Abstract
Zero-order drug release that releases drugs at a constant rate is beneficial to prolong the therapeutic effect and avoid the side effects of drugs. However, due to the weak interaction between the drug and the carrier, it is particularly challenging to achieve zero-order release of water-soluble drugs. Inspired by the marker pen, which stores the water-based ink in the sponge core and releases a constant amount of ink from the tip for writing, we explore the marker pen as a drug delivery platform to achieve zero-order release of water-soluble drugs. Through the capillary interaction between the material and water, the pen core can absorb the aqueous drug solution to encapsulate and store the water-soluble drug model sodium fluorescein (SF) and can release the encapsulated SF by moving the pen tip across the surface. The results show that the marker pen can release a constant amount of SF at the nanogram level per unit length of the line drawn with the pen, and the cumulative SF release amount has a linear relationship with the length of the line. In addition, the amount of released SF is linear with respect to the SF concentration in the aqueous solution. Moreover, the SF-filled marker pen has excellent long-term stability as evidenced by that the amount of SF released from the pen remains constant within two weeks after filling.
Introduction
Water solubility is one of the most important parameters to determine the drug distribution and absorption to achieve the required concentration of drug for a desired pharmacological response. In order to be absorbed, the drug must be present in the form of a solution at the site of absorption.1 Many protein-based drugs (such as insulin and growth factors) are water-soluble.2−4 Almost all therapeutic nucleic acids (such as RNA and DNA) and antibiotics are water-soluble.5−7 However, on the other hand, good water solubility tends to distribute the drug into the aqueous biological environment of the human body, leading to rapid clearance from the systemic circulation or a local site of administration.8 The unexpected drug distribution limits therapeutic efficacy and causes adverse side effects.9
The pharmacokinetics and biodistribution profile of water-soluble drugs can be improved by encapsulating them in delivery systems that allow sustained drug release.8 Three strategies have been documented to obtain the encapsulation and controlled release of water-soluble drugs: (i) physical encapsulation in liposomes/polymersomes.10 Liposomes and polymersomes, core–shell structured hollow particles, can efficiently encapsulate water-soluble drugs into the aqueous pockets with a relatively high loading efficiency. The hydrophobic shells prevent leakage of hydrophilic drugs, achieving sustained release. (ii) Physical adsorption through electronic interaction, which refers to the physical entrap of charged molecules in oppositely charged carriers.11 (iii) Covalent conjugation. Water-soluble drugs are covalently bonded to the polymer backbone via the hydrolysable chemical bond.12 The drug can be released via the hydrolysis of the chemical bond, and the release rate can be tuned by adjusting the hydrophobicity of the polymer backbone. Although these three strategies can efficiently encapsulate water-soluble drugs and obtain sustained drug release, they release the drug in a “bell-shaped” concentration–time profile. The drug concentration increases to a peak concentration and then decreases. The concentration of the drug is maintained for a relatively short period in the therapeutic window, while a large portion of the drug is released at ineffective or toxic concentrations. This may manifest as burst drug release and systemic toxicity, especially problematic with ultra-potent drugs.
Zero-order drug release is an ideal way to improve the therapeutic effect and avoid the side effects of the drug.13,14 The drug is released from the carrier at a constant rate; therefore, the drug concentration–time profile is flat. The drug concentration can always be maintained within the therapeutic window, without the drug waste and side effects. However, achieving zero-order drug release is challenging, especially for water-soluble drugs, where the interaction between the drug and the carrier is weak.
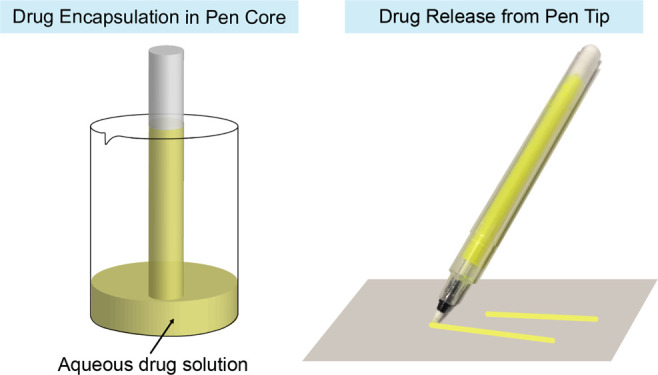
Inspired by the marker pen, which stores the water-based ink in the sponge core and always releases a constant amount of ink from the tip through capillary action for writing, we hypothesize that the marker pen should be able to encapsulate the water-soluble drugs in the pen core by absorbing the drug aqueous solution and release the encapsulated drug through the pen tip under the zero-order mechanism.
Results
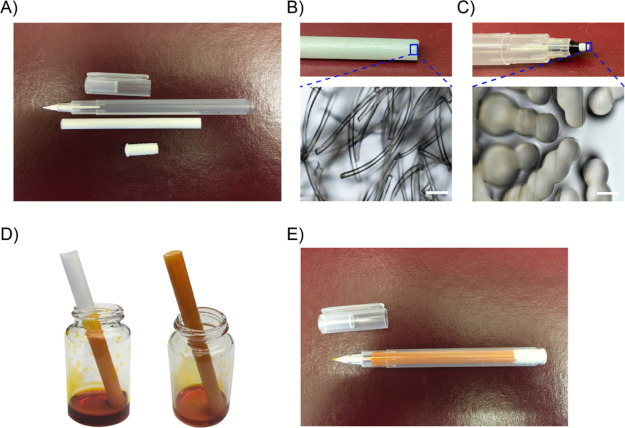
Kuretake Karappo empty felt tip pen is an empty pen that can be filled with custom dyes (Figure 1A). The core and tip of the pen are composed of micron-sized fibers and channels, so they can absorb and maintain water like a sponge by the capillary action (Figure 1B,C). To fill the pen with the drug, place one end of the pen core into a container with the aqueous drug solution (Figure 1D). Due to capillary action, the aqueous drug solution slowly rises to the core (Figure 1D). After the core is full, it is pulled out. Any drug solution from the outside of the core is wiped off. Then, insert the core into the pen’s outer holder, followed by inserting the stopper (Figure 1E).
Figure 1.
Setting of the Kuretake Karappo empty felt tip pen. (A) Five solid parts of the pen: cap, outer holder, tip, core, and stopper. (B) Photo and microscope image of pen core. Scale bar = 100 μm. (C) Photo and microscope image of the pen tip. (D) Photo of the pen core absorbing aqueous drug solution. (E) Photo of the assembled drug-filled pen.
The diameter of the pen core is 0.7 cm, the length is 8.5 cm, and the filling volume of the aqueous drug solution is about 2.5 mL. The drug loading can be adjusted by changing the concentration of the aqueous drug solution, and the maximum drug loading is determined by the water solubility of the drug. In this study, sodium fluorescein (SF) is used as a water-soluble model drug. Four different concentrations of SF aqueous solutions were prepared: 0.5, 1, 5, and 10 mg/mL. Therefore, the four corresponding SF loads in the pen are 1.25, 2.5, 12.5, and 25 mg, respectively.
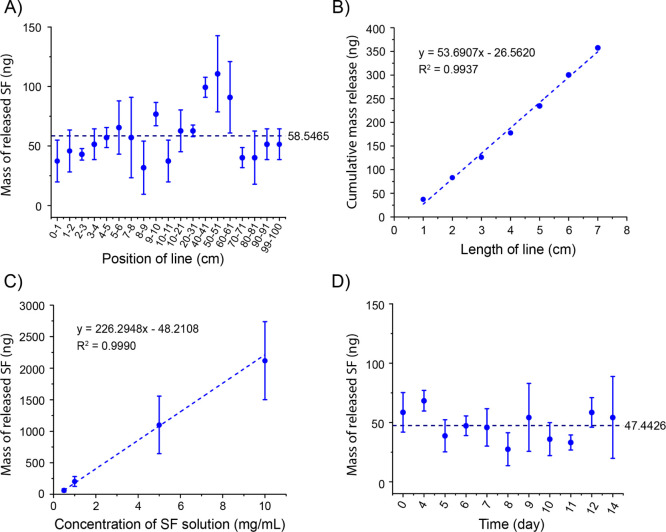
The pen has a tip of 0.4 mm in diameter. Inside the pen tip, there are multiple micron channels (Figure 1C). Through capillary action, these micron channels can transfer the aqueous drug solution from the pen core to the pen tip and then from the center of the pen tip to the surface of the pen tip. The loaded SF can be released onto a surface by moving the tip across the surface. In order to quantitatively evaluate the pen’s drug release control, a line with a width of 0.4 mm and a length of 1 m was drawn on the brand copy and print paper (Figure S1). Then, the paper with a 1 cm-long line was cut out and incubated in deionized water for 24 h. Because the drug is water-soluble, the drug was fully dissolved in the water after 24 h. The SF concentration in the aqueous solution was measured with an absorbance microplate reader. As shown in Figure 2A, regardless of the position, the marker pen filled with 0.5 mg/mL SF solution releases 58.6 ± 22.0 ng SF per unit length (1 cm) of the line. These results indicate that the marker pen can accurately release a stable amount of drug at the nanogram level. There are fluctuations in different positions of the line, which may be caused by the inconsistent manual drawing speed. We draw the lines by hand to imitate the patient self-administration in practical applications. We can only control the speed to approximately but not exactly at 1 cm/s. Because trademark copy paper can absorb aqueous solutions through capillary action, a slower drawing speed will cause more solution to be released from the pen tip. When drawing a line on the human skin, this fluctuation can be reduced because human skin is not as porous as trademark copy paper. The cumulative amount of SF release is linear with respect to the length of the line, R2 = 0.9937 (Figure 2B).
Figure 2.
In vitro release profiles of SF from the pen by moving the pen tip across the paper. (A) Mass of SF released per unit length (1 cm) of the line at different positions of the line drawn with a pen filled with the 0.5 mg/mL SF solution. (B) Linear relationship between the cumulative mass release of SF and the length of the line drawn with a pen filled with the 0.5 mg/mL SF solution. (C) Liner relationship between the mass of SF released in a 1 cm-long line and the SF concentration of the filling solution. (D) Mass of SF released on a 1 cm line drawn with the pen filled with the 0.5 mg/mL SF solution on different days after the pen is filled.
The amount of SF released by pens filled with four different concentrations of SF solution was compared. Figure 2C shows that the mass of SF released in a 1 cm-long line has a good linear relationship with the SF concentration of the filling solution, R2 = 0.9990. This indicates that the mass of the drug released by the pen can be adjusted by changing the SF concentration of the filling solution, which can vary from 0 to saturated concentration. Therefore, depending on the solubility of the drug, the target amount of drug released by the pen can range from sub-nanogram to gram.
The drug release control property of the pen is stable for a long time. After filling with 0.5 mg/mL SF solution, the pen was left in the dark at room temperature. A 1 m-long line was drawn on the paper with the pen every day. During the 2 weeks of the test, the mass of the SF released in the drawn 1 cm-long line remained stable at 47.5 ± 12.6 ng (Figure 2D). Over time, the amount of drug released did not increase or decrease. This long-term stability would benefit the management of chronic diseases.
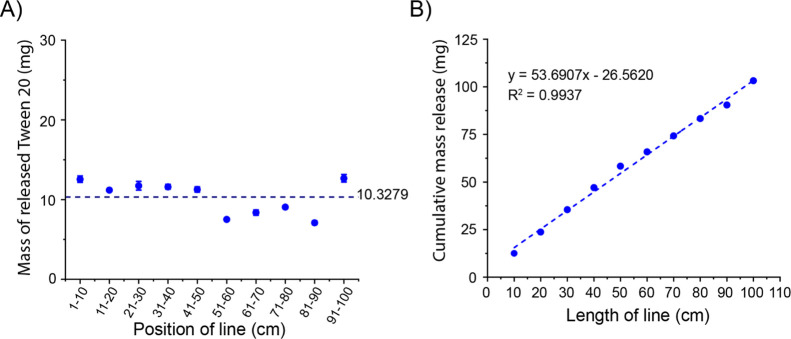
One possible practical application of the marker pen platform is topical and transdermal drug delivery, which applies drugs to body surfaces such as the skin or mucous membranes. However, some water-soluble drugs may have low skin permeability due to low lipophilicity or high molecular weight (>600 Da). In order to increase the skin permeability of these drugs, it may be necessary to co-deliver a chemical permeation enhancer (CPE) with the water-soluble drugs by the marker pen.12,15 Because surfactants are an important type of CPE, we examined the model surfactant Polysorbate 20 (Tween 20) released by the marker pen. The marker pen was filled with the aqueous solution of Tween 20 (100 mg/mL), and the loaded Tween 20 was released onto the Kimtech Science Kimwipes by moving the pen tip across the surface. As shown in Figure 3A, no matter where the position is, the marker pen releases 10.3 ± 2.1 mg Tween 20 per unit length (10 cm) of the line. In addition, the cumulative amount of Tween 20 release has a linear relationship with the length of the line, R2 = 0.9937 (Figure 3B). These results show that, just like water-soluble drugs, the marker pen can constantly release a stable amount of surfactant. This feature of the marker pen is also ideal for drug combinations, which enables the marker pen to release two drugs at a constant ratio to ensure the best synergy between the drugs.
Figure 3.
In vitro release profiles of Tween 20 from the pen by moving the pen tip on the Kimtech Science Kimwipes. (A) Mass of Tween 20 released in the line of per unit length (10 cm) at different positions of the line drawn with a pen filled with the 100 mg/mL Tween 20 solution. (B) Linear relationship between the cumulative mass release of Tween 20 and the length of the line drawn with a pen filled with the 100 mg/mL Tween 20 solution.
Discussion
In addition to achieving the zero-order release of water-soluble drugs, the marker pen platform also has many other features that can benefit its potential clinical applications: (1) there is no undesired drug leakage during storage because the pen core can retain the drug through capillary action, and the pen stopper can prevent the drug from leaking out of the pen holder; (2) there is no waste of drug during the encapsulation process because the aqueous drug solution can be reused until all is absorbed into the pen core; (3) no organic solvents are involved in the encapsulation and release process; (4) since the drug is dissolved in an aqueous solution and the concentration of the drug can be reduced to the desired level by diluting the solution, the amount of the drug released per treatment can be nanograms or less. This feature is extremely important for the ultrapotent drugs; (5) the marker pen can deliver multiple drugs or ingredients by simply co-dissolving them in a filling solution. Moreover, the release mass ratio between different drugs and ingredients can be maintained at the designed ratio to maximize the synergistic effect of drugs; (6) the marker pen is a simple and low-cost strategy. The simple procedure also makes it highly reproducible, which minimizes batch-to-batch variation between products; (7) the marker pen can also be applied to poorly water-soluble drugs if they can be uniformly dispersed in water or their solubility in water is improved; (8) if the size of nanoparticles is within the range that does not block the micro-channels of the pen tip and the nanoparticles have a stable suspension in water, the marker pen can be used as a carrier for nanoparticles.
The features of the marker pen make it have good potential applications in local drug delivery, especially in topical and transdermal drug administration. For example, the marker pen can be used to deliver local anesthetics on-demand to manage skin pain. Local anesthetics can be repeatedly applied to the painful area with the marker pen. This allows patients to control the frequency, intensity, and duration of anesthesia according to changing needs and conditions. Likewise, the marker pen can be used to deliver antibiotics to treat skin infections such as otitis media, eczema, and psoriasis.
In addition to the marker pen, many micro- and nanotechnologies can be used for drug loading and controlled release through capillary action. For example, the electrospun matrices manufactured by electrospinning have unique nano- to micrometer-sized fibers. The fiber morphology produces extremely high surface-to-volume ratios, complex porous structures, and excellent pore interconnectivity.16,17 These characteristics make the electrospun matrix a good platform for encapsulation and controlled drug release through capillary action. The electrospun matrices loaded with drugs can be used as drug reservoirs and scaffolds for tissue regeneration. Likewise, nanotubes, nanosheets, mesoporous silica nanoparticles can be used for drug delivery platforms based on capillary action, because all these materials have a large surface-to-volume ratio that can enhance the capillary action between materials and liquids.18,19
Conclusions
A marker pen platform for drug delivery is presented. The marker pen achieves zero-order controlled release of a water-soluble drug, for example, the SF release amount can be linearly controlled by changing the length of a line drawn with the pen and the SF concentration of the filling solution. The marker pen uses capillary action to encapsulate and release drugs. The capillary action is a different approach from the existing water-soluble drug delivery technologies. The drug delivery platforms based on capillary action have many unique characteristics in structure and function, which would be beneficial to clinical practices that cannot be achieved using existing technologies. It is expected that with the progress of related research, capillary action will become an important choice for drug delivery platform design and will play an important role in improving the efficacy of drugs, reducing the side effects of drugs and ultimately improving human health.
Materials
Fluorescein sodium salt was purchased from VWR International Ltd (Radnor, PA). Kuretake Karappo empty felt tip pen was purchased from Kuretake Co. Ltd. (Nara, Japan).
Methods
Drug Loading into the Pen
SF was dissolved in deionized water at four different concentrations: 0.5, 1, 5, and 10 mg/mL. Tween 20 was dissolved in deionized water at a concentration of 100 mg/mL. To fill the pen with the drug, place one end of the pen core into a container with 3 mL of the drug solution. The pen core was left in the solution for 3 min. After the core is full, it is pulled out. Any drug solution from the outside of the core was wiped off. Then, insert the core into the pen, followed by inserting the stopper.
Determination of the Drug Release
Use the SF-filled marker pen to draw a line with a width of 0.4 mm and a length of 1 m on the brand copy and print paper. The drawing speed is about 1 cm/s. Then, cut out a piece of paper with a line of 1 cm long on it. Incubate the piece of paper in 1–2 mL of deionized water for 24 h. The SF concentration in the solution is measured with an absorbance microplate reader (EMax Plus, Molecular Devices). Briefly, put 200 μL of the solution into a 96-well plate. Each sample has 3–6 duplicates. Measure the optical density (OD) value at a wavelength of 492 nm. The standard curve is measured from the solutions with the known SF concentrations. (y = 8.4503x – 0.264, R2 = 0.9988; y, concentration in μg/mL; x, the OD value read from the microplate reader). Use the Tween 20-filled marker pen to draw a line with a width of 0.4 mm and a length of 1 m on the Kimtec Science Kimwipes. The drawing speed is about 1 cm/s. Then, cut out a piece of Kimwipe with a line of 10 cm long on it. Incubate the piece of paper in 3 mL of deionized water for 24 h to fully dissolve the Tween 20. The Tween 20 concentration in the solution is measured with UV–vis Spectrophotometers (UV-3100PC, VWR). Briefly, 2 mL of the solution was measured each time at a wavelength of 240 nm with three duplicates. The standard curve is measured from the solutions with the known Tween 20 concentrations. (y = 51.916x – 3.297, R2 = 0.9987; y, concentration in mg/mL; x, the OD value read from the UV–vis spectra).
Observation of the Morphology of Core and Tip of the Pen
Cut the pen core and tip along the cross-section with a lancet and observe using an optical microscope (Ts2R, Nikon) with a 20× magnification lens.
Acknowledgments
C.Z. acknowledges the support of start-up package and the Level 1- Standard RGC Project/RGC Seed Project (GR14901) from The University of Alabama. C.Z. is a recipient of the National Institutes of Health Research Enhancement Award (R15GM139193).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c01141.
Lines drawn on the brand copy and print paper using the pen filled with the sodium fluorescein solution (5 mg/mL) (PDF)
Author Contributions
X.L. and Q.L. contributed equally. X.L. and C.Z. conceived the presented idea and planned the experiments. X.L., Q.L., and C.Z. carried out the experiments. X.L., Q.L., and C.Z. analyzed the data. X.L., Q.L., and C.Z. wrote the paper. C.Z. supervised the project.
The authors declare no competing financial interest.
Supplementary Material
References
- Savjani K. T.; Gajjar A. K.; Savjani J. K. Drug solubility: importance and enhancement techniques. ISRN Pharm. 2012, 2012, 195727. 10.5402/2012/195727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.; Zhao C.; Hu R.; Lin W.; Wang Q.; Zhao J.; Bilinovich S. M.; Leeper T. C.; Li L.; Cheung H. M.; Chen S.; Zheng J. Probing the weak interaction of proteins with neutral and zwitterionic antifouling polymers. Acta Biomater. 2014, 10, 751–760. 10.1016/j.actbio.2013.09.038. [DOI] [PubMed] [Google Scholar]
- Zhao C.; Li X.; Li L.; Gong X.; Chang Y.; Zheng J. Mimicking the binding and unbinding of Fe3+ with transferrin using a single biomimetic nanochannel. Chem. Commun. 2013, 49, 9317–9319. 10.1039/c3cc44249g. [DOI] [PubMed] [Google Scholar]
- Pace C. N.; Treviño S.; Prabhakaran E.; Scholtz J. M. Protein structure, stability and solubility in water and other solvents. Philos. Trans. R. Soc. London, Ser. B 2004, 359, 1225–1234. 10.1098/rstb.2004.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]; ; discussion 1234-5
- Duffy K.; Arangundy-Franklin S.; Holliger P. Modified nucleic acids: replication, evolution, and next-generation therapeutics. BMC Biol. 2020, 18, 112. 10.1186/s12915-020-00803-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihata T.; Rytting J. H.; Higuchi T.; Caldwell L. J.; Selk S. J. Enhancement of rectal absorption of water-soluble antibiotics in dogs. Int. J. Pharm. 1984, 21, 239–248. 10.1016/0378-5173(84)90098-x. [DOI] [Google Scholar]
- Touraki M.; Rigas P.; Kastritsis C. Liposome mediated delivery of water soluble antibiotics to the larvae of aquatic animals. Aquaculture 1995, 136, 1–10. 10.1016/0044-8486(95)01039-4. [DOI] [Google Scholar]
- Li Q.; Li X.; Zhao C. Strategies to Obtain Encapsulation and Controlled Release of Small Hydrophilic Molecules. Front. Bioeng. Biotechnol. 2020, 8, 437. 10.3389/fbioe.2020.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H.; Jung H.; Li X. Drug Delivery Approaches in Addressing Clinical Pharmacology-Related Issues: Opportunities and Challenges. AAPS J. 2015, 17, 1327–1340. 10.1208/s12248-015-9814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomorony A.; Santamaria C. M.; Zhao C.; Rwei A. Y.; Mehta M.; Zurakowski D.; Kohane D. S. Prolonged Duration Local Anesthesia by Combined Delivery of Capsaicin- and Tetrodotoxin-Loaded Liposomes. Anesth. Analg. 2019, 129, 709. 10.1213/ane.0000000000004108. [DOI] [PubMed] [Google Scholar]
- Liu Q.; Santamaria C. M.; Wei T.; Zhao C.; Ji T.; Yang T.; Shomorony A.; Wang B. Y.; Kohane D. S. Hollow Silica Nanoparticles Penetrate the Peripheral Nerve and Enhance the Nerve Blockade from Tetrodotoxin. Nano Lett. 2018, 18, 32–37. 10.1021/acs.nanolett.7b02461. [DOI] [PubMed] [Google Scholar]
- Zhao C.; Liu A.; Santamaria C. M.; Shomorony A.; Ji T.; Wei T.; Gordon A.; Elofsson H.; Mehta M.; Yang R.; Kohane D. S. Polymer-tetrodotoxin conjugates to induce prolonged duration local anesthesia with minimal toxicity. Nat. Commun. 2019, 10, 2566. 10.1038/s41467-019-10296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y.; Kao W. J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Delivery 2010, 7, 429–444. 10.1517/17425241003602259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodkin S.; Tucker F. E. Linear Drug Release from Laminated Hydroxypropyl Cellulose-Polyvinyl Acetate Films. J. Pharma Sci. 1975, 64, 1289–1294. 10.1002/jps.2600640806. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Quan P.; Liu X.; Wang M.; Fang L. Novel chemical permeation enhancers for transdermal drug delivery. Asian J. Pharm. Sci. 2014, 9, 51–64. 10.1016/j.ajps.2014.01.001. [DOI] [Google Scholar]
- Feng X.; Li J.; Zhang X.; Liu T.; Ding J.; Chen X. Electrospun polymer micro/nanofibers as pharmaceutical repositories for healthcare. J. Controlled Release 2019, 302, 19–41. 10.1016/j.jconrel.2019.03.020. [DOI] [PubMed] [Google Scholar]
- Ding J.; Zhang J.; Li J.; Li D.; Xiao C.; Xiao H.; Yang H.; Zhuang X.; Chen X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. 10.1016/j.progpolymsci.2019.01.002. [DOI] [Google Scholar]
- Qi B.; Wang C.; Ding J.; Tao W. Editorial: Applications of Nanobiotechnology in Pharmacology. Front. Pharmacol. 2019, 10, 1451. 10.3389/fphar.2019.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y.; Chen J.; Wang C.; Ding J.; Tao W. Editorial: Emerging Micro- and Nanotechnologies for Medical and Pharmacological Applications. Front. Pharmacol. 2021, 12, 102. 10.3389/fphar.2021.648749. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.