Abstract
Importance
Spitz nevi are benign melanocytic neoplasms that classically present in childhood. Isolated Spitz nevi have been associated with oncogenic gene fusions in approximately 50% of cases. The rare agminated variant of Spitz nevi, thought to arise from cutaneous genetic mosaicism, is characterized by development of clusters of multiple lesions in a segmental distribution, which can complicate surgical removal. Somatic single-nucleotide variants in the HRAS oncogene have been described in agminated Spitz nevi, most of which were associated with an underlying nevus spilus. The use of targeted medical therapy for agminated Spitz nevi is not well understood.
Observations
A girl aged 30 months presented with facial agminated Spitz nevi that recurred rapidly and extensively after surgery. Owing to the morbidity of further surgery, referral was made to a molecular tumor board. The patient’s archival nevus tissue was submitted for extended immunohistochemical analysis and genetic sequencing. Strong ROS1 protein expression was identified by immunohistochemistry. Consistent with this, analysis of whole-genome sequencing data revealed GOPC-ROS1 fusions. These results indicated likely benefit from the oral tyrosine kinase inhibitor crizotinib, which was administered at a dosage of 280 mg/m2 twice daily. An excellent response was observed in all lesions within 5 weeks, with complete flattening after 20 weeks.
Conclusions and Relevance
Given the response following crizotinib treatment observed in this case, the kinase fusion was believed to be functionally consequential in the patient’s agminated Spitz nevi and likely the driver mutational event for growth of her nevi. The repurposing of crizotinib for GOPC-ROS1 Spitz nevi defines a new treatment option for these lesions, particularly in cases for which surgery is relatively contraindicated.
Key Points
Question
Is there an effective targeted systemic therapy for unresectable, agminated Spitz nevi?
Findings
A 3-year-old girl with inoperable facial agminated Spitz nevi containing GOPC-ROS1 fusions displayed an impressive response to tyrosine kinase inhibition with crizotinib.
Meaning
These findings suggest the GOPC-ROS1 kinase fusion as a cause of agminated Spitz nevi, potentially expanding the spectrum of use of crizotinib as a therapeutic option for unresectable agminated Spitz nevi.
We present a case of extensive and aggressively recurrent facial agminated Spitz nevi associated with a GOPC-ROS1 kinase fusion and the response to systemic treatment with crizotinib.
Introduction
Spitz nevi are benign melanocytic neoplasms that classically present in childhood. They are initiated by oncogenic gene fusions in approximately 50% of cases.1 The rare agminated variant of Spitz nevi is characterized by development of multiple lesions in a localized cutaneous distribution. Lesions may continue to develop within the affected segment and recur after surgical excision; however, to our knowledge, there are no reports of malignant transformation.
Agminated Spitz nevi arise from cutaneous genetic mosaicism. Several reports2,3 have demonstrated somatic pathogenic HRAS variants in nevus spilus–associated agminated Spitz nevi. Recently, a complex translocation involving TRPM1, PUM1, and LCK has also been implicated4; however, to our knowledge, no other genetic variants of agminated Spitz nevi have been reported. Herein, we present a case of extensive and aggressively recurrent facial agminated Spitz nevi associated with GOPC-ROS1 kinase fusions and the response following systemic treatment with crizotinib.
Report of a Case
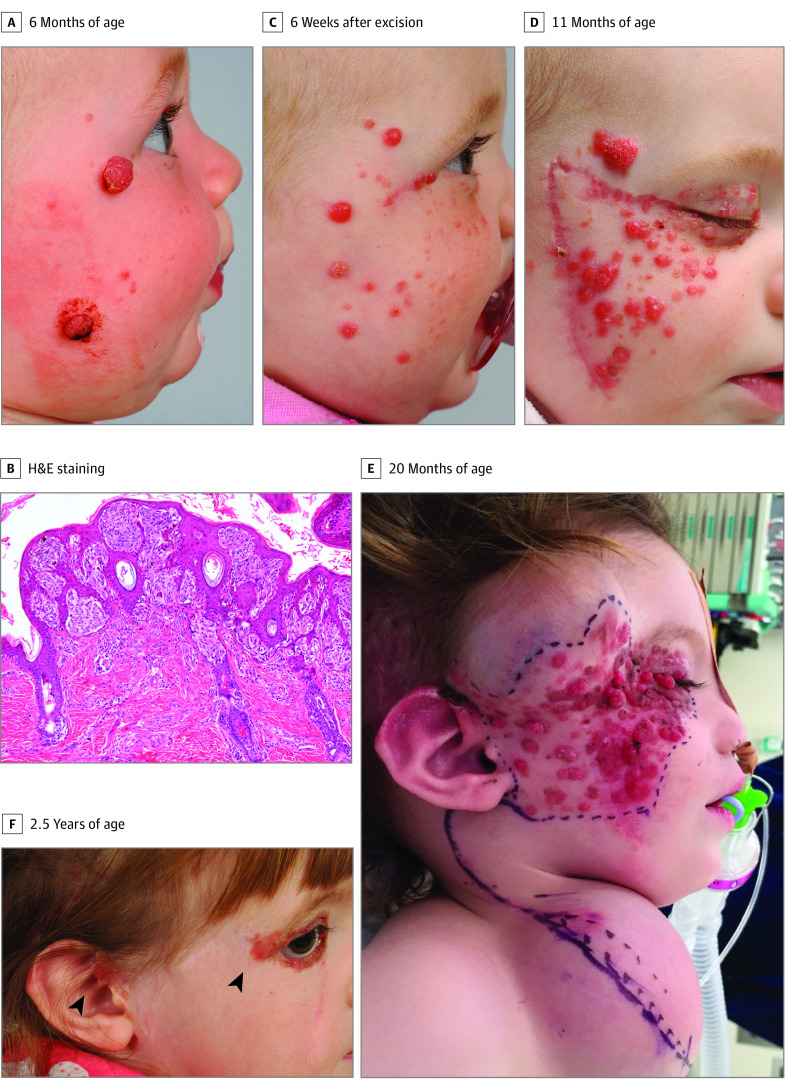
An otherwise healthy girl aged 6 months presented with a 3-month history of progressive facial lesions. On examination, 2 large, exophytic, erythematous, ulcerated nodules were present on the right temple and the right cheek. Smaller, erythematous papules were also observed on the right cheek, temple, and upper eyelid (Figure 1A). The larger lesions were excised and revealed histologically identical, symmetrical, and polypoid intradermal and junctional clusters of plump epithelioid and spindle nevus cells with occasional Kamino bodies, consistent with Spitz nevi. No malignant features were detected (Figure 1B).
Figure 1. Clinical Progression of Agminated Spitz Nevi.
A, At presentation, 2 large exophytic, erythematous, hemorrhagic nodules affected the right cheek, and smaller erythematous papules were observed on the right temple, cheek, and upper eyelid. B, Hematoxylin-eosin (H&E) staining revealed a polypoid, symmetrical lesion comprising intradermal and junctional clusters of plump epithelioid and spindle nevus cells with occasional Kamino bodies. C, Six weeks after excision of the 2 larger lesions, the smaller erythematous papules enlarged substantially, and more lesions appeared on the right cheek and temple. D, By 11 months of age and despite multiple further excisions, more lesions continued to arise in a segmental distribution involving the right cheek, temple, and the upper and lower eyelids. E, Preoperative appearance at age 20 months showing extensive lesions involving the right cheek, with tissue expanders in situ at the right temple and right neck placed previously to facilitate reconstructive surgery. F, The patient at 2.5 years of age after resection of most of the affected tissue from the right cheek and temple and cervicofacial flap reconstruction. Residual periocular and auricular lesions (arrows) had increased in size.
Six weeks later, the smaller papular lesions had enlarged, and a number of new lesions had developed in the same facial segment (Figure 1C). Two months later, further excisions were performed; the histologic findings were again consistent with Spitz nevi. When the child reached age 11 months, the progressive lesions demonstrated rapid growth within a segmental distribution affecting the right cheek, temple, and eyelids, consistent with the agminated variant of Spitz nevi (Figure 1D).
Because of the substantial tumor burden, en bloc excision was performed and repaired with a cervicofacial flap (Figure 1E). Subsequently, by 2.5 years of age, the patient had further development of lesions on the right periorbital skin, right ear, and right postauricular scalp (Figure 1F). These lesions continued to progress, becoming irritated and ulcerated. The patient was referred for extended immunohistochemical evaluation and whole-genome sequencing of the resected lesions (eMethods in the Supplement).
Results
Identification of GOPC-ROS1 Fusions
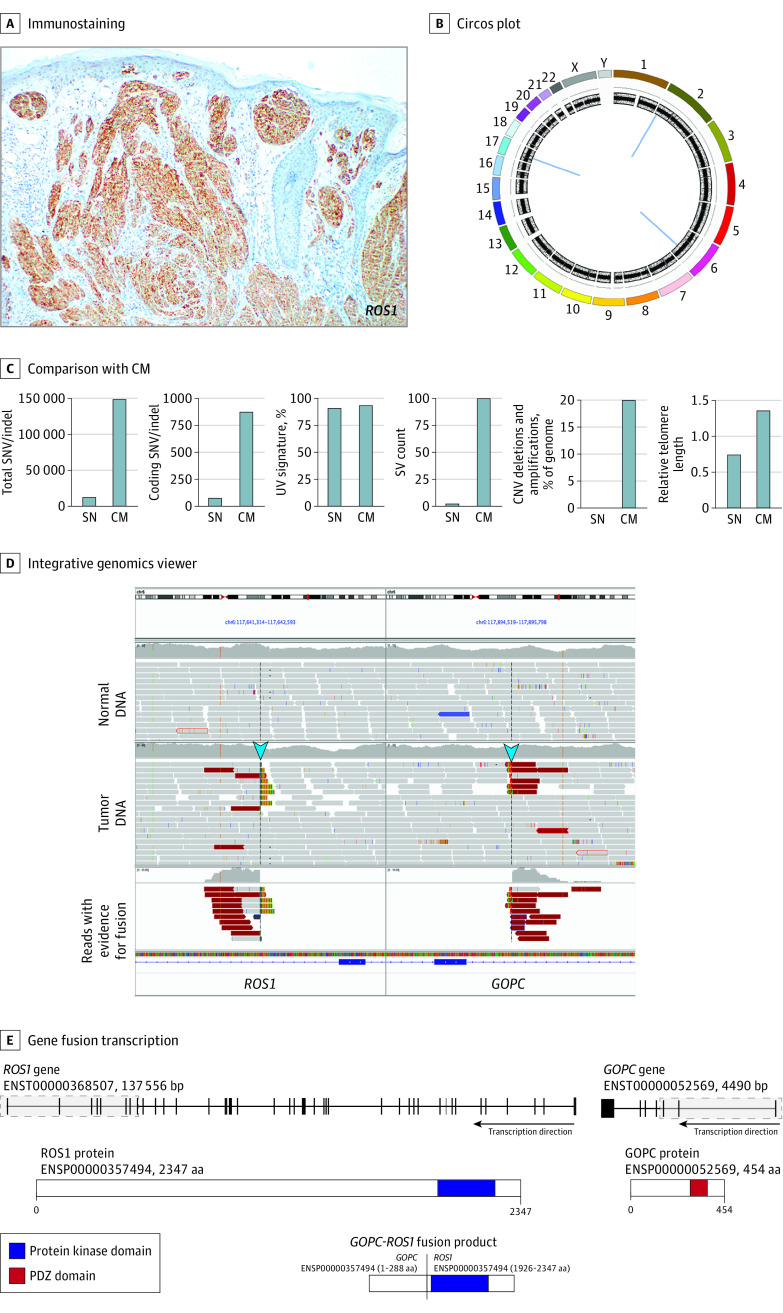
Because isolated Spitz nevi were linked with receptor tyrosine kinase gene fusions,1,5 resected lesions were labeled with an anti–receptor tyrosine kinase antibody cocktail to evaluate for targetable oncogenic driver proteins, including ROS1, ALK, and TrK family members. Positive staining results indicated expression of 1 or more of these proteins, which was subsequently shown to be ROS1 by single-antibody labeling (Figure 2A).
Figure 2. Immunophenotypic and Genetic Characterization of a Resected Agminated Spitz Nevus.
A, Immunostaining for ROS1 expression. B, Circos plot showing copy number and structural variants. Chromosomes are colored in the outer ring; copy number and B allele frequency changes are shown in the inner rings. Structural rearrangements are shown in the middle (blue lines) and include events on chromosomes 1, 6, and 17. C, Comparison of genomic features of the case (SN) compared with related published mean values in cutaneous melanomas (CM).6 CNV indicates copy number variant; indel, insertion/deletion; SNV, single-nucleotide variant; SV, structural variant. D, Integrative genomics viewer image shows sequence reads with evidence of the structural rearrangement on chromosome 6 that fused the ROS1 and GOPC genes. Blue arrows show reads that indicated the fusion. E, The gene fusion event and predicted protein product of the ROS1-GOPC gene fusion. The gray shaded parts of each transcript indicate the regions of each gene that were fused. The protein products of ROS1 and GOPC and the GOPC-ROS1 fusion product are shown. aa indicates amino acids; bp, base pairs.
Analysis of whole-genome sequencing data indicated that the tumor DNA was substantially structurally intact, with only 3 somatic rearrangements evident on chromosomes 2, 6, and 17 (Figure 2B), none of which was present in DNA extracted from blood. The burden of single-nucleotide variants in the lesion was low (101 variants in coding regions), copy number variants were negligible, and the relative lengths of telomeric elements decreased compared with typical profiles of cutaneous melanomas (Figure 2C).6 Single-nucleotide variants were not observed in HRAS or known melanoma oncogenes.
The intrachromosomal rearrangements on chromosomes 2 and 17 deleted 210– and 192–base pair segments within intergenic regions, respectively. These deletions did not affect gene loci and were of unknown and unlikely clinical significance. In contrast, a 257-kb rearrangement affecting chromosome 6 deleted the DCBLD1 locus and joined contiguous segments of the ROS1 and GOPC genes (Figure 2D). This rearrangement predicted an in-frame translation of a protein in which the PDZ domain of GOPC was substituted for the kinase domain of ROS1 (Figure 2E), with expected constitutive expression of the latter. The presence of a GOPC-ROS1 fusion was validated by an orthogonal method that sequenced multiplex polymerase chain reaction–amplified complementary DNA reverse transcribed from formalin-fixed paraffin-embedded RNA extracted from the lesion.7,8
Response Following Crizotinib Treatment
The identification of a GOPC-ROS1 fusion by complementary approaches (Figure 2B-D) and the expression of ROS1 protein (Figure 2A) in the lesions were considered by a molecular tumor board, which recommended treatment with crizotinib.9 A dose of 280 mg/m2 twice daily10 was given via a nasogastric tube with the antiemetic ondansetron hydrochloride owing to refusal of oral treatment by the patient.
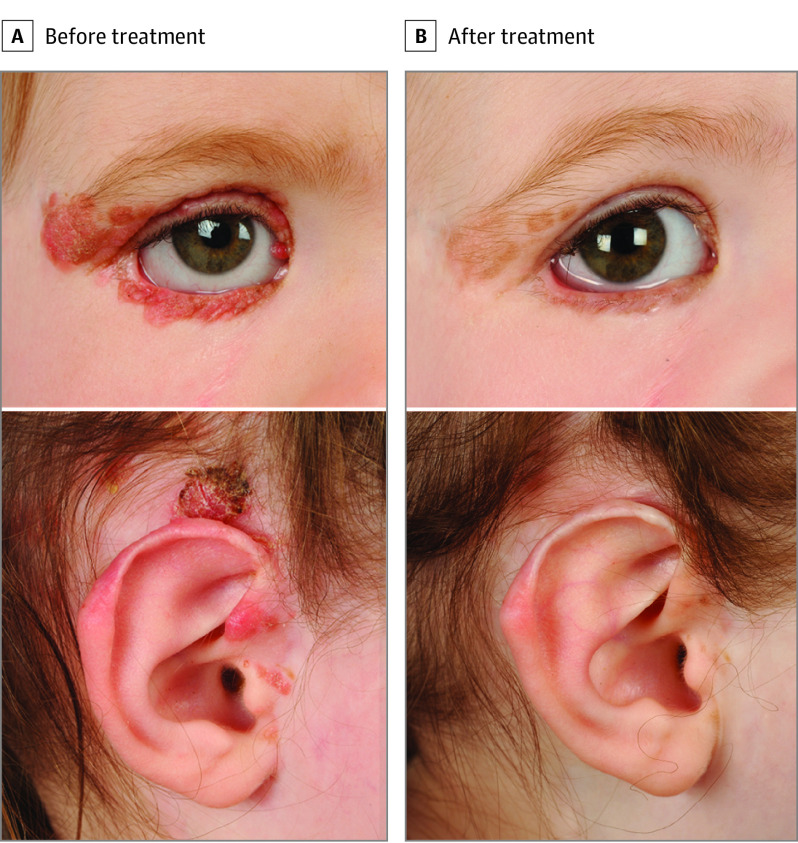
Crizotinib was well tolerated apart from grade 1 nausea and vomiting. The patient had 1 episode of impetigo and 2 viral upper respiratory tract infections, both unrelated to crizotinib. After 20 weeks of crizotinib treatment, the patient at 3 years of age showed complete flattening of the periocular, scalp, and auricular lesions to faint pigmented macules (Figure 3) and no evidence of new lesions.
Figure 3. Response of GOPC-ROS1 Fusion Agminated Spitz Nevi Following Crizotinib Treatment.
A, Before treatment, multiple coalescent erythematous and pigmented papules of recurrent Spitz nevi were seen involving the right upper and lower eyelids, including a medial canthus nevus adjacent to the right upper punctum pressing on the globe, and lesions affecting the right tragus, crus of the helix, and just superior to the right ear. B, After 20 weeks of crizotinib treatment, dramatic reduction and flattening were demonstrated of all periorbital and auricular lesions to faint pigmented macules.
Although the treatment was well tolerated, the ongoing requirement for administration via nasogastric tube prompted the patient’s parents to cease treatment. Subsequently, slow regrowth of lesions was observed.
Discussion
The present case of agminated Spitz nevi was associated with a fusion involving the proto-oncogene receptor tyrosine kinase ROS1. GOPC-ROS1 fusions lead to the formation of chimeric tyrosine kinase proteins with constitutive activity and activation of oncogenic pathways such as MAPK/ERK and PI3K/AKT/MTOR, as demonstrated in functional studies of melanocytes with ROS1 fusions.1 ROS1 fusions are implicated in a variety of human cancers, including non–small cell lung cancer, cholangiocarcinoma, gastric cancer, ovarian cancer, and glioblastoma multiforme.11
ROS1 fusions are also found across a spectrum of spitzoid neoplasms, including Spitz nevi, atypical spitzoid tumors, and spitzoid melanomas.1,5 Approximately 50% of Spitz neoplasms have genomic rearrangements involving ALK, ROS1, NTRK1, RET, MET, or BRAF,5 with approximately 17% having ROS1 fusions.1 ROS1 rearrangements were found in 26% of Spitz nevi1 and are considered mitogenic drivers that trigger initial rapid proliferation of these lesions followed by growth arrest through tumor-suppressive mechanisms.5 We posit that the GOPC-ROS1 fusion was functionally consequential in our patient’s agminated Spitz nevi because of the excellent response following treatment with crizotinib and because no other oncogenic driver mutations were identified by whole-genome sequencing.
Systemic treatment options for ROS1 fusion-driven tumors include the orally available, small molecule inhibitor crizotinib, a multitargeted tyrosine kinase inhibitor with activity against ALK, MET, and ROS1 kinases. Crizotinib has established efficacy with minimal toxic effects in adult cancers, particularly non–small cell lung cancer, that harbor ROS1 and ALK rearrangements.9 Use of crizotinib in children with anaplastic large cell lymphoma, inflammatory myofibroblastic tumors, and neuroblastoma has shown encouraging results.10 Our report extends the potential use of crizotinib to unresectable agminated Spitz nevi with oncogenic ROS1 fusions.
Limitations
This study has some limitations. Because pediatric Spitz nevi are benign lesions,12 their treatment with systemic therapies should only be considered in circumstances in which intervention is necessary and surgical excision is not feasible. Although the safety and durability of response to extended use of crizotinib have been demonstrated in children with anaplastic large cell lymphoma and inflammatory myofibroblastic tumor,13 the consequences of long-term use are not known. The most common reported adverse reactions to crizotinib in adults are visual disturbances, gastrointestinal tract upset, peripheral edema, neutropenia/lymphopenia, raised alanine aminotransferase levels, and hypophosphatemia.14 The most common adverse effect in long-term pediatric use of crizotinib was neutropenia13; however, a public safety warning was issued recently regarding the risk of severe ocular toxic effects in young patients with anaplastic large cell lymphoma.15
Although crizotinib was the only inhibitor available to us, we expect that other ROS1-targeting agents16 also would have been effective. However, because crizotinib has been tested broadly in pediatric populations, guidance is available regarding dosing and expected tolerability. Owing to the need in our case to cease crizotinib therapy because of poor patient tolerance, we are not able to report long-term safety and antitumor outcomes. However, the slow regrowth of lesions indicates that additional treatment might be required in the future.
Conclusions
We present a case of agminated Spitz nevi caused by GOPC-ROS1 fusions wherein tyrosine kinase inhibition with crizotinib was associated with regression of the lesions. Our case highlights the roles of precision medicine and repurposing of established cancer therapies for novel neoplastic indications.
eMethods. Histologic and Genetic Analysis
eReferences.
References
- 1.Wiesner T, He J, Yelensky R, et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat Commun. 2014;5:3116. doi: 10.1038/ncomms4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarin KY, Sun BK, Bangs CD, et al. Activating HRAS mutation in agminated Spitz nevi arising in a nevus spilus. JAMA Dermatol. 2013;149(9):1077-1081. doi: 10.1001/jamadermatol.2013.4745 [DOI] [PubMed] [Google Scholar]
- 3.Porubsky C, Teer JK, Zhang Y, Deschaine M, Sondak VK, Messina JL. Genomic analysis of a case of agminated Spitz nevi and congenital-pattern nevi arising in extensive nevus spilus. J Cutan Pathol. 2018;45(2):180-183. doi: 10.1111/cup.13082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goto K, Pissaloux D, Durand L, Tirode F, Guillot B, de la Fouchardière A. Novel three-way complex rearrangement of TRPM1-PUM1-LCK in a case of agminated Spitz nevi arising in a giant congenital hyperpigmented macule. Pigment Cell Melanoma Res. 2020;33(5):767-772. doi: 10.1111/pcmr.12884 [DOI] [PubMed] [Google Scholar]
- 5.Wiesner T, Kutzner H, Cerroni L, Mihm MC Jr, Busam KJ, Murali R. Genomic aberrations in spitzoid melanocytic tumours and their implications for diagnosis, prognosis and therapy. Pathology. 2016;48(2):113-131. doi: 10.1016/j.pathol.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayward NK, Wilmott JS, Waddell N, et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545(7653):175-180. doi: 10.1038/nature22071 [DOI] [PubMed] [Google Scholar]
- 7.Zheng Z, Liebers M, Zhelyazkova B, et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat Med. 2014;20(12):1479-1484. doi: 10.1038/nm.3729 [DOI] [PubMed] [Google Scholar]
- 8.Murphy DA, Ely HA, Shoemaker R, et al. Detecting gene rearrangements in patient populations through a 2-step diagnostic test comprised of rapid IHC enrichment followed by sensitive next-generation sequencing. Appl Immunohistochem Mol Morphol. 2017;25(7):513-523. doi: 10.1097/PAI.0000000000000360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non–small-cell lung cancer. N Engl J Med. 2014;371(21):1963-1971. doi: 10.1056/NEJMoa1406766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mossé YP, Lim MS, Voss SD, et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children’s Oncology Group phase 1 consortium study. Lancet Oncol. 2013;14(6):472-480. doi: 10.1016/S1470-2045(13)70095-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies KD, Doebele RC. Molecular pathways: ROS1 fusion proteins in cancer. Clin Cancer Res. 2013;19(15):4040-4045. doi: 10.1158/1078-0432.CCR-12-2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartenstein DW, Fisher JM, Stamoulis C, et al. Clinical features and outcomes of spitzoid proliferations in children and adolescents. Br J Dermatol. 2019;181(2):366-372. doi: 10.1111/bjd.17450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mossé YP, Voss SD, Lim MS, et al. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: a Children’s Oncology Group Study. J Clin Oncol. 2017;35(28):3215-3221. doi: 10.1200/JCO.2017.73.4830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non–small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13(10):1011-1019. doi: 10.1016/S1470-2045(12)70344-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ASCO Post Staff. FDA approves crizotinib for children and young adults with relapsed or refractory systemic ALK-positive ALCL. Published January 25, 2021. Accessed February 10, 2021. https://ascopost.com/issues/january-25-2021/fda-approves-crizotinib-for-children-and-young-adults-with-relapsed-or-refractory-systemic-alk-positive-alcl/
- 16.Lin JJ, Shaw AT. Recent advances in targeting ROS1 in lung cancer. J Thorac Oncol. 2017;12(11):1611-1625. doi: 10.1016/j.jtho.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Histologic and Genetic Analysis
eReferences.