Abstract
Background:
Studies indicate that female cannabis users progress through the milestones of cannabis use disorder (CUD) more quickly than male users, likely due to greater subjective craving response in women relative to men. While studies have reported sex-related differences in subjective craving, differences in neural response and the relative contributions of neural and behavioral response remain unclear.
Methods:
We examined sex-related differences in neural and behavioral response to cannabis cues and cannabis use measures in 112 heavy cannabis users (54 females). We used principal component analysis to determine the relative contributions of neural and behavioral response and cannabis use measures.
Results:
We found that principal component (PC) 1, which accounts for the most variance in the dataset, was correlated with neural response to cannabis cues with no differences between male and female users (p = 0.21). PC2, which accounts for the second-most variance, was correlated with subjective craving such that female users exhibited greater subjective craving relative to male users (p = 0.003). We also found that CUD symptoms correlated with both PC1 and PC2, corroborating the relationship between craving and CUD severity.
Conclusions:
These results indicate that neural activity primarily underlies response to cannabis cues and that a complex relationship characterizes a convergent neural response and a divergent subjective craving response that differs between the sexes. Accounting for these differences will increase efficacy of treatments through personalized approaches.
Keywords: Cannabis, Sex-related differences, Cue-elicited craving, Neural response, Subjective craving, Principal component analysis
1. Introduction
Cannabis use incidence has generally been higher in men; however, recent trends suggest growing use in women (Center for Behavioral Health Statistics and Quality, 2017; Hasin, 2018; Lopez and Blanco, 2019; United Nations Office on Drugs and Crime, 2018). This trend is of particular importance given evidence for sex-related differences in substance use that are critical in continued use, development of abuse and dependence, and relapse (Becker and Koob, 2016; Bobzean et al., 2014; Chauchard et al., 2013; Hernandez-Avila et al., 2004; Perry et al., 2016). From a translational perspective, it is important to identify how sex-related differences affect factors that not only impact clinical interventions, but also any therapeutic effects of cannabis (Cooper and Craft, 2018).
Cannabis use disorders (CUDs) are difficult to treat (Sherman and McRae-Clark, 2016; Walker et al., 2015) and rates of treatment-seeking in both men and women are very low (Khan et al., 2013). Although women in general start using cannabis at a later age than men, they progress more quickly through the milestones of CUD and ultimately enter into treatment programs at a younger age, a phenomenon known as telescoping (Hernandez-Avila et al., 2004; Kerridge et al., 2018; Khan et al., 2013). Women also report higher abuse-related effects, suggesting increased sensitivity to cannabis that may promote its continued use (Cooper and Haney, 2014; Kerridge et al., 2018). In treatment-seeking individuals with CUD, women report greater withdrawal symptoms and higher negative impact of withdrawal (Herrmann et al., 2015; Sherman et al., 2017) with similar results in non-treatment-seeking cannabis users (Copersino et al., 2010; Schlienz et al., 2017). These considerations have important clinical relevance as studies report poorer outcomes for women in psychological and pharmacological treatments (Ali et al., 2015; Bassir Nia et al., 2018; DeVito et al., 2014; Ketcherside et al., 2016; Litt et al., 2015; McHugh et al., 2018; Sherman et al., 2017; Wetherill et al., 2015).
Cue-exposure elicits subjective craving and activates neural pathways related to reward processing (Filbey et al., 2009) that underlie the development of CUD and cannabis-related problems. While numerous studies have examined cue-induced craving in cannabis users (Cousijn et al., 2012; Filbey et al., 2016, 2014, 2009; Lundahl and Johanson, 2011; McRae-Clark et al., 2011), the few that have investigated sex-related differences have examined behavioral and neural response separately; thus, potential interactions between sex and measure of response are unknown. In a behavioral study, Lundahl and colleagues reported no significant sex-related differences in behavioral response during a cannabis cue-exposure task (Lundahl and Johanson, 2011). In a functional MRI (fMRI) study, Wetherill and colleagues also found no sex-related differences in neural response to cannabis cues in cannabis-dependent adults (Wetherill et al., 2015). However, women exhibited a positive correlation between neural response to cannabis cues in the bilateral insula and craving and a negative correlation in the left lateral orbitofrontal cortex (OFC), while men exhibited a positive correlation between cannabis craving and neural responses to cannabis cues in the striatum. The authors suggest that this difference may signify a top-down treatment approach in women with increased higher-order processing and that different treatment approaches can improve clinical outcomes in men and women.
We examined sex-related differences in brain perfusion and metabolism during rest in a subset of the data included in the present study and found that female cannabis users had increased global cerebral blood flow compared to male users. However, regionally, men had increased cerebral blood flow in the right insula and women had increased cerebral blood flow in the left posterior cingulate and bilateral precuneus (Filbey et al., 2018a). We also found that female users had increased cerebral metabolic rate of oxygen compared to male users. These differences, however, were found during rest and were not task-related. To our knowledge, no studies have examined sex-related differences in the combined and relative contributions of behavioral and neural response to cannabis cues in a cue-exposure task.
Evidence from animal models supports sex-related differences in the subjective effects of cannabis. The endocannabinoid system modulates the endocrine system (Battista et al., 2012) and cannabis use is postulated to interact with hormones in a bi-directional manner (Brown and Dobs, 2002; Gorzalka and Dang, 2012; Maria et al., 2014). Δ-9-tetrahydrocannabinol (THC) affects the hypothalamic pituitary gonadal (HPG) and hypothalamic pituitary adrenal (HPA) axes and is modulated by cannabinoid (CB1) receptors (Brown and Dobs, 2002; Crane et al., 2013; Ketcherside et al., 2016; López, 2010; Mendelson et al., 1984). THC also alters the release of gonadotropin-releasing hormone (GnRH) from the hypothalamus, leading to differential downstream effects in men and women (Battista et al., 2012; Brents, 2016). For example, similar to humans, female rats exhibit increased sensitivity to the acute effects of cannabis (Craft et al., 2013; Farquhar et al., 2019). Fattore and colleagues have found that ovarian hormones regulate reinforcement of cannabinoids such that female rats exhibited increased learned associations between cannabis-related stimuli and effects of cannabis (Fattore et al., 2007) and that ovariectomized female rodents displayed reduced cue-elicited cannabinoid-seeking behavior compared to intact female rodents (Fattore et al., 2010). A study in gonadectomized rats found that replaced testosterone reduced withdrawal symptoms, while both estradiol and progesterone increased withdrawal symptoms (Marusich et al., 2015). More recently, Farquhar and colleagues found that repeated exposure to THC increased downregulation and desensitization of CB1 receptors in female rats (Farquhar et al., 2019). These preclinical studies indicate that ovarian hormones influence cannabis reinforcement and provide potential mechanisms underlying sex differences (Crane et al., 2013; Lynch et al., 2002).
The aim of this study was to examine the interaction between sex and measures of craving and use in heavy cannabis users. We used principal component analysis (PCA), a multivariate approach, to determine whether the different behavioral, neural, and use variables may be combined based on common dimensions. Specifically, PCA aims to describe and summarize patterns of data by grouping together correlated variables into components that account for the most variance possible in the original variables. This approach also limits the number of variables to be analyzed in subsequent analyses (Tabachnik and Fidell, 2013). This data reduction is achieved by identifying the underlying structure in the data, plotting the data points in a multidimensional space, and creating components that can be thought of as lines in the multidimensional space in the directions where there is the most variance. In doing so, PCA provides a more nuanced approach to understanding sex-related differences compared to group comparisons via traditional statistical methods (Halai et al., 2017). We hypothesized comparable neural response to cannabis cues, but increased subjective craving in female cannabis users compared to male users.
2. Material and methods
2.1. Participants
We analyzed data from 141 heavy cannabis users recruited for a study that examined the effects of cannabis use on neural response (Filbey et al., 2016). These data were collected as part of a larger project that included heavy cannabis users and non-using controls and have resulted in prior publications (Filbey et al., 2018a, 2018b, 2016; Ketcherside et al., 2017). Filbey et al., 2018a examined sex-related differences in resting brain perfusion and metabolism in a subset of the data included in the present study. The study found some sex-related differences in global and regional cerebral blood flow and cerebral metabolic rate of oxygen. These differences were found during rest and were not task-related. Participants were included if they were right-handed, spoke English as their primary language, had no history of neurological or psychiatric diagnoses, and no MRI contraindications. Participants were excluded for regular tobacco use (> 1 pack of cigarettes per month) or current alcohol dependence based on the Structured Clinical Interview for DSM-IV (First et al., 2002). We defined heavy cannabis use as at least 5000 lifetime uses (Gruber et al., 2003; Kouri and Pope, 2000; Pope et al., 2002, 2001) and verified use via quantification of THC metabolites in urine using gas chromatography/mass spectrometry (GC/MS) normalized by creatinine (Cr) concentration (Huestis and Cone, 1998) from Quest Diagnostics (https://www.questdiagnostics.com). The Institutional Review Boards (IRB) of the University of Texas at Dallas and the University of Texas Southwestern Medical Center approved the protocol. All participants provided their written informed consent in compliance with the World Medical Association Declaration of Helsinki.
Of the 141 participants, 28 were excluded due to missing fMRI data and one was excluded due to a positive drug screen for amphetamines, resulting in a sample of 112 participants with 54 female users (mean age: 29.9 ± 7.4 years; range: 19–47 years) and 58 male users (mean age: 31.1 ± 7.5 years; range: 21–55 years). See Table 1 for demographic information. Participants completed a baseline session consisting of behavioral measures and a 72 -h abstinent MRI session. We verified abstinence through self-report and a reduction in THC/Cr level after the 72 -h abstinence period compared to baseline (Filbey et al., 2016, 2009).
Table 1.
Participant demographics (mean ± SD).
| Female cannabis users | Male cannabis users | p-value | |
|---|---|---|---|
| N | 54 | 58 | - |
| Age (years) | 30.4 ± 7.4 (range: 19–47) | 31.6 ± 7.5 (range 21–55) | 0.43 |
| Years of education | 13.0 ± 3.5 | 13.2 ± 2.2 | 0.17 |
| Ethnicity | |||
| Hispanic/Latino | 10 | 11 | 0.95 |
| Non-Hispanic/Latino | 44 | 47 | |
| Race | |||
| Caucasian | 33 | 32 | 0.92 |
| African American | 14 | 15 | |
| Asian | 1 | 1 | |
| Other | 6 | 10 | |
| Number of alcohol drinking days in preceding 60 days | 8.7 ± 10.8 (range: 0–60) | 15.7 ± 18.3 (range: 0–60) | 0.018* |
| Amount of alcohol (# of drinks) | 28.2 ± 39.4 (range: 0–240) | 63.5 ± 86.9 (range: 0–381) | 0.0064* |
| Average number of drinks per drinking day | 2.9 ± 1.7 (range: 0–6.7) | 3.4 ± 2.7 (range: 0–14) | 0.20 |
| Number of smoking days in preceding 60 days | 1.8 ± 5.4 (range: 0–30) | 0.7 ± 2.0 (range: 0–10) | 0.16 |
| Number of cannabis use days in preceding 60 days | 59.6 ± 1.6 (range: 49–60) | 58.7 ± 6.8 (range: 47–60) | 0.31 |
| Amount of cannabis use in preceding 60 days (grams) | 1.9 ± 1.3 (range: 0.19–5.0) | 4.1 ± 12.9 (range: 0.16–12.14) | 0.23 |
| Age of first cannabis use (years) | 16.0 ± 4.5 (range: 7–37) | 16.1 ± 3.2 (range: 9–25) | 0.86 |
| Duration of regular cannabis use (years) | 14.8 ± 7.6 (range: 3.8–33.3) | 15.7 ± 7.9 (range: 2.7–41.1) | 0.54 |
| Time since last cannabis use (hours) | 78.7 ± 6.7 (range: 72.0–107.2) | 79.0 ± 6.4 (range: 72.5–101.0) | 0.81 |
| Number of participants meeting criteria for cannabis abuse (current / lifetime) | 11/15 | 23/30 | 0.025*/0.025* |
| Number of participants meeting criteria for cannabis dependence (current / lifetime) | 17/24 | 14/19 | 0.18/0.44 |
| MPS | 3.4 ± 4.5 (range: 0–24) | 2.6 ± 2.8 (range: 0–13) | 0.26 |
| Pre-fMRI MCQ | 283.1 ± 159.8 (range: 0–500) | 208.1 ± 187.5 (range: 0–500) | 0.026* |
| Post-fMRI MCQ | 322.4 ± 169.1 (range: 0–500) | 196.3 ± 159.9 (range: 0–500) | < 0.001* |
| Average craving rating to cannabis cue during cue-exposure task | 5.5 ± 3.3 (range: 0–10) | 4.6 ± 3.2 (range: 0–10) | 0.18 |
| MWC | 11.0 ± 8.4 (range: 0–29) | 6.9 ± 6.1 (range: 0–28) | 0.087 |
| THC/Cr level | 21.2 ± 135.6 (range: 0–150) | 54.0 ± 222.7 (range: 0–250) | 0.35 |
MPS, Marijuana Problem Scale; MCQ, Marijuana Craving Questionnaire; MWC, Marijuana Withdrawal Checklist; THC, Δ-9-tetrahydrocannabinol; Cr, creatinine.
2.2. Behavioral measures
We used the Timeline Followback (TLFB) (Sobell and Sobell, 1992) to assess cannabis, alcohol, and cigarette use in the two months preceding the baseline session. In addition, we used the Marijuana Craving Questionnaire (MCQ) (Heishman et al., 2009) to measure subjective craving before and after the fMRI. We also used the Marijuana Withdrawal Checklist (MWC) (Budney et al., 1999) to measure withdrawal prior to the fMRI scan and the Marijuana Problem Scale (MPS) (Stephens et al., 2002) to measure the effect of cannabis use on daily functioning.
2.3. MRI acquisition
We collected the MRI data at the Advanced Imaging Research Center, University of Texas Southwestern Medical Center using a 3T Philips whole body scanner equipped with Quasar gradient subsystem (40 m T/m amplitude, a slew rate of 220 m T/m/ms). We collected structural MRI scans with a MPRAGE sequence with the following parameters: TR/TE/TI = 8.2/3.70/1100 ms, flip angle = 12°, FOV = 256 × 256 mm, slab thickness = 160 mm (along left-right direction), voxel size = 1 × 1 × 1 mm, total scan time =3 min 57 s. We collected fMRI scans using a gradient echo, echo-planar sequence with the intercomissural line (AC-PC) as a reference (TR: 2.0 s, TE: 29 ms, flip angle: 75 degrees, matrix size: 64 × 64, 39 slices, voxel size: 3.44 × 3.44 × 3.5 mm3).
Participants completed a cannabis cue-exposure task to measure the blood oxygen level dependent (BOLD) response to cannabis, appetitive, and neutral cues (Filbey et al., 2016). We presented this task in two separate EPI runs of 18 pseudorandom tactile presentations of the participants’ most commonly used cannabis paraphernalia (MJ cue × 6 trials), preferred fruit (appetitive cue × 6 trials), or a pencil (neutral cue × 6 trials). Each trial started with a cue-exposure period when participants were presented with both tactile (cue placed in the participant’s left hand) and visual (images of themselves holding the cue) exposure. Participants then had a 5-second urge rating period in which they were asked to “Please rate your level of urge to use marijuana right now” on a scale of 1 (no urge) to 10 (high urge) via button presses. There was a washout period with a fixation cross in between each trial. There were 405 trials per run with a task duration of 28 min for the entire experiment. We presented the task using back-projection to a mirror system mounted on the head coil and delivered the stimulus presentation using E-Prime 2.0 (Psychology Software Tools, Pittsburgh, PA) synchronized with trigger pulses from the scanner.
2.4. fMRI data analyses
We analyzed the participants’ fMRI data in FSL version 6.0 (FMRIB Software Library, Analysis Group, FMRIB, Oxford, UK) as described in (Filbey et al., 2016). We used FSL’s FEAT (FMRI Expert Analysis Tool) for standard pre-processing steps, including brain extraction (Smith, 2002), spatial smoothing (Gaussian kernel of FWHM 6 mm), high-pass temporal filtering (sigma = 100.0 s), slice timing correction, and motion correction. We co-registered functional images to their anatomical MPRAGE image and subsequently to Montreal Neurological Institute (MNI) standard space using FSL’s FLIRT (Jenkinson et al., 2002; Jenkinson and Smith, 2001) and FNIRT (Andersson et al., 2007). We used multiple regression procedures from FSL’s FILM (FMRIB’s Improved Linear Model) (Woolrich et al., 2001) to convolve the onsets of cannabis and appetitive cues using a double gamma hemodynamic response function. We used a fixed effects analysis to determine the mean activation of the two fMRI runs. We created masks for regions of interest in which cannabis users exhibited increased activation during exposure to the cannabis cue compared to the appetitive cue that were identified in our previous study (Filbey et al., 2016) as well as other studies (Wetherill et al., 2015) using the Harvard-Oxford cortical and subcortical atlases with a 50 % probability threshold. The regions of interest were the orbitofrontal cortex (OFC; 1695 voxels), anterior cingulate cortex (ACC; 1531 voxels), posterior cingulate cortex (PCC; 1300 voxels), nucleus accumbens (NAc; 126 voxels), dorsal striatum (2418 voxels), amygdala (520 voxels), insula (1080 voxels), and dorsolateral prefrontal cortex (dlPFC; 1488 voxels). We then extracted the mean z-statistic within each region for the cannabis cue > appetitive cue contrast following the fixed effects analysis. We focused on the cannabis cue > appetitive cue contrast to examine neural response to a cannabis cue beyond a response to a naturally rewarding cue.
2.5. Principal component analysis
Principal component analysis (PCA) is a multivariate statistical technique that linearly transforms the original dataset into new orthogonal (i.e., uncorrelated) variables (principal components; PCs) and identifies which original variables account for the most variance (Abdi and Williams, 2010; Hotelling, 1933). We included BOLD response to cannabis cue > appetitive cue during the cue-exposure task in the eight a priori identified regions of interest in the dataset. We also included behavioral variables, i.e., pre-fMRI MCQ, post-fMRI MCQ, average craving rating to the cannabis cue during the cue-exposure task, MWC, MPS, amount of cannabis used in the past two months (in grams), age of onset of cannabis use, years of cannabis use, and THC/Cr level for a total of 17 variables. We centered and normalized the dataset and ran PCA using a custom script in MATLAB 9.2 (MathWorks, Natick, MA). The PCA algorithm computed PCs and each participant had corresponding values for these PCs, i.e., factor scores. We also calculated factor loadings that represent the correlation between the original variables and the PCs and provide insight into which original variables contributed the most variance in the data. Although the original variables may be correlated with multiple PCs, we only retained each variable once in the PC with which it had the highest absolute significant correlation coefficient, as is standard (Osborne et al., 2008). To determine the number of PCs to retain, we calculated the variance accounted for by each PC and used a fixed effect analysis to reconstruct the dataset using the first M components and obtain an estimated dataset. We then calculated the residual sum of squares (RSS) between the original dataset and the estimated dataset to determine the importance of each PC. To estimate the generalizability of the model, we used a random effect analysis through the leave-one-out cross-validation method (Abdi and Williams, 2010; Efron, 1979). This procedure iteratively withholds the observations for each participant and the remaining data are used to estimate the observation that was left out, resulting in a dataset of predicted observations. We then calculated a predicted residual sum of squares (PRSS) to compare the predicted dataset with the original dataset. We selected the number of PCs to retain based on the variance, RSS, and PRSS values (Abdi and Williams, 2010), which indicated the importance of each PC.
To determine whether there were significant differences between the PC scores of the male and female participants, we conducted separate independent t-tests for each PC. To account for the multiple comparisons, we defined significance at p < 0.01. We also correlated the PC scores with cannabis abuse and dependence symptom counts to determine if the PC scores were associated with CUD severity.
2.6. Exploratory analysis
We conducted an exploratory secondary analysis after separating the female participants into two a priori groups based on self-reported menstrual cycle phase, resulting in 13 female participants in the follicular phase of their menstrual cycle (days 0–13) and 22 female participants in the luteal phase of their menstrual cycle (days 16–29). We estimated menstrual cycle phase by counting the number of days between the participant’s self-reported last menstrual cycle and the date of data collection. Nineteen female participants who were taking birth control, post-menopausal, breastfeeding, more than 29 days from their last menstrual period, or did not provide their menstrual cycle information were excluded from this exploratory analysis. To determine whether there were significant differences between the PC scores of the female participants in the follicular and luteal phases and male participants, we conducted separate one-way ANOVAs for each PC on the three groups. We decomposed any significant effects using a post-hoc Bonferroni correction for multiple comparisons.
3. Results
3.1. Participants
There were no significant differences in age, ethnicity, race, or education levels between the male and female cannabis users. There were also no significant differences in the number of tobacco smoking days and cannabis use days in the preceding 60 days, but male participants had a significantly greater alcohol drinking days (male: 15.7 ± 18.3; female: 8.7 ± 10.8; p = 0.018) and number of drinks (male: 63.5 ± 86.9; female: 28.2 ± 39.4; p = 0.0064) compared to female participants. However, the average drinks per drinking day was not significantly different between the groups (p = 0.20). All participants were required to abstain from cannabis for 72 h prior to the fMRI scan and there was no difference in hours since last cannabis use between the groups (p = 0.81). Eleven female users and 23 male users met criteria for current cannabis abuse (p = 0.025), 15 female users and 30 male users met criteria for lifetime cannabis abuse (p = 0.025), 17 female users and 14 male users met criteria for current cannabis dependence (p = 0.18), and 24 female users and 19 male users met criteria for lifetime cannabis dependence (p = 0.44).
3.2. Sex-related differences
The RSS (error associated with the fixed effect model) and PRSS (error associated with the random effect model) values associated with each PC are depicted in Table 2. The RSS for the model based on the first five PCs was 2.0 % and the PRSS was 2.2 %, with both suggesting greater than 97 % accuracy. Based on these values, we extracted the first five PCs, which accounted for 70.3 % of the variance in the data (Fig. 1), for further analysis.
Table 2.
RSS and PRSS values associated with each PC. The first five PCs were extracted for further analysis.
| PC | RSS | PRSS |
|---|---|---|
| PC1 | 0.046 | 0.020 |
| PC2 | 0.036 | 0.021 |
| PC3 | 0.029 | 0.014 |
| PC4 | 0.024 | 0.042 |
| PC5 | 0.020 | 0.022 |
| PC6 | 0.016 | 0.056 |
| PC7 | 0.014 | 0.025 |
| PC8 | 0.011 | 0.024 |
| PC9 | 0.0092 | 0.040 |
| PC10 | 0.0072 | 0.019 |
| PC11 | 0.0056 | 0.013 |
| PC12 | 0.0042 | 0.024 |
| PC13 | 0.0029 | 0.048 |
| PC14 | 0.0017 | 0.013 |
| PC15 | 0.00094 | 0.032 |
| PC16 | 0.00044 | 0.016 |
| PC17 | 8.7 × 10−32 | 0.031 |
PC, principal component; RSS, residual sum of squares; PRSS, predicted residual sum of squares.
Fig. 1.
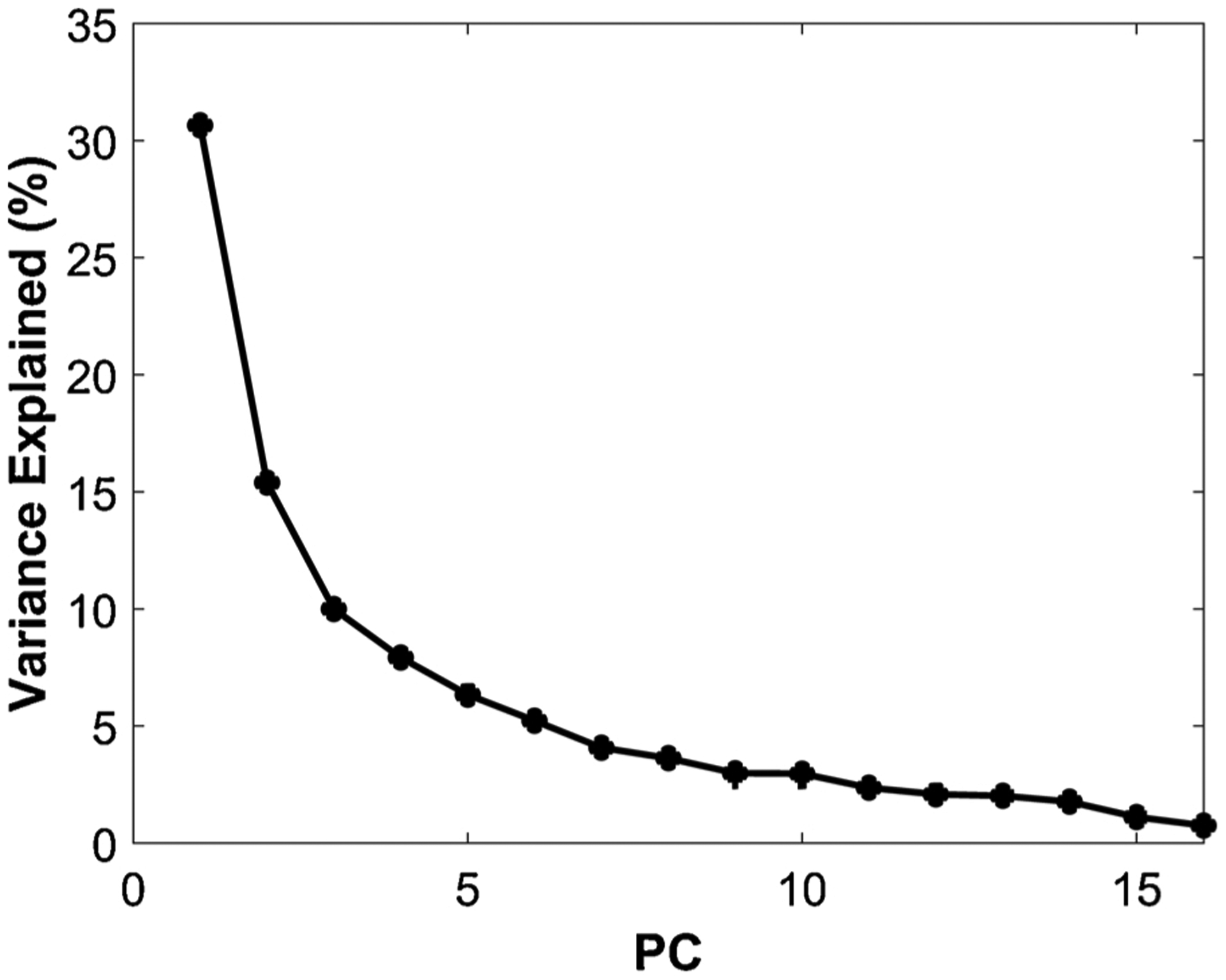
The variance explained by each principal component (PC). The first five PCs accounted for 70.3 % of the variance and were extracted for further analysis.
The PC loadings (Fig. 2) indicated that PC1 was strongly correlated with neural response in all eight regions of interest (OFC, r = 0.82, p < 0.001; ACC, r = 0.91, p < 0.001; PCC, r = 0.77, p < 0.001; NAc, r = 0.55, p < 0.001; dorsal striatum, r = 0.90, p < 0.001; amygdala, r = 0.75, p < 0.001; insula, r = 0.88, p < 0.001; dlPFC, r = 0.67, p < 0.001). PC2 was strongly correlated with subjective craving (pre-fMRI MCQ, r = 0.79, p < 0.001; post-fMRI MCQ, r = 0.85, p < 0.001; average craving rating to cannabis cues, r = 0.76, p < 0.001). PC3 was correlated with amount used (r = 0.41, p < 0.001). PC4 was correlated with age of onset (r = 0.81, p < 0.001) and years of use (r = −0.71, p < 0.001). Finally, PC5 was correlated with MPS (r = −0.60, p < 0.001) and THC/Cr level (r = 0.61, p < 0.001).
Fig. 2.
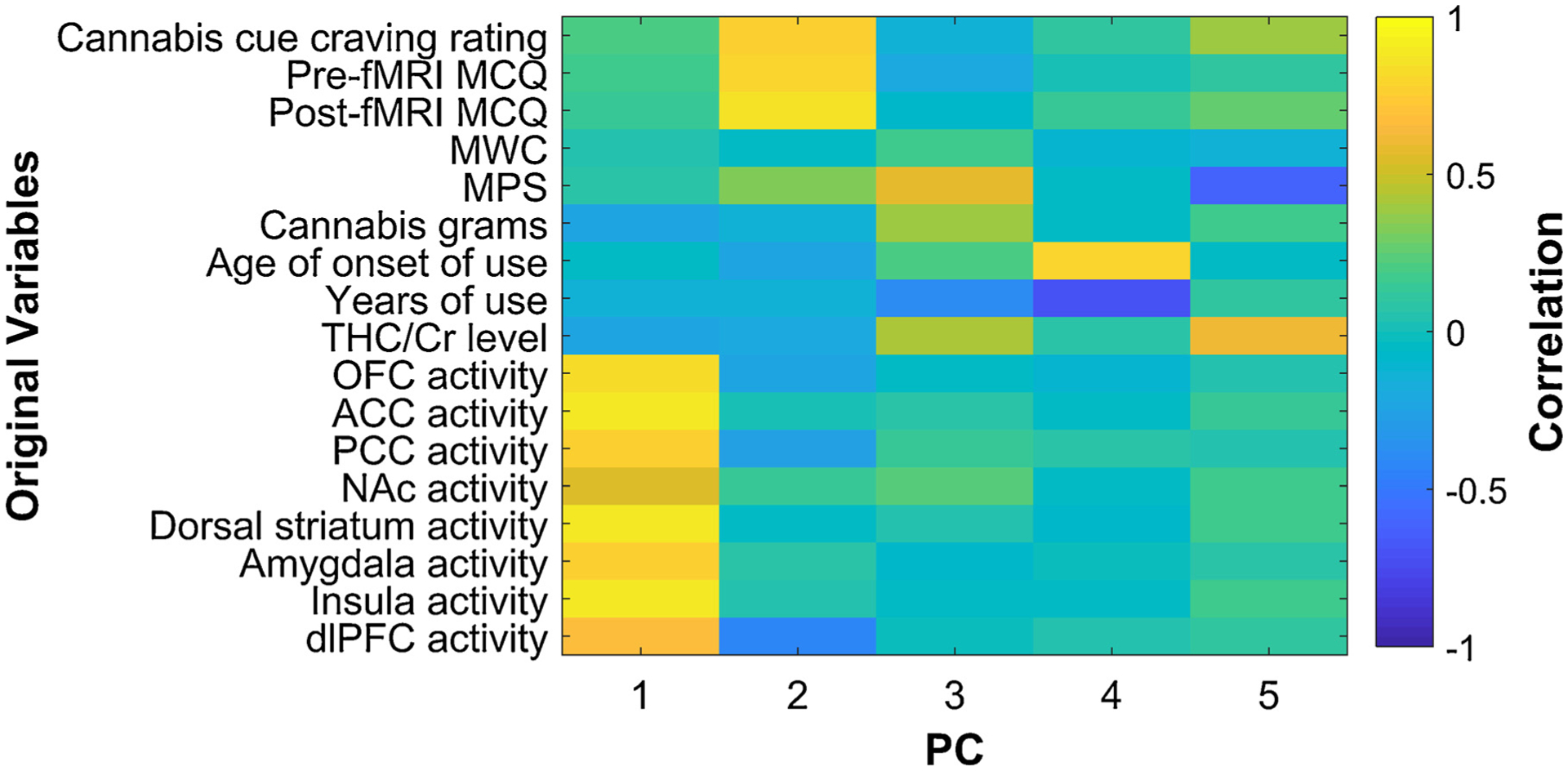
Principal component (PC) loadings show the correlation between PCs 1–5 and the original variables. MCQ, Marijuana Craving Questionnaire; MWC, Marijuana Withdrawal Checklist; MPS, Marijuana Problem Scale; THC, Δ-9-tetrahydrocannabinol; Cr, creatinine; OFC, orbitofrontal cortex; ACC, anterior cingulate cortex; PCC, posterior cingulate cortex; NAc, nucleus accumbens; dlPFC, dorsolateral prefrontal cortex.
There were significant differences between male and female users in subjective craving (PC2, p = 0.003, Cohen’s d = 0.58), but no differences in neural response (PC1, p = 0.21, Cohen’s d = 0.24), PC3 (p = 0.73, Cohen’s d = 0.38), PC4 (p = 0.55, Cohen’s d = 0.11), or PC5 (p = 0.99, Cohen’s d = 0.01; Fig. 3).
Fig. 3.
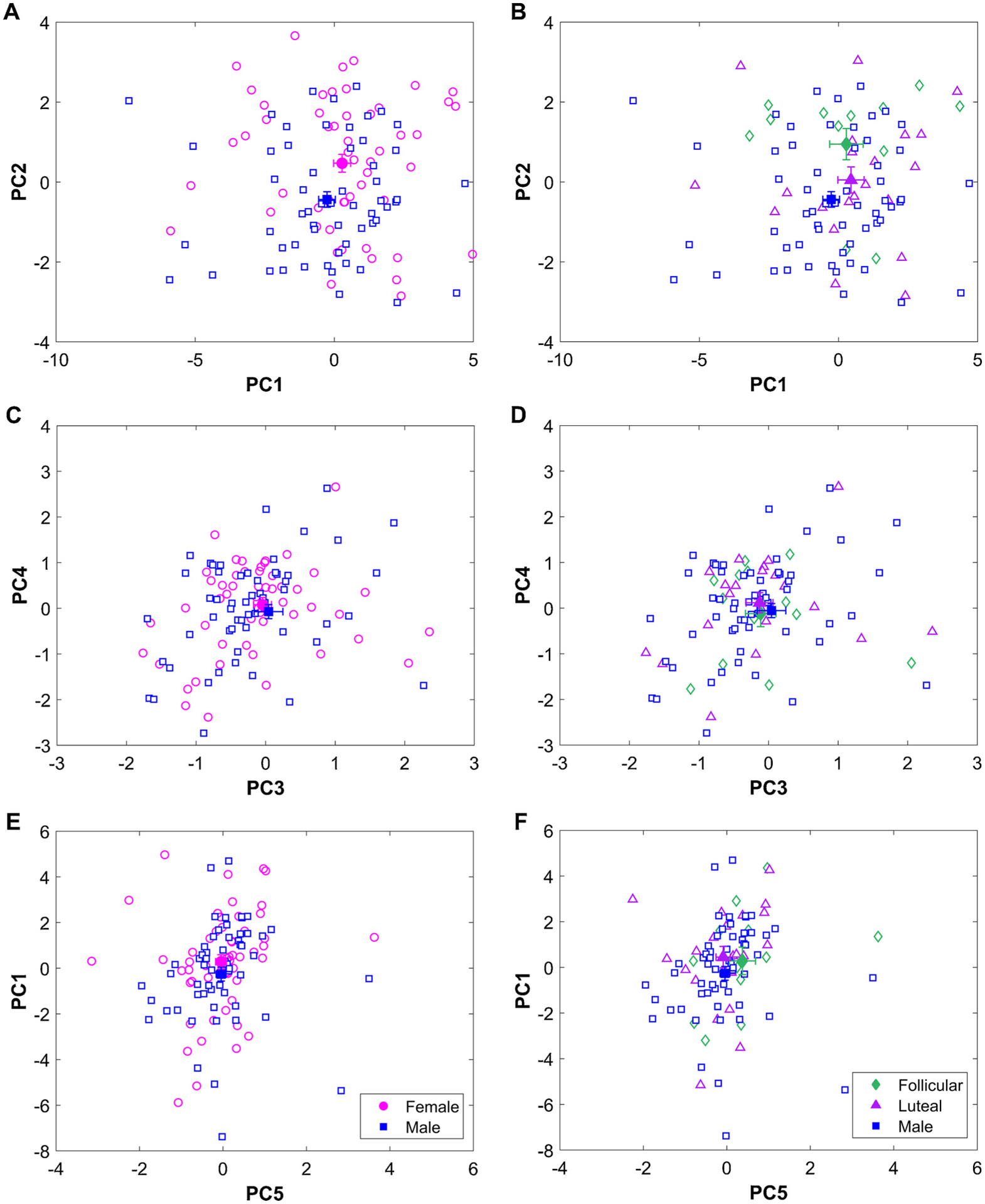
Principal component (PC) scores for male and female participants in the left column (A, C, and E) and menstrual cycle phase in the right column (B, D, and F). Open pink circles represent individual female participants and the open blue squares represent individual male participants. The filled pink circle and blue square represent the mean score for the female and male participants, respectively. The open green diamonds represent individual female participants in the follicular phase and open purple triangles represent individual female participants in the luteal phase and the filled green diamonds and purple triangles represent the group mean respectively. Error bars indicate standard error. Note that in C and D, the filled markers are overlapping and thus are difficult to differentiate.
PC1, PC2, and PC3 were positively correlated with lifetime cannabis dependence symptom count (PC1: r = 0.21, p = 0.033; PC2: r = 0.22, p = 0.023; PC3: r = 0.26, p = 0.008) and PC2 and PC3 were positively correlated with current cannabis dependence symptom count (PC2: r = 0.30, p = 0.003; PC3: r = 0.25, p = 0.015). PC5 was negatively correlated with cannabis abuse symptom count (current: r = −0.23, p = 0.032; lifetime: r = −0.23, p = 0.018). There were no significant correlations with PC4.
3.3. Exploratory menstrual cycle phase effects
Our exploratory analysis found significant differences between the groups separated by menstrual cycle in PC2 (i.e., subjective craving) only (F(2,92) = 4.8, p = 0.010, η2 = 0.097) with female participants in the follicular phase exhibiting greater PC2 scores compared to male participants (p = 0.009, Cohen’s d = 0.94). Fig. 3 displays the PC scores for PC 1–5 separated by sex (Fig. 3A, C, and E) and menstrual cycle phase (Fig. 3B, D, and F).
4. Discussion
We investigated sex-related differences in neural and behavioral responses to cannabis cues in heavy cannabis users. We found that while neural response accounted for most of the variance in the dataset, there were no sex-related differences; however, there were differences in subjective craving, which accounted for the second-most variance. The exploratory analysis suggested that this difference may be driven by increased estrogen in the follicular phase in female cannabis users, but must be investigated further. These findings indicate that neural and behavioral response provide complementary information such that women experience more intense subjective craving compared to men, despite similar neural response. Both neural and behavioral measures must be considered to understand underlying mechanisms of substance use and determine appropriate treatment interventions that may differ for men and women.
4.1. Sex-related differences in subjective, but not neural, response to cannabis cues
We found that neural response to cannabis cues in the regions of interest contributed to the most variance in the dataset with no sex-related differences. We did find sex-related differences in subjective craving such that female users exhibited increased subjective craving response that was a combination of pre-fMRI MCQ, post-fMRI MCQ, and response to the cannabis-related cue during the cue-exposure task compared to male users. When we separated the female users into groups by menstrual cycle phase, we found that female users in the follicular phase (when estrogen levels increase) exhibited an increased craving response compared to male users. It is important to emphasize that this finding is preliminary and it is unclear whether this increase may have contributed to the sex-related differences. While some studies have found no differences in effects of cannabis across menstrual cycle phase (Griffin et al., 1986; Lex et al., 1984) and no changes in hormone concentrations with cannabis use in both men and women (Block et al., 1991), additional studies are required to further investigate the interaction between cannabis and menstrual cycles (Terner and de Wit, 2006).
It is important to note that our findings are inconsistent with a previous study by Lundahl and colleagues that found no significant differences in subjective craving during a cue-exposure task (Lundahl and Johanson, 2011). There were some key differences in the demographics of the participants between the study by Lundahl and Johanson and the current study, as average years of cannabis use in the Lundahl and colleagues study was approximately 6.7 years and in the current study was around 15 years. This difference indicates that behavioral differences between the sexes may emerge with continued use. There were also differences in sample size in the studies (N = 32 in Lundahl and Johanson, 2011 and N = 112 in the present study) that may have contributed to the different results. Combined with the smaller sample size, there may have been a lack of power to detect differences using the three-way ANOVA as performed by Lundahl and colleagues. However, our findings were consistent with the study by Wetherill and colleagues that found no sex-related differences in neural response to a cannabis cue-exposure task (Wetherill et al., 2015). The sample size in the current study (N = 112) was higher than that in the study by Wetherill and colleagues (N = 44), providing further evidence for lack of differences in neural response between the sexes. Additionally, unlike the present study, the participants included in the Wetherill et al. study included users who were cannabis-dependent, suggesting that even when meeting criteria for dependence, there are no sex-related differences in neural response to cannabis cues. In contrast to our findings in resting brain perfusion and metabolism in a subset of these data (Filbey et al., 2018a), we did not find sex-related differences in task-related neural response. Future studies may consider examining how cerebral blood flow during rest translates to neural response in the cue-exposure task.
Our key finding of the absence of sex-related differences in neural response, but presence in subjective craving, indicates divergent behavioral response even with convergent neural activity. This suggests that there are interactions with hormones downstream of neural processing that influence behavioral response such that subjective craving is heightened in female users. A variety of factors including biological, psychological, and sociocultural processes have been postulated to underlie subjective craving (Abrams, 2000; Skewes and Gonzalez, 2013). These factors may explain how subjective craving emerges from a montage of processes that underlie individual differences, resulting in dimorphic behavior and supports a role of hormones downstream of neural processing that affect behavioral response. As such, a potential factor that may have a differential downstream effect is stress. Studies suggest that an increased response to stress is related to heightened subjective craving (Hartwell and Ray, 2013; Koob, 2008; Sinha, 2008) via interactions between THC, the HPA axis, and sex hormones (López, 2010; Volkow et al., 2017). Additionally, impairments in self-awareness and interoception in substance use (DeWitt et al., 2015; Goldstein et al., 2009; Paulus and Stewart, 2014; Prashad et al., 2018) may affect how men and women self-report craving differentially (Perkins, 1996). Further studies are needed to disentangle the potential interactions and additive effects of these factors.
While there are no other studies in cannabis users that have examined sex-related differences in neural and behavioral responses to a cue-exposure task, findings in other substances of abuse may provide some context to these findings. In cocaine users, studies have reported that women reported increased subjective craving after exposure to drug cues (Elman et al., 2001; Kennedy et al., 2013; Kosten et al., 1996; Robbins et al., 1999). Similar results have been reported in nicotine with women reporting increased subjective craving to smoking-related cues (Dickmann et al., 2009; Doran, 2014; Faulkner et al., 2018) and some studies suggested that these differences may be related to sex hormones (Franklin et al., 2019, 2015; Sofuoglu et al., 2009, 2001). However, studies in nicotine have also suggested sex-related differences in neural response in women (Franklin et al., 2019, 2015; McClernon et al., 2008). In alcohol users, studies have found that women report increased subjective craving compared to men (Hartwell and Ray, 2013; Rubonis et al., 1994; Seo et al., 2011). Interestingly, Kaag et al. reported no differences in subjective craving in male and female alcohol drinkers, but increased neural response in the dorsal striatum in male drinkers compared to female drinkers (Kaag et al., 2018). These studies provide an important context for our findings; however, they exhibit the same limitations as studies in cannabis users, i.e., they do not consider the interaction between sex and subjective and neural measures of craving together. Further examinations of these differences will shed light on whether patterns of sex-related differences are similar or different across substances of abuse.
4.2. Amount of cannabis use did not differ between male and female users
Given that measures of cannabis use were similar for male and female users, consistent with some previous research (Griffin et al., 1986), we found no sex-related differences in the PCs that correlated with cannabis use, suggesting that negative consequences relating to cannabis use and amounts of cannabis used were comparable among male and female users. This similarity in amount of cannabis use between the male and female participants was expected in this sample as per the inclusion criteria as our focus was to investigate differences in neural and behavioral subjective craving when amount of cannabis use was similar among male and female users. It is important to note that other studies have found that men use more cannabis more frequently and in higher quantities than women (Cuttler et al., 2016; Lopez and Blanco, 2019).
4.3. Clinical implications and relationship with CUD
Exposure to cannabis cues promotes continued use (Milton and Everitt, 2012; Volkow and Morales, 2015) by activating reward pathways that underlie craving (Cousijn et al., 2012; Filbey et al., 2016, 2014, 2009; Lundahl and Johanson, 2011; McRae-Clark et al., 2011; Wetherill et al., 2015) and the development of CUD. In this study, we found that female cannabis users exhibited increased subjective craving compared to male users. Further, PC1 and PC2 correlated with neural response and subjective craving, respectively, as well as lifetime cannabis dependence symptom count. Interestingly, we found that PC1 was correlated with lifetime cannabis dependence symptom count, but not current symptom count, and PC2 was correlated with both lifetime and current symptom count. This suggests that changes in neural response (i.e., PC1) are associated with the long-term chronic use of cannabis, but is not sensitive to current use, while subjective craving (i.e., PC2) is sensitive to both lifetime and current use. Studies in other substances have reported similar findings including alcohol dependence where lifetime alcohol dependence was correlated with neural and subjective response in a cue-exposure task (Sjoerds et al., 2014; Williams et al., 2009) as well as cocaine dependence (Brewer et al., 2008) and nicotine dependence (McClernon et al., 2008). This correlation supports previous findings that increased craving may contribute to the telescoping effect whereby female users progress through the milestones of CUD more rapidly than male users and may affect treatment response. Thus, it is important for clinicians to consider these results to determine the most effective treatment approaches for individuals.
4.4. Limitations and future directions
The findings in this study must be interpreted within the context of its limitations. The focus of this study was sex-related differences and we did not quantify ovarian hormone levels. We calculated menstrual cycle phases based on self-reported date of last menstrual period that provided a highly cursory estimate of the current cycle phase. Our preliminary findings support the role of menstrual cycle in a differential subjective craving response in men and women, but future studies may examine this hypothesis by quantifying ovarian hormones to determine the cycle phase more accurately. Further, our sample contained male cannabis users that had significantly higher alcohol use, but no difference in average drinks per drinking day between the groups, suggesting that some of changes in neural response may have been due to overall increased alcohol use, which affects the same regions and pathways. Despite women drinking less alcohol overall, alcohol may have differences in potency between the sexes, that may differentially affect the neural and behavior response results. In addition, more male participants met criteria for cannabis abuse compared to female participants. Replicating and extending the present findings will also support scientific rigor and prevent reporting bias in the sex-related differences literature (David et al., 2018).
4.5. Conclusions
This study demonstrates that both neural and behavioral response to cue-elicited craving are critical for understanding the underlying neurobiological mechanisms of cannabis use. Neural and subjective craving response together accounted for the most variance in the data, but only subjective craving exhibited sex-related differences, indicating a divergent behavioral response between men and women even with convergent neural activity. The differences in men and women in continued cannabis use and development of CUD underscore their impact on interventions and treatments and propensity for relapse. A better understanding of the clinical relevance of sex-related differences in cannabis use and response will help account for individual differences and improve clinical outcomes for women.
Acknowledgement
This work was supported by the National Institutes of Health [grant number R01 DA030344].
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abdi H, Williams LJ, 2010. Principal component analysis: principal component analysis. Wiley Interdiscip. Rev. Comput. Stat 2, 433–459. 10.1002/wics.101. [DOI] [Google Scholar]
- Abrams DB, 2000. Transdisciplinary concepts and measures of craving: commentary and future directions. Addiction 95, 237–246. 10.1046/j.1360-0443.95.8s2.12.x. [DOI] [PubMed] [Google Scholar]
- Ali B, Seitz-Brown CJ, Daughters SB, 2015. The interacting effect of depressive symptoms, gender, and distress tolerance on substance use problems among residential treatment-seeking substance users. Drug Alcohol Depend. 148, 21–26. 10.1016/j.drugalcdep.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JL, Jenkinson M, Smith S, 2007. Non-linear Registration, Aka Spatial Normalisation, FMRIB Technical Report TR07JA2. FMRIB Centre, Oxford, United Kingdom. [Google Scholar]
- Bassir Nia A, Mann C, Kaur H, Ranganathan M, 2018. Cannabis use: neurobiological, behavioral, and sex/gender considerations. Curr. Behav. Neurosci. Rep 5, 271–280. 10.1007/s40473-018-0167-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista N, Meccariello R, Cobellis G, Fasano S, Di Tommaso M, Pirazzi V, Konje JC, Pierantoni R, Maccarrone M, 2012. The role of endocannabinoids in gonadal function and fertility along the evolutionary axis. Mol. Cell. Endocrinol 355, 1–14. 10.1016/j.mce.2012.01.014. [DOI] [PubMed] [Google Scholar]
- Becker JB, Koob GF, 2016. Sex differences in animal models: focus on addiction. Pharmacol. Rev 68, 242–263. 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block RI, Farinpour R, Schlechte JA, 1991. Effects of chronic marijuana use on testosterone, luteinizing hormone, follicle stimulating hormone, prolactin and cortisol in men and women. Drug Alcohol Depend. 28, 121–128. 10.1016/0376-8716(91)90068-A. [DOI] [PubMed] [Google Scholar]
- Bobzean SAM, DeNobrega AK, Perrotti LI, 2014. Sex differences in the neurobiology of drug addiction. Exp. Neurol 259, 64–74. 10.1016/j.expneurol.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Brents LK, 2016. Marijuana, the endocannabinoid system and the female reproductive system. Yale J. Biol. Med 89, 175–191. [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN, 2008. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biol. Psychiatry 64, 998–1004. 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, Dobs AS, 2002. Endocrine effects of marijuana. J. Clin. Pharmacol 42, 90S–96S. 10.1002/j.1552-4604.2002.tb06008.x. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Novy PL, Hughes JR, 1999. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction 94, 1311–1322. [DOI] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality, 2017. Results From the 2016 National Survey on Drug Use and Health: Detailed Tables. Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- Chauchard E, Levin KH, Copersino ML, Heishman SJ, Gorelick DA, 2013. Motivations to quit cannabis use in an adult non-treatment sample: are they related to relapse? Addict. Behav 38, 2422–2427. 10.1016/j.addbeh.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Craft RM, 2018. Sex-dependent effects of Cannabis and cannabinoids: a translational perspective. Neuropsychopharmacology 43, 34–51. 10.1038/npp.2017.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Haney M, 2014. Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug Alcohol Depend. 136, 85–91. 10.1016/j.drugalcdep.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, Simmons MS, Gorelick DA, 2010. Sociodemographic characteristics of Cannabis smokers and the experience of Cannabis withdrawal. Am. J. Drug Alcohol Abuse 36, 311–319. 10.3109/00952990.2010.503825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Goudriaan AE, Ridderinkhof KR, van den Brink W, Veltman DJ, Wiers RW, 2012. Neural responses associated with cue-reactivity in frequent cannabis users: brain activity to cannabis cues. Addict. Biol 18, 570–580. 10.1111/j.1369-1600.2011.00417.x. [DOI] [PubMed] [Google Scholar]
- Craft RM, Marusich JA, Wiley JL, 2013. Sex differences in cannabinoid pharmacology: a reflection of differences in the endocannabinoid system? Life Sci. 92, 476–481. 10.1016/j.lfs.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane NA, Schuster RM, Fusar-Poli P, Gonzalez R, 2013. Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Neuropsychol. Rev 23, 117–137. 10.1007/s11065-012-9222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttler C, Mischley LK, Sexton M, 2016. Sex differences in cannabis use and effects: a cross-sectional survey of cannabis users. Cannabis Cannabinoid Res. 1, 166–175. 10.1089/can.2016.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Naudet F, Laude J, Radua J, Fusar-Poli P, Chu I, Stefanick ML, Ioannidis JPA, 2018. Potential reporting bias in neuroimaging studies of sex differences. Sci. Rep 8. 10.1038/s41598-018-23976-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Babuscio TA, Nich C, Ball SA, Carroll KM, 2014. Gender differences in clinical outcomes for cocaine dependence: randomized clinical trials of behavioral therapy and disulfiram. Drug Alcohol Depend. 145, 156–167. 10.1016/j.drugalcdep.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt SJ, Ketcherside A, McQueeny TM, Dunlop JP, Filbey FM, 2015. The hyper-sentient addict: an exteroception model of addiction. Am. J. Drug Alcohol Abuse 41, 374–381. 10.3109/00952990.2015.1049701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickmann PJ, Mooney ME, Allen SS, Hanson K, Hatsukami DK, 2009. Nicotine withdrawal and craving in adolescents: effects of sex and hormonal contraceptive use. Addict. Behav 34, 620–623. 10.1016/j.addbeh.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran N, 2014. Sex differences in smoking cue reactivity: craving, negative affect, and preference for immediate smoking. Am. J. Addict 23, 211–217. 10.1111/j.1521-0391.2014.12094.x. [DOI] [PubMed] [Google Scholar]
- Efron B, 1979. Bootstrap methods: another look at the jackknife. Ann. Stat 7, 1–26. [Google Scholar]
- Elman I, Karlsgodt KH, Gastfriend DR, 2001. Gender differences in cocaine craving among non-treatment-seeking individuals with cocaine dependence. Am. J. Drug Alcohol Abuse 27, 193–202. 10.1081/ADA-100103705. [DOI] [PubMed] [Google Scholar]
- Farquhar CE, Breivogel CS, Gamage TF, Gay EA, Thomas BF, Craft RM, Wiley JL, 2019. Sex, THC, and hormones: effects on density and sensitivity of CB1 cannabinoid receptors in rats. Drug Alcohol Depend. 194, 20–27. 10.1016/j.drugalcdep.2018.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W, 2007. Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. Br. J. Pharmacol 152, 795–804. 10.1038/sj.bjp.0707465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Spano M, Altea S, Fadda P, Fratta W, 2010. Drug- and cue-induced re-instatement of cannabinoid-seeking behaviour in male and female rats: influence of ovarian hormones: sex differences in relapse to cannabinoid-seeking. Br. J. Pharmacol 160, 724–735. 10.1111/j.1476-5381.2010.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner P, Petersen N, Ghahremani DG, Cox CM, Tyndale RF, Hellemann GS, London ED, 2018. Sex differences in tobacco withdrawal and responses to smoking reduced-nicotine cigarettes in young smokers. Psychopharmacology (Berl.) 235, 193–202. 10.1007/s00213-017-4755-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE, 2009. Marijuana craving in the brain. Proc. Natl. Acad. Sci 106, 13016–13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Aslan S, Calhoun VD, Spence JS, Damaraju E, Caprihan A, Segall J, 2014. Long-term effects of marijuana use on the brain. Proc. Natl. Acad. Sci 111, 16913–16918. 10.1073/pnas.1415297111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Dunlop J, Ketcherside A, Baine J, Rhinehardt T, Kuhn B, DeWitt S, Alvi T, 2016. fMRI study of neural sensitization to hedonic stimuli in long-term, daily cannabis users: reward cue-reactivity in marijuana users. Hum. Brain Mapp 37, 3431–3443. 10.1002/hbm.23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Aslan S, Lu H, Peng S-L, 2018a. Residual effects of THC via novel measures of brain perfusion and metabolism in a large group of chronic cannabis users. Neuropsychopharmacology 43, 700–707. 10.1038/npp.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Gohel S, Prashad S, Biswal BB, 2018b. Differential associations of combined vs. isolated cannabis and nicotine on brain resting state networks. Brain Struct. Funct 223, 3317–3326. 10.1007/s00429-018-1690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition. (SCID-I/NP) Biometrics Research, New York State Psychiatric Institute, New York. [Google Scholar]
- Franklin TR, Jagannathan K, Wetherill RR, Johnson B, Kelly S, Langguth J, Mumma J, Childress AR, 2015. Influence of menstrual cycle phase on neural and craving responses to appetitive smoking cues in naturally cycling females. Nicotine Tob. Res 17, 390–397. 10.1093/ntr/ntu183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Jagannathan K, Ketcherside A, Spilka N, Wetherill RR, 2019. Menstrual cycle phase modulates responses to smoking cues in the putamen: preliminary evidence for a novel target. Drug Alcohol Depend. 198, 100–104. 10.1016/j.drugalcdep.2019.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Craig A.D. (Bud), Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND, 2009. The neurocircuitry of impaired insight in drug addiction. Trends Cogn. Sci 13, 372–380. 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorzalka BB, Dang SS, 2012. Minireview: endocannabinoids and gonadal hormones: bidirectional interactions in physiology and behavior. Endocrinology 153, 1016–1024. 10.1210/en.2011-1643. [DOI] [PubMed] [Google Scholar]
- Griffin ML, Mendelson JH, Mello NK, Lex BW, 1986. Marihuana use across the menstrual cycle. Drug Alcohol Depend. 18, 213–224. 10.1016/0376-8716(86)90053-0. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Pope HG, Hudson JI, Yurgelun-Todd D, 2003. Attributes of long-term heavy cannabis users: a case control study. Psychol. Med 33, 1415–1422. 10.1017/S0033291703008560. [DOI] [PubMed] [Google Scholar]
- Halai AD, Woollams AM, Lambon Ralph MA, 2017. Using principal component analysis to capture individual differences within a unified neuropsychological model of chronic post-stroke aphasia: revealing the unique neural correlates of speech fluency, phonology and semantics. Cortex 86, 275–289. 10.1016/j.cortex.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell EE, Ray LA, 2013. Sex moderates stress reactivity in heavy drinkers. Addict. Behav 38, 2643–2646. 10.1016/j.addbeh.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Hasin DS, 2018. US epidemiology of Cannabis use and associated problems. Neuropsychopharmacology 43, 195–212. 10.1038/npp.2017.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, Gorelick DA, 2009. Reliability and validity of a short form of the Marijuana Craving Questionnaire. Drug Alcohol Depend. 102, 35–40. 10.1016/j.drugalcdep.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR, 2004. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 74, 265–272. 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Herrmann ES, Weerts EM, Vandrey R, 2015. Sex differences in cannabis withdrawal symptoms among treatment-seeking cannabis users. Exp. Clin. Psychopharmacol 23, 415–421. 10.1037/pha0000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotelling H, 1933. Analysis of a complex of statistical variables into principal components. J. Educ. Psychol 24, 417–441. [Google Scholar]
- Huestis MA, Cone EJ, 1998. Urinary excretion half-life of 11-nor-9-carboxy-Δ9-tetrahydrocannabinol in humans. Ther. Drug Monit 20, 570–576. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S, 2001. A global optimisation method for robust affine registration of brain images. Med. Image Anal 5, 143–156. 10.1016/S1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S, 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17, 825–841. 10.1006/nimg.2002.1132. [DOI] [PubMed] [Google Scholar]
- Kaag AM, Wiers RW, de Vries TJ, Pattij T, Goudriaan AE, 2018. Striatal alcohol cue-reactivity is stronger in male than female problem drinkers. Eur. J. Neurosci 10.1111/ejn.13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AP, Epstein DH, Phillips KA, Preston KL, 2013. Sex differences in cocaine/heroin users: drug-use triggers and craving in daily life. Drug Alcohol Depend. 132, 29–37. 10.1016/j.drugalcdep.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerridge BT, Pickering R, Chou P, Saha TD, Hasin DS, 2018. DSM-5 cannabis use disorder in the national epidemiologic survey on alcohol and related Conditions-III: gender-specific profiles. Addict. Behav 76, 52–60. 10.1016/j.addbeh.2017.07.012. [DOI] [PubMed] [Google Scholar]
- Ketcherside A, Baine J, Filbey F, 2016. Sex effects of marijuana on brain structure and function. Curr. Addict. Rep 3, 323–331. 10.1007/s40429-016-0114-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketcherside A, Noble LJ, McIntyre CK, Filbey FM, 2017. Cannabinoid receptor 1 gene by Cannabis use interaction on CB1 receptor density. Cannabis Cannabinoid Res. 2, 202–209. 10.1089/can.2017.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SS, Secades-Villa R, Okuda M, Wang S, Pérez-Fuentes G, Kerridge BT, Blanco C, 2013. Gender differences in cannabis use disorders: results from the National Epidemiologic Survey of Alcohol and Related Conditions. Drug Alcohol Depend. 130, 101–108. 10.1016/j.drugalcdep.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, 2008. A role for brain stress systems in addiction. Neuron 59, 11–34. 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Kosten TA, McDougle CJ, Hameedi FA, McCance EF, Rosen MI, Oliveto AH, Price LH, 1996. Gender differences in response to intranasal cocaine administration to humans. Biol. Psychiatry 39, 147–148. 10.1016/0006-3223(95)00386-X. [DOI] [PubMed] [Google Scholar]
- Kouri EM, Pope HG Jr., 2000. Abstinence symptoms during withdrawal from chronic marijuana use. Exp. Clin. Psychopharmacol 8, 483–492. 10.1037//1064-1297.8.4.483. [DOI] [PubMed] [Google Scholar]
- Lex BW, Mendelson JH, Bavli S, Harvey K, Mello NK, 1984. Effects of acute marijuana smoking on pulse rate and mood states in women. Psychopharmacology (Berl.) 84, 178–187. 10.1007/BF00427443. [DOI] [PubMed] [Google Scholar]
- Litt MD, Kadden RM, Tennen H, 2015. Network Support treatment for alcohol dependence: gender differences in treatment mechanisms and outcomes. Addict. Behav 45, 87–92. 10.1016/j.addbeh.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López HH, 2010. Cannabinoid–hormone interactions in the regulation of motivational processes. Horm. Behav 58, 100–110. 10.1016/j.yhbeh.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Lopez M, Blanco C, 2019. Epidemiology of Cannabis use disorder. In: Montoya ID, Weiss SRB (Eds.), Cannabis Use Disorders. Springer International Publishing, Cham, pp. 7–12. 10.1007/978-3-319-90365-1_2. [DOI] [Google Scholar]
- Lundahl LH, Johanson C-E, 2011. Cue-induced craving for marijuana in cannabis-dependent adults. Exp. Clin. Psychopharmacol 19, 224–230. 10.1037/a0023030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch W, Roth M, Carroll M, 2002. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl.) 164, 121–137. 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Maria MMM-S, Flanagan J, Brady K, 2014. Ovarian hormones and drug abuse. Curr. Psychiatry Rep 16, 511. 10.1007/s11920-014-0511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Craft RM, Lefever TW, Wiley JL, 2015. The impact of gonadal hormones on cannabinoid dependence. Exp. Clin. Psychopharmacol 23, 206–216. 10.1037/pha0000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Rose JE, 2008. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology 33, 2148–2157. 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- McHugh RK, Votaw VR, Sugarman DE, Greenfield SF, 2018. Sex and gender differences in substance use disorders. Clin. Psychol. Rev 66, 12–23. 10.1016/j.cpr.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark AL, Carter RE, Price KL, Baker NL, Thomas S, Saladin ME, Giarla K, Nicholas K, Brady KT, 2011. Stress- and cue-elicited craving and reactivity in marijuana-dependent individuals. Psychopharmacology (Berl.) 218, 49–58. 10.1007/s00213-011-2376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Cristofaro P, Ellingboe J, Benedikt R, 1984. Acute effects of marijuana on pituitary and gonadal hormones during the periovulatory phase of the menstrual cycle. Proc. 46th Annu. Sci. Meet. Comm. Probl. Drug Depend. Inc 10.1037/e475312004-001. [DOI] [PubMed] [Google Scholar]
- Milton AL, Everitt BJ, 2012. The persistence of maladaptive memory: addiction, drug memories and anti-relapse treatments. Neurosci. Biobehav. Rev 36, 1119–1139. 10.1016/j.neubiorev.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Osborne JW, Costello AB, Kellow JT, 2008. Best practices in exploratory factor analysis. In: Osborne JW (Ed.), Best Practices in Quantitative Methods. SAGE Publications, Inc., Thousand Oaks, pp. 86–99. [Google Scholar]
- Paulus MP, Stewart JL, 2014. Interoception and drug addiction. Neuropharmacology 76, 342–350. 10.1016/j.neuropharm.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, 1996. Sex differences in nicotine versus nonnicotine reinforcement as determinants of tobacco smoking. Exp. Clin. Psychopharmacol 4, 166–177. [Google Scholar]
- Perry AN, Westenbroek C, Becker JB, 2016. Sex differences and addiction. In: Shanksy RM (Ed.), Sex Differences in the Central Nervous System. Academic Press, New York, pp. 129–147. [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D, 2001. Neuropsychological performance in long-term cannabis users. Arch. Gen. Psychiatry 58, 909–915. [DOI] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D, 2002. Cognitive measures in long-term Cannabis users. J. Clin. Pharmacol 42, 41S–47S. 10.1002/j.1552-4604.2002.tb06002.x. [DOI] [PubMed] [Google Scholar]
- Prashad S, Dedrick ES, To WT, Vanneste S, Filbey FM, 2018. Testing the role of the posterior cingulate cortex in processing salient stimuli in cannabis users: an rTMS study. Eur. J. Neurosci 10.1111/ejn.14194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP, 1999. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 53, 223–230. 10.1016/S0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- Rubonis AV, Colby SM, Monti PM, Rohsenow DJ, Gulliver SB, Sirota AD, 1994. Alcohol cue reactivity and mood induction in male and female alcoholics. J. Stud. Alcohol 55, 487–494. 10.15288/jsa.1994.55.487. [DOI] [PubMed] [Google Scholar]
- Schlienz NJ, Budney AJ, Lee DC, Vandrey R, 2017. Cannabis withdrawal: a review of neurobiological mechanisms and sex differences. Curr. Addict. Rep 4, 75–81. 10.1007/s40429-017-0143-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Jia Z, Lacadie CM, Tsou KA, Bergquist K, Sinha R, 2011. Sex differences in neural responses to stress and alcohol context cues. Hum. Brain Mapp 32, 1998–2013. 10.1002/hbm.21165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman BJ, McRae-Clark AL, 2016. Treatment of Cannabis use disorder: current science and future outlook. Pharmacother. J. Hum. Pharmacol. Drug Ther 36, 511–535. 10.1002/phar.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman BJ, McRae-Clark AL, Baker NL, Sonne SC, Killeen TK, Cloud K, Gray KM, 2017. Gender differences among treatment-seeking adults with cannabis use disorder: clinical profiles of women and men enrolled in the achieving cannabis cessation-evaluating N-acetylcysteine treatment (ACCENT) study: ACCENT-Gender Differences and CUD. Am. J. Addict 26, 136–144. 10.1111/ajad.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, 2008. Chronic stress, drug use, and vulnerability to addiction. Ann. N. Y. Acad. Sci 1141, 105–130. 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoerds Z, van den Brink W, Beekman ATF, Penninx BWJH, Veltman DJ, 2014. Cue reactivity is associated with duration and severity of alcohol dependence: an fMRI study. PLoS One 9, e84560. 10.1371/journal.pone.0084560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skewes MC, Gonzalez VM, 2013. The biopsychosocial model of addiction. Principles of Addiction. Elsevier, pp. 61–70. 10.1016/B978-0-12-398336-7.00006-1. [DOI] [Google Scholar]
- Smith SM, 2002. Fast robust automated brain extraction. Hum. Brain Mapp 17, 143–155. 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, 1992. Timeline follow-back. In: Litten RZ, Allen JP (Eds.), Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press, Totowa, NJ, pp. 41–72. [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK, 2001. Progesterone treatment during the early follicular phase of the menstrual cycle: effects on smoking behavior in women. Pharmacol. Biochem. Behav 69, 299–304. 10.1016/S0091-3057(01)00527-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Mooney M, 2009. Progesterone effects on subjective and physiological responses to intravenous nicotine in male and female smokers. Hum. Psychopharmacol. Clin. Exp 24, 559–564. 10.1002/hup.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens RS, Babor TF, Kadden R, Miller M, 2002. The Marijuana Treatment Project: rationale, design and participant characteristics. Addiction 97, 109–124. [DOI] [PubMed] [Google Scholar]
- Tabachnik BG, Fidell LS, 2013. Using Multivariate Statistics, 6th ed. Pearson, Boston, MA. [Google Scholar]
- Terner JM, de Wit H, 2006. Menstrual cycle phase and responses to drugs of abuse in humans. Drug Alcohol Depend. 84, 1–13. 10.1016/j.drugalcdep.2005.12.007. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime, 2018. World Drug Report 2018. United Nations publication Sales No. E.18.XI.9. [Google Scholar]
- Volkow ND, Morales M, 2015. The brain on drugs: from reward to addiction. Cell 162, 712–725. 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hampson AJ, Baler RD, 2017. Don’t worry, be happy: endocannabinoids and cannabis at the intersection of stress and reward. Annu. Rev. Pharmacol. Toxicol 57, 285–308. 10.1146/annurev-pharmtox-010716-104615. [DOI] [PubMed] [Google Scholar]
- Walker DD, Stephens RS, Towe S, Banes K, Roffman R, 2015. Maintenance check-ups following treatment for Cannabis dependence. J. Subst. Abuse Treat 56, 11–15. 10.1016/j.jsat.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Jagannathan K, Hager N, Childress AR, Franklin TR, 2015. Sex differences in associations between cannabis craving and neural responses to cannabis cues: implications for treatment. Exp. Clin. Psychopharmacol 23, 238–246. 10.1037/pha0000036. [DOI] [PubMed] [Google Scholar]
- Williams TM, Davies SJC, Taylor LG, Daglish MRC, Hammers A, Brooks DJ, Nutt DJ, Lingford-Hughes A, 2009. Brain opioid receptor binding in early abstinence from alcohol dependence and relationship to craving: an [11C]diprenorphine PET study. Eur. Neuropsychopharmacol 19, 740–748. 10.1016/j.euroneuro.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM, 2001. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage 14, 1370–1386. 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]


