Summary
This is the second of a three-part series that charts the history of minimal access surgery from antiquity to current times. Although rapid developments in laparoscopic and robotic surgery have transformed surgical care over the last 30 years, our predecessors made significant advances in their time which set the principles for modern practice. Part I of this series described how ancient medical practitioners developed simple instruments, from metal or wood, for viewing body cavities. Improvements in the use of metal, glass and lighting allowed for inspection of deeper parts of the body. This second part of the series will show how advances in electrical technology allowed the development of improved lighting for endoscopy and laparoscopy along with the use of electrocautery for a wide range of therapeutic procedures.
Keywords: History of medicine, surgery, general surgery
Part II – 1850–1990
Technological developments
The late 19th century was a time of many scientific discoveries and social changes. Innovative scientists and physicians applied the new technological discovery of electricity to various clinical scenarios. In 1855, the British urologist George Robinson attempted to use electricity to treat bladder stones.1 He performed an experiment using a Leyden jar as the source of electricity connected to two copper wires which were inserted into a water-filled bladder via a catheter.2 By pulsing electricity along these wires, he was able to crush many types of stones. He called his discovery electrolithotripsy, but he was unable to persuade any practising surgeons to use his device. A similar system using platinum wires and metal tube inside a glass tube for insultation had previously been devised by Dr Franz von Paula Gruithuisen (1997–1852) in Bavaria.2
A few years later, the German physician Maximilian Nitze (1848–1906) (Figure 1) performed multiple cystoscopies using a variety of instruments, which he developed with his colleagues and collaborators. One of the most important was the Bruck-Nitze-Leiter or Nitze-Leiter cystoscope, which was reported in 1878. Bruck was a German dentist who used white hot platinum wire inside a tube to inspect the bladder and the rectum. Leiter was an instrument maker in Vienna who collaborated with Nitze in the development of instruments for visualisation of the urinary tract – initially using Bruck’s principle of heated platinum wire. They included an irrigation system along with an electrically heated incandescent platinum wire as an internal lighting source, the first of its kind.3
Figure 1.
Maximilian Carl-Friedrich Nitze (1848–1906).
The move from candlelight to electric light, following Edison’s invention of the light bulb (patented in 1879), induced the paradigm shifts in the development of minimal access surgery. Initially used for urological procedures, the Nitze-Leiter cystoscope was adapted for examination of the upper gastrointestinal tract. Nitze later fell out with Joseph Leiter, but both continued to develop their instruments in their separate institutions. Nitze summarised his achievements and made a remarkably prescient comment about the developments which were to come in the following century:
… this writing presents only a framework, the complete construction of which will be accomplished over the course of years through the joint work of numerous researchers. We are dealing here with a large new field of work which assuredly harbours untold treasures of knowledge …
(Berlin, December 1887)4
Although most of our knowledge of the history of minimal access surgery from 1800 onwards comes from Europe or America, there is evidence of the development of minimal access technology in Russia towards the end of the 19th century. The ‘St Petersburg Trio’ of Alexander Ebermann, Alfred Couriard and Benjamin Tarnowsky made a number of advances in the fields of electrosurgery and instrument design. In the 1860s, Alexander Ebermann (1830–1902) adapted an electrical device to improve illumination at endoscopy by either attaching the device to the endoscope directly, or by means of a cumbersome headlight. He also developed a punch biopsy forceps which was used to excise urethral polyps and strictures. Alfred Couriard improved the lighting of cystoscopies by placing a convex lens directly adjacent to the kerosene lamp in order to produce a brighter and more precise beam of light. He also modified the shape of the distal end of the shaft of the scope in order to reduce distortion of the image and improved the cylindrical shape of the shaft which allowed the light to penetrate more deeply into the cavity. Couriard and Benjamin Tarnowsky were the first to split the essential components of cystoscopes into their component parts for greater ease of handling. Up to this time, scopes had comprised a light source, a lens system and the scope tube. These were often mounted on a base to keep them stable in relation to each other, but this made the devices difficult to manoeuvre and cumbersome to use. By separating these three components into separate parts, Couriard and Tarnowsky improved not only the portability and handling of these devices but also the image quality since the lenses were no longer incorporated within the scope tube, and thus, the image was directly visualised rather than reflected.5
The little known Russian gynaecologist Dmitrij Oscarovic Ott (1855–1929) performed numerous diagnostic and therapeutic endoscopic procedures, which were the forerunners of both laparoscopic abdominal surgery and Natural Orifice Transluminal Endoscopic Surgery.6 He used a completely different approach from his European contemporaries, namely ventroscopy. The patient was placed in an extreme Trendelenburg position under general anaesthetic and the vagina incised such that two speculae could be inserted to open the incision. By means of an incandescent electric light and reflecting mirrors, the abdominal cavity could be inspected and various procedures performed. He was able to inspect virtually all the pelvic and abdominal organs.7 He later published a series of 606 patients with gynaecological conditions such as ovarian cysts and bleeding after laparotomies.8 The mortality rate of this series was 2.14%, compared to 11.11% in his laparotomy patients. Subsequently he published a series of over 1000 ventroscopies in which he diagnosed 64 ectopic pregnancies, 60 cases with pelvic inflammatory disease, 15 cases of adhesions and performed three appendicectomies (the very first Natural Orifice Transluminal Endoscopic Surgery appendicectomy). The mortality in this huge series was only 1.48%, and his average inspection time was just 3–5 min.9
Developments in laparoscopic inspection of the larger body cavities – abdomen and thorax – came in the early 20th century. Time and space do not permit a detailed account of every individual development, but some of the strategic and illustrative advances are described here. For further detailed history, the reader is referred to some of the earlier works.10–16
In 1901, Georg Kelling (1866–1945) (Figure 2) performed what is generally thought to be the first laparoscopy (coelioscopy) in a dog. His main interest was actually in gastroscopy as he believed that gastric distention could be used as a treatment for gastric haemorrhage. He modified the rigid Nitze/Leiter cystoscope with a longer flexible end in order to access the stomach and insufflate it thus raising the intragastric pressure. After performing multiple such gastroscopies, he wished to investigate what effect raising the intragastric pressure might have on the internal organs; therefore, he undertook his famous canine laparoscopy and reported that the abdominal organs looked smaller and paler than expected after insufflation of intraperitoneal air.17 Kelling was a visionary who realised that laparoscopy would lead to improved diagnosis of abdominal conditions, more accurate staging of cancers, fewer complications than exploratory laparotomy and its possible use as a day-case procedure. He even addressed the issue of training, by practising laparoscopy on corpses which were the only available ‘models’ in his day.
Figure 2.
Georg Kelling (1866–1945).
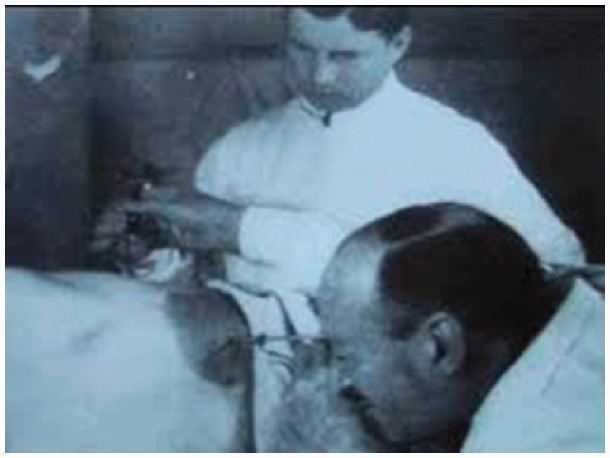
He was closely followed by Hans Christian Jacobaeus (1879–1937) who performed the first laparoscopy in humans (Figure 3). He used a cystoscope to diagnose abdominal complaints in 19 patients, performed thoracoscopy, recognised the risks of iatrogenic injury and cautioned about the use of this technique in the only two non-ascitic patients in his published series.18 He subsequently reported a second series of 97 patients, including eight without ascites. He also moved on from diagnostic laparoscopy to thoracoscopy, along with Ludoph Brauer, a chest specialist from Hamburg. They treated patients with tuberculosis with thoracoscopy for either inducement of therapeutic pneumothorax (of which Brauer was a great advocate) or division of adhesions around the lung to prevent atelectasis.19,20 This thoracoscopic approach was widely practised until the discovery of streptomycin in 1945 transformed the medical treatment of tuberculosis.
Figure 3.
Jacobaeus performs the first human abdominal laparoscopy.
The following year, 1911, Bertram Bernheim from Johns Hopkins Hospital, Boston, performed the first laparoscopy in the USA. He examined the peritoneal cavity of a jaundiced patient by using a proctoscope inserted via an epigastric incision.
The first half of the 20th century saw a huge proliferation in both uptake and development of minimally invasive techniques, particularly in Europe and North America. The field was led mainly by gynaecologists, and advances were made in the visualisation of different organs, the range of indications for laparoscopic surgery, the formation and nature of the pneumoperitoneum, electrocautery, visualisation and optics.10–16
Creation and constitution of pneumoperitoneum
Many of the aforementioned surgeons realised that it was necessary to induce a pneumoperitoneum in order to avoid iatrogenic damage to abdominal organs; thus, the blunt ‘mandrin’ type of trocar was used by Kelling and Jacobaeus. In 1918, Otto Goetze developed an automatic needle which could be safely introduced into the peritoneal cavity for radiologic and laparoscopic procedures.21
The discipline of interventional radiology was also emerging at this time, and in the USA, the radiologist William Stewart and his gynaecologist colleague Arthur Stein used an anaesthetic bag to inflate the abdominal cavity with air or oxygen via a spinal needle in order to outline the abdominal viscera on plain abdominal films.
It was recognised that air or oxygen within the peritoneal cavity would preclude the use of electrocautery for therapeutic procedures because of the risk of explosion and also that its presence could cause pain for several days due to slow absorption. The American doctor Walter Alvarez (1921) and Richard Zollikofer, a Swiss gynaecologist (1924), were the first to describe the use of carbon dioxide rather than air for the pneumoperitoneum in these radiological and laparoscopic procedures.
During the 1920s, the American radiologist Bejamin Ordnoff published a series of 42 peritoneoscopies including cases of peritonitis, ascites, ectopic pregnancy and tumours of the ovary, stomach, pancreas and other organs.22 He accessed the peritoneum under local anaesthetic using a spinal needle to insufflate oxygen, performed fluoroscopy in order to avoid damaging the organs and then used a sharp pyramidal trocar in order to insert his ‘peritoneoscope’, a modification of Jacobaeus’s instrument. Although he presented his findings to the Loyola Research Society in Chicago and the Omaha Roentgen Society in Nebraska in 1920, his work was not recognised for its seminal importance, and he devoted the rest of his long and distinguished career to other aspects of radiology.23
Janos Veress (1903–1979) (Figure 4) developed his eponymous spring-loaded needle for access to the thorax initially, although it continues in widespread use (with minor modifications) as one of the most common instruments for accessing the peritoneum to this day.24 Veress described the problem he was trying to address:
Undoubtedly, an important moment of pleural and peritoneal puncture is the moment when the needle punctures the pleura or the peritoneum, because the vulnerable lungs or intestines are exposed to injuries with the pointed instrument. The sharp tip on the puncture instrument, to penetrate the thoracic or abdominal wall easily, is of great importance, but only till the moment when the needle reaches the cavity: then, in the cavity, the sharp tip is superfluous, even dangerous.
Veress originally designed his small bore (2 mm) needle with a spring-loaded obturator to enter the thoracic cavity in order to induce a pneumothorax in patients with tuberculosis while avoiding iatrogenic injury to the underlying lung. On passing through the chest wall (or abdominal wall in the case of peritoneal access), the obturator ‘clicks’ into place to cover the needle tip. There is the possibility that a ‘click’ may also occur if the needle tip enters a hollow viscus, so appropriate care should be taken on placement, despite the rarity of this complication.25 In modern laparoscopic abdominal surgery, needles based on Veress’ original design are by far the most common in current use and are available from multiple commercial sources (Figure 5).
Figure 4.
Janos Veress (1903–1979).
Figure 5.
Tip of modern Veress needle with retractable tip (Mölnlycke®).
In 1971, Harrith Hasson described the technique of ‘open laparoscopy’ whereby the initial entrance to the peritoneal cavity was performed under direct vision, thus reducing the risk of iatrogenic perforation.26 His eponymous technique is now one of the most commonly used laparoscopic access procedures used in worldwide surgical practice.
Diathermy
In 1933, Karl Fervers used electrocautery to divide adhesions at laparoscopy. However, he also described ‘explosions’ and light flashes due to the interaction of oxygen with the high-frequency electric current.
The American cardiologist John Ruddock (1891–1964) changed specialty and developed a combined approach to gastric cancer. He designed a peritoneoscope to inspect the gastric surface and combined this with a ‘special stomach tube’ (Rehfuss tube) which had a light at its tip and through which air was insufflated to distend the stomach. Ruddock also used urology biopsy forceps to take specimens from the liver, spleen, stomach, omentum and peritoneum via his peritoneoscope. The Ruddock Peritoneoscope comprises six parts,27 most of which can be found in modern laparoscopic sets:
Sheath;
Bistoury-tipped obturator (bistoury = narrow surgical blade);
Telescope (14 inches) – airtight fit within sheath;
Biopsy forceps;
Fluid evacuator;
Pneumoperitoneum needle and a special Rehfuss tube with an electric light at the tip.
He reported over 2000 cases and used electrocautery to coagulate all wounds with monopolar current. This was the forerunner of many interventional laparoscopic procedures over the next half century. He also published a list of the causes of failure of peritoneoscopic examination that have stood the test of time as the causes of such failures today:
Lack of basic knowledge of anatomy, pathology and physiology;
Improper technique of examination;
Incorrect interpretation;
Incomplete examinations;
Lack of familiarly with instrument;
Poor selection of cases.
There is indeed much that current surgeons could (re)learn from the pioneers of surgical history.
Lighting and lenses
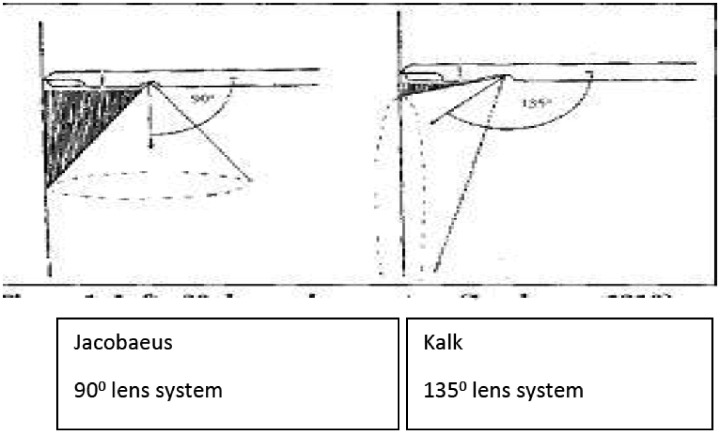
The next major development was the development of a 135° lens system and double trocar by Heinz Kalk (1895–1973), who is sometimes referred to as the father of German laparoscopic surgery.28 As a gastroenterologist, he was particularly interested in liver and gallbladder disease. He had recognised the high fatality rate of blind liver biopsy and used laparoscopy to take liver biopsies under direct vision. Endemic hepatitis spread through the German troops during the early 1940s leading to a dramatic increase in his clinical practice.29 He performed over 2000 liver punctures under local anaesthesia and other procedures such as division of adhesions. He published prolifically,30 and his achievements in laparoscopy were recognised throughout Europe in the following years. He collaborated with the Heynemann Company to produce a laparoscope with a 135° lens system rather than the 90° lens as per Jacobaeus’ original design (Figure 6).
Figure 6.
Diagram of Jacobaeus (90°) and Kalk (135°) lens systems.12
From the 1930s onwards, the cinema and television industries began to flourish, and enterprising doctors transferred their technology into medical and surgical practice. Initially, the Japanese led the way when Mori and Yamadori used a glass fibre hysteroscope to film the birth of a live infant by placing a camera on the inside of the uterine wall. At the Hayashida Hospital, Uji, Suginara and Fukami developed the ‘gastrocamera’ – one of the earliest endoscopic cameras – in 1950. This was closely followed by Cohen and Guterman who produced the Cameron cavicamera, which was able to take both still and moving pictures in 1953.
The British physicist Harold Hopkins (1918–1994) (Figure 7) transformed the practice of urology, and many other branches of minimal access surgery and endoscopy, by his invention of flexible fibre-optic lighting systems.31,32 He had a doctorate in optical physics, and among his many achievements, was the development of the zoom lens, which allowed the televising of major sporting events, and was first used in 1948 for a cricket match at Lord’s. His lack of avarice and self-interest was demonstrated by the fact that he took a one-off £2000 payment rather than percentage fee for this invention that revolutionised the broadcasting of sports and other live events. A chance meeting with the gastroenterologist Hugh Gainsborough in 1951 diverted Hopkin’s efforts into medical applications of optical physics. The existing gastroscopes were fairly rigid instruments which risked perforation of the patient’s stomach or oesophagus while also failing to examine every part of the organs. Hopkins and his research student Narinder Kapany were the first to produce a bundle of narrow glass fibres which transmitted light and was also flexible enough to transmit light round corners, which he published in Nature in 1954.33 Although this publication generated much interest from the medical fraternity, Hopkins was unable to secure funding to develop his fibrescope beyond the prototype stage within the UK. However, the South African Basil Hirschowitz, who was practising gastroenterology in Michigan, recognised the potential of this development. He met Hopkins and collaborated to develop the flexible gastroscope by initially performing his own endoscopy before taking it into his clinical practice in collaboration with both Hopkins and the physicists Peter and Curtiss at Ann Arbor in Michigan. They presented this model at a meeting in Colorado Springs in 1957 and subsequently published a report in The Lancet.34
Figure 7.

Harold Hopkins (1918–1994).
Despite his disappointment that he had to develop his gastroscope outside of the UK, Hopkins was persuaded by the eminent British urologist James Gow to collaborate on developments in cystoscopy. Gow was based in Liverpool, and he was keen to be able to take photographs during cystoscopy, but this was not possible as the extant cystoscopes were rigid tubes with a light at one end and a series of glass lenses along the air-filled tube to relay the image. Hopkins’ solution was to reverse the components such that the tube contained a series of glass rods that transmitted the light more efficiently, interspersed with thin air ‘lenses’. This so-called ‘glass rod lens system’ had multiple advantages, including improved light transmission and image quality, reduced light scatter, and easier mounting and better stability of the cystoscope. In 1961, Hopkins and Gow presented the first photographs taken with this system to the Société Internationale d’Urologie meeting in Rio de Janeiro.35 Again, Hopkins failed to obtain interest or investment from any British companies, but after a presentation in Cologne in 1965, Hopkins was contacted by Karl Storz who, at that time, was running a small instrument company in Germany. Storz added his own ‘cold light’ to Hopkins’ ‘rod lens’, and when they presented their new instrument to the Société Internationale d’Urologie meeting in Munich in 1967, it was immediately taken up, not only by urologists, but deployed in all fields of endoscopic and minimal access surgery worldwide. Its applications are too numerous to recount but suffice it to say that virtually all endoscopic and laparoscopic procedures since 1967 have used devices incorporating the Hopkins and Storz technology.
The ability to broadcast and publicise these technological advances expanded rapidly in the 1940s–1960s. Brubaker and Holinger presented moving pictures from bronchoscopy in 1945. Their lighting system was bulky and awkward to use and generated excessive heat, so it did not catch on widely. French endoscopists first started to film and broadcast their procedures in the early 1950s, and in 1955, Palmer (see below) published the first live laparoscopy in colour. In the same year, the French doctors Soulas and Dubois de Montreynaud broadcast films of bronchoscopy procedures.36 In 1958, Frangenheim (see below) produced a film of gynaecological laparoscopic surgery which caught the imagination of the gynaecologic world and transformed clinical practice in gynaecology.
By the mid-1960s, the advance of technological developments in gynaecological laparoscopic surgery slowed down, and it again took the vision and single-mindedness of a gifted individual to introduce a paradigm shift in the specialty. Camran Nezhat realised that despite the obvious benefits of laparoscopic surgery in terms of lesser physiological insult, improved cosmesis and shorter length of stay, many surgeons found it difficult to perform minimal access procedures due to the need to remain hunched over the apparatus while squinting down the eye piece. Although some of his forebears had contemplated using television screens as teaching attachments, he realised that it would be much easier if endoscopic images could be projected onto a monitor. The Hopkins rod lens system, which was in common use at the time, did not generate enough light for useful images to be transmitted to a monitor. Nezhat experimented with different combinations of monitor, lighting, scopes, etc. and initially used a single-tube camera attached to a monitor (Medical Dynamics, Synvision model) which provided an adequate image, although at low light level. He set up collaborations with commercial partners including Karl Storz and revolutionised the practice of laparoscopic gynaecological surgery. Once video monitoring of sufficient resolution to allow live operating had developed, Nezhat began to develop suturing techniques that had been virtually impossible while peering down a tube scope. Surgeons took up these techniques in huge numbers, and the early 1990s saw a revival in many types of laparoscopic surgery paving the way for today’s clinical practice.
Expanding the repertoire
For the first part of the 20th century, minimally invasive surgery was generally confined to urology, gynaecology, gastroscopy, diagnostic laparoscopy and some otorhinolaryngology. It was very much the preserve of enthusiasts who performed large series of individual cases, but general enthusiasm waxed and waned, particularly during the years of World War II. There was caution over the appropriateness of this technology due to reports of iatrogenic perforations and air embolism, along with concerns about the cost of such procedures. After a proliferation of reports in the literature during the 1920s–1930s, there was a significant decline in reports on laparoscopic work from 1940 to 1960. However, Raoul Palmer (1905–1985) (Figure 8), a gynaecologist in Paris, resurrected interest in laparoscopic gynaecological surgery by his industrious work and sheer determination to bring laparoscopy into the mainstream of gynaecological practice. He addressed every aspect of the service from the clinical indications, to operative technique, to instrument design to training issues.14 The fact that Paris was under occupation by German forces during much of this time only serves to highlight his remarkable achievements.
Figure 8.
Raoul Palmer (1905–1985).
Palmer’s initial clinical experience was in the field of infertility. He was concerned that performing exploratory laparotomies was causing iatrogenic damage and adhesions and in 1943 moved to ‘preoperative exploratory coelioscopy’ despite the criticism of his peers. He performed a range of gynaecological operations including drainage of cysts, adhesiolysis and electrocautery. In 1961, Palmer and his colleague Klein were the first to report laparoscopic retrieval (using a cystoscope) of an oocyte.37 He also led the field of laparoscopic sterilisation by tubal ligation experimenting with mono polar and later bipolar diathermy.
Palmer experimented with different surgical techniques, including the transvaginal culdoscopic approach, but subsequently settled on the transabdominal approach with the patient in a deep Trendelenberg position. He was also one of the first to recognise the importance of controlling the intra-abdominal pressure during laparoscopy. There had been previous reports of death due to air embolism, and Palmer defined 25 mmHg as the maximum safe pressure for these procedures. He also realised that carbon dioxide was much safer than oxygen for insufflation and determined that the insufflation speed should be 400–500 cc per min. He was also one of the first to realise that it was safer to introduce the insufflator and first trocar in the lower, rather than upper, abdomen in order to reduce iatrogenic damage and to facilitate a better view of the pelvic organs.
He developed a set of electrocautery forceps to take ovarian biopsies and to perform tubal ligations. This instrument became very popular, particularly in the USA, thus enhancing Palmer’s reputation as a leader in the field of gynaecological surgery. He was also able to adapt technologies invented for the movie industry for use in minimal access surgery. For instance, when the quartz rod lighting system was developed in 1952, Palmer realised it could be applied to improve lighting at laparoscopy and developed a small (5 mm) scope which contained a powerful lighting system. He also adapted photography and recording devices in order to make films of live pelvic surgery.
He recognised the importance of teaching and, after World War II, set up a school to which surgeons and gynaecologists from all over the world came to observe his techniques and develop their own range of skills. Many of these went on to become leaders of operative gynaecology, laparoscopic surgery and fertility treatment in their own countries during the 1960s–1980s. Famous alumni included Melvin Cohen from Chicago. Robert Neuwirth from New York, the Canadians Jacques Rioux and Victor Gomel and Patrick Steptoe from the UK, who, together with his colleague Robert Edwards, adapted Palmer’s technique for oocyte retrieval to bring about the birth of the first test tube baby, Louise Brown, in 1978.
Hans Frangenheim was a fertility specialist in Wuppertal Germany, contemporaneous with Palmer. He too struggled with lack of resources in the postwar years, since German doctors and academics were cut off from colleagues in the rest of Europe. In 1955, he met Palmer and they became friends who shared ideas and supported each other when their immediate colleagues poured cold water on their more visionary ideas. Culdoscopy, as popularised by Ott and Decker, was practised quite widely by gynaecologists in Germany, but Frangenheim was convinced of the superiority of laparoscopy, and in 1958, he reported over 350 such cases.38 He also developed an improved carbon dioxide insufflator and modified quartz rods to improve optics. However, he may be best remembered for publishing the first textbook of Gynaecologic Laparoscopy.39 His work continued for the next quarter of a century, and he published prolifically until well into the 1980s.
During the 1980s, Nezhat and his brothers continued their collaborative efforts with like-minded surgeons and gynaecologists and expanded the range of procedures performed laparoscopically. He struggled to get his work published initially as there was resistance within both the clinical and academic communities to his ideas, his commercial collaborations and private practice. His main clinical interest was endometriosis, but he also had wider collaborations with colorectal surgeons and urologists. During the early 1990s, Nezhat reported many ‘firsts’ including laparoscopic bowel resection and repair for advanced endometriosis,40,41 laparoscopic ureteric resection and ureteroureterostomy,42 laparoscopic radical hysterectomy with para-aortic and pelvic node dissection,43 laparoscopic bladder resection,44 laparoscopic vesicovaginal repair,45 laparoscopic ovarian cystectomy during pregnancy,46 laparoscopic-assisted myomectomy47 and laparoscopic sacral colpopexy.48 Nezhat’s work attracted controversy39 from both the medical profession and the lay media leading to a formal investigation by Stanford University, the U.S. State Supreme Court and the State Medical Boards of California and Georgia. After detailed investigations, he was acquitted of any misconduct, and subsequent experience and publications by legions of surgeons have proven his ideas to be visionary and transformative.
Kurt Semm (1927–2003), a German gynaecologist who practised in Kiel, is widely recognised as being the first to perform a laparoscopic appendicectomy on 12 September 1980. In his own words, he stated that
The technique, recommended only for non-acute cases, consisted of an extracorporeal ligation of the mesoappendix with endoscopic ligation of the appendix with a pretied loop. The appendix was transected across its base with electrocautery. Laparoscopy subsequently became a practical and popular technique for the evaluation and treatment of right lower quadrant pain in females, utilized by general and gynecologic surgeons alike.
He also was a technical genius developing a new and safer insufflator, better thermocoagulation devices, developed techniques for intra- and extracorporeal knot tying and invented a loop applicator, a tissue morcellator and an aqua-purator which could switch between aspiration and insufflation of irrigation fluid. He published widely on these developments and many more.
His technique49 and his approach to widening the range and impact of laparoscopic surgery drew admiration and admonition in equal measure from colleagues and the medical fraternity more generally. He robustly defended his position in person and in print and entered into combative debates with any who disagreed with him, including his old colleague and mentor Frangenheim. However, he did much to bring laparoscopic surgery into the arena where it is today, and history has vindicated many of his assertions.
The most seminal expansion in the repertoire of laparoscopic general surgery came in the mid-1980s with the advent of laparoscopic cholecystectomy. In 1985, Erich Műhe (1938–2006) (Figure 9) performed what is thought to have been the first laparoscopic cholecystectomy. He personally designed an instrument which he called a ‘gallscope’, which he used alongside Semm’s instruments and monitors to remove a gallbladder in less than 2 hours. By 1987, he had performed over 100 laparoscopic cholecystectomies. However, when he presented his work at the German Surgical Society in 1986, he was met with scepticism, and his probity was called into question leading to a lawsuit against him for ‘improper surgical conduct’. It was not until 1992 that the German Surgical Society exonerated his work. He is therefore rarely recognised for this achievement, and it was the French gynaecologist Phillippe Mouret (1938–2008) (Figure 10) who produced a video of the procedure which was highly publicised and laparoscopic cholecystectomy rapidly gained popularity worldwide. Despite the initially high incidence of bile duct injuries, the benefits of less pain, shorter hospital stay, quicker recovery and return to work were obvious to both patients and surgeons, and many current aspiring surgeons rarely see an open cholecystectomy during their training. The rapid adoption of laparoscopic cholecystectomy led to the uptake of laparoscopic techniques by most general surgeons, and operations on virtually every abdominal and thoracic organ can now be performed laparoscopically.
Figure 9.
Erich Muhe (1938–2006).
Figure 10.
Phillippe Mouret (1938–2008).
Conclusion
Between 1850 and 1990, minimal access surgery made significant advances due to the ingenuity of individual surgeons and their ability to apply technology from other disciplines. Advances in glass making, electrical engineering, cinematography and broadcasting were just some of the technical developments that were modified and applied to minimal access surgery. This period also highlighted not only the vision and determination of many individual surgeons and scientists but also the scepticism and opposition of the medical profession which they encountered.
Part III of this series will bring us up to date with more recent developments in minimal access surgery, particularly with regard to organisational issues and the rise of robotic technology.
Footnotes
Acknowledgement: The author would like to thank Dr Jane Lane for proofreading the manuscript.
Provenance: Not commissioned; peer-reviewed by David Webster.
ORCID iD: Rachel Hargest https://orcid.org/0000-0001-9830-3832
Declarations
Competing Interests: None declared.
Funding: None declared.
Ethical Approval: No ethical approval required.
Guarantor: RH.
Contributorship: Sole authorship.
References
- 1.Hargest R. Five Thousand Years of Minimal Access Surgery: 3000BC to 1850: Early Instruments for viewing body cavities. Journal of the Royal Society of Medicine. [DOI] [PMC free article] [PubMed]
- 2.European Museum of Urology. Electrohydraulic Lithotripsy. http://history.uroweb.org/history-of-urology/early-urological-interventions/lithotripsy/the-early-history-of-lithotripsy/.
- 3.Meyer W. On Cystoscopy and the New Cystoscope of Nitze and Leiter. With a Demonstration of the Same (Reprinted from the New York Medical Journal, April 21 1888). London: Forgotten Books, 2018.
- 4.Newell O. The endoscopic instruments of Joseph Leiter of Vienna and the present development of endoscopy. Boston Med J 1887; 117: 528–530. [Google Scholar]
- 5.Reuter A, Reuter H, Engel R. History of Endoscopy. Vol I–IV 1999; Vol I, Stuttgart, Germany: Max Nitze Museum. [Google Scholar]
- 6.Hatzinger M, Fesenko A, Sohn M. The first human laparoscopy and NOTES operation: Dmitrij Oscarovic Ott (1855–1929). Urologia Int 2014; 92: 387–391. [DOI] [PubMed] [Google Scholar]
- 7.Ott D. Die Beleuchtung der Bauchhöhle (Ventroskopie) als Methode bei vaginaler Köliotomie. Zentralbl Gynakol 1902; 26: 817–820. [Google Scholar]
- 8.Ott D. Die unmittelbare Beleuchtung der Bauchhöhle, der Harnblase, des Dickdarms und der Gebärmutter zu diagnostischen und operativen Zwecken. Monatsschr Geburtshilfe Gynakol 1903; 18: 645–670. [Google Scholar]
- 9.Ott D. Die Resultate der Anwendung der direktenBeleuchtung der Bauchhöhle, des Dickdarms und der Harnblase bei den Operationen und zu diagnostischen Zwecken. Zentralbl Gynakol 1909; 33: 1–8. [Google Scholar]
- 10.Schollmeyer T, Mettler L, Ruther D, Alkatout I. Practical Manual for Laparoscopic & Hysteroscopic Gynecological Surgery, 2nd edn. London: Jaypee Brothers Medical Publishers Ltd., 2013. . See https://books.google.co.uk/books?id=pA0FrBYqDo4C&pg=PA3&lpg=PA3&dq=archigenes+and+mirror&source=bl&ots=HaWqflxBBj&sig=ACfU3U31Tk9u4S4_LuuSUxX8fNiCrs1JGQ&hl=en&sa=X&ved=2ahUKEwj_4LXWgdToAhWPfMAKHcHJAlkQ6AEwD3oECAsQNA#v=onepage&q=archigenes%20and%20mirror&f=false. [Google Scholar]
- 11.Nezhat C. Neshat’s History of Endoscopy. An Analysis of Endoscopy’s Ascension since Antiquity, Tuttlingen, Germany: EndoPress, 2011. . See https://books.google.co.uk/books?id=_-dPJbOLjj0C&pg=PA35&lpg=PA35&dq=Segalas+and+John+fisher&source=bl&ots=AzhswEuEAG&sig=ACfU3U1YFrn9FEi37Q3Kp68q2oc6u_0azQ&hl=en&sa=X&ved=2ahUKEwjb3sP4j9noAhVNhlwKHSIbDGIQ6AEwBHoECAkQOQ#v=onepage&q=Segalas%20and%20John%20fisher&f=false. [Google Scholar]
- 12.Litynski G. Highlights in the History of Laparoscopy, Frankfurt/Main, Germany: Barbara Berneit Verlag, 1996. [Google Scholar]
- 13.Nezhat F. Triumphs and controversies in laparoscopy: the past, the present, and the future. J Soc Lap Robot Surg 2003; 7: 1–5. [PMC free article] [PubMed] [Google Scholar]
- 14.Nezhat C. Nezhat’s History of Endoscopy. Let There be Light: A Historical Analysis of Endoscopy’s Ascension since Antiquity, Tuttlingen, Germany: EndoPress, 2005. See https://laparoscopy.blogs.com/endoscopyhistory/. [Google Scholar]
- 15.Hockstein NG, Gourin CG, Faust RA, Terris DJ. A history of robots: from science fiction to surgical robotics. J Robot Surg 2007; 1: 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antoniou SA, Antoniou GA, Antoniou AI, Granderath F-A. Past, present and future of minimally invasive abdominal surgery. J Soc Lap Robot Surg 2015; 19: e2015.00052–e2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Litynski GS. Laparoscopy – the early attempts: spotlighting George Kelling and Hans Christian Jacobaeus. J Soc Lap Robot Surg 1997; 1: 83–85. [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobaeus HC. Über die Möglichkeit die Zystoskopie bei Untersuchung seröser Höhlungen anzuwenden [On the possibility to perform cystoscopy in examinations of serous cavities]. Münchner Medize Wochen 1910; 57: 2090–2092. [Google Scholar]
- 19.Jacobaeus HC. The cauterization of adhesions in pneumothorax treatment of tuberculosis. Surg Gyn Obst 1912; 32: 493–500. [Google Scholar]
- 20.Braimbridge MV. The history of thoracoscopic surgery. Ann Thor Surg 1993; 56: 610–614. [DOI] [PubMed] [Google Scholar]
- 21.Goetze O. Die neues Verfabren der Gasfüllung für das pneumoperitoneum. Münchner Mediz Wochen 1921; I: 233–234. [Google Scholar]
- 22.Ordnoff BH. The peritoneoscope in diagnosis of disease of the abdomen. J Radiol 1920; 1: 307–325. [Google Scholar]
- 23.Morgenstern L. An unlikely pioneer in laparoscopy: Benjamin Henry Ordnoff. Surg Innov 2003; 1: 5–6. [DOI] [PubMed] [Google Scholar]
- 24.Veress J. Neues instrument zur Ausführung von Brust oder Bauch Punktionen und Pneumothoraxbehandlung. Deutsche Mediz Wochen 1938; 64: 480–481. [Google Scholar]
- 25.Schäfer M, Lauper M, Krähenbühl L. Trocar and Veress needle injuries during laparoscopy. Surg Endo 2001; 15: 275–280. [DOI] [PubMed] [Google Scholar]
- 26.Hasson H. A modified instrument and method for laparoscopy. Am J Obstet Gynecol 1971; 110: 886–887. [DOI] [PubMed] [Google Scholar]
- 27.Ruddock J. The application and evaluation of peritoneoscopy. Califor Med 1959; 71: 110–116. [PMC free article] [PubMed] [Google Scholar]
- 28.Kalk H. Erfahrungen mit det laparoskopie (zugleich mit der beschreibung eines neuen instruments). Zeitschrift für Klinische Medzin 1929; 111: 304–348. [Google Scholar]
- 29.Litynski G. Laparoscopy between the world wars: the barriers to trans-Atlantic exchange. J Soc Lap Robot Surg 1997; 1: 185–188. [PMC free article] [PubMed] [Google Scholar]
- 30.Kalk H, Bruhl W, Sieke W. Die gezielte leberpunktion. Deutsche Mediz Wochen 1943; 69: 693–695. [Google Scholar]
- 31.Bhatt J, Jones A, Foley S, Shah Z, Malone P, Fawcett, et al. Harold Horace Hopkins: a short biography. Br J Uro Int 2010; 106: 1425–1430. [DOI] [PubMed] [Google Scholar]
- 32.Whitaker M. Saints and sinners – Harold Hopkins. Bull R Coll Surg Eng 2012; 94: 168–170. [Google Scholar]
- 33.Hopkins H, Kapany N. A flexible fibrescope, using static scanning. Nature 1954; 173: 39–41. [Google Scholar]
- 34.Hirschowitz B. A fibre optic flexible Œsophagoscope. Lancet 1963; 282: 388–388. [DOI] [PubMed] [Google Scholar]
- 35.Gow J. Harold Hopkins and optical systems for urology – an appreciation. Urol 1998; 52: 152–152. [DOI] [PubMed] [Google Scholar]
- 36.Soulas A, Dubois de Montreynaud J, Edwards R, Gladu AJ. Bronchoscopy and television. Dis Chest 1957; 31: 580–584. [DOI] [PubMed] [Google Scholar]
- 37.Klein R, Palmer R. Technique of sampling human ova by follicular puncture under celioscopy. Compt Rendus Séances Soc Biol Filiales 1961; 155: 1919–1921. [PubMed] [Google Scholar]
- 38.Frangenheim H. Die bedeutung der laparoskopie fur die gynakologische diagnostik. Fortschr Med 1958; 76: 451–452. [Google Scholar]
- 39.Frangenheim H. Die Laparoskopie und die Culdoskopie in der Gynakologie, Stuttgart, Germany: Georg Thieme Verlag, 1959. [Google Scholar]
- 40.Nezhat C, Pennington E, Nezhat F, Silfen S. Laparoscopically assisted anterior rectal wall resection and reanastomosis for deeply infiltrating endometriosis. Surg Laparosc Endosc 1991; 1: 106–108. Retraction in: Surg Laparosc Endosc Percutan Tech 2001; 11: 1. [PubMed] [Google Scholar]
- 41.Nezhat F, Nezhat C, Pennington E. Laparoscopic proctectomy for infiltrating endometriosis of the rectum. Fertil Steril 1992; 57: 1129–1132. [DOI] [PubMed] [Google Scholar]
- 42.Nezhat C, Nezhat F, Green B. Laparoscopic treatment of obstructed ureter due to endometriosis by resection and ureteroureterostomy: a case report. J Urol 1992; 148: 865–868. [DOI] [PubMed] [Google Scholar]
- 43.Nezhat CR, Burrell MO, Nezhat FR, Benigno B, Welander C. Laparoscopic radical hysterectomy with paraaortic and pelvic node dissection. Am J Obstet Gynecol 1992; 166: 864–865. [DOI] [PubMed] [Google Scholar]
- 44.Nezhat C, Nezhat F. Laparoscopic segmental bladder resection for endometriosis: a report of two cases. Obstet Gynecol 1993; 81: 882–884. [PubMed] [Google Scholar]
- 45.Nezhat CH, Nezhat F, Nezhat C, Rottenberg H. Laparoscopic repair of a vesicovaginal fistula: a case report. Obstet Gynecol 1994; 83: 899–901. [PubMed] [Google Scholar]
- 46.Nezhat F, Nezhat C, Silfen S, Fehnel SH. Laparoscopic ovarian cystectomy during pregnancy. J Laparoendosc Surg 1991; 1: 161–164. [DOI] [PubMed] [Google Scholar]
- 47.Nezhat C, Nezhat F, Silfen SL, Schaffer N, Evans D. Laparoscopic myomectomy. Int J Fertil 1991; 36: 275–280. [PubMed] [Google Scholar]
- 48.Nezhat C, Nezhat F, Nezhat C. Laparoscopic sacral colpopexy for vaginal vault prolapse. Obstet Gynecol 1994; 84: 885–888. [PubMed] [Google Scholar]
- 49.Semm K. Endoscopic appendectomy. Endoscopy 1983; 15: 59–64. [DOI] [PubMed] [Google Scholar]