Abstract
The periodontal ligament (PDL) plays a critical role in providing immediate response to abrupt high loads during mastication while also facilitating slow remodeling of the alveolar bone. The PDL exceptional functionality is permitted by the unique nonuniform structure of the tissue. Two distinct areas that are critical to PDL function were previously identified: the furcation and the dense collar. Despite their hypothesized functions in tooth movement and maintenance, these 2 regions have not yet been compared within the context of their native environment. Therefore, the objective of this study is to elucidate the extracellular matrix (ECM) structure, composition, and biomechanical function of the furcation and the collar regions while maintaining the 3-dimensional (3D) structure in the murine PDL. We identify significant difference between the collar and furcation regions in both structure and mechanical properties. Specifically, we observed unique longitudinal structures in the dense collar that correlate with type VI collagen and LOX, both of which are associated with increased type I collagen density and tissue stiffness and are therefore proposed to function as scaffolds for tooth stabilization. We also found that the collar region is stiffer than the furcation region and therefore suggest that the dense collar acts as a suspense structure of the tooth within the bone during physiological loading. The furcation region of the PDL contained more proteins associated with reduced stiffness and higher tissue remodeling, as well as a dual mechanical behavior, suggesting a critical function in loads transfer and remodeling of the alveolar bone. In summary, this work unravels the nonuniform nature of the PDL within the 3D structural context and establishes understanding of regional PDL function, which opens new avenues for future studies of remodeling, regeneration, and disease.
Keywords: dense collar, furcation, structure, immunohistochemistry, mass spectrometry, mechanics
Introduction
The periodontal ligament (PDL) has a central role in the adaptation mechanism of teeth to loading. It provides immediate response to abrupt high mastication loads and facilitates slow adaptation to gradual morphological changes. To perform this unique function, the 3-dimensional (3D) structure of the PDL must be both strong and flexible. In contrast to other ligaments that function primarily in tension, the PDL endures multiaxial loading and is therefore programmed to function under compression, tension, shear, and torsion. The PDL is composed of a rich extracellular matrix (ECM) including different types of collagens, proteoglycans, and, unlike other ligaments, a vast vascular network (McCulloch et al. 2000; Dangaria et al. 2009; Nanci 2017). Furthermore, the organization and structure of this unique ECM are not uniform, which allows the PDL to optimally function in such a complicated loading environment (Kaneko et al. 2001; Hurng et al. 2011; Naveh et al. 2013, 2018). However, none of the studies were able to associate regional ECM composition with regional biomechanical properties, which is vital to deciphering the function of the PDL.
The furcation region, the area where the roots split in a multirooted tooth, functions under compression in high loads such as encountered during mastication as well as low loads such as orthodontic forces (Qian et al. 2009; Naveh, Lev-Tov Chattah, et al. 2012; Naveh, Shahar, et al. 2012; Naveh et al. 2013; Naveh and Weiner 2015; Naveh et al. 2018; Maria et al. 2019). In a pig model, it was shown that the bone in the furcation region has a unique isotropic disoriented structure that is neither cortical nor trabecular; well structured for functioning under multidirectional compression loads (Ben-Zvi et al. 2019; Maria et al. 2019). Furthermore, the PDL in this region has a sparse disorganized collagen network and large blood vessels in contrast to typically poorly avascular ligaments (Jang et al. 2015; Naveh et al. 2018). However, there have been limited studies to date that have measured the mechanical function of the PDL furcation region, and detailed compositional analyses of furcation ECM have yet to be performed.
Naveh et al. (2018) identified another structure within the PDL, the dense collar (Fig. 1). This region was identified in mice as the most coronal 150 to 200 µm of the PDL and was proposed to function primarily under tensile forces acting to stabilize the tooth within the bone and control apical movement. Early work showed that the turnover rate of the PDL in the coronal region is slower compared to other regions in the PDL, consistent with other ligaments throughout the body and suggesting increased collagen crosslinks (Rippin 1976, 1978). The latest 3D structural studies using focused ion beam scanning electron microscopy (FIB/SEM) and micro–computed tomography (CT) found that this dense collar region consists of short horizontal fibers oriented in the direction of the natural drift of the tooth (Naveh et al. 2018; Hirashima, Kanazawa et al. 2019; Hirashima, Ohta et al. 2019). These unique structural features allude to a distinct function of the dense collar region that differs significantly from the furcation region.
Figure 1.
Periodontal ligament regions scheme. (A) Sagittal section of 3-dimensional volume rendering of an in situ mouse first mandibular molar from a micro–computed tomography (CT) scan showing the tooth, alveolar bone, and interconnecting collagenous component of the PDL. Imaging was done on a fresh sample using a humidity chamber and phase enhancement by micro-CT according to our published method (Naveh et al. 2013, 2018). Colored regions highlight the furcation, dense collar, and apical regions as defined in this study for sample collection analysis. (B) Digital graphic depicting the transverse view of the PDL and surrounding structures at the section level used for mechanical analyses. Dense collar region was analyzed only on the buccal surface for biomechanics but analyzed from lingual and buccal surfaces for all other analyses.
The objective of this study is therefore to elucidate the ECM structure, composition, and biomechanical function of the collar and furcation regions while maintaining the 3D structure of murine PDL. We employed a novel in situ atomic force microscopy (AFM)-based nanomechanics to perform dynamic mechanical testing. Nontargeted proteomics from fresh samples allowed for identification of key differences in regional ECM composition. Finally, we used targeted immunohistochemistry to reveal regionally dependent composition and structure of critical ECM components within the furcation and collar regions. We show for the first time that indeed the structural as well as functional properties of the collar and the furcation regions are significantly different.
Materials and Methods
All animal experiments were performed in compliance with National Institute of Health’s Guide for the Care and Use of Laboratory Animals and guidelines from the Harvard University Institutional Animal Care and Use Committee (protocol no. 01840).
Sample Preparation
Young adult C57BL/6 mice (male) at 9 wk of age (n = 32) were sacrificed and their mandibles were dissected and split into hemimandibles through the fibrous symphysis. Fresh samples were then embedded and used for mechanical testing, proteomic analysis, and immunohistochemistry as described in the Appendix.
Mechanical Testing
After sectioning (as described in the Appendix), samples were thawed in 1× phosphate-buffered saline (PBS) and then affixed to the testing stage from the crown side using a light-cured dental restorative composite material Z100 (3M ESPE). Indentations were performed on the transverse surface of each unfixed sample while maintaining the 3D structure of the collar and furcation regions. Samples were immersed in PBS for the duration of the experiments. For mechanical evaluation, indentations were performed in the dense collar on the buccal side and furcation regions (Fig. 1B). Testing was performed using our custom-built nanoscale rheology system coupled to a commercial atomic force microscope using polystyrene spherical probe tips with a 25-μm diameter (Polysciences). Further details can be found in the Appendix and previous publications (Connizzo and Grodzinsky 2017, 2018).
For each of the regions, indentations were performed 3 times in 3 locations and averaged within a single region. Data from the mesial and distal buccal regions of the dense collar were pooled for the purposes of these analyses. Some of the data were not normally distributed, and therefore statistical comparisons were made for each mechanical parameter using nonparametric Mann-Whitney tests between collar and furcation regions. Significance was set at P < 0.05 and a trend set at P < 0.10.
Mass Spectrometry
Sections were thawed and dried inside a desiccator for 30 min. Thereafter, stickers with dried specimens were loaded onto the metal frame of designated slides for microdissection microscopes (#415190-9101; Zeiss). Specimens were collected with a laser microdissection microscope equipped with a pulsed nitrogen laser (PALM CombiSystem; Zeiss) using a 20× lens with a cutting speed of 14 and cutting energy pulse set to 72 to 78 pulses/s. Samples from collar, furcation, and apex were collected into different collection tubes and further analyzed by liquid chromatography–tandem mass spectrometry (LC-MS/MS). Further details can be found in the Appendix.
Results
Teeth sustain loads in various directions and frequencies; using a high-frequency AFM-based technique, we characterized the dynamic mechanical response to a full range of functional loading rates. The magnitude of the dynamic modulus (Fig. 2A) was significantly increased in the dense collar region compared to the furcation region at all frequencies (Fig. 2B, C) with a distinct ligament-like behavior with a 2-peak phase response in both regions (Fig. 2D). Low-frequencies peak is indicative of flow-independent tissue viscoelasticity associated with macromolecule interactions and inherent matrix viscoelasticity (Connizzo and Grodzinsky 2018; Oftadeh et al. 2018). A small but statistically significant difference in the viscoelastic peak frequency favored increased viscoelasticity in the collar (Fig. 2E). The higher frequencies peak is attributed to fluid flow-dependent poroelastic mechanisms and was slightly increased (trend only, Fig. 2F) in the furcation region compared to the dense collar region.
Figure 2.
Periodontal ligament biomechanics. (A) Magnitude of the dynamic modulus was increased in the collar region (red) compared to the furcation region (blue) at all frequencies. Quantification demonstrated that these differences were statistically significant at (B) low frequencies and (C) high frequencies. (D) Phase angle of the dynamic modulus showed a distinctive ligament-like 2-peak phase response, with viscoelastic (left peak) and poroelastic (right peak) for each group depicted with corresponding colored arrows. (E) Viscoelastic peak frequency was slightly but significantly higher in the collar than in the furcation region, while (F) poroelastic peak frequency was higher in the furcation region (trend only). Data are presented as mean ± 95% CI. *P < 0.05. #P < 0.10.
Nontargeted proteomics analysis showed the presence in a total of 122 proteins according to cellular component ontology (GOCC, http://www.geneontology.org/) (Appendix Table 1). Among the 122 proteins, 21 are matrisome associated (Hynes and Naba 2012) (Appendix Table 2). The amount of tissue collected from the samples enabled 2 replicates from each region, and therefore patterns can be deduced but no statistics are presented. The most abundant matrisome-associated protein in all regions in the PDL (upper 10%, log2-normalized intensity >27 in Appendix Table 1) was collagen I. Specific examination of the different regions revealed that the collar also showed high abundance of collagen XII and tenascin N (TNN). Looking at relative abundance (Fig. 3), collagen VI (α3 chain) was found as the highest relative abundant protein in the collar region when compared to both furcation and apex (see Appendix Fig. 1 for scatterplots). Ribosomal protein L47 (Mrpl47) was found in the highest relative abundance in the furcation when compared to the collar and apex, and myelin proteins (Mpz, Mbp) were found only in the apex. Interestingly, heat shock protein HSP 90β was found in relative high abundancies uniquely in the furcation. Collagen XII, collagen V, and TNN were also found in relative higher abundance in the collar when compared to apex and furcation, respectively (Fig. 3). Interestingly, LOX, TGM (1 and 3), Serpinh1, and collagen III were found in relative high abundancies in the furcation and apex compared to the collar.
Figure 3.
Ratio of protein abundancies by intensity fold change (>2) in the different regions: collar (C), furcation (F), and apex (A). Highlighted proteins are matrisome-associated proteins. Full gene names and intensities are found in Appendix Table 1. Intensities are in log2 scale. Red = extracellular matrix (ECM)–associated proteins; highlighted blue = ECM space-associated proteins (according to GOCC definition); blue = ECM organelle-associated proteins.
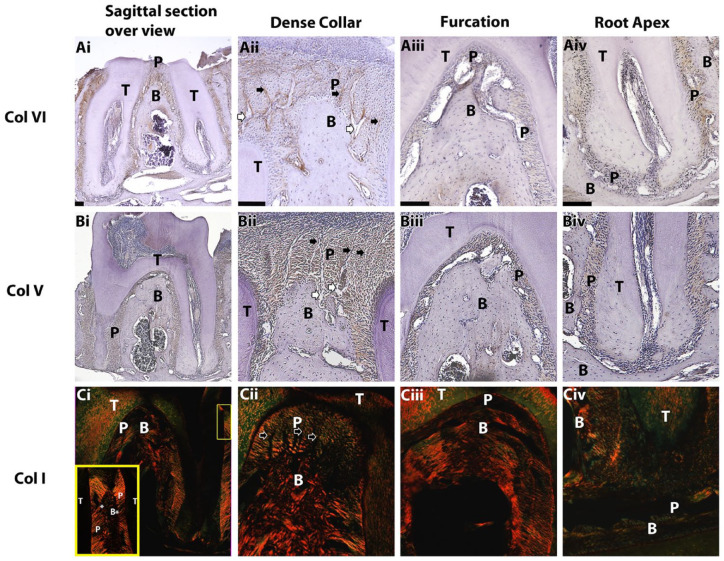
To identify the location of the proteins within the tissue and to confirm our proteomic findings, immunohistochemistry was performed for the identified proteins that were previously associated with biomechanical function. Staining for collagen VI was abundant in the dense collar region and more specifically was linked to longitudinal structures found there (black arrows, Fig. 4Aii). It was not stained in the apical region and, to a lesser extent, in the furcation region (Fig. 4Aiii, iv). Collagen V was observed uniformly in the PDL except in the apical region (Fig. 4Biv). Direction and density of type I collagen demonstrated a nonuniform distribution throughout the PDL with dense horizontal collagen fibers in the collar region. The fibers in the collar region were oriented mainly horizontally, perpendicular to the long axis of the tooth, with some directed obliquely in interproximal (IP) regions (Fig. 4Dii). In these instances, an abrupt directional shift of ~90 degrees in the collagen fibers was seen 150 to 200 µm below the alveolar crest, which is where the dense collar region ended. In the furcation and apical regions, collagen fibers were hardly visible using the polarizing filter, indicating disorganization or collagen remodeling events (Fig. 4Ciii, iv).
Figure 4.
Immunohistochemistry, sagittal slices. Brown staining for collagen VI (Ai–Aiv) and V (Bi–Biv) represents higher protein concentration. Polarized light and picrosirius red staining were used for collagen I (Ci–Civ); yellow represents higher collagen density and alignment along the sectioning plain, whereas green stain is indicative of orientation perpendicular to the sectioning plain. Yellow frame in Ci: inset of yellow rectangle in top right. Black arrows point to longitudinal structures in the dense collar, and white arrows show interstitial spaces forming next to alveolar bone. B, alveolar bone; P, periodontal ligament; T, tooth. *Abrupt change of periodontal ligament dense collar fibers direction. Scale bars: 100 µm.
We observed higher annexin V abundance in the furcation region relative to the rest of the PDL (Fig. 5A). The longitudinal structures inside the dense collar regions did not stain positively for annexin V (Fig. 5Aii). TNN was observed to a lesser extent in the furcation and apical regions (Fig. 5B). Interestingly, TNN was found in the dense collar but not in the longitudinal structures of the dense collar (Fig. 5Bii). Our results show the presence of LOX throughout the PDL (Fig. 5C). Darker staining, indicative of relative higher concentration, was observed in the furcation region around the blood vessels and apical region, and it was associated with the longitudinal structures in the dense collar region. Finally, TGM1 was present in the furcation and the dense collar at similar intensity levels (Fig. 5D). The staining in the apical region was slightly more abundant mainly within the canal opening (Fig. 5Dv), and there was no evident staining of the longitudinal structure in the collar (Fig. 5Dii).
Figure 5.
Immunohistochemistry, sagittal slices. Brown staining represents higher protein concentration. Black arrows indicate longitudinal structures in the dense collar. B, alveolar bone; P, periodontal ligament; T, tooth. Scale bars: 100 µm.
Discussion
In this study, we investigated the nonuniformity of the PDL while focusing on the structure and function of 2 unique regions: the furcation and the dense collar. Previous studies showed that the collagen network density in the PDL is not uniform and is correlated to regional function of the tissue (Naveh et al. 2013, 2018). Specifically, the furcation region was found to be populated with sparse collagen network functioning under mainly compression (Naveh, Shahar et al. 2012; Naveh et al. 2013, 2018; Ben-Zvi et al. 2019), while the collar region exhibited a dense collagen network and has been proposed as working mostly under tension and directing tooth physiological drift (Naveh et al. 2018). We therefore hypothesized that the difference in collagen density might be indicative of other structural and compositional differences contributing to the nonuniformity, as well as to local biomechanical properties.
Structurally, the dense collar is the only portion of the PDL with close similarity to a typical ligament, with well-organized parallel dense collagen fibers. Rippin (1976, 1978) showed a low remodeling rate of the coronal region of the PDL, which is typical of other tendons and ligaments, and it correlates with the dense collar region. Using regional in situ AFM measurements on nonfixed samples, we found that the collar region is stiffer and more viscoelastic than the furcation. This suggests that the dense collar acts as a 3D support structure primarily subjected to tensile loads while maintaining the tooth alignment. Together with the correlation found between physiological tooth drift and fiber direction within the dense collar region in our previous study (Naveh et al. 2018), these data provide solid support for the central role of the dense collar in PDL function and tooth movement.
Structurally and by proteomics, we were able to associate high density of type VI collagen with the collar region, particularly in the longitudinal structures. Collagen VI, microfibril-forming collagen, is found in a wide range of tissues and is shown to increase the organization of type I collagen fibers and tissue stiffness (Lamandé and Bateman 2018). It is also associated with anchoring the basement membrane of blood vessels, nerves, and muscles to the ECM (Timpl and Chu 1994). A recent study found higher abundance for collagen VI in the cervical region of the PDL relative to more apical regions of the PDL, which further supports our findings (Denes et al. 2020). The authors also found a high abundance of type XII collagen in the collar, similar to our nontargeted proteomics results. Collagen XII is a member of the fibril-associated collagens with the interrupted triple helices (FACIT) group of collagens (Shaw and Olsen 1991). FACITs are associated with dense networks of fibrillar collagens such as type I collagen. It has been implicated previously that collagen XII forms combined complexes with collagen VI in the ECM (Izu et al. 2016).
The longitudinal structures (Fig. 4) within the dense collar are lined with a single layer of cells and exhibit narrow lumen size. Therefore, they might be part of the system of interstitial lymphatic or blood vessels (Levy and Bernick 1968; Staszyk et al. 2005). However, these cells did not stain for CD31, a marker for endothelial cells (Appendix Fig. 2), and are not adjacent to the alveolar bone crypts, where blood vessels in the PDL are normally located; therefore, these structures are unlikely to be blood vessels. Thus, one could speculate that these are lymphatic vessels, as their size increases toward the apical region. However, in many instances, these structures are lacking of any lumen. The longitudinal structures in the dense collar region were also stained positive for LOX, which is a protein that contributes to collagen crosslinking, resulting in increased tissue stiffness, and was recently associated with increased loading in the PDL (Sulakshana et al. 2019). Based on these findings, we propose that the longitudinal structures in the collar region serve as a physical scaffold, providing anchorage and reinforcement to the ECM structures. We also suggest that the lack of lumen in these structures could be indicative of the high tissue density and stiffness of the collar region. This notion can explain the association between higher interstitial and blood volume and reduction in collagen density and tissue stiffness in periodontal disease (Jabłońska-Trypuć et al. 2016; Solomonov et al. 2016). However, these remain avenues for further investigation.
Interestingly, our proteomic results show high abundance of LOX and TGM (1,3) in the furcation and apex relative to the collar. Like LOX, the transglutaminase family generates crosslinks in ECM proteins. There are 2 types of crosslinks in the PDL, reducible and irreducible, which contribute differently to tissue stiffness and resistance to degradation (Kaku et al. 2016). Crosslinked collagen fibers usually increase tissue stiffness, but high LOX and TGM presence might also be indicative of high tissue damage and remodeling rate and smaller-sized fiber production, as seen in periodontal disease (Moore Rosset et al. 2020). The furcation region incurs high and repetitive compression forces that can cause tissue damage and therefore requires rapid remodeling. Distinctive structural characteristics of the PDL in the furcation, such as sparse disoriented collagen network and large blood vessels, are indicative of such functions (Naveh et al. 2018). Our proteomic analysis further supports this notion, as we found proteins that are associated with high collagen and bone remodeling (mrpl47, hsps, serpinh1, nucb1, ywhag). Annexin V (anxa5) was found in higher abundance in the furcation relative to both apex and collar, which was also supported by the immunohistochemistry (IHC). Annexin V, also called anchorin CII, is a collagen binding protein found to be associated with Ca2+ uptake into matrix vesicles and is therefore considered a mediator for mineralization (Kirsch et al. 2000; Heino 2007). We speculate that annexin V in the PDL promotes mineralization of the alveolar bone to maintain the furcation bone level (Kalamajski and Oldberg 2010). Direct force transduction from the teeth to the alveolar bone during mastication, as well as orthodontic loads, was proposed in different models (Mühlemann 1954; Picton 1965; Asundi and Kishen 2000; Naveh, Shahar et al. 2012; Naveh and Weiner 2015; Ben-Zvi et al. 2019). Our biomechanical findings indicate relatively lower compressive modulus in the furcation region of the PDL as well as increased poroelasticity. A lower compressive modulus is consistent with higher tissue compressibility, especially at low frequencies. However, the higher poroelasticity of the furcation also indicates an ability to resist compression at higher loading frequencies by resisting intratissue flow. This suggests the furcation has a complicated dual function, likely due to the need for resisting damage from high mastication loads while also allowing for nutrient transport into the tissue in lower loads. This unique regional function is critical for load transfer and subsequent remodeling of the alveolar bone. In contrast to the furcation, the apical region showed high abundance of trypsin (Try10), which is associated with collagen remodeling and myelin proteins as this is the region where innervation fibers enter the tooth. However, further biomechanical investigation is warranted to elucidate the function of the apical region.
This study is not without limitations. Our mechanical analyses relied on top-down microscale optics in the commercial AFM to visualize tissue; thus, we were unable to identify the apical region and specific tissue structures. Therefore, while we are confident that our data are representative of the 2 PDL regions and we were able to verify ligament-like tissue properties, it is possible that some indentations were of large blood vessels that are present in the furcation region and have never been tested using our testing setup. In addition, although we were able to find differential protein composition between the collar and furcation in the nontargeted proteomics, the total number of proteins identified was low. Given the small size of tissue specimens, we were most likely able to detect only those proteins found at higher concentrations in each region, therefore providing partial representation of the proteins in the different regions. Future studies will increase sample size and tissue size to pool protein concentration for more accurate estimates. Finally, immunohistochemical staining is inherently qualitative, and we are currently working on new techniques to better quantify regional variations in protein concentration.
This article set out to perform a comprehensive regional analysis of the ECM structure, composition, and mechanical function of the murine PDL, specifically focusing on differences between the dense collar and furcation regions. We identified several functional and compositional differences between the 2 regions. We propose, based on our findings, that the dense collar of the PDL functions as the primary stabilizer of the tooth within the bone with lower remodeling potential and acts as a critical regulator of tooth movement. Furthermore, our studies demonstrate a unique dual function of the furcation region, which we propose acts as a significant remodeling region and cushion for apical tooth translation during low loads and a transducer of masticatory forces to the underlying alveolar bone remodeling in high loads. In summary, this work shows the nonuniformity of the PDL’s 3D structure and function and the critical importance of regional analysis within the 3D context in future studies.
Author Contributions
B.K. Connizzo, I. Solomonov, I. Sagi, A.J. Grodzinsky, G.R.S. Naveh, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; L. Sun, contributed to data acquisition, critically revised the manuscript; N. Lacin, contributed to data acquisition and interpretation, drafted and critically revised the manuscript; A. Gendelman, contributed to data acquisition, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034520962455 for Nonuniformity in Periodontal Ligament: Mechanics and Matrix Composition by B.K. Connizzo, L. Sun, N. Lacin, A. Gendelman, I. Solomonov, I. Sagi, A.J. Grodzinsky and G.R.S. Naveh in Journal of Dental Research
Acknowledgments
The authors thank the Smoler proteomics center at the Technion, Israel, for their analysis support. Figure 1 was generated with BioRender.com.
Footnotes
A supplemental appendix to this article is available online.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Institutes of Health (NIH) (National Institute of Dental and Craniofacial Research R00-DE 025053, principal investigator [PI]: Naveh; National Institute on Aging K99-AG063896, PI: Connizzo) and the National Science Foundation (CMMI-1536233; PI: Grodzinsky), NINDS P30 Core Center Grant NS072030 for the neuroimaging facility at Harvard Medical School.
ORCID iD: N. Lacin  https://orcid.org/0000-0002-1576-2281
https://orcid.org/0000-0002-1576-2281
References
- Asundi A, Kishen A. 2000. A strain gauge and photoelastic analysis of in vivo strain and in vitro stress distribution in human dental supporting structures. Arch Oral Biol. 45(7):543–550. [DOI] [PubMed] [Google Scholar]
- Ben-Zvi Y, Maria R, Pierantoni M, Brumfeld V, Shahar R, Weiner S. 2019. Response of the tooth-periodontal ligament-bone complex to load: a microCT study of the minipig molar. J Struc Biol. 205(2):155–162. [DOI] [PubMed] [Google Scholar]
- Connizzo BK, Grodzinsky AJ. 2017. Tendon exhibits complex poroelastic behavior at the nanoscale as revealed by high-frequency AFM-based rheology. J Biomech. 54:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connizzo BK, Grodzinsky AJ. 2018. Multiscale poroviscoelastic compressive properties of mouse supraspinatus tendons are altered in young and aged mice. J Biomech Eng. 140(5):0510021–0510028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangaria SJ, Ito Y, Walker C, Druzinsky R, Luan X, Diekwisch TG. 2009. Extracellular matrix–mediated differentiation of periodontal progenitor cells. Differentiation. 78(2–3):79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denes BJ, Ait-Lounis A, Wehrle-Haller B, Kiliaridis S. 2020. Core matrisome protein signature during periodontal ligament maturation from pre-occlusal eruption to occlusal function. Front Physiol. 11:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heino J. 2007. The collagen family members as cell adhesion proteins. Bioessays. 29(10):1001–1010. [DOI] [PubMed] [Google Scholar]
- Hirashima S, Kanazawa T, Ohta K, Nakamura KI. 2019. Three-dimensional ultrastructural imaging and quantitative analysis of the periodontal ligament. Anat Sci Int. 95(1):1–11. [DOI] [PubMed] [Google Scholar]
- Hirashima S, Ohta K, Kanazawa T, Togo A, Kakuma T, Kusukawa J, Nakamura KI. 2019. Three-dimensional ultrastructural and histomorphological analysis of the periodontal ligament with occlusal hypofunction via focused ion beam/scanning electron microscope tomography. Sci Rep. 9(1):9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurng JM, Kurylo MP, Marshall GW, Webb SM, Ryder MI, Ho SP. 2011. Discontinuities in the human bone–PDL–cementum complex. Biomaterials. 32(29):7106–7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO, Naba A. 2012. Overview of the matrisome—an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol. 4(1):a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izu Y, Ezura Y, Koch M, Birk DE, Noda M. 2016. Collagens VI and XII form complexes mediating osteoblast interactions during osteogenesis. Cell Tissue Res. 364(3):623–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabłońska-Trypuć A, Matejczyk M, Rosochacki S. 2016. Matrix metalloproteinases (MMPS), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzyme Inhib Med Chem. 31(suppl 1):177–183. [DOI] [PubMed] [Google Scholar]
- Jang AT, Merkle AP, Fahey KP, Gansky SA, Ho SP. 2015. Multiscale biomechanical responses of adapted bone–periodontal ligament–tooth fibrous joints. Bone. 81:196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku M, Rosales Rocabado JM, Kitami M, Ida T, Akiba Y, Yamauchi M, Uoshima K. 2016. Mechanical loading stimulates expression of collagen cross-linking associated enzymes in periodontal ligament. J Cell Physiol. 231(4):926–933. [DOI] [PubMed] [Google Scholar]
- Kalamajski S, Oldberg Å. 2010. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol. 29(4):248–253. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Ohashi K, Soma K, Yanagishita M. 2001. Occlusal hypofunction causes changes of proteoglycan content in the rat periodontal ligament.J Period Res. 36(1):9–17. [DOI] [PubMed] [Google Scholar]
- Kirsch T, Harrison G, Golub EE, Nah H-D. 2000. The roles of annexins and types II and X collagen in matrix vesicle-mediated mineralization of growth plate cartilage. J Biol Chem. 275(45):35577–35583. [DOI] [PubMed] [Google Scholar]
- Lamandé SR, Bateman JF. 2018. Collagen VI disorders: insights on form and function in the extracellular matrix and beyond. Matrix Biol. 71–72:348–367. [DOI] [PubMed] [Google Scholar]
- Levy BM, Bernick S. 1968. Studies on the biology of the periodontium of marmosets: V. Lymphatic vessels of the periodontal ligament. J Dent Res. 47(6):1166–1175. [DOI] [PubMed] [Google Scholar]
- Maria R, Ben-Zvi Y, Rechav K, Klein E, Shahar R, Weiner S. 2019. An unusual disordered alveolar bone material in the upper furcation region of minipig mandibles: a 3D hierarchical structural study. J Struc Biol. 206(1):128–137. [DOI] [PubMed] [Google Scholar]
- McCulloch CA, Lekic P, McKee MD. 2000. Role of physical forces in regulating the form and function of the periodontal ligament. Periodontol. 24:56–72. [DOI] [PubMed] [Google Scholar]
- Moore Rosset E, Trombetta-eSilva J, Hepfer G, Chen P, Yao H, Bradshaw AD. 2020. Inhibition of transglutaminase activity in periodontitis rescues periodontal ligament collagen content and architecture. J Periodontal Res. 55(1):107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlemann HR. 1954. Tooth mobility. J Periodontol. 25:128–137. [Google Scholar]
- Nanci A. 2017. Ten Cate’s oral histology-e-book: development, structure, and function. Cambridge (MA): Elsevier Health Sciences. [Google Scholar]
- Naveh GRS, Brumfeld V, Shahar R, Weiner S. 2013. Tooth periodontal ligament: direct 3D microCT visualization of the collagen network and how the network changes when the tooth is loaded. J Struc Biol. 181(2):108–115. [DOI] [PubMed] [Google Scholar]
- Naveh GRS, Foster JE, Silva Santisteban TM, X Yang, Olsen BR. 2018. Nonuniformity in ligaments is a structural strategy for optimizing functionality. Proc Natl Acad Sci. 115(36):9008–9013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveh GRS, Lev-Tov Chattah N, Zaslansky P, Shahar R, Weiner S. 2012. Tooth-PDL-bone complex: response to compressive loads encountered during mastication—a review. Arch Oral Biol. 57(12):1575–1584. [DOI] [PubMed] [Google Scholar]
- Naveh GRS, Shahar R, Brumfeld V, Weiner S. 2012. Tooth movements are guided by specific contact areas between the tooth root and the jaw bone: a dynamic 3D microCT study of the rat molar. J Struct Biol. 177(2):477–483. [DOI] [PubMed] [Google Scholar]
- Naveh GRS, Weiner S. 2015. Initial orthodontic tooth movement of a multirooted tooth: a 3D study of a rat molar. Orthodon Cranio Res. 18(3):134–142. [DOI] [PubMed] [Google Scholar]
- Oftadeh R, Connizzo BK, Nia HT, Ortiz C, Grodzinsky AJ. 2018. Biological connective tissues exhibit viscoelastic and poroelastic behavior at different frequency regimes: application to tendon and skin biophysics. Acta Biomater. 70:249–259. [DOI] [PubMed] [Google Scholar]
- Picton DC. 1965. On the part played by the socket in tooth support. Arch Oral Biol. 10(6):945–955. [DOI] [PubMed] [Google Scholar]
- Qian L, Todo M, Morita Y, Matsushita Y, Koyano K. 2009. Deformation analysis of the periodontium considering the viscoelasticity of the periodontal ligament. Dent Mater. 25(10):1285–1292. [DOI] [PubMed] [Google Scholar]
- Rippin J. 1976. Collagen turnover in the periodontal ligament under normal and altered functional forces: I. Young rat molars. J Period Res. 11(2):101–107. [DOI] [PubMed] [Google Scholar]
- Rippin J. 1978. Collagen turnover in the periodontal ligament under normal and altered functional forces: II. Adult rat molars. J Period Res. 13(2):149–154. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Olsen BR. 1991. Facit collagens: diverse molecular bridges in extracellular matrices. Trends Biochem Sci. 16(5):191–194. [DOI] [PubMed] [Google Scholar]
- Solomonov I, Zehorai E, Talmi-Frank D, Wolf SG, Shainskaya A, Zhuravlev A, Kartvelishvily E, Visse R, Levin Y, Kampf N. 2016. Distinct biological events generated by ECM proteolysis by two homologous collagenases. Proc Natl Acad Sci. 113(39):10884–10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staszyk C, Duesterdieck KF, Gasse H, Bienert A. 2005. Immunohistochemical identification of lymphatic vessels in the periodontium of equine cheek teeth. J Vet Dent. 22(4):227–232. [DOI] [PubMed] [Google Scholar]
- Sulakshana K, Vijayaraghavan N, Krishnan V. Time-dependent variation in expression patterns of Lysyl Oxidase, Type I Collagen and tropoelastin mRNA in response to orthodontic force application. Arch Oral Biol. 2019;102:218–224. [DOI] [PubMed] [Google Scholar]
- Timpl R, Chu M-L. 1994. Microfibrillar collagen type VI. In: Yurchenco PD, Birk DE, Mecham RP, editors. Extracellular matrix assembly and structure. San Diego (CA): Academic Press. p. 207–242. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034520962455 for Nonuniformity in Periodontal Ligament: Mechanics and Matrix Composition by B.K. Connizzo, L. Sun, N. Lacin, A. Gendelman, I. Solomonov, I. Sagi, A.J. Grodzinsky and G.R.S. Naveh in Journal of Dental Research