Abstract
BACKGROUND
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease and affects approximately 25% of the general global adult population. The prognosis of NAFLD patients with advanced liver fibrosis is known to be poor. It is difficult to assess disease progression in all patients with NAFLD; thus, it is necessary to identify patients who will show poor prognosis.
AIM
To investigate the efficacy of non-invasive biomarkers for predicting disease progression in patients with NAFLD.
METHODS
We investigated biomarkers associated with mortality in patients with NAFLD who visited the Kawasaki Medical School General Medical Center from 1996 to 2018 and underwent liver biopsy and had been followed-up for > 1 year. Cumulative overall mortality and liver-related events during follow-up were calculated using the Kaplan-Meier analysis and compared using log-rank testing. We calculated the odds ratio and performed receiver operating characteristic curve analysis with logistic regression analysis to determine the optimal cut-off value with the highest prognostic ability.
RESULTS
We enrolled 489 patients who were followed-up for a period of 1-22.2 years. In total, 13 patients died (2.7% of total patients enrolled); 7 patients died due to liver-related causes. Poor prognosis was associated with liver fibrosis on histological examination but not with inflammation or steatosis. Blood biomarkers associated with mortality were platelet counts, albumin levels, and type IV collagen 7S levels. The optimal cutoff index for predicting total mortality was a platelet count of 15 × 104/μL, albumin level of 3.5 g/dL, and type IV collagen 7S level of 5 mg/dL. In particular, only one-factor patients with NAFLD presenting with platelet counts ≤ 15 × 104/μL, albumin levels ≤ 3.5 g/dL, or type IV collagen 7S ≥ 5 mg/dL showed 5-year, 10-year, and 15-year survival rates of 99.7%, 98.3%, and 94%, respectively. However, patients with two factors had lower 5-year and 10-year survival rates of 98% and 43%, respectively. Similarly, patients with all three factors showed the lowest 5-year and 10-year survival rates of 53% and 26%, respectively.
CONCLUSION
A combination of the three non-invasive biomarkers is a useful predictor of NAFLD prognosis and can help identify patients with NAFLD who are at a high risk of all-cause mortality.
Keywords: Non-alcoholic fatty liver disease, Non-alcoholic steatohepatitis, Platelet count, Albumin, Type IV collagen 7S, All-cause mortality
Core Tip: We investigated biomarkers associated with mortality in non-alcoholic fatty liver disease (NAFLD) patients who underwent liver biopsy. Blood biomarkers associated with mortality were platelet count, albumin levels, and type IV collagen 7S levels. In particular, 5-year and 10-year survival rates were reduced for patients with all three factors: platelet counts below 15 × 104/μL, albumin levels below 3.5 g/dL, and type IV collagen 7S levels more 5 ng/dL. In summary, the combination of the three non-invasive biomarkers is a useful predictor of NAFLD prognosis and helps identify patients with NAFLD who are at high risk of death from all causes.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease and affects approximately 25% of the general global adult population[1]. The development of NAFLD is associated with lifestyle-related diseases, such as obesity, type 2 diabetes, hypertension, and dyslipidemia. Cardiovascular disease is the leading cause of death among NAFLD patients[2,3]. However, liver-related diseases are also a major cause of death among patients with NAFLD, and liver-specific and all-cause mortality rates are higher for these patients than for the general population NAFLD, and liver-specific and all-cause mortality rates are higher for these patients than for the general population[1]. The incidence of liver-specific and all-cause mortality among patients with NAFLD is generally 0.77 and 11.77 per 1000 years, respectively, while it is 15.44 and 25.56 per 1000 years, respectively, for patients with non-alcoholic steatohepatitis (NASH)[1].
The prognosis of NAFLD patients with advanced liver fibrosis is known to be poor[1,4-8]. Progression of liver fibrosis in patients with NAFLD is associated with mortality from various non-liver-related causes[6].
Liver biopsy is typically performed for diagnosing advanced fibrosis in patients with other liver diseases, such as NASH; however, it is not a practical tool for the diagnosis of NAFLD. In addition, the limitations of liver biopsies, such as invasiveness, poor patient tolerance, sampling variability, and high costs, are well known. Thus, there is increasing interest in developing and validating non-invasive methods for measuring liver stiffness, such as imaging and elastography techniques based on ultrasonography or magnetic resonance imaging[3,4,9-14]. However, a limitation of these methods is that the images are visualized using an instrument that is not available in many institutions. Therefore, serum biomarkers that can assess the progression of liver fibrosis in patients with NAFLD may serve as important tools for identifying patients with advanced fibrosis. Some biomarkers of interest, such as procollagen type III N-terminal propeptide, type IV collagen 7S, hyaluronic acid, and Mac-2 binding protein [WFA(+)-M2BP] levels, and cytokeratin-18 have been used for identifying patients with NAFLD with advanced fibrosis. Other studies have used different biomarker scores, such as the BARD score, NAFLD fibrosis score, FIB-4 (fibrosis-4) index, aspartate aminotransaminase (AST) to alanine aminotransaminase (ALT) ratio, AST to platelet ratio index, FibroTest, and Enhanced Liver Fibrosis score, for the assessment of liver fibrosis[3,4,11,13,14-24]. However, none of these scores predict the prognosis of NAFLD patients. Hence, we aimed to investigate the efficacy of non-invasive biomarkers for predicting disease progression in patients with NAFLD.
MATERIALS AND METHODS
Patients
We retrospectively identified patients with NAFLD who underwent liver biopsy at the Kawasaki Medical School General Medical Center from 1996 to 2018 (Table 1). The exclusion criteria were as follows: history of other liver diseases including hepatitis B virus or hepatitis C virus infections, autoimmune liver diseases, drug-induced liver injury, metabolic liver diseases, or history of alcohol intake (men, ≥ 30 g/d and women, ≥ 20 g/d). Blood tests were performed before the liver biopsy, and we examined the prognostic factors based on the blood test results. The study protocol complied with the guidelines of the 1975 Helsinki Declaration and was approved by the Institutional Research Ethics Committee. Written informed consent was obtained from all the patients.
Table 1.
Clinical and histological characteristics of the patient population (n = 489)
|
Characteristics
|
Values
|
| Age | 50.1 (14-82) |
| Male sex, % | 54.6 |
| Body mass index, kg/m2 | 26.9 (20.8-49.5) |
| Fibrosis stage, 0/1/2/3/4 | 65/173/111/122/18 |
| Grade, 0/1/2/3 | 45/204/178/62 |
| Steatosis, 0/1/2/3 | 13/158/228/90 |
| NAFLD activity score, < 4/≥ 5 | 265/224 |
| ALT, IU/L | 69 (2-563) |
| AST, IU/L | 43 (13-312) |
| γ-GTP, IU/L | 60 (12-736) |
| Total bilirubin, mg/dL | 0.8 (0.04-2.7) |
| Total cholesterol, ng/dL | 198 (102-317) |
| Cholinesterase, IU/L | 205 (90-337) |
| Platelet count, × 104/μL | 20.8 (6.6-44.7) |
| Albumin, g/dL | 4.5 (2.5-5.4) |
| HOMA-IR | 2.9 (0.7-22.4) |
| Iron, μg/dL | 119 (13-295) |
| Ferritin, ng/dL | 149 (3.9-983) |
| Leptin, ng/dL | 9.3 (1.1-59.3) |
| Adiponectin, μg/mL | 5.5 (2.0-27.5) |
| High-sensitivity CRP, mg/dL | 0.117 (0.01-1.92) |
| P-III-P, U/mL | 0.7 (0.28-3.8) |
| Type IV collagen 7S, ng/mL | 4.1 (1.9-15) |
| Hyaluronic acid, ng/mL | 28 (9-619) |
| Fibrosis-4 index | 1.29 (0.17-1.29) |
NAFLD: Non-alcoholic fatty liver disease; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; γ-GTP: Gamma-glutamyl transpeptidase; HOMA-IR: Homeostatic model assessment of insulin resistance; CRP: C-reactive protein; P-III-P: Procollagen-III peptide.
Clinical, biochemical, and histological parameters
We investigated the mortality rate and causes of death among the enrolled patients. We also investigated the development of any complications during the follow-up period. The start date of the follow-up period was defined as the date of liver biopsy and the end date of the follow-up period was defined as the date of last follow-up for surviving patients or the date of death for patients who died during the follow-up period. All NAFLD patients visited our hospital once every 3-6 mo. The following clinical parameters were included in the analysis: age at diagnosis of NAFLD; sex; body mass index calculated as weight (in kg) divided by height (in meters squared); and the presence of diabetes mellitus, hyperlipidemia, and dyslipidemia. We also included the following biochemical parameters in the analysis: platelet count, levels of albumin, total bilirubin, AST, ALT, gamma glutamyl transpeptidase, total cholesterol, cholinesterase, serum iron, ferritin, leptin, adiponectin, and high-sensitivity C-reactive protein, and homeostasis model assessment insulin resistance. The FIB-4 index was calculated as follows: age (years) × AST (U/L)/platelet count (× 104/μL) × √AST (U/L)[13,16,17]. Type IV collagen 7S and procollagen III peptide (P-III-P) were used as indicators of liver fibrosis.
Liver biopsy and histological analysis
All liver biopsies were performed using 16G or 17G biopsy needles with ultrasound guidance or using 14G needles with laparoscopic guidance. The histological examinations were performed by two experienced liver pathologists who were blinded to the patient details. The histological parameters included fibrosis, inflammation, steatosis, hepatocyte ballooning, and the NAFLD activity score (NAS) system[25]. The individual histological features of NAFLD were assessed using the following NAS system proposed by the NASH Clinical Research Network (NASH CRN): lobular inflammation (0-3), steatosis (0-3), and hepatocellular ballooning (0-2)[26,27]. The liver fibrosis stages were assessed according to Brunt’s criteria.
Statistical analysis
The cumulative all-cause mortality and liver-related events during follow-up were assessed using the Kaplan-Meier method and compared using the log-rank test. The Kaplan-Meier analysis included the following variables: steatosis grade, ballooning grade, NAS category, fibrosis stage, albumin, platelet counts, type IV collagen 7S levels, and FIB-4 index. We also calculated the odds ratio and performed receiver operating characteristic (ROC) curve analysis with logistic regression analysis to determine the cutoff values with the highest predictive ability. The optimal cut-off value was determined based on the Youden index. The prognostic performance of the optimal cutoff value was expressed as the diagnostic specificity, sensitivity, positive predictive value, and negative predictive value, using area under the ROC (AUROC) curve analysis. In univariate (unadjusted) and multivariate (adjusted) analyses, the hazard rate ratio estimates (relative risk) for outcomes were calculated using Cox proportional hazard regression analysis to control for the effect of potential risk factors (confounders) while considering the different follow-up durations. A P value < 0.05 was considered significant. All statistical analyses were performed using JMP (version 14.2, SAS system, United States). The statistical methods of this study were reviewed by Akiyoshi Izumi from Asahigawaso Rehabilitation and Medical Center, Okayama.
RESULTS
Survival rate
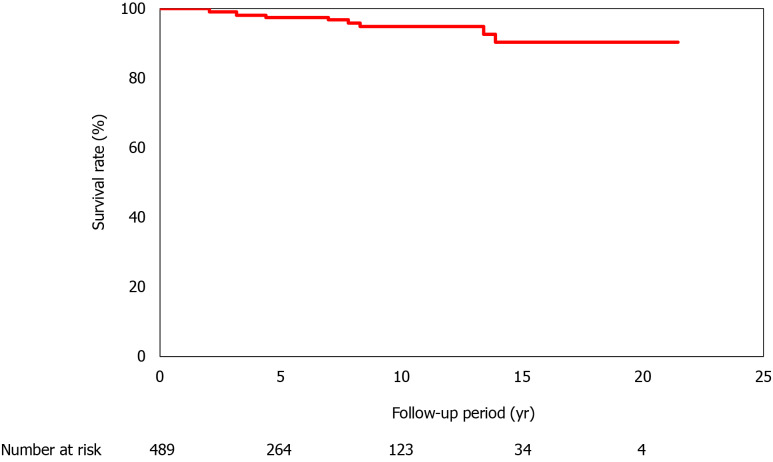
In total, 489 patients were enrolled in the present study; the 5-year survival rate was 98.5%, and the 10-year, 15-year, and 20-year survival rates were 95.4%, 91.9%, and 91.9%, respectively. The follow-up period varied between 1 and 22.2 years (Figure 1). In total, 13 (2.7%) patients died; of these, 7 patients died of liver-related causes [hepatocellular carcinoma (HCC) was observed in 1 patient; Table 2]. The complications that developed during the follow-up period were HCC (n = 12), other organ cancers (n = 13), and cerebrovascular disorders (n = 9).
Figure 1.
Survival of the 489 patients with non-alcoholic fatty liver disease. The follow-up period varied between 1 yr and 21.2 yr, and all-cause mortality was considered. The survival rates are 98.5% at 5 yr, 95.4% at 10 yr, 91.9% at 15 yr, and 91.9% at 20 yr.
Table 2.
Summary of the causes of death
|
|
n
(%)
|
| All deaths | 13 (2.7) |
| Liver-related events | 7 (1.4) |
| HCC + liver failure | 3 |
| HCC only | 1 |
| Liver failure | 3 |
| Cerebrovascular disease | 1 (0.2) |
| Non-liver cancers | 4 (0.8) |
| Pancreatic cancer | 2 |
| Bile duct cancer | 2 |
| Infection | 1 (0.2) |
HCC: Hepatocellular carcinoma.
Liver histological findings
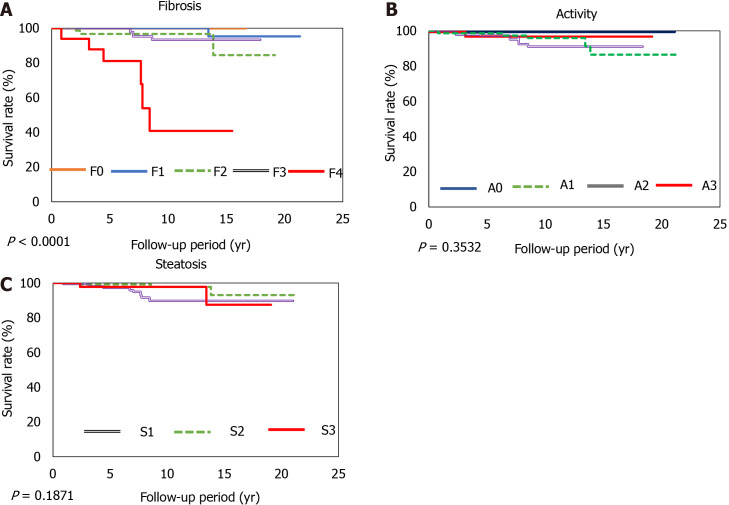
Patients presenting with progression of advanced liver fibrosis after liver biopsy had increased mortality. The 5-year and 10-year survival rates of patients with NASH CRN Stage 4 disease were 81% and 41%, respectively. However, the degree of inflammation or steatosis was not associated with poor prognosis. The optimal area under the curve for albumin was 3.8 and 3.5 with specificities of 47% and 39%, sensitivities of 95% and 99%, positive predictive values of 98% and 98%, and negative predictive values of 21% and 56% (Figure 2).
Figure 2.
Survival rates according to the grading of fibrosis, inflammation, and steatosis. The overall survival rates for stage 4 liver fibrosis are 81% at 5 yr and 41% at 10 yr. A: Fibrosis (F0-4); B: Inflammation (A0-3); C: Steatosis (S1-3).
Blood test factors
A univariate Cox hazard model was used for analyzing factors associated with mortality at the time of diagnosis of the NASH Clinical Research Network. We found that the ALT levels, platelet counts, albumin levels, and levels of liver fibrosis markers (P-III-P, type IV collagen 7S and FIB-4 index) were significantly associated with mortality (Table 3).
Table 3.
Factors associated with mortality among the patients with non-alcoholic fatty liver disease (n = 489)
|
|
AUROC
|
Odds ratio
|
95%CI
|
P
value
|
| AST | 0.57 | 1.00 | 0.99-1.02 | 0.9841 |
| ALT | 0.71 | 0.97 | 0.95-0.99 | 0.0026 |
| γ-GTP | 0.521 | 1.00 | 0.99-1.01 | 0.4259 |
| Platelet count | 0.748 | 0.78 | 0.69-0.88 | < 0.0001 |
| Total bilirubin | 0.588 | 1.10 | 0.55-1.39 | 0.3208 |
| Total cholesterol | 0.580 | 0.99 | 0.98-1.01 | 0.2 |
| Iron | 0.553 | 1.01 | 1.02 | 0.1801 |
| Albumin | 0.815 | 0.093 | 0.04-0.20 | < 0.0001 |
| Ferritin | 0.527 | 1.00 | 1.00-1.00 | 0.7651 |
| Leptin | 0.565 | 1.00 | 0.94-1.06 | 0.7441 |
| HOMA-IR | 0.731 | 1.04 | 1.009-1.06 | 0.0182 |
| P-III-P | 0.786 | 5.58 | 2.27-11.6 | 0.0014 |
| Type IV collagen 7S | 0.863 | 1.48 | 1.28-1.67 | < 0.0001 |
| Fibrosis-4 index | 0.914 | 1.799 | 1.44-2.23 | < 0.0001 |
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; CI: Confidence interval; γ-GTP: Gamma-glutamyl transpeptidase; HOMA-IR: Homeostatic model assessment of insulin resistance; P-III-P: Procollagen-III peptide; AUROC: Area under the receiver operating characteristic curve.
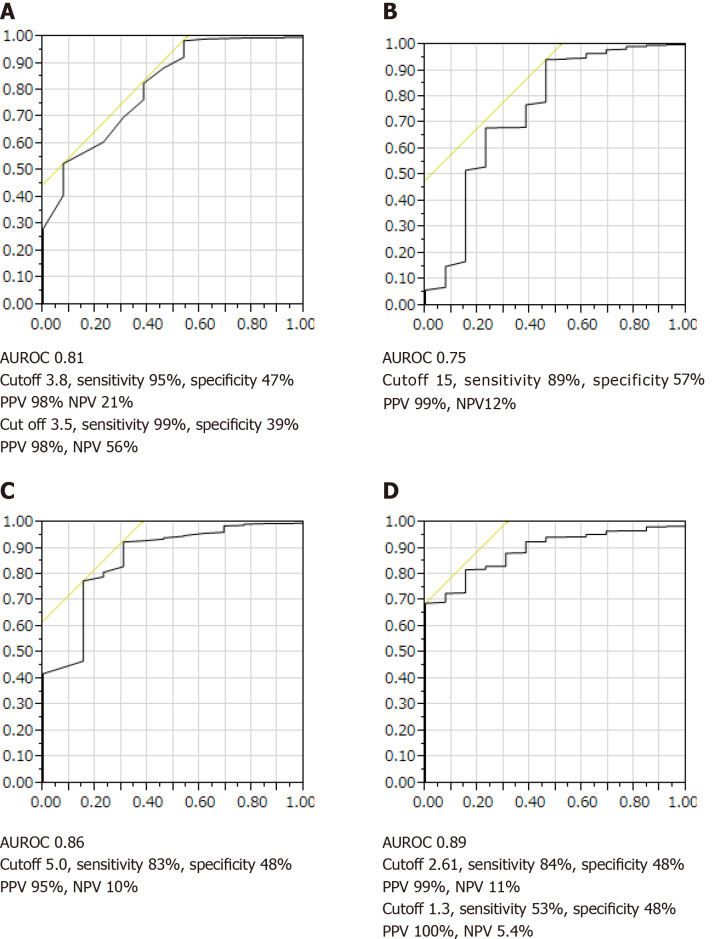
Survival curves were created using the following biomarkers: type IV collagen 7S, platelet count, albumin, and FIB-4 index. ALT was not included as a biomarker because the levels frequently varied. To investigate the predictive performance of these biomarkers with respect to NAFLD mortality, an optimal COI for type IV collagen 7S level, platelet count, albumin level, and FIB-4 index was determined based on the ROC curve analysis of all 489 patients with NAFLD. As shown in Figure 3A-D, the cutoff values for the platelet count, albumin level, type IV collagen 7S concentration and the FIB-4 index were set at 15 × 104, 3.8 g/dL, and 3.5 mg/dL, 5.0 ng/mL, and 1.3 and 2.61, respectively.
Figure 3.
Receiver-operating characteristic curves for survival among patients with non-alcoholic fatty liver disease. A: Albumin concentration; B: Platelet count; C: Type IV collagen 7S concentration; D: Fibrosis-4 index. AUROC: Area under the receiver operating characteristic curve, PPV: Positive predictive value, NPV: Negative predictive value.
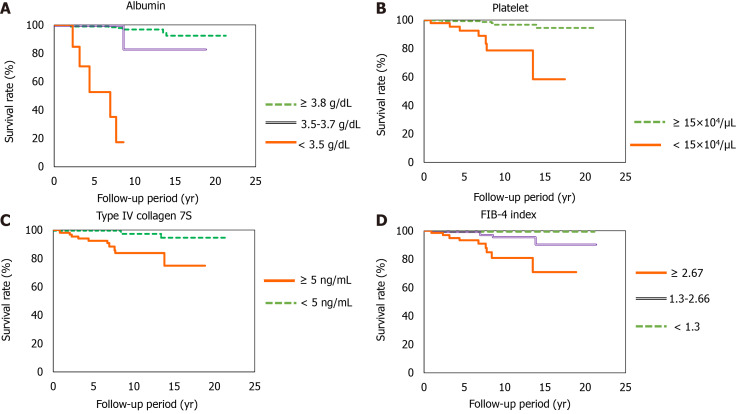
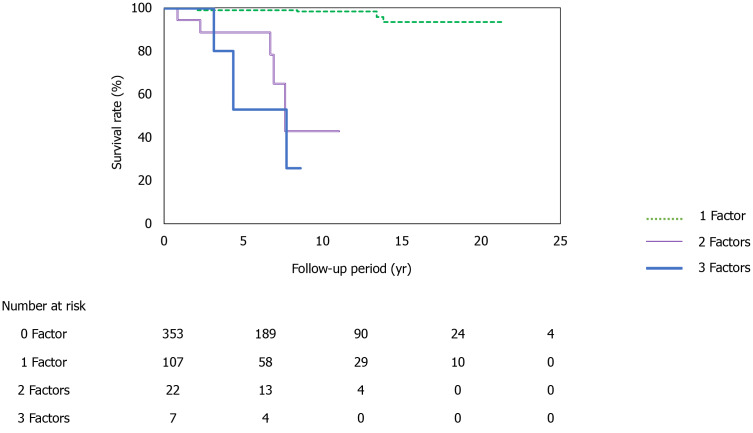
At the time of NASH diagnosis, patients with albumin levels < 3.5 mg/dL, platelet counts < 15 × 104, type IV collagen 7S levels ≥ 5 ng/dL, and FIB-4 indexes ≥ 2.67 clearly showed reduced survival (Figure 4A-D). Furthermore, we investigated the prognosis by combining type IV collagen 7S, which had a high AUROC among liver fibrosis markers (type IV collagen 7S, P-III-P, and FIB-4 index), the albumin level, and platelet count. Albumin level < 3.5 mg/dL, platelet count < 15 × 104/µL, and type IV collagen 7S levels ≥ 5 ng/dL were examined individually and in combination. The 5-year, 10-year, and 15-year survival rates for patients with only one factor were 99.7%, 98.3%, and 94%, respectively. However, survival rates were low for patients who presented with more than one factor. For these individuals, the 5-year and 10-year survival rates were 98% and 43%, respectively. For those who presented with two factors, the 5-year and 10-year survival were 53% and 26%, respectively, and for those presenting with three factors (Figure 5).
Figure 4.
Survival rates. A: Albumin concentration (albumin ≥ 3.8 g/dL vs 3.5-3.7 g/dL; P < 0.001, albumin ≥ 3.8 g/dL vs < 3.5 g/dL; P < 0.0001, albumin 3.5-3.7 g/dL vs < 3.5; P < 0.0001); B: Platelet count (platelet ≥ 15 × 104/µL vs < 15 × 104/µL; P < 0.0001); C: Type IV collagen 7S concentration (type IV collagen 7S ≥ 5 ng/mL vs < 5 ng/mL; P < 0.0001); D: Fibrosis-4 index (Fibrosis-4 index ≥ 2.67 vs 1.3-2.67; P < 0.001, Fibrosis-4 index C1.3-2.67 vs < 1.3; P < 0.0001, Fibrosis-4 index ≥ 2.67 vs < 2.67; P < 0.0001). FIB: Fibrosis.
Figure 5.
Survival rates according to positivity for the different biomarkers. Patients with only one risk factor have relatively good survival rates at 5 yr (99.7%), 10 yr (98.3%), and 15 yr (94%). However, patients with two risk factors have lower survival rates at 5 yr (98%) and 10 yr (43%), and patients with all three risk factors have even lower survival rates at 5 yr (53%) and 10 yr (26%) (1 factor vs 2 factors, P < 0.0001; 1 factor vs 3 factors, P < 0.0001; 2 factors vs 3 factors; P < 0.05).
DISCUSSION
To the best of our knowledge, this study is the first study to evaluate the predictors of the prognosis of NAFLD based on the results of a blood test. We found that a combination of three non-invasive biomarkers, namely, platelet count, albumin level, and type IV collagen 7S level, is a useful predictor of NAFLD prognosis. The major causes of death in patients with NAFLD are cardiovascular events, organ cancers other than liver cancer, and liver-related disease. Among Japanese patients with NAFLD, the reported mortality rates associated with NAFLD are low during the follow-up period. The causes of death are more likely to be cancers of other organs and cerebral cardiovascular events than liver-related pathologies[28].
The most important predictor of outcomes among patients with NAFLD is the progression of liver fibrosis[1,5-7]. Angulo et al[6] retrospectively analyzed the long-term outcomes of 619 patients diagnosed with NAFLD in the United States, Europe, and Thailand during 1975-2005[6] and reported that only liver fibrosis, among various longitudinal histological features, was associated with disease prognosis. Only liver fibrosis was independently associated with long-term all-cause mortality, liver transplantation, and liver-related events. Meta-analyses have also reported that liver fibrosis is an important risk factor for liver-related mortality[1,7]. Compared with NAFLD patients without fibrosis, NAFLD patients with fibrosis were at an increased risk of all-cause mortality, and the risk increased as fibrosis progressed[7]. In our study, patients with advanced liver fibrosis, especially cirrhosis, also showed poor prognosis; however, an association with inflammation, steatosis, or ballooning was not noted. Our findings further confirm that the progression of fibrosis markedly affects the prognosis of patients with NAFLD.
Several biomarkers can be used to evaluate liver fibrosis in patients with NAFLD[3,4,11,13,14-25,29]; however, previous studies have not examined disease prognosis using blood biomarker levels recorded at the time of NAFLD diagnosis using liver biopsy.
NAFLD may progress rapidly in some patients and slowly in other patients. Singh et al[5] performed a systematic review and meta-analysis of 11 paired biopsy cohort studies that included 411 patients with > 2145 person-years of follow-up data and reported that approximately 30% of the patients developed advanced fibrosis and 70% of the patients remained stable or the stage of fibrosis in these patients improved. Furthermore, the annual fibrosis progression rates were 0.07 stages for patients with NAFLD and 0.14 stages for patients with NASH. Nasr et al[30] conducted a biochemical, clinical, and histological analysis of 129 patients with NAFLD who were enrolled between 1988 and 1993 in a prospective cohort study and followed them for 19.8 years. They reported that end-stage liver disease developed in 12 (9.3%) patients and advanced fibrosis developed in 34% of the patients. Furthermore, among the 113 patients with low baseline fibrosis (stage 3), 16% of the patients developed advanced fibrosis. No differences in clinical, histological, or biochemical variables were observed between patients who developed liver fibrosis and those who did not. These studies did not examine the association of PNPLA3 polymorphisms with menopause. Although the difference in the progression of NASH and NAFLD is not clear, racial differences and genetic factors, including PNPLA3 expression[31], weight gain, onset and deterioration of diabetes[32], sex differences, and menopausal factors, affect prognosis[33].
It is necessary to consider the various factors that affect disease progress in each case of NAFLD. Although several studies have reported on the evaluation of biomarkers and elastography methods that can predict the progression of liver fibrosis[3,4,11,13,14-25], non-invasive biomarkers that can easily predict the prognosis of NAFLD have not been identified to date.
Our results indicate that patients with NAFLD who present with a combination of albumin level < 3.5 g/dL, platelet count < 15 × 104/µL, and type IV collagen 7S level ≥ 5 ng/mL show poor prognosis. In particular, the 10-year survival rate was only 43% for patients who presented with all three factors. We observed that type IV collagen 7S was a more useful indicator of advanced liver fibrosis than other biomarkers (Table 3). Yoneda et al[24] reported that the type IV collagen 7S level is a more useful marker of prognosis for patients with advanced fibrosis associated with NASH than for patients with mild fibrosis. Furthermore, a scoring system that uses type IV collagen 7S and AST levels, named the CA index, has been reported to predict NASH and fibrosis associated with NAFLD with sufficient accuracy, thus allowing for convenient diagnosis and screening of NASH and associated fibrosis[21]. The same index was found to be useful in 400 Japanese patients from 18 institutes with biopsy-proven NAFLD and advanced liver fibrosis due to CA or FA fibrosis. The CA index is a combination of AST and type IV collagen 7S levels, and the FM fiber index includes type IV collagen 7S and hyaluronic acid levels and vascular cell adhesion[25]. The type IV collagen 7S level is useful for determining advanced fibrosis in patients with NASH and was found to be more sensitive and specific than other fibrosis markers assessed in our study.
Albumin is also an important biomarker for predicting the prognosis of HCC in patients with NAFLD. Kawaguchi et al[34] analyzed the factors affecting survival by performing a random forest analysis for 247 NAFLD-HCC patients diagnosed between 2000 and 2014 and recruited from 17 medical institutions in Japan. The results showed that the best prognostic profile for patients with NAFLD-HC comprised treatment for HCC and serum albumin levels > 3.7 g/dL.
There are some limitations of this study. We did not classify prognosis according to all-cause mortality; moreover, the study population comprised patients from at a single center. Nevertheless, it is significant that the study followed a long-term course of up to 20 years.
In our study, the platelet count, albumin level, type IV collagen 7S level, and the FIB-4 index were important prognostic factors at the time of diagnosis of NAFLD. Our findings suggest that these factors should be recorded in patients with NAFLD at the time of diagnosis to determine future treatment strategies.
Studies conducted in the future should focus on assessing these biomarkers further and examining long-term prognosis using Fibroscan and magnetic resonance elastography. Further research is also needed to confirm these findings in other populations.
CONCLUSION
This study may prove useful in clinical practice because simple predictors of NAFLD progression, namely, albumin level, platelet count, and type IV collagen 7S level, were identified; all these parameters are can be easily assessed in daily practice.
ARTICLE HIGHLIGHTS
Research background
Non-alcoholic steatohepatitis has few symptoms until it progresses; thus, it is necessary to identify non-alcoholic fatty liver disease (NAFLD) patients who will show poor prognosis.
Research motivation
The limitations of liver biopsies, such as invasiveness, poor patient tolerance, sampling variability, and high costs, are well known. Thus, there is increasing interest in developing and validating non-invasive methods for measuring liver stiffness. However, many current methods involve instruments that are not available in many institutions.
Research objectives
Serum biomarkers that can assess the progression of liver fibrosis in patients with NAFLD may serve as important tools for identifying patients with advanced fibrosis. We aimed to investigate the efficacy of non-invasive biomarkers for predicting disease progression in patients with NAFLD.
Research methods
We investigated biomarkers with predictable prognosis for NAFLD patients who underwent liver biopsy. All patients were followed-up for > 1 year.
Research results
The combination of three non-invasive biomarkers involved in NAFLD prognosis comprised platelet counts, albumin levels, and type IV collagen 7S. Our results indicate that patients with NAFLD who present with a combination of albumin levels < 3.5 g/dL, platelet counts < 15 × 104/µL, and type IV collagen 7S levels ≥ 5 ng/mL show poor prognosis. In particular, the 10-year survival rate was only 43% for patients who presented with all three factors.
Research conclusions
The combination of platelet count, albumin level, and type IV collagen 7S was useful in further predicting the prognosis of NAFLD.
Research perspectives
Studies conducted in the future should focus on assessing these biomarkers further and examining long-term prognosis.
Footnotes
Institutional review board statement: The study protocol complied with guidelines of the 1975 Helsinki Declaration and was approved by the Institutional Research Ethics Committee (approval No. 3027).
Informed consent statement: Written informed consent was obtained from all the patients.
Conflict-of-interest statement: The authors declare that they have no competing interests.
Manuscript source: Unsolicited manuscript
Peer-review started: December 31, 2020
First decision: February 13, 2021
Article in press: April 22, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chai J S-Editor: Gao CC L-Editor: A P-Editor: Wang LL
Contributor Information
Miwa Kawanaka, Department of General Internal Medicine, Kawasaki Medical School, Okayama 700-8505, Japan. m.kawanaka@med.kawasaki-m.ac.jp.
Ken Nishino, Department of General Internal Medicine, Kawasaki Medical School, Okayama 700-8505, Japan.
Katsunori Ishii, Department of General Internal Medicine, Kawasaki Medical School, Okayama 700-8505, Japan.
Tomohiro Tanikawa, Department of General Internal Medicine, Kawasaki Medical School, Okayama 700-8505, Japan.
Noriyo Urata, Department of General Internal Medicine, Kawasaki Medical School, Okayama 700-8505, Japan.
Mitsuhiko Suehiro, Department of General Internal Medicine, Kawasaki Medical School, Okayama 700-8505, Japan.
Takako Sasai, Department of General Internal Medicine, Kawasaki Medical School, Okayama 700-8505, Japan.
Ken Haruma, Department of General Internal Medicine, Kawasaki Medical School, Okayama 700-8505, Japan.
Hirofumi Kawamoto, Department of General Internal Medicine, Kawasaki Medical School, Okayama 700-8505, Japan.
Data sharing statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59:1121–1140. doi: 10.1007/s00125-016-3902-y. [DOI] [PubMed] [Google Scholar]
- 3.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 4.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 5.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2015; 13: 643-54. :quiz e39–40. doi: 10.1016/j.cgh.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2015; 149: 389-97. :e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, Sebastiani G, Ekstedt M, Hagstrom H, Nasr P, Stal P, Wong VW, Kechagias S, Hultcrantz R, Loomba R. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65:1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, Kechagias S. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67:1265–1273. doi: 10.1016/j.jhep.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 9.Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, Fujita K, Yoneda M, Taguri M, Hyogo H, Sumida Y, Ono M, Eguchi Y, Inoue T, Yamanaka T, Wada K, Saito S, Nakajima A. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology 2016; 150: 626-637. :e7. doi: 10.1053/j.gastro.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 10.Ferraioli G, Wong VW, Castera L, Berzigotti A, Sporea I, Dietrich CF, Choi BI, Wilson SR, Kudo M, Barr RG. Liver Ultrasound Elastography: An Update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound Med Biol. 2018;44:2419–2440. doi: 10.1016/j.ultrasmedbio.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Vilar-Gomez E, Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: Clinical prediction rules and blood-based biomarkers. J Hepatol. 2018;68:305–315. doi: 10.1016/j.jhep.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Younossi ZM, Loomba R, Anstee QM, Rinella ME, Bugianesi E, Marchesini G, Neuschwander-Tetri BA, Serfaty L, Negro F, Caldwell SH, Ratziu V, Corey KE, Friedman SL, Abdelmalek MF, Harrison SA, Sanyal AJ, Lavine JE, Mathurin P, Charlton MR, Goodman ZD, Chalasani NP, Kowdley KV, George J, Lindor K. Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology. 2018;68:349–360. doi: 10.1002/hep.29721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology. 2017;66:1486–1501. doi: 10.1002/hep.29302. [DOI] [PubMed] [Google Scholar]
- 14.Kawanaka M, Nishino K, Nakamura J, Urata N, Oka T, Goto D, Suehiro M, Kawamoto H, Yamada G. Correlation between serum cytokeratin-18 and the progression or regression of non-alcoholic fatty liver disease. Ann Hepatol. 2015;14:837–844. doi: 10.5604/16652681.1171767. [DOI] [PubMed] [Google Scholar]
- 15.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, Lindor K, Sanderson SO, Lenzi M, Adams LA, Kench J, Therneau TM, Day CP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 16.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 17.Wong VW, Adams LA, de Lédinghen V, Wong GL, Sookoian S. Noninvasive biomarkers in NAFLD and NASH - current progress and future promise. Nat Rev Gastroenterol Hepatol. 2018;15:461–478. doi: 10.1038/s41575-018-0014-9. [DOI] [PubMed] [Google Scholar]
- 18.Sumida Y, Yoneda M, Hyogo H, Itoh Y, Ono M, Fujii H, Eguchi Y, Suzuki Y, Aoki N, Kanemasa K, Fujita K, Chayama K, Saibara T, Kawada N, Fujimoto K, Kohgo Y, Yoshikawa T, Okanoue T Japan Study Group of Nonalcoholic Fatty Liver Disease (JSG-NAFLD) Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012;12:2. doi: 10.1186/1471-230X-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srivastava A, Gailer R, Tanwar S, Trembling P, Parkes J, Rodger A, Suri D, Thorburn D, Sennett K, Morgan S, Tsochatzis EA, Rosenberg W. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J Hepatol. 2019;71:371–378. doi: 10.1016/j.jhep.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 21.Okanoue T, Ebise H, Kai T, Mizuno M, Shima T, Ichihara J, Aoki M. A simple scoring system using type IV collagen 7S and aspartate aminotransferase for diagnosing nonalcoholic steatohepatitis and related fibrosis. J Gastroenterol. 2018;53:129–139. doi: 10.1007/s00535-017-1355-9. [DOI] [PubMed] [Google Scholar]
- 22.Anstee QM, Lawitz EJ, Alkhouri N, Wong VW, Romero-Gomez M, Okanoue T, Trauner M, Kersey K, Li G, Han L, Jia C, Wang L, Chen G, Subramanian GM, Myers RP, Djedjos CS, Kohli A, Bzowej N, Younes Z, Sarin S, Shiffman ML, Harrison SA, Afdhal NH, Goodman Z, Younossi ZM. Noninvasive Tests Accurately Identify Advanced Fibrosis due to NASH: Baseline Data From the STELLAR Trials. Hepatology. 2019;70:1521–1530. doi: 10.1002/hep.30842. [DOI] [PubMed] [Google Scholar]
- 23.Kamada Y, Ono M, Hyogo H, Fujii H, Sumida Y, Yamada M, Mori K, Tanaka S, Maekawa T, Ebisutani Y, Yamamoto A, Takamatsu S, Yoneda M, Kawada N, Chayama K, Saibara T, Takehara T, Miyoshi E Japan Study Group of Nonalcoholic Fatty Liver Disease (JSG‐NAFLD) Use of Mac-2 binding protein as a biomarker for nonalcoholic fatty liver disease diagnosis. Hepatol Commun. 2017;1:780–791. doi: 10.1002/hep4.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoneda M, Mawatari H, Fujita K, Yonemitsu K, Kato S, Takahashi H, Kirikoshi H, Inamori M, Nozaki Y, Abe Y, Kubota K, Saito S, Iwasaki T, Terauchi Y, Togo S, Maeyama S, Nakajima A. Type IV collagen 7s domain is an independent clinical marker of the severity of fibrosis in patients with nonalcoholic steatohepatitis before the cirrhotic stage. J Gastroenterol. 2007;42:375–381. doi: 10.1007/s00535-007-2014-3. [DOI] [PubMed] [Google Scholar]
- 25.Itoh Y, Seko Y, Shima T, Nakajima T, Mizuno K, Kawamura Y, Akuta N, Ito K, Kawanaka M, Hiramatsu A, Sakamoto M, Harada K, Goto Y, Nakayama T, Kumada H, Okanoue T. Accuracy of non-invasive scoring systems for diagnosing non-alcoholic steatohepatitis-related fibrosis: Multicenter validation study. Hepatol Res. 2018;48:1099–1107. doi: 10.1111/hepr.13226. [DOI] [PubMed] [Google Scholar]
- 26.Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA NASH Clinical Research Network (CRN) Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810–820. doi: 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 28.Bedossa P FLIP Pathology Consortium. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology. 2014;60:565–575. doi: 10.1002/hep.27173. [DOI] [PubMed] [Google Scholar]
- 29.Tada T, Kumada T, Toyoda H, Mizuno K, Sone Y, Akita T, Tanaka J. Progression of liver fibrosis is associated with non-liver-related mortality in patients with nonalcoholic fatty liver disease. Hepatol Commun. 2017;1:899–910. doi: 10.1002/hep4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nasr P, Ignatova S, Kechagias S, Ekstedt M. Natural history of nonalcoholic fatty liver disease: A prospective follow-up study with serial biopsies. Hepatol Commun. 2018;2:199–210. doi: 10.1002/hep4.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seko Y, Sumida Y, Tanaka S, Mori K, Taketani H, Ishiba H, Hara T, Okajima A, Umemura A, Nishikawa T, Yamaguchi K, Moriguchi M, Kanemasa K, Yasui K, Imai S, Shimada K, Itoh Y. Development of hepatocellular carcinoma in Japanese patients with biopsy-proven non-alcoholic fatty liver disease: Association between PNPLA3 genotype and hepatocarcinogenesis/fibrosis progression. Hepatol Res. 2017;47:1083–1092. doi: 10.1111/hepr.12840. [DOI] [PubMed] [Google Scholar]
- 32.Jarvis H, Craig D, Barker R, Spiers G, Stow D, Anstee QM, Hanratty B. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of population-based observational studies. PLoS Med. 2020;17:e1003100. doi: 10.1371/journal.pmed.1003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klair JS, Yang JD, Abdelmalek MF, Guy CD, Gill RM, Yates K, Unalp-Arida A, Lavine JE, Clark JM, Diehl AM, Suzuki A Nonalcoholic Steatohepatitis Clinical Research Network. A longer duration of estrogen deficiency increases fibrosis risk among postmenopausal women with nonalcoholic fatty liver disease. Hepatology. 2016;64:85–91. doi: 10.1002/hep.28514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawaguchi T, Tokushige K, Hyogo H, Aikata H, Nakajima T, Ono M, Kawanaka M, Sawada K, Imajo K, Honda K, Takahashi H, Mori K, Tanaka S, Seko Y, Nozaki Y, Kamada Y, Fujii H, Kawaguchi A, Takehara T, Yanase M, Sumida Y, Eguchi Y, Seike M, Yoneda M, Suzuki Y, Saibara T, Karino Y, Chayama K, Hashimoto E, George J, Torimura T. A Data Mining-based Prognostic Algorithm for NAFLD-related Hepatoma Patients: A Nationwide Study by the Japan Study Group of NAFLD. Sci Rep. 2018;8:10434. doi: 10.1038/s41598-018-28650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.