Abstract
BACKGROUND
Breast cancer is the most common cause of the majority of cancer-related deaths in women, among which triple-negative breast cancer is the most aggressive type of breast cancer diagnosed with limited treatment options. Thymoquinone (TQ), the main bioactive constituent of Nigella sativa, has been extensively studied as a potent anticancer molecule against various types of cancers. Honeybee products such as the royal jelly (RJ), the nutritive secretion fed to honeybee queens, exhibit a variety of biological activities besides its anticancer effect. However, the anticancer activity of the combination of TQ and RJ against breast cancer is still unknown.
AIM
To investigate cytotoxicity of RJ in FHs 74 Int cells and the anticancer effects of TQ, RJ, and their combinations in the MDA-MB-231 cell line.
METHODS
Cells were treated with TQ, RJ, and their combinations for 24 h. Using 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, we determined the half-maximal inhibitory concentration of TQ. Trypan blue and 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays were then performed to assess the cell viability in response to different treatment conditions. Cell death and cycle regulation were investigated using propidium iodide deoxyribonucleic acid staining followed by flow cytometry in response to a single dose of TQ, RJ, and their combination. Immunostaining for cleaved caspase 3 and Ki67 expression was used to determine apoptosis induction and changes in cell proliferation.
RESULTS
TQ alone inhibited cell viability in a dose-dependent manner at concentrations below and above the half-maximal inhibitory concentration. RJ exhibited relatively nontoxic effects against MDA-MB-231 cells and FHs 74 Int small intestinal cells at concentrations below 5 µg/mL. High doses of RJ (200 µg/mL) had greater toxicity against MDA-MB-231 cells. Interestingly, the inhibition of cell viability was most pronounced in response to 15 µmol/L TQ and 5 µg/mL RJ. A dose of 15 µmol/L TQ caused a significant increase in the PreG1 population, while a more pronounced effect on cell viability inhibition and PreG1 increase was observed in response to TQ and RJ combinations. TQ was the main inducer of caspase 3-dependent apoptosis when applied alone and in combination with RJ. In contrast, no significant regulation of Ki67 expression was observed, indicating that the decrease in cell viability was due to apoptosis induction rather than to inhibition of cell proliferation.
CONCLUSION
This study is the first to report enhanced anticancer effects of TQ and RJ combination against MDA-MB-231 breast cancer cells, which could confer an advantage for cancer therapy.
Keywords: Anticancer activity, Breast cancer cells, Drug combination, Natural products, Royal jelly, Thymoquinone
Core Tip: Royal jelly enhances thymoquinone (TQ) anticancer activity against breast cancer. TQ induces the apoptotic response in breast cancer cells while royal jelly when combined with TQ potentiates the reduction in cell viability more than each drug alone.
INTRODUCTION
According to the global cancer project GLOBOCAN 2020, 19.3 million new cancer cases and 10 million cancer deaths have occurred in 2020[1]. The World Health Organization predicted that the global burden of cancer would double to about 29-37 million new cancer cases by 2040. Their report estimated the trend of future cancer cases by which breast cancer will account for 2778850 cases in 2040[2]. Breast cancer is the most common cancer among women worldwide[3] and is responsible for the majority of cancer-related deaths among women[4]. Invasive ductal carcinoma (IDC) is the most frequent type of breast tumor, followed by invasive lobular carcinoma (ILC). Together they make up 90% of breast cancers, while the remaining 10% is caused by particular types of none-ILC/none-IDC tumors[5-7]. Triple-negative breast cancer is an aggressive type of breast cancer with limited treatment options[8,9].
The human breast cancer cell line MDA-MB-231, a highly invasive and poorly differentiated triple-negative breast cancer (TNBC), was employed in our study. Being one of the most commonly used breast cancer cell lines in medical research, MDA-MB-231 derives from pleural effusion in metastatic mammary adenocarcinomas[10]. The absence of estrogen receptor, progesterone receptor, and human epidermal receptor 2 expression renders them nonresponsive to hormonal treatments[11]. However, these cells possess high invasive capacity and metastatic potential as they degrade the extracellular matrix of tissues and metastasize into lung, bone, or brain-specific cancers[12-14]. Conventional treatment of breast cancer comprises surgical procedures, radiotherapy, chemotherapy, endocrine (hormonal) therapy, and targeted and immunotherapies[15]. Despite the therapeutic impact of conventional treatments, they exert numerous side effects. Thus, extensive research has been conducted on alternative treatments utilizing plant-derived natural products with relatively non-toxic effects and high therapeutic potential.
Thymoquinone (TQ), the main bioactive constituent of Nigella sativa L. (Ranunculaceae), modulates the hallmarks of cancer[16] in addition to its cytoprotective[17,18], immunomodulatory, anti-oxidant, and anti-inflammatory activities[19,20]. Previous studies reported that TQ alone and in combination with natural and chemical agents act to inhibit breast cancer[21-23]. For instance, TQ in combination with piperine, lowered vascular endothelial growth factor expression, enhanced serum interferon-γ levels and apoptosis induction, and shifted the immune response toward T helper1 responses against EMT6 epithelial breast cancer[24]. TQ was shown to induce apoptosis when used alone or combined with amoxifen and suppressed the growth, viability, and invasion of breast cancer cell lines[25,26] through the regulation of the Akt signaling pathway[27].
Royal jelly (RJ), the nutritive secretion secreted from the mandibular and hypo-pharyngeal glands of worker bees Apis mellifera (Hymenoptera, Apidae), is the only food of the queen bee at larval and adult life and is responsible for fertility and prolonged life span[28,29]. Similar to TQ, RJ exerts various biological activities, including wound healing[30], anti-oxidant[26,31], immunomodulatory, and anti-inflammatory[32] activities, and anti-hypercholesterolemic[33,34], anti-hypertensive[35], anti-aging[36,37], and anticancer activities[38,39]. RJ was found to inhibit the proliferation of estradiol-induced cell proliferation of MCF-7 breast cancer cells[38] and reduce the volume of the 4T1 breast mammary tumor[39]. RJ also inhibited the proliferation of human colorectal adenocarcinoma cells[40], neuroblastoma[41], and vascular endothelial growth factor-induced migration, proliferation, and tube formation in human umbilical vein endothelial cells[42].
The poor prognosis of TNBC and its ability to resist chemotherapy and metastasize[43,44] made combination therapy a necessary option. TQ was successfully combined with several agents to enhance its anticancer therapeutic efficacy; however, TQ anticancer activity was not tested in combination with RJ. In a previous study, we showed that TQ exerted a dose-dependent antitumor effect against a panel of human colon cancer cell lines with minimal cytotoxicity against FHs 74 Int non-tumorigenic human intestinal cells. Here, to assess the cytotoxic effects of RJ, FHs 74 Int intestinal cell line was used as a model of non-tumorigenic epithelial cells. In our study, we investigated the anticancer activity of TQ and RJ combination in vitro against the MDA-MB-231 human TNBC cell line. Significant inhibitory effect of TQ and RJ combination was revealed by the enhanced cell death effects in MDA-MB-231 cell line. TQ was the main inducer of apoptosis mediating cell death mechanisms by inducing caspase 3 dependent apoptosis in a dose-dependent manner.
MATERIALS AND METHODS
Materials
MDA-MB-231 human breast cancer and FHs 74 Int human small intestinal cell lines were purchased from ATCC (Manassas, VA, United States). Dulbecco’s Modified Eagle Medium (DMEM) and DMEM-F12 cell culture media were purchased from Lonza (Verviers, Belgium). TQ, trypsin-ethylenediamine tetraacetic acid, Dulbecco’s phosphate-buffered saline (PBS), horse serum, fetal bovine serum (FBS), penicillin-streptomycin, dimethyl sulfoxide (DMSO), 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), trypan blue and methanol were purchased from Sigma-Aldrich (St. Louis, MO, United States). Insulin used for FHs 74 Int cell line culture (Actrapid 100 IU/mL) was purchased from the pharmacy at the American University of Beirut Medical Center. 4′,6-diamidino-2-phenylindole stain was purchased from Abcam (Cambridge, United Kingdom). Rabbit caspase 3 polyclonal antibody (9662) was purchased from Cell Signaling Technology (Danvers, MA, United States). Goat anti-rabbit polyclonal secondary antibody, Alexa Fluor 568 (A11011) was purchased from Invitrogen, Thermo Fisher Scientific (Carlsbad, CA, United States). Rabbit Ki67 monoclonal primary antibody (Cell Marque 275R-15) and donkey anti-rabbit Cy3 secondary antibody (Jackson 711-165-152) were provided by Dr. Noel Ghanem, Professor of Biology, American University of Beirut. Crude RJ was purchased from the bee farm at Rashaya al-Wadi, Lebanon, located at 1200-1600 m above sea level. A variety of seasonal plants predominate at this altitude and contribute to the diet of bees, among which are Brassicaceae (Nasturtium), Anacardiaceae (Rhus), Ulmaceae (Ulmus), Rosaceae (Rosa), and Apiaceae (Eryngium). RJ was collected during the summer season of 2018-19 and stored at -20 ºC.
Cell culture conditions
MDA-MB-231 was used as the model of an aggressive breast cancer cell line, while FHs 74 Int cell line was used as the model of a non-tumorigenic epithelial cell line. Both cell lines were cultured in their respective media in two-dimensional monolayer conditions. MDA-MB-231 cells were grown in DMEM cell culture media supplemented with 10% heat-inactivated FBS, 1% penicillin/streptomycin (penicillin-streptomycin with penicillin at 10000 units and streptomycin at 10 mg/mL), and 10 μg/mL insulin was added to grow FHs 74 Int cells. All cells were maintained in a humidified atmosphere of 5% CO2 at 37 ºC.
Dissolution of RJ
Fresh RJ was supplied to our lab in the form of a solid extract. Knowing that RJ contains both polar and non-polar compounds, we considered mixing the crude RJ with DMSO, a polar aprotic solvent that is miscible with dH2O and that is capable of dissolving hydrophilic and hydrophobic compounds[45,46]. Different proportions of dH2O to DMSO were used to determine the ratio that produces the best solubility. Complete solubility was only obtained upon dissolving 20 mg of RJ in a solution of 800 μL dH2O and 200 μL DMSO solution at 37 ºC for 30 min along with vortexing every 10 min. Therefore, this protocol was used to prepare fresh RJ stocks prior to every treatment. Fresh RJ stock was then used to prepare dilutions needed for experiments.
Drug preparation and treatment
Directly before use, fresh stock of the purified synthetic compound TQ of 0.1 mol/L concentration was prepared by dissolving 16.4 mg of TQ crystals in 1 mL methanol. TQ stock was then diluted in respective media to obtain different TQ concentrations ranging from 1 μmol/L to 100 μmol/L used in cell treatment. RJ fresh stock was prepared by dissolving 20 mg in 200 μL DMSO mixed with 800 μL distilled water solution at 37 ºC for 30 min. Intermediate concentrations of RJ ranging between 0.01 and 200 μg/mL were then prepared by serial dilutions from stock and used in cell treatment. In all experiments, treatment with TQ and RJ each alone or in combination was performed at 50% cell confluency. Treatment with TQ-RJ combination was done by adding TQ and RJ, each alone in wells containing their respective media and incubating cells with this mixture at different concentrations for 24 h.
MTT cell viability assay
All cells were seeded in 96-well plates at a density of 10000 cells/well, then treated for 24 h. Cell viability was then assessed by MTT that measures the ability of metabolically active cells to convert tetrazolium salt into violet formazan crystals. Cells were incubated with 120 μL of MTT solution (5 mg/mL prepared in 1 × PBS) for 3 h at 37 °C. Afterward, the solution containing the MTT dye was removed and replaced with 100 μL isopropanol to dissolve the formazan crystal. MTT optical density was then measured using a microplate reader enzyme-linked immuno-sorbent assay at 595 nm. Cellular viability was expressed as a percentage of metabolically active cells in treated conditions relative to control. Cell viability was reported as an average of three independent experiments, each condition in sextuplicate. RJ inhibitory effect was not determined by MTT due to its interference with the colorimetric absorbance measures of the MTT assay (data not shown).
Trypan blue exclusion assay
FHs 74 Int cells were seeded in 24-well-plates at a density of 70000 cells/well, while MDA-MB-231 were seeded in 12-well plates at a density of 20000 cells/well. Following treatment of cells for 24 h, alive and dead cells were collected. Samples were centrifuged at 1300 rpm for 5 min. Then, pellets were resuspended in DMEM growth medium, and trypan blue was added to the cell suspension in a 1:1 ratio. Next, cells were counted using a hemocytometer under the Axiovert inverted microscope at 10 × magnification. Cells stained blue were counted as dead, and results are expressed as a percentage of total cells. Cell viability was reported as an average of three independent experiments, each condition in duplicates.
Combination index analysis
The interaction between TQ and RJ was assessed using the Chou-Talalay plot[47]. Combination indices (CI) were calculated from the mean affected fraction at each drug combination using CompuSyn software (CompuSyn, Inc. Paramus, NJ, United States). CI > 1, CI = 1, and CI < 1 indicate antagonistic, additive, and synergistic effects, respectively.
Cell cycle analysis
MDA-MD-231 cells were seeded in 6-well plates at a density of 80000 cells/well. Cells were treated with 0.1 µg/mL RJ and 15 µmol/L TQ each alone. After 24 h, cells were collected and washed twice with 1 × PBS, fixed in 70% ice-cold ethanol, and stored at −20 °C for at least 1 d. Subsequently, cells were washed twice with 1 × PBS and incubated for 30 min at 37 °C with 100 μL of propidium iodide (PI) solution [6 μL RNase, 30 μL PI (1 mg/mL)]. Supernatants were then transferred to flow tubes with 200 μL PBS added. Cell cycle analysis was performed using the Fluorescence-activated cell sorting scan flow cytometer (Becton Dickinson, Franklin Lakes, NJ, United States) and the Cell Quest software (Becton-Dickinson) was used to analyze the distribution of cells in the different phases of the cell cycle.
Immunofluorescence assay
MDA-MB-231 cells were plated on coverslips in 12-well plates at a density of 60000 cells/well. The medium was then removed, and the cells were treated with either TQ, RJ, or combinations. After treatment, the cells were washed twice with 1 × PBS and fixed at room temperature for 20 min in 4% formaldehyde. The formaldehyde was then removed, and the cells were washed three times in PBS before permeabilization in 0.5% Triton solution for 5 min. After two successive washes in PBS, cells were blocked in blocking buffer with FBS for 1 h at room temperature. Apoptosis was assessed using the caspase 3 antibody, which was subsequently diluted (1:500) in 3% bovine serum albumin and incubated separately with the cells overnight at 4 ºC. The primary antibody was removed the next day, and the cells were washed three times in PBS supplemented with 0.1% Tween 20 before incubation for 1 h with goat anti-rabbit secondary antibody diluted (1:200) in 3% bovine serum albumin at room temperature. Finally, the secondary antibody was removed, and the cells were washed three times in PBS with 0.1% Tween 20 before staining the nuclei with 4′,6-diamidino-2-phenylindole and mounting on a glass slide. To evaluate cell proliferation, the same immunostaining protocol was followed for Ki67 immunofluorescence with minor modifications, including the preparation of Ki67 primary antibody and donkey anti-rabbit Cy3 secondary antibody solutions in blocking buffer with donkey serum at dilution 1:500 and 1:200 ratios, respectively. Also, cells were washed three times in PBS only after the removal of the primary and secondary antibodies. Imaging and visualization were performed using the microscope Zeiss Axio. For cleaved caspase 3 and Ki67 biomarkers, an equal number of representative images were taken for each slide in all conditions. The percentage of apoptotic cells expressing cleaved caspase 3 was then calculated while Ki67 immunofluorescence intensity was measured by ZEN lite Digital Imaging Software.
Statistical analysis
Unless otherwise stated, data are presented as mean ± standard error of the mean of three independent experiments with statistical analysis performed by one way analysis of variance (non-parametric) multiple comparison test on Graph Pad Prism V.7. Software (La Jolla, CA, United States). Statistical significance was set with a 95% confidence interval at P < 0.05.
Confocal Imaging
Cells were visualized and imaged by Axiovert inverted microscope from Zeiss at 10 × magnification. Confocal images were taken on Confocal Microscope Zeiss LSM710 at 40 × oil immersion magnification.
RESULTS
Cytotoxicity of TQ and RJ on human breast cancer cells
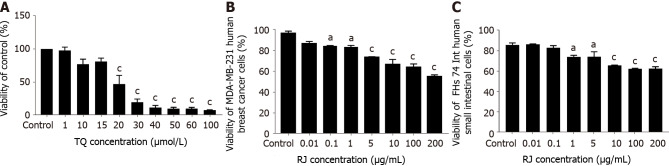
In a previous study, we showed that TQ doses up to 60 µmol/L exert minimal cytotoxic effects on normal intestinal cells[48]. Post 24 h treatment, TQ at concentrations below 15 µmol/L did not exert any statistically significant toxicity on MDA-MB-231 cell line relative to the control. Cell viability decreased remarkably, reaching 47% at 20 µmol/L, revealing that the half-maximal inhibitory concentration (IC50) value was around 20 µmol/L (Figure 1A). The decline in cell viability was more pronounced with increasing TQ concentrations, indicating that TQ exhibited significant anticancer activity against MDA-MB-231 human breast cancer cells in a dose dependent manner. RJ exerted mild inhibitory effects at low doses of RJ (below 5 μg/mL) on FHs 74 Int non-tumorigenic human intestinal cells and MDA-MB-231 human breast cancer cells. However, at higher doses ranging from 10 μg/mL to 200 μg/mL of RJ, a more pronounced decrease in cell viability was observed, suggesting that very high doses of RJ are toxic to FHs 74 Int non-tumorigenic cells with a greater toxicity being exerted on breast cancer cells (Figure 1B and C). The IC50 of RJ was estimated to be 216 µg/mL in MDA-MB-231 cells, while it was much higher (292 µg/mL) in the FHs 74 Int cell line. Based on these results, relatively non-toxic doses of RJ ranging between 0.1-5.0 μg/mL were combined with TQ in subsequent experiments.
Figure 1.
The inhibitory effect of thymoquinone and royal jelly on the viability of MDA-MB-231 and FHs74 Int cell line. A: 3-(4,5-dmethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay showing the percentage viability of MDA-MB-231 cell line and the half-maximal inhibitory concentration of thymoquinone (TQ) on MDA-MB-231 human breast cancer cell line after 24 h of treatment with different TQ concentrations. Cell viability was estimated by measuring the absorbance of the cell suspension after incubation with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; B: Trypan blue exclusion assay showing the percentage cell viability after 24 h of treatment with different royal jelly concentrations on FHs74 Int; C: MDA-MB-231 cell lines. Data shown are an average of 3 independent experiments for panels A and B, and 2 independent experiments for panel C, respectively, expressed as mean ± standard error of the mean. Asterisks represent statistically significant results compared to the control, (aP < 0.05, bP < 0.01, cP < 0.001). RJ: Royal jelly; TQ: Thymoquinone.
Anticancer effect of TQ, RJ, and their combinations against breast cancer
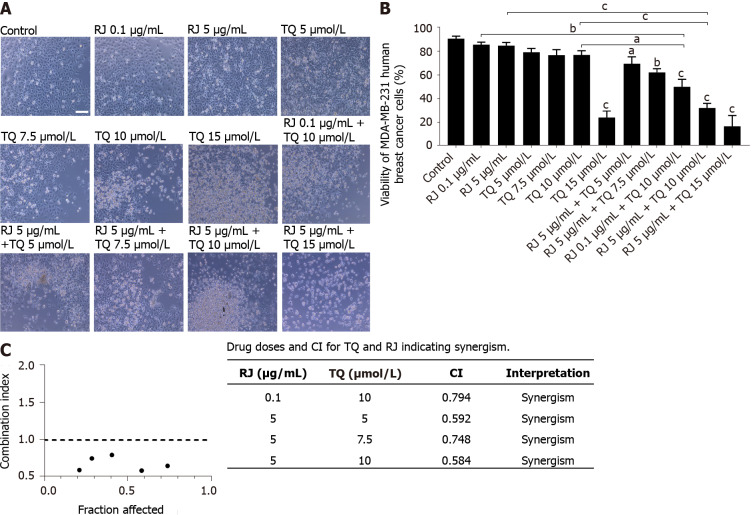
Next, we determined the effects of the combination of increasing doses of both RJ and TQ on cell viability. As shown in Figure 2A and B, no significant reduction in MDA-MB-231 viability was detected upon the treatment with RJ alone at doses of 0.1 µg/mL and 5 µg/mL or with TQ alone at doses below 10 µmol/L. Treatment with 5 µg/mL RJ, when combined with 5 µmol/L or 7.5 µmol/L of TQ caused 21% and 29% inhibition in cell viability, respectively (Figure 2B). The anti-tumor effects were more pronounced upon treatment with higher TQ doses. A dose of 10 µmol/L TQ in combination with 0.1 µg/mL RJ or 5 µg/mL RJ yielded a significant decrease in MDA-MB-231 cell viability by 40% and 58%, respectively. Treatment with 5 µg/mL RJ in combination with 10 µmol/L or 15 µmol/L of TQ decreased cell viability by 58% and 74%, respectively. These findings confirm the more potent anti-tumor effects upon combination treatment with higher RJ doses compared to each drug alone. The inhibition of cell viability (58% inhibition) by this combination treatment was greater than the sum of inhibition observed by each compound alone (6% and 12% inhibition by TQ and RJ alone, respectively), suggesting a synergistic effect. CIs were then calculated using CompuSyn software, confirming the synergistic interaction between both compounds in all the combinations tested with a CI value < 1. Anti-tumor effect was most pronounced (CI = 0.584) upon the combination of 5 µg/mL RJ with 10 µmol/L TQ (Figure 2C). Therefore, RJ enhances TQ anti-tumor activity against breast cancer by inducing dose dependent cell death effects.
Figure 2.
Royal jelly and thymoquinone combinations enhanced the inhibition of MDA-MB-231 human breast cancer cell viability. A: Representative light microscopy images of MDA-MB-231 viability in response to different treatments. Cells were visualized by Axiovert inverted microscope from Zeiss at 10 × magnification with scale bar = 10 µmol/L; B: Trypan blue exclusion assay showing the percentage viability of MDA-MB-231 cell line after 24 h of treatment with different concentrations of royal jelly (RJ), thymoquinone, and combinations. Data shown are an average of three independent experiments expressed as mean ± standard error of the mean. Asterisks represent statistically significant results compared to the control and treatment conditions, (aP < 0.05, bP < 0.01, cP < 0.001); C: Fraction affected-combination index plot showing combination index (CI) values plotted as a function of fraction affected values corresponding to the % cell death of five different combinations of thymoquinone (5 µmol/L, 7.5 µmol/L, 10 µmol/L and 15 µmol/L) and royal jelly (0.1 and 5 µg/mL) in MDA-MB-231 cells. The dotted line is the reference line, where CI value is equal to 1; circles in black represent CI values at different Fa. CI > 1, CI = 1, and CI < 1 indicate antagonistic, additive, and synergistic effects, respectively. CI: Combination index; RJ: Royal jelly; TQ: Thymoquinone.
TQ alone and in combination with RJ increases PreG1 population in breast cancer cells
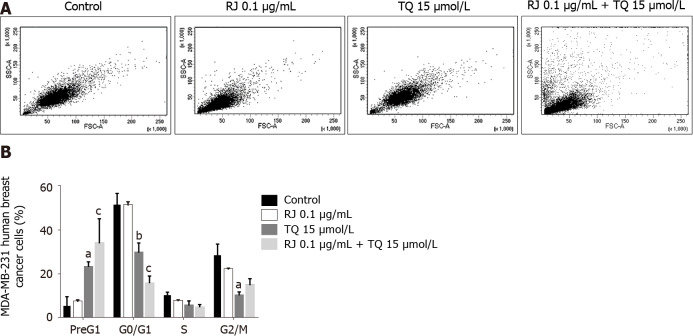
To confirm further cell death and determine whether the inhibition of cell viability by RJ and TQ treatment of MDA-MB-231 cells was associated with changes in cell cycle regulation, cell cycle analysis using PI deoxyribonucleic acid staining with flow cytometry was performed. Cell death was significantly enhanced in response to TQ alone and when TQ was combined with 0.1 µg/mL RJ, a relatively non-cytotoxic dose (Figure 3A and B). In comparison with the control, the PreG1 population increased significantly upon the treatment with 15 µmol/L TQ alone, while a greater elevation was obtained when this dose of TQ was combined with 0.1 µg/mL RJ (Figure 3B). The increase in the PreG1 population was associated with a notable reduction in the G0/G1 and G2/M populations.
Figure 3.
Cell death is enhanced by thymoquinone alone and by the combination thymoquinone and royal jelly. A: Representative density plots showing MDA-MB-231 cell distribution as a function of side scatter area and forward scatter area in the control and post-treatment with 15 µmol/L thymoquinone (TQ) and 0.1 µg/mL royal jelly alone and in combination for 24 h; B: Propidium iodide staining with flow cytometry showing the increase in Pre G1 upon treatment with TQ alone and the combination of TQ and royal jelly. Data shown are an average of three independent experiments expressed as mean ± standard error of the mean and analyzed by a two-way analysis of variance test followed by multiple comparisons test. Asterisks represent statistically significant results compared to the control, (aP < 0.05, bP < 0.01, cP < 0.001). RJ: Royal jelly; TQ: Thymoquinone.
TQ and RJ combinations induce apoptotic cell death in breast cancer cells
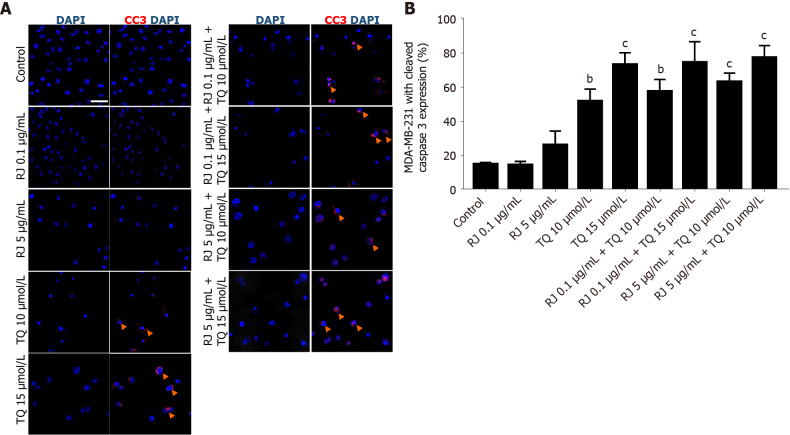
To identify the mechanism of action responsible for the enhanced cell death effect of TQ and RJ combination treatment, we assessed the apoptotic effects of each compound alone and their combinations in MDA-MB-231 cell line. Insignificant increase in apoptosis levels was reported upon treatment with 0.1 µg/mL or 5 µg/mL of RJ. Confocal micrographs showed enhancement of apoptosis in response to the TQ and combination treatment, as evidenced by the increase in apoptotic nuclear bodies in MDA-MB-231 cells (Figure 4A). A significant increase in active caspase 3 expression was observed in response to treatment with 10 µmol/L and 15 µmol/L TQ alone, yielding a respective increase of 52% and 73% of caspase 3 expression in MDA-MB-231 cells (Figure 4B). Similar results were obtained upon treatment with 0.1 µg/mL RJ in combination with 10 µmol/L and 15 µmol/L TQ, while a more pronounced apoptotic effect was observed in response to treatment with 5 µg/mL RJ in combination with 10 µmol/L and 15 µmol/L TQ. Cell proliferation was then evaluated after 24 h of treatment by measuring the intensity of Ki67 fluorescence, a sensitive and specific proliferation biomarker. A minimal non-significant decrease in Ki67 expression was observed in response to all doses of RJ and TQ combinations as compared to the control (data not shown). This effect was confirmed by confocal imaging showing the modest change in the nuclear expression of Ki67 in response to the different treatments in MDA-MB-231 cells (data not shown). Therefore, RJ alone and TQ alone or their combinations did not modulate the expression of Ki67 in MDA-MB-231 human TNBC.
Figure 4.
Effect of royal jelly, thymoquinone and combinations on caspase 3 cleavage in MDA-MB-231 human breast cancer cells. A: Immunofluorescence micrographs of cleaved caspase 3 expression at 24 h after treatment. Red indicates cleaved caspase 3 expression and blue indicates nuclei counter stained by 4′,6-diamidino-2-phenylindole. Arrows indicate apoptotic nuclei. Nuclei were visualized by confocal Zeiss Axio microscope, 40 × oil immersion with scale bar = 50 µmol/L; B: Quantification of cleaved caspase 3 in MDA-MB-231 cells at 24 h of treatment with different concentrations of royal jelly, thymoquinone, and their combinations. Data shown are an average of 3 independent experiments expressed as mean ± standard error of the mean. Asterisks represent statistically significant results, (aP < 0.05, bP < 0.01, cP < 0.001). RJ: Royal jelly; TQ: Thymoquinone.
DISCUSSION
Breast cancer, the most common cancer among women, is identified as a heterogeneous disease arising from the differential expression of hormonal receptors along with genomic and intratumoral heterogeneity[5]. Despite the tremendous improvement in the therapeutic approaches, conventional treatments of breast cancer, including systemic therapy, exert organ-specific toxicity along with various side effects[49]. The interest in alternative treatments relying on relatively non-toxic and cost-effective natural resources has surged over the past decades, particularly from medicinal plants and honeybee products. TQ, the main constituent of Nigella sativa essential oil, and the nutritious honeybee secretions of RJ were shown to have potent anticancer activities against many types of cancers, including breast cancer[50,51].
Our study is the first to investigate the anticancer activity of both TQ and RJ alone and in combination against the triple-negative MDA-MB-231 human metastatic breast cancer cell line. TQ has been shown to possess potent anticancer activities against various cancer types, including colon cancer, with minimal cytotoxic effects on normal intestinal cells[48]. In the context of breast cancer, previous studies reported TQ-mediated induction of apoptosis, growth inhibition, in addition to suppression of viability and invasion of MDA-MB-231 and MCF7 cell lines mainly through the inhibition of Akt phosphorylation[23,25]. In line with these studies, we further confirmed TQ’s anticancer activity in MDA-MB-231 cell line as evidenced by the dose-dependent cell death effects at concentrations below and above the IC50 value of 20 µmol/L. Our results showed that RJ exhibited minimal toxicity on FHs74 Int cell line at doses below 5 µg/mL, while a more pronounced inhibitory effect was observed at higher doses with a clear saturation effect obtained at doses equal or greater than 100 µg/mL. This indicates that RJ is relatively nontoxic to the non-tumorigenic human small intestinal cell line at doses equal or below 5 µg/mL. In line with the previously published studies[38,39], our findings demonstrate that RJ inhibits the viability of breast cancer cells. RJ exerted low to mild dose-dependent inhibitory effects on the viability of MDA-MB-231cell line at doses below 5 µg/mL. Cell death was more pronounced in MDA-MB-231 at 200 μg/mL RJ, suggesting the greater toxicity of RJ to breast cancer cells with the IC50 estimated to be 1.4 fold greater in FHs 74 Int cell line compared to that of MDA-MB-231 cell line.
Combination therapy is usually used to enhance the therapeutic response and overcome any possible drug resistance in cancer patients[52]. To assess for any possible anticancer synergy (or additive effects), concentrations that are not highly cytotoxic to cells (i.e. less than 50% cell death) should be used. Therefore, the anticancer effect of TQ in combination with RJ was assessed using drug doses below the IC50 values (i.e. 15 µmol/L). We documented an enhanced anticancer activity of TQ when combined with RJ against MDA-MB-231 cell line. Cancer cell viability decreased significantly in response to different combinations as compared to the treatment with each drug alone. Cell death was amplified by 3- and 5-fold in response to the combination of 5 µg/mL of RJ with 10 µmol/L and 15 µmol/L of TQ, respectively, with the lowest combination index obtained upon the combination of 5 µg/mL RJ with 10 µmol/L TQ, suggesting synergistic interaction. These results are consistent with the previous studies that reported the synergism of TQ in combination with different agents including melatonin[53] and piperine[24] against breast cancer, diosgenin on squamous cell carcinoma[54], docetaxel against prostate cancer[55], in addition to arsenic and interferon-alpha against human T-cell leukemia/lymphoma[56].
Cell cycle analysis using propidium iodide staining was performed to confirm further cell death and to examine whether TQ and RJ alone or in combination affect the cell cycle progression of breast cancer cells. In accordance with our findings using trypan blue exclusion assay, RJ alone at a dose of 0.1 µg/mL did not exert significant changes in cell viability compared to the control. Consistent with previous studies reporting the inhibitory activity of TQ[48,57,58], our study reports 4-fold increase in the PreG1 cell population, which was associated with a significant decrease in G0/G1 and G2/M cell populations in response to 15 µmol/L of TQ, further confirming TQ-mediated cell death. Interestingly, combining 15 µmol/L of TQ with 0.1 µg/mL RJ yielded a more pronounced cell death effect evidenced by the 6-fold increase in the PreG1 population. These results indicate that the cell death effect is enhanced upon the combination of TQ with RJ compared to single treatments with each compound alone. This indicates that RJ enhances the cell death effects of TQ in metastatic breast cancer.
To understand the underlying mechanism of the observed reduction in the viability of metastatic breast cancer cells upon the different treatments, we investigated apoptosis induction as a possible mechanism of cell death. Enhanced induction of apoptosis was evidenced by the increase in caspase 3 cleavage in response to the increasing TQ doses alone or in combination with RJ. On the other hand, treatment with RJ alone did not induce any significant apoptotic effect compared to the control. Apoptotic cell death was increased by 4-fold in response to the combination of 10 µmol/L of TQ with 0.1 µg/mL and 5 µg/mL RJ, while a 5-fold increase was obtained upon combining 15 µmol/L of TQ with both RJ doses. Our results indicate that TQ is the main inducer of apoptosis, although an augmented apoptotic response was observed upon the combination with RJ, indicating that RJ could modestly potentiate the anticancer activity of TQ in TNBC. Our findings are consistent with previous studies showing induction of apoptosis upon treatment with TQ alone and in combination with other agents against cancer cells[24,56,59,60]. In line with previously published data[59], our study shows minimal changes in Ki67 intensity in response to TQ. Therefore, cell death of MDA-MB-231 cells was not due to the inhibition of proliferation but rather to apoptosis induction, as evidenced by the enhanced caspase 3 cleavage.
CONCLUSION
In conclusion, RJ and TQ, each being relatively non-toxic to normal cells, exhibit enhanced anti-tumor activities against human metastatic breast cancer when combined. Although TQ and RJ combination enhances apoptotic cell death, TQ appears to act as the main inducer of apoptosis mediating cell death by inducing caspase 3 dependent apoptosis in a dose-dependent manner. The combination of these two natural compounds deserves further investigation to identify the key molecules responsible for this enhanced anticancer activity.
ARTICLE HIGHLIGHTS
Research background
Despite the tremendous improvement in therapeutic approaches, triple-negative breast cancer has poor prognosis. Thymoquinone (TQ), the main constituent of Nigella sativa seeds and royal jelly (RJ), the honeybee secretion fed to honeybee queens, are effective against cancer. However, the anticancer activity of the combination of TQ and RJ against aggressive human breast cancer cells is yet unknown.
Research motivation
To establish novel treatments for breast cancer using natural, relatively non-toxic compounds with significant therapeutic value. We focused on investigating the anticancer activity of TQ and RJ combinations against triple-negative breast cancer.
Research objectives
This study aimed to characterize the anticancer activity of TQ and RJ alone and their combination in vitro against human triple-negative breast cancer.
Research methods
The inhibitory effect of TQ on triple-negative breast cancer cells was assessed by 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Trypan blue exclusion assay was used to evaluate cell viability in response to different treatment conditions. Propidium iodide deoxyribonucleic acid staining followed by flow cytometry was performed to evaluate possible cell cycle regulation and cell death effects. Apoptosis and cell proliferation were determined using immunofluorescence assays for cleaved caspase 3 and Ki67 expression, respectively. The interaction between TQ and RJ and combination indices were evaluated using CompuSyn software.
Research results
TQ inhibited MDA-MB-231 breast cancer cell viability in a dose-dependent manner. RJ at low doses was relatively nontoxic to non-tumorigenic FHs 74 Int small intestinal epithelial cells, while at high doses greater toxicity against MDA-MB-231 breast cancer cells was observed. Inhibition of cell viability and cell death effects were more pronounced in response to TQ and RJ combinations compared to each drug alone. The reduction in breast cancer cell viability was mainly due to TQ-mediated caspase 3-dependent apoptosis.
Research conclusions
RJ and TQ are relatively non-toxic to normal cells and exhibited pronounced anticancer effects against human metastatic breast cancer. Although our findings demonstrate the potent pro-apoptotic activity of TQ compared to that of RJ, this is the first report of a significant enhancement in TQ’s anticancer activity when combined with RJ.
Research perspectives
The reduction in breast cancer cell viability and enhanced cell death effects upon TQ and RJ combinations highlights their potential therapy for human triple-negative breast cancer.
ACKNOWLEDGEMENTS
We are grateful to all members of the Gali-Muhtasib Laboratory and the staff of the core facilities in the DTS Building at the American University of Beirut for their help and support.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interest for this manuscript.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Manuscript source: Invited manuscript
Peer-review started: January 28, 2021
First decision: March 1, 2021
Article in press: April 26, 2021
Specialty type: Oncology
Country/Territory of origin: Lebanon
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dahmen U S-Editor: Liu M L-Editor: Filipodia P-Editor: Yuan YY
Contributor Information
Maya M Moubarak, Department of Biology, American University of Beirut, Beirut 1107-2020, Lebanon.
Nour Chanouha, Department of Biology, American University of Beirut, Beirut 1107-2020, Lebanon.
Najwa Abou Ibrahim, Rammal Rammal Laboratory (ATAC group), Faculty of Sciences I, Hadath 1003, Lebanon.
Hala Khalife, Rammal Rammal Laboratory (ATAC group), Faculty of Sciences I, Hadath 1003, Lebanon.
Hala Gali-Muhtasib, Department of Biology and Center for Drug Discovery, American University of Beirut, Beirut 1107-2020, Lebanon. amro@aub.edu.lb.
Data sharing statement
No additional data are available.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021 doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO report on cancer: setting priorities, investing wisely and providing care for all [Publications]. Geneva: World Health Organization; 2020 Available from: https://apps.who.int/iris/handle/10665/330745 .
- 3.Tao Z, Shi A, Lu C, Song T, Zhang Z, Zhao J. Breast Cancer: Epidemiology and Etiology. Cell Biochem Biophys. 2015;72:333–338. doi: 10.1007/s12013-014-0459-6. [DOI] [PubMed] [Google Scholar]
- 4.Grieco MP, Simonacci F, Bertozzi N, Grignaffini E, Raposio E. Breast reconstruction with breast implants. Acta Biomed. 2019;89:457–462. doi: 10.23750/abm.v89i4.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertos NR, Park M. Breast cancer - one term, many entities? J Clin Invest. 2011;121:3789–3796. doi: 10.1172/JCI57100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li CI, Uribe DJ, Daling JR. Clinical characteristics of different histologic types of breast cancer. Br J Cancer. 2005;93:1046–1052. doi: 10.1038/sj.bjc.6602787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weigelt B, Geyer FC, Reis-Filho JS. Histological types of breast cancer: how special are they? Mol Oncol. 2010;4:192–208. doi: 10.1016/j.molonc.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peddi PF, Ellis MJ, Ma C. Molecular basis of triple negative breast cancer and implications for therapy. Int J Breast Cancer. 2012;2012:217185. doi: 10.1155/2012/217185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wahba HA, El-Hadaad HA. Current approaches in treatment of triple-negative breast cancer. Cancer Biol Med. 2015;12:106–116. doi: 10.7497/j.issn.2095-3941.2015.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cailleau R, Olivé M, Cruciger QV. Long-term human breast carcinoma cell lines of metastatic origin: preliminary characterization. In Vitro. 1978;14:911–915. doi: 10.1007/BF02616120. [DOI] [PubMed] [Google Scholar]
- 11.Chavez KJ, Garimella SV, Lipkowitz S. Triple negative breast cancer cell lines: one tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010;32:35–48. doi: 10.3233/BD-2010-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordón-Cardo C, Guise TA, Massagué J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 13.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massagué J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmieri D, Smith QR, Lockman PR, Bronder J, Gril B, Chambers AF, Weil RJ, Steeg PS. Brain metastases of breast cancer. Breast Dis. 2006-2007;26:139–147. doi: 10.3233/bd-2007-26112. [DOI] [PubMed] [Google Scholar]
- 15.Palumbo MO, Kavan P, Miller WH Jr, Panasci L, Assouline S, Johnson N, Cohen V, Patenaude F, Pollak M, Jagoe RT, Batist G. Systemic cancer therapy: achievements and challenges that lie ahead. Front Pharmacol. 2013;4:57. doi: 10.3389/fphar.2013.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider-Stock R, Fakhoury IH, Zaki AM, El-Baba CO, Gali-Muhtasib HU. Thymoquinone: fifty years of success in the battle against cancer models. Drug Discov Today. 2014;19:18–30. doi: 10.1016/j.drudis.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Aycan IÖ, Tüfek A, Tokgöz O, Evliyaoğlu O, Fırat U, Kavak GÖ, Turgut H, Yüksel MU. Thymoquinone treatment against acetaminophen-induced hepatotoxicity in rats. Int J Surg. 2014;12:213–218. doi: 10.1016/j.ijsu.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Abulfadl YS, El-Maraghy NN, Ahmed AAE, Nofal S, Badary OA. Protective effects of thymoquinone on D-galactose and aluminum chloride induced neurotoxicity in rats: biochemical, histological and behavioral changes. Neurol Res. 2018;40:324–333. doi: 10.1080/01616412.2018.1441776. [DOI] [PubMed] [Google Scholar]
- 19.Asaduzzaman Khan M, Tania M, Fu S, Fu J. Thymoquinone, as an anticancer molecule: from basic research to clinical investigation. Oncotarget. 2017;8:51907–51919. doi: 10.18632/oncotarget.17206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaterzadeh-Yazdi H, Noorbakhsh MF, Hayati F, Samarghandian S, Farkhondeh T. Immunomodulatory and Anti-inflammatory Effects of Thymoquinone. Cardiovasc Hematol Disord Drug Targets. 2018;18:52–60. doi: 10.2174/1871529X18666180212114816. [DOI] [PubMed] [Google Scholar]
- 21.Alobaedi OH, Talib WH, Basheti IA. Antitumor effect of thymoquinone combined with resveratrol on mice transplanted with breast cancer. Asian Pac J Trop Med. 2017;10:400–408. doi: 10.1016/j.apjtm.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 22.Bashmail HA, Alamoudi AA, Noorwali A, Hegazy GA, AJabnoor G, Choudhry H, Al-Abd AM. Thymoquinone synergizes gemcitabine anti-breast cancer activity via modulating its apoptotic and autophagic activities. Sci Rep. 2018;8:11674. doi: 10.1038/s41598-018-30046-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganji-Harsini S, Khazaei M, Rashidi Z, Ghanbari A. Thymoquinone Could Increase The Efficacy of Tamoxifen Induced Apoptosis in Human Breast Cancer Cells: An In Vitro Study. Cell J. 2016;18:245–254. doi: 10.22074/cellj.2016.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talib WH. Regressions of Breast Carcinoma Syngraft Following Treatment with Piperine in Combination with Thymoquinone. Sci Pharm. 2017;85 doi: 10.3390/scipharm85030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Attoub S, Sperandio O, Raza H, Arafat K, Al-Salam S, Al Sultan MA, Al Safi M, Takahashi T, Adem A. Thymoquinone as an anticancer agent: evidence from inhibition of cancer cells viability and invasion in vitro and tumor growth in vivo. Fundam Clin Pharmacol. 2013;27:557–569. doi: 10.1111/j.1472-8206.2012.01056.x. [DOI] [PubMed] [Google Scholar]
- 26.Kocot J, Kielczykowska M, Luchowska-Kocot D, Kurzepa J, Musik I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxid Med Cell Longev. 2018;2018:7074209. doi: 10.1155/2018/7074209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajput S, Kumar BN, Dey KK, Pal I, Parekh A, Mandal M. Molecular targeting of Akt by thymoquinone promotes G(1) arrest through translation inhibition of cyclin D1 and induces apoptosis in breast cancer cells. Life Sci. 2013;93:783–790. doi: 10.1016/j.lfs.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Kamakura M. [Royalactin induces queen differentiation in honeybees] Seikagaku. 2012;84:994–1003. [PubMed] [Google Scholar]
- 29.Melliou E, Chinou I. Chemistry and bioactivity of royal jelly from Greece. J Agric Food Chem. 2005;53:8987–8992. doi: 10.1021/jf051550p. [DOI] [PubMed] [Google Scholar]
- 30.Taniguchi Y, Kohno K, Inoue S, Koya-Miyata S, Okamoto I, Arai N, Iwaki K, Ikeda M, Kurimoto M. Oral administration of royal jelly inhibits the development of atopic dermatitis-like skin lesions in NC/Nga mice. Int Immunopharmacol. 2003;3:1313–1324. doi: 10.1016/s1567-5769(03)00132-2. [DOI] [PubMed] [Google Scholar]
- 31.Liu JR, Yang YC, Shi LS, Peng CC. Antioxidant properties of royal jelly associated with larval age and time of harvest. J Agric Food Chem. 2008;56:11447–11452. doi: 10.1021/jf802494e. [DOI] [PubMed] [Google Scholar]
- 32.Kohno K, Okamoto I, Sano O, Arai N, Iwaki K, Ikeda M, Kurimoto M. Royal jelly inhibits the production of proinflammatory cytokines by activated macrophages. Biosci Biotechnol Biochem. 2004;68:138–145. doi: 10.1271/bbb.68.138. [DOI] [PubMed] [Google Scholar]
- 33.Kamakura M, Moriyama T, Sakaki T. Changes in hepatic gene expression associated with the hypocholesterolaemic activity of royal jelly. J Pharm Pharmacol. 2006;58:1683–1689. doi: 10.1211/jpp.58.12.0017. [DOI] [PubMed] [Google Scholar]
- 34.Chiu HF, Chen BK, Lu YY, Han YC, Shen YC, Venkatakrishnan K, Golovinskaia O, Wang CK. Hypocholesterolemic efficacy of royal jelly in healthy mild hypercholesterolemic adults. Pharm Biol. 2017;55:497–502. doi: 10.1080/13880209.2016.1253110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan P, Han B, Feng M, Fang Y, Zhang L, Hu H, Hao Y, Qi Y, Zhang X, Li J. Functional and Proteomic Investigations Reveal Major Royal Jelly Protein 1 Associated with Anti-hypertension Activity in Mouse Vascular Smooth Muscle Cells. Sci Rep. 2016;6:30230. doi: 10.1038/srep30230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zargar HR, Hemmati AA, Ghafourian M, Arzi A, Rezaie A, Javad-Moosavi SA. Long-term treatment with royal jelly improves bleomycin-induced pulmonary fibrosis in rats. Can J Physiol Pharmacol. 2017;95:23–31. doi: 10.1139/cjpp-2015-0451. [DOI] [PubMed] [Google Scholar]
- 37.Pyrzanowska J, Piechal A, Blecharz-Klin K, Joniec-Maciejak I, Graikou K, Chinou I, Widy-Tyszkiewicz E. Long-term administration of Greek Royal Jelly improves spatial memory and influences the concentration of brain neurotransmitters in naturally aged Wistar male rats. J Ethnopharmacol. 2014;155:343–351. doi: 10.1016/j.jep.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 38.Nakaya M, Onda H, Sasaki K, Yukiyoshi A, Tachibana H, Yamada K. Effect of royal jelly on bisphenol A-induced proliferation of human breast cancer cells. Biosci Biotechnol Biochem. 2007;71:253–255. doi: 10.1271/bbb.60453. [DOI] [PubMed] [Google Scholar]
- 39.Zhang S, Shao Q, Geng H, Su S. The effect of royal jelly on the growth of breast cancer in mice. Oncol Lett. 2017;14:7615–7621. doi: 10.3892/ol.2017.7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Filipič B, Gradišnik L, Rihar K, Šooš E, Pereyra A, Potokar J. The influence of royal jelly and human interferon-alpha (HuIFN-αN3) on proliferation, glutathione level and lipid peroxidation in human colorectal adenocarcinoma cells in vitro. Arh Hig Rada Toksikol. 2015;66:269–274. doi: 10.1515/aiht-2015-66-2632. [DOI] [PubMed] [Google Scholar]
- 41.Gismondi A, Trionfera E, Canuti L, Di Marco G, Canini A. Royal jelly lipophilic fraction induces antiproliferative effects on SH-SY5Y human neuroblastoma cells. Oncol Rep. 2017;38:1833–1844. doi: 10.3892/or.2017.5851. [DOI] [PubMed] [Google Scholar]
- 42.Izuta H, Chikaraishi Y, Shimazawa M, Mishima S, Hara H. 10-Hydroxy-2-decenoic acid, a major fatty acid from royal jelly, inhibits VEGF-induced angiogenesis in human umbilical vein endothelial cells. Evid Based Complement Alternat Med. 2009;6:489–494. doi: 10.1093/ecam/nem152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nedeljković M, Damjanović A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge. Cells. 2019;8 doi: 10.3390/cells8090957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao Y, Chu Y, Xu B, Hu Q, Song Q. Risk factors for distant metastasis of patients with primary triple-negative breast cancer. Biosci Rep. 2019;39 doi: 10.1042/BSR20190288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galvao J, Davis B, Tilley M, Normando E, Duchen MR, Cordeiro MF. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J. 2014;28:1317–1330. doi: 10.1096/fj.13-235440. [DOI] [PubMed] [Google Scholar]
- 46.Cevallos AM, Herrera J, López-Villaseñor I, Hernández R. Differential Effects of Two Widely Used Solvents, DMSO and Ethanol, on the Growth and Recovery of Trypanosoma cruzi Epimastigotes in Culture. Korean J Parasitol. 2017;55:81–84. doi: 10.3347/kjp.2017.55.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 48.El-Najjar N, Chatila M, Moukadem H, Vuorela H, Ocker M, Gandesiri M, Schneider-Stock R, Gali-Muhtasib H. Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis. 2010;15:183–195. doi: 10.1007/s10495-009-0421-z. [DOI] [PubMed] [Google Scholar]
- 49.Bodai BI, Tuso P. Breast cancer survivorship: a comprehensive review of long-term medical issues and lifestyle recommendations. Perm J. 2015;19:48–79. doi: 10.7812/TPP/14-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmad S, Campos MG, Fratini F, Altaye SZ, Li J. New Insights into the Biological and Pharmaceutical Properties of Royal Jelly. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21020382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Imran M, Rauf A, Khan IA, Shahbaz M, Qaisrani TB, Fatmawati S, Abu-Izneid T, Imran A, Rahman KU, Gondal TA. Thymoquinone: A novel strategy to combat cancer: A review. Biomed Pharmacother. 2018;106:390–402. doi: 10.1016/j.biopha.2018.06.159. [DOI] [PubMed] [Google Scholar]
- 52.Palmer AC, Sorger PK. Combination Cancer Therapy Can Confer Benefit via Patient-to-Patient Variability without Drug Additivity or Synergy. Cell. 2017;171:1678–1691.e13. doi: 10.1016/j.cell.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Odeh LH, Talib WH, Basheti IA. Synergistic effect of thymoquinone and melatonin against breast cancer implanted in mice. J Cancer Res Ther. 2018;14:S324–S330. doi: 10.4103/0973-1482.235349. [DOI] [PubMed] [Google Scholar]
- 54.Das S, Dey KK, Dey G, Pal I, Majumder A, MaitiChoudhury S, Kundu SC, Mandal M. Antineoplastic and apoptotic potential of traditional medicines thymoquinone and diosgenin in squamous cell carcinoma. PLoS One. 2012;7:e46641. doi: 10.1371/journal.pone.0046641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dirican A, Atmaca H, Bozkurt E, Erten C, Karaca B, Uslu R. Novel combination of docetaxel and thymoquinone induces synergistic cytotoxicity and apoptosis in DU-145 human prostate cancer cells by modulating PI3K-AKT pathway. Clin Transl Oncol. 2015;17:145–151. doi: 10.1007/s12094-014-1206-6. [DOI] [PubMed] [Google Scholar]
- 56.Houssein M, Fatfat M, Habli Z, Ghazal N, Moodad S, Khalife H, Khalil M, Gali-Muhtasib H. Thymoquinone synergizes with arsenic and interferon alpha to target human T-cell leukemia/lymphoma. Life Sci. 2020;251:117639. doi: 10.1016/j.lfs.2020.117639. [DOI] [PubMed] [Google Scholar]
- 57.Gali-Muhtasib H, Kuester D, Mawrin C, Bajbouj K, Diestel A, Ocker M, Habold C, Foltzer-Jourdainne C, Schoenfeld P, Peters B, Diab-Assaf M, Pommrich U, Itani W, Lippert H, Roessner A, Schneider-Stock R. Thymoquinone triggers inactivation of the stress response pathway sensor CHEK1 and contributes to apoptosis in colorectal cancer cells. Cancer Res. 2008;68:5609–5618. doi: 10.1158/0008-5472.CAN-08-0884. [DOI] [PubMed] [Google Scholar]
- 58.Khalife R, Hodroj MH, Fakhoury R, Rizk S. Thymoquinone from Nigella sativa Seeds Promotes the Antitumor Activity of Noncytotoxic Doses of Topotecan in Human Colorectal Cancer Cells in Vitro. Planta Med. 2016;82:312–321. doi: 10.1055/s-0035-1558289. [DOI] [PubMed] [Google Scholar]
- 59.Gali-Muhtasib H, Ocker M, Kuester D, Krueger S, El-Hajj Z, Diestel A, Evert M, El-Najjar N, Peters B, Jurjus A, Roessner A, Schneider-Stock R. Thymoquinone reduces mouse colon tumor cell invasion and inhibits tumor growth in murine colon cancer models. J Cell Mol Med. 2008;12:330–342. doi: 10.1111/j.1582-4934.2007.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roepke M, Diestel A, Bajbouj K, Walluscheck D, Schonfeld P, Roessner A, Schneider-Stock R, Gali-Muhtasib H. Lack of p53 augments thymoquinone-induced apoptosis and caspase activation in human osteosarcoma cells. Cancer Biol Ther. 2007;6:160–169. doi: 10.4161/cbt.6.2.3575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.