Abstract
Objectives:
To assess the association between poor handgrip strength (HGS) determined by clinical criterion and incidence of falls in older women.
Methods:
The cohort included 195 women (68.1±6.2 years) who were assessed for HGS (Jamar Dynamometer) at baseline and were prospectively followed for 18 months. FNIH Sarcopenia threshold of HGS adjusted for body mass index (<0.56) was used for clinical determination of poor HGS. Association between poor HGS and incidence of falls was analyzed using Cox hazard models in the total cohort and in a stratified analysis by balance status.
Results:
During the follow-up, 53 (27%) women experienced at least one fall. In a multivariable model, poor HGS was associated with approximately 3-fold increased risk for falls [Hazard Ratio (HR)=2.73, 95% Confidence Interval (CI)=1.28–5.82, p=0.009]. In a stratified analysis, women with impaired balance exhibited even greater risk for falls (HR=3.85, 95%CI=1.47–10.12, p=0.011), although no association was found in women with normal balance (p=0.459).
Conclusions:
Poor HGS based on clinical criterion is independently associated with higher risk of falls in older women, particularly in those with impaired balance. These results suggest potential prognostic value of FNIH Sarcopenia threshold for risk stratification and referring high-risk individuals to fall prevention programs.
Keywords: Accidental falls, Aging, Hand Strength, Muscle weakness, Sarcopenia
Introduction
Falls in older age are a major public health issue and the leading cause of fatal and nonfatal injuries among adults 65 years and older[1,2]. A fall is often defined as “inadvertently coming to rest on the ground, floor or other lower level, excluding intentional change in position to rest in furniture, wall or other objects”[3]. The annual incidence of falls in older population ranges from 28% to 42% and the consequences of falls can be devastating[3]. Extensive body of literature demonstrated that falls caused fractures (64%), hospital admissions (32%), becoming dependent (32%), confusion (22%), severe disability as bed-bounded (12%), negative psychological consequences and reduced activity of daily living and poor quality of life[2,3]. Additionally, falls are considered as a main reason for a nursing home admission, accounting for 40% of all injury death among the older people[3].
Compared to men, women are more likely to experience nonfatal falls and injuries[2,3]. Large population-based studies reported that women had 40% to 60% higher overall injury rate, more than double the risk for fractures and 80% increased risk for hospitalization due to fall injury[4,5]. Falls are also associated with tremendous economic burden. In the United States, the direct medical costs for falls are estimated to be $50 billion per year[6], where the expenses among women approximately 71% of the total medical costs[7].
Falls have a multifactorial etiology and muscle weakness is the most significant risk factor[2,3,8-12]. The World Health Organization reported that individuals with muscle weakness are five-times more likely to fall compared to those with normal muscle strength[3]. A meta-analysis of prospective studies found that upper limb muscle weakness (primarily measured by handgrip strength (HGS)) was associated with 53% higher risk for falls[8]. Although previous studies consistently demonstrated an increased risk of falls among individuals with poor HGS, these studies have limitations in terms of inconsistent methodology for determining poor HGS, lack of consideration for physiological differences between sexes and absence of appropriate anthropometric adjustments[8,9,12,13]. For instance, individuals exhibited the poorest HGS (lowest quartile or quantile or 10th percentile) were compared to individuals with the highest HGS (highest quartile or quantile or 10th percentile), while a clinical criterion for muscle weakness has not been applied[8,9,12]. Additionally, women compared to men, have lower muscle strength and body size, where the latter is correlated with lower absolute muscle strength[13]. These important considerations and appropriate adjustments have not been taken in previous studies and require further exploration[8,9,12].
To address these shortfalls, Foundation for the National Institutes of Health (FNIH) Sarcopenia Project was implemented, aiming at using multiple data-set sources to identify cutoff values for clinically meaningful muscle weakness. These thresholds, however, needs to be further explored and in different older populations before can be used in clinical practice. Therefore, the current study aimed to assess the association between poor HGS determined by clinical criterion from FNIH Sarcopenia Project and the incidence of falls in older women.
Methods
Design and participants
The study was conducted at the University of Brasília, Brasília, Brazil and has been previously described[14,15]. In brief, a prospective study among community-dwelling women, assessing the relationship between physiological functions and health-related outcomes was conducted between July 2015 to January 2018. Potential participants were contacted through phone calls, flyers, and visits to local community social groups. A comprehensive questionnaire, the Mini-Mental State Examination and the Katz index were used to verify their medical history and eligibility prior to enrolment[16,17]. Physical inactivity and sedentary behavior were evaluated using the short version of the International Physical Activity Questionnaire[18]. Knee extensors isometric peak torque relative to body weight was measured using an isokinetic dynamometer (Biodex 4, Biodex Medical, Inc., New York, United States)[14]. The QuickScreen© Clinical Falls Risk Assessment was utilized for identifying established risk factors for falls[19]. The instrument assesses falls in the last 12 months, usage of four or more medications per day (excluding vitamins and minerals), any psychotropic medication use, low contrast visual acuity, peripheral sensation, postural balance, reaction time, and lower limb muscle strength. The total number of risk factors provides four levels probability for future falls (0-1 risk factors=7% probability, 2-3=13%, 4-5=27%, or 6+=49%)[19].
All participants provided written informed consent, and the study was approved by the University of Brasilia Research Ethics Committee (protocol 1.223.636). Eligibly criteria were volunteering to participate in the study, age between 60 and 85 years and ability to walk without assistance. Those who had conditions that could interfere with the study measures such as severe musculoskeletal disorders, neurological illness, diagnosis of cancer, 6-month postoperative condition, cognitive impairment, or functional dependency were excluded.
Exposure (handgrip strength)
Handgrip strength was measured using a calibrated Jamar hydraulic hand dynamometer (Sammons Preston, Bolingbrook, USA) according to the American Society of Hand Therapists guidelines[20]. All measurements were performed in the sitting position, elbow joint at 90°, forearm in neutral position, and wrist between 0° and 30° of extension[20]. Three trials of maximal dynamometer squeezing were performed using both right and left hands. The average of these trials in both hands was used for HGS analyses. The average HGS was divided by body mass index according to the FNIH Sarcopenia Project recommendations, and a clinical criterion of muscle weakness (<0.56) was applied dichotomously[21]. This threshold was determined by pooling data from nine sources of community-dwelling older adults and a series of six clinical trials and reviewed by representatives from academia, professional organizations, government, and the private sector who have an interest in aging and muscle[21]. Participants with HGS adjusted for body mass index below the threshold (<0.56) were determined as having poor muscle strength, whereas participants with values ≥0.56 were considered as having normal muscle strength[21].
Postural balance status was evaluated by near tandem stand test, which measures the time that participants can stand with their eyes closed and their bare feet in a near tandem position (feet parallel and separated laterally by 2.5 cm, and the heel of the front foot 2.5 cm anterior to the great toe of the back foot)[19]. The inability to maintain this position for at least 10 seconds was determined as impaired balance status, whereas ability to maintain this position for 10 seconds or more was considered as normal balance status[19].
Outcome ascertainment (incidence of falls)
Incidence of falls was assessed according to procedures described elsewhere[14,15]. In brief, a fall was defined as ‘‘an unexpected event in which the participants come to rest on the ground, floor, or lower level’’[3,22]. This definition was explained to the participants during the baseline assessment, and incident falls were recorded at the end of the follow-up period through telephone surveys with the question “During the past 18 months, did you fall any time?”. Responses to the question were recorded as a dichotomous variable of whether or not participants experienced falling down at least once during the follow-up. All telephone calls were made by the same researcher experienced with telephone surveys. At least ten contact attempts were made to each participant with missed calls.
Statistical analysis
Descriptive data of continuous variables were expressed as mean and standard deviation, while categorical variables were expressed as absolute numbers and proportion. To test for normality of the data distribution, the Shapiro-Wilk test was performed. For exploratory analysis, correlation between HGS and knee extensors peak torque was assessed using Pearson’s correlation test. Between-group comparisons were conducted using independent t-test and Fisher’s exact test for the continuous and categorical data, respectfully. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated using Cox proportional hazard analysis both in the total cohort and stratified by balance status. Model 1 was adjusted for age. Model 2 was adjusted for age, diabetes, osteoporosis, lower limb injury, physical inactivity, sedentary behaviour, alcohol consumption, smoking status, low contrast visual acuity, peripheral sensation, balance status and number of risk factors for falls. Kaplan-Meier curves were constructed for cumulative incidence of falls and analyzed using and a log-rank test. Statistical analyses were conducted using Statistical Package for Social Sciences software version 20.0 (SPSS Inc, Chicago, Illinois). The significance level was set at p<0.05.
Results
Five hundred potential participants were initially contacted and 335 agreed to participate in the study. After exclusion criteria were applied, a total of 246 women underwent baseline assessment. From the baseline sample, 195 volunteers were successfully tracked for ascertainment of falls over the 18-month follow-up period and were included in the current analyses (Figure 1). Participants with poor HGS had lower knee extensors peak torque and higher prevalence of impaired postural balance compared to participants with normal HGS (Table 1). There were no significant differences in all characterization variables between the final sample and the participants who were lost to follow-up (all p>.05).
Figure 1.
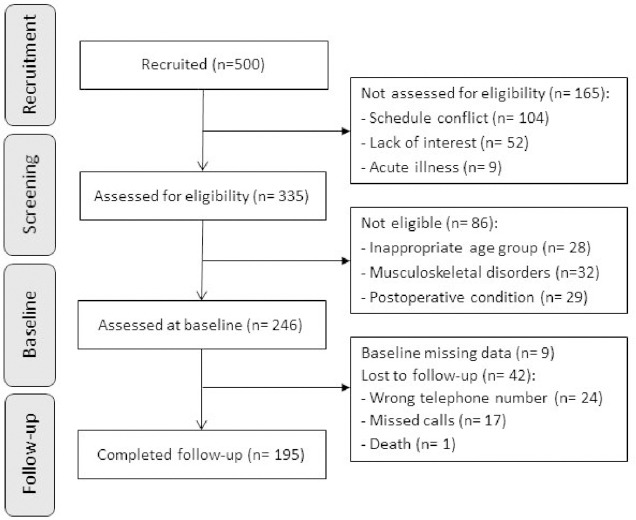
Flowchart diagram of participants in the study.
Table 1.
Characteristics of the sample.
| Characteristic | Total cohort (n=195) | Normal handgrip strength (n=174) | Poor handgrip strength (n= 21) | p |
|---|---|---|---|---|
| Age (years) | 68.0 (6.0) | 67.6 (5.7) | 70.1 (7.3) | .069 § |
| Body mass index - bmi (kg/m2) | 27.7 (4.4) | 27.3 (4.0) | 31.3 (5.9) | .006 § |
| Handgrip strength (kgf) | 21.7 (5.2) | 22.7 (4.5) | 14.1 (3.6) | < .001 § |
| Adjusted handgrip strength (kgf/ bmi) | 0.80 (0.22) | 0.85 (0.19) | 0.46 (0.10) | < .001 § |
| Knee extensors peak torque (Nm/ kg) | 157.7 (47.0) | 161.5 (45.8) | 123.3 (49.3) | < .001 § |
| Use of 4 or more medications per day | 71 (36.4) | 64 (36.8) | 7 (33.3) | .815 £ |
| Any psychotropic medication use | 35 (17.9) | 31 (17.8) | 4 (19.0) | 1.000 £ |
| Low contrast visual acuity | 14 (7.2) | 14 (8.0) | 0 (0.0) | .371 £ |
| Low peripheral sensation | 23 (11.8) | 20 (11.5) | 3 (14.3) | .720 £ |
| Impaired postural balance | 87 (44.6) | 72 (41.4) | 15 (71.4) | .011 £ |
| Slow reaction time | 74 (37.9) | 62 (35.6) | 12 (57.1) | .061 £ |
| Poor lower-limb muscle strength | 66 (33.8) | 60 (34.5) | 6 (28.6) | .808 £ |
| Number of risk factors for falls | 2.2 (1.5) | 2.2 (1.6) | 2.6 (1.5) | .217 § |
| Hypertension | 106 (54.6) | 95 (54.9) | 11 (52.4) | .822 £ |
| Diabetes mellitus | 29 (14.9) | 26 (15.0) | 3 (14.3) | 1.000 £ |
| Osteoporosis | 42 (21.8) | 38 (22.1) | 4 (19.0) | 1.000 £ |
| Lower-limb injury | 60 (30.8) | 51 (29.3) | 9 (42.9) | .217 £ |
| Alcohol consumption | 65 (33.3) | 60 (34.5) | 5 (23.8) | .463 £ |
| Current smoker | 7 (3.6) | 6 (3.5) | 1 (4.8) | .560 £ |
| Physical inactivity a | 142 (72.8) | 125 (71.8) | 17 (81.0) | .477 £ |
| Sedentary behaviour b | 40 (20.5) | 33 (19.0) | 7 (33.3) | .151 £ |
Note: Continuous data presented as mean and standard deviation. Categorical data presented as absolute number and proportion.
Physical activity level was evaluated using the short version of the International Physical Activity Question-naire – IPAQ.
bSitting time ≥8 hours/day.
Independent t-test;
Fisher’s exact test.
During the follow up, 53 (27%) participants experienced incident of fall. Both in the age-adjusted and the multivariable models, participants with poor HGS had a higher risk of falls (HR=2.20, 95%CI=1.09–4.44, p=.027), (HR=2.73, 95%CI: 1.28–5.82, p=.009), respectively (Table 2). Kaplan Meir curve showed that poor HGS was associated with higher incidence of falls (p=0.015; Figure 2). In a stratified analysis by balance status, poor HGS was associated with falls only in women with impaired postural balance (Figure 3). The corresponding HR were 2.44 (95%CI: 1.07–5.60, p=.035) and 3.85 (95%CI: 1.47–10.12, p=.006) for age-adjusted and fully adjusted models, respectively. Exploratory analysis showed that HGS adjusted for BMI was moderately correlated (r=0.55, p<.001) with knee extensors peak torque relative to body weight.
Table 2.
Age-adjusted and multivariable risk models of falls in older women.
| Variable | HR (95% CI) | p |
|---|---|---|
| Age-adjusted analysis | ||
| Poor handgrip strength | 2.20 (1.09-4.44) | .027 |
| Low contrast visual acuity | 1.69 (0.71-4.04) | .237 |
| Low peripheral sensation | 0.77 (0.32-1.82) | .547 |
| Postural balance impairment | 1.40 (0.79-0.97) | .245 |
| Number of risk factors for falls | 1.35 (1.12-1.62) | .001 |
| Diabetes | 0.99 (0.46-2.10) | .973 |
| Osteoporosis | 0.87 (0.45-1.67) | .679 |
| Lower-limb injury | 0.99 (0.56-1.77) | .982 |
| Alcohol consumption | 0.68 (0.37-1.24) | .210 |
| Current smoker | 2.00 (0.62-6.51) | .247 |
| Physical inactivity | 1.97 (0.97-3.94) | .055 |
| Sedentary behavior | 1.27 (0.67-2.40) | .470 |
| Multivariable analysis | ||
| Poor handgrip strength | 2.73 (1.28-5.82) | .009 |
| Low contrast visual acuity | 1.68 (0.61-4;66) | .320 |
| Low peripheral sensation | 0.46 (0.16-1.29) | .139 |
| Postural balance impairment | 0.84 (0.43-1.61) | .592 |
| Number of risk factors for falls | 1.51 (1.20-1.91) | <.001 |
| Diabetes | 0.53 (0.22-1.27) | .156 |
| Osteoporosis | 0.45 (0.20-1.01) | .052 |
| Lower-limb injury | 0.71 (0.37-1.37) | .308 |
| Alcohol consumption | 0.71 (0.37-1.35) | .292 |
| Current smoker | 1.17 (0.32-4.27) | .809 |
| Physical inactivity | 1.87 (0.87-4.03) | .109 |
| Sedentary behavior | 0.77 (0.37-1.60) | .481 |
Abbreviations: HR= hazard ratio; CI= confidence interval.
Figure 2.
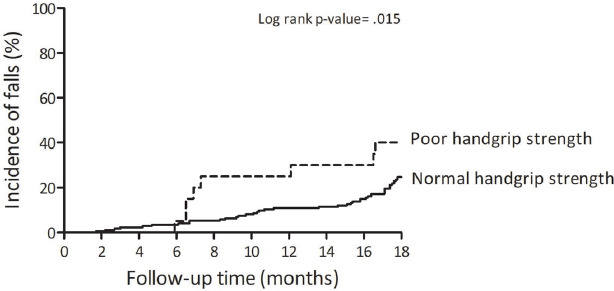
Comparison of cumulative incidence of falls between poor and normal handgrip strength in older women.
Figure 3.
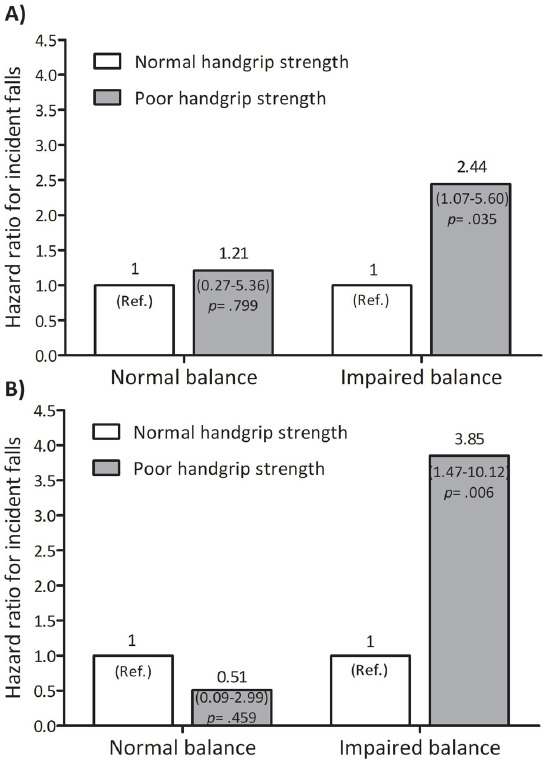
Fall incidence risk of poor handgrip strength stratified by balance status in older women. Chart (A) presents age-adjusted models. Chart (B) presents multivariable model. Balance status was evaluated by near tandem stand test.
Discussion
The present study examined the association between poor HGS, determined by FNIH clinical criterion, and the incidence of falls in older women. The findings showed that poor HGS is independently associated with significantly higher risk of falls, particularly in women with impaired balance. Although large prospective studies of FNIH Sarcopenia criterion are still warranted[21], these results support the potential prognostic value of this method for fall risk stratification in clinical and research settings. Individuals with poor HGS could be referred to fall prevention programs such as strength, balance and functional training[23].
The findings of the present study are consistent with previous reports with respect to the association between poor HGS and falls in older population[2,3,8,9,12]. A meta-analysis found that poor HGS is associated with 53% increased risk of falling[8]. Other study reported that a greater decline compared to normal age-related decline in HGS was associated with 25% higher risk for falls[12]. Recently, a study showed that poor HGS is a significant predictor of fractures due to falling[9]. Our findings also align with the well-established data with respect to the associations between poor HGS, general morbidity and mortality[24-26].
The relationship observed in the current study is stronger than the observed in these earlier reports and add novel and important information that have clinical and public health implications for risk stratification and falls prevention programs. To our knowledge, the current study is the first to demonstrate the risk association between poor HGS determined by the FNIH Sarcopenia clinical criterion and incidence of falls in older women[21]. Although previous studies have demonstrated an increased risk of falls among individuals with poor HGS, these studies have limitations in terms of inconsistent methodology for determining poor HGS, lack consideration for physiological differences between sexes and absence anthropometric adjustments[8,9,12,13]. The current study overcame these limitations by utilizing an established clinical criterion of muscle weakness from FNIH Sarcopenia project[21], which has specific threshold for women and is adjusted for body mass index[21]. Since both poor muscle strength and higher body mass index are important risk factors for falls[27], combing these variables into an index seems like improving the risk stratification and prognostic value. The results showed that poor HGS is independently associated with higher risk of falls in the total cohort and in a stratified analysis by balance status.
Although the exact mechanisms involved in the association between poor HGS and falls have yet to be fully understood, several potential explanations should be mentioned. Muscle weakness is a well-established health marker associated with many chronic conditions, mortality outcomes and falls in older people[2,3,8,24-26,28,29]. Stronger skeletal muscles, on the other hand, have been consistently demonstrated to be associated with better clinical outcomes and lower mortality risk, especially among older people[25,26,28,29]. The correlation between HGS and knee extensors peak torque observed in the current study further suggests the potential representation of HGS of other muscles groups and overall body strength, particularly when expressed relative to body weight or body mass index[30]. This aligns with established literature that relative rather than absolute muscle strength is a more sensitive prognostic marker for falls, supporting the utilization of FNIH Sarcopenia threshold in the clinical and research fields[11,14].
The study has strengths and limitations. The strengths include application of clinical criterion for poor HGS, prospective evaluation of the outcomes (incidence of falls), relatively lengthy follow up time (18 months), and analysis of both age-adjustment and multivariable models for extracting the independent association between exposure and outcome. Certain limitations are also acknowledged. First, although the number of participants and cases of falls were sufficient to conduct a multivariable analysis, the sample size was relatively small and thus additional research is required. Second, the study cohort included women participants only and the mean age was 68±6 years; thus, the application for men and older populations needs to be examined in future studies. Finally, although mental examination was performed at baseline for excluding cognitive impairments, recall bias of falls incidence in utilizing a retrospective telephone surveys could affect the results.
Conclusions
In summary, poor HGS determined by the FNIH Sarcopenia clinical criterion is independently associated with higher risk of falls in older women, particularly in those with impaired postural balance. Given the simplicity and the low-cost of the applied method, the findings support the utilization FNIH criterion as risk stratification for falls and refer individuals having poor HGS to fall prevention programs.
Acknowledgements
The authors acknowledge Ana Luiza Correia, André Gadelha and Juscélia Cristina Pereira for assistance in data collection. SGRN receives research funding from the Productivity Research Program of the Estácio de Sá University. RML receives research founding from the Brazilian National Council for Scientific and Technological Development.
Footnotes
Edited by: Dawn Skelton
Authors’ contributions
SGRN, RML and BV conceived the study and contributed to the study design. SGRN coordinated the data collection with assistance from RML. SGRN, RML, HSR and BV contributed to data analysis and interpretation writing and drafting the manuscript. All authors contributed to revision of the manuscript and approved the final version. SGRN is the guarantor for the study.
References
- 1.Tinetti ME. Preventing falls in elderly persons. N N Engl J Med. 2003;348(1):42–49. doi: 10.1056/NEJMcp020719. [DOI] [PubMed] [Google Scholar]
- 2.National Center for Injury Prevention and Control. Centers for Disease Control and Prevention. Preventing Falls:a guide to implementing effective community-based fall prevention programs. 2nd ed. 2015. [Accessed 20 August 2020]. https: //www.cdc.gov/homeandrecreationalsafety/falls/community_preventfalls.html .
- 3.World Health Organization. A global report on falls prevention. Epidemiology of falls. 2007. [Accessed 20 August 2020]. https: //www.who.int/ageing/projects/1.Epidemiology%20of%20falls%20in%20older%20age.pdf .
- 4.Stevens JA, Sogolow ED. Gender differences for non-fatal unintentional fall related injuries among older adults. Inj Prev. 2005;11(2):115–119. doi: 10.1136/ip.2004.005835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Do MT, Chang VC, Kuran N, Thompson W. Fall-related injuries among Canadian seniors, 2005-2013:an analysis of the Canadian Community Health Survey. Health Promot Chronic Dis Prev Can. 2015;35(7):99–108. doi: 10.24095/hpcdp.35.7.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Florence CS, Bergen G, Atherly A, Burns E, Stevens J, Drake C. Medical costs of fatal and nonfatal falls in older adults. J Am Geriatr Soc. 2018;66(4):693–698. doi: 10.1111/jgs.15304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns ER, Stevens JA, Lee R. The direct costs of fatal and non-fatal falls among older adults - United States. J Safety Res. 2016;58:99–103. doi: 10.1016/j.jsr.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreland JD, Richardson JA, Goldsmith CH, Clase CM. Muscle weakness and falls in older adults:a systematic review and meta-analysis. J Am Geriatr Soc. 2004;52(7):1121–1129. doi: 10.1111/j.1532-5415.2004.52310.x. [DOI] [PubMed] [Google Scholar]
- 9.Balogun S, Winzenberg T, Wills K, et al. Prospective associations of low muscle mass and function with 10-year falls risk, incident fracture and mortality in community-dwelling older adults. J Nutr Health Aging. 2017;21(7):843–848. doi: 10.1007/s12603-016-0843-6. [DOI] [PubMed] [Google Scholar]
- 10.Horlings CG, van Engelen BG, Allum JH, Bloem BR. A weak balance: the contribution of muscle weakness to postural instability and falls. Nat Clin Pract Neurol. 2008;4(9):504–515. doi: 10.1038/ncpneuro0886. [DOI] [PubMed] [Google Scholar]
- 11.Gadelha AB, Neri SGR, Bottaro M, Lima RM. The relationship between muscle quality and incidence of falls in older community-dwelling women:An 18-month follow-up study. Exp Gerontol. 2018;110:241–246. doi: 10.1016/j.exger.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Xue Q-L, Walston JD, Fried LP, Beamer BA. Prediction of risk of falling, physical disability, and frailty by rate of decline in grip strength:the women's health and aging study. Arch Intern Med. 2011;171(12):1119–1121. doi: 10.1001/archinternmed.2011.252. [DOI] [PubMed] [Google Scholar]
- 13.Kenney WL, Wilmore JH, Costill DL. 5th ed. Champaign, IL: Human Kinetics; 2012. Physiology of sport and exercise. [Google Scholar]
- 14.Neri SG, Harvey LA, Tiedemann A, Gadelha AB, Lima RM. Obesity and falls in older women:mediating effects of muscle quality, foot loads and postural control. Gait Posture. 2020;77:138–143. doi: 10.1016/j.gaitpost.2020.01.025. [DOI] [PubMed] [Google Scholar]
- 15.Neri SGR, Tiedemann A, Gadelha AB, Lima RM. Body fat distribution in obesity and the association with falls:a cohort study of Brazilian women aged 60 years and over. Maturitas. 2020;139:64–68. doi: 10.1016/j.maturitas.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Bertolucci PH, Brucki S, Campacci SR, Juliano Y. O mini-exame do estado mental em uma populaçäo geral:impacto da escolaridade. Arq Neuropsiquiatr. 1994;52(1):1–7. [PubMed] [Google Scholar]
- 17.Shelkey M, Wallace M. Katz index of independence in activities of daily living (ADL) Gerontologist. 2000;10(1):20–30. [PubMed] [Google Scholar]
- 18.Matsudo S, Araújo T, Marsudo V, Andrade D, Andrade E, Braggion G. Questinário internacional de atividade f1sica (IPAQ):estudo de validade e reprodutibilidade no Brasil. Rev Bras Ativ Fís Saúde. 6(2):05–18. [Google Scholar]
- 19.Tiedemann A, Lord SR, Sherrington C. The development and validation of a brief performance-based fall risk assessment tool for use in primary care. J Gerontol A Bio Sci Med Sci. 2010;65(8):896–903. doi: 10.1093/gerona/glq067. [DOI] [PubMed] [Google Scholar]
- 20.Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg. 1984;9(2):222–226. doi: 10.1016/s0363-5023(84)80146-x. [DOI] [PubMed] [Google Scholar]
- 21.Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project:rationale, study description, conference recommendations, and final estimates. J Gerontol A Bio Sci Med Sci. 2014;69(5):547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamb SE, Jørstad-Stein EC, Hauer K, Becker C. Development of a common outcome data set for fall injury prevention trials:the Prevention of Falls Network Europe consensus. J Ame Geriatr Soc. 2005;53(9):1618–1622. doi: 10.1111/j.1532-5415.2005.53455.x. [DOI] [PubMed] [Google Scholar]
- 23.Sherrington C, Fairhall N, Wallbank G, et al. Exercise for preventing falls in older people living in the community:an abridged Cochrane systematic review. Br J Sports Med. 2020;54(15):885–891. doi: 10.1136/bjsports-2019-101512. [DOI] [PubMed] [Google Scholar]
- 24.Leong DP, Teo KK, Rangarajan S, et al. Prognostic value of grip strength:findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386(9990):266–273. doi: 10.1016/S0140-6736(14)62000-6. [DOI] [PubMed] [Google Scholar]
- 25.Rijk JM, Roos PR, Deckx L, van den Akker M, Buntinx F. Prognostic value of handgrip strength in people aged 60 years and older:A systematic review and meta-analysis. Geriatr Gerontol Int. 2016;16(1):5–20. doi: 10.1111/ggi.12508. [DOI] [PubMed] [Google Scholar]
- 26.Cooper R, Kuh D, Hardy R, Mortality Review G Falcon, Teams HAS. Objectively measured physical capability levels and mortality:systematic review and meta-analysis. BMJ. 2010;341:c4467. doi: 10.1136/bmj.c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neri SGR, Oliveira JS, Dario AB, Lima RM, Tiedemann A. Does obesity increase the risk and severity of falls in people aged 60 years and older?A systematic review and meta-analysis of observational studies. Gerontol A Bio Sci Med Sci. 2020;75(5):952–960. doi: 10.1093/gerona/glz272. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Hermoso A, Cavero-Redondo I, Ramirez-Velez R, et al. Muscular strength as a predictor of all-cause mortality in an apparently healthy population:a systematic review and meta-analysis of data from approximately 2 million men and women. Arch Phys Med Rehabil. 2018;99(10):2100–2113. e2105. doi: 10.1016/j.apmr.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Volaklis KA, Halle M, Meisinger C. Muscular strength as a strong predictor of mortality:A narrative review. Eur J Intern Med. 2015;26(5):303–310. doi: 10.1016/j.ejim.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 30.American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. 10th ed. Philadelphia: Wolters Kluwer/Lippincott Williams &Wilkins Health; 2018. [Google Scholar]


