Abstract
Background:
Early studies have reported various electrolyte abnormalities at admission in patients who progress to the severe form of coronavirus disease 2019 (COVID-19). As electrolyte imbalance may not only impact patient care, but provide insight into the pathophysiology of COVID-19, we aimed to analyze all early data reported on electrolytes in COVID-19 patients with and without severe form.
Methods:
An electronic search of Medline (PubMed interface), Scopus, and Web of Science was performed for articles comparing electrolytes (sodium, potassium, chloride, and calcium) between COVID-19 patients with and without severe disease. A pooled analysis was performed to estimate the weighted mean difference (WMD) with 95% confidence interval.
Results:
Five studies with a total sample size of 1415 COVID-19 patients. Sodium was significantly lower in patients with severe COVID-19 (WMD: −0.91 mmol/L [95% CI: −1.33 to −0.50 mmol/L]). Similarly, potassium was also significantly lower in COVID-19 patients with severe disease (WMD: −0.12 mmol/L [95%CI: −0.18 to −0.07 mmol/L], I2=33%). For chloride, no statistical differences were observed between patients with severe and non-severe COVID-19 (WMD: 0.30 mmol/L [95%CI: −0.41 to 1.01 mmol/L]). For calcium, a statistically significant lower concentration was noted in patients with severe COVID-19 (WMD: −0.20 mmol/L [95% CI: −0.25 to −0.20 mmol/L]).
Conclusions:
This pooled analysis confirms that COVID-19 severity is associated with lower serum concentrations of sodium, potassium, and calcium. We recommend electrolytes be measured at initial presentation and serially monitored during hospitalization in order to establish timely and appropriate corrective actions.
Keywords: sodium, potassium, chloride, calcium, coronavirus, SARS-CoV-2, COVID-19
Introduction
Initial case reports and cohort studies have described many clinical characteristics of patients with coronavirus disease 2019 (COVID-19), an emerging infectious disorder caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In early COVID-19 studies, some evidence has been provided that electrolyte disorders may also be present upon patients’ presentation, including sodium, potassium, chloride and calcium abnormalities (1,2). Others have postulated that patients with more severe COVID-19 tend to display a higher proportion of hypokalemia at baseline compared to those with less severe forms of disease (3). Such electrolyte disturbances have important implications not only for patient management, but also for identifying potential pathophysiologic mechanisms underlying COVID-19, that could drive novel therapeutic opportunities. Nevertheless, limited sample sizes and heterogeneity in electrolyte reporting have limited interpretations to-date. Therefore, we aimed to provide a pooled analysis comparing sodium, potassium, chloride and calcium serum concentration between patients with severe disease versus those with less severe disease, to enhance our current understanding of electrolyte imbalance in COVID-19.
Methods
A search of Medline (PubMed interface), Scopus, and Web of Science was performed, employing the keywords “sodium” OR “potassium” OR “chloride” OR “calcium” AND “coronavirus 2019” OR “COVID-19” OR “2019-nCoV” OR “SARS-CoV-2” in all fields, from December 1, 2019 and through March 23, 2020. No language or date restrictions were applied. Identified studies were screened by title, abstract, and full text. Articles reporting mean (and standard deviation) or median (and interquartile range) data on serum electrolytes in laboratory-confirmed adult COVID-19-positive patients (>18 years of age), with or without severe illness were included in a pooled analysis. A clinically valid definition of “severe disease” (i.e., patients requiring mechanical ventilation, vital life support, intensive care unit admission) in accordance with the "New Coronavirus Infected Pneumonia Diagnosis and Treatment Program (Trial Sixth Edition)" issued by the National Health Commission of the People's Republic of China was needed for inclusion (4). No other specific eligibility criteria were applied. The reference list of all identified studies was also hand-searched (through forward and backward citation tracking), for detecting additional eligible studies. As the acquired data set was limited, no study risk of bias or publication bias evaluation was performed. When not available, mean and standard deviation of a given electrolytes were extrapolated from sample size, median and interquartile range (IQR), according to Hozo et al (5).
A pooled analysis was performed to estimate the weighted mean difference (WMD) with 95% confidence interval for each electrolyte (sodium, potassium, chloride and calcium) in laboratory-confirmed COVID-19 patients with or without (i.e., versus) severe disease. Statistical analysis was performed using MetaXL, software Version 5.3 (EpiGear International Pty Ltd., Sunrise Beach, Australia) employing an inverse variance model. When heterogeneity was high (I2>50%), a leave-one-out sensitivity analysis or analysis excluding studies with unclear lab time point measure was performed. This study was performed in compliance with the declaration of Helsinki and local legislation, without need for ethics committee approval.
Results
Following removal of duplicates, a total of 36 articles were initially identified. Among these, 33 were excluded because they were review articles (n=13), did not report data on COVID-19 disease (n=11), did not provide extractable data on electrolytes (n=7), or were editorials (n=2). Two additional studies were identified from hand-search of reference lists. Thus, the final pooled analysis included 5 studies, with a total sample size of 1415 COVID-19 patients, 244 (17.2%) with the severe form (1,2,6–8). Three studies reported laboratory data at hospital admission, whilst in the remaining two studies the timing of blood collection was not clearly defined (6,8). All 5 studies reported data on sodium and potassium, whereas 3 studies (n=1239) reported data on chloride (1,6,8), and only 2 studies (n=140) reported data on calcium (6,8).
The results of the pooled analysis are presented in Figure 1. Sodium was significantly lower in patients with severe COVID-19 compared to those with non-severe disease (WMD: −0.91 mmol/L [95% CI: −1.33 to −0.50 mmol/L], I2=77%). A leave-one-out sensitivity analysis found no statistically significant difference when the largest study of Guan et al. (1) was excluded (WMD: −0.50 mmol/L [95% CI: −0.98 to −0.02 mmol/L], nor when studies with unclear time points were excluded (WMD: −0.85 mmol/L [95% CI: −1.28 to −0.43 mmol/L]. Potassium was also significantly lower in COVID-19 patients with severe disease compared to those without severe disease, with substantially less heterogeneity than observed for sodium (WMD: −0.12 mmol/L [95% CI: −0.18 to −0.07 mmol/L], I2=33%). No heterogeneity was observed for chloride, with no significant difference between severe and non-severe patients (WMD: 0.30 mmol/L [95% CI: −0.41 to 1.01 mmol/L], I2=0%). For calcium, a statistically significant lower concentration was noted in patients with severe COVID-19 compared to those with less-severe disease, with no observed heterogeneity between studies (WMD: −0.20 mmol/L [95% CI: −0.25 to −0.20 mmol/L], I2=0%).
Figure 1.
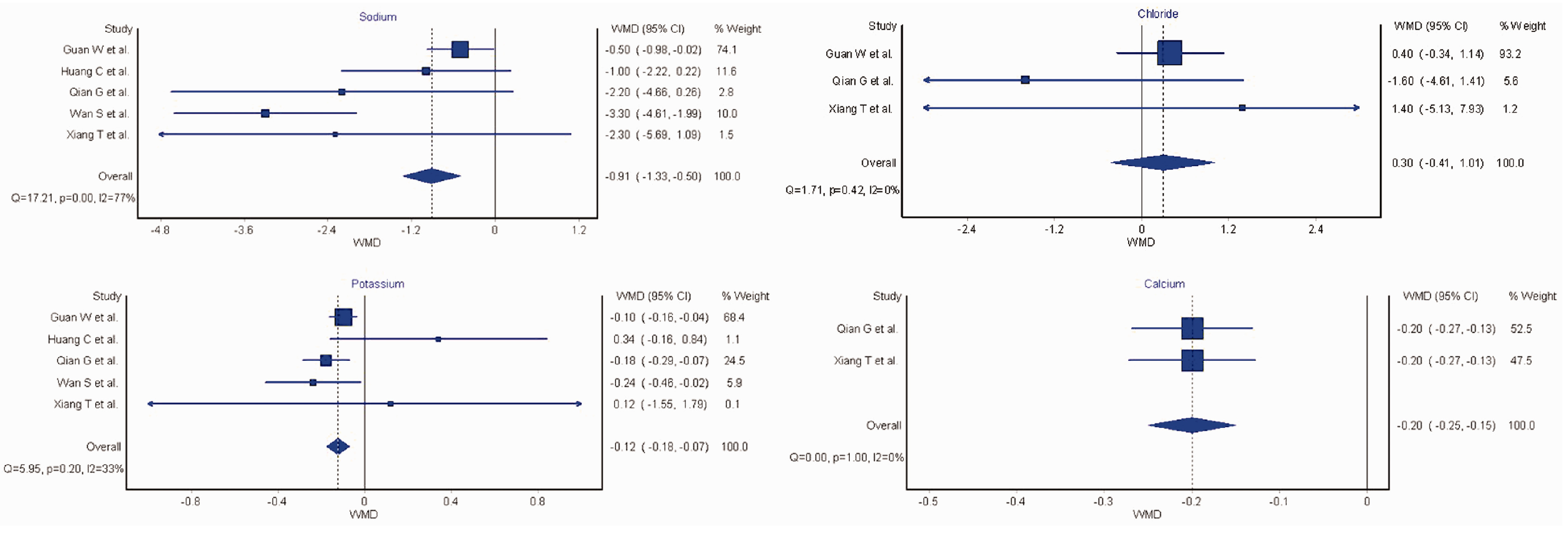
Forest plots comparing sodium, potassium, chloride and calcium concentrations between coronavirus disease 2019 (COVID-19) patients with or without severe disease. Individual weighted mean differences indicated by squares and 95% CI. Dashed vertical lines and diamonds indicate weighted mean difference, with diamonds further including the 95% CI. WMD: weighted mean difference.
Discussion
In summary, this pooled analysis confirms that COVID-19 severity is associated with lower serum concentrations of sodium, potassium, and calcium. However, it is not yet known if there is a higher risk of hyponatremia, hypokalemia, or hypocalcemia associated with COVID-19, and additional clinical information including fluid status and serum albumin and ionized calcium concentrations would be necessary to interpret these abnormalities. These disturbances, in particular hypokalemia, may have clinically significant implications for patient management, and potentially contribute to unraveling pathogenetic mechanisms underlying COVID-19. Hypokalemia is known to exacerbate acute respiratory distress syndrome (ARDS) and acute cardiac injury, which are common complications in COVID-19, especially in patients with underlying lung or heart disease. Hypokalemia also provides a pathophysiologic clue; SARS-CoV-2 binds to its host receptor, angiotensin-converting enzyme 2 (ACE2), and likely reduces ACE2 expression, thus leading to increased angiotensin II, which can cause increased potassium excretion by the kidneys, ultimately leading to hypokalemia (3,9). Increased plasma angiotensin II concentration has been described in patients with COVID-19, possibly acting as mediator of acute lung injury, as earlier confirmed in SARS-CoV animal models (9,10). A second potential contributor to hypokalemia and other electrolyte imbalance in some COVID-19 patients may be gastrointestinal losses, with diarrhea and nausea present in as many as 34.0% and 3.9% of cases, respectively (11). Further research, based on larger prospective cohort studies, is necessary to confirm the findings presented in this study and to establish their clinical significance. In the meanwhile, we suggest that it may be advisable to assess the electrolyte status upon patient presentation and to serial monitor electrolyte disturbances throughout the course of illness, in order to establish timely and appropriate corrective actions.
Funding:
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors
Footnotes
Conflict of interest: None
Ethical approval: Not applicable
Guarantor: BMH
References
- 1.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020. February 28;382: 1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020. February 15;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen D, Li X, Song Q, Hu C, Su F, Dai J. Hypokalemia and Clinical Implications in Patients with Coronavirus Disease 2019 (COVID-19). medRxiv. 2020. February 29;2020.02.27.20028530.
- 4.National Health Board of Health People’s Republic of China. Novel coronavirus infection pneumonia treatment program (Trial Sixth Edition) [Internet]. Medical Administration and Hospital Authority; 2020. Available from: http://www.nhc.gov.cn/yzygj/s7652m/202002/54e1ad5c2aac45c19eb541799bf637e9.shtml
- 5.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology. 2005. April 20;5(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qian G-Q, Yang N-B, Ding F, Ma AHY, Wang Z-Y, Shen Y-F, et al. Epidemiologic and Clinical Characteristics of 91 Hospitalized Patients with COVID-19 in Zhejiang, China: A retrospective, multi-centre case series. QJM. 2020. March 17; DOI: 10.1093/qjmed/hcaa089 [DOI] [PMC free article] [PubMed]
- 7.Wan S, Xiang Y, Fang W, Zheng Y, Li B, Hu Y, et al. Clinical Features and Treatment of COVID-19 Patients in Northeast Chongqing. J Med Virol. 2020. March 21; DOI: 10.1002/jmv.25783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiang T, Liu J, Xu F, Cheng N, Liu Y, Qian K. [Analysis of clinical features of 49 patients with new type of coronavirus pneumonia in Jiangxi]. Chinese Journal of Respiratory and Critical Care. 2020;19: 154–160 [Google Scholar]
- 9.Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020. March 1;63(3):364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005. August;11(8):875–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan L, Mu M, Yang P, Sun Y, Yan J, Li P, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. American Journal of Gastroenterology. 2020. 115: 766–773 [DOI] [PMC free article] [PubMed] [Google Scholar]


