, et al. The autoinflammatory side of recurrent pericarditis: Enlightening the pathogenesis for a more rational treatment. Trends Cardiovasc Med. 2020. 10.1016/j.tcm.2020.04.006. [DOI] [PubMed]
- 29.Dolhnikoff M, Ferreira FJ, de Almeida Monteiro RA, et al. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet Child Adolesc Health. 2020;4642(20):1–5. doi: 10.1016/S2352-4642(20)30257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, Baldanti F, Paolucci S, Pelenghi S, Iotti GA, Mojoli F, Arbustini E. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22(5):911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Remmelink M, De Mendonça R, D’Haene N, et al. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit Care. 2020;24(1):495. doi: 10.1186/s13054-020-03218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farina A, Uccello G, Spreafico M, Bassanelli G, Savonitto S. SARS-CoV-2 detection in the pericardial fluid of a patient with cardiac tamponade. Eur J Intern Med. 2020;76:100–101. doi: 10.1016/j.ejim.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danilczyk U, Penninger JM. Angiotensin-converting enzyme II in the heart and the kidney. Circ Res. 2006;98:463–471. doi: 10.1161/01.RES.0000205761.22353.5f. [DOI] [PubMed] [Google Scholar]
- 34.Tucker NR, Chaffin M, Bedi KC, et al. Myocyte-specific upregulation of ACE2 in cardiovascular disease: Implications for SARS-CoV-2-mediated myocarditis. Circulation. 2020;142:708–710. doi: 10.1161/CIRCULATIONAHA.120.047911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel AB, Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA - J Am Med Assoc. 2020;323:1769–1770. doi: 10.1001/jama.2020.4812. [DOI] [PubMed] [Google Scholar]
- 36.Miró Ò, Llorens P, Jiménez S, Piñera P, Burillo-Putze G, Martín A, Martín-Sánchez FJ, González del Castillo J, Spanish Investigators in Emergency Situations TeAm (SIESTA) network Frequency of five unusual presentations in patients with COVID-19: results of the UMC-19-S1. Epidemiol Infect. 2020;148:e189. doi: 10.1017/S0950268820001910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanley B, Naresh KN, Roufosse C, et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1(6):e245–e253. doi: 10.1016/s2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;2019:1–9. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.•• Brito D, Meester S, Yanamala N, et al. High prevalence of pericardial involvement in college student athletes recovering from COVID-19. JACC Cardiovasc Imaging. 2020. 10.1016/j.jcmg.2020.10.023This paper reports the prevalence of pericardial involvement and imaging findings in athletes. [DOI] [PMC free article] [PubMed]
- 40.•• Eiros R, Barreiro-Perez M, Martin-Garcia A, et al. Pericarditis and myocarditis long after SARS-CoV-2 infection: a cross-sectional descriptive study in health-care workers. medrxiv. n.d.. 10.1101/2020.07.12.20151316Descriptive study assessing the long-term effects of SARS-CoV2 infection on heart. It presents the prevalence of pericarditis, myocarditis, myopericarditis, and perimyocareditis in healthcare workers recovering from COVID19.
- 41.Kumar S, Paz D. Clinical case and assessment databases in periodontology created from virtual clinic rotation. J Dent Educ. 2020:jdd.12293. 10.1002/jdd.12293. [DOI] [PubMed]
- 42.Ortiz-Martínez Y, Cabeza-Ruiz LD, Vásquez-Lozano SH, Villamil-Gómez WE, Rodriguez-Morales AJ. Pericarditis in a young internal medicine resident with COVID-19 in Colombia. Travel Med Infect Dis. 2020;37:101863. doi: 10.1016/j.tmaid.2020.101863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Purohit R, Kanwal A, Pandit A, et al. Acute myopericarditis with pericardial effusion and cardiac tamponade in a patient with COVID-19. Am J Case Rep. 2020;21:1–4. doi: 10.12659/AJCR.925554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asif T, Kassab K, Iskander F, Alyousef T. Acute pericarditis and cardiac tamponade in a patient with COVID-19: a therapeutic challenge. Eur J Case Reports Intern Med. 2020;7(6):001701. doi: 10.12890/2020_001701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fox K, Prokup JA, Butson K, Jordan K. Acute effusive pericarditis: a late complication of COVID-19. Cureus. 2020;12(7):e9074. doi: 10.7759/cureus.9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker C, Peyko V, Farrell C, Awad-Spirtos J, Adamo M, Scrocco J. Pericardial effusion and cardiac tamponade requiring pericardial window in an otherwise healthy 30-year-old patient with COVID-19: a case report. J Med Case Rep. 2020;14(1):158. doi: 10.1186/s13256-020-02467-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blagojevic NR, Bosnjakovic D, Vukomanovic V, Arsenovic S, Lazic JS, Tadic M. Acute pericarditis and severe acute respiratory syndrome coronavirus 2: case report. Int J Infect Dis. 2020;101:180–182. doi: 10.1016/j.ijid.2020.09.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sauer F, Dagrenat C, Couppie P, Jochum G, Leddet P. Pericardial effusion in patients with COVID-19: case series. Eur Hear J - Case Rep. 2020;4(FI1):1–7. doi: 10.1093/ehjcr/ytaa287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar R, Kumar J, Daly C, Edroos SA. Acute pericarditis as a primary presentation of COVID-19. BMJ Case Rep. 2020;13(8):1–3. doi: 10.1136/bcr-2020-237617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dabbagh MF, Aurora L, D’Souza P, Weinmann AJ, Bhargava P, Basir MB. Cardiac tamponade secondary to COVID-19. JACC Case Rep. 2020;2(9):1326–1330. doi: 10.1016/j.jaccas.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tung-Chen Y. Acute pericarditis due to COVID-19 infection: an underdiagnosed disease? Med Clin (Barc) 2020;155:44–45. doi: 10.1016/j.medcli.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karadeniz H, Yamak BA, Özger HS, Sezenöz B, Tufan A, Emmi G. Anakinra for the treatment of COVID-19-associated pericarditis: a case report. Cardiovasc Drugs Ther. 2020;34:883–885. doi: 10.1007/s10557-020-07044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raymond TT, Das A, Manzuri S, Ehrett S, Guleserian K, Brenes J. Pediatric COVID-19 and pericarditis presenting with acute pericardial tamponade. World J Pediatr Congenit Heart Surg. 2020;11(6):802–804. doi: 10.1177/2150135120949455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reddy A, Nethercott S, Duehmke R, Nair S, Abdul-Samad O. ‘Dry’ pericarditis with rapid progression to tamponade as a feature of COVID-19. Eur Med J. 2021. 10.33590/emj/20-00244.
- 55.Bao C, Liu X, Zhang H, Li Y, Liu J. Coronavirus disease 2019 (COVID-19) CT findings: a systematic review and meta-analysis. J Am Coll Radiol. 2020;17(6):701–709. doi: 10.1016/j.jacr.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Naderi N, Ansari Ramandi MM, Baay M, et al. Cardiovascular patients in COVID-19 era, a case series, an experience from a tertiary cardiovascular center in Tehran, Iran. Clin Case Rep. 2020;(May):1–7. 10.1002/ccr3.3163. [DOI] [PMC free article] [PubMed]
- 57.Derveni V, Kaniaris E, Toumpanakis D, Potamianou E, Ioannidou I, Theodoulou D, Kyriakoudi A, Kyriakopoulou M, Pontikis K, Daganou M. Acute life-threatening cardiac tamponade in a mechanically ventilated patient with COVID-19 pneumonia. IDCases. 2020;21:e00898. doi: 10.1016/j.idcr.2020.e00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allam HH, Kinsara AJ, Tuaima T, Alfakih S. Pericardial fluid in a COVID-19 patient: is it exudate or transudate? Eur J Case Reports Intern Med. 2020;7(6):001703. doi: 10.12890/2020_001703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shah ZS, Kumar SA, Patel AA. Myocarditis and pericarditis in patients with COVID-19. Hear Views. 2020;21(3):209. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_154_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R, Adamo M, Ammirati E, Sinagra G, Lombardi CM, Metra M. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2019;2020(7):819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cizgici AY, Zencirkiran AH, Yildiz M. COVID-19 myopericarditis: IT should be kept in mind in today’s conditions. Am J Emerg Med. 2020;38(7):1547.e5–1547.e6. doi: 10.1016/j.ajem.2020.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Naneishvili T, Khalil A, O’Leary R, Prasad N. Fulminant myocarditis as an early presentation of SARS-CoV-2. BMJ Case Rep. 2020;13(9):e237553. doi: 10.1136/bcr-2020-237553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Newton-Cheh C, Zlotoff DA, Hung J, Rupasov A, Crowley JC, Funamoto M. Case 24-2020: A 44-year-old woman with chest pain, dyspnea, and shock. N Engl J Med. 2020;383(5):475–484. doi: 10.1056/NEJMcpc2004975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hua A, O’Gallagher K, Sado D, Byrne J. Life-threatening cardiac tamponade complicating myo-pericarditis in COVID-19. Eur Heart J. 2020;41(22):2130. doi: 10.1093/eurheartj/ehaa253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li A, Garcia-Bengochea Y, Stechel R, Azari BM. Management of COVID-19 myopericarditis with reversal of cardiac dysfunction after blunting of cytokine storm: a case report. Eur Hear J - Case Rep. 2020;4(FI1):1–6. doi: 10.1093/ehjcr/ytaa224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, Brucato A, Gueret P, Klingel K, Lionis C, Maisch B, Mayosi B, Pavie A, Ristić AD, Sabaté Tenas M, Seferovic P, Swedberg K, Tomkowski W, ESC Scientific Document Group. Achenbach S, Agewall S, al-Attar N, Angel Ferrer J, Arad M, Asteggiano R, Bueno H, Caforio ALP, Carerj S, Ceconi C, Evangelista A, Flachskampf F, Giannakoulas G, Gielen S, Habib G, Kolh P, Lambrinou E, Lancellotti P, Lazaros G, Linhart A, Meurin P, Nieman K, Piepoli MF, Price S, Roos-Hesselink J, Roubille F, Ruschitzka F, Sagristà Sauleda J, Sousa-Uva M, Uwe Voigt J, Luis Zamorano J, Zamorano JL, Aboyans V, Achenbach S, Agewall S, Badimon L, Barón-Esquivias G, Baumgartner H, Bax JJ, Bueno H, Carerj S, Dean V, Erol Ç, Fitzimons D, Gaemperli O, Kirchhof P, Kolh P, Lancellotti P, Lip GYH, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Roffi M, Torbicki A, Vaz Carneiro A, Windecker S, Shuka N, Sisakian H, Mascherbauer J, Isayev E, Shumavets V, van Camp G, Gatzov P, Hanzevacki JS, Moustra HH, Linhart A, Møller JE, Aboleineen MW, Põder P, Lehtonen J, Antov S, Damy T, Schieffer B, Dimitriadis K, Kiss RG, Rafnsson A, Arad M, Novo S, Mirrakhimov E, Stradinš P, Kavoliuniene A, Codreanu A, Dingli P, Vataman E, el Hattaoui M, Samstad SO, Hoffman P, Lopes LR, Dimulescu DR, Arutyunov GP, Pavlovic M, Dúbrava J, Sauleda JS, Andersson B, Müller H, Bouma BJ, Abaci A, Archbold A, Nesukay E. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases. Eur Heart J. 2015;36(42):2921–2964. doi: 10.1093/eurheartj/ehv318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chetrit M, Xu B, Verma BR, Klein AL. Multimodality imaging for the assessment of pericardial diseases. Curr Cardiol Rep. 2019;21:41. doi: 10.1007/s11886-019-1115-y. [DOI] [PubMed] [Google Scholar]
- 70.Brucato A, Imazio M, Gattorno M, et al. Effect of anakinra on recurrent pericarditis among patients with colchicine resistance and corticosteroid dependence. JAMA. 2016;316(18):1906. doi: 10.1001/jama.2016.15826. [DOI] [PubMed] [Google Scholar]
- 71.Klein A, Imazio M, Brucato A, et al. RHAPSODY: Rationale for and design of a pivotal phase 3 trial to assess efficacy and safety of rilonacept, an IL-1α and IL-1β trap, in patients with recurrent pericarditis. Am Heart J. 2020;228:81–90. doi: 10.1016/j.ahj.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 72.Imazio M, Lazaros G, Picardi E, Vasileiou P, Carraro M, Tousoulis D, Belli R, Gaita F. Intravenous human immunoglobulins for refractory recurrent pericarditis. J Cardiovasc Med. 2016;17(4):263–269. doi: 10.2459/JCM.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 73.Vianello F, Cinetto F, Cavraro M, Battisti A, Castelli M, Imbergamo S, Marcolongo R. Azathioprine in isolated recurrent pericarditis: a single centre experience. Int J Cardiol. 2011;147(3):477–478. doi: 10.1016/j.ijcard.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 74.Day M. Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020;368:m1086. doi: 10.1136/bmj.m1086. [DOI] [PubMed] [Google Scholar]
- 75.Sodhi M, Etminan M. Safety of ibuprofen in patients with COVID-19: causal or confounded? Chest. 2020;158:55–56. doi: 10.1016/j.chest.2020.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chow JH, Khanna AK., Kethireddy S., et al. Aspirin use is associated with decreased mechanical ventilation, ICU admission, and in-hospital mortality in hospitalized patients with COVID-19. vol. Publish Ah. 2020. [DOI] [PubMed]
- 77.Sahai A, Bhandari R, Koupenova M, et al. SARS-CoV-2 Receptors are expressed on human platelets and the effect of aspirin on clinical outcomes in COVID-19 patients. Res Sq. n.d.. 10.21203/RS.3.RS-119031/V1.
- 78.The use of non-steroidal anti-inflammatory drugs (NSAIDs) in patients with COVID-19. Available at: https://www.who.int/news-room/commentaries/detail/the-use-of-non-steroidal-anti-inflammatory-drugs-(nsaids)-in-patients-with-covid-19. Accessed October 26, 2020.
- 79.FDA advises patients on use of non-steroidal anti-inflammatory drugs (NSAIDs) for COVID-19 | FDA. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-advises-patients-use-non-steroidal-anti-inflammatory-drugs-nsaids-covid-19. Accessed October 26, 2020.
- 80.Lazaros G, Imazio M, Brucato A, Tousoulis D. Untying the Gordian knot of pericardial diseases: a pragmatic approach. Hell J Cardiol. 2016;57(5):315–322. doi: 10.1016/j.hjc.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 81.• Lopes MI, Bonjorno LP, Giannini MC, et al. Beneficial effects of colchicine for moderate to severe COVID-19: an interim analysis of a randomized, double-blinded, placebo controlled clinical trial. MedRxiv. 2020:2020.08.06.20169573. 10.1101/2020.08.06.20169573This interim report from a randomized control trial evaluates the beneficial role of colchicine in COVID19 patients.
- 82.Dalili N, Dalili N, Kashefizadeh A, et al. Adding colchicine to the antiretroviral medication-lopinavir/ritonavir (Kaletra) in hospitalized patients with non-severe Covid-19 pneumonia: a structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21:489. doi: 10.1186/s13063-020-04455-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deftereos SG, Giannopoulos G, Vrachatis DA, et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial. JAMA Netw Open. 2020;3(6):e2013136. doi: 10.1001/jamanetworkopen.2020.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lamontagne F, Agoritsas T, MacDonald H, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. doi: 10.1136/bmj.m3379. [DOI] [PubMed] [Google Scholar]
- 85.Huet T, Beaussier H, Voisin O, Jouveshomme S, Dauriat G, Lazareth I, Sacco E, Naccache JM, Bézie Y, Laplanche S, le Berre A, le Pavec J, Salmeron S, Emmerich J, Mourad JJ, Chatellier G, Hayem G. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(7):e393–e400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Imazio M, Brucato A, Lazaros G, Andreis A, Scarsi M, Klein A, de Ferrari GM, Adler Y. Anti-inflammatory therapies for pericardial diseases in the COVID-19 pandemic: safety and potentiality. J Cardiovasc Med. 2020;21(9):625–629. doi: 10.2459/JCM.0000000000001059. [DOI] [PubMed] [Google Scholar]
- 87.Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, Avezum A, Lopes RD, Bueno FR, Silva MVAO, Baldassare FP, Costa ELV, Moura RAB, Honorato MO, Costa AN, Damiani LP, Lisboa T, Kawano-Dourado L, Zampieri FG, Olivato GB, Righy C, Amendola CP, Roepke RML, Freitas DHM, Forte DN, Freitas FGR, Fernandes CCF, Melro LMG, Junior GFS, Morais DC, Zung S, Machado FR, Azevedo LCP, COALITION COVID-19 Brazil III Investigators Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA - J Am Med Assoc. 2020;324(13):1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]