Key Points
Question
Is opioid agonist treatment (OAT) associated with risk of overall and cause-specific mortality among people with opioid dependence?
Findings
In this systematic review and meta-analysis, risk of all-cause, overdose, suicide, alcohol-related, cancer, and cardiovascular-related mortality was significantly lower for people with opioid dependence during OAT.
Meaning
These findings suggest that increasing access to OAT and retention in treatment are critical for reducing rates of preventable mortality among people with opioid dependence.
This systematic review and meta-analysis investigates the association of opioid agonist treatment with risk of overall and cause-specific mortality among people with opioid dependence.
Abstract
Importance
Mortality among people with opioid dependence is higher than that of the general population. Opioid agonist treatment (OAT) is an effective treatment for opioid dependence; however, there has not yet been a systematic review on the relationship between OAT and specific causes of mortality.
Objective
To estimate the association of time receiving OAT with mortality.
Data Sources
The Embase, MEDLINE, and PsycINFO databases were searched through February 18, 2020, including clinical trial registries and previous Cochrane reviews.
Study Selection
All observational studies that collected data on all-cause or cause-specific mortality among people with opioid dependence while receiving and not receiving OAT were included. Randomized clinical trials (RCTs) were also included.
Data Extraction and Synthesis
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. Data on study, participant, and treatment characteristics were extracted; person-years, all-cause mortality, and cause-specific mortality were calculated. Crude mortality rates and rate ratios (RRs) were pooled using random-effects meta-analyses.
Main Outcomes and Measures
Overall all-cause and cause-specific mortality both by setting and by participant characteristics. Methadone and buprenorphine OAT were evaluated specifically.
Results
Fifteen RCTs including 3852 participants and 36 primary cohort studies including 749 634 participants were analyzed. Among the cohort studies, the rate of all-cause mortality during OAT was more than half of the rate seen during time out of OAT (RR, 0.47; 95% CI, 0.42-0.53). This association was consistent regardless of patient sex, age, geographic location, HIV status, and hepatitis C virus status and whether drugs were taken through injection. Associations were not different for methadone (RR, 0.47; 95% CI, 0.41-0.54) vs buprenorphine (RR, 0.34; 95% CI, 0.26-0.45). There was lower risk of suicide (RR, 0.48; 95% CI, 0.37-0.61), cancer (RR, 0.72; 95% CI, 0.52-0.98), drug-related (RR, 0.41; 95% CI, 0.33-0.52), alcohol-related (RR, 0.59; 95% CI, 0.49-0.72), and cardiovascular-related (RR, 0.69; 95% CI, 0.60-0.79) mortality during OAT. In the first 4 weeks of methadone treatment, rates of all-cause mortality and drug-related poisoning were almost double the rates during the remainder of OAT (RR, 2.01; 95% CI, 1.55-5.09) but not for buprenorphine (RR, 0.58; 95% CI, 0.18-1.85). All-cause mortality was 6 times higher in the 4 weeks after OAT cessation (RR, 6.01; 95% CI, 4.32-8.36), remaining double the rate for the remainder of time not receiving OAT (RR, 1.81; 95% CI, 1.50-2.18). Opioid agonist treatment was associated with a lower risk of mortality during incarceration (RR, 0.06; 95% CI, 0.01-0.46) and after release from incarceration (RR, 0.09; 95% CI, 0.02-0.56).
Conclusions and Relevance
This systematic review and meta-analysis found that OAT was associated with lower rates of mortality. However, access to OAT remains limited, and coverage of OAT remains low. Work to improve access globally may have important population-level benefits.
Introduction
Opioid dependence is increasing in many countries, particularly in North America, where there have been substantial increases in opioid-related health harms, specifically overdose.1,2 In the US during the COVID-19 pandemic, opioid overdoses have increased in some states by up to 30% in 2020 compared with those in 2019.3 Population-level increases in “deaths of despair,” including suicides, injuries, and liver disease, have also been observed.4 People with opioid dependence are at an elevated risk of a range of causes of death beyond deaths of despair, including other acute and systemic causes such as unintentional opioid and suicide-related death, and all liver-related, alcohol-related, cancer-related, chronic respiratory–related, digestive-related, HIV-related, influenza- and pneumonia-related, and injection-related injuries.2,5
Methadone and buprenorphine are classified by the World Health Organization as essential medicines for opioid agonist treatment (OAT) for opioid dependence.6 There is robust evidence from a recent systematic review that during OAT, overdose and all-cause mortality are reduced among people with opioid dependence.7 That review also found that people who cease OAT are at the highest risk of all-cause and overdose mortality in the first 4 weeks after treatment cessation7 and that risk of mortality is elevated in the first 4 weeks of OAT compared with the remainder of time receiving OAT.7
To our knowledge, there has not been a systematic examination of (1) the evidence on the potential association of OAT with other causes of death or (2) OAT provided in alternative settings, including during and immediately after incarceration. In this review, we aim to (1) examine and compare all-cause and cause-specific crude mortality rates (CMRs) during and out of OAT, for both randomized clinical trials (RCTs) and observational studies; (2) examine these rates according to specific periods during and after treatment; (3) examine and compare all-cause and cause-specific CMRs for OAT provided during incarceration, after release from incarceration while receiving OAT, and according to the amount of time receiving and not receiving OAT after release from incarceration; and (4) examine the association between risk of mortality during and out of OAT by participant and treatment characteristics.
Methods
This systematic review and meta-analysis was conducted from January 15 to February 18, 2020. As this was a review, this study was not approved by an institutional review board. We reported on published, peer-reviewed data. Each included study obtained approval from their respective jurisdictions. This study followed the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER),8 Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline,9 and Meta-analysis of Observational Studies in Epidemiology (MOOSE) guideline10 (eTables 1 and 2 in the Supplement). The protocol was registered on PROSPERO.11
Search Strategy and Inclusion Criteria
We systematically searched 3 peer-reviewed databases (Medline, Embase, and PsycINFO) without language limitation; database searches were completed on February 18, 2020. Search terms included exploded MeSH terms and keywords for opioid dependence, OAT, and mortality (eAppendix in the Supplement). We consulted experts and investigators with ongoing studies of mortality among people with opioid dependence, authors of studies included in a previous review7 of OAT and mortality, systematic reviews of OAT and opioid dependence, and clinical registries.
Eligible studies had to report mortality data for people with opioid dependence during and out of OAT (prespecified exclusion criteria and excluded studies are in the eAppendix and eTable 3 in the Supplement). We included both observational studies and RCTs. Observational studies that reported any form of mortality and person-time data for time during OAT and out of OAT were included. Randomized clinical trials were required to report mortality data for participants allocated to OAT and those allocated to control or comparator interventions separately. Authors of studies that did not report mortality or person-time data during and after OAT separately were contacted for additional information.
Study Selection and Data Extraction
Two reviewers independently reviewed the titles and abstracts identified in the search and retrieved articles to determine eligibility; full texts were also independently reviewed (T.S., B.C., L.T.T.). Conflicts were resolved by consensus with a third reviewer (L.D., J.G., G.C.). If additional data were required for the primary analyses in any setting or the secondary analyses (eg, medication type, time spent in or out of treatment, and participant characteristics), then the study authors were contacted.
We extracted information on participant and study characteristics, treatment modality characteristics, number of deaths, person-years at risk, and all-cause and cause-specific mortality rates during follow-up periods during and after treatment cessation (eTables 5, 6, and 7 in the Supplement). All extracted data were confirmed by a second person (T.S., B.C., or L.T.T.).
We assessed risk of bias using the Risk Of Bias In Nonrandomized Studies–of Interventions (ROBINS-I) tool for observational studies12; and Risk of Bias 2 (RoB-2)13 tool for RCTs. Two reviewers (T.S., B.C., or L.T.T.) assessed each study independently with conflicts resolved by a third party (L.D., J.G., or G.C.).
Classifying Causes of Mortality
We reported on multiple causes of death using the primary cause of death assigned to each fatality. To standardize the definitions for cause-specific deaths included in each study, we contacted study authors asking them to provide data using specified International Classification of Diseases, Ninth Revision (ICD-9) and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10)14 codes (eTable 4 in the Supplement).
Statistical Analysis
For RCTs, log-transformed rate ratios (RRs) were calculated by comparing binary death and participant totals using the Mantel-Haenszel fixed method. For observational cohorts, observed number of deaths were divided by person-years (PYs) to calculate crude mortality rates (CMRs) for all and specific causes during periods in and out of treatment. Unadjusted log-transformed RRs for each study were calculated by comparing CMRs for time during and out of treatment. Crude mortality rates for each period and RRs were pooled using random-effects meta-analyses with exact 95% CIs assuming a Poisson distribution. Heterogeneity was quantified using the I2 statistic. Analyses were conducted in Stata, version 16.1,15 using the metan16 command for meta-analyses and the metareg17 command for meta-regressions (StataCorp). Significance was set at P < .05, and all P values were 2-sided.
Separate analyses of mortality risk were conducted for studies of patients who were incarcerated, observational studies of people released from prison with or without OAT, and studies that monitored time in or out of OAT after release from prison. Pooled analyses were conducted for all-cause and cause-specific mortality for the first 4 weeks during OAT and after OAT cessation and the remainder of time during and out of OAT. Crude mortality rates were calculated for each of the 4 periods, and RRs were calculated with the remainder of time in treatment as the reference period. We performed sensitivity analyses by excluding studies with participants who had no record of OAT during the study, studies at high risk of bias owing to missing data or loss to follow-up, and any studies of participants who were positive for HIV and released from prison.
Results
Of the 7980 studies identified, 72 publications fulfilled inclusion criteria (eFigure 1 in the Supplement; a list of articles excluded during full-text review is in eTable 3 in the Supplement). A total of 15 RCTs including 3852 participants and 36 primary cohort studies including 749 634 participants were eligible for analysis. An additional 21 articles were not included analyses despite fulfilling the inclusion criteria due to participant overlap: 3 were included in subanalyses (C. Bharat, personal communication, 2021; eTable 8 in the Supplement for secondary publications).15,18
Randomized Clinical Trials
Characteristics of Included Studies
Characteristics of RCTs are presented in eAppendix 4 (eTables 7-15 in the Supplement). Eight of 15 RCTs (53%)19,20,21,22,23,24,25,26 were conducted in North America and at single clinics (8 [53%]19,20,21,22,23,24,25,26). Buprenorphine was studied in 7 of 15 of the RCTs (47%),19,22,23,27,28,29,30 and the most common comparator was detoxification alone (5 [33%]20,25,27,29,31). Most RCTs (12 of 15 [80%]19,20,21,22,25,26,27,28,29,31,32,33) commenced before 2010 and lasted 6 months or less (9 [60%]19,20,23,24,25,26,28,29,30). Eight of the RCTs (53%)19,20,23,26,27,29,30,31 had greater than 20% of participants lost to follow-up.
All-Cause Mortality During and Out of OAT
In total, 45 deaths were reported across RCTs; 7 of 15 RCTs (47%) reported 0 deaths.19,20,23,25,28,29,30 There was no significant difference in all-cause mortality for patients allocated to OAT compared with comparison groups (RR, 0.86; 95% CI, 0.59-1.23) (eFigure 3 in the Supplement). Three of 15 RCTs (20%) evaluated the administration of OAT to patients who were incarcerated; no deaths were reported (eFigure 3 in the Supplement).19,21,24
Observational Studies
Characteristics of Included Studies
Characteristics of observational studies are presented in eAppendix 4 (eTables 7-15 in the Supplement). Cohorts ranged from 110 to 306 786 participants and included people from Europe (20 of 36 studies [58%]34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53; 278 977 people), Australia (6 studies [17%]54,55,56,57,58,59; 103 715 people), Asia (5 studies60,61,62,63,64 [14%]; 311 658 people), and North America (5 studies65,66,67,68,69 [14%]; 110 631 people). Seventeen studies (47%)34,35,39,41,42,45,47,48,49,50,57,58,61,64,66,68,69 concluded follow-up in 2010 or later. Sixteen studies (44%)34,36,37,38,39,41,42,45,50,51,54,57,58,66,68,69 used OAT registry data. Mortality data during and after OAT were stratified by methadone treatment for 28 of 36 studies (78%)37,38,39,40,42,43,44,45,46,48,49,52,53,54,55,56,57,58,60,61,62,63,64,65,66,67,69,70 and by buprenorphine treatment for 8 of 36 studies (22%).41,45,54,55,57,58,59,69 Type of OAT was unspecified in 6 of 36 studies (17%).35,36,47,50,51,68
All-Cause Mortality During and Out of OAT
The pooled all-cause CMR while enrolled in any form of OAT was 11.00 deaths per 1000 PY (95% CI, 9.20-13.16) compared with 23.97 deaths per 1000 PY when not receiving OAT (95% CI, 19.92-28.83). Being in OAT was associated with more than half the risk of mortality (RR, 0.47; 95% CI, 0.42-0.53; 30 of 36 studies [83%]34,35,36,38,39,41,42,43,44,45,48,49,50,52,53,54,55,56,57,58,59,61,62,63,64,65,66,67,68,69; 562 714 patients) (eFigure 2 in the Supplement). The association was not different for methadone (RR, 0.47; 95% CI, 0.41-0.54; 23 of 36 studies [64%]38,39,40,42,43,44,45,46,48,49,52,53,54,56,57,61,62,63,64,65,66,67,69) or buprenorphine (RR, 0.34; 95% CI, 0.26-0.45; 8 of 36 studies [22%]41,45,54,55,57,58,59,69) (Figure).
Figure. Studies on the Association of Opioid Agonist Treatment (OAT) With All-Cause Mortality From Randomized Clinical Trials and Cohort Studies by Administration of Buprenorphine or Methadone.
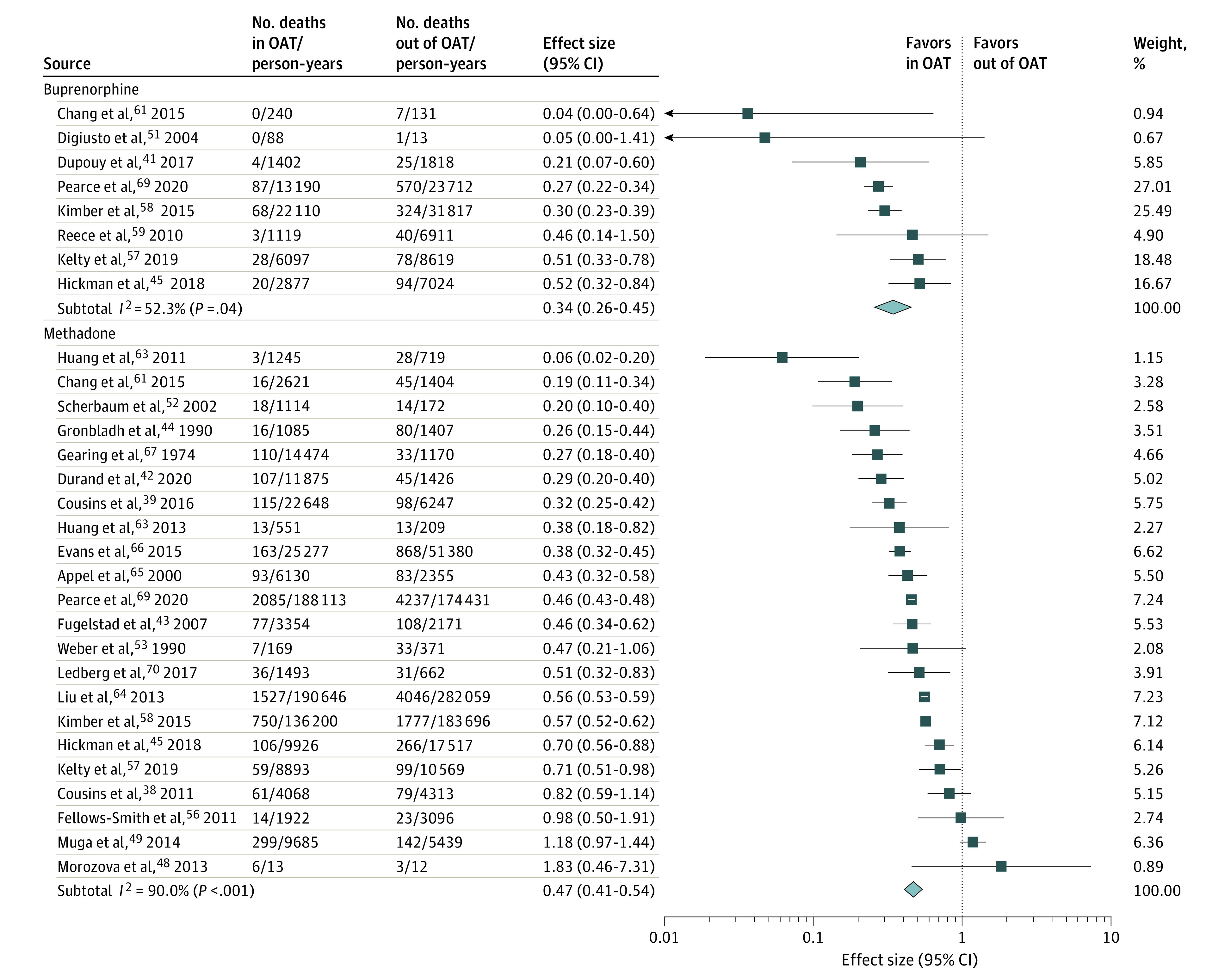
Weights are from random-effects analysis.
Table 1 shows mortality rates and RRs for time in and out of OAT according to participant characteristics, treatment administrators, study methodologies, and region. Although absolute mortality rates during and after OAT varied (Table 1), the RRs for time during and out of OAT were similar across subgroups of people with opioid dependence (eg, by sex, age group, HIV or hepatitis C virus [HCV] status, and taking drugs via injection). Study findings were consistent across study design, mortality ascertainment method, and region. Meta-regressions found no evidence for variation of RR by study year, sample size, follow-up time, age, proportion of women, proportion of people who inject drugs, or people with HIV or HCV (Table 1; eTable 16 in the Supplement).
Table 1. Findings From Observational Studies on the Association of Time During Opioid Agonist Treatment (OAT) and Out of OAT With All-Cause Mortality According to Demographic, Clinical, and Study-Level Variables.
| Strata | References | No. of studies | Person-years | CMR per 1000 person-years during OAT (95% CI) | I2, % | CMR per 1000 person-years out of OAT (95% CI) | I2, % | Rate ratio (95% CI) | I2, % | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| During OAT | Out of OAT | |||||||||||
| Participant characteristics | ||||||||||||
| Sex | ||||||||||||
| Women | 38, 39, 41, 42, 45, 49, 50, 57, 58, 61, 63, 65, 69, 70 | 14 | 204 366 | 237 402 | 9.56 (7.30-12.53) | 94 | 15.49 (11.62-20.65) | 97 | 0.56 (0.45-0.69) | 77 | ||
| Men | 38, 39, 41, 42, 45, 49, 50, 57, 58, 61, 63, 65, 69, 70 | 14 | 254 166 | 253 977 | 9.85 (7.00-13.86) | 98 | 23.32 (16.10-33.78) | 99 | 0.45 (0.38-0.53) | 80 | ||
| Age, y | ||||||||||||
| <35 | 38, 39, 41, 42, 45, 49, 50, 57, 58, 61, 63, 69, 70 | 13 | 255 219 | 301 704 | 6.09 (3.72-9.97) | 98 | 13.69 (10.65-17.60) | 97 | 0.48 (0.34-0.68) | 93 | ||
| ≥35 | 38, 39, 41, 42, 45, 49, 50, 57, 58, 61, 63, 69, 70 | 13 | 194 443 | 190 105 | 14.27 (10.86-18.75) | 95 | 28.74 (22.41-36.85) | 97 | 0.48 (0.38-0.61) | 88 | ||
| People who inject drugs | 49, 50, 61, 63, 69 | 5 | 144 795 | 125 822 | 14.31 (8.08-25.37) | 98 | 27.56 (17.39-43.70) | 97 | 0.52 (0.28-0.94) | 96 | ||
| People who do not inject drugs | 49, 50, 61, 69 | 4 | 75 134 | 90 922 | 6.83 (3.88-12.04) | 83 | 10.36 (5.24-20.48) | 89 | 0.32 (0.09-1.17) | 91 | ||
| HIV positive | 41, 49, 50, 61, 63, 64, 69 | 7 | 35 878 | 35 697 | 33.72 (25.26-45.02) | 93 | 58.46 (49.58-68.94) | 80 | 0.56 (0.38-0.82) | 92 | ||
| HCV positive | 49, 50, 57, 61, 63, 69 | 6 | 37 100 | 30 641 | 14.41 (6.81-30.50) | 98 | 28.28 (11.83-67.63) | 99 | 0.53 (0.36-0.77) | 85 | ||
| Treatment administrator | ||||||||||||
| Specialist | 34, 36, 39, 41-44, 49, 61, 63, 67, 69 | 12 | 108 200 | 53 796 | 13.41 (9.75-18.45) | 97 | 34.54 (28.91-41.19) | 90 | 0.39 (0.28-0.54) | 93 | ||
| General practitioner, mixed, or other | 35, 38, 39, 41, 42, 45, 52, 53, 55, 56, 58, 59, 62, 64, 68, 69 | 16 | 507 753 | 643 343 | 9.09 (7.48-11.05) | 95 | 20.98 (16.02-27.47) | 99 | 0.47 (0.41-0.55) | 85 | ||
| Geographic coverage | ||||||||||||
| Single clinic | 35, 44, 49, 53, 59 | 5 | 13 347 | 15 021 | 15.98 (8.29-30.81) | 90 | 29.48 (13.41-64.79) | 98 | 0.50 (0.23-1.07) | 89 | ||
| City or state | 38, 42, 43, 50, 52, 54, 56-58, 63, 65-69 | 15 | 579 229 | 653 601 | 10.20 (8.22-12.65) | 98 | 21.79 (16.22-29.29) | 0.48 (0.42-0.54) | 85 | |||
| National | 34, 36, 39, 41, 45, 48, 55, 61, 62, 64 | 10 | 277 189 | 336 357 | 0.40 (0.31-0.51) | 88 | 23.93 (18.55-30.86) | 96 | 0.40 (0.31-0.51) | 88 | ||
| GBD region | ||||||||||||
| Australasia | 54-59 | 6 | 304 121 | 372 567 | 5.96 (5.69-6.24) | 0 | 9.54 (8.18-11.12) | 90 | 0.57 (0.47-0.70) | 78 | ||
| East Asia | 61-64 | 4 | 195 303 | 284 522 | 8.00 (4.37-14.66) | 86 | 32.09 (15.84-65.02) | 97 | 0.24 (0.10-0.58) | 91 | ||
| Europe | 34-36, 38, 39, 41-45, 48-50, 52, 53 | 15 | 119 889 | 79 458 | 15.06 (10.85-20.91) | 97 | 29.20 (21.62-39.45) | 97 | 0.50 (0.38-0.64) | 89 | ||
| North America | 65-69 | 5 | 250 452 | 268 433 | 10.72 (7.98-14.41) | 95 | 29.03 (19.72-42.73) | 99 | 0.40 (0.34-0.46) | 60 | ||
| Death verification | ||||||||||||
| Comprehensive death registers | 34-36, 38, 39, 41-45, 49, 50, 52, 54, 56-59, 61-63, 66, 68, 69 | 24 | 658 065 | 718 992 | 9.68 (7.82-11.98) | 98 | 21.93 (17.10-26.77) | 99 | 0.47 (0.42-0.54) | 89 | ||
| Other death verification | 48, 53, 55, 64, 65, 67 | 6 | 211 700 | 285 987 | 22.68 (12.80-40.18) | 97 | 49.11 (23.79-101.36) | 98 | 0.41 (0.26-0.66) | 86 | ||
Abbreviations: CMR, crude mortality rate; GBD, Global Burden of Disease; HCV, hepatitis C virus.
Cause-Specific Mortality During and Out of OAT
Results of all-cause and cause-specific mortality analyses are presented in Table 2. While receiving OAT, people with opioid dependence were at lower risk of all injury and poisoning (pooled RR, 0.34; 95% CI, 0.27-0.42), suicide (pooled RR, 0.48; 95% CI, 0.37-0.61), cancer (pooled RR, 0.72; 95% CI, 0.54-0.98), and alcohol-related (pooled RR, 0.59; 95% CI, 0.49-0.72) and cardiovascular-related (pooled RR, 0.69; 95% CI, 0.60-0.79) mortality. The strongest association with lower mortality risk while receiving OAT was observed for deaths related to injury or poisoning, specifically, unintentional drug-related death (pooled RR, 0.41; 95% CI, 0.33-0.52) and suicide (pooled RR, 0.48; 95% CI, 0.37-0.61). Liver disease–related deaths in general were lower during OAT, but there was an association between OAT and viral hepatitis mortality (RR, 1.35; 95% CI, 1.15-1.60). Forest plots for each cause of death are presented in eFigures 4-24 in the Supplement.
Table 2. Findings From Observational Studies on the Pooled All-Cause and Cause-Specific Crude Mortality Rates, and Mortality Rate Ratios Among People With Opioid Dependence According to Time Spent During and Out of OAT.
| Variable | References | No. of cohortsa | No. of people | CMR with treatment | CMR without treatment | Rate ratio with OAT/without OAT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PY | Deaths | Pooled CMR per 1000 PY (95% CI) | I2, % | PY | Deaths | Pooled CMR per 1000 PY (95% CI) | I2, % | Rate ratio (95% CI) | I2, % | ||||
| All-cause | 34-36, 38, 39, 41-45, 48-50, 52-59, 61-69 | 30 | 562 714 | 869 497 | 7438 | 11.00 (9.20-13.16) | 97.8 | 1 004 979 | 16 349 | 23.97 (19.92-28.83) | 99.1 | 0.47 (0.42-0.53) | 88.6 |
| All injury and poisoning | 36, 43-45, 49, 50, 52, 54, 55, 57-59, 61, 63, 65, 69, 70 | 17 | 179 184 | 563 194 | 2014 | 4.73 (3.80-5.90) | 93.9 | 607 523 | 5677 | 15.31 (11.70-20.03) | 98.6 | 0.34 (0.27-0.42) | 90.2 |
| Drug-induced deaths | 34, 36-40, 42-45, 50, 51, 53-55, 57-59, 63, 65, 67, 69, 70 | 22 | 364 971 | 908 947 | 2351 | 3.03 (2.52-3.63) | 92.8 | 808 982 | 5412 | 7.90 (6.07-10.28) | 98.7 | 0.41 (0.33-0.52) | 92.0 |
| Unintentional drug-induced deaths | 44, 45, 50, 54, 55, 57-59, 63, 69, 70 | 11 | 166 044 | 509 209 | 1026 | 2.14 (1.78-2.56) | 78.2 | 577 366 | 3674 | 7.32 (4.89-10.94) | 99.1 | 0.35 (0.27-0.46) | 89.3 |
| Unintentional opioid deaths | 44, 45, 50, 54, 55, 57-59, 63, 65, 68-70 | 13 | 185 166 | 511 428 | 772 | 1.96 (1.32-2.91) | 95.2 | 590 416 | 3203 | 7.14 (4.55-11.20) | 99.2 | 0.31 (0.23-0.43) | 88.1 |
| Suicide | 36, 43-45, 50, 53-55, 57-59, 63, 69, 70 | 14 | 175 032 | 542 285 | 293 | 0.79 (0.53-1.17) | 86.1 | 598 393 | 723 | 1.36 (1.03-1.80) | 85.2 | 0.48 (0.37-0.61) | 37.0 |
| Violence | 44, 45, 50, 53-55, 57-59, 63, 65, 69, 70 | 13 | 167 885 | 493 943 | 89 | 0.30 (0.10-0.86) | 95.1 | 528 904 | 150 | 0.58 (0.22-1.55) | 96.4 | 0.72 (0.39-1.33) | 66.3 |
| Motor vehicle and transport injuries | 44, 45, 50, 54, 55, 57-59, 63, 70 | 10 | 110 697 | 306 359 | 115 | 0.63 (0.27-1.48) | 91.1 | 385 455 | 133 | 0.39 (0.24-1.97) | 94.7 | 0.90 (0.56-1.45) | 31.1 |
| Falls, fires, burns, drownings | 44, 45, 50, 54, 55, 57-59, 63, 70 | 10 | 110 697 | 301 278 | 26 | 0.16 (0.07-0.39) | 68.9 | 372 786 | 37 | 0.17 (0.08-0.37) | 75.4 | 0.88 (0.52-1.48) | 0.0 |
| All liver-related | 44, 45, 49, 50, 53-55, 57-59, 61, 63, 69, 70 | 14 | 169 002 | 519 613 | 597 | 1.19 (0.80-1.77) | 91.3 | 589 295 | 702 | 1.16 (0.78-1.72) | 94.2 | 0.89 (0.67-1.19) | 57.7 |
| Viral hepatitis | 43-45, 49, 50, 54, 55, 57-59, 61, 63, 69, 70 | 14 | 169 553 | 521 162 | 340 | 0.75 (0.44-1.27) | 93.2 | 583 020 | 262 | 0.45 (0.29-0.71) | 87.8 | 1.35 (1.15-1.60) | 0.0 |
| All alcohol-related | 43-45, 49, 50, 54, 55, 57-59, 61, 63, 65, 69, 70 | 15 | 171 097 | 525 967 | 174 | 0.34 (0.13-0.87) | 96.4 | 579 300 | 296 | 0.67 (0.34-1.30) | 95.9 | 0.59 (0.49-0.72) | 0.0 |
| Cancer | 43-45, 49, 50, 54, 55, 57-59, 61, 63, 69, 70 | 14 | 169 553 | 524 023 | 522 | 0.97 (0.57-1.66) | 95.1 | 591 466 | 958 | 0.99 (0.56-1.74) | 97.5 | 0.72 (0.54-0.98) | 63.2 |
| Cardiovascular disease | 43-45, 49, 50, 54, 55, 57-59, 61, 63, 69, 70 | 14 | 169 553 | 524 023 | 429 | 0.82 (0.55-1.22) | 88.8 | 592 224 | 677 | 1.14 (0.74-1.76) | 93.9 | 0.69 (0.60-0.79) | 4.1 |
| Chronic respiratory disease | 44, 45, 49, 50, 54, 55, 57-59, 61, 63, 69, 70 | 13 | 168 705 | 497 738 | 176 | 0.35 (0.16-0.74) | 92.3 | 581 398 | 199 | 0.28 (0.14-0.58) | 92.1 | 0.95 (0.78-1.17) | 0.0 |
| Digestive disorders | 44, 45, 49, 50, 54, 55, 57-59, 61, 63, 69, 70 | 13 | 168 705 | 502 819 | 74 | 0.20 (0.11-0.34) | 72.1 | 551 954 | 106 | 0.30 (0.18-0.52) | 84.7 | 0.72 (0.41-1.26) | 50.0 |
| HIV related | 43-45, 49, 50, 53-55, 57-59, 61, 63, 69, 70 | 15 | 169 850 | 493 493 | 383 | 1.51 (0.38-6.02) | 99.3 | 565 738 | 307 | 1.14 (0.38-3.40) | 98.5 | 1.14 (0.75-1.74) | 72.9 |
| Influenza and pneumonia | 43-45, 49, 50, 53-55, 57-59, 61, 63, 69, 70 | 15 | 169 850 | 512 728 | 101 | 0.20 (0.09-0.44) | 87.9 | 572 650 | 115 | 0.29 (0.17-0.62) | 88.1 | 0.92 (0.70-1.20) | 0.0 |
| Injection-related injuries and diseases | 44, 45, 49, 50, 54, 55, 57-59, 61, 63, 69, 70 | 13 | 168 705 | 500 359 | 144 | 0.36 (0.19-0.69) | 87.9 | 585 263 | 178 | 0.41 (0.20-0.84) | 93.0 | 0.90 (0.72-1.12) | 0.0 |
| Endocarditis | 44, 45, 49, 50, 54, 55, 57-59, 61, 63, 69, 70 | 13 | 168 705 | 487 813 | 74 | 0.23 (0.10-0.52) | 88.9 | 565 575 | 102 | 0.25 (0.09-0.69) | 94.9 | 0.80 (0.59-1.08) | 0.0 |
| Bacteremia or sepsis | 44, 45, 49, 50, 53-55, 57-59, 61, 63, 69, 70 | 14 | 169 002 | 499 274 | 43 | 0.09 (0.05-0.18) | 64.1 | 558 128 | 51 | 0.17 (0.08-0.36) | 81.8 | 0.93 (0.62-1.40) | 0.0 |
| Skin or soft-tissue infections | 44, 45, 49, 50, 54, 55, 57-59, 61, 63, 69, 70 | 13 | 168 705 | 486 728 | 23 | 0.06 (0.02-0.17) | 80.5 | 546 515 | 23 | 0.08 (0.02-0.27) | 87.0 | 1.17 (0.65-2.09) | 0.0 |
Abbreviations: CMR, crude mortality rates; OAT, opioid agonist treatment; PY, person-years of follow-up.
Mortality by Specific Periods During OAT and After OAT Cessation
Stratified OAT time interval and all-cause mortality data were available for 1438,39,41,42,45,49,50,54,58,61,62,65,69,70 cohort studies (39%; 175 213 people). The remainder of time spent in OAT (after the first 4 weeks of treatment) was the period of lowest mortality risk overall. In the first 4 weeks of OAT, all-cause mortality was almost double that in the remainder of time spent in treatment (RR, 1.92; 95% CI, 1.10-3.35). Compared with the remainder of time spent in OAT, all-cause mortality was 6 times higher in the first 4 weeks after OAT cessation (RR, 6.01; 95% CI, 4.32-8.36) and remained at double the rate for the remainder of time spent while not receiving OAT (RR, 1.81; 95% CI, 1.50-2.18) (Figure; eFigure 26 in the Supplement). A similar pattern emerged for drug-induced and unintentional opioid-related deaths.
Acute causes of mortality that may be associated with OAT are listed in eTable 17 in the Supplement; these causes were stratified by times in and out of OAT (forest plots are presented in eFigures 27-33 in the Supplement; data on other causes of death stratified by OAT time period are presented in eTable 18 in the Supplement; and analyses using first 2-week time periods are presented in eTable 19 in the Supplement). Cause-specific mortality rates were lowest after the first 4 weeks in OAT (ie, remainder in OAT; eTable 17 in the Supplement). For pooled RRs, rate of mortality during the remainder of time receiving OAT was used as the referent. Because of insufficient mortality data (eg, more than half the included studies reported 0 deaths for a specific cause), RRs were not reported for less common causes of death.
Mortality During and After Incarceration for People Receiving OAT
Table 3 presents results of analyses during and after incarceration for people receiving OAT compared to those not receiving OAT. Only 1 study18 that examined the OAT during incarceration was identified, with OAT associated with reduced all-cause (RR, 0.06; 95% CI, 0.01-0.46), drug-related, and suicide-related (RR, 0.11; 95% CI, 0.01-0.85) mortality during the first 4 weeks of treatment and the entire time spent in incarceration.
Table 3. Findings From Observational Studies on the Pooled Cause-Specific Rates Among People Receiving OAT, by Time During Incarceration and After Release From Incarceration.
| Settinga | No. of cohorts | No. of people | With OAT | Without OAT | Rate ratio (with OAT/without OAT) | I2, % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deaths | PY | Pooled CMR (95% CI) | I2, % | Deaths | PY | Pooled CMR (95% CI) | I2, % | |||||
| Mortality during incarceration 18 , b | ||||||||||||
| First 4 wk in prison | ||||||||||||
| All-cause | 1 | 16 715 | 1 | 1308 | 0.77 (0.11-5.43) | NA | 16 | 1263 | 12.67 (7.76-20.68) | NA | 0.06 (0.01-0.46) | NA |
| Drug-related | 1 | 16 715 | 0 | 1308 | NA | NA | 1 | 1263 | 0.79 (0.11-5.62) | NA | NAc | NA |
| Suicide | 1 | 16 715 | 1 | 1308 | 0.77 (0.11-5.43) | NA | 9 | 1263 | 7.13 (3.71-13.70) | NA | 0.11 (0.01-0.85) | NA |
| Remainder in prison | ||||||||||||
| All-cause | 1 | 16 715 | 10 | 15 132 | 0.66 (0.36-1.23) | NA | 24 | 13 285 | 1.81 (1.21-2.70) | NA | 0.37 (0.17-0.76) | NA |
| Drug-related | 1 | 16 715 | 0 | 15 132 | NA | NA | 5 | 13 285 | 0.38 (0.16-0.90) | NA | NAc | NA |
| Suicide | 1 | 16 715 | 2 | 15 132 | 0.13 (0.03-0.53) | NA | 7 | 13 285 | 0.53 (0.25-1.11) | NA | 0.25 (0.05-1.21) | NA |
| Total time in prison | ||||||||||||
| All-cause | 1 | 16 715 | 11 | 16 440 | 0.67 (0.37-1.21) | NA | 40 | 14 548 | 2.75 (2.02-3.75) | NA | 0.24 (0.12-0.47) | NA |
| Drug-related | 1 | 16 715 | 0 | 16 440 | NA | NA | 6 | 14 548 | 0.41 (0.19-0.92) | NA | NAc | NA |
| Suicide | 1 | 16 715 | 3 | 16 440 | 0.18 (0.06-0.57) | NA | 16 | 14 548 | 1.10 (0.67-1.80) | NA | 0.17 (0.05-0.57) | NA |
| Mortality after release from incarceration d | ||||||||||||
| First 4 wk after release47,71 | ||||||||||||
| All-cause | 2 | 28 713 | 25 | 2856 | 8.76 (5.92-12.96) | 0 | 95 | 2578 | 36.85 (30.14-45.06) | 0 | 0.24 (0.15-0.37) | 0 |
| Drug-related | 2 | 28 713 | 10 | 2856 | 3.55 (1.91-6.61) | 0 | 47 | 2578 | 21.11 (10.74-41.52) | 79.5 | 0.19 (0.10-0.37) | 0 |
| Suicide | 2 | 28 713 | 0 | 2856 | NA | NA | 3 | 2578 | 1.23 (0.40-3.82) | 0 | NAc | 0 |
| Remainder after release47,71 | ||||||||||||
| All-cause | 2 | 28 713 | 212 | 21 812 | 9.98 (7.99-12.46) | 60.4 | 245 | 19 828 | 12.42 (10.96-14.07) | 0 | 0.82 (0.58-1.15) | 65.3 |
| Drug-related | 2 | 28 713 | 66 | 21 812 | 3.13 (0.70-13.91) | 97.1 | 95 | 19 828 | 5.29 (2.75-10.20) | 90.4 | 0.59 (0.25-1.36) | 84.7 |
| Suicide | 2 | 28 713 | 18 | 21 812 | 0.87 (0.20-3.87) | 89.2 | 13 | 19 828 | 0.73 (0.32-1.64) | 55.5 | 1.19 (0.58-2.47) | 0 |
| Total time after release | ||||||||||||
| All-cause47,60,71 | 3 | 29 004 | 279 | 25 275 | 18.92 (6.57-54.46) | 98.6 | 360 | 22 666 | 23.83 (12.66-44.87) | 95.6 | 0.72 (0.52-1.00) | 67.1 |
| Drug-related47,71 | 2 | 28 713 | 76 | 24 669 | 3.30 (0.87-12.47) | 97.0 | 142 | 22 407 | 6.98 (3.97-12.29) | 91.1 | 0.47 (0.22-1.01) | 85.9 |
| Suicide47,71 | 2 | 28 713 | 18 | 24 669 | 0.78 (0.17-3.60) | 89.8 | 16 | 22 407 | 0.79 (0.37-1.70) | 58.7 | 0.97 (0.45-2.08) | 17.7 |
| Mortality after release from incarceration e | ||||||||||||
| First 4 wk after release71 | ||||||||||||
| All-cause | 1 | 16 453 | 17 | 2129 | 7.98 (4.96-12.84) | NA | 79 | 2172 | 36.37 (29.17-45.35) | NA | 0.22 (0.13-0.37) | NA |
| Drug-related | 1 | 16 453 | 6 | 2129 | 2.82 (1.27-6.27) | NA | 33 | 2172 | 15.19 (10.80-21.37) | NA | 0.19 (0.08-0.44) | NA |
| Suicide | 1 | 16 453 | 0 | 2129 | NA | NA | 2 | 2172 | 0.92 (0.23-3.68) | NA | NAc | NA |
| Remainder after release71 | ||||||||||||
| All-cause | 1 | 16 453 | 46 | 13 309 | 3.46 (2.59-4.61) | NA | 276 | 16 333 | 16.90 (15.02-19.02) | NA | 0.20 (0.15-0.28) | NA |
| Drug-related | 1 | 16 453 | 9 | 13 309 | 0.68 (0.35-1.30) | NA | 68 | 16 333 | 4.16 (3.28-5.28) | NA | 0.16 (0.08-0.33) | NA |
| Suicide | 1 | 16 453 | 4 | 13 309 | 0.30 (0.11-0.80) | NA | 9 | 16 333 | 0.55 (0.29-1.06) | NA | 0.55 (0.17-1.77) | NA |
| Total time after release | ||||||||||||
| All-cause62,71 | 2 | 18 435 | 66 | 16 684 | 3.98 (3.13-5.07) | 0 | 382 | 18 890 | 36.21 (10.16-129.00) | 97.6 | 0.09 (0.02-0.56) | 88.0 |
| Drug-related71 | 1 | 16 453 | 15 | 16 453 | 0.97 (0.59-1.61) | NA | 101 | 18 505 | 5.46 (4.49-6.63) | NA | 0.18 (0.10-0.31) | NA |
| Suicide71 | 1 | 16 453 | 4 | 16 453 | 0.30 (0.10-0.69) | NA | 11 | 18 505 | 0.59 (0.33-1.07) | NA | 0.44 (0.14-1.37) | NA |
Abbreviations: CMR, crude mortality rates; NA, not available; OAT, opioid agonist treatment; PY, person-years of follow-up.
“First 4 wk” refers to the first 4 weeks in or out of incarceration; “remainder” refers to remainder of time in incarceration or after release; “total time” refers to total time spent in incarceration, or total time after release up to 12 months after release from incarceration. Additional causes of mortality and for all forest plots pertaining to this table are displayed in eAppendix 8 in the Supplement.
“With OAT” data represent time spent with OAT during incarceration; “Without OAT” data represent time spent without OAT during incarceration.
Rate ratios are suppressed where at least half of the studies reported 0 deaths for 1 of the time periods involved.
“With OAT” data represent individuals who left incarceration on OAT; “Without OAT” data represent individuals who left incarceration not on OAT.
“With OAT” data represent time spent with OAT after release from incarceration: “Without OAT” data represent time spent without OAT after release from incarceration.
Three studies47,60,71 reported mortality after release from prison for people who initiated OAT during incarceration, compared with those who had opioid dependence and were incarcerated but had not received OAT. All-cause (RR, 0.24; 95% CI, 0.15-0.37) and drug-related (RR, 0.19; 95% CI, 0.10-0.37) mortality was lower during the first 4 weeks after release from prison for people who left incarceration still receiving OAT compared with those who were released but were not receiving OAT.
Two studies62,71 followed all-cause mortality rates while receiving and not receiving OAT after release from incarceration. Time spent in OAT after release was associated with lower all-cause (RR, 0.09; 95% CI, 0.09-0.56) and drug-related mortality (RR, 0.18; 95% CI, 0.10-0.31) in 1 study71 (eFigures 34-36 and eTable 20 in the Supplement).
Study Quality and Risk of Bias
Twelve RCTs (80%) were assessed as having high risk of bias largely due to missing outcome data and measurement of outcome (eTables 44 and 45 in the Supplement).20,22,23,25,26,27,28,29,30,31,32,33 Assessments of observational studies using the ROBINS-I tool included in all-cause mortality analyses found that most studies were at moderate risk of bias (26 of 30 [87%]).34,35,36,37,38,39,40,41,42,43,44,45,49,51,54,56,57,58,59,61,63,66,67,68,69,70 Some studies were at serious risk of bias (4 [13%]),50,55,64,65 and 1 study (3%) was at critical risk of bias owing to measurement of mortality data and participant loss to follow-up.48 The sole study of OAT for patients who were incarcerated was at low risk of bias.18 The other studies with follow-up time during or out of OAT after incarceration were at moderate risk of bias (eFigures 39-43 in the Supplement).47,60,62,71
Nineteen observational studies conducted multivariable analyses adjusting for a range of potential confounders on all-cause (and in some cases overdose-related) mortality (Table 4).18,34,38,39,40,41,42,45,47,51,54,60,62,63,64,66,68,69,70 There was minimal change in the RRs after controlling for confounding, regardless of the variables included (eTable 21 in the Supplement).
Table 4. Summary of Studies That Adjusted for Confounding in Observational Studies of the Association of OAT With Mortality.
| Sourcea | No. of people | OAT setting | Variables included | Association of OAT with all-cause mortality, estimate (95% CI) | |
|---|---|---|---|---|---|
| Bivariate | Adjusted | ||||
| Abrahamsson et al,34 2017 | 4501 | Community OAT | Sex, age, inpatient treatment for previous (1) mental disorders, (2) nonfatal overdoses, and (3) suicide attempts | HR, 0.56 (0.45-0.69) | AHR, 0.55 (0.44-0.68) |
| Ledberg et al,70 2017b,c,d | 441 | Community OAT | Sex, age, year of treatment entry | NR | AHR, 0.47 (0.29-0.77) |
| Bazazi et al,60 2018 | 291 | Released from incarceration with OAT | Age, marital status, ethnicity, employment, education, prison sentence, dependence severity, injecting history, HIV symptoms, study randomization phase | HR, 1.32 (0.58-3.03) | AHR, 1.07 (0.44-2.61) |
| Cousins et al,38 2011b | 3162 | Community OAT | Sex, age, social class, comorbidity, methadone dose, No. of methadone treatment episodes, psychiatric admission, benzodiazepine prescription, urine drug tests, overuse of methadone | HR, 1.19 (0.70-2.04) [drug-related only] | NR [drug-related only] |
| Cousins et al,39 2016b | 6983 | Community OAT | Time period of treatment, sex, age, No. of treatment episodes, methadone dose, supervised consumption, median comorbidity score, co-prescriptions | RR, 0.32 (0.18-0.57) | ARR, 0.27 (0.16-0.47) |
| Davoli et al,40 2007 | 10 258 | Community OAT | Age, sex, psychiatric comorbidity, HIV status, previous nonfatal overdose, route of administration, length of use. | HR, 0.09 (0.04-0.19) [overdose only] | AHR, 0.09 (0.04-0.19) [overdose only] |
| Dupouy et al,41 2017b | 713 | Community OAT | Age, sex, Charlson Comorbidity Index, psychiatric diagnoses recorded as chronic diseases or during a hospitalization, dispensed psychoactive medications, socioeconomic covariates (ecologic estimator of social deprivation, the status of the beneficiary, and universal insurance coverage) | HR, 0.03 (0.01-0.09) | AHR, 0.03 (0.01-0.09) |
| Durand et al,42 2020b | 2899 | Community OAT | Sex, age, incarceration history, methadone dose, mental disorders, disease categories | RR, 0.57 (0.38-0.97) | ARR, 0.49 (0.29-0.83) |
| Evans et al,66 2015 | 32 322 | Community OAT | Primary drug, age, sex, race/ethnicity, disability, labor force status, Medi-Cal beneficiary, mentally ill, hepatitis C status, tuberculosis status, hospitalization in past 30 d, HIV testing status, educational attainment, years from first primary drug use to first treatment episode, criminal justice system involvement, secondary drug type | NA | AHR, 0.27 (0.23-0.32) |
| Hickman et al,45 2018b | 11 033 | Community OAT | Sex, age, calendar year, comorbidity score, geographic region, benzodiazepine coprescription, gabapentinoid coprescription, number of OAT patients per GP practice, number of GPs prescribing per practice, history of recorded self-harm, overdose poisoning, alcohol problems, imprisonment, or homelessness; propensity scores based on the probability of being prescribed buprenorphine were incorporated into models as inverse probability weights | IRR, 0.45 (0.37-0.55) | AIRR, 0.36 (0.31-0.41) |
| Huang et al,62 2011 | 1982 | OAT after release | Age, sex, area of residence, HIV status prior to release | NA | AHR, 0.07 (0.02-0.21) |
| Huang et al,63 2013b | 1616 | Community OAT | Sex, age, living with partner, education, cardiovascular disease, pneumonia/bronchitis/asthma, diabetes, HBV antigen, HCV antibody, HIV antibody, syphilis antibody, psychiatric treatment, multiple illicit substance use, alcohol disorder, previous drug overdose, previous suicide attempts, methadone dose, duration heroin use | HR, 0.32 (0.14-0.72) | AHR, 0.32 (0.13-0.80) |
| Degenhardt et al,71 2014b,c,d | 16 453 | Released from incarceration with OAT | Sex, age, indigenous status, juvenile offending history, length of prison episode, No. of prior prison episodes, prior property offense, prior drug offense, prior violent offense | First 4 wk after release: | |
| OAT after release | HR, 0.32 (0.21-0.50) | AHR, 0.06 (0.01-0.30)c | |||
| Total time after release: | |||||
| HR, 0.22 (0.13-0.37) | AHR, 0.25 (0.12-0.53) | ||||
| Larney et al,18 2014c,d | 16 715 | OAT during incarceration | Sex, indigenous status, age, incarceration history (number and duration), offending history (drug, property, and violent offenses) | HR, 0.25 (0.13-0.48) | AHR, 0.26 (0.13-0.5) |
| Larochelle et al,68 2018 | 17 568 | Community OAT | Age, sex, anxiety diagnosis, depression diagnosis, receipt of methadone, buprenorphine, opioid, and benzodiazepine prescriptions in the 12 mo before index nonfatal opioid overdose, benzodiazepine prescriptions, detoxification episode and short- and long-term residential treatments | NA | M-AHR, 0.37 (0.24-0.59) |
| B-AHR, 0.35 (0.23-0.53) | |||||
| Liu et al,64 2013 | 306 786 | Community OAT | Age, sex, marital status, education, ethnicity, route of administration, needle sharing, methadone dose, HIV status | HR, 0.53 (0.50-0.56) | AHR, 0.56 (0.53-0.59) |
| Marsden et al,47 2017b | 12 260 | Released from incarceration with OAT | Age, injection drug use, self-reported problem alcohol use, nonmedical benzodiazepine use, cocaine use, demographic and clinical covariates, prison transfer, community treatment | HR, 0.25 (0.10-0.60) | AHR, 0.25 (0.09-0.64) |
| Pearce et al,69 2020b | 55 347 | Community OAT | Age, sex, medication type (buprenorphine/naloxone only, methadone only), OAT period | HR, 0.45 (0.43-0.49) | AHR, 0.40 (0.34-0.46) |
| Pierce et al,51 2016 | 151 983 | Community OAT | Age, sex, patient-reported injecting status, patient-reported problematic use of alcohol, benzodiazepines, crack cocaine, cocaine powder, amphetamines; referred from the criminal justice system | HR, 0.64 (0.58-0.71) [drug-related only] | AHR, 0.58 (0.52-0.64) [drug-related only] |
Abbreviations: AHR, adjusted hazard ratio; AIRR, adjusted incidence rate ratio; ARR, adjusted relative risk; HBV, hepatitis B virus; HCV, hepatitis C virus; HR, hazard ratio; IRR, incidence rate ratio; NA, not available; NR, not reported; OAT, opioid agonist treatment; RR, relative risk; Rx, treatment.
Authors sent additional information.
There was an interaction with time since release with the protective association extremely marked in first few days and then decaying.
These are secondary studies from which some data have been used in subanalyses. Secondary studies are listed directly below the corresponding primary article.
Sensitivity analyses are reported in eFigures 46-49 in the Supplement. None of the analyses yielded results that changed the direction or size of associations reported in the main findings.
Discussion
To our knowledge, this systematic review and meta-analysis is the first to document the association of OAT across different settings with both all-cause and cause-specific mortality. We synthesized 36 observational cohort studies that assessed mortality risk during and out of OAT, which represented a 3-fold increase from a previous review of all-cause mortality7 that included 19 cohorts. Our findings suggest a potential public health benefit of OAT, which was associated with a greater than 50% lower risk of all-cause mortality, drug-related deaths, and suicide and was associated with significantly lower rates of mortality for other causes. The association was consistent across a range of participant and study characteristics.
Our results suggest that RCTs of OAT were underpowered to examine mortality risk. There was no significant association between OAT and mortality risk in the pooled community RCTs; in 7 studies,19,20,23,25,28,29,30 no deaths were reported. None of the 3 RCTs conducted on the effect of OAT during or after incarceration could be used to guide inferences.19,21,24
Several examples of discrepant findings and conclusions on the benefits of treatment between RCTs and observational studies, such as hormone replacement treatment on cardiovascular outcomes72 and vitamin, mineral, or fish oil supplements on noncommunicable disease,73 exist. Discrepancies between findings from RCTs and observational studies and mortality outcomes described in the current review are not the same because (1) associations between OAT and all-cause mortality (as well as overdose and suicide) were strong, and (2) there was little difference between adjusted and unadjusted estimates; therefore, it was unlikely that unmeasured confounding would alter the findings.
People with opioid dependence were at substantially lower risk of suicide, cancer, drug-related, alcohol-related, and cardiovascular-related mortality during OAT compared with time while not receiving OAT. The association between OAT and a lower risk of overdose was identified in previous reviews.7 The reductions in alcohol-related, cardiovascular-related, and cancer-related mortality may be due to reductions in alcohol use during OAT; similarly, reduced stimulant use may be associated with reduced cardiovascular mortality. The reductions in cardiovascular and cancer-related mortality may also reflect greater access to screening, early intervention, and treatment as a result of improved engagement with treatment administrators.
By contrast, viral hepatitis mortality was higher among those who received OAT. This may reflect an issue of competing risks74 (with people retained in OAT living longer and therefore at higher risk of HCV infection). However, we believe this may have been due to misclassification. As we show in eAppendix 11, eFigure 49 in the Supplement, 8 cohorts45,49,50,54,57,58,69,70 reported both overall liver-related and viral hepatitis mortality during and out of OAT. There was an elevated risk of mortality due to viral hepatitis, suggesting that OAT status may have affected coronial decisions around attribution of the liver-related cause of death. Until very recently, there were no highly effective treatments for HCV, and coverage availability of HCV treatment has been historically low. There may be an opportunity to further reduce mortality through increasing coverage of HCV treatment as 1 strategy to reduce viral hepatitis mortality in this population.
Despite a hypothesized relationship between OAT and mortality risk due to injection-related injuries and diseases, such as bacterial infections, no such relationship was identified. This may reflect the way in which we operationalized exposure to OAT as overall exposure and according to specific periods of time, but not specifically measured as long-term and stable retention in OAT. The potential for OAT to have an influence on more chronic consequences of dependent opioid use would not be expected except insofar as OAT was being delivered at a certain level of quality and intensity. Although we extracted information on the context of OAT provision where reported, there was often very limited information on the manner of OAT delivery and the other services available to, and received by, people in the cohorts. It is likely that additional interventions will be required to reduce risk of mortality due to injection-related injuries and diseases.
There is a need for more detailed investigation and intervention development to minimize mortality risk during induction of OAT and to maintain patients on OAT who are in critical need of such therapy. A clinical decision support system, stratifying clients’ risk of dropout in real time, may facilitate the identification of those in need of service enhancements to increase engagement and prevent dropout. This will be challenging given limited evidence to support the influence of a number of factors on retention, including age, substance use, OAT dose, legal issues, and attitudes toward OAT.75 There is evidence that people with multiple treatment episodes continue receiving OAT for progressively longer periods in later treatment episodes.39,76 Findings from a Scottish cohort suggest that survival benefits increase with cumulative exposure to OAT.77
Evidence from previous studies suggests a strong association between OAT and lower risk of mortality when incarcerated, when released from incarceration while receiving OAT, and when receiving OAT after release from incarceration, with particularly lower risk of suicide and overdose mortality. Opioid agonist treatment was also found to be cost-saving as an intervention to reduce the risk of mortality after release from incarceration.78 Guidelines have been developed in the UK recommending and informing the use of OAT in carceral settings and after release79; international guidelines are also needed.
Future RCTs that withhold OAT are no longer ethical, and it is unlikely that future trials of OAT will be large enough to detect differences in mortality outcomes. The evidence base can be transformed and improved in 2 ways: (1) investing in research that tests how alternative ways of delivering OAT (and different medications) and adjunct interventions can improve retention, minimize elevated risk of mortality at initiation and maximize cost-effectiveness and (2) investing in data linkage studies that can create better-emulated trials and natural experiments of OAT delivery and combinations of interventions on mortality risk.
Limitations
This study had some limitations. The current evidence base in general was unrefined, lacking detail on clinical characteristics of patient history, intervention delivery, and consistent measures of confounders. For example, most cohorts did not specify whether treatment ceased because of dropout or completion. Those that did specify suggested that most patients dropped out of treatment as opposed to having a planned completion date.76,80 Morbidity was strongly associated with mortality risk and may also have been associated with retention.
There have been divergent findings according to which comorbidities are considered. One study found retention was higher among people with greater comorbidity (measured as the number of chronic diseases)76; another study suggested no association of HIV or HCV status with retention81; and an Australian study suggested that depression and other substance use disorders were associated with increased retention, whereas psychosis was associated with reduced retention (C. Bharat, personal communication, 2021). Moreover, cohort studies that have adjusted for comorbidity did not find changes in the estimated mortality risk by time during and out of OAT.
The evidence depends on observational studies that were subject to bias, confounding, and selection of participants. In most cohorts, there was little bias in measuring the outcome, but there were problems with missing data in some studies. However, we did find consistent data and little difference between adjusted and unadjusted estimates (Table 4). The evidence base could be substantially strengthened if future studies linked more detailed clinical information with mortality records so that more refined adjustments could be made for patterns and severity of opioid dependence, comorbidity, and environmental hazards on OAT retention and mortality risk.
Studies were largely limited to those who had received OAT for opioid dependence at some point. It is possible that mortality risk is higher for those who never receive OAT than it is for out-of-OAT periods among those who have some OAT experience, leading to an underestimate of the association between OAT and risk of mortality.
Despite the large numbers of people in the identified cohorts, for some causes of death, we had limited power, particularly when evaluating association during specific periods in and out of OAT. Similarly, there were insufficient studies to examine potential differences between buprenorphine and methadone by specific periods in and out of treatment.
Although associations were seen in this review, there have been too few studies of the influence of OAT during incarceration and after release. More studies using linked data that follow people through OAT during incarceration and after release, as conducted previously,18,71 are needed.
Conclusions
The results of this systematic review meta-analysis suggest that OAT is an important intervention for people with opioid dependence, with the capacity to reduce multiple causes of death. Despite this positive association, access to OAT remains limited in many settings, and in the US and globally, coverage for this type of treatment is low. Future work to increase access could have important population-level benefits.
eTable 1. GATHER Checklist
eTable 2. PRISMA Checklist
eAppendix. Peer-Reviewed Literature Search
eFigure 1. PRISMA Flow Diagram
eTable 3. List of Studies Excluded at Full-Text Review Stage and Reasons for Exclusion
eTable 4. Cause of Death Categories
eTable 5. Variables
eTable 6. Key Extracted and Used Data
eTable 7. Characteristics of Eligible Randomized Controlled Trials and Observational Studies That Reported on the Impact of Opioid Agonist Treatment (OAT) on Mortality
eTable 8. List of All Eligible Observational Studies
eTable 9. List of Included RCTs
eTable 10. Features of OAT in Included Primary Observational Studies
eTable 11. Features of OAT in Included RCTs
eTable 12. Characteristics of Included Primary Observational Studies
eTable 13. Characteristics of Included RCTs
eTable 14. Characteristics of Participants in Included Primary Observational Studies
eTable 15. Characteristics of Participants in Included RCTs
eFigure 2. All-Cause Mortality In OAT Compared to Out of OAT
eFigure 3. All-Cause Mortality In and Out of OAT in RCTs
eTable 16. Meta-Regression of Potential Sources of Heterogeneity in the Pooled All-Cause Crude Mortality Rate Ratio (In vs Out of OAT)
eFigure 4. Cause-Specific Mortality: All Injury and Poisoning
eFigure 5. Cause-Specific Mortality: Drug-Induced
eFigure 6. Cause-Specific Mortality: Accidental Drug-Induced
eFigure 7. Cause-Specific Mortality: Accidental Opioid
eFigure 8. Cause-Specific Mortality: Suicide
eFigure 9. Cause-Specific Mortality: Violence
eFigure 10. Cause-Specific Mortality: Motor Vehicle and Transport Accidents
eFigure 11. Cause-Specific Mortality: Falls, Fires, Burns, and Drownings
eFigure 12. Cause-Specific Mortality: All Liver-Related
eFigure 13. Cause-Specific Mortality: Viral Hepatitis
eFigure 14. Cause-Specific Mortality: All Alcohol-Related
eFigure 15. Cause-Specific Mortality: Cancer
eFigure 16. Cause-Specific Mortality: Cardiovascular Disease
eFigure 17. Cause-Specific Mortality: Chronic Respiratory Disease
eFigure 18. Cause-Specific Mortality: Digestive Disorders
eFigure 19. Cause-Specific Mortality: HIV-Related
eFigure 20. Cause-Specific Mortality: Influenza and Pneumonia
eFigure 21. Cause-Specific Mortality: Injection Related Injury and Disease
eFigure 22. Cause-Specific Mortality: Endocarditis
eFigure 23. Cause-Specific Mortality: Bacteraemia and Sepsis
eFigure 24. Cause-Specific Mortality: Skin and Soft Tissue Infections
eTable 17. Findings From Observational Studies on the Pooled Cause-Specific Mortality Rates, and Mortality Rate Ratios in People Receiving OAT, by Specific Time Periods In and Out of Treatment
eFigure 25. Studies on the Association of Opioid Agonist Treatment (OAT) With All-Cause Mortality by specific time periods in and out of treatment from observational studies, stratified by buprenorphine (B) or methadone (M)
eTable 18. Additional Causes of Death by Time Period In or Out of OAT
eFigure 26. All-Cause Mortality According to Time Period In and Out of OAT
eFigure 27. All Injury & Poisoning Mortality According to Time Period In and Out of OAT
eFigure 28. Drug-Induced Mortality According to Time Period In and Out of OAT
eFigure 29. Accidental Drug-Induced Mortality According to Time Period In and Out of OAT
eFigure 30. Accidental Opioid Mortality According to Time Period In and Out of OAT
eFigure 31. Suicide Mortality According to Time Period In and Out of OAT
eFigure 32. Motor Vehicle and Transport Accident Mortality According to Time Period In and Out of OAT
eFigure 33. Injection-Related Injury and Disease Mortality According to Time Period In and Out of OAT
eTable 19: Pooled all-cause and drug-induced mortality rates in people receiving OAT, by time in and out of treatment (first two weeks in and out of OAT)
eFigure 34. All-Cause and Cause-Specific Mortality During Incarceration According to OAT Status, Stratified by the First Four Weeks and Remainder of Time in Incarceration
eFigure 35. All-Cause and Cause-Specific Mortality Post-Release According to OAT Status in Incarceration, Stratified by the First Four Weeks and Remainder of Time (up to 1 year) Post-Release From Incarceration
eFigure 36. All-Cause and Cause-Specific Mortality Post-Release From Incarceration According to OAT Status Post-Release, Stratified by the First Four Weeks and Remainder of Time (up to 18 months) Post-Release From Incarceration
eTable 20. Additional Pooled Cause-Specific Rates in People Receiving OAT, by Time During Incarceration and Post-Release From Incarceration
eFigure 37. ROBINS-I for Community-Based Observational Studies Used in Analyses
eFigure 38. ROBINS-I for Community-Based Observational Studies – Pooled Domain Scores Weighted by Person Years Contributed by Each Study
eFigure 39. ROBINS-I for Studies Used in Analyses of the Impact of OAT Provided During Incarceration on Mortality During Incarceration
eFigure 40. ROBINS-I for Studies Used in Analyses of the Impact of OAT Provided During Incarceration Upon Post-Release Mortality
eFigure 41. ROBINS-I for Studies of OAT Impact During Incarceration – Pooled Domain Scores Weighted by Person Years Contributed by Each Study
eFigure 42. ROBINS-I for Studies Used in Analyses of the Effect of Post-Release OAT on Mortality
eFigure 43. ROBINS-I for OAT Impact Post-Release – Pooled Domain Scores Weighted by Person Years Contributed by each Study
eTable 21. Summary of Studies That Adjusted for Confounding in Observational Studies of the Impact of OAT on Mortality
eFigure 44. ROB-2 for RCTs
eFigure 45. ROB-2 RCTs—Pooled Domain Scores Weighted by Contribution of Each Study to Pooled Mortality Estimate
eFigure 46. Sensitivity Analysis Restricted to Those Who Ever Enter OAT
eFigure 47. Sensitivity Analysis for Studies of OAT Post-Release From Incarceration
eFigure 48. Sensitivity Analysis Excluding Studies With Serious Risk of Bias Due to Missing Data
eFigure 49. Sensitivity Analysis- All Liver-Related and Viral Hepatitis RRs Restricted to the Same Cohorts
eReferences
References
- 1.Irvine MA, Kuo M, Buxton JA, et al. Modelling the combined impact of interventions in averting deaths during a synthetic-opioid overdose epidemic. Addiction. 2019;114(9):1602-1613. doi: 10.1111/add.14664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Degenhardt L, Grebely J, Stone J, et al. Global patterns of opioid use and dependence: harms to populations, interventions, and future action. Lancet. 2019;394(10208):1560-1579. doi: 10.1016/S0140-6736(19)32229-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wen LS, Sadeghi NB. The opioid crisis and the 2020 US election: crossroads for a national epidemic. Lancet. 2020;396(10259):1316-1318. doi: 10.1016/S0140-6736(20)32113-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brignone E, George DR, Sinoway L, et al. Trends in the diagnosis of diseases of despair in the United States, 2009-2018: a retrospective cohort study. BMJ Open. 2020;10(10):e037679. doi: 10.1136/bmjopen-2020-037679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larney S, Tran LT, Leung J, et al. All-cause and cause-specific mortality among people using extramedical opioids: a systematic review and meta-analysis. JAMA Psychiatry. 2020;77(5):493-502. doi: 10.1001/jamapsychiatry.2019.4170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . WHO Model list of essential medicines. Accessed February 15, 2020. https://www.who.int/groups/expert-committee-on-selection-and-use-of-essential-medicines/essential-medicines-lists
- 7.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens GA, Alkema L, Black RE, et al. ; GATHER Working Group . Guidelines for Accurate and Transparent Health Estimates Reporting: the GATHER statement. Lancet. 2016;388(10062):e19-e23. doi: 10.1016/S0140-6736(16)30388-9 [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269, W64. doi: 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 10.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 11.Examining the effect of opioid agonist treatment on all-cause and cause-specific mortality. PROSPERO identifier: CRD42020171949. Updated April 28, 2020. Accessed April 21, 2021. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=171949
- 12.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919-i. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterne JA, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ.2019;366:I4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed]
- 14.Randall D, Roxburgh A, Gibson A, Degenhardt L. Mortality among people who use illicit drugs: a toolkit for classifying major causes of death. Accessed January 23, 2010. https://ndarc.med.unsw.edu.au/sites/default/files/ndarc/resources/Randall%20et%20al%20Classifying%20causes%20of%20death%202009%20corrected.pdf
- 15.StataCorp . Stata Statistical Software: Release 16. StataCorp, LLC; 2019. [Google Scholar]
- 16.Harris RJ, Deeks JJ, Altman DG, Bradburn MJ, Harbord RM, Sterne JA. Metan: fixed-and random-effects meta-analysis. SJ.2008;8(1):3-28.
- 17.Harbord R, Higgins J.. Metareg: Stata Module to Perform Meta-Analysis Regression. Boston College Department of Economics; 2009.
- 18.Larney S, Gisev N, Farrell M, et al. Opioid substitution therapy as a strategy to reduce deaths in prison: retrospective cohort study. BMJ Open. 2014;4(4):e004666. doi: 10.1136/bmjopen-2013-004666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon MS, Kinlock TW, Schwartz RP, Fitzgerald TT, O'Grady KE, Vocci FJ. A randomized controlled trial of prison-initiated buprenorphine: prison outcomes and community treatment entry. Drug Alcohol Depend. 2014;142:33-40. doi: 10.1016/j.drugalcdep.2014.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruber VA, Delucchi KL, Kielstein A, Batki SL. A randomized trial of 6-month methadone maintenance with standard or minimal counseling versus 21-day methadone detoxification. Drug Alcohol Depend. 2008;94(1-3):199-206. doi: 10.1016/j.drugalcdep.2007.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinlock TW, Gordon MS, Schwartz RP, Fitzgerald TT, O'Grady KE. A randomized clinical trial of methadone maintenance for prisoners: results at 12 months postrelease. J Subst Abuse Treat. 2009;37(3):277-285. doi: 10.1016/j.jsat.2009.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi: 10.1016/S0140-6736(17)32812-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ling W, Casadonte P, Bigelow G, et al. Buprenorphine implants for treatment of opioid dependence: a randomized controlled trial. JAMA. 2010;304(14):1576-1583. doi: 10.1001/jama.2010.1427 [DOI] [PubMed] [Google Scholar]
- 24.Rich JD, McKenzie M, Larney S, et al. Methadone continuation versus forced withdrawal on incarceration in a combined US prison and jail: a randomised, open-label trial. Lancet. 2015;386(9991):350-359. doi: 10.1016/S0140-6736(14)62338-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strain E, Stitzer M, Liebson I, Bigelow G. Dose-response effects of methadone in the treatment of opioid dependence. Ann Intern Med. 1993;119(1):23-27. doi: 10.7326/0003-4819-119-1-199307010-00004 [DOI] [PubMed] [Google Scholar]
- 26.Yancovitz SR, Des Jarlais DC, Peyser NP, et al. A randomized trial of an interim methadone maintenance clinic. Am J Public Health. 1991;81(9):1185-1191. doi: 10.2105/ajph.81.9.1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kakko J, Svanborg KD, Kreek MJ, Heilig M. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo-controlled trial. Lancet. 2003;361(9358):662-668. doi: 10.1016/S0140-6736(03)12600-1 [DOI] [PubMed] [Google Scholar]
- 28.Krook AL, Brors O, Dahlberg J, et al. A placebo-controlled study of high dose buprenorphine in opiate dependents waiting for medication-assisted rehabilitation in Oslo, Norway. Addiction. 2002;97(5):533-542. doi: 10.1046/j.1360-0443.2002.00090.x [DOI] [PubMed] [Google Scholar]
- 29.Schottenfeld RS, Chawarski MC, Mazlan M. Maintenance treatment with buprenorphine and naltrexone for heroin dependence in Malaysia: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371(9631):2192-2200. doi: 10.1016/S0140-6736(08)60954-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanum L, Solli KK, Latif ZE, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74(12):1197-1205. doi: 10.1001/jamapsychiatry.2017.3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newman R, Whitehill W. Double-blind comparison of methadone and placebo maintenance treatments of narcotic addicts in Hong Kong. Lancet. 1979;2(8141):485-488. doi: 10.1016/s0140-6736(79)91550-2 [DOI] [PubMed] [Google Scholar]
- 32.Gunne LM, Grönbladh L. The Swedish methadone maintenance program: a controlled study. Drug Alcohol Depend. 1981;7(3):249-256. doi: 10.1016/0376-8716(81)90096-x [DOI] [PubMed] [Google Scholar]
- 33.Metzger DS, Donnell D, Celentano DD, et al. Expanding substance use treatment options for HIV prevention with buprenorphine-naloxone: HIV Prevention Trials Network 058. J Acquir Immune Defic Syndr. 2015;68(5):554-561. doi: 10.1097/QAI.0000000000000510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abrahamsson T, Berge J, Öjehagen A, Håkansson A. Benzodiazepine, z-drug and pregabalin prescriptions and mortality among patients in opioid maintenance treatment—a nation-wide register-based open cohort study. Drug Alcohol Depend. 2017;174:58-64. doi: 10.1016/j.drugalcdep.2017.01.013 [DOI] [PubMed] [Google Scholar]
- 35.Bakker A, Streel E. Benzodiazepine maintenance in opiate substitution treatment: good or bad? a retrospective primary care case-note review. J Psychopharmacol. 2017;31(1):62-66. doi: 10.1177/0269881116675508 [DOI] [PubMed] [Google Scholar]
- 36.Bukten A, Stavseth MR, Clasuen T. From restrictive to more liberal: variations in mortality among patients in opioid maintenance treatment over a 12-year period. BMC Health Serv Res. 2019;19(1):553. doi: 10.1186/s12913-019-4382-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buster MC, van Brussel GH, van den Brink W. An increase in overdose mortality during the first 2 weeks after entering or re-entering methadone treatment in Amsterdam. Addiction. 2002;97(8):993-1001. doi: 10.1046/j.1360-0443.2002.00179.x [DOI] [PubMed] [Google Scholar]
- 38.Cousins G, Teljeur C, Motterlini N, McCowan C, Dimitrov BD, Fahey T. Risk of drug-related mortality during periods of transition in methadone maintenance treatment: a cohort study. J Subst Abuse Treat. 2011;41(3):252-260. doi: 10.1016/j.jsat.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 39.Cousins G, Boland F, Courtney B, Barry J, Lyons S, Fahey T. Risk of mortality on and off methadone substitution treatment in primary care: a national cohort study. Addiction. 2016;111(1):73-82. doi: 10.1111/add.13087 [DOI] [PubMed] [Google Scholar]
- 40.Davoli M, Bargagli AM, Perucci CA, et al. ; VEdeTTE Study Group . Risk of fatal overdose during and after specialist drug treatment: the VEdeTTE study, a national multi-site prospective cohort study. Addiction. 2007;102(12):1954-1959. doi: 10.1111/j.1360-0443.2007.02025.x [DOI] [PubMed] [Google Scholar]
- 41.Dupouy J, Palmaro A, Fatséas M, et al. Mortality associated with time in and out of buprenorphine treatment in French office-based general practice: a 7-year cohort study. Ann Fam Med. 2017;15(4):355-358. doi: 10.1370/afm.2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Durand L, O'Driscoll D, Boland F, et al. Do interruptions to the continuity of methadone maintenance treatment in specialist addiction settings increase the risk of drug-related poisoning deaths? a retrospective-cohort study. Addiction.2020;115(10):1867-1877. doi: 10.1111/add.15004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fugelstad A, Stenbacka M, Leifman A, Nylander M, Thiblin I. Methadone maintenance treatment: the balance between life-saving treatment and fatal poisonings. Addiction. 2007;102(3):406-412. doi: 10.1111/j.1360-0443.2006.01714.x [DOI] [PubMed] [Google Scholar]
- 44.Grönbladh L, Ohlund LS, Gunne LM. Mortality in heroin addiction: impact of methadone treatment. Acta Psychiatr Scand. 1990;82(3):223-227. doi: 10.1111/j.1600-0447.1990.tb03057.x [DOI] [PubMed] [Google Scholar]
- 45.Hickman M, Steer C, Tilling K, et al. The impact of buprenorphine and methadone on mortality: a primary care cohort study in the United Kingdom. Addiction. 2018;113(8):1461-1476. doi: 10.1111/add.14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Langendam MW, van Brussel GH, Coutinho RA, van Ameijden EJ. The impact of harm-reduction-based methadone treatment on mortality among heroin users. Am J Public Health. 2001;91(5):774-780. doi: 10.2105/AJPH.91.5.774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marsden J, Stillwell G, Jones H, et al. Does exposure to opioid substitution treatment in prison reduce the risk of death after release? a national prospective observational study in England. Addiction. 2017;112(8):1408-1418. doi: 10.1111/add.13779 [DOI] [PubMed] [Google Scholar]
- 48.Morozova O, Dvoryak S, Altice FL. Methadone treatment improves tuberculosis treatment among hospitalized opioid dependent patients in Ukraine. Int J Drug Policy. 2013;24(6):e91-e98. doi: 10.1016/j.drugpo.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muga R, Rivas I, Faure E, et al. Sex-specific disease outcomes of HIV-positive and HIV-negative drug users admitted to an opioid substitution therapy program in Spain: a cohort study. BMC Infect Dis. 2014;14:504. doi: 10.1186/1471-2334-14-504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pavarin RM, Fioritti A, Sanchini S. Mortality trends among heroin users treated between 1975 and 2013 in Northern Italy: results of a longitudinal study. J Subst Abuse Treat. 2017;77:166-173. doi: 10.1016/j.jsat.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 51.Pierce M, Bird SM, Hickman M, et al. Impact of treatment for opioid dependence on fatal drug-related poisoning: a national cohort study in England. Addiction. 2016;111(2):298-308. doi: 10.1111/add.13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scherbaum N, Specka M, Hauptmann G, Gastpar M. Does maintenance treatment reduce the mortality rate of opioid addicts?. Article written in German. Fortschr Neurol Psychiatr. 2002;70(9):455-461. doi: 10.1055/s-2002-33758 [DOI] [PubMed] [Google Scholar]
- 53.Weber R, Ledergerber B, Opravil M, Siegenthaler W, Lüthy R. Progression of HIV infection in misusers of injected drugs who stop injecting or follow a programme of maintenance treatment with methadone. BMJ. 1990;301(6765):1362-1365. doi: 10.1136/bmj.301.6765.1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Degenhardt L, Randall D, Hall W, Law M, Butler T, Burns L. Mortality among clients of a state-wide opioid pharmacotherapy program over 20 years: risk factors and lives saved. Drug Alcohol Depend. 2009;105(1-2):9-15. doi: 10.1016/j.drugalcdep.2009.05.021 [DOI] [PubMed] [Google Scholar]
- 55.Digiusto E, Shakeshaft A, Ritter A, O’Brien S, Mattick RP; NEPOD Research Group . Serious adverse events in the Australian National Evaluation of Pharmacotherapies for Opioid Dependence (NEPOD). Addiction. 2004;99(4):450-460. doi: 10.1111/j.1360-0443.2004.00654.x [DOI] [PubMed] [Google Scholar]
- 56.Fellows-Smith J. Opioid-dependent error processing. J Opioid Manag. 2011;7(6):443-449. [PubMed] [Google Scholar]
- 57.Kelty E, Joyce D, Hulse G. A retrospective cohort study of mortality rates in patients with an opioid use disorder treated with implant naltrexone, oral methadone or sublingual buprenorphine. Am J Drug Alcohol Abuse. 2019;45(3):285-291. doi: 10.1080/00952990.2018.1545131 [DOI] [PubMed] [Google Scholar]
- 58.Kimber J, Larney S, Hickman M, Randall D, Degenhardt L. Mortality risk of opioid substitution therapy with methadone versus buprenorphine: a retrospective cohort study. Lancet Psychiatry. 2015;2(10):901-908. doi: 10.1016/S2215-0366(15)00366-1 [DOI] [PubMed] [Google Scholar]
- 59.Reece AS. Favorable mortality profile of naltrexone implants for opiate addiction. J Addict Dis. 2010;29(1):30-50. doi: 10.1080/10550880903435988 [DOI] [PubMed] [Google Scholar]
- 60.Bazazi AR. Characterizing and Responding to the Epidemics of HIV and Injection Drug Use in Malaysia. Dissertation. Yale University; 2018.
- 61.Chang KC, Lu TH, Lee KY, Hwang JS, Cheng CM, Wang JD. Estimation of life expectancy and the expected years of life lost among heroin users in the era of opioid substitution treatment (OST) in Taiwan. Drug Alcohol Depend. 2015;153:152-158. doi: 10.1016/j.drugalcdep.2015.05.033 [DOI] [PubMed] [Google Scholar]
- 62.Huang YF, Kuo HS, Lew-Ting CY, et al. Mortality among a cohort of drug users after their release from prison: an evaluation of the effectiveness of a harm reduction program in Taiwan. Addiction. 2011;106(8):1437-1445. doi: 10.1111/j.1360-0443.2011.03443.x [DOI] [PubMed] [Google Scholar]
- 63.Huang CL, Lee CW. Factors associated with mortality among heroin users after seeking treatment with methadone: a population-based cohort study in Taiwan. J Subst Abuse Treat. 2013;44(3):295-300. doi: 10.1016/j.jsat.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 64.Liu E, Rou K, McGoogan JM, et al. ; China’s National Working Group on Methadone Maintenance Treatment . Factors associated with mortality of HIV-positive clients receiving methadone maintenance treatment in China. J Infect Dis. 2013;208(3):442-453. doi: 10.1093/infdis/jit163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Appel PW, Joseph H, Richman BL. Causes and rates of death among methadone maintenance patients before and after the onset of the HIV/AIDS epidemic. Mt Sinai J Med. 2000;67(5-6):444-451. [PubMed] [Google Scholar]
- 66.Evans E, Li L, Min J, et al. Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006-10. Addiction. 2015;110(6):996-1005. doi: 10.1111/add.12863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gearing FR, Schweitzer MD. An epidemiologic evaluation of long-term methadone maintenance treatment for heroin addiction. Am J Epidemiol. 1974;100(2):101-112. doi: 10.1093/oxfordjournals.aje.a112012 [DOI] [PubMed] [Google Scholar]
- 68.Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137-145. doi: 10.7326/M17-3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pearce LA, Min JE, Piske M, et al. Opioid agonist treatment and risk of mortality during opioid overdose public health emergency: population based retrospective cohort study. BMJ. 2020;368:m772. doi: 10.1136/bmj.m772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ledberg A. Mortality related to methadone maintenance treatment in Stockholm, Sweden, during 2006-2013. J Subst Abuse Treat. 2017;74:35-41. doi: 10.1016/j.jsat.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 71.Degenhardt L, Larney S, Kimber J, et al. The impact of opioid substitution therapy on mortality post-release from prison: retrospective data linkage study. Addiction. 2014;109(8):1306-1317. doi: 10.1111/add.12536 [DOI] [PubMed] [Google Scholar]
- 72.Lawlor DA, Davey Smith G, Ebrahim S. Commentary: the hormone replacement–coronary heart disease conundrum: is this the death of observational epidemiology? Int J Epidemiol. 2004;33(3):464-467. doi: 10.1093/ije/dyh124 [DOI] [PubMed] [Google Scholar]
- 73.Zhang FF, Barr SI, McNulty H, Li D, Blumberg JB. Health effects of vitamin and mineral supplements. BMJ. 2020;369:m2511. doi: 10.1136/bmj.m2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pintilie M.Competing Risks: A Practical Perspective: John Wiley & Sons; 2006. doi: 10.1002/9780470870709 [DOI] [Google Scholar]
- 75.O’Connor AM, Cousins G, Durand L, Barry J, Boland F. Retention of patients in opioid substitution treatment: a systematic review. PLoS One. 2020;15(5):e0232086. doi: 10.1371/journal.pone.0232086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nosyk B, MacNab YC, Sun H, et al. Proportional hazards frailty models for recurrent methadone maintenance treatment. Am J Epidemiol. 2009;170(6):783-792. doi: 10.1093/aje/kwp186 [DOI] [PubMed] [Google Scholar]
- 77.Kimber J, Copeland L, Hickman M, et al. Survival and cessation in injecting drug users: prospective observational study of outcomes and effect of opiate substitution treatment. BMJ. 2010;341:c3172. doi: 10.1136/bmj.c3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gisev N, Shanahan M, Weatherburn DJ, et al. A cost-effectiveness analysis of opioid substitution therapy upon prison release in reducing mortality among people with a history of opioid dependence. Addiction. 2015;110(12):1975-1984. doi: 10.1111/add.13073 [DOI] [PubMed] [Google Scholar]
- 79.Department of Health. Drug misuse and dependence. Accessed April 15, 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/673978/clinical_guidelines_2017.pdf
- 80.Cornish R, Macleod J, Strang J, Vickerman P, Hickman M. Risk of death during and after opiate substitution treatment in primary care: prospective observational study in UK General Practice Research Database. BMJ. 2010;341(7779):c5475. doi: 10.1136/bmj.c5475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang L, Zou X, Zhang D, Li X, Zhao P, Ling L. Investigation of repeat client drop-out and re-enrolment cycles in fourteen methadone maintenance treatment clinics in Guangdong, China. PLoS One.2015;10(10):e0139942. doi: 10.1371/journal.pone.0139942 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. GATHER Checklist
eTable 2. PRISMA Checklist
eAppendix. Peer-Reviewed Literature Search
eFigure 1. PRISMA Flow Diagram
eTable 3. List of Studies Excluded at Full-Text Review Stage and Reasons for Exclusion
eTable 4. Cause of Death Categories
eTable 5. Variables
eTable 6. Key Extracted and Used Data
eTable 7. Characteristics of Eligible Randomized Controlled Trials and Observational Studies That Reported on the Impact of Opioid Agonist Treatment (OAT) on Mortality
eTable 8. List of All Eligible Observational Studies
eTable 9. List of Included RCTs
eTable 10. Features of OAT in Included Primary Observational Studies
eTable 11. Features of OAT in Included RCTs
eTable 12. Characteristics of Included Primary Observational Studies
eTable 13. Characteristics of Included RCTs
eTable 14. Characteristics of Participants in Included Primary Observational Studies
eTable 15. Characteristics of Participants in Included RCTs
eFigure 2. All-Cause Mortality In OAT Compared to Out of OAT
eFigure 3. All-Cause Mortality In and Out of OAT in RCTs
eTable 16. Meta-Regression of Potential Sources of Heterogeneity in the Pooled All-Cause Crude Mortality Rate Ratio (In vs Out of OAT)
eFigure 4. Cause-Specific Mortality: All Injury and Poisoning
eFigure 5. Cause-Specific Mortality: Drug-Induced
eFigure 6. Cause-Specific Mortality: Accidental Drug-Induced
eFigure 7. Cause-Specific Mortality: Accidental Opioid
eFigure 8. Cause-Specific Mortality: Suicide
eFigure 9. Cause-Specific Mortality: Violence
eFigure 10. Cause-Specific Mortality: Motor Vehicle and Transport Accidents
eFigure 11. Cause-Specific Mortality: Falls, Fires, Burns, and Drownings
eFigure 12. Cause-Specific Mortality: All Liver-Related
eFigure 13. Cause-Specific Mortality: Viral Hepatitis
eFigure 14. Cause-Specific Mortality: All Alcohol-Related
eFigure 15. Cause-Specific Mortality: Cancer
eFigure 16. Cause-Specific Mortality: Cardiovascular Disease
eFigure 17. Cause-Specific Mortality: Chronic Respiratory Disease
eFigure 18. Cause-Specific Mortality: Digestive Disorders
eFigure 19. Cause-Specific Mortality: HIV-Related
eFigure 20. Cause-Specific Mortality: Influenza and Pneumonia
eFigure 21. Cause-Specific Mortality: Injection Related Injury and Disease
eFigure 22. Cause-Specific Mortality: Endocarditis
eFigure 23. Cause-Specific Mortality: Bacteraemia and Sepsis
eFigure 24. Cause-Specific Mortality: Skin and Soft Tissue Infections
eTable 17. Findings From Observational Studies on the Pooled Cause-Specific Mortality Rates, and Mortality Rate Ratios in People Receiving OAT, by Specific Time Periods In and Out of Treatment
eFigure 25. Studies on the Association of Opioid Agonist Treatment (OAT) With All-Cause Mortality by specific time periods in and out of treatment from observational studies, stratified by buprenorphine (B) or methadone (M)
eTable 18. Additional Causes of Death by Time Period In or Out of OAT
eFigure 26. All-Cause Mortality According to Time Period In and Out of OAT
eFigure 27. All Injury & Poisoning Mortality According to Time Period In and Out of OAT
eFigure 28. Drug-Induced Mortality According to Time Period In and Out of OAT
eFigure 29. Accidental Drug-Induced Mortality According to Time Period In and Out of OAT
eFigure 30. Accidental Opioid Mortality According to Time Period In and Out of OAT
eFigure 31. Suicide Mortality According to Time Period In and Out of OAT
eFigure 32. Motor Vehicle and Transport Accident Mortality According to Time Period In and Out of OAT
eFigure 33. Injection-Related Injury and Disease Mortality According to Time Period In and Out of OAT
eTable 19: Pooled all-cause and drug-induced mortality rates in people receiving OAT, by time in and out of treatment (first two weeks in and out of OAT)
eFigure 34. All-Cause and Cause-Specific Mortality During Incarceration According to OAT Status, Stratified by the First Four Weeks and Remainder of Time in Incarceration
eFigure 35. All-Cause and Cause-Specific Mortality Post-Release According to OAT Status in Incarceration, Stratified by the First Four Weeks and Remainder of Time (up to 1 year) Post-Release From Incarceration
eFigure 36. All-Cause and Cause-Specific Mortality Post-Release From Incarceration According to OAT Status Post-Release, Stratified by the First Four Weeks and Remainder of Time (up to 18 months) Post-Release From Incarceration
eTable 20. Additional Pooled Cause-Specific Rates in People Receiving OAT, by Time During Incarceration and Post-Release From Incarceration
eFigure 37. ROBINS-I for Community-Based Observational Studies Used in Analyses
eFigure 38. ROBINS-I for Community-Based Observational Studies – Pooled Domain Scores Weighted by Person Years Contributed by Each Study
eFigure 39. ROBINS-I for Studies Used in Analyses of the Impact of OAT Provided During Incarceration on Mortality During Incarceration
eFigure 40. ROBINS-I for Studies Used in Analyses of the Impact of OAT Provided During Incarceration Upon Post-Release Mortality
eFigure 41. ROBINS-I for Studies of OAT Impact During Incarceration – Pooled Domain Scores Weighted by Person Years Contributed by Each Study
eFigure 42. ROBINS-I for Studies Used in Analyses of the Effect of Post-Release OAT on Mortality
eFigure 43. ROBINS-I for OAT Impact Post-Release – Pooled Domain Scores Weighted by Person Years Contributed by each Study
eTable 21. Summary of Studies That Adjusted for Confounding in Observational Studies of the Impact of OAT on Mortality
eFigure 44. ROB-2 for RCTs
eFigure 45. ROB-2 RCTs—Pooled Domain Scores Weighted by Contribution of Each Study to Pooled Mortality Estimate
eFigure 46. Sensitivity Analysis Restricted to Those Who Ever Enter OAT
eFigure 47. Sensitivity Analysis for Studies of OAT Post-Release From Incarceration
eFigure 48. Sensitivity Analysis Excluding Studies With Serious Risk of Bias Due to Missing Data
eFigure 49. Sensitivity Analysis- All Liver-Related and Viral Hepatitis RRs Restricted to the Same Cohorts
eReferences


