Dear Editor,
Since its beginning, the evolving Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2)/Coronavirus Disease 2019 (COVID-19) pandemic has shaded many other ongoing epidemics worldwide. Such is the case of many arboviruses, like Dengue virus (DENV). This has generated concern amongst travel medicine practitioners and public health authorities particularly after the pandemic started to hit and spread across DENV endemic regions worldwide. Now, concurrent overlapping infections have increasingly been reported from Brazil, Ecuador, Colombia and Peru [1,2]. Furthermore, occurrence of co-infections in Mayotte (Comoros archipelago, France), Argentina and other places [3,4] have been reported, including many of them in returning travelers. Herein, we report a case of COVID-19 and Dengue co-infection in Colombia and discuss the potential clinical and epidemiological challenges of current syndemics.
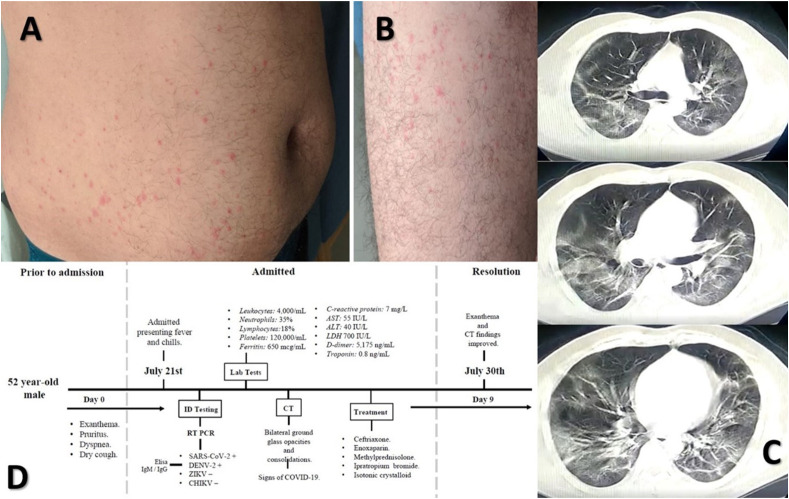
A 52-year-old male, bus driver from Barranquilla, Atlantico, Colombia, with no significant past-medical history (and no past history of dengue) presented on July 21st, 2020, with flu-like symptoms and worsening fever, chills and pruritus. On admission, his temperature was 41.0 °C accompanied by increasing arthralgia, myalgia, dorso-lumbar pain, asthenia and adynamia. He also referred dyspnea and dry cough days prior to admission. Physical examination revealed blood pressure of 110/70 mmHg, a heart rate of 100 beats per minute, respiratory frequency of 40 breaths per minute, and oxygen saturation of 90–92% -ambient air-, his body mass index was 31.7 kg/m2 (weight 95 kg, height 1.73 m). Pulmonary auscultation revealed bibasilar crackles extending to both mid pulmonary fields. Examination of the skin revealed a roseoliform maculopapular rash located to trunk and limbs, which rapidly evolved into a scarlatiniform-like rash. Lesions later progressed and coalesced to form rounded areas spared by unaffected skin (commonly referred to as “white islands in a red sea” in context DENV infection) (Fig. 1 ). There was neither mucosal nor hand and feet involvement. Initial pruritus quickly ceased.
Fig. 1.
Rash in the abdomen (A) and lower limbs (B), related mostly to dengue; CT-scan showing bilateral glass opacities and consolidations compatible with COVID-19 (C); and the timeline of the case (D).
During the hospital course, laboratory findings revealed leukocytosis 4,000 cells/mL (normal range 4,500–11,000 cells/mL), with 35% neutrophils, 18% lymphocytes, and a 120,000 cells/mL platelet count. Inflammatory markers included a C-reactive protein of 7 mg/l, LDH 700 IU/l, and ferritin 650 mcg/mL. The D-dimer was elevated 5,175 ng/mL (with no clinical or pulmonary imaging signs of thromboembolism), with a troponin 0.8 ng/mL. AST and ALT were 55 IU/l and 40 IU/l respectively. Other laboratory findings, including arterial blood gases, are presented in Table 1 . An ECG failed to reveal ischemic changes or other abnormalities. Cardiac monitoring remained normal during the course of disease. Given the non-specific, overlapping constellation of symptoms, RT-PCR testing was performed to further determine the presence of both viruses. A nasopharyngeal swab tested for presence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) resulted positive. In addition, nucleic acid amplification tests for Chikungunya and Zika viruses were negative, but positive for Dengue 2 (DENV-2). Serologic testing performed by ELISA further confirmed positive DENV IgM and IgG, 10 days after the RT-PCR. Findings on computerized tomography (CT) scan of the chest showed bilateral reticular ground-glass opacities and patchy consolidations compatible with COVID-19 (Fig. 1).
Table 1.
Relevant markers of inflammation and in the patient.
| Date |
|||
|---|---|---|---|
| Markers | July 21, 2021 | July 31, 2021 | Normal values |
| LDH (U/L) | 700 | 220 | 140–280 |
| Ferritin (ng/mL) | 650 | 250 | 20–250 |
| D-dimer (ng/mL) | 5175 | 0.4 | <0.5 |
| Troponin (ng/mL) | 0.8 | 0.4 | 0–0.4 |
| Fibrinogen (g/L) | 7 | 5 | 2.0–4.0 |
| Platelets (cells x103/mL) |
120 |
100 |
150–400 |
| Arterial blood gases (at sea level) | |||
| pH | 7.42 | 7.2 | 7.38–7.42 |
| PCO2 (mmHg) | 35.6 | 30.0 | 38–42 |
| PO2 (mmHg) | 90 | 94 | 75–100 |
| HCO3 (mEq/L) | 19 | 20 | 22–28 |
| SatO2 (%) | 97 | 98 | 94–100 |
| Lactate (mmol/L) | 1 | 0.42 | 0.5–1 |
Bold, abnormal values.
The patient was diagnosed with dengue and warning signs of moderate COVID-19 with pulmonary compromise. He was treated with ceftriaxone, enoxaparin (1.5 mg/kg), methylprednisolone, ipratropium bromide, isotonic crystalloid 10 mL/kg, and supplementary oxygen by nasal cannula and CPAP, with notable improvement. Patient was discharged a week after admission, and after 9 days following discharge, the exanthema completely cleared, and CT findings had significantly improved (Fig. 1).
This case report highlights the complexity in the distinction between the shared clinical features of dengue and COVID-19, including their dermatological manifestations [[1], [2], [3], [4]]. Up to June 3, 2021, Colombia had reported 3,488,046 COVID-19 cases, with 90,353 deaths (2.59%). While, a total of 78,979 dengue cases, with 55 deaths (0.07%), were officially informed for 2020, and in 2021 a total of 13,316 cases have been reported up to May 22, 2021, with 7 confirmed deaths (0.053%). With all the focus on SARS-CoV-2, arbovirus infections such as Dengue (DENV), Chikungunya (CHIKV) and Zika (ZIKV) are likely being under-recognized in most endemic regions of the world. This, in part to the significant overlap of signs and symptoms between SARS-CoV-2 and endemic arboviruses. Moreover, at a clinical level, the endotheliotropic nature of DENV and SARS-CoV-2, as well as their shared pathophysiological mechanism involving an immune mediated cytokine storm among a wide constellation of other systemic drivers, further exacerbate the clinical mimics between both pathogens, making it hard to differentiate from a clinical diagnostic standpoint. To date, little has been published on how SARS-CoV-2 and DENV coinfection may influence clinical outcomes. A recent study from Carosella et al. suggests that co-infection by these viruses does not lead to worsening symptoms when compared to SARS-CoV-2 or DENV infections alone [4]. Such findings may be explained in part by the opposed pro and anticoagulant states triggered by SARS-CoV-2 and DENV respectively, as well as to other potential causes such as viral interference [5]. Moreover, computational docking studies have shown that human DENV antibodies can bind to the receptor-binding (RBD) domain of SARS-CoV-2 spike protein conferring some degree of protection leading to reduced disease severity [6]. In addition, experimental evidence suggesting that blockage of AT1 and inhibition of ACE receptor may lead to decreased DENV cell internalization [[4], [5], [6]] provides further insights on how interaction between these two viruses may modulate disease outcome. Further studies aimed to provide a better understanding on how viral interaction and pathogenic mechanisms amongst these viruses modulate disease are urgently needed to better define disease prognosis in DENV and other arboviral endemic areas.
Finally, from a laboratory perspective, serological cross-reactivity has become an ever-increasing challenge in DENV endemic regions, leading to a potential number of false-positives [6]. This clearly underscores the need for implementing a more comprehensive testing strategy inclusive of other circulating and endemic viruses in order to better guide patient care as well as prevention and control measures.
Funding
This research received no external funding.
Authors' contributions
IRT, LEPV, LACT, MAMM, JDMR, and WEVG, contributed to the attention of the patient. WEVG, AJRM and AEPM conceived the manuscript, developed the preliminary search strategy, and drafted the manuscript. All authors critically reviewed the manuscript for relevant intellectual content. All authors have read and approved the final version of the paper.
Informed consent statement
Written consent inform was obtained from the patient.
Declaration of competing interest
All authors report no potential conflicts.
Acknowledgements
None.
References
- 1.Cardona-Ospina J.A., Arteaga-Livias K., Villamil-Gomez W.E., Perez-Diaz C.E., Katterine Bonilla-Aldana D., Mondragon-Cardona A., et al. Dengue and COVID-19, overlapping epidemics? An analysis from Colombia. J Med Virol. 2021;93(1):522–527. doi: 10.1002/jmv.26194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mascarenhas M.D.M., Batista F.M.A., Rodrigues M.T.P., Barbosa O.A.A., Barros V.C. Simultaneous occurrence of COVID-19 and dengue: what do the data show? Cad Saúde Pública. 2020;36 doi: 10.1590/0102-311X00126520. e00126520. [DOI] [PubMed] [Google Scholar]
- 3.Epelboin L., Blonde R., Nacher M., Combe P., Collet L. COVID-19 and dengue co-infection in a returning traveller. J Trav Med. 2020;27(6):taaa114. doi: 10.1093/jtm/taaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carosella L.M., Pryluka D., Maranzana A., Barcan L., Cuini R., Freuler C., Martinez A., Equiza T.R., Peria C.R., Yahni D., Stryjewski M.E., Covidengue Study Group1 Characteristics of patients Co-infected with severe acute respiratory syndrome coronavirus 2 and dengue virus, buenos aires, Argentina, march-June 2020. Emerg Infect Dis. 2021 Feb;27(2):348–351. doi: 10.3201/eid2702.203439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang P.C., Chen K.Y., Huang C.H., Chang K., Lu P.L., Yeh M.L., Huang C.F., Huang C.I., Hsieh M.H., Dai C.Y., Lin Z.Y., Chen S.C., Chuang W.L., Chen Y.H., Huang J.F., Yu M.L. Viral interference between dengue virus and Hepatitis C virus infections. Open Forum Infect Dis. 2020;7(8) doi: 10.1093/ofid/ofaa272. ofaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nath H., Mallick A., Roy S., Sukla S., Biswas S. Computational modelling supports that dengue virus envelope antibodies can bind to SARS-CoV-2 receptor binding sites: is pre-exposure to dengue virus protective against COVID-19 severity? Comput Struct Biotechnol J. 2021;19:459–466. doi: 10.1016/j.csbj.2020.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]