Abstract
Following a request from the European Commission, the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) was asked to deliver an opinion on the relationship between alpha‐lipoic acid (ALA) and the risk of insulin autoimmune syndrome (IAS). The Panel was also asked to advise on the dose below which ALA added to foods is not expected to cause IAS. A review of all possible adverse effects associated with consumption of ALA was not requested. This mandate refers to the procedure under Article 8(2) of Regulation (EC) No 1925/2006 on addition of vitamins, minerals and certain other substances to foods. No pre‐established rule exists for the evaluation of the safety of foods when classical toxicity tests cannot be used, e.g. for autoimmune diseases. Published scientific evidence was retrieved through comprehensive literature searches, particularly 49 case reports in which IAS developed following ALA consumption. In all cases, IAS resolved after a few weeks to months when ALA was discontinued. No publication linking the intake of ALA naturally occurring in foods to IAS was identified. The Panel concludes that the consumption of ALA added to foods, including food supplements, is likely to increase the risk of developing IAS in individuals with certain genetic polymorphisms, who cannot be readily identified without genetic testing. The plausible mechanism of such an effect has not yet been fully elucidated. The incidence of IAS in Europe is low and likely lower than in Japan where it has been estimated to be 0.017 per 100,000 inhabitants in 2017–2018. Considering the limited data available, the risk associated with the development of IAS following ALA consumption cannot be quantified precisely. An ALA dose below which IAS is not expected to occur is likely to vary between individuals and cannot be determined from the available data.
Keywords: Thioctic acid, Hirata's disease, insulin autoimmune syndrome, comprehensive literature search, genetic determinants, case report, food supplement
1. Introduction
1.1. Background
The Danish authorities requested the Commission to initiate the procedure under Article 8 of Regulation (EC) No 1925/2006 on the addition of vitamins and minerals and of certain other substances to foods1 for the intake of alpha‐lipoic acid in food supplements because of the potential risk to health associated with the intake of this substance. Safety concerns associated with the use of alpha‐lipoic acid in food supplements have been outlined in a scientific opinion by the Danish National Food Institute (DTU) on the safety of alpha‐lipoic acid use in food supplements,2 and in an expert opinion on the safety of placing dietary supplements with alpha‐lipoic acid on the market for the general population3 by the Belgian Superior Health Council.
The above‐mentioned scientific assessments lay out the possible harmful effects associated with the use of alpha‐lipoic acid in food supplements, in particular a potential risk for Insulin Autoimmune Syndrome and reports in clinical studies of several adverse effects.
Consequently, the Commission has initiated the procedure under Article 8 (2) of Regulation (EC) No 1925/2006 on the addition of vitamins and minerals and of certain other substances to foods, for the intake of alpha‐lipoic acid in food supplements.
1.2. Terms of Reference as provided by the requestor
In accordance with Article 29(1)(a) of Regulation (EC) No 178/20024, the European Commission asks EFSA to:
-
–
Review the existing scientific data on the possible link between the intake of alpha‐lipoic acid and Insulin Autoimmune Syndrome.
-
–
Provide advice on a dietary intake of alpha‐lipoic acid intentionally added to foods that does not give rise to concerns about Insulin Autoimmune Syndrome for the general population, and as appropriate, for vulnerable subgroups of the population.
1.3. Interpretation of the Terms of Reference
The Panel understands that it is expected to provide information on the relationship between oral consumption of alpha‐lipoic acid (ALA, or thioctic acid) that is added to food, including food supplements, and insulin autoimmune syndrome (IAS, or Hirata's disease).
The Panel is also expected to provide advice on the dose below which ALA added to foods is not expected to cause IAS in the general population or in vulnerable subgroups thereof.
In line with the mandate, it is out of the scope to review possible adverse effects other than IAS associated with the oral consumption of ALA.
Also out of scope of the mandate are the assessment of metabolic/beneficial effect(s) of oral consumption of ALA, a risk–benefit analysis of ALA supplementation and an exposure assessment of ALA in the European population.
1.4. Context of the assessment
Article 8 of Regulation (EC) No 1925/2006 provides for a procedure for the regulatory management of substances other than vitamins or minerals added to foods that may present a potential risk to consumers. Upon its own initiative or on the basis of information provided by Member States, the European Commission may ask EFSA for a scientific assessment of the safety of such a substance.4 On the basis of EFSA's assessment, the European Commission together with the Member States may decide either to allow the use of the substance (with or without restrictions) in food, to prohibit the use of the substance in food or to put the substance under scrutiny.
1.5. Previous assessments
ALA is used as an active ingredient in medicinal products mainly for the treatment of diabetic neuropathy. It is also available as a food supplement.
Several scientific bodies in the area of food or medicinal products have published scientific assessments on ALA within the European Union (EU), in particular in relation to the risk of IAS, that are summarised in chronological order below.
In 2008, the French Food Safety Agency (AFSSA, 2008) (now called ANSES) published an opinion on a draft regulatory text from the French risk management authorities, about the use of substances with nutritional or physiological effect and plants or plant preparations in food supplements. Several substances were considered, including ALA, for which no maximal dose was proposed in the draft regulatory text. The French Food Safety Agency discussed amongst others a paper describing an acute and a subacute toxicity study in rats (Cremer et al., 2006b). Regarding data in humans, the French Food Safety Agency reviewed references on tolerance of ALA treatment for patients with diabetic neuropathy and three published case reports of IAS (Furukawa et al., 2007; Ishida et al., 2007; Takeuchi et al., 2007). Overall, it concluded that the risk of occurrence of this syndrome following consumption of ALA cannot be excluded, but the risk is very low in the French population. Similar considerations were repeated in another opinion of the French Food Safety Agency in 2011 on the assessment of the risks associated with substances with nutritional or physiological effects with a view to restricting or prohibiting their use in foodstuffs (ANSES, 2011).
The Superior Health Council of Belgium (2015) noted that ALA may be sold as a medicinal product (e.g. in Germany) used for the treatment of diabetic neuropathy, and that adverse effects of treatment with this substance have been observed without further details in the report. The Council recommended that ALA should be used as a medicinal product instead of a food supplement and consumed under medical supervision (as ALA was available as food supplement in Belgium at the time when these conclusions were drawn).
The Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) published recommendations in 2015 for an update of the product information for medicinal products containing thioctic acid (a synonym for ALA) and occurrence of IAS (EMA, 2015). It was explained that the summary of product's characteristics should be updated to indicate the following:
-
–
Under ‘special warnings and precautions for use’ ‘Cases of Insulin Autoimmune Syndrome (IAS) have been reported during treatment with thioctic acid. Patients with human leukocyte antigen genotype such as HLA‐DRB1*04:06 and HLA‐DRB1*04:03 alleles, are more susceptible to develop IAS when treated with thioctic acid. HLA‐DRB1*04:03 allele (susceptibility to IAS odds ratio: 1.6) is especially found in Caucasians, with a higher prevalence in southern than in northern Europe and HLA‐DRB1*04:06 allele (susceptibility to IAS odds ratio: 56.6) is especially found in Japanese and Korean patients. IAS should be considered in the differential diagnosis of spontaneous hypoglycaemia in patients using thioctic acid […].’
-
–
Under ‘undesirable effects’ ‘Immune system disorders Frequency unknown: insulin autoimmune syndrome’’ Also, it was recommended that the package leaflet should indicate the following:
-
–
‘Patients with a certain human leukocyte antigen genotype (which is more frequent in Japanese and Korean patients, but is also found in Caucasians) are more prone to development of insulin autoimmune syndrome (disorder of the blood glucose regulating hormones with pronounced lowering of blood sugar levels) when treated with thioctic acid.’
In 2017, the Danish National Food Institute (DTU) (DTU Food, 2017) described a number of adverse effects other than IAS related to the consumption of ALA by humans, such as allergic skin reactions, stomach ache, nausea, vomiting, diarrhoea and dizziness. DTU discussed a paper describing an acute and a subacute toxicity study in rats (Cremer et al., 2006b) as well as another paper on a chronic toxicity study also in rats (Cremer et al., 2006a). From these studies on endpoints unrelated to IAS, DTU concluded that ‘a dose of 60 mg alpha‐lipoic acid per kg body weight per day is considered a no observed adverse effect level (NOAEL)’. Using an uncertainty factor of 100 to take into account inter and intra‐species variability, DTU derived from the NOAEL ‘an upper limit of safe intake of alpha‐lipoic acid’ of ‘0.6 mg/kg body weight per day’, corresponding to ‘a maximum daily dose of 42 mg alpha‐lipoic acid for a person weighing 70 kg’. This NOAEL did not apply to the risk of developing IAS. In this relation, DTU mentioned that ‘it is known that alpha‐lipoic acid can cause insulin autoimmune syndrome’, and that ‘since it is an immunological response (autoimmune response), it is assumed that the dose consumed does not play a crucial role in the development of the disease’. DTU also discussed data on the ‘strong genetic element in the development of IAS’. Finally, DTU stated the following: ‘Based on a calculated upper safe intake of 42 mg alpha‐lipoic acid per person per day determined from two rat studies and a general risk of increased incidence of IAS (irrespective of dosage level and due to increased exposure of the population), DTU FOOD concludes that a supplement with a recommended daily intake of 150–200 mg alpha‐lipoic acid (as proposed by the applicants) gives rise to safety concern’.
2. Data and methodologies
2.1. Data
For this scientific assessment, a protocol (Appendix B) has been developed in line with existing methodology (EFSA, 2020).5
This Scientific Opinion is based on data that were retrieved through comprehensive literature searches in Embase and PubMed on 4 September 2020 (and in March 2021, see further down) for relevant publications in one of the EU languages. The Panel is aware that case reports published only in non‐European languages were not taken into account in the assessment. However, the Panel considers, in view of the previous assessments conducted by other bodies, and the number of published case reports identified in EU languages for this assessment, that such an exclusion did not impact the overall assessment and conclusions.
The searches were conducted without applying limits to the date of publication. The database searches were complemented by searches on websites of relevant institutions and authorities, such as the US Food and Drug Administration (FDA), Health Canada or Food Standards Australia New Zealand (FSANZ). The hits on these websites were added to the ones obtained from the databases and used in particular for the general sections of this opinion.
Three searches were set up a priori. Only data in humans were considered for this assessment, as no animal or in vitro model of IAS was identified.
Through search 1, EFSA retrieved case reports published in peer‐reviewed journals of IAS associated with ALA consumption. In some instances, these case reports were accompanied by reviews of the evidence. The information provided in the review section of these papers was also considered.
The purpose of search 2 was to retrieve literature reviews on IAS.
Search 3 was focussed on retrieving clinical trials in which ALA was administered either alone or in combination. The original aim of search 3 was to identify clinical trials in humans in which IAS or symptoms indicative of IAS have been reported as adverse effects. Following a protocol amendment (No 1), search 3 was only used to retrieve pharmacokinetic studies in humans as well as studies specifically designed to investigate safety of ALA in humans. While relevant pharmacokinetic studies were identified, no relevant safety studies were found. The list of references of the retrieved studies were screened for additional publications. Some reviews that were found in search 3 were kept as background information and were also used in the opinion.
Another protocol amendment (No 2) concerned signal data (published or unpublished) from vigilance databases that were originally planned to be retrieved in the protocol but were not used (for explanation, see Section 3.6).
The title and abstract screening was carried out in duplicate in Distiller SR®. Title and abstract screening was done by EFSA staff members in parallel. Full‐text screening was performed by a single EFSA staff member (protocol amendment No 3). Any uncertainties were resolved by the Working Group. Data from case reports were extracted in tabular format in Microsoft Excel® by one EFSA staff member and double‐checked by another.
While all pertinent case reports have been included in the assessment, data coming from reviews and pharmacokinetic studies were only described as appropriate. Previous assessments from other scientific bodies were used for hand search in their lists of references, applying the inclusion criteria of the present assessment.
No author was contacted to collect missing information.
An additional non‐systematic search was conducted on 1 March 2021 after the public consultation of the Opinion in Scopus and Embase, in order to retrieve evidence on the pharmacokinetics of dihydrolipoic acid in humans, the reduced form of ALA. This was done following a comment received during the public consultation to expand on the absorption, distribution, metabolism and excretion of this form (in addition to the information already present in the opinion for ALA). The title, abstract and full‐text screening was carried out by a single reviewer in Distiller SR®. A hand search of the retrieved publications was also performed.
The PRISMA flow charts of the four searches are included in Appendix A. A total of 3,094 titles and abstracts were screened, of which 85 papers were identified as pertinent.
The eligibility criteria of the searches are reported in the protocol as Appendix B of this Scientific Opinion. The search strings of the three initial searches are available in Appendix C. The search strings for the additional search carried out after the public consultation are reported in Appendix D.
2.2. Methodologies
There is no pre‐established rule for the evaluation of the safety of foods/substances in cases where classical toxicity tests cannot be used as a basis of the assessment, such as for autoimmune diseases. There is no guidance document available on how to perform such an assessment. In the present case, the Panel relied on published case reports that linked the consumption of ALA to the development of IAS.
In line with EFSA's policy on openness and transparency, and in order for EFSA to receive comments from the scientific community and stakeholders, the draft Scientific Opinion was released for public consultation. The outcome of the public consultation is described in a technical report published as Annex A to this Opinion.
2.3. Protocol amendments
Search 3 was only used to retrieve pharmacokinetic studies in humans as well as studies specifically designed to investigate safety of ALA in humans instead of all clinical trials conducted with ALA in humans.
Signal data from vigilance databases were not used in the assessment (for explanation, see Section 3.6).
Full text screening was done by a single EFSA staff member instead of performing it in duplicate. Any uncertainties were resolved by the Working Group.
Data on oral administration of ALA analogues, derivatives or metabolites were not included in the assessment, as no case report was retrieved in which consumption of an ALA analogue, derivative or metabolite was linked to the development of IAS.
An additional search was carried out on the pharmacokinetics of dihydrolipoic acid.
3. Assessment
3.1. Identity of alpha‐lipoic acid
ALA (also called thioctic acid, C8H14O2S2, CAS Number: 1200‐22‐2 (racemic: 1077‐28‐7), IUPAC name: 5‐[(3R)‐1,2‐dithiolan‐3‐yl]pentanoic acid, molecular weight 206.3 g/mol) is an eight‐carbon fatty acid (Evans et al., 2002) that has a chiral centre in its 1,2‐dithiolane ring (Ikuta et al., 2016). Therefore, ALA exists in the form of two enantiomers: R‐(+) and S‐(–), for which formulas are presented in Figure 1. The R‐(+)‐enantiomer is the naturally occurring form of ALA (Hermann et al., 2014). It can be synthesised in the body from octanoic acid and cysteine (Bilska and Wlodek, 2005), while the S‐(–) form cannot (Ikuta et al., 2016). The S‐(–) form is formed during the industrial production of ALA by chemical synthesis (Yoon et al., 2016) (See Section 3.2.).
Figure 1.
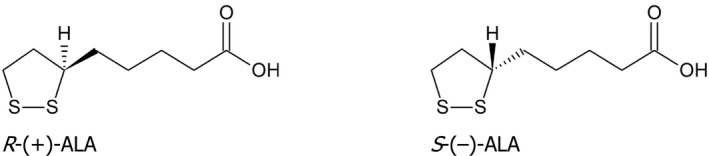
Stereochemistry of alpha‐lipoic acid
3.2. Sources of alpha‐lipoic acid, production process, stability and type of formulations available
In its natural form, ALA occurs in foods of animal and plant origin as R‐(+)‐enantiomer, with the highest content in tissues with a high metabolic activity such as the heart and lower contents in muscle tissue. For example, pig hearts contain 1.1–1.6 mg/kg and calf muscles 0.07–0.15 mg/kg ALA (Biewenga et al., 1997).
For the industrial production, several ways exist to produce ALA synthetically, as reviewed by the US Food and Drug Administration (FDA) (Zhang et al., 2018). In particular, one high‐yield synthetic production process is cited in which dihydrolipoic acid, containing two sulfhydryl groups (see Section 3.5.4) is produced from cyclohexanone, vinyl ethyl ether and thiourea and finally oxidised into ALA. The Panel assumes that most industrially produced ALA is a racemic mixture rather than the pure R‐(+)‐enantiomer.6
Dihydrolipoic acid can be a residue of the ALA synthesis or generated from the photolysis of ALA. FDA states that impurities from the production process likely include, apart from dihydrolipoic acid, oligomers resulting from its polymerisation and trace amounts of solvents and reagents. The European Pharmacopoeia monograph for (RS)‐ALA (Ph. Eur. 10.0, 4020‐4021) identifies as specified impurities 5‐[(4RS)‐1,2,3‐trithian‐4‐yl]pentoic acid and α‐hydro‐ω‐hydroxypoly[sulfanediyl(3‐sulfanyl‐8‐oxooctane‐1,8‐diyl)], the latter being a mixture of ALA polymers. Limit for total impurities set in the European Pharmacopoeia is 0.3% (HPLC‐UV). No information was found in the retrieved literature on potential contamination or adulteration of products containing ALA (see Appendix B, Section B.1.1).
ALA is light‐ and heat‐sensitive. The FDA review indicates that it is likely to be stable in solid formulations when protected from light and heat, but less stable in liquid formulations. The R‐(+) form is less stable than the racemic mixture (Zhang et al., 2018).
The Superior Health Council of Belgium (2015) reports that ALA as food supplement is mainly sold in tablet or capsule form with recommended intakes usually between 300 and 600 mg/day.
The Panel notes that in the retrieved evidence there was no information on whether production processes used to produce ALA for foods including food supplements substantially differ from those used to produce ALA for use in medicinal products, or whether impurities or degradation products may be different in nature or amount between food supplements and medicinal products. However, considering that IAS has been observed to occur in conjunction with the intake of ALA as food supplement as well as with its intake as medicinal product, the Panel considers that impurities or degradation products were unlikely to play a role in the development of IAS (see Appendix B).
3.3. Biochemistry
ALA is present in all prokaryotic and eukaryotic cells. Together with its reduced form, dihydrolipoic acid, it acts as a redox couple (Biewenga et al., 1997). ALA is covalently bound to a lysine of the E2 (dihydrolipoate acyltransferase) subunit of several 2‐oxo acid dehydrogenase multienzyme complexes (Teichert et al., 2003; Mignini et al., 2011) and acts as a cofactor that catalyses oxidative decarboxylation of pyruvate, α‐ketoglutarate and branched‐chain α‐ketoacids, formed during transamination of leucine, isoleucine and valine. ALA is also an element of a mitochondrial complex involved in glycine synthesis and degradation (Bilska and Wlodek, 2005). ALA is found both in hydrophilic (cytoplasm, extracellular matrix) and hydrophobic (plasma membranes) environments (Brufani and Figliola, 2014).
3.4. Absorption, distribution, metabolism and excretion of alpha‐lipoic acid
It has been suggested that R‐(+)‐ALA from natural sources is absorbed as lipoyllysine7 and is not found unbound in humans. In contrast, supplemental ALA (racemic mixture or R‐(+) form) is absorbed as such and then found in its free form in the circulation (Biewenga et al., 1997). Free ALA in circulation may also origin from the endogenous production of ALA. Apart from its free form and lipoyllysine, ALA is also circulating as weakly bound to proteins via hydrogen bonds (Khan et al., 2015).
Supplemental ALA is readily absorbed. The Panel is not aware of any data on the potential mechanisms of absorption (i.e. passive or active absorption) or on a potential interaction with other substances. In the fasting state, several studies report mean time to maximum plasma concentrations (Tmax) in adults mostly in the range of 0.5–1.0 h for both enantiomers (Gleiter et al., 1996; Hermann et al., 1996, 2014; Teichert et al., 1998; Breithaupt‐Grögler et al., 1999; Evans et al., 2002; Zheng et al., 2014; Rhee et al., 2018). Mignini et al. (2007) found a mean Tmax of up to 2 h, depending on the formulation (i.e. solid or liquid). In the fed state, absorption is delayed. Gleiter et al. (1996) observed mean Tmax of about 2.5 h and 1 h in the fed state and in the fasting state in the same subjects, respectively. Liquid formulations seem to be absorbed more rapidly than solid formulations (Hermann et al., 1996, 2014).
R‐(+)‐ALA is generally more bioavailable than the S‐(‐) form (Gleiter et al., 1996; Hermann et al., 1996, 2014; Breithaupt‐Grögler et al., 1999). Breithaupt‐Grögler et al. (1999) reported the bioavailability of R‐(+)‐ALA as being 40–50% higher than S‐(‐)‐ALA, and Hermann et al. (2014) a bioavailability of R‐(+)‐ALA being twice that of the S‐(‐) form (both based on maximum plasma concentrations (Cmax)). It has been speculated that this difference might be attributed to different intestinal uptake mechanisms between R‐(+)‐ALA and S‐(‐)‐ALA (i.e. active, transporter‐mediated absorption for the R‐(+)‐form vs. passive or less effective active absorption for the S‐(‐) form) (Hermann et al., 2014).
Hermann et al. (2014) observed that, after body weight normalisation of data, ALA was consistently more bioavailable in females than in males (n = 12 per sex) both for R‐(+)‐ALA and S‐(‐)‐ALA. Areas under the curve (AUCs) were on average 40% higher in females compared with males. Differences in Tmax and Cmax, although higher in females, did not reach statistical significance. This sex‐specific effect was consistent for all formulations studied (i.e. liquid and various doses of solid formulations).
Pharmacokinetic parameters have been demonstrated to react in a linear and proportional dose‐dependent manner in the dose range of oral intakes of ALA of 50–600 mg (Breithaupt‐Grögler et al., 1999).
ALA is rapidly removed from circulation. The mean half‐life of ALA in plasma is generally described to be about 30 min (Hermann et al., 1996; Biewenga et al., 1997; Teichert et al., 1998, 2003; Breithaupt‐Grögler et al., 1999; Zheng et al., 2014; Rhee et al., 2018), though Mignini et al. (2007) reported a mean half‐life of around 6 h. Breithaupt‐Grögler et al. (1999) found that there was no difference in half‐life between R‐(+)‐ALA and S‐(‐)‐ALA. A significant first pass effect (Teichert et al., 2003; Zhang et al., 2018), in which total plasma clearance of ALA is in the same range as the plasma flow in the liver, is responsible for the relatively low bioavailability of around 20–40%, depending on the isomer and the formulation (i.e. liquid or solid) (Biewenga et al., 1997; Teichert et al., 2003).
Following absorption, ALA is taken up into cells (Bustamante et al., 1998) and reduced to dihydrolipoic acid. Both are predominantly metabolised via β‐oxidation. 4,6‐Bis(methylthio)hexanoic acid is the main metabolite in plasma (Teichert et al., 1998) and excreted in the urine (Teichert et al., 2003; Zhang et al., 2018).
Only limited data are available on the concentrations of dihydrolipoic acid in human plasma and no publication has been retrieved on its pharmacokinetics. Khan et al. (2011) reported mean (standard deviation, SD) concentrations in plasma of 15 healthy volunteers (aged 22–25 years) of 173 (4.26) ng/mL for dihydrolipoic acid and of 35 (5.64) ng/mL for ALA. Whether volunteers had received ALA supplements or not, was not explicitly stated. Teichert and Preiss (1992) found dihydrolipoic acid concentrations in plasma of six healthy non‐supplemented volunteers of 33–145 ng/mL and ALA concentration of 1–25 ng/mL after acid hydrolysis. In contrast, Haj‐Yehia et al. (2000) showed in a chromatogram that concentrations of dihydrolipoic acid in plasma of a volunteer who had received supplemental ALA were lower than ALA concentrations (numeric values not reported). This is similar to what was shown by Khan et al. (2015) for free endogenous ALA from plasma of a most likely non‐supplemented individual. The Panel notes that these data on the plasma ratio of dihydrolipoic acid/ALA are limited and insufficient to conclude on which is the major circulating form.
3.5. Insulin autoimmune syndrome
3.5.1. Definition of insulin autoimmune syndrome
IAS is a an autoimmune disease characterised by spontaneous hypoglycaemic episodes due to high titres of insulin autoantibodies (IAA), which determine a marked increase in total serum insulin, and free insulin concentrations often within the normal range (Archambeaud‐Mouveroux et al., 1989). C‐peptide and proinsulin concentrations are either elevated or within the normal range (depending partly on whether IAA are also able to bind C‐peptide and proinsulin and partly on the laboratory assay used) (Censi et al., 2018b). IAS has been considered to occur in individuals that have not been exposed to exogenous insulin, even though more recently, some cases of IAS in diabetic subjects who received exogenous insulin have been reported (Cappellani et al., 2020).
Antibodies are mostly polyclonal IgG with kappa light chains (Cooper, 1999) and to a minor extent with lambda light chains. However, the presence of IgA, IgM and monoclonal IgG has also been described (Archambeaud‐Mouveroux et al., 1989; Censi et al., 2018b).
3.5.2. Epidemiology of insulin autoimmune syndrome
IAS is rare: a survey conducted in the years 2017–2018, in Japanese hospitals with more than 300 beds (Yamada et al., 2020), identified 22 cases of IAS out of 785 patients with endogenous hyperinsulinaemic hypoglycaemia who required treatment (i.e. 2.8%). Based on the assumption that these 22 IAS cases were all the cases that had occurred in Japan in the years 2017–2018, the authors calculated an incidence of IAS in the general population in Japan of 0.017 cases per 100.000 inhabitants in these years. In an older study (Takayama‐Hasumi et al., 1990), conducted from 1979 to 1981 also in Japan, IAS was identified as the third cause (11.7% of cases) of severe spontaneous hypoglycaemias treated in hospitals (after insulinoma and extrapancreatic neoplasms).
The incidence of IAS in Caucasians seems to be lower than in the Japanese population (Cappellani et al., 2020). However, the number of case reports regarding Caucasians has been increasing in recent years (Bresciani et al., 2011; Gullo et al., 2014; Michalopoulou Alevras et al., 2015; Lio et al., 2016; Ferreira et al., 2017; Bolayir et al., 2018; Cappellani et al., 2018; Veltroni et al., 2018; Alagüney et al., 2019; Moffa et al., 2019; Cambria et al., 2020; Okuroglu et al., 2020; Yukina et al., 2020). It is, however, difficult to estimate the actual incidence or conclude on a true increase in the disease incidence among Caucasians, because of a possible underestimation of the occurrence of the syndrome linked to a possible unawareness of the disease and its subsequent underdiagnosis and underreporting (Cappellani et al., 2020).
3.5.3. Genetic determinants of insulin autoimmune syndrome
The presence of the Human Leukocyte Antigen HLA‐DR4 (Uchigata et al., 2010), and in particular the alleles DRB1*04:06 (most of the Asian cases), DRB1*04:03 (most of the Caucasian cases) and DRB1*04:07 (Patel et al., 2020) and to a lesser extent DRB1*04:15 (Cappellani et al., 2018; Cambria et al., 2020) are associated with an increased risk of developing the disease.
Glutamate at position 74 in all the alleles DRB1*04:03, DRB1*04:06 and DRB1*04:07 and serine at position 37 (unique to DRB1*04:06) have been proposed to be responsible for this increased predisposition for developing IAS (Yukina et al., 2020). DRB1*04:03 can be considered as the ancestral allele from which DRB1*04:06 and DRB1*04:07 developed independently by nucleotide substitution or by gene conversion (in case of DRB1*04:07) (Uchigata et al., 2000). The Panel was unable to retrieve data on the amino acids that are present at position 74 and 37 in the allele DRB1*04:15.
As reported in the Allele Frequency Net Database (Gonzalez‐Galarza Faviel et al., 2019),8 DRB1*04:03 was found to occur in various populations worldwide.9 In populations living in Europe, the frequency is reported to range from 0.4% to 3.9% (data from Austria (1.5%), Germany (0.6–1.8%), Greece (6%), Ireland (0.4%), Italy (1.0–2.1%), the Netherlands (2.2%), Poland (1.3%), Slovenia (0.7–1%), Spain (3.1–4%), UK (0.7–3.9%), (Gonzalez‐Galarza Faviel et al., 2019) and France (1.9%) (Uchigata et al., 2000)). In comparison, in Japan and South Korea, the prevalence is 1.6–12.3% (Uchigata et al., 2000; Gonzalez‐Galarza Faviel et al., 2019).
DRB1*04:06 is mostly present in East Asian populations (e.g. prevalence in Japan between 5.3% and 13.2%), while, in Europe, it is between 0.1 and 1% (data from studies in Italy (1%), the Netherlands (0.2%), Poland (0.1%) and Spain (0.5–0.6%)(Gonzalez‐Galarza Faviel et al., 2019) and France (0.3%) (Uchigata et al., 2000).
The prevalence of DRB1*04:07 in Europe has been observed to be in the range of 0.5–3.4%; in Japan and South Korea, it is 0.3–2.8% (Uchigata et al., 2000; Gonzalez‐Galarza Faviel et al., 2019).
The prevalence of DRB1*04:15 was investigated only in a limited number of studies and countries. The only study which detected DRB1*04:15 was a study in Poland which found DRB1*04:15 in one participant among 23,595 individuals. In none of the other studies, an individual carrying the DRB1*04:15 was detected.
3.5.4. Pathophysiology of insulin autoimmune syndrome
IAS has been described as an autoimmune disease which may develop after the intake of substances containing a sulfhydryl group (such as methimazole or dihydrolipoic acid; see Section 3.2). However, IAS can also be triggered by viral infections and, in some cases, the trigger cannot be identified. It may be sporadic, or may occur together with other autoimmune diseases (Archambeaud‐Mouveroux et al., 1989).
It has been proposed that substances containing sulfhydryl groups may cleave one disulfide bond of insulin, resulting in structural modification and increased immunogenicity (Cappellani et al., 2020). The resulting peptides have been shown to bind to gene products of DRB1*04:06. This leads to the insulin‐specific proliferation of T cells (Ito et al., 1993), and the subsequent production of IAA by B cells.
Wasada et al. (1988) performed a study in which methimazole was incubated together with biosynthetic recombinant human insulin. Authors concluded that insulin appeared not to be structurally changed, although they did not directly investigate cleavage of disulfide bonds. Even if in vitro antibody binding of insulin that had been incubated with methimazole was not observed, the Panel notes that this does not allow to conclude on the inability of the incubated insulin to induce the production of IAA.
Most authors hypothesise that IAA bind to endogenous insulin that is released from beta‐cells in response to a glucose stimulus. Insufficient free insulin is therefore available, causing a temporary hyperglycaemia. This hyperglycaemia stimulates insulin secretion further. When the IAA‐insulin complexes dissociate post‐prandially, biologically active insulin is released into circulation, resulting in an excess of insulin and a subsequent hypoglycaemia (Ismail, 2016; Censi et al., 2018a; Cappellani et al., 2020). Hypoglycaemia typically occurs within 2–6 h post‐prandially with varying severity, as the half‐life of insulin in IAS is increased from minutes to hours. The severity of hypoglycaemia depends on the antibody characteristics (i.e. binding capacity and affinity that determines the dissociation rate) and their titres (Ismail, 2016). High capacity/low affinity antibodies are more likely to cause hypoglycaemia (Redmon and Nuttall, 1999).
The hypothesis mentioned above was supported by Dozio et al. (1998) who administered 125I‐labelled insulin intravenously to a patient with IAS and a healthy volunteer. In the healthy subject, radiolabelled insulin was quickly removed from plasma and taken up by the liver and the kidney. In the IAS patient, radioactivity remained in the blood with hardly any uptake by the liver and kidney; 93.6% of the 125I‐labelled insulin was bound to antibodies.
In some parts of the population, IAA are present in serum and are not associated with adverse effects. For example, Sodoyez et al. (1990) reported the presence (> mean + 3SD) of IAA in 1% of 2,200 healthy blood donors. Hattori et al. (2014) found IAA in 2.7% (of 263) of type 2 diabetics never having received insulin. Cooper (1999) cited a study in which 6% (of 206) of patients treated with methimazole (a substance associated with an increased risk of developing IAS) had IAA without developing symptoms of IAS. In that study, IAA insulin‐binding capacity was lower than usually observed in IAS; the IAA concentrations peaked 2–3 months after methimazole administration and declined thereafter to almost undetectable levels. In addition, Cooper (1999) mentioned another report in which also 6% (of 95) patients treated with methimazole or carbimazole had IAA. The Panel notes that this indicates that IAA with different affinities exist. Another possible explanation could be that there is a threshold below which IAA do not cause adverse effects.
The Panel notes that there is a plausible mechanism by which ALA may increase the risk of developing IAS in individuals with certain genetic polymorphisms: cleavage of endogenous insulin, insulin‐specific proliferation of T cells, subsequent production of IAA by B cells, binding of IAA to endogenous insulin, spontaneous dissociation of IAA‐insulin complexes with an excess of insulin in circulation. However, this mechanism has not yet been fully elucidated.
3.5.5. Symptoms of insulin autoimmune syndrome
IAS is most often associated with post‐prandial hypoglycaemia (Okuroglu et al., 2020). The nature of the symptoms is neuroglycopenic (e.g. behavioural changes, confusion, fatigue, seizures and loss of consciousness), neurogenic (e.g. palpitations, tremor, anxiety) or cholinergic (e.g. sweating, hunger, paresthesia) (Davi et al., 2017; Censi et al., 2018b). Symptoms typically resolve with food intake. Fasting hypoglycaemia occurred in only a few cases (Cappellani et al., 2020).
IAS usually resolves within a few months once the trigger (see Section 3.5.4) is removed (hence the symptoms disappear). However, some patients require pharmacological treatment (Cappellani et al., 2020). When the trigger is re‐introduced, the syndrome may reappear (Bae et al., 2013); see Table 1.
Table 1.
Summary of case reports reporting on insulin autoimmune syndrome (IAS) related to consumption of alpha‐lipoic acid (ALA, or thioctic acid) (published in a EU language, date of search 4/9/2020, chronological order)
| Reference (year of 1st publication for reviews) | Country of diagnosis | Amount mg/d Duration | Sex Age (years) Ethnicity (if reported) | Health status | Other concomitant medication | Symptoms | Time to remission | Treatment of IAS | ALA as medicinal product or food supplement | HLA class DRB1* |
|---|---|---|---|---|---|---|---|---|---|---|
| Bae et al. (2013) from review (2003) | JP | NR | F 55 | NR | NR | NR | NR | NR | NR | 04:06 |
| Bae et al. (2013) from review (2006) | JP | NR | F 32 | NR | NR | NR | NR | NR | NR | 04:06 |
| Bae et al. (2013) from review (2006) | JP | NR | F 34 | NR | NR | NR | NR | NR | NR | 04:06 |
| Bae et al. (2013) from review (2006) | JP | NR | F 44 | NR | NR | NR | NR | NR | NR | 04:06 |
| Bae et al. (2013) from review (2006) | JP | NR | F 49 | NR | NR | NR | NR | NR | NR | 04:06 |
| Bae et al. (2013) from review (2006) | JP | NR | F 64 | NR | NR | NR | NR | NR | NR | DR4 |
| Bae et al. (2013) from review (2006) | JP | NR | M 66 | NR | NR | NR | NR | NR | NR | 04:06 |
| Bae et al. (2013) from review (2006) | JP | NR | F 67 | NR | NR | NR | NR | NR | NR | NR |
| Bae et al. (2013) from review (2007) | JP | NR | F 32 | NR | NR | NR | NR | NR | NR | 04:06 |
| Bae et al. (2013) from review (2007) | JP | NR | F 34 | NR | NR | NR | NR | NR | NR | 04:06 |
| Bae et al. (2013) from review (2007) | JP | NR | M 35 | NR | NR | NR | NR | NR | NR | 04:06 |
| Bae et al. (2013) from review (2007) | JP | NR | F 36 | NR | NR | NR | NR | NR | NR | NR |
| Bae et al. (2013) from review (2007) | JP | NR | F 36 | NR | NR | NR | NR | NR | NR | NR |
| Bae et al. (2013) from review (2007) | JP | NR | F 40 | NR | NR | NR | NR | NR | NR | 04:03 |
| Bae et al. (2013) from review (2007) | JP | NR | F 41 | NR | NR | NR | NR | NR | NR | NR |
| Bae et al. (2013) from review (2007) | JP | NR | F 45 | NR | NR | NR | NR | NR | NR | 04:03 |
| Bae et al. (2013) from review (2007) | JP | NR | F 48 | NR | NR | NR | NR | NR | NR | 04:06 |
| Bae et al. (2013) from review (2007) | JP | NR | M 55 | NR | NR | NR | NR | NR | NR | 04:06 |
| Furukawa et al. (2007) | JP | 200 Unclear | F 44 | Oligomenorrhoea | Norgesterel/ethinylestradiol, Q10 and carnitin | Recurrent attacks of weakness and malaise | Shortly after withdrawal of ALA | None | Food supplement | 04:06 |
| Ishida et al. (2007) | JP | 200 2 mo | F 32 | Diabetes | None | Pre‐prandial weariness | Within around 3 wk | None (only discontinuation of ALA) | Food supplement | 04:06 |
| Yamada et al. (2007) | JP | 1 mo | F 45 | NR | NR | Hypoglycaemic coma | NR | NR | Food supplement | 04:03 |
| Takeuchi et al. (2007) | JP | 225 1 wk | M 55 | History of obesity, hypertension and hyperuricaemia; slight liver dysfunction, hyperuricaemia | NR | Hunger, sweating, palpitations and tremor, lost consciousness | Few wk | Fractionate meals | Food supplement | 04:06 |
| Bae et al. (2013) from review | KR | NR | F 71 | NR | NR | NR | NR | NR | NR | 04:06 |
| Bresciani et al. (2011) | IT | 600 17 d | F 70 Caucasian | Healthy | None | Recurrent episodes of sweating, weariness and fainting occurring both fasting and postprandial | 1 wk from first dose of diazoxide (hypoglycaemia persisted after prednisone) | Prednisone and then diazoxide | Food supplement | 04:06 |
| Vimalraj et al. (2011) | IN | NR | F 28 | NR | NR | Repeated episodes of weakness, sweating, palpitations, hunger and tremor; lost consciousness | 4 wk | None | Medicinal product | NR |
| Bae et al. (2013) | KR | 600 2 wk Re‐challenge | F 67 | T2DM | Gliclazide | Repeated episodes of hunger, hand tremor, cold sweat and dizziness 3 to 4 hr after a meal, but symptoms improved after eating snacks | 2 mo | Prednisolone | Medicinal product (thioctacid) | 04:06 |
| Bae et al. (2013) from review (2013) | KR | NR | F 67 | NR | NR | NR | NR | NR | NR | 04:06 |
| Gullo et al. (2014) | IT | 600 120 d | F 40 Caucasian | Disk hernia | None | Dizziness, tachycardia, sweating, loss of consciousness | Within 3 mo | Oral sucrose and/or repeated high carbohydrate snacks for 2–5 d | NR | 04:03 |
| Gullo et al. (2014) | IT | 600 45 d | M 53 Caucasian | Membrano‐proliferative glomerulo‐nephritis | Ramipril | Lost consciousness | Within 3 mo | Prednisone (100 d) + oral sucrose and/or repeated high carbohydrate snacks for 2–5 d | NR | 04:03 |
| Gullo et al. (2014) | IT | 600 30 d | M 56 Caucasian | Disk hernia, hypertension | Losartan; omeprazole; acetylsalicylic acid | Sweating, trembling, dizziness, lost consciousness | Within 3 mo | Prednisone (20 d) + oral sucrose and/or repeated high carbohydrate snacks for 2–5 d | NR | 04:03 |
| Gullo et al. (2014) | IT | 600 70 d | F 70 Caucasian | Depression, hypertension, osteoporosis, autoimmune thyroiditis | Candesartan, omeprazole; L‐thyroxine; calcidiol, hydrochlorothiazide | Trembling, sweating, weakness | Within 3 mo | Prednisone (60 d) + oral sucrose and/or repeated high carbohydrate snacks for 2–5 d | Food supplement (see Gatti et al. (2020) | 04:03 |
| Gullo et al. (2014) | IT | 600 30 d | M 75 Caucasian | Renal disease, hypertension, asthmatic bronchitis, rheumatoid arthritis | Furosemide; candesartan; leflunomide; celecoxib | Lost consciousness | Within 3 mo | Prednisone (60 d) + oral sucrose and/or repeated high carbohydrate snacks for 2–5 d + IV 10% dextrose | Food supplement (see Gatti et al. (2020) | 04:06 |
| Gullo et al. (2014) | IT | 600 60 d | F 77 Caucasian | Hypothyroidism, hypertension, atrial fibrillation, osteoporosis, diabetes | Acenocoumarol, omeprazole; atorvastatin; bisoprolol; lisinopril; doxazosin; L‐thyroxine; | Confusion, lost consciousness | Within 3 mo | Prednisone (100 d) + oral sucrose and/or repeated high carbohydrate snacks for 2–5 d | Food supplement (see Gatti et al. (2020) | 04:03 |
| Michalopoulou Alevras et al. (2015) | ES | 200 15 d | F 55 Caucasian | Allergy to iodinated contrast | No habitual medication | Adrenergic and neuroglycopenic symptoms, both in fasting and postprandial states | Unclear | Fractionated diet and 200 g of iv glucose + IV Prednisone | Food supplement | NR |
| Lio et al. (2016) | IT | 300–600 3 wk | F 68 | Rheumatoid arthritis, hypertensive cardiomyopathy, one episode of atrial fibrillation with proper electrical cardioversion, cholecystectomy | Prednisone at very low doses, hydroxychloroquine, cholecalciferol, NSAIDs, paracetamol; bisoprolol, amlodipine, ASA, pantoprazole | Recurrent episodes of impaired consciousness, diaphoresis and non‐diabetic spontaneous, symptomatic, both fasting and postprandial hypoglycaemia with sweating, tremors, instability | Within 3 mo | IV 10% glucose, prednisone | Food supplement | 04:03 |
| Pavithran et al. (2016) | IN | NR | F 69 | T2DM | NR | NR | 6 wk | Prednisolone, short‐acting insulin before breakfast and lunch | Food supplement | NR |
| Ferreira et al. (2017) | PT | Few wk | F 57 Caucasian (supposedly) | Behcet disease | Tapenthadol, flupirtine | Hypoglycaemic episodes 2.5 hr after breakfast, abdominal discomfort, tremors and blurred vision‐symptoms resolved after sugar ingestion | NR | Hydrocortisone (the patient did not tolerate well prednisone) | Food supplement | NR |
| Bolayir et al. (2018) | TR | NR NR (until 3 wk before hospitalisation) | F 62 | Hypertension and hyperlipidaemia | Indapamide, nebivolol and atorvastatin | Recurrent episodes of sweating, weariness, heart palpitations and anxiety occurring both fasting and postprandial | NR | Diet with low carbohydrate and frequent small meals was planned | Food supplement | NR |
| Cappellani et al. (2018) | IT | 2 wk | F 35 Asian | Endometriosis | Oestroprogestins | Asthenia, blurred vision, aphasia, loss of coordination and partial amnesia | 15 mo | High frequent and low‐caloric meals, rich in simple sugars + flash glucose monitoring system FreeStyle Libre | Food supplement | 04:15 |
| Izzo et al. (2018) | IT | 800 1 mo + 10 d after a suspension of 15 d | F 66 Presumably Caucasian | Negative clinical anamnesis | NR | Repeated episodes of hand tremor and hunger that appeared 3–4h after a meal. Malaise and sweating | NR | Continuous iv 5% dextrose for 10 d | Unclear (therapy for joint pain) | 04:03 |
| Prabhakar and Dass (2018) | IN | 9 d NR | F 59 | Recent bilateral knee pain | NR | Episodic sudden severe fatigue, profuse sweating and palpitations | 8 wk | IV Hydrocortisone initially, then Prednisolone orally, dietary modifications | Food supplement | DRB1*04, DRB1*15, DRB4, DRB5 |
| Veltroni et al. (2018) | IT | 600 2 wk | F 56 Caucasian | Healthy, obese, surgical transsphenoidal removal of a prolactin‐secreting pituitary adenoma 20 y before, carpal tunnel decompression surgery 1 mo before | NR | Blurred vision, diaphoresis and confusion, mainly occurring 2–3 h after meal and resolving with food intake; lost consciousness, asthenia, dizziness | 3 mo | 1 wk infusion with 20% glucose, recommended small frequent meals, avoiding simple sugars and increasing complex carbohydrates. Prednisone 50 mg/d for 6 mo | Food supplement | 04:03 |
| Alagüney et al. (2019) | TR | NR | M 50 | Without pathology | Proton pump inhibitors (esomeprazole and rabeprazole), pregabalin | Fever, sweating and palpitations, symptoms aggravated with hunger and 2–3 h after the meal; food cravings 2 or 3 times a night | NR | NR | Food supplement | NR |
| Moffa et al. (2019) | IT | 600 Until 1 wk before hospitalisation | F 66 Caucasian | No history of major chronic diseases; hypertension | Ramipril | Sweating, hunger, palpitations and tremors that occurred 2 or 3 h after meals and during the night | Unclear, but Prednisone suspended after 4 mo | Prednisone | NR | 04:03 |
| Moffa et al. (2019) | IT | 300 NR | F 82 Caucasian | No history of alcohol abuse or diabetes and no previous exposure to diabetes medications | Bisoprolol, irbesartan, aspirin | Lost consciousness | Unclear, but Prednisone suspended after 9 mo | Prednisone | NR | 04:03 |
| Cambria et al. (2020) | IT | Few wk | F 76 | T2DM | Metformin until the first hypoglycaemic episode | Recurrent hypoglycaemic episodes | Unclear but diazoxide was suspended only 4 months later | diazoxide | Food supplement | 04:15 |
| Okuroglu et al. (2020) | TR | 600 1 mo | F 62 | Hypertension and chronic obstructive lung disease. No previous diagnosis of diabetes | Telmisartan/hydrochlorothiazide and budesonide plus formoterol | Recurrent dizziness, malaise and fatigue | After discharge (hospitalised for 72 hr) she did not experience any hypoglycaemia episodes | Advised to avoid ALA, eat small meals but frequently, eat low‐carbohydrate foods and avoid fasting | NR | 04:03 |
| Patel et al. (2020) from review (NR) | IN | 6 wk | F 50 | NR | NR | NR | NR | Prednisolone | NR | NR |
| Yukina et al. (2020) | RU | NR 1 mo | F 46 | Grade II obesity, dyslipidaemia, hyperuricaemia, cholelithiasis, hiatal hernia, mixed gastritis (superficial and erosive). In 2016, surgical removal of uterus and ovaries for bilateral contained pyosalpinx, ovarian abscess and endomyometritis. In September 2017, surgery for discitis, followed by a 2‐mo antibacterial therapy incl. ciprofloxacin, doxycycline and metronidazole. | NR | Dizziness, sense of fear and ‘creeping’ sensations occurring 2–3 hr after meals | ≈3 mo (from December 2017 to February 2018) | ALA avoidance, no medicinal therapy | Medicinal product | DRB1*03‐DQA1*05:01‐ DQB1*02/DRB1*04‐DQA1*03:01‐DQB1*03:02 |
AR: Argentina; d: day(s); ASA: acetylsalicylic acid; ES: Spain; F: female; hr: hour(s); HLA: human leukocyte antigen; IN: India; IT: Italy; JP: Japan; KR: Korea, Republic of; LK: Sri Lanka; M: male; mo: month(s); NR: not reported; NSAIDs: non‐steroidal anti‐inflammatory drugs; PT: Portugal; RU: Russia Federation; T2DM: type 2 diabetes mellitus; TR: Turkey; wk: week(s); y: year(s).
3.6. Summary of case reports linking alpha‐lipoic acid intake with insulin autoimmune syndrome
From the comprehensive literature search, 49 cases of IAS linked to the consumption of ALA as medicinal product or as food supplement were retrieved that were published as case reports or part of reviews published in English (see Table 1). Case reports only published in non‐European languages were not considered as part of this Opinion (see Section 2.1). In all cases, authors confirmed that circulating IAA were present. However, in the case report series described by Gullo et al. (2014) (n = 6), the assay that was used to determine IAA was not specific for IAA.
Of the 49 cases identified in the literature, 20 occurred in Europe, out of which 19 were presumably Caucasians (one was a woman of Sri Lankan origin). Most Caucasian cases were reported for Italy (n = 13). One case was reported in Spain and one in Portugal, three in Turkey and one in Russia. Outside Europe, the majority of cases were observed in Japan (n = 22), three were reported in South Korea and four in India (supposedly all of South and East Asian origin, i.e. 30 cases in individuals with Asian ethnicity in total (including the woman of Sri Lankan origin diagnosed in Italy)).
There was a predominance of female cases (41 out of 49) reported. Ages ranged from 28 to 82 years.
For 18 of the 49 cases in Table 1, the amount of ALA was reported and for 22 cases the duration of intake was given. The intake varied between 200 and 800 mg/day. The time to onset in the investigated case reports ranged from 1 week to 4 months (7–120 days). There was no obvious association between dose and time to onset. However, for several cases, information on the dose or duration of consumption or both was not available (e.g. 24 cases reporting neither the dose nor the duration of consumption).
Among the cases, one South Korean 67‐year‐old woman underwent two accidental re‐challenges with ALA. In all three instances, in which she had consumed ALA in amounts of 600 mg/day, she developed IAS, which completely resolved before each re‐challenge. Also in other cases, IAS resolved after a few weeks to months when ALA was discontinued (see Table 1).
Out of the 49 cases, data on health status, other possible concomitant medication and HLA class DRB1* genotype were available for 26, 20 and 38 cases, respectively. The type of signs and symptoms occurring were reported for 27 cases. In 12 of those cases, subjects lost consciousness or went into hypoglycaemic coma. Other symptoms reported were mostly sweating, tremors, dizziness, fatigue, weakness, confusion, hunger and palpitations.
Nineteen individuals were identified as carrying the DRB1*04:06 allele, 14 had DRB1*04:03, two DRB1*04:15 and three individuals were identified as subtypes DRB1*04. No cases associated with DRB1*04:07 were found. The predominant allele in the 19 cases of Caucasian origin diagnosed in Europe was DRB1*04:03 (11 cases). The alleles DRB1*04:06 and DRB1*04:15 were found in two cases and one case, respectively. One case was identified as DRB1*04 and in four cases no information was available.
In 44 cases, no concomitant intake of other substances that are potential triggers of IAS, was reported. In four cases, omeprazole was taken and in one case gliclazide. For both substances, only one case report each exists in which the development of IAS was associated with the use of the substance (as reported in the review by Cappellani et al. (2020)). The woman who had consumed gliclazide (Bae et al., 2013) had done this prior to the ingestion of ALA and symptoms of IAS started only after the intake of ALA. In addition, she developed two additional episodes of IAS upon re‐exposure to ALA (see South Korean case described above). Given the wide‐spread use of these substances, the Panel considers it unlikely that gliclazide or omeprazole were involved in the development of IAS in these cases.
The Panel notes that the one case (Bae et al., 2013) in which evidence of a double re‐challenge was available allows to attribute the development of IAS with a high probability to the consumption of ALA. In addition, similar signs and symptoms occurred in the 27 cases for which this information was available. The development of symptoms has always been preceded by the consumption of ALA either as food supplement or medicinal product, in 44 cases without concomitant intake of other substances that have been reported to be a potential trigger of IAS. The reported times to onset of IAS are compatible with the emergence of an autoimmune disease. IAS resolved upon withdrawal of ALA after several weeks to months. These observations are in line with the plausible mechanism that has been put forward by which consumption of ALA could increase the risk of developing IAS (see Section 3.5.4).
The Panel considers that there is an association between the consumption of ALA and an increased risk of development of IAS in individuals with certain polymorphisms in the HLA region (see Section 3.5.3 on genetic determinants).
As conclusions on an association between the consumption of ALA and an increased risk of development of IAS could be drawn from published case reports, the data retrieval from vigilance databases and adverse event reports from clinical trials was not further pursued as this would have not changed the conclusions of the Panel derived from the case reports. The same applies to sales data and data from food business operators’ post‐marketing surveillance systems.
3.7. Dose below which insulin autoimmune syndrome is not expected to occur
The lowest ALA intake that was associated with the development of IAS in the case reports described in Section 3.6 was reported to be 200 mg/day. However, no data are available that would allow a judgement to be made on whether IAS also occurs at lower doses. The Panel notes that generally the susceptibility of individuals to triggers of autoimmune diseases varies. It is therefore likely that this is also the case for IAS.
With respect to the NOAEL (0.6 mg/kg body weight per day) proposed by DTU, the Panel notes that this was based on toxicological endpoints unrelated to IAS and therefore does not necessarily protect from the development of IAS. In addition, the Panel notes that standard toxicity tests are not suitable for determining a threshold below which an autoimmune disease is unlikely to occur (see Section 2.2).
The Panel considers that, based on the data available, a dose below which IAS is not expected to occur cannot be derived, neither for the general population nor for vulnerable sub‐groups thereof.
4. Conclusions
The Panel concludes that the consumption of ALA added to foods, including food supplements, is likely to lead to an increased risk of development of IAS in individuals with certain genetic polymorphisms. The plausible mechanism by which ALA may increase this risk has not yet been fully elucidated. These conclusions are based on the review of 49 case reports in which IAS developed following ALA consumption. In the reviewed cases, IAS resolved a few weeks to months after ALA was discontinued.
The Panel has not identified any publication linking the intake of ALA naturally occurring in foods to IAS.
The prevalence of the three main HLA alleles associated with an increased risk of development of IAS has been reported to be around 0.1–3.9% in Europe, depending on the alleles and the country (Uchigata et al., 2000; Gonzalez‐Galarza Faviel et al., 2019). The Panel notes that the individuals carrying the relevant polymorphisms cannot be readily identified without genetic testing.
The incidence of IAS (of all causes) for the years 2017–2018 in the general population in Japan has been estimated to be 0.017 per 100,000 inhabitants (i.e. a total of 22 cases in these years) (Yamada et al., 2020). The incidence in Europe is likely to be lower, considering that the prevalence of alleles associated with an increased risk of development of IAS is less frequent in populations with Caucasian than Asian ethnicity. However, based on the limited data available, the Panel concludes that the incidence of IAS in Europe and the risk associated with the development of IAS following consumption of ALA cannot be quantified either for the general population or for sub‐groups or individuals with genetic susceptibility.
The Panel also concludes that an ALA dose below which IAS is not expected to occur is likely to vary between individuals and can therefore not be determined based on the data that are available.
5. Uncertainties
The following sources of uncertainties have been detected:
There is a lack of information on whether the industrial production of ALA that is used in food supplements differs from the one of ALA used in medicinal products, i.e. whether ALA for foods and ALA for pharmaceutical production are interchangeable;
Publications in non‐EU languages have not been considered;
It is likely that not all cases of IAS following ALA consumption in Europe have been published;
There is likely a possible underestimation of the occurrence of IAS that is linked to a possible unawareness of the disease and its subsequent underdiagnosis and underreporting.
A plausible mechanism by which ALA may trigger IAS in individuals with certain genetic polymorphisms has been suggested, but it still needs to be demonstrated.
Even though data on allele frequency is available for a number of European countries, data covering a sufficiently large representative sample of the whole EU population is missing.
The Panel acknowledges these uncertainties, but they do not diminish the scientific conclusions.
6. Recommendations
In order to reduce the uncertainties, the Panel has identified the following recommendations for research:
Elucidation of the mechanism of action by which ALA increases the risk of developing IAS;
Investigation of the incidence of IAS in the EU also in conjunction with the use of ALA;
Investigation of the prevalence of HLA alleles associated with an increased risk of development of IAS in a representative sample of the EU population.
7. Documentation as provided to EFSA
The following documentation was provided together with the mandate from the European Commission:
Abbreviations
- AFSSA
French Food Safety Agency
- ALA
alpha‐lipoic acid
- ANSES
French Agency for Food, Environmental and Occupational Health and Safety
- ASA
acetyl salicylic acid
- CAS
Chemical Abstracts Service
- Cmax
maximum plasma concentrations
- DTU
Danish National Food Institute
- EINECS
European Inventory of Existing Chemical Substances
- EKE
expert knowledge elicitation
- EMA
European Medicines Agency
- FDA
US Food and Drug Administration
- FSANZ
Food Safety Australia New Zealand
- HLA
human leukocyte antigen
- HPLC
high‐performance liquid chromatography
- IAA
insulin autoantibodies
- IAS
insulin autoimmune syndrome
- IgA
immunoglobulin A
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- IUPAC
International Union of Pure and Applied Chemistry
- NDA
EFSA Panel on Nutrition, Novel Foods and Food Allergens
- NOAEL
no observed adverse effect level
- Ph. Eur.
European Pharmacopeia
- PRAC
Pharmacovigilance Risk Assessment Committee
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
- SD
standard deviation
- Tmax
mean time to maximal plasma concentrations
- UV
ultraviolet
Appendix A – PRISMA10 flow charts for the four literature searches
1.
PRISMA flow chart search 1
Aimed at identifying case reports that link the development of IAS to the intake of ALA

PRISMA flow chart search 2
Aimed at identifying reviews on IAS
PRISMA flow chart search 3
Aimed at identifying pharmacokinetic studies and safety studies in humans
PRISMA flow chart search 4
Aimed at identifying studies on pharmacokinetics of dihydrolipoic acid in humans
Appendix B – Protocol for the assessment of the relationship between intake of alpha‐lipoic acid (thioctic acid) and the risk of insulin autoimmune syndrome
B.1. Problem formulation and planned approach towards the evidence retrieval from the scientific literature
The following protocol has been developed in line with existing methodology (EFSA, 2020).
B.1.1. Assessment questions and sub‐questions
In order to answer the Terms of Reference (ToR) as interpreted in Section 1.3, the Panel considers that the following questions and subquestions need to be answered.
-
1
Identity of the ALA
-
o
What are the chemical structure, the stereochemistry, the nomenclature (e.g. IUPAC chemical name, CAS number, EINECS number)?
-
o
What are the synonyms that are used to refer to the same substance?
-
o
Analogues, derivatives, metabolites that are on the market?
-
o
Is the substance occurring also naturally in food or is it only produced synthetically?
-
o
How is ALA that is used in food supplements produced (e.g. source material, process)?
-
o
Is there any difference between ALA used for food supplements and the one used for medicines (structure, purity, production, source etc.)?
-
o
How stable is ALA throughout shelf‐life, are there any degradation products that are produced that need to be considered?
-
o
Are there impurities or contaminants in industrial ALA? If yes, are they related to the mode of production? Is there data in the literature pertaining on contamination or adulteration of ALA?
-
o
-
2
How is ALA absorbed, distributed, metabolised, excreted?
-
o
Is ALA absorbed as such or are there any modifications in the gastro‐intestinal tract before absorption?
-
o
What is its bioavailability? Are there bioavailability variations between individuals?
-
o
What are the mechanisms regulating ALA absorption and distribution: transporters or passive absorption, interaction with other substances/nutrients, circulating forms? What are the metabolites of ALA?
-
o
Which metabolic pathways are involved?
-
o
Are there any interindividual differences in metabolism (that could lead to a higher susceptibility of certain individuals than others)?
-
o
How long is the half‐life of ALA and its metabolites in blood circulation?
-
o
Can ALA be accumulated in any form in the organism?
-
o
How are ALA and its metabolites excreted?
-
o
-
3
What is IAS?
-
o
What are the prevalence and incidence of the disease?
-
o
Which is the aetiology of the disease (are there any other causes than ALA that would need to be considered when evaluating the data on the link between ALA and the disease)?
-
o
Which polymorphisms have been associated with a higher risk of developing the disease and what are the reasons?
-
o
Are there any vulnerable sub‐populations that can be readily defined? Are there subpopulations with certain recognisable characteristics (with no need for genetic testing) that have a higher susceptibility for the disease? Are children, elderly, lactating or pregnant women at a higher/lower risk?
-
o
What is the definition and pathophysiology of the disease?
-
o
What are symptoms of the disease and its differential diagnosis?
-
o
-
4
What is the association between ALA and IAS in humans?
-
o
Which previous assessments from official scientific bodies are available and what are the conclusions?
-
o
Which are documented case reports in humans available in the literature?
-
o
Are there any additional reports in European Nutravigilance databases of European Member States or the EMA Eudravigilance database, not yet published in the literature?
-
o
IAS reported in clinical trials.
-
o
How strong is the association (how reliable was the ascertainment of the disease, how reliable was the exposure assessment, are there data on challenge‐re-challenge)?
-
o
Has a causal relationship between ALA and IAS been established?
-
o
What are the mechanisms of action involved in the detrimental effects of ALA?
-
o
-
5
What is the minimum eliciting dose?
-
o
At which doses of ALA and exposure durations was IAS observed in well documented cases?
-
o
Is there any health‐based guidance value that could be set from the data?
-
o
B.1.2. Formulation of the sub‐questions
For each of the questions above, the objectives of the sub‐questions are as follows:
-
1
Identity of the ALA
-
o
Objective 1: Map the main characteristics of ALA (chemical structure, enantiomer, natural or synthetic occurrence)
-
o
Objective 2: Identify synonyms for ALA
-
o
Objective 3: Identify the production process of ALA for food supplements
-
o
Objective 4: Compare the production/source of ALA for medical or food purpose
-
o
Objective 5: Identify the shelf‐life of ALA and whether degradation products could have effects
-
o
-
2
How is ALA absorbed, distributed, metabolised, excreted?
-
o
Objective 1: Identify the mechanisms of absorption, distribution, metabolisation, storage and excretion of ALA
-
o
-
3
What is IAS?
-
o
Objective 1: Map the main characteristics of IAS (symptoms, pathophysiology, causes of the disease)
-
o
Objective 2: Identify genetical predisposition to developing IAS
-
o
Objective 3: Identify vulnerable sub‐populations
-
o
Objective 4: Prevalence and incidence of the disease
-
o
-
3
What is the association between ALA and IAS in humans?
-
o
Objective 1: Identify the link between the intake of ALA and IAS.
-
o
-
4
What is the minimum eliciting dose?
-
o
Objective 1: based on the literature mentioned before, identify, if possible, a no observed adverse effect level (NOAEL) of ALA intentionally added to foods regarding IAS for the general population and vulnerable sub‐groups.
-
o
B.1.3. Definition of the search strategy and eligibility criteria for study selection (i.e. evidence needs)
Questions 1 and 2 (Section B.1.1) will be answered following a narrative approach (i.e. no comprehensive search, no data extraction forms), while questions 3, 4 and 5 will be answered based on a comprehensive literature search, further described afterwards, and considering also other types of data (e.g. reports from relevant scientific institutions and authorities, such as FDA, Health Canada, Australia or New Zealand authorities). Information for questions 1 and 2 will be retrieved from relevant websites, books and publications.
For question 4 (What is the association between ALA and IAS in humans?), three types of data are expected:
-
o
Published trials that would report IAS as an adverse effect: PICO framework11: (p) general population (i) consuming ALA or other drugs/substances vs. (c) placebo or another comparator (o) developing IAS.
-
o
Published case reports of IAS that would report the occurrence of IAS in a limited number of individuals and the investigation to identify the cause (e.g. previous exposure to ALA or another drug)
PO framework12: (p) the individual(s) that is/are described in the case reports, (o) that developed IAS.
-
o
Other types of data (e.g. relevant institutions and authorities, such as FDA, Health Canada, Australia or New Zealand authorities)
In order to answer the questions 3, 4 and 5 identified above (section B.1.1 of the protocol), the following dedicated comprehensive literature searches will be conducted by EFSA's information specialist, in Embase and PubMed, with no limitation on the date of publication and limiting the search to EU languages. The search strings that will be used can be found in Appendix C.
The following inclusion and exclusion criteria will be applied:
-
–
Search 1 related to Q4 (What is the association between ALA and IAS in humans?): Papers (intervention or observational studies) on oral consumption of ALA in humans and IAS
-
Inclusion:
-
▪
Trials (also single arm) on oral consumption of ALA as food, supplement or medicinal product, also in combination with other substances, in humans that report on IAS
-
▪
Observational studies, in particular case reports, on the occurrence of IAS in a limited number of individuals (that may report on the investigation to identify the cause, possibly ALA, only in the full text)
-
▪
Abstracts of conferences on potential case reports
-
▪
Statements/opinions from competent authorities e.g. US FDA, Health Canada, etc.
-
▪
All population independently of age, healthy/disease status, ethnicity, etc.
-
▪
ALA in different forms i.e. tablets, capsules, etc. (even with the same dose)
-
▪
-
Exclusion:
-
▪
Protocols
-
▪
Studies on animals or cells as no animal or in vitro model of IAS was identified
-
▪
Reviews/meta-analyses
-
▪
Studies not on ALA and/or not on IAS
-
▪
Studies not on oral consumption (e.g. injection of ALA)
-
▪
-
-
–
Search 2 related to Q3 (What is IAS?): Reviews (systematic or narrative) on IAS
-
Inclusion: review (narrative or systematic including also meta‐analysis) on IAS (whatever aspects of this disease) or autoimmune reaction related to insulin or hypoglycaemia or autoimmune polyendocrine syndrome.
-
▪
NB: The purpose of this question is to identify plausible mechanisms (in humans, and possibly also from data on animal or cell cultures), if reported in the reviews. This will be discussed in a dedicated section of the opinion.
-
▪
-
Exclusion:
-
▪
All types of intervention or observational studies
-
▪
Protocols, abstracts of conference/congress/symposium
-
▪
Reviews not on IAS or autoimmune reaction related to insulin or hypoglycaemia or autoimmune polyendocrine syndrome
-
▪
-
-
–
Search 3: Clinical trials on oral consumption of ALA
Trials on oral consumption of ALA, alone or in combination, as food, supplement or medicinal product that report on side effects.
-
Exclusion:
-
▪
Protocols, abstracts of conference/congress/symposium
-
▪
Reviews/meta‐analyses
-
▪
Studies not on ALA
-
▪
Studies not on oral consumption
-
▪
Regarding the grey literature, additional searches will be performed on the websites of relevant institutions and authorities, such as FDA, Health Canada, Australia or New Zealand authorities. Websites will be browsed or searched to retrieve published reports on the relationship of ALA and IAS. Methods to retrieve the information will be reported. Vigilance databases will also be searched to retrieve case reports not published elsewhere.
B.1.4. Definition of the methods for selecting studies for inclusion/exclusion
For questions 3, 4 and 5 of section B.1.1, the screening will be done in duplicate by EFSA staff members using DistillerSR (Evidence Partners, Ottawa, Canada), at the level of the title and abstract and then at the level of the full text. Conflicts that might arise will be discussed first amongst the two reviewers of that study and if the conflict is not solved all reviewers will be involved. The references will then be exported to EndNote®.
B.1.5. Definition of the methods for extracting data from studies included from the systematic literature search
Data will be extracted from case reports and clinical trials. Extraction will be done in Microsoft Excel®, where one reviewer will do the extraction, and another will validate it.
The items extracted will cover, e.g. RefID from DistillerSR® (Evidence Partners, Ottawa, Canada), extraction date, author name, year, location, ALA dose, ALA as medicinal product or food supplement, subject characteristics, duration of ALA consumption.
B.1.6. Definition of the methods for appraising evidence
No appraisal is foreseen.
B.1.7. Preliminary identification of the sources of uncertainty and definition of the methods for prioritising them
For all questions identified in Section B.1.1, limited answers may be the consequence of lack of data. Uncertainty analysis of the scientific assessment, i.e. identifying possible limitations in scientific knowledge and assessing their implications for scientific conclusions, will be discussed briefly, based on the EFSA guidance document on uncertainty (EFSA Scientific Committee, 2018). This implies in particular identifying the sources of uncertainty affecting the assessment, prioritising these sources based on their expected influence on the outcome/results and final overall discussions and planning how the uncertainty analysis will be handled. It is expected that the following identified sources of uncertainties will be discussed qualitatively (descriptive method) and no quantitative analysis will be undertaken.
Uncertainties that might be encountered for question 1: Identity of ALA?, question 2: How is ALA absorbed, distributed, metabolised and excreted? and question 3: what is IAS? (identified in Section B.1.1):
Lack of published data
Uncertainties that might be encountered for question 4: What is the association between ALA and IAS in humans? and question 5: What is the minimum eliciting dose? (identified in Section B.1.1):
-
o
Exposure/compliance of subjects (in case of trials), i.e.
uncertainty of dose inducing IAS leading to possible over/underestimation.
-
o
Insufficient reporting on the assessment/diagnosis of the adverse effects by investigators
-
▪
Potential misclassification of adverse effects
-
▪
-
o
Limited information on genetical susceptibility and impossibility to identify populations at risk.
-
o
Publication bias
-
▪
Case reports may not all be published
-
▪
Adverse effects not adequately/extensively reported in trials
-
▪
-
o
Precision of the measurements made (if any)
-
o
Power (for trials)
-
o
Heterogeneity of the dataset
-
o
Language of the SR
-
▪
Case reports published in non‐EU languages will not be considered.
-
o
Appraisal
-
▪
Due to time constrains it might not be feasible to conduct one.
-
o
Representativity
-
▪
Relevance for the EU population will be assessed by expert judgement, as the case reports are expected to be from a limited number of countries.
-
▪
B.1.8. Definition of the methods for synthesising evidence within the sub‐question
Question 4 (section B.1.1). What is the association between ALA and IAS in humans?
It will depend on the evidence that will be obtained. Possibly qualitative.
Question 5 (section B.1.1). What is the minimum eliciting dose?
It will depend on the evidence that will be obtained. Possibly semi‐quantitative. Expert knowledge elicitation (EKE) might be needed, taking into account the relevant EFSA guidance (EFSA, 2014).
B.1.9. Definition of the methods for analysing uncertainties individually and combined
Uncertainties will be identified at each step of the assessment, but no formal uncertainty assessment is foreseen.
Appendix C – Search strings for the three initially foreseen literature searches
1.
Search 1
Embase
Date of the search 4‐9‐2020
| Set | Query | Results |
|---|---|---|
| #8 | #5 AND #6 AND ([basque]/lim OR [bulgarian]/lim OR [catalan]/lim OR [croatian]/lim OR [czech]/lim OR [danish]/lim OR [dutch]/lim OR [english]/lim OR [estonian]/lim OR [finnish]/lim OR [french]/lim OR [german]/lim OR [greek]/lim OR [hungarian]/lim OR [irish gaelic]/lim OR [italian]/lim OR [latvian]/lim OR [lithuanian]/lim OR [macedonian]/lim OR [norwegian]/lim OR [polish]/lim OR [polyglot]/lim OR [portuguese]/lim OR [romanian]/lim OR [scottish gaelic]/lim OR [serbian]/lim OR [slovak]/lim OR [slovenian]/lim OR [spanish]/lim OR [swedish]/lim) | 154 |
| #7 | #5 AND #6 | 160 |
| #6 | ‘hirata disease’/exp OR ‘hypoglycemia’/exp OR ‘insulin autoimmune syndrome’/exp OR aih:ti,ab,kw OR (‘insulin’/exp AND (‘immunopathology’/de OR ‘immune system’/de OR ‘autoimmune disease’/de)) OR ((hirata* NEAR/3 disease*):ti,ab,kw) OR hypoglycaemia:ti,ab,kw OR hypoglycaemic:ti,ab,kw OR hypoglycemia:ti,ab,kw OR hypoglycemic:ti,ab,kw OR ias:ti,ab,kw OR ((insulin NEAR/5 autoimmune):ti,ab,kw) | 134,940 |
| #5 | #3 OR #4 | 10,181 |
| #4 | alipure:ti,ab,kw OR ‘alpha‐lipogamma’:ti,ab,kw OR ‘alphalipogamma’:ti,ab,kw OR ‘alpha‐lipon’:ti,ab,kw OR alphalipon:ti,ab,kw OR ‘alpha liponaure’:ti,ab,kw OR alphaliponaure:ti,ab,kw OR alphaliponsaure:ti,ab,kw OR ‘alpha liponsaure’:ti,ab,kw OR ‘alpha lippon’:ti,ab,kw OR alphalippon:ti,ab,kw OR ‘alpha vibolex’:ti,ab,kw OR alphavibolex:ti,ab,kw OR azulipont:ti,ab,kw OR berlition:ti,ab,kw OR berlithione:ti,ab,kw OR biletan:ti,ab,kw OR ‘biomo lipon’:ti,ab,kw OR biomolipon:ti,ab,kw OR ‘byodinoral r’:ti,ab,kw OR ‘coenzyme compositum’:ti,ab,kw OR ‘discus compositum’:ti,ab,kw OR duralipon:ti,ab,kw OR ‘espa lipon’:ti,ab,kw OR espalipon:ti,ab,kw OR fenint:ti,ab,kw OR ‘hepar compositium’:ti,ab,kw OR heparlipon:ti,ab,kw OR juthiac:ti,ab,kw OR ‘ledum compositum’:ti,ab,kw OR ‘lycopodium compositum’:ti,ab,kw OR liposan:ti,ab,kw OR ‘liponsaure‐ratiopharm’:ti,ab,kw OR liponsaureratiopharm:ti,ab,kw OR lipothion:ti,ab,kw OR mtwalphaliponsaure:ti,ab,kw OR neurium:ti,ab,kw OR octolipen:ti,ab,kw OR oktolipen:ti,ab,kw OR ‘pleomix alpha’:ti,ab,kw OR ‘quamtrax ala’:ti,ab,kw OR thioctacid:ti,ab,kw OR thioctacide:ti,ab,kw OR thioctan:ti,ab,kw OR thioctsan:ti,ab,kw OR thiogamma:ti,ab,kw OR thiotacid:ti,ab,kw OR tromlipon:ti,ab,kw OR tioctacid:ti,ab,kw OR tioctan:ti,ab,kw OR tioctidasi:ti,ab,kw OR ‘ubichinon compositum’:ti,ab,kw OR ‘verla lipon’:ti,ab,kw OR verlalipon:ti,ab,kw OR zeel:ti,ab | 141 |
| #3 | #1 OR #2 | 10,122 |
| #2 | ((‘1 2 dithiolan 3 pentanic’ OR 12dithiolan3pentanic OR ‘1 2 dithiolane 3 pentanoic’ OR ‘12dithiolane3pentanoic’ OR ‘1 2 dithiolane 3 valeric’ OR ‘12dithiolane3valeric’ OR ‘5 dithiolan 3 yl valeric’ OR ‘5dithiolan3ylvaleric’ OR ‘5 1 2 dithiolan 3 yl valeric’ OR 512ditiolan3ylvaleric OR 68thioctic OR ‘6 8thioctic acid’ OR αlipoic OR alipoic OR lipoic OR liponic OR thioctic OR tioctic) NEAR/3 (acid OR acids)):ti,ab,kw | 6,833 |
| #1 | ‘thioctic acid’/exp OR ‘1077 28 7’:ti,ab,kw OR 1077287:ti,ab,kw | 9,259 |
PubMed
Date of the search: 04‐09‐2020
| Search | Query | Results |
|---|---|---|
| #6 | Search: #5 AND (“bulgarian”[Language] OR “catalan”[Language] OR “croatian”[Language] OR “czech”[Language] OR “danish”[Language] OR “dutch”[Language] OR “english”[Language] OR “estonian”[Language] OR “finnish”[Language] OR “french”[Language] OR “german”[Language] OR “greek modern”[Language] OR “hungarian”[Language] OR “italian”[Language] OR “latvian”[Language] OR “lithuanian”[Language] OR “multiple languages”[Language] OR “norwegian”[Language] OR “polish”[Language] OR “portuguese”[Language] OR “romanian”[Language] OR “scottish gaelic”[Language] OR “serbian”[Language] OR “slovak”[Language] OR “slovenian”[Language] OR “spanish”[Language] OR “swedish”[Language] OR “undetermined”[Language] OR “welsh”[Language]) Sort by: Most Recent | 54 |
| #5 | Search: #3 AND #4 | 59 |
| #4 | Search: (“Insulin”[Mesh] AND (“Immune System Diseases”[Mesh:noexp] OR “Immune System”[Mesh:noexp] OR “Autoimmune Diseases”[Mesh:noexp])) OR “Hypoglycemia”[Mesh] OR aih[tiab] OR (hirata*[tiab] AND disease*[tiab]) OR Hypoglycaemia[tiab] OR Hypoglycaemic[tiab] OR Hypoglycemia[tiab] OR Hypoglycemic[tiab] OR ias[tiab] OR (Insulin[tiab] AND autoimmune[tiab]) | 76,375 |
| #3 | Search: #1 OR #2 Sort by: Most Recent | 6,051 |
| #2 | Search: “Thioctic Acid”[Mesh] OR “1077‐28‐7”[tiab] OR 1077287[tiab] OR “1,2‐Dithiolan‐3‐pentanic acid”[tiab] OR “1,2‐Dithiolan‐3‐pentanic acids”[tiab] OR “1 2 dithiolane 3 pentanoic acid”[tiab] OR “12dithiolane3pentanoic acid”[tiab] OR “1 2 dithiolane 3 valeric acid”[tiab] OR ‘“2dithiolane3valeric acid”[tiab] OR “5 dithiolan 3 yl valeric acid”[tiab] OR “5dithiolan3ylvaleric acid”[tiab] OR “5 1 2 dithiolan 3 yl valeric acid”[tiab] OR “512ditiolan3ylvaleric acid”[tiab] OR “1 2 dithiolane 3 pentanoic acids”[tiab] OR “12dithiolane3pentanoic acids”[tiab] OR “1 2 dithiolane 3 valeric acids”[tiab] OR ‘“2dithiolane3valeric acids”[tiab] OR “5 dithiolan 3 yl valeric acids”[tiab] OR “5dithiolan3ylvaleric acids”[tiab] OR “5 1 2 dithiolan 3 yl valeric acids”[tiab] OR “512ditiolan3ylvaleric acids”[tiab] OR “68thioctic acid”[tiab] OR “68thioctic acids”[tiab] OR “6 8thioctic acid”[tiab] OR “6 8thioctic acids”[tiab] OR “αlipoic acid”[tiab] OR “αlipoic acids”[tiab] OR “lipoic acid”[tiab] OR “lipoic acids”[tiab] OR “liponic acid”[tiab] OR “liponic acids”[tiab] OR “thioctic acid”[tiab] OR “thioctic acids”[tiab] OR “tioctic acid”[tiab] OR “tioctic acids”[tiab] | 6.014 |
| #1 | Search: Alipure[tiab] OR “Alpha‐Lipogamma”[tiab] OR “AlphaLipogamma”[tiab] OR “Alpha‐Lipon”[tiab] OR AlphaLipon[tiab] OR “alpha Liponaure”[tiab] OR alphaLiponaure[tiab] OR AlphaLiponsaure[tiab] OR “Alpha‐Liponsaure”[tiab] OR “Alpha Lippon”[tiab] OR AlphaLippon[tiab] OR “Alpha Vibolex”[tiab] OR alphaVibolex[tiab] OR Azulipont[tiab] OR Berlithion[tiab] OR Berlithione[tiab] OR Biletan[tiab] OR “Biomo lipon”[tiab] OR Biomolipon[tiab] OR “Byodinoral R”[tiab] OR “coenzyme compositum”[tiab] OR “discus compositum”[tiab] OR Duralipon[tiab] OR “Espa‐lipon”[tiab] OR Espalipon[tiab] OR Fenint[tiab] OR “Hepar compositum”[tiab] OR Heparlipon[tiab] OR Juthiac[tiab] OR “Lycopodium compositum”[tiab] OR “Ledum compositum”[tiab] OR Liposan[tiab] OR “Liponsaure‐ratiopharm”[tiab] OR Liponsaureratiopharm[tiab] OR Lipothion [tiab] OR MTWAlphaliponsaure[tiab] OR Neurium[tiab] OR Octolipen[tiab] OR Oktolipen[tiab] OR “Pleomix Alpha”[tiab] OR “Quamtrax ALA”[tiab] OR Thioctacid[tiab] OR Thioctacide[tiab] OR Thioctan[tiab] OR Thioctsan[tiab] OR Thiogamma[tiab] OR Thiotacid[tiab] OR Tromlipon[tiab] OR Tioctacid[tiab] OR Tioctidasi[tiab] OR Tioctan[tiab] OR Tioctidasi[tiab] OR “Ubichinon compositum”[tiab] OR “Verla Lipon”[tiab] OR VerlaLipon[tiab] OR Zeel[tiab] | 78 |
Search 2
Embase
Date of the search: 4‐9‐2020
| Set | Query | Results |
|---|---|---|
| #6 | #5 AND #4 | 123 |
| #5 | ‘meta analysis’/exp OR ’meta analysis (topic)’/exp OR ’review’/exp OR 'systematic review (topic)’/exp OR ’biomedical technology assessment’/exp OR (((systematic* OR methodologic* OR quantitative OR research OR integrative OR collaborative) NEAR/3 overview*):ti,ab) OR review*:ti,ab,kw OR ((pool* NEAR/3 analy*):ti,ab) | 36,427,795 |
| #4 | #3 AND ([basque]/lim OR [bulgarian]/lim OR [catalan]/lim OR [croatian]/lim OR [czech]/lim OR [danish]/lim OR [dutch]/lim OR [english]/lim OR [estonian]/lim OR [finnish]/lim OR [french]/lim OR [german]/lim OR [hungarian]/lim OR [norwegian]/lim OR [polish]/lim OR [polyglot]/lim OR [portuguese]/lim OR [romanian]/lim OR [scottish gaelic]/lim OR [serbian]/lim OR [slovak]/lim OR [slovenian]/lim OR [spanish]/lim OR [swedish]/lim) | 699 |
| #3 | #1 OR #2 | 775 |
| #2 | ((autoimmune NEAR/3 (hypoglycaemia OR hypoglycemia)):ti,ab,kw) OR (((‘insulin autoimmune’ OR ‘autoimmune insulin’) NEAR/3 (syndrom* OR disease*)):ti,ab,kw) OR ((endogenous NEAR/3 hyperinsulinemic NEAR/3 (hypoglycaemia OR hypoglycemia)):ti,ab,kw) OR ((hirata* NEAR/3 disease*):ti,ab,kw) OR ((insulin NEAR/3 autoimmune NEAR/3 (hypoglycaemia OR hypoglycemia)):ti,ab,kw) OR ‘insulin autoimmunity’:ti,ab,kw OR ((spontaneous NEAR/3 (hypoglycaemia OR hypoglycemia) NEAR/3 (attack* OR insulin)):ti,ab,kw) OR ((insulinNEAR/3 (‘autoimmune disease’ OR ’autoimmune diseases’ OR ’autoimmune syndrome’ OR ’autoimmune syndromes’)):ti,ab,kw) | 761 |
| #1 | ‘hirata disease’/exp OR ‘insulin autoimmune syndrome’/exp | 107 |
PubMed
Date of the search: 4‐9‐2020
| Search | Query | Results |
|---|---|---|
| #5 | Search: #4 AND #3 | 187 |
| #4 | Search: “review”[sb] OR “systematic”[sb] OR “meta‐analysis”[pt] OR “meta‐analysis as topic”[Mesh] OR “meta‐analysis”[Mesh] OR meta analy*[tw] OR metanaly*[tw] OR metaanaly*[tw] OR met analy*[tw] OR integrative research[tiab] OR review*[tiab] OR integrative overview*[tiab] OR research integration*[tiab] OR research overview*[tiab] OR comparative efficacy[tiab] OR comparative effectiveness[tiab] OR outcomes research[tiab] OR indirect comparison*[tiab] OR Embase*[tiab] OR Cinahl*[tiab] OR systematic overview*[tiab] OR methodological overview*[tiab] OR methodologic overview*[tiab] OR methodological review*[tiab] OR methodologic review*[tiab] OR quantitative review*[tiab] OR quantitative overview*[tiab] OR quantitative synthes*[tiab] OR pooled analy*[tiab] OR Cochrane[tiab] OR Medline[tiab] OR Pubmed[tiab] OR Medlars[tiab] OR handsearch*[tiab] OR hand search*[tiab] OR meta‐regression*[tiab] OR metaregression*[tiab] OR data synthes*[tiab] OR data extraction[tiab] OR data abstraction*[tiab] OR mantel haenszel[tiab] OR peto[tiab] OR der‐simonian[tiab] OR dersimonian[tiab] OR fixed effect*[tiab] OR “Cochrane Database Syst Rev”[Journal:__jrid21711] | 3,795,869 |
| #3 | Search: #1 AND #2 Sort by: Most Recent | 936 |
| #2 | Search: (“bulgarian”[Language] OR “catalan”[Language] OR “croatian”[Language] OR “czech”[Language] OR “danish”[Language] OR “dutch”[Language] OR “english”[Language] OR “estonian”[Language] OR “finnish”[Language] OR “french”[Language] OR “german”[Language] OR “greek modern”[Language] OR “hungarian”[Language] OR “italian”[Language] OR “latvian”[Language] OR “lithuanian”[Language] OR “multiple languages”[Language] OR “norwegian”[Language] OR “polish”[Language] OR “portuguese”[Language] OR “romanian”[Language] OR “scottish gaelic”[Language] OR “serbian”[Language] OR “slovak”[Language] OR “slovenian”[Language] OR “spanish”[Language] OR “swedish”[Language] OR “undetermined”[Language] OR “welsh”[Language]) Sort by: Most Recent | 29,780,296 |
| #1 | Search: (autoimmune hypoglycaemia[tiab] OR autoimmune hypoglycaemia[tiab] OR ((“insulin autoimmune”[tiab] OR “autoimmune insulin”[tiab]) AND syndrom*[tiab])OR “insulin autoimmune disease”[tiab] OR “insulin autoimmune diseases”[tiab] OR (endogenous[tiab] AND hyperinsulinemic[tiab] AND (hypoglycaemia[tiab] OR hypoglycemia[tiab])) OR (hirata*[tiab] AND disease*[tiab]) OR (insulin[tiab] AND autoimmune[tiab] AND (hypoglycaemia[tiab] OR hypoglycemia[tiab])) OR “insulin autoimmunity”[tiab] OR (spontaneous[tiab] AND (hypoglycaemia[tiab] OR hypoglycemia[tiab]) AND (attack*[tiab] OR insulin[tiab])) OR (insulin[tiab] AND (“autoimmune syndrome”[tiab] OR “autoimmune syndromes”[tiab])) OR (“insulin autoimmune disease”[tiab] OR “insulin autoimmune diseases”[tiab])) | 981 |
Search 3
Embase
Date of the search: 4‐9‐2020
| Set | Query | Results |
|---|---|---|
| #17 | #16 NOT #17 AND [english]/lim | 2,203 |
| #18 | #16 NOT #17 | 2,400 |
| #17 | (rat:ti OR rats:ti OR mouse:ti OR mice:ti OR swine:ti OR porcine:ti OR murine:ti OR sheep:ti OR lambs:ti OR pigs:ti OR piglets:ti OR rabbit:ti OR rabbits:ti OR cat:ti OR cats:ti OR dog:ti OR dogs:ti OR cattle:ti OR bovine:ti OR monkey:ti OR monkeys:ti OR trout:ti OR marmoset*:ti) AND ’animal experiment’/de | 1,077,288 |
| #16 | #14 NOT #15 | 2,405 |
| #15 | ‘animal experiment’/de NOT (‘human experiment’/de OR ‘human’/de) | 2,272,297 |
| #14 | #5 AND #13 | 3,014 |
| #13 | #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 | 5,520,026 |
| #12 | ((data NEAR/1 (synthes* OR extraction* OR abstraction*)):ti,ab) OR handsearch*:ti,ab OR ‘hand search’:ti,ab OR ’hand searches’:ti,ab OR ’hand searching’:ti,ab OR ‘mantel haenszel’:ti,ab OR peto:ti,ab OR ‘der simonian’:ti,ab OR dersimonian:ti,ab OR ‘fixed effect’:ti,ab OR ‘fixed effects’:ti,ab OR ‘latin square’:ti,ab OR ‘latin squares’:ti,ab OR ‘meta analysis’:ti,ab OR ‘meta analyses’:ti,ab OR ‘met analysis’:ti,ab OR ‘met analyses’:ti,ab OR metaanaly*:ti,ab OR metanaly*:ti,ab OR ‘meta regression’:ti,ab OR ‘meta regressions’:ti,ab OR metaregression*:ti,ab OR medline:ti,ab OR cochrane:ti,ab OR pubmed:ti,ab OR medlars:ti,ab OR embase:ti,ab OR cinahl:ti,ab OR cochrane:jt OR ‘evidence report’:jt OR ((comparative NEAR/3 (efficacy OR effectiveness)):ti,ab) OR ‘outcomes research’:ti,ab OR ‘relative effectiveness’:ti,ab OR (((indirect OR ‘indirect treatment’ OR ‘mixed treatment’) NEAR/3 comparison):ti,ab) | 475,049 |
| #11 | ((systematic* NEAR/3 (review* OR overview*)):ti,ab) OR ((methodologic* NEAR/3 (review* OR overview*)):ti,ab) OR ((quantitative NEAR/3 (review* OR overview* OR synthes*)):ti,ab) OR ((research NEAR/3 (integrati* OR overview*)):ti,ab) OR ((integrative NEAR/3 (review* OR overview*)):ti,ab) OR ((collaborative NEAR/3 (review* OR overview*)):ti,ab) OR ((pool* NEAR/3 analy*):ti,ab) | 283,769 |
| #10 | ‘meta analysis’/exp OR ’meta analysis (topic)’/exp OR 'systematic review’/exp OR 'systematic review (topic)’/exp OR ’biomedical technology assessment’/exp | 415,886 |
| #9 | ‘crossover procedure’/exp OR (((crossover OR ’cross over’) NEAR/10 (study OR studies OR design* OR method* OR procedure OR comparison)):ti,ab) | 94,585 |
| #8 | ((singl* OR doubl* OR trebl* OR tripl*) NEAR/10 (mask* OR blind* OR dumm*)):ti,ab | 252,294 |
| #7 | ‘clinical trial (topic)’/exp OR ’double blind procedure’/exp OR 'single blind procedure’/exp OR ’triple blind procedure’/exp | 542,371 |
| #6 | ‘clinical trial’/exp OR ’randomization’/exp OR randomized:ti,ab OR randomised:ti,ab OR placebo:ti,ab OR randomly:ti,ab OR trial:ti,ab OR groups:ti,ab | 4,935,446 |
| #5 | #3 OR #4 | 10,181 |
| #4 | alipure:ti,ab,kw OR ‘alpha‐lipogamma’:ti,ab,kw OR ‘alphalipogamma’:ti,ab,kw OR ‘alpha‐lipon’:ti,ab,kw OR alphalipon:ti,ab,kw OR ‘alpha liponaure’:ti,ab,kw OR alphaliponaure:ti,ab,kw OR alphaliponsaure:ti,ab,kw OR ‘alpha liponsaure’:ti,ab,kw OR ‘alpha lippon’:ti,ab,kw OR alphalippon:ti,ab,kw OR ‘alpha vibolex’:ti,ab,kw OR alphavibolex:ti,ab,kw OR azulipont:ti,ab,kw OR berlition:ti,ab,kw OR berlithione:ti,ab,kw OR biletan:ti,ab,kw OR ‘biomo lipon’:ti,ab,kw OR biomolipon:ti,ab,kw OR ‘byodinoral r’:ti,ab,kw OR ‘coenzyme compositum’:ti,ab,kw OR ‘discus compositum’:ti,ab,kw OR duralipon:ti,ab,kw OR ‘espa lipon’:ti,ab,kw OR espalipon:ti,ab,kw OR fenint:ti,ab,kw OR ‘hepar compositium’:ti,ab,kw OR heparlipon:ti,ab,kw OR juthiac:ti,ab,kw OR ‘ledum compositum’:ti,ab,kw OR ‘lycopodium compositum’:ti,ab,kw OR liposan:ti,ab,kw OR ‘liponsaure‐ratiopharm’:ti,ab,kw OR liponsaureratiopharm:ti,ab,kw OR lipothion:ti,ab,kw OR mtwalphaliponsaure:ti,ab,kw OR neurium:ti,ab,kw OR octolipen:ti,ab,kw OR oktolipen:ti,ab,kw OR ‘pleomix alpha’:ti,ab,kw OR ‘quamtrax ala’:ti,ab,kw OR thioctacid:ti,ab,kw OR thioctacide:ti,ab,kw OR thioctan:ti,ab,kw OR thioctsan:ti,ab,kw OR thiogamma:ti,ab,kw OR thiotacid:ti,ab,kw OR tromlipon:ti,ab,kw OR tioctacid:ti,ab,kw OR tioctan:ti,ab,kw OR tioctidasi:ti,ab,kw OR ‘ubichinon compositum’:ti,ab,kw OR ‘verla lipon’:ti,ab,kw OR verlalipon:ti,ab,kw OR zeel:ti,ab | 141 |
| #3 | #1 OR #2 | 10,112 |
| #2 | ((‘1 2 dithiolan 3 pentanic’ OR 12dithiolan3pentanic OR ’1 2 dithiolane 3 pentanoic’ OR ’12dithiolane3pentanoic’ OR ’1 2 dithiolane 3 valeric’ OR ’12dithiolane3valeric’ OR ’5 dithiolan 3 yl valeric’ OR ’5dithiolan3ylvaleric’ OR ’5 1 2 dithiolan 3 yl valeric’ OR 512ditiolan3ylvaleric OR 68thioctic OR ’6 8thioctic acid’ OR αlipoic OR alipoic OR lipoic OR liponic OR thioctic OR tioctic) NEAR/3 (acidOR acids)):ti,ab,kw | 6,833 |
| #1 | ‘thioctic acid’/exp OR ’1077 28 7’:ti,ab,kw OR 1077287:ti,ab,kw | 9,259 |
PubMed
Date of the search: 4‐9‐2020
| Search | Query | Results |
|---|---|---|
| #12 | Search: “english”[Language] AND #11 | 772 |
| #11 | Search: #9 NOT #10 | 843 |
| #10 | Search: (rat[ti] OR rats[ti] OR mouse[ti] OR mice[ti] OR swine[ti] OR porcine[ti] OR murine[ti] OR sheep[ti] OR lambs[ti] OR pigs[ti] OR piglets[ti] OR rabbit[ti] OR rabbits[ti] OR cat[ti] OR cats[ti] OR dog[ti] OR dogs[ti] OR cattle[ti] OR bovine[ti] OR monkey[ti] OR monkeys[ti] OR trout[ti] OR marmoset*[ti]) | 2,021,263 |
| #9 | Search: #7 NOT #8 | 934 |
| #8 | Search: (“Animals”[Mesh] NOT (“Animals”[Mesh] AND “Humans”[Mesh])) | 4,731,720 |
| #7 | Search: #6 AND #3 | 1,511 |
| #6 | Search: #4 OR #5 | 3,788,408 |
| #5 | Search: “clinical trial”[pt] OR “Random Allocation”[Mesh] OR randomized[tiab] OR randomised[tiab] OR placebo[tiab] OR randomly[tiab] OR trial[tiab] OR groups[tiab] OR “Clinical Trials as Topic”[Mesh] OR “Double‐Blind Method”[Mesh] OR “Single‐Blind Method”[Mesh] OR ((singl*[tiab] OR doubl*[tiab] OR trebl*[tiab] OR tripl*[tiab]) AND (mask*[tiab] OR blind*[tiab] OR dumm*[tiab])) OR “Cross‐Over Studies”[Mesh] OR ((crossover[tiab] OR “cross over”[tiab]) AND (study[tiab] OR studies[tiab] OR design*[tiab] OR method*[tiab] OR procedure[tiab] OR comparison[tiab])) | 3,516,499 |
| #4 | Search: systematic[sb] OR meta‐analysis[pt] OR meta‐analysis as topic[Mesh] OR meta‐analysis[Mesh] OR meta analy*[tw] OR metanaly*[tw] OR metaanaly*[tw] OR met analy*[tw] OR integrative research[tiab] OR integrative review*[tiab] OR integrative overview*[tiab] OR research integration*[tiab] OR research overview*[tiab] OR collaborative review*[tiab] OR collaborative overview*[tiab] OR systematic review*[tiab] OR comparative efficacy[tiab] OR comparative effectiveness[tiab] OR outcomes research[tiab] OR indirect comparison*[tiab] OR Embase*[tiab] OR Cinahl*[tiab] OR systematic overview*[tiab] OR methodological overview*[tiab] OR methodologic overview*[tiab] OR methodological review*[tiab] OR methodologic review*[tiab] OR quantitative review*[tiab] OR quantitative overview*[tiab] OR quantitative synthes*[tiab] OR pooled analy*[tiab] OR Cochrane[tiab] OR Medline[tiab] OR Pubmed[tiab] OR Medlars[tiab] OR handsearch*[tiab] OR hand search*[tiab] OR meta‐regression*[tiab] OR metaregression*[tiab] OR data synthes*[tiab] OR data extraction[tiab] OR data abstraction*[tiab] OR mantel haenszel[tiab] OR peto[tiab] OR der‐simonian[tiab] OR dersimonian[tiab] OR fixed effect*[tiab] OR “Cochrane Database Syst Rev”[Journal:__jrid21711] | 433,871 |
| #3 | Search: #1 OR #2 Sort by: Most Recent | 6,014 |
| #2 | Search: “Thioctic Acid”[Mesh] OR “1077‐28‐7”[tiab] OR 1077287[tiab] OR “1,2‐Dithiolan‐3‐pentanic acid”[tiab] OR “1,2‐Dithiolan‐3‐pentanic acids”[tiab] OR “1 2 dithiolane 3 pentanoic acid”[tiab] OR “12dithiolane3pentanoic acid”[tiab] OR “1 2 dithiolane 3 valeric acid”[tiab] OR ‘“2dithiolane3valeric acid”[tiab] OR “5 dithiolan 3 yl valeric acid”[tiab] OR “5dithiolan3ylvaleric acid”[tiab] OR “5 1 2 dithiolan 3 yl valeric acid”[tiab] OR “512ditiolan3ylvaleric acid”[tiab] OR “1 2 dithiolane 3 pentanoic acids”[tiab] OR “12dithiolane3pentanoic acids”[tiab] OR “1 2 dithiolane 3 valeric acids”[tiab] OR ‘“2dithiolane3valeric acids”[tiab] OR “5 dithiolan 3 yl valeric acids”[tiab] OR “5dithiolan3ylvaleric acids”[tiab] OR “5 1 2 dithiolan 3 yl valeric acids”[tiab] OR “512ditiolan3ylvaleric acids”[tiab] OR “68thioctic acid”[tiab] OR “68thioctic acids”[tiab] OR “6 8thioctic acid”[tiab] OR “6 8thioctic acids”[tiab] OR “αlipoic acid”[tiab] OR “αlipoic acids”[tiab] OR “lipoic acid”[tiab] OR “lipoic acids”[tiab] OR “liponic acid”[tiab] OR “liponic acids”[tiab] OR “thioctic acid”[tiab] OR “thioctic acids”[tiab] OR “tioctic acid”[tiab] OR “tioctic acids”[tiab] | 5,970 |
| #1 | Search: Alipure[tiab] OR “Alpha‐Lipogamma”[tiab] OR “AlphaLipogamma”[tiab] OR “Alpha‐Lipon”[tiab] OR AlphaLipon[tiab] OR “alpha Liponaure”[tiab] OR alphaLiponaure[tiab] OR AlphaLiponsaure[tiab] OR “Alpha‐Liponsaure”[tiab] OR “Alpha Lippon”[tiab] OR AlphaLippon[tiab] OR “Alpha Vibolex”[tiab] OR alphaVibolex[tiab] OR Azulipont[tiab] OR Berlithion[tiab] OR Berlithione[tiab] OR Biletan[tiab] OR “Biomo lipon”[tiab] OR Biomolipon[tiab] OR “Byodinoral R”[tiab] OR “coenzyme compositum”[tiab] OR “discus compositum”[tiab] OR Duralipon[tiab] OR “Espa‐lipon”[tiab] OR Espalipon[tiab] OR Fenint[tiab] OR “Hepar compositum”[tiab] OR Heparlipon[tiab] OR Juthiac[tiab] OR “Lycopodium compositum”[tiab] OR “Ledum compositum”[tiab] OR Liposan[tiab] OR “Liponsaure‐ratiopharm”[tiab] OR Liponsaureratiopharm[tiab] OR Lipothion [tiab] OR MTWAlphaliponsaure[tiab] OR Neurium[tiab] OR Octolipen[tiab] OR Oktolipen[tiab] OR “Pleomix Alpha”[tiab] OR “Quamtrax ALA”[tiab] OR Thioctacid[tiab] OR Thioctacide[tiab] OR Thioctan[tiab] OR Thioctsan[tiab] OR Thiogamma[tiab] OR Thiotacid[tiab] OR Tromlipon[tiab] OR Tioctacid[tiab] OR Tioctidasi[tiab] OR Tioctan[tiab] OR Tioctidasi[tiab] OR “Ubichinon compositum”[tiab] OR “Verla Lipon”[tiab] OR VerlaLipon[tiab] OR Zeel[tiab] | 78 |
Appendix D – Search strings for the fourth literature search performed after public consultation
1.
Embase
Date of the search 1/3/1021
| Set | Query | Results | Comments |
|---|---|---|---|
| #6 | #1 AND #4 AND [humans]/lim | 271 | Dihydrolipoic acid AND ADME AND Humans |
| #5 | #1 AND #4 | 674 | Dihydrolipoic acid AND ADME |
| #4 | #2 OR #3 | 15,430,136 | ADME |
| #3 | adme:ti,ab,kw OR admet:ti,ab,kw OR activat*:ti,ab,kw OR administ*:ti,ab,kw OR absorpt*:ti,ab,kw OR bioavailab*:ti,ab,kw OR bioconcentrat*:ti,ab,kw OR biotransform*:ti,ab,kw OR clear*:ti,ab,kw OR concentrat*:ti,ab,kw OR diffus*:ti,ab,kw OR distribut*:ti,ab,kw OR eliminat*:ti,ab,kw OR excret*:ti,ab,kw OR ’half life’:ti,ab,kw OR metaboli*:ti,ab,kw OR pbk:ti,ab,kw OR pbpk:ti,ab,kw OR pharmacokinetic*:ti,ab,kw OR pharmacodynamic*:ti,ab,kw OR resorpt*:ti,ab,kw OR 'systemic circulation’:ti,ab,kw OR toxicokinetic*:ti,ab,kw OR transfer*:ti,ab,kw OR transport*:ti,ab,kw | 10,873,475 | ADME 2 |
| #2 | ‘pharmacokinetics’/exp OR ’absorption’/de OR ’distribution’/exp OR ’metabolism’/exp OR ’excretion’/exp OR ’pharmacodynamics’/exp | 9,045,217 | ADME 1 |
| #1 | ‘dihydrolipoate’/exp OR ‘462 20 4’:ti,ab,kw OR dihydrolipoate:ti,ab,kw OR (((dihydrolipoic OR dihydrothioctic OR ‘dihydro thioctic’ OR ‘6 8 dimercaptooctanoic’ OR ‘6 8 dimercapto octane’ OR ‘6 8 dimercaptooctane’ OR ‘6 8 dimercaptocaprylic’ OR ‘6 8 dithiooctanoic’ OR ‘dihydro α lipoic’ OR ‘dihydro alpha lipoic’ OR dihydroalphalipoic OR ‘reduced lipoic’ OR ‘reduced thioctic’ OR ‘γ lipoic’ OR γlipoic OR ‘gamma lipoic’ OR gammalipoic OR thioctanic) NEAR/3 acid*):ti,ab,kw) | 805 | Dihydrolipoic acid |
Scopus
Date of the search 1/3/1021
| Set | Search | Results | Comments |
|---|---|---|---|
| #8 | ((((CASREGNUMBER (462‐20‐4)) OR (TITLE‐ABS‐KEY (dihydrolipoate OR ((dihydrolipoic OR dihydrothioctic OR “dihydro Thioctic” OR “6 8 Dimercaptooctanoic” OR “6 8 dimercapto octane” OR “6 8 dimercaptooctane” OR “6 8 dimercaptocaprylic” OR “6 8 dithiooctanoic” OR “dihydro α lipoic” OR “dihydro a lipoic” OR “dihydro alpha lipoic” OR dihydroalphalipoic OR “reduced lipoic” OR “lipoic reduced” OR “reduced thioctic” OR “thioctic reduced” OR “γ lipoic” OR γlipoic OR “gamma lipoic” OR gammalipoic OR thioctanic) W/3 acid*)))) AND (TITLE‐ABS‐KEY (adme OR admet OR activat* OR administ* OR absorpt* OR bioavailab* OR bioconcentrat* OR biotransform* OR clear* OR concentrat* OR diffus* OR distribut* OR eliminat* OR excret* OR “half life” OR metaboli* OR pbk OR pbpk OR pharmacokinetic* OR pharmacodynamic* OR resorpt* OR “systemic circulation” OR toxicokinetic* OR transfer* OR transport*))) AND ((INDEXTERMS (humans OR human)))) OR ((((CASREGNUMBER (462‐20‐4)) OR (TITLE‐ABS‐KEY (dihydrolipoate OR ((dihydrolipoic OR dihydrothioctic OR “dihydro Thioctic” OR “6 8 Dimercaptooctanoic” OR “6 8 dimercapto octane” OR “6 8 dimercaptooctane” OR “6 8 dimercaptocaprylic” OR “6 8 dithiooctanoic” OR “dihydro α lipoic” OR “dihydro a lipoic” OR “dihydro alpha lipoic” OR dihydroalphalipoic OR “reduced lipoic” OR “lipoic reduced” OR “reduced thioctic” OR “thioctic reduced” OR “γ lipoic” OR γlipoic OR “gamma lipoic” OR gammalipoic OR thioctanic) W/3 acid*)))) AND (TITLE‐ABS‐KEY (adme OR admet OR activat* OR administ* OR absorpt* OR bioavailab* OR bioconcentrat* OR biotransform* OR clear* OR concentrat* OR diffus* OR distribut* OR eliminat* OR excret* OR “half life” OR metaboli* OR pbk OR pbpk OR pharmacokinetic* OR pharmacodynamic* OR resorpt* OR “systemic circulation” OR toxicokinetic* OR transfer* OR transport*))) AND (TITLE‐ABS‐KEY ((human* OR patient* OR women OR woman OR men OR man OR child OR children OR baby OR babies OR newborn OR newborns)))) | 281 document results | (Dihydrolipoic acid AND ADME AND Humans 1) OR (Dihydrolipoic acid AND ADME AND Humans 2) |
| #7 | (((CASREGNUMBER (462‐20‐4)) OR (TITLE‐ABS‐KEY (dihydrolipoate OR ((dihydrolipoic OR dihydrothioctic OR “dihydro Thioctic” OR “6 8 Dimercaptooctanoic” OR “6 8 dimercapto octane” OR “6 8 dimercaptooctane” OR “6 8 dimercaptocaprylic” OR “6 8 dithiooctanoic” OR “dihydro α lipoic” OR “dihydro a lipoic” OR “dihydro alpha lipoic” OR dihydroalphalipoic OR “reduced lipoic” OR “lipoic reduced” OR “reduced thioctic” OR “thioctic reduced” OR “γ lipoic” OR γlipoic OR “gamma lipoic” OR gammalipoic OR thioctanic) W/3 acid*)))) AND (TITLE‐ABS‐KEY (adme OR admet OR activat* OR administ* OR absorpt* OR bioavailab* OR bioconcentrat* OR biotransform* OR clear* OR concentrat* OR diffus* OR distribut* OR eliminat* OR excret* OR “half life” OR metaboli* OR pbk OR pbpk OR pharmacokinetic* OR pharmacodynamic* OR resorpt* OR “systemic circulation” OR toxicokinetic* OR transfer* OR transport*))) AND (TITLE‐ABS‐KEY ((human* OR patient* OR women OR woman OR men OR man OR child OR children OR baby OR babies OR newborn OR newborns))) …View More | 281 document results | Dihydrolipoic acid AND ADME AND Humans 2 |
| #6 | (((CASREGNUMBER (462‐20‐4)) OR (TITLE‐ABS‐KEY (dihydrolipoate OR ((dihydrolipoic OR dihydrothioctic OR “dihydro Thioctic” OR “6 8 Dimercaptooctanoic” OR “6 8 dimercapto octane” OR “6 8 dimercaptooctane” OR “6 8 dimercaptocaprylic” OR “6 8 dithiooctanoic” OR “dihydro α lipoic” OR “dihydro a lipoic” OR “dihydro alpha lipoic” OR dihydroalphalipoic OR “reduced lipoic” OR “lipoic reduced” OR “reduced thioctic” OR “thioctic reduced” OR “γ lipoic” OR γlipoic OR “gamma lipoic” OR gammalipoic OR thioctanic) W/3 acid*)))) AND (TITLE‐ABS‐KEY (adme OR admet OR activat* OR administ* OR absorpt* OR bioavailab* OR bioconcentrat* OR biotransform* OR clear* OR concentrat* OR diffus* OR distribut* OR eliminat* OR excret* OR “half life” OR metaboli* OR pbk OR pbpk OR pharmacokinetic* OR pharmacodynamic* OR resorpt* OR “systemic circulation” OR toxicokinetic* OR transfer* OR transport*))) AND ((INDEXTERMS (humans OR human))) | 243 document results | Dihydrolipoic acid AND ADME AND Humans 1 |
| #5 | TITLE‐ABS‐KEY ((human* OR patient* OR women OR woman OR men OR man OR child OR children OR baby OR babies OR newborn OR newborns)) | 25,473,800 document results | Humans 2 |
| #4 | (INDEXTERMS (humans OR human)) | 21,158,270 document results | Humans 1 |
| #3 | ((CASREGNUMBER (462‐20‐4)) OR (TITLE‐ABS‐KEY (dihydrolipoate OR ((dihydrolipoic OR dihydrothioctic OR “dihydro Thioctic” OR “6 8 Dimercaptooctanoic” OR “6 8 dimercapto octane” OR “6 8 dimercaptooctane” OR “6 8 dimercaptocaprylic” OR “6 8 dithiooctanoic” OR “dihydro α lipoic” OR “dihydro a lipoic” OR “dihydro alpha lipoic” OR dihydroalphalipoic OR “reduced lipoic” OR “lipoic reduced” OR “reduced thioctic” OR “thioctic reduced” OR “γ lipoic” OR γlipoic OR “gamma lipoic” OR gammalipoic OR thioctanic) W/3 acid*)))) AND (TITLE‐ABS‐KEY (adme OR admet OR activat* OR administ* OR absorpt* OR bioavailab* OR bioconcentrat* OR biotransform* OR clear* OR concentrat* OR diffus* OR distribut* OR eliminat* OR excret* OR “half life” OR metaboli* OR pbk OR pbpk OR pharmacokinetic* OR pharmacodynamic* OR resorpt* OR “systemic circulation” OR toxicokinetic* OR transfer* OR transport*)) | 682 document results | Dihydrolipoic acid AND ADME |
| #2 | TITLE‐ABS‐KEY (adme OR admet OR activat* OR administ* OR absorpt* OR bioavailab* OR bioconcentrat* OR biotransform* OR clear* OR concentrat* OR diffus* OR distribut* OR eliminat* OR excret* OR “half life” OR metaboli* OR pbk OR pbpk OR pharmacokinetic* OR pharmacodynamic* OR resorpt* OR “systemic circulation” OR toxicokinetic* OR transfer* OR transport*) | 23,905,987 document results | ADME |
| #1 | (CASREGNUMBER (462‐20‐4)) OR (TITLE‐ABS‐KEY (dihydrolipoate OR ((dihydrolipoic OR dihydrothioctic OR “dihydro Thioctic” OR “6 8 Dimercaptooctanoic” OR “6 8 dimercapto octane” OR “6 8 dimercaptooctane” OR “6 8 dimercaptocaprylic” OR “6 8 dithiooctanoic” OR “dihydro α lipoic” OR “dihydro a lipoic” OR “dihydro alpha lipoic” OR dihydroalphalipoic OR “reduced lipoic” OR “lipoic reduced” OR “reduced thioctic” OR “thioctic reduced” OR “γ lipoic” OR γlipoic OR “gamma lipoic” OR gammalipoic OR thioctanic) W/3 acid*))) | 920 document results | Dihydrolipoic acid |
Annex A – Outcome of a public consultation on the Scientific Opinion of the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) on the relationship between intake of alpha‐lipoic acid (thioctic acid) and the risk of insulin autoimmune syndrome
1.
Annex A can be found in the online version of this output (in the ‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2021.6577
Supporting information
Outcome of a public consultation on the Scientific Opinion of the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) on the relationship between intake of alpha‐lipoic acid (thioctic acid) and the risk of insulin autoimmune syndrome
Uriach CHC Position Statement Lipoic Acid AIS
Lipoic Acid ESFA Public consultation
Suggested citation: EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , Turck D, Castenmiller J, de Henauw S, Hirsch‐Ernst KI, Kearney J, Knutsen HK, Mangelsdorf I, McArdle HJ, Naska A, Pelaez C, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M, Cappellani D, Ijzerman R, Van Loveren H, Titz A and Maciuk A, 2021. Scientific opinion on the relationship between intake of alpha‐lipoic acid (thioctic acid) and the risk of insulin autoimmune syndrome. EFSA Journal 2021;19(6):6577, 46 pp. 10.2903/j.efsa.2021.6577
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00457
Panel members: Dominique Turck, Jacqueline Castenmiller, Stefaan De Henauw, Karen Ildico Hirsch‐Ernst, John Kearney, Helle Katrine Knutsen, Alexandre Maciuk, Inge Mangelsdorf, Harry J McArdle, Androniki Naska, Carmen Pelaez, Kristina Pentieva, Alfonso Siani, Frank Thies, Sophia Tsabouri and Marco Vinceti.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: The Panel also wishes to thank the following EFSA staff members for the support provided to this scientific output: Fabio Alfieri, Janusz Ciok, Ester Artau Cortacans, Ionut Craciun, Céline Dumas, Lucia Fabiani, Leonard Matijevic, Irene Muñoz Guajardo and Charlotte Salgaard Nielsen.
Adopted: 8 April 2021
The Outcome of the public consultation is available under the Supporting Information section .
Notes
OJ L 404, 30.12.2006, p. 26.
‘Safety of alpha‐lipoic acid use in food supplements’, Danish National Food Institute, DTU Doc nr. 17/14450, 10.10.2017.
Avis du Conseil Supérieur de la Sante N. 9274, ‘Innocuité de l'acide alpha‐lipoïque dans les compléments alimentaires’, 4.6.2015.
To date EFSA has delivered five scientific opinions in the context of Article 8(2) of Regulation (EU) No 1925/2006, i.e. Scientific Opinions the safety of 1) Ephedra species, 2) Yohimbe (Pausinystalia yohimbe (K. Schum.) Pierre ex Beille, 3) hydroxyanthracene derivatives, 4) green tea catechins, and 5) monacolins in red yeast rice: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2013.3467, https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2013.3302, https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2018.5090, https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2018.5239, https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2018.5368
i.e. bound to the amino acid lysine.
As case reports on IAS associated with ALA are mainly available for Europe as well as Japan and South Korea, allele frequencies are only reported for these two regions.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta‐Analyses.
Population (p), Intervention (i), Comparator (c) and Outcome (o) in a question about an intervention effect.
Population (p) and Outcome (o) in a descriptive question.
References
- AFSSA , 2008. AVIS de l'Agence française de sécurité sanitaire des aliments relatif à l’évaluation d'un projet d'arrêté relatif à l'emploi de substances à but nutritionnel ou physiologique et de plantes et préparations de plantes dans la fabrication des compléments alimentaires. Saisine n° 2007‐SA-0231.
- Alagüney ES, Efe B, Yorulmaz G, Acu B and Durmuş I, 2019. Hypoglycemia due to the presence of anti‐insulin antibodies: a case report. Turkish Journal of Endocrinology and Metabolism, 23, 72–75. [Google Scholar]
- ANSES (French Agency for Food, Environmental and Occupational Health & Safety), 2011. Opinion on the assessment of the risks associated with substances with nutritional or physiological effects with a view to restricting or prohibiting their use in foodstuffs. ANSES – Request No. 2007‐SA-0314, 34 pp.
- Archambeaud‐Mouveroux F, Huc MC, Nadalon S, Fournier MP and Canivet B, 1989. Autoimmune insulin syndrome. Biomedicine and Pharmacotherapy, 43, 581–586. 10.1016/0753-3322(89)90036-x [DOI] [PubMed] [Google Scholar]
- Bae SM, Bae MN, Kim EY, Kim IK, Seo MW, Shin JK, Cho SR and Jeong GH, 2013. Recurrent insulin autoimmune syndrome caused by α‐lipoic acid in type 2 diabetes. Endocrinology and Metabolism, 28, 326–330. 10.3803/EnM.2013.28.4.326 Epub 2013 Dec 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biewenga GP, Haenen GRMM and Bast A, 1997. The pharmacology of the antioxidant lipoic acid. General Pharmacology: The Vascular System, 29, 315–331. 10.1016/S0306-3623(96)00474-0 [DOI] [PubMed] [Google Scholar]
- Bilska A and Wlodek L, 2005. Lipoic acid ‐ the drug of the future? Pharmacological reports : PR, 57, 570–577. [PubMed] [Google Scholar]
- Bolayir B, Karakoc MA, Deniz MS, Altinova A, Akturk M, Yurumez B, Toruner F and Yetkin I, 2018. A rare cause of hypoglycemia: insulin autoimmune syndrome in a Turkish patient taking Alpha lipoic acid. Presented at Society for Endocrinology BES 2017, Harrogate, UK. Endocrine Abstracts 56 P325; 10.1530/endoabs.56.P325 [DOI]
- Breithaupt‐Grögler K, Niebch G, Schneider E, Erb K, Hermann R, Blume HH, Schug BS and Belz GG, 1999. Dose‐proportionality of oral thioctic acid–coincidence of assessments via pooled plasma and individual data. European Journal of Pharmaceutical Sciences, 8, 57–65. 10.1016/s0928-0987(98)00061-x [DOI] [PubMed] [Google Scholar]
- Bresciani E, Bussi A, Bazzigaluppi E and Balestrieri G, 2011. Insulin autoimmune syndrome induced by α‐lipoic acid in a Caucasian woman: case report. Diabetes Care, 34. 10.2337/dc11-0600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brufani M and Figliola R, 2014. (R)‐α‐lipoic acid oral liquid formulation: pharmacokinetic parameters and therapeutic efficacy. Acta bio‐medica: Atenei Parmensis, 85, 108–115. Available online: http://europepmc.org/abstract/MED/25245645 [PubMed] [Google Scholar]
- Bustamante J, Lodge JK, Marcocci L, Tritschler HJ, Packer L and Rihn BH, 1998. α‐Lipoic acid in liver metabolism and disease. Free Radical Biology and Medicine, 24, 1023–1039. 10.1016/S0891-5849(97)00371-7 [DOI] [PubMed] [Google Scholar]
- Cambria V, Beccuti G, Gatti F, Bona C, Maccario M and Gasco V, 2020. HLA DRB1*0415: a new possible genetic susceptibility factor for Hirata's disease. Endocrine, 67, 729–732. 10.1007/s12020-019-02132-3 Epub 2019 Nov 16. [DOI] [PubMed] [Google Scholar]
- Cappellani D, Sardella C, Campopiano MC, Falorni A, Marchetti P and Macchia E, 2018. Spontaneously remitting insulin autoimmune syndrome in a patient taking alpha‐lipoic acid. Endocrinology, Diabetes and Metabolism Case Reports. 10.1530/EDM-18-0122. Epub 2018 Dec 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellani D, Macchia E, Falorni A and Marchetti P, 2020. Insulin Autoimmune Syndrome (Hirata Disease): a comprehensive review fifty years after its first description. Diabetes, Metabolic Syndrome and Obesity, 13, 963–978. 10.2147/DMSO.S219438.eCollection [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censi S, Albergoni MP, Gallo N, Plebani M, Boscaro M and Betterle C, 2018a. Insulin autoimmune syndrome (Hirata's disease) in an Italian patient: a case report and review of the literature. Clinical Chemistry and Laboratory Medicine, 56, 889–895. 10.1515/cclm-2017-0392 [DOI] [PubMed] [Google Scholar]
- Censi S, Mian C and Betterle C, 2018b. Insulin autoimmune syndrome: from diagnosis to clinical management. Annals of Translational Medicine, 6, 335. 10.21037/atm.2018.07.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DS, 1999. The side effects of antithyroid drugs. Endocrinologist, 9, 457–467. [Google Scholar]
- Cremer DR, Rabeler R, Roberts A and Lynch B, 2006a. Long‐term safety of α‐lipoic acid (ALA) consumption: a 2‐year study. Regulatory Toxicology and Pharmacology, 46, 193–201. 10.1016/j.yrtph.2006.06.003 [DOI] [PubMed] [Google Scholar]
- Cremer DR, Rabeler R, Roberts A and Lynch B, 2006b. Safety evaluation of alpha‐lipoic acid (ALA). Regulatory Toxicology and Pharmacology, 46, 29–41. 10.1016/j.yrtph.2006.06.004 [DOI] [PubMed] [Google Scholar]
- Davi MV, Pia A, Guarnotta V, Pizza G, Colao A and Faggiano A, 2017. The treatment of hyperinsulinemic hypoglycaemia in adults: an update. Journal of Endocrinological Investigation, 40, 9–20. 10.1007/s40618-016-0536-3 Epub 2016 Sep 13. [DOI] [PubMed] [Google Scholar]
- Dozio N, Scavini M, Beretta A, Sarugeri E, Sartori S, Belloni C, Dosio F, Savi A, Fazio F, Sodoyez JC and Pozza G, 1998. Imaging of the buffering effect of insulin antibodies in the autoimmune hypoglycemic syndrome. The Journal of Clinical Endocrinology & Metabolism, 83, 643–648. 10.1210/jcem.83.2.4599 [DOI] [PubMed] [Google Scholar]
- DTU Food , 2017. Safety of alpha‐lipoic acid use in food supplements. DTU Doc nr. 17/14450, 5 pp. [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Guidance on expert knowledge elicitation in food and feed safety risk assessment. EFSA Journal 2014;1831–4732, 3734 pp. Available online: https://efsa.onlinelibrary.wiley.com/doi/abs/10.2903/j.efsa.2014.3734
- EFSA Scientific Committee , Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Martino L, Merten C, Mosbach‐Schulz O and Hardy A, 2018. Guidance on uncertainty analysis in scientific assessments. EFSA Journal 2018;1831–4732, e05123 pp. Available online: https://efsa.onlinelibrary.wiley.com/doi/abs/10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed]
- EFSA (European Food Safety Authority), Martino L, Aiassa E, Halldórsson Þ, Koutsoumanis KP, Naegeli H, Baert K, Baldinelli F, Devos Y, Lodi F, Lostia A, Manini P, Merten C, Messens W, Rizzi V, Tarazona J, Titz A and Vos S, 2020. Draft framework for protocol development for EFSA's scientific assessments. EFSA Journal 2020;2397–8325, 1843E pp. Available online: https://efsa.onlinelibrary.wiley.com/doi/abs/10.2903/sp.efsa.2020.EN-1843
- EMA (European Medicines Agency), 2015. PRAC recommendation on signals. EMA/PRAC/590240/2015, 9 pp.
- Evans JL, Heymann CJ, Goldfine ID and Gavin LA, 2002. Pharmacokinetics, tolerability, and fructosamine‐lowering effect of a novel, controlled‐release formulation of alpha‐lipoic acid. Endocr Pract, 8, 29–35. 10.4158/EP.8.1.29 [DOI] [PubMed] [Google Scholar]
- Ferreira AG, Luiz HV, Silva TN, Manita I, Cordeiro MC and Portugal JR, 2017. Autoimmune hypoglycemia possibly associated with alpha lipoic acid on a caucasian woman. Endocrine Reviews, 38. [Google Scholar]
- Furukawa N, Miyamura N, Nishida K, Motoshima H, Taketa K and Araki E, 2007. Possible relevance of alpha lipoic acid contained in a health supplement in a case of insulin autoimmune syndrome. Diabetes Research and Clinical Practice, 75, 366–367. 10.1016/j.diabres.2006.07.005 Epub 2006 Sep 11. [DOI] [PubMed] [Google Scholar]
- Gatti M, Ippoliti I, Poluzzi E, Antonazzo IC, Moro PA, Moretti U, Menniti‐Ippolito F, Mazzanti G, De Ponti F and Raschi E, 2020. Assessment of adverse reactions to α‐lipoic acid containing dietary supplements through spontaneous reporting systems. Clinical Nutrition. 10.1016/j.clnu.2020.07.028 [DOI] [PubMed] [Google Scholar]
- Gleiter CH, Schug BS, Hermann R, Elze M, Blume HH and Gundert‐Remy U, 1996. Influence of food intake on the bioavailability of thioctic acid enantiomers. European Journal of Clinical Pharmacology, 50, 513–514. 10.1007/s002280050151 [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Galarza Faviel F, McCabe A, Santos Eduardo JMd, Jones J, Takeshita L, Ortega‐Rivera Nestor D, Cid‐Pavon Glenda MD, Ramsbottom K, Ghattaoraya G, Alfirevic A, Middleton D and Jones Andrew R, 2019. Allele frequency net database (AFND) 2020 update: gold‐standard data classification, open access genotype data and new query tools. Nucleic Acids Research, 48, D783–D788. 10.1093/nar/gkz1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullo D, Evans JL, Sortino G, Goldfine ID and Vigneri R, 2014. Insulin autoimmune syndrome (Hirata Disease) in European Caucasians taking α‐lipoic acid. Clinical Endocrinology, 81, 204–209. 10.1111/cen.12334 Epub 2013 Oct 23. [DOI] [PubMed] [Google Scholar]
- Haj‐Yehia AI, Assaf P, Nassar T and Katzhendler J, 2000. Determination of lipoic acid and dihydrolipoic acid in human plasma and urine by high‐performance liquid chromatography with fluorimetric detection. Journal of Chromatography A, 870, 381–388. 10.1016/S0021-9673(99)00857-2 [DOI] [PubMed] [Google Scholar]
- Hattori N, Duhita MR, Mukai A, Matsueda M and Shimatsu A, 2014. Development of insulin antibodies and changes in titers over a long‐term period in patients with type 2 diabetes. Clinica Chimica Acta, 433, 135–138. 10.1016/j.cca.2014.03.008 [DOI] [PubMed] [Google Scholar]
- Hermann R, Niebch G, Borbe HO, Fieger‐Büschges H, Ruus P, Nowak H, Riethmüller‐Winzen H, Peukert M and Blume H, 1996. Enantioselective pharmacokinetics and bioavailability of different racemic α‐lipoic acid formulations in healthy volunteers. European Journal of Pharmaceutical Sciences, 4, 167–174. [Google Scholar]
- Hermann R, Mungo J, Cnota PJ and Ziegler D, 2014. Enantiomer‐selective pharmacokinetics, oral bioavailability, and sex effects of various alpha‐lipoic acid dosage forms. Clinical Pharmacology, 6, 195–204. 10.2147/CPAA.S71574. eCollection [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta N, Okamoto H, Furune T, Uekaji Y, Terao K, Uchida R, Iwamoto K, Miyajima A, Hirota T and Sakamoto N, 2016. Bioavailability of an R‐α‐lipoic acid/γ‐cyclodextrin complex in healthy volunteers. International Journal of Molecular Sciences, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Ohara T, Okuno Y, Ito T, Hirota Y, Furukawa K, Sakaguchi K, Ogawa W and Kasuga M, 2007. Alpha‐lipoic acid and insulin autoimmune syndrome. Diabetes Care, 30, 2240–2241. 10.2337/dc07-0689 Epub 2007 Jun 22. [DOI] [PubMed] [Google Scholar]
- Ismail AA, 2016. The insulin autoimmune syndrome (IAS) as a cause of hypoglycaemia: an update on the pathophysiology, biochemical investigations and diagnosis. Clinical Chemistry and Laboratory Medicine, 54, 1715–1724. 10.1515/cclm-2015-1255 [DOI] [PubMed] [Google Scholar]
- Ito Y, Nieda M, Uchigata Y, Nishimura M, Tokunaga K, Kuwata S, Obata F, Tadokoro K, Hirata Y and Omori Y, 1993. Recognition of human insulin in the context of HLA‐DRB1*0406 products by T cells of insulin autoimmune syndrome patients and healthy donors. The Journal of Immunology, 151, 5770–5776. [PubMed] [Google Scholar]
- Izzo V, Greco C, Corradini D, Infante M, Staltari MT, Romano M, Bellia A, Lauro D and Uccioli L, 2018. Insulin autoimmune syndrome in an Argentine woman taking α‐lipoic acid: a case report and review of the literature. SAGE Open Med Case Rep, 6, 2050313x18819601. 10.1177/2050313X18819601. eCollection 2018. [DOI] [PMC free article] [PubMed]
- Khan A, Iqbal Z, Watson DG, Khan A, Khan I, Muhammad N, Muhammad S, Nasib HA, Iqbal N, Faizur R and Kashif M, 2011. Simultaneous determination of lipoic acid (LA) and dihydrolipoic acid (DHLA) in human plasma using high‐performance liquid chromatography coupled with electrochemical detection. Journal of Chromatography B, 879, 1725–1731. 10.1016/j.jchromb.2011.04.017 [DOI] [PubMed] [Google Scholar]
- Khan MI, Iqbal Z and Khan A, 2015. Simultaneous determination of the endogenous free α‐lipoic acid and dihydrolipoic acid in human plasma and erythrocytes by RP‐HPLC coupled with electrochemical detector. In: Armstrong D (ed). Methods in Molecular Biology. Springer, New York Heidelberg Dordrecht London, Humana Press Inc.. pp. 345–360. [DOI] [PubMed] [Google Scholar]
- Lio S, Gibin P, Della Mea P and De Bastiani P, 2016. Insulin autoimmune syndrome in patients with rheumatoid arthritis and lipoic acid intake. Endocrine Reviews, 37. [Google Scholar]
- Michalopoulou Alevras T, Guerrero Gual M, Villabona Artero C and Pérez‐Maraver M, 2015. Autoimmune hypoglycemia syndrome associated with α lipoic acid consumption. Medicina Clinica, 144, 93–94. 10.1016/j.medcli.2014.02.013 Epub 2014 Apr 1. [DOI] [PubMed] [Google Scholar]
- Mignini F, Streccioni V, Tomassoni D, Traini E and Amenta F, 2007. Comparative crossover, randomized, open‐label bioequivalence study on the bioequivalence of two formulations of thioctic acid in healthy volunteers. Clinical and Experimental Hypertension, 29, 575–586. 10.1080/10641960701744111 [DOI] [PubMed] [Google Scholar]
- Mignini F, Capacchietti M, Napolioni V, Reggiardo G, Fasani R and Ferrari P, 2011. Single dose bioavailability and pharmacokinetic study of a innovative formulation of α‐lipoic acid (ALA600) in healthy volunteers. Minerva Medica, 102, 475–482. [PubMed] [Google Scholar]
- Moffa S, Improta I, Rocchetti S, Mezza T and Giaccari A, 2019. Potential cause‐effect relationship between insulin autoimmune syndrome and alpha lipoic acid: two case reports. Nutrition, 57, 1–4. 10.1016/j.nut.2018.04.010 Epub 2018 May 25. [DOI] [PubMed] [Google Scholar]
- Okuroglu N, Sertbas M, Akkoz C, Sertbas Y, Sancak S and Ozdemir A, 2020. Insulin autoimmune syndrome ‐ time to remember. Endokrynologia Polska, 71, 204–205. [DOI] [PubMed] [Google Scholar]
- Patel M, Shah R, Ramteke‐Jadhav S, Patil V, Patel SK, Lila A, Shah N and Bandgar T, 2020. Management of insulin autoimmune hypoglycaemia: single‐centre experience from western india with systematic review of world literature. Clinical Endocrinology, 92, 409–420. 10.1111/cen.14174 Epub 2020 Feb 26. [DOI] [PubMed] [Google Scholar]
- Pavithran PV, Bhavani N, Jayakumar RV, Menon AS, Kumar H, Menon VU, Nair V and Abraham N, 2016. Autoantibodies to insulin and dysglycemia in people with and without diabetes: an underdiagnosed association. Clinical Diabetes, 34, 164–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar K and Dass AS, 2018. Hirata's disease due to alpha lipoic acid in an Indian patient. Indian Journal of Endocrinology and Metabolism, 22, S25. [Google Scholar]
- Redmon JB and Nuttall FQ, 1999. Autoimmune hypoglycemia. Endocrinology and Metabolism Clinics of North America, 28, 603–618, vii. 10.1016/s0889-8529(05)70090-6 [DOI] [PubMed] [Google Scholar]
- Rhee SJ, Lee H, Ahn LY, Lim KS and Yu KS, 2018. Lack of a clinically significant pharmacokinetic interaction between pregabalin and thioctic acid in healthy volunteers. Clinical Therapeutics, 40, 1720–1728.e1722. 10.1016/j.clinthera.2018.08.016 Epub 2018 Sep 18. [DOI] [PubMed] [Google Scholar]
- Sodoyez JC, Sodoyez‐Goffaux F, Koch M, Sondag D, Bouillenne C, Francois‐Gerard C and Bosi E, 1990. Clonally restricted insulin autoantibodies in a cohort of 2200 healthy blood donors. Diabetologia, 33, 719–725. 10.1007/BF00400341. [DOI] [PubMed] [Google Scholar]
- Superior Health Council of Belgium , 2015. Innocuité de l'acide alpha‐lipoïque dan les compléments alimentaires. Avis du Conseil Supérieur de la Santé No 9274, 10 pp.
- Takayama‐Hasumi S, Eguchi Y, Sato A, Morita C and Hirata Y, 1990. Insulin autoimmune syndrome is the third leading cause of spontaneous hypoglycemic attacks in Japan. Diabetes Research and Clinical Practice, 10, 211–214. 10.1016/0168-8227(90)90063-y [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Miyamoto T, Kakizawa T, Shigematsu S and Hashizume K, 2007. Insulin Autoimmune Syndrome possibly caused by alpha lipoic acid. Internal Medicine, 46, 237–239. 10.2169/internalmedicine.46.1893 Epub 2007 Mar 1. [DOI] [PubMed] [Google Scholar]
- Teichert J and Preiss R, 1992. HPLC‐methods for determination of lipoic acid and its reduced form in human plasma. International Journal of Clinical Pharmacology, Therapy, and Toxicology, 30, 511–512. [PubMed] [Google Scholar]
- Teichert J, Kern J, Tritschler HJ, Ulrich H and Preiß R, 1998. Investigations on the pharmacokinetics of α‐lipoic acid in healthy volunteers. International Journal of Clinical Pharmacology and Therapeutics, 36, 625–628. [PubMed] [Google Scholar]
- Teichert J, Hermann R, Ruus P and Preiss R, 2003. Plasma kinetics, metabolism, and urinary excretion of alpha‐lipoic acid following oral administration in healthy volunteers. The Journal of Clinical Pharmacology, 43, 1257–1267. 10.1177/0091270003258654 [DOI] [PubMed] [Google Scholar]
- Uchigata Y, Hirata Y, Omori Y, Iwamoto Y and Tokunaga K, 2000. Worldwide differences in the incidence of insulin autoimmune syndrome (Hirata disease) with respect to the evolution of HLA‐DR4 alleles. Human Immunology, 61, 154–157. 10.1016/S0198-8859(99)00144-5 [DOI] [PubMed] [Google Scholar]
- Uchigata Y, Hirata Y and Iwamoto Y, 2010. Insulin autoimmune syndrome (Hirata disease): epidemiology in Asia, including Japan. Diabetology International, 1, 21–25. [Google Scholar]
- Veltroni A, Zambon G, Cingarlini S and Davì MV, 2018. Autoimmune hypoglycaemia caused by alpha‐lipoic acid: a rare condition in Caucasian patients. Endocrinology, Diabetes and Metabolism Case Reports. 10.1530/EDM-18-0011. Epub 2018 Dec 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimalraj V, Jeswanth S and Surendran R, 2011. A case of hyper insulinemic hypoglycaemia. Pancreatology, 11, 56. [Google Scholar]
- Wasada T, Eguchi Y, Takayana S, Yao K and Hirata Y, 1988. Reverse phase high performance liquid chromatographic analysis of circulating insulin in the insulin autoimmune syndrome. The Journal of Clinical Endocrinology & Metabolism, 66, 153–158. 10.1210/jcem-66-1-153 [DOI] [PubMed] [Google Scholar]
- Yamada T, Imai J, Ishigaki Y, Hinokio Y, Oka Y and Katagiri H, 2007. Possible relevance of HLA‐DRB1*0403 haplotype in insulin autoimmune syndrome induced by alpha‐lipoic acid, used as a dietary supplement. Diabetes Care, 30. 10.2337/dc07-1636 [DOI] [PubMed] [Google Scholar]
- Yamada Y, Kitayama K, Oyachi M, Higuchi S, Kawakita R, Kanamori Y and Yorifuji T, 2020. Nationwide survey of endogenous hyperinsulinemic hypoglycemia in Japan (2017‐2018): congenital hyperinsulinism, insulinoma, non‐insulinoma pancreatogenous hypoglycemia syndrome and insulin autoimmune syndrome (Hirata's disease). Journal of Diabetes Investigation, 11, 554–563. 10.1111/jdi.13180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J, Moon SJ, Lee KO, Yoon SH, Jang IJ, Yu KS and Lee SH, 2016. Comparison of R(+)‐α‐lipoic acid exposure after R(+)‐α‐lipoic acid 200 mg and 300 mg and thioctic acid 600 mg in healthy Korean male subjects. Translational and Clinical Pharmacology, 24, 137–142. [Google Scholar]
- Yukina M, Nuralieva N, Solovyev M, Troshina E and Vasilyev E, 2020. Insulin autoimmune syndrome. Endocrinology, Diabetes and Metabolism Case Reports, 2020. 10.1530/EDM-19-0159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Chan Y‐M, Harrouk W, Brave M and Hankla E, 2018. FDA review of alpha lipoic acid, 30 pp.
- Zheng R, Song HH, Kwon YE and Kim BH, 2014. Pharmacokinetic evaluation of a newly developed piperazine dithioctate formulation in healthy volunteers. International Journal of Clinical Pharmacology and Therapeutics, 52, 1083–1092. 10.5414/CP202162 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Outcome of a public consultation on the Scientific Opinion of the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) on the relationship between intake of alpha‐lipoic acid (thioctic acid) and the risk of insulin autoimmune syndrome
Uriach CHC Position Statement Lipoic Acid AIS
Lipoic Acid ESFA Public consultation


