Abstract
Oral cancer patients often have severe, chronic, and mechanically induced pain at the site of the primary cancer. Oral cancer pain is initiated and maintained in the cancer microenvironment and attributed to release of mediators that sensitize primary sensory nerves. This study was designed to investigate the histopathology associated with painful oral cancers in a preclinical model. The relationship of pain scores with pathologic variables was also investigated in a cohort of 72 oral cancer patients. Wild-type mice were exposed to the carcinogen, 4-nitroquinoline 1-oxide (4NQO). Nociceptive (pain) behavior was measured with the dolognawmeter, an operant device and assay for measuring functional and mechanical allodynia. Lesions developed on the tongues and esophagi of the 4NQO-treated animals and included hyperkeratoses, papillomas, dysplasias, and cancers. Papillomas included lesions with benign and dysplastic pathological features. Two histologic subtypes of squamous cell carcinomas (SCCs) were identified—SCCs with exophytic and invasive components associated with papillary lesions (pSCCs) and invasive SCCs without exophytic histology (iSCCs). Only the pSCC subtype of tongue cancer was associated with nociceptive behavior. Increased tumor size was associated with greater nociceptive behavior in the mouse model and more pain experienced by oral cancer patients. In addition, depth of invasion was associated with patient-reported pain. The pSCC histology identifies 4NQO-induced tongue cancers that are expected to be enriched for expression and release of nociceptive mediators.
Keywords: oral SCC, 4-nitroquinoline 1-oxide, oral carcinogenesis, oral cancer pathology, nociception, nociceptive pain
Introduction
Cancer patients variably have pain at the site of the cancer and/or at metastatic sites. Sensitization and activation of peripheral nerves innervating the cancer microenvironment occurs via release of algesic substances from the cancer cells and cells of the cancer microenvironment, including inflammatory mediators from immune cell infiltrate. Cancer pain can also be attributed to damage to peripheral nerves that can result in neuropathy and persistent pain (Schmidt et al. 2010).
Oral cancer pain is unique (Viet and Schmidt 2012). Oral cancer patients report severe chronic and mechanically induced pain at the site of the cancer (Connelly and Schmidt 2004). Often pain is the first symptom that motivates patients to seek care. Patients with precancerous oral lesions (dysplasia) do not report pain (Lam and Schmidt 2011; Bhattacharya et al. 2020). Clinical findings and preclinical studies indicate that cancer pain is initiated and maintained in the cancer microenvironment. Most oral cancer patients do not demonstrate central sensitization (i.e., neuropathic pain) (Viet and Schmidt 2012). Pain regresses after surgical resection of the cancer (Kolokythas et al. 2007). Nociceptive mediators have been identified and nociceptive behavior reduced or eliminated by antagonizing specific mediators (Viet and Schmidt 2012). Not all oral cancer patients, however, report pain (Connelly and Schmidt 2004). Oral cancers are heterogeneous—in their pathology, molecular lesions, and with respect to response to therapy (Schmidt et al. 2010). These observations raise the question as to why pain is associated with some oral cancers.
We use the 4-nitroquinoline 1-oxide (4NQO) carcinogenesis murine model to study pain and test strategies to alleviate pain (Lam et al. 2012). The model recapitulates oral cancer progression and heterogeneity. With time following exposure to 4NQO, multiple lesions arise in the oral cavity, primarily on the tongue, and esophagus. Animals develop field changes, with precancers (oral epithelial dysplasia), papillomas, carcinoma in situ (CIS), and squamous cell carcinoma (SCC). The mice also develop functional allodynia (Lam et al. 2012). The objective of this study was to determine whether a greater number of lesions or specific lesion types were associated with increased nociceptive behavior in this model.
Materials and Methods
Detailed methods are provided in the Appendix data.
Mouse Studies
4NQO Treatment
Animals were housed in a temperature-controlled, pathogen-free room on a 12:12 light/dark cycle (6 a.m.–6 p.m.) with ad libitum access to food and water. Procedures involving animals were approved by the New York University Institutional Animal Care and Use Committee (IACUC) under protocol 160908-01, in accordance with the Guide for the Care and Use of Laboratory Animals (U.S. Institute for Laboratory Animal Research, 8th edition), and ARRIVE guidelines. For all experiments, animals were habituated to handling prior to testing. Estrous cycles were not monitored. Forty C57BL/6 female mice (stock #000664; Jackson Laboratories) were offered drinking water with 100 µg/mL 4NQO (catalog number N0250; TCI America) for 16 wk. Following withdrawal of 4NQO, the mice consumed tap water and were followed until 28 wk after introduction of 4NQO. Animals were sacrificed in accordance with IACUC recommendations. Cervical dislocation was performed after anesthesia by isoflurane inhalation.
Dolognawmeter Assay
Nociceptive behavior was measured using the dolognawmeter device, a validated operant assay for measuring mechanical functional allodynia (Dolan et al. 2010), in which the time for mice to gnaw through dowels to escape confinement is measured. The assay was modified to ensure that the mice would complete the task as cancer developed by using a lower durometer (hardness) second dowel (Dolan et al. 2010). The final nociception score for each animal was calculated as the median of the percentage change in gnaw time from baseline (100 × (session gnaw time – baseline gnaw time)/baseline gnaw time) over the last 4 sessions for the first dowel.
Pathologic Analysis of Harvested Mouse Tissues
At the time of sacrifice, tongues were excised, examined clinically and under a stereo microscope (magnification 80× to 100×, Leica MZ12; Leica Microsystems) for the presence of visible lesions prior to fixation. Tissues were fixed in 10% formalin and sectioned and stained with hematoxylin and eosin (H&E). Whole-slide scanning (magnification 400×) was performed for pathologic analysis. Histologic diagnoses were rendered using established criteria (Abbey et al. 1995; Warnakulasuriya et al. 2008). Hyperkeratoses were characterized by a thickened keratinized layer, with or without a thickened spinous layer (acanthosis), and an absence of nuclear or cellular atypia. Exophytic papillary lesions without stromal invasion were called papillomas. Lesions that showed frank invasion into the underlying connective tissue stroma were considered SCCs. Dysplasias were characterized as lesions that showed histopathologic alterations, including enlarged nuclei and cells, large and/or prominent nucleoli, increased nuclear to cytoplasmic ratio, hyperchromatic nuclei, dyskeratosis, increased and/or abnormal mitotic figures, bulbous or teardrop-shaped rete ridges, loss of polarity, and loss of typical epithelial cell cohesiveness. All lesions showing cytologic atypia but lacking evidence of invasion were grouped under the single category of dysplasia (Hasina et al. 2009). Dysplasia was not graded because of the subjective nature of epithelial dysplasia grading and its limited ability to predict biological progression (Abbey et al. 1995; Warnakulasuriya et al. 2008; Hasina et al. 2009). Moreover, in our experience, 100% of the mice exposed to 4NQO harbor field dysplastic changes dispersed through the tongue epithelium. Therefore, grading dysplasia would not contribute to differentiating groups. Depth of tumor invasion (DOI) and tumor size (greatest dimension) of SCCs were measured following established guidelines (Berdugo et al. 2019) using ImageJ software (National Institutes of Health). Perineural invasion (PNI) was defined as the invasion of cancer into or around 33.3% of the circumference of the nerve (Liebig et al. 2009; Chi et al. 2016). Lymphovascular invasion (LVI) was defined as foci of tumor surrounded by a clear space and with a well-visualized endothelial lining on H&E-stained sections (Larson et al. 2020). The inflammatory infiltrate was identified by inflammatory cell morphology in H&E-stained sections. The reviewing pathologist (AB) was blinded to the pain scores during pathological evaluation.
Human Studies
In a previous study (Bhattacharya et al. 2020), we assembled a cohort of human oral cancer patients (n = 72) for study of the association of neck lymph node metastasis with pathologic and clinical features, including pain scores. Pathological data were retrieved from surgical pathology reports. Patient-reported pain was evaluated with the validated University of California Oral Cancer Pain Questionnaire (UCSFOCPQ). The study was approved by the New York University (NYU) School of Medicine Institutional Review Board (IRB 10-01261) and was carried out in accordance with the NYU School of Medicine Policies and Procedures for Human Subjects Research Protection. All patients consented to participate in the study.
Data Analysis
Pearson correlations were computed between the presence and number of lesion types and pain scores. Mean pain scores were compared between cancerous and noncancerous lesions with an independent samples t test. Analyses were computed using IBM SPSS (v24; SPSS, Inc.) and GraphPad Prism 8 for MacOS v8.3.1 (GraphPad Software). Exact P values were given for all tests when those values were ≥0.001; otherwise, P < 0.001 was used.
Results
Multiple Pathological Lesions Developed on the Tongues and Esophagi of 4NQO-Treated Mice
Tongue Lesions
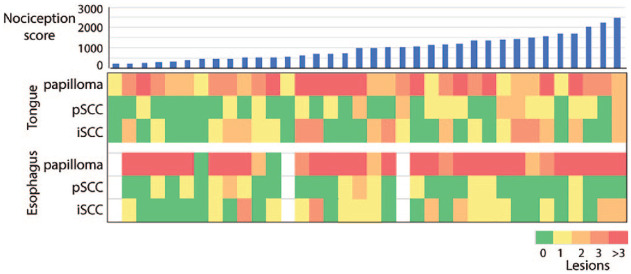
Pathological analysis of 36 tongues revealed multiple lesions on all tongues (Fig. 1A), consistent with previous reports (Hawkins et al. 1994; Tang et al. 2004; Hasina et al. 2009). Papillomas were present in all tongues and included papillary exophytic lesions with benign histology, as well as those showing varying grades of dysplasia. The papillomas were either sessile (directly attached to the epithelium; Fig. 1B) or pedunculated (attached to the epithelium by a stalk; Fig. 1C). Papillary exophytic lesions with invasive features (Fig. 1A, D) were identified in 17 mice. We called these lesions pSCCs to distinguish them from SCCs lacking papillary features (invasive SCCs [iSCCs]; Fig. 1E). Cancers were present in 72% (n = 26) of tongues (Fig. 1D, E). Hyperkeratoses (Fig. 1F) and dysplasias (Fig. 1G, H) were observed in all tongues. Among the iSCCs, we observed in 2 mice deeply invasive SCCs (diSCCs) that had invaded >2 mm in depth and involving >75% of the lateral or dorsoventral thickness of the tongue (Fig. 2A, B). Individual tongues harbored more than 1 cancer (Fig. 3), including multiple pSCCs (n = 5 tongues), iSCCs (n = 17 tongues), or both iSCCs and pSCCs in their tongues (n = 11 tongues). Papillomas and SCCs were observed more frequently on the dorsal surface (124 dorsal vs. 45 ventral lesions; Appendix Table 1) consistent with previous reports (Tang et al. 2004). Ten mice did not develop cancers. Accurate diagnosis required histologic analysis. For example, of the 67 SCC lesions identified by the pathologist, 39 were also identified by clinical and stereomicroscopic examination (sensitivity = 0.58, positive predictive value = 0.60).
Figure 1.
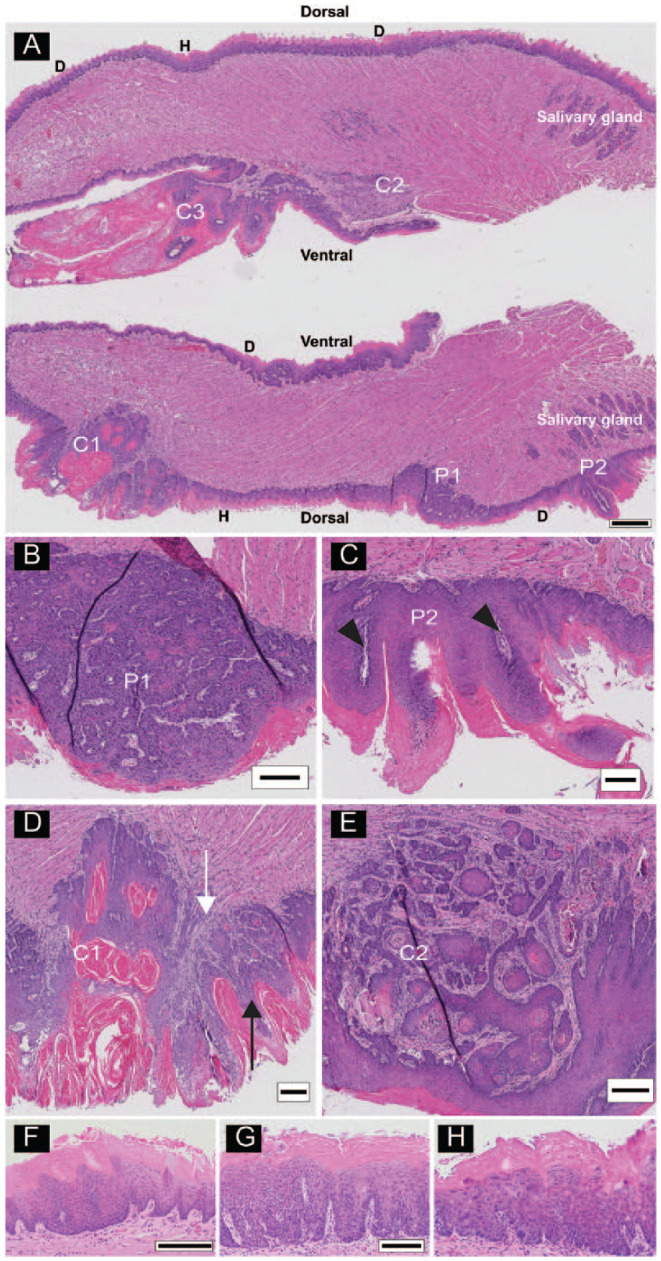
Multiple lesions on a tongue from a 4-nitroquinoline 1-oxide (4NQO)–treated animal. (A) Longitudinal sections (anterior to the left) of a bisected tongue. Tongues were sectioned from the center outward. The scanned images are from slide 40, so that the top and bottom portions of the tongue are separated by 390 µm. Lesions include acanthosis, hyperkeratosis (H), field dysplastic changes (D), papillomas (P1, P2), and cancers (C1, C2, C3). (B) High-power image of P1, a dorsal sessile papilloma. (C) High-power image of P2, a dorsal pedunculated papilloma with finger-like epithelial proliferations with fibrovascular cores (arrowheads). (D) High-power image of C1, a dorsal papillary squamous cell carcinoma (pSCC) with exophytic (black arrow) and invasive (white arrow) components. (E) High-power image of C2, a ventral invasive squamous cell carcinoma (iSCC). High-power images of additional lesion types from other tongue sections. (F) Acanthosis and hyperkeratosis. (G) Hyperkeratosis with low-grade dysplasia and (H) high-grade dysplasia. Scale bars: A = 500 µm, B–E = 250 µm, F = 200 µm, G and H shown in G = 100 µm.
Figure 2.
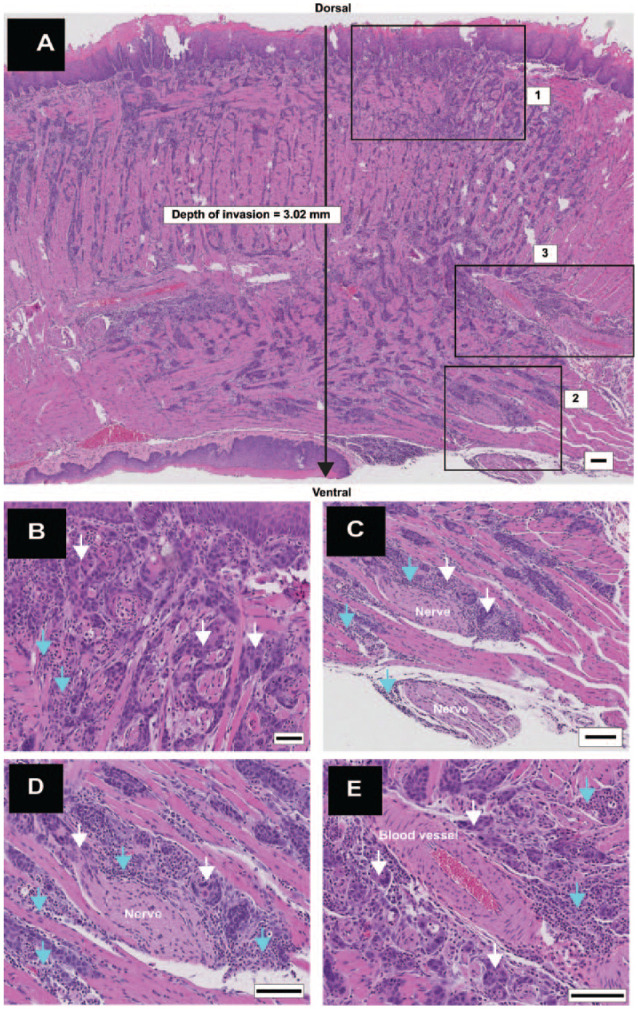
Deeply invasive squamous cell carcinoma (diSCC) with aggressive features. (A) Longitudinal section (anterior to the left) shows a diSCC involving the entire dorsoventral thickness of the tongue with depth of invasion greater than 2 mm. High-power views of inset boxes 1, 2, and 3 shown in panels B–D. (B) Inset box 1: islands of diSCC (white arrows) invading stroma with interspersed inflammatory cells (turquoise arrows). (C) Inset box 2: Perineural invasion (PNI) with cancer (white arrows) surrounding greater than 50% of a nerve at multiple foci. Inflammation (turquoise arrows) is interspersed between nerves and cancer. (D) High-power image of PNI, cancer (white arrows), and inflammation (turquoise arrows). (E) Inset box 3: large blood vessels surrounded by cancer islands (white arrows) and inflammation (turquoise arrows). Scale bars: A = 100 µm; B–E = 50 µm.
Figure 3.
Tongues from 4-nitroquinoline 1-oxide (4NQO)–treated animals harbor multiple lesions. Shown are individual mice in columns (arranged in order of pain scores from left to right) and with numbers of indicated lesions in rows.
Metastasis, PNI, LVI, and inflammation are pathological features associated with poor prognosis in human cancer (Arora et al. 2017). Metastasis was not observed (n = 36 mice). Perineural invasion and LVI were rarely observed; PNI was noted in 3 mice that harbored iSCCs, including multifocal PNI associated with 1 diSCC (Fig. 2C, D), and LVI was present in 2 mice with iSCCs. Areas suspicious for LVI were noted in 2 other tongues. Inflammation was variably present in the 4NQO-treated tongues. Minimal inflammation was present in 2 of the tongues that lacked cancers. Inflammation was more prominent in tongues with cancers (Fig. 2A, B and Appendix Table 1), in areas of PNI (Fig. 2C, D), and adjacent to large blood vessels (Fig. 2E). Detailed analysis of inflammation with immune cell markers was not performed.
Esophagus Lesions
Esophagi, harvested from 33 animals, harbored multiple lesions throughout the length of the esophagus (Appendix Fig. 1). Hyperkeratoses, dysplastic field changes, and benign and dysplastic papillomas were observed in all harvested esophagi, similar to tongue lesions. SCCs were present in 22 esophagi and included 26 iSCCs and 14 pSCCs. Esophageal lesion subtypes (papillomas and cancers) occurred with similar frequency as tongue lesions. Stromal inflammation similar to that seen in tongue lesions was observed. Twelve mice harbored both pSCCs and iSCCs in their esophagi. We did not detect PNI and LVI in H&E-stained sections of esophagi.
SCCs at Other Oral Sites
Four mice harbored SCCs (iSCCs or pSCCs ) at oral sites other than tongue (Appendix Fig. 2, hard palate, mandibular gingiva-submandibular region, lip, and buccal mucosa). The low frequency is consistent with other reports (Tang et al. 2004; Vered et al. 2005; Kanojia and Vaidya 2006).
Presence of pSCCs Correlates with Nociceptive Behavior
Dolognawmeter testing throughout the 28-wk experimental period was completed by 36 of 40 mice. Four animals died early in the experiment from unknown causes before the completion of 4NQO administration. Nociception scores ranged from 211.5% to 2471.0% (mean ± SD = 981.9% ± 591.7%). Univariate analyses were performed to determine the correlation of pathologic variables with nociception (Appendix Table 2). We found pain scores were correlated with the total number of tongue pSCCs (r = 0.42, P = 0.01), with presence of any tongue pSCC (r = 0.39, P = 0.02) or an increasing number of tongue pSCCs (r = 0.41, P = 0.01). Moreover, pain scores were 50% higher in mice with a tongue pSCC than without a pSCC (mean ± SD = 1,232.8% ± 594.4% vs. 802.6% ± 533.2%, P = 0.03). Pain scores did not correlate with types and numbers of esophageal lesions.
Pain Correlates with Tumor Size in Cancer Patients and in the Mouse Model
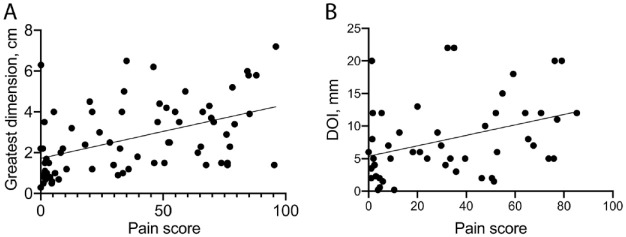
To determine how nociception and histopathological features of mouse pSCCs compared with patient-reported pain and histopathology of human oral cancers, we compared features that could be measured in the mouse model and in a cohort of 72 oral cancer patients (Bhattacharya et al. 2020). Pain scores, pathology, and clinical information were available for these patients (Appendix Table 3). Pathological assessment of human oral cancer specimens includes evaluation of DOI, greatest tumor dimension, and presence of PNI, LVI, and nodal metastasis. Metastasis (n = 0), PNI (n = 3), and LVI (n = 2) could not be analyzed in the mouse model due to the small number of observations. Therefore, we investigated whether DOI and greatest tumor dimension correlated with pain in the mouse model. To account for presence of multiple pSCCs on some mouse tongues, we summed the measures for DOI and greatest lesion dimension for pSCC lesions on each tongue. We found pain score correlated with pSCC size (n = 17, r = 0.52, P = 0.03) in the mouse and with greatest tumor dimension in human cancers (n = 71, r = 0.47, P < 0.001, Fig. 4A). Pain correlated with DOI (n = 49, r = 0.37, P = 0.01, Fig. 4B) in human cancers, and there was a trend for association with DOI (r = 0.43, P = 0.08) in the mouse pSCCs. In addition, in the human cohort (Appendix Table 3), we determined that pain correlated with sex (women reported greater pain than men, r = −0.24, P = 0.05), as reported previously (Scheff et al. 2018); lymph node metastasis (n = 64, r = 0.27, P = 0.03); PNI (n = 69, r = 0.33, P = 0.01); pT stage (n = 72, r = 0.38, P < 0.01); and cT stage (n = 72, r = 0.32, P = 0.01).
Figure 4.
Scatterplots showing correlation of pain scores (x-axis) and pathologic variables (y-axis) in the human cohort of 72 oral cancer patients. (A) Correlation of pain scores with tumor size (greatest dimension, n = 71 patients, r = 0.47, P < 0.001). (B) Correlation of pain scores with depth of invasion (DOI; n = 49 patients, r = 0.37, P = 0.01). r = Pearson’s coefficient of correlation; P is significant at P ≤ 0.05.
Discussion
We evaluated nociceptive behavior and tongue lesions in 4NQO-treated wild-type mice. The animals developed tongue and esophageal cancers that could be separated into 2 subtypes (iSCC and pSCC). Presence of tongue pSCCs correlated with mechanical functional allodynia as measured with the dolognawmeter assay. Size of the pSCC lesion (greatest dimension) also correlated with pain score. We found that tumor size and DOI of human cancers correlate with pain.
Most human SCCs are preceded by dysplasia (Hunter et al. 2005). Papillary lesions are rarely seen in human cancer (Thompson 2003; Ding et al. 2013). In rodent carcinogenesis models, however, papillomas appear to be a morphological feature reflective of a path to cancer. Papillomas are characteristic histopathology of the 2-stage 7,12-dimethylbenz [a]-anthracene/12-O-tetradecanoylphorbol-13-acetate (DMBA/TPA) skin carcinogenesis model, as well as the 4NQO model (Tang et al. 2004; Vitale-Cross et al. 2012; Huang and Balmain 2014; Page et al. 2016). Papillomas with invasive components, similar to pSCCs reported here, were noted in at least 1 study using the 2-stage DMBA/TPA skin carcinogenesis model (Page et al. 2016). The skin model longitudinal study of papillomas revealed that some papillomas regress and others progress to cancer. Skin papilloma multiplicity and incidence appear to be determined at least in part genetically and/or by therapeutic intervention, suggesting that incidence and multiplicity of papillomas reflect selection for different routes to cancer.
There is a lack of consensus regarding the role of papillomas in cancer progression in the 4NQO model. Authors have omitted papillomas from the spectrum of 4NQO-induced lesions (Vered et al. 2007), described papillomas as a stage in progression to cancer (Tang et al. 2004), and designated papillomas as cancer (Vitale-Cross et al. 2012). Longitudinal studies of 4NQO-induced tongue and esophageal papillomas are not possible; however, papillomas with dysplasia have been reported at earlier times after 4NQO treatment than cancers (Hasina et al. 2009; Wang et al. 2019), suggesting they are precursor lesions to cancer, possibly the pSCC subtype. The 4NQO-induced papillomas harbor genomic alterations (e.g., cancer-associated mutations and copy number alterations; unpublished observations), suggesting that the lesions are poised for malignant transformation. We propose that the histopathology of the 4NQO pSCCs is a phenotypic characteristic identifying lesions that progress to cancer by acquisition of genetic alterations favoring expression of pain mediators.
Oral cancer patients have function-related mechanical pain (i.e., the highest pain scores were associated with eating and talking) (Connelly and Schmidt 2004; Bhattacharya et al. 2020). The dolognawmeter is a validated operant assay and device that measures functional mechanical allodynia and takes advantage of the normal behavior of rodents to escape from confinement (Dolan et al. 2010). There are technical advantages associated with use of the dolognawmeter, including automation and capability to run multiple devices concurrently overnight during the natural time of rodent activity. Measurements are objective and therefore less prone to observer bias. There are, however, limitations that include possible effects of nonnociceptive factors on gnawing activity (e.g., noise that might distract an animal from gnawing), systemic illness, or cancer-related cachexia, which can weaken an animal and increase gnaw time, as well as failure of an animal to complete the task (loss of data). To address the last point, we modified the device to make the task easier to complete (use of a second dowel of lower durometer).
Our study design has 2 strengths. First, we modified the dolognawmeter assay to reduce the difficulty of the task. The animals spent less time in the device and experienced less stress, ensuring their survival for histopathologic analysis as they developed cancer. This modification enabled collection of behavior data from 95% of the animals. In addition, twice-weekly testing of the animals increased the number of data points for robust statistical analyses of behavior and histology. Second, we performed a complete histologic evaluation of the tongues and esophagi. The comprehensive analysis allowed us to identify differences in histopathology occurring in lesions in sections separated by 100 µm in depth, for example. Clinical and stereomicroscopic examination did not accurately identify lesions (Appendix Table 1). Moreover, it is only possible to assess aggressive features (e.g., DOI, PNI, LVI) and distinguish between lesion types (e.g., papillomas and pSCCs) by histopathology.
There are limitations to this study. Only female mice were included. Sex differences have been reported in cancer pain. Females mice exhibit greater nociceptive behavior in the 4NQO model (Scheff et al. 2018). Women with oral SCC report greater pain than men (Reyes-Gibby et al. 2014; Allen-Ayodabo et al. 2019). Future studies are required to validate our observations and investigate sex as a biologic variable. Other pathological features associated with pain were not studied in depth by analysis of the H&E sections, including inflammation, PNI, and LVI. We may have underreported PNI and LVI. Use of immunohistochemical stains for PNI and LVI increases detection (Schmitd et al. 2018; Larson et al. 2020), and immune profiling is needed to determine the nature and potential function of the inflammatory infiltrate. Cancer neural interactions, not defined as PNI by current criteria (Chi et al. 2016; Schmitd et al. 2018), including sprouting of neurites into the cancer microenvironment (Schmidt 2015; Faulkner et al. 2019), should be evaluated. Neuronal density has been associated with pain in pancreatic adenocarcinoma (Bapat et al. 2011; Demir et al. 2015). Neck lymph node metastasis, a significant predictor of survival in oral cancer, is associated with greater patient-reported pain (Connelly and Schmidt 2004; Bhattacharya et al. 2020). Metastasis, however, could not be studied. The 4NQO model rarely yields metastatic lesions (Li et al. 2013). A high rate of metastasis has been reported in mice genetically engineered to overexpress Pik3ca (Du et al. 2016), suggesting that cancer associated nociception and metastasis could be studied in such genetically engineered mice (Allen-Ayodabo et al. 2019) or in xenograft mouse models (Judd et al. 2012; Shirako et al. 2015).
Conclusion
We found that presence of pSCCs, a specific pathological lesion, is associated with increased mechanical and functional allodynia in the 4NQO model. Cancers with pSCC histology are expected to overexpress and release mediators that sensitize primary afferent nociceptors.
Author Contributions
K. Naik, contributed to conception, design, and data acquisition, drafted and critically revised the manuscript; M.N. Janal, D.G. Albertson, contributed to design, data analysis, and interpretation, drafted and critically revised the manuscript; J. Chen, D. Bandary, B. Brar, S. Zhang, contributed to design and data acquisition, drafted the manuscript; J.C. Dolan, B.L. Schmidt, contributed to design, data acquisition, and interpretation, drafted the manuscript; A. Bhattacharya, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034520961020 for The Histopathology of Oral Cancer Pain in a Mouse Model and a Human Cohort by K. Naik, M.N. Janal, J. Chen, D. Bandary, B. Brar, S. Zhang, J.C. Dolan, B.L. Schmidt, D.G. Albertson and A. Bhattacharya in Journal of Dental Research
Acknowledgments
We thank Nguyen Huu Tu and Zinaida Dubeykovskaya for guidance on the dolognawmeter assays and animal care. We thank Mark Alu for histopathology support and the NYU Langone Health Experimental Pathology Laboratory for assistance with histotechnology and slide scanning. This shared resource is partially funded by the Laura and Isaac Perlmutter Cancer Center support grant, P30CA016087.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a research grant from the American Academy of Oral and Maxillofacial Pathology (AAOMP) and by the New York University Goddard Junior Faculty Fellowship to Aditi Bhattacharya. This work was partially supported by National Institutes of Health (NIH) grants R01CA113833, R21CA163019, 3UL1TR000004-07S1, R01DE019796, R01DE0 26806, and R21DE026964. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
ORCID iDs: S. Zhang  https://orcid.org/0000-0003-1389-7120
https://orcid.org/0000-0003-1389-7120
A. Bhattacharya  https://orcid.org/0000-0002-5099-0968
https://orcid.org/0000-0002-5099-0968
A supplemental appendix to this article is available online.
References
- Abbey LM, Kaugars GE, Gunsolley JC, Burns JC, Page DG, Svirsky JA, Eisenberg E, Krutchkoff DJ, Cushing M. 1995. Intraexaminer and interexaminer reliability in the diagnosis of oral epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 80(2):188–191. [DOI] [PubMed] [Google Scholar]
- Allen-Ayodabo CO, Eskander A, Davis LE, Zhao H, Mahar AL, Karam I, Singh S, Gupta V, Bubis LD, Moody L, et al. 2019. Symptom burden among head and neck cancer patients in the first year after diagnosis: association with primary treatment modality. Oral Oncol. 99:104434. [DOI] [PubMed] [Google Scholar]
- Arora A, Husain N, Bansal A, Neyaz A, Jaiswal R, Jain K, Chaturvedi A, Anand N, Malhotra K, Shukla S. 2017. Development of a new outcome prediction model in early-stage squamous cell carcinoma of the oral cavity based on histopathologic parameters with multivariate analysis: the aditi-nuzhat lymph-node prediction score (ANLPS) system. Am J Surg Pathol. 41(7):950–960. [DOI] [PubMed] [Google Scholar]
- Bapat AA, Hostetter G, Von Hoff DD, Han H. 2011. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 11(10):695–707. [DOI] [PubMed] [Google Scholar]
- Berdugo J, Thompson LDR, Purgina B, Sturgis CD, Tuluc M, Seethala R, Chiosea SI. 2019. Measuring depth of invasion in early squamous cell carcinoma of the oral tongue: positive deep margin, extratumoral perineural invasion, and other challenges. Head Neck Pathol. 13(2):154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A, Janal MN, Veeramachaneni R, Dolgalev I, Dubeykovskaya Z, Tu NH, Kim H, Zhang S, Wu AK, Hagiwara M, et al. 2020. Oncogenes overexpressed in metastatic oral cancers from patients with pain: potential pain mediators released in exosomes. Sci Rep. 10(1):14724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi AC, Katabi N, Chen HS, Cheng YL. 2016. Interobserver variation among pathologists in evaluating perineural invasion for oral squamous cell carcinoma. Head Neck Pathol. 10(4):451–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly ST, Schmidt BL. 2004. Evaluation of pain in patients with oral squamous cell carcinoma. J Pain. 5(9):505–510. [DOI] [PubMed] [Google Scholar]
- Demir IE, Friess H, Ceyhan GO. 2015. Neural plasticity in pancreatitis and pancreatic cancer. Nat Rev Gastroenterol Hepatol. 12(11):649–659. [DOI] [PubMed] [Google Scholar]
- Ding Y, Ma L, Shi L, Feng J, Liu W, Zhou Z. 2013. Papillary squamous cell carcinoma of the oral mucosa: a clinicopathologic and immunohistochemical study of 12 cases and literature review. Ann Diagn Pathol. 17(1):18–21. [DOI] [PubMed] [Google Scholar]
- Dolan JC, Lam DK, Achdjian SH, Schmidt BL. 2010. The dolognawmeter: a novel instrument and assay to quantify nociception in rodent models of orofacial pain. J Neurosci Methods. 187(2):207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Chen X, Cao Y, Lu L, Zhang F, Bornstein S, Li Y, Owens P, Malkoski S, Said S, et al. 2016. Overexpression of PIK3CA in murine head and neck epithelium drives tumor invasion and metastasis through PDK1 and enhanced TGFβ signaling. Oncogene. 35(35):4641–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner S, Jobling P, March B, Jiang CC, Hondermarck H. 2019. Tumor neurobiology and the war of nerves in cancer. Cancer Discov. 9(6):702–710. [DOI] [PubMed] [Google Scholar]
- Hasina R, Martin LE, Kasza K, Jones CL, Jalil A, Lingen MW. 2009. ABT-510 is an effective chemopreventive agent in the mouse 4-nitroquinoline 1-oxide model of oral carcinogenesis. Cancer Prev Res (Phila). 2(4):385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BL, Heniford BW, Ackermann DM, Leonberger M, Martinez SA, Hendler FJ. 1994. 4NQO carcinogenesis: a mouse model of oral cavity squamous cell carcinoma. Head Neck. 16(5):424–432. [DOI] [PubMed] [Google Scholar]
- Huang PY, Balmain A. 2014. Modeling cutaneous squamous carcinoma development in the mouse. Cold Spring Harb Perspect Med. 4(9):a013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter KD, Parkinson EK, Harrison PR. 2005. Profiling early head and neck cancer. Nat Rev Cancer. 5(2):127–135. [DOI] [PubMed] [Google Scholar]
- Judd NP, Winkler AE, Murillo-Sauca O, Brotman JJ, Law JH, Lewis JS, Jr, Dunn GP, Bui JD, Sunwoo JB, Uppaluri R. 2012. ERK1/2 regulation of CD44 modulates oral cancer aggressiveness. Cancer Res. 72(1):365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanojia D, Vaidya MM. 2006. 4-Nitroquinoline-1-oxide induced experimental oral carcinogenesis. Oral Oncol. 42(7):655–667. [DOI] [PubMed] [Google Scholar]
- Kolokythas A, Connelly ST, Schmidt BL. 2007. Validation of the University of California San Francisco oral cancer pain questionnaire. J Pain. 8(12):950–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DK, Dang D, Zhang J, Dolan JC, Schmidt BL. 2012. Novel animal models of acute and chronic cancer pain: a pivotal role for PAR2. J Neurosci. 32(41):14178–14183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DK, Schmidt BL. 2011. Orofacial pain onset predicts transition to head and neck cancer. Pain. 152(5):1206–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson AR, Kemmer J, Formeister E, El-Sayed I, Ha P, George J, Ryan W, Chan E, Heaton C. 2020. Beyond depth of invasion: adverse pathologic tumor features in early oral tongue squamous cell carcinoma. Laryngoscope. 130(7):1715–1720. [DOI] [PubMed] [Google Scholar]
- Li J, Liang F, Yu D, Qing H, Yang Y. 2013. Development of a 4-nitroquinoline-1-oxide model of lymph node metastasis in oral squamous cell carcinoma. Oral Oncol. 49(4):299–305. [DOI] [PubMed] [Google Scholar]
- Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. 2009. Perineural invasion in cancer: a review of the literature. Cancer. 115(15):3379–3391. [DOI] [PubMed] [Google Scholar]
- Page A, Navarro M, Suarez-Cabrera C, Alameda JP, Casanova ML, Paramio JM, Bravo A, Ramirez A. 2016. Protective role of p53 in skin cancer: carcinogenesis studies in mice lacking epidermal p53. Oncotarget. 7(15):20902–20918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Gibby CC, Anderson KO, Merriman KW, Todd KH, Shete SS, Hanna EY. 2014. Survival patterns in squamous cell carcinoma of the head and neck: pain as an independent prognostic factor for survival. J Pain. 15(10):1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff NN, Bhattacharya A, Dowse E, Dang RX, Dolan JC, Wang S, Kim H, Albertson DG, Schmidt BL. 2018. Neutrophil-mediated endogenous analgesia contributes to sex differences in oral cancer pain. Front Integr Neurosci. 12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BL. 2015. What pain tells us about cancer. Pain. 156(suppl 1):S32–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BL, Hamamoto DT, Simone DA, Wilcox GL. 2010. Mechanism of cancer pain. Mol Interv. 10(3):164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitd LB, Beesley LJ, Russo N, Bellile EL, Inglehart RC, Liu M, Romanowicz G, Wolf GT, Taylor JMG, D’Silva NJ. 2018. Redefining perineural invasion: integration of biology with clinical outcome. Neoplasia. 20(7):657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirako Y, Taya Y, Sato K, Chiba T, Imai K, Shimazu Y, Aoba T, Soeno Y. 2015. Heterogeneous tumor stromal microenvironments of oral squamous cell carcinoma cells in tongue and nodal metastatic lesions in a xenograft mouse model. J Oral Pathol Med. 44(9):656–668. [DOI] [PubMed] [Google Scholar]
- Tang XH, Knudsen B, Bemis D, Tickoo S, Gudas LJ. 2004. Oral cavity and esophageal carcinogenesis modeled in carcinogen-treated mice. Clin Cancer Res. 10:301–313. [DOI] [PubMed] [Google Scholar]
- Thompson LDR. 2003. Squamous cell carcinoma variants of the head and neck. Mini symposium: Head and neck pathology. Curr Diagn Pathol. 9(6):384–396. [Google Scholar]
- Vered M, Allon I, Buchner A, Dayan D. 2007. Stromal myofibroblasts and malignant transformation in a 4NQO rat tongue carcinogenesis model. Oral Oncol. 43(10):999–1006. [DOI] [PubMed] [Google Scholar]
- Vered M, Yarom N, Dayan D. 2005. 4nqo oral carcinogenesis: animal models, molecular markers and future expectations. Oral Oncol. 41(4):337–339. [DOI] [PubMed] [Google Scholar]
- Viet CT, Schmidt BL. 2012. Biologic mechanisms of oral cancer pain and implications for clinical therapy. J Dent Res. 91(5):447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale-Cross L, Molinolo AA, Martin D, Younis RH, Maruyama T, Patel V, Chen W, Schneider A, Gutkind JS. 2012. Metformin prevents the development of oral squamous cell carcinomas from carcinogen-induced premalignant lesions. Cancer Prev Res (Phila). 5(4):562–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Li F, Qiang D, Hu Z, Meng Y, Shi L, Zhao E, Niu Y. 2019. Impact of immunodeficiency on lingual carcinogenesis and lymph node metastasis in mice. J Oral Pathol Med. 48(9):826–831. [DOI] [PubMed] [Google Scholar]
- Warnakulasuriya S, Reibel J, Bouquot J, Dabelsteen E. 2008. Oral epithelial dysplasia classification systems: predictive value, utility, weaknesses and scope for improvement. J Oral Pathol Med. 37(3):127–133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034520961020 for The Histopathology of Oral Cancer Pain in a Mouse Model and a Human Cohort by K. Naik, M.N. Janal, J. Chen, D. Bandary, B. Brar, S. Zhang, J.C. Dolan, B.L. Schmidt, D.G. Albertson and A. Bhattacharya in Journal of Dental Research