Abstract
Background
Chronic kidney disease (CKD) is one of the pathologies with the greatest impact on the public health system. Over the last few decades, the relevance of CKD in Mexico has increased, with associated overwhelming costs for care of renal disease. There are no reliable CKD statistics in Mexico.
Methodology
In June 2018, the government of Aguascalientes called on all Health Institutions to create a state registry of treated end-stage renal disease (ESRD). In the same system, a renal biopsy result registry included all the native kidney biopsies obtained in the state of Aguascalientes since 2012. We herein describe the prevalence, incidence and characteristics of the patients included in the CKD and renal biopsy registry in the state of Aguascalientes.
Results
As of April 2020, the state has documented 2827 patients on renal replacement therapy (RRT), 1877 on dialysis and 950 that have been transplanted. The prevalence of patients on dialysis is 1326 per million population (p.m.p.), and if transplanted individuals are included, it is 1997 p.m.p. The incidence of treated ESRD in 2019 was 336 p.m.p. (n = 474) in individuals with an average age of 45.6 years (standard deviation ±18), and in a higher proportion of men (61%). There is a bimodal distribution of the age at which RRT was initiated. The first and the most significant peaks are between the ages of 20 and 40 years and are usually the result of CKD of unknown cause (73%). The second peak is between 50 and 70 years of age, and CKD is usually the result of diabetes mellitus and systemic arterial hypertension (59.6%). Since January 2012, 423 biopsies have been recorded. The patient’s ages were between 20 and 30 years (n = 112), and the most frequent diagnosis was focal segmental glomerulosclerosis (FSGS) (54%).
Conclusions
The prevalence of treated ESRD in the state of Aguascalientes is high. The disease mostly afflicts young people between 20 and 40 years of age, and there is a clear male predominance. In this age group, the main clinical diagnosis is CKD of unknown origin, and the most frequent biopsy diagnosis was FSGS.
Keywords: chronic kidney disease, chronic kidney disease of unknown origin, chronic kidney disease registry, focal segmental glomerulosclerosis, renal biopsy registry
INTRODUCTION
Chronic kidney disease (CKD) is one of the main public health challenges in our country. The number of patients with this condition has increased worldwide due to the increased prevalence of metabolic diseases such as obesity, systemic arterial hypertension (SAH) and Type 2 diabetes mellitus (DM2) [1, 2].
In Mexico, it is estimated that there are 12.8 million patients with DM2, with a prevalence of 13.5%, while that of obesity and overweight are 28.9 and 64.9%, respectively [1, 2]. The chronic nature of these pathologies combined with greater access to health services has resulted in an increased number of patients with long-term complications, including CKD.
The impact of CKD lies beyond being one of the leading causes of death. CKD and DM are the main causes of lost years of life and years lived with disabilities in Mexico. Specifically, CKD resulting from DM and CKD of unknown origin have the greatest impact in terms of lost years of life and the overall number of years lived with disability [3, 4].
Furthermore, the care costs of this pathology are overwhelming in any health system. In 2014, the Mexican Social Security Institute (IMSS), the health institute covering the largest number of individuals, paid 996 US Million Dollars (USMD) for the treatment of end-stage renal disease (ESRD), representing 15% of the institution's total annual expenditures. In 2017, CKD was in third place in terms of expenses (546 USMD), below only DM (2188 USMD) and SAH (1180 USMD); in Mexico, the reported number of patients with treated ESRD was 69 267, there were 2.8 million patients with DM and 4.5 million with SAH. Again, that year, the cost per ESRD patient was the highest, reported at 7899 USD, followed by breast cancer at 2822 USD [5].
To understand CKD and its impact on the country, the first step is to develop a statistical registry. Unfortunately, in Mexico, we sorely lack a national CKD registry. In the state of Jalisco, some data have been reported to the United States Renal Data System (USRDS) for almost two decades [6]. The aim of this report is to describe the information obtained from the CKD and renal biopsy registry as the initial essential tool to understand CKD in Aguascalientes, and suggest approaches to face this overwhelmingly common entity in the state and in the country.
MATERIALS AND METHODS
Aguascalientes is a state located in northern central Mexico; it has a surface area of 5616 km2 and a population of 1.41 million inhabitants. In June 2018, the State Registry of CKD and Renal Biopsy was developed and instituted. Under the direction of the Aguascalientes State Health Services Institute, the various public (Instituto Mexicano del Seguro Social, Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado y el Insituto de Servicios de Salud del Estado de Aguascalientes ) and private institutions agreed and committed to report patients who are currently on renal replacement therapy (RRT; hemodialysis, peritoneal dialysis and transplantation). The requested data were collected in a single population registry (essential to avoid duplication of cases) and included place of birth and residence, cause of CKD, date of initiation of RRT and RRT modality (transplant, peritoneal dialysis or hemodialysis).
For follow-up purposes, changes in RRT and the date of change were added, as well as the date and cause of death. Each institution verified their data in their electronic files. The causes of CKD were categorized as secondary to DM if the patient had a history of the disease, SAH in cases with a long-standing history of the disease, and glomerulopathies if a biopsy was obtained and established the diagnosis of a primary or secondary glomerulopathy (excluding DM). Patients with no history of DM, glomerulopathy, long-standing SAH or any identifiable cause (abnormalities of the urinary tract or polycystic kidney disease) were classified as kidney disease of unknown origin. The renal biopsy registry was managed by the only nephropathologist in the state, and also included basic demographic data: age, gender, place of birth and residence, in addition to the histological diagnosis. In this report, only patients residing in the state of Aguascalientes were included. Descriptive analyses of light microscopy findings in focal segmental glomerulosclerosis (FSGS) biopsies were performed and recorded.
Registration is conducted online on a platform designed to be used with an electronic address (SQL server Microsoft Visual Studio 2010). Each institution has a unique access code and is responsible for adding and modifying the data in a timely manner. Each of the institutional managers is responsible for the management of personal data and must sign a letter of commitment to properly use this information.
Renal biopsies
All biopsies were analyzed by a certified nephropathologist. The biopsy cores were dissected into two portions, one for light microscopy and the other for immunofluorescence. The core for immunofluorescence was placed in Michel’s fixative, and that for light microscopy in 10% neutral buffered formalin.
For light microscopy, the sample was embedded in paraffin, sections were cut serially every 3 μm and stained with hematoxylin and eosin, periodic acid–Schiff, silver methenamine and Masson trichrome. For immunofluorescence, sections of snap-frozen biopsies were cut every 2 μm in a Cryostat set a −30°C, and stained with fluorescein-tagged polyclonal rabbit antibodies against immunoglobulin A (IgA), IgG, IgM, complement (C1q, C3c and C4d), kappa and lambda light chain and fibrinogen (Genetex®).
Global sclerosis was expressed as the median and interquartile range (IQR) , and categorized with relative and absolute frequencies. Segmental glomerular sclerosis was diagnosed if synechiae from the Bowman capsule to the glomerular basement membrane and/or segmental glomerular sclerosis were detected. These findings were classified based on the Columbia classification of FSGS. Glomerulomegaly was defined as at least one glomerulus with a diameter of ≥150% of the mean glomerular diameter in the sample, measured in a 10× field. Interstitial fibrosis, tubular atrophy and interstitial inflammation were classified on the basis of qualitative observation and expressed as the median and IQR, and categorized in relative and absolute frequencies.
Tubulitis was defined as the presence of inflammatory cell infiltration (lymphocytes or polymorphonuclear cells) in least one transverse tubule and categorized as mild 0–5 (infiltrating cells), moderate (5–10 cells) or severe (>10 cells). Mesangial proliferation was defined as more than three cells in an individual glomerular mesangial region, away from the vascular pole. Vascular changes were described in medium-size arteries. Subintimal fibrosis was defined as mild (narrowing <25%), moderate (25–50%) and severe (>50%). Median hyperplasia was defined by the qualitative observation of smooth muscle hyperplasia (present or not). In arterioles, hyalinosis was qualitatively described as present or not present.
Statistical analysis
Descriptive statistics were performed based on the level of measurement. For continuous variables with a normal distribution, mean [standard deviation (SD)] were reported. Relative and absolute frequency were used for dichotomous or ordinal variables. Prevalence was calculated with the number of live patients per million. The incidence was calculated as the number of patients who initiated RRT in 2019, divided by a million inhabitants. Survival analysis of incident patients was calculated in 2018 and 2019 with Kaplan–Meier curves. Between-group comparisons were performed with the log-rank method, and Wilcoxon–Breslow adjustment for early differences. A P < 0.05 was considered significant. A descriptive analysis of FSGS biopsies on light microscopy was performed on all available material. Microsoft Excel, STATA version 11.1 and GraphPad version 5.01 were used for the analysis.
RESULTS
At the time of this report (16 April 2020), there are 2827 live patients with an average age of 45.6 years (±18) and predominantly male (60.9%), included in the registry.
In the state, there are 1877 patients on dialysis and 950 are kidney transplant recipients. The prevalence of treated ESRD cases on dialysis is 1326 per million population (p.m.p.). If kidney transplant recipients are included, the prevalence is 1997 p.m.p. The state municipality with the highest prevalence of RRT is Calvillo, with 192 patients on RRT and a prevalence of 3096 p.m.p.; it is followed by the capital’s municipality, with 2404 patients and a prevalence of 2533 p.m.p. Together, the remaining counties account for 231 patients, with a prevalence of 574 p.m.p.
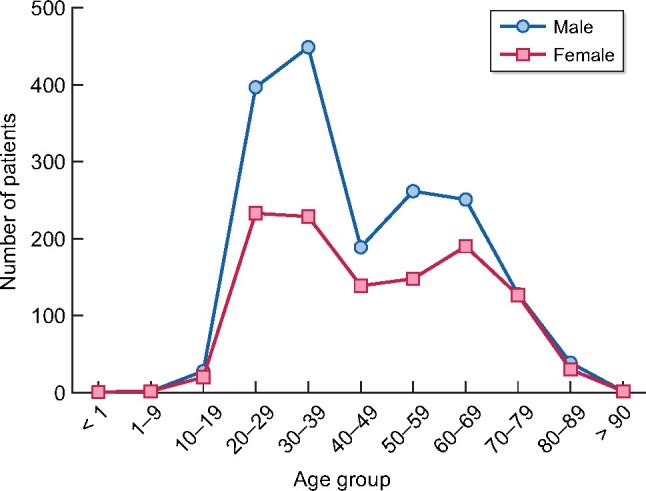
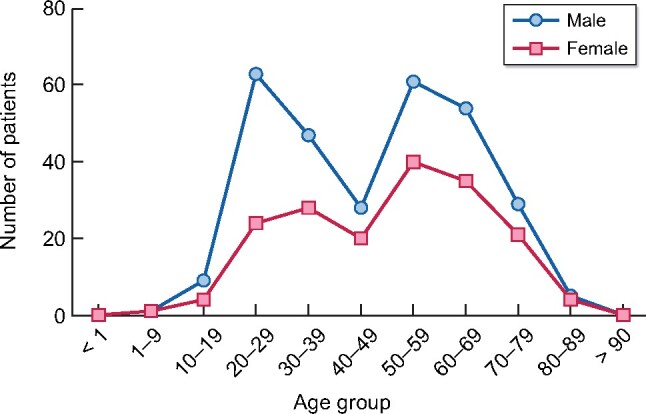
The prevalence in men is 2505 p.m.p. and in women, it is 1543 p.m.p. The distribution of patients on RRT by age group has a bimodal distribution, whereby the highest peak is in patients between the ages of 20 and 40 years, followed by the group between 50 and 70 years (Figure 1).
FIGURE 1: Distribution of prevalent alive patients with treated ESRD (dialysis and transplant) in the state of Aguascalientes by age group and sex.
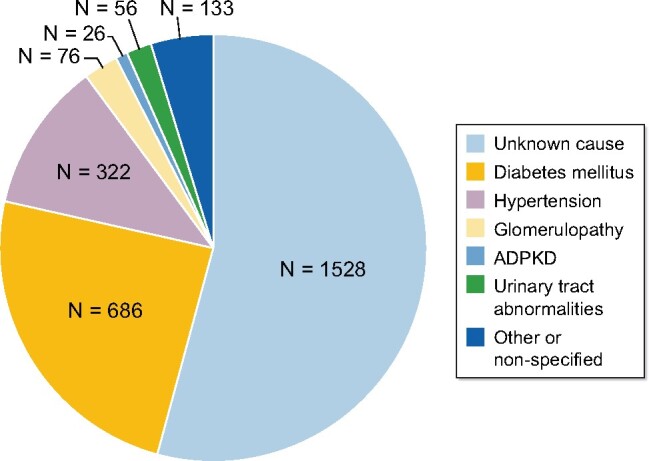
The most frequent etiology of treated ESRD is CKD of unknown origin (54%), followed by DM (24%) and SAH (11.3%) (Figure 2).
FIGURE 2: Causes of CKD of prevalent patients with treated ESRD (dialysis and transplant) in the state of Aguascalientes (April 2020): unknown origin 54%, DM 24.2%, hypertension 11.3%, glomerulopathy 2.6%, urinary tract abnormalities 1.9%, ADPKD 0.9% and other or unspecified 4.7%. ADPKD, autosomal dominant polycystic kidney disease.
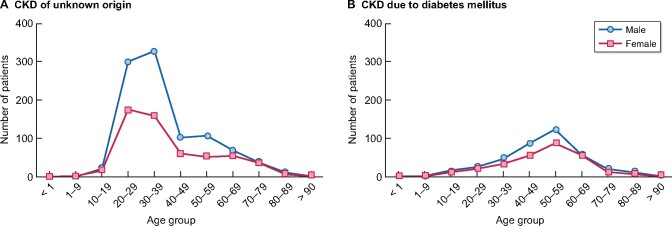
The causes of CKD differ according to the affected age group. The average age of patients with CKD of unknown origin is 39.1 years, while in patients with DM, it is 59 years (Figure 3).
FIGURE 3:
Age and sex distribution according to the most frequent CKD etiologies in the state of Aguascalientes. Patients included were prevalent and alive with treated ESRD in April 2020.
Throughout 2019, we recorded 474 new patients with treated CKD, reflecting an incidence of 336 p.m.p. The absolute distribution by age and sex is bimodal, with peaks encompassing the 20–30 and the 50–60 age groups (Figure 4).
FIGURE 4: Incident patients with treated ESRD in 2019, distributed by age and sex. Patients included were on dialysis or transplant.
The incidence and prevalence by age groups are shown in Table 1, reflecting the predominance of patients between 20 and 39 years (114.8 and 921 p.m.p.) in age-unadjusted values, and 60–79 years (1209 and 5924 p.m.p.) in age-adjusted values (Table 1).
Table 1.
Incidence (2019) and prevalence (April 2020) of treated ESRD by age groups
| Age groups, years | No. of population | No. of ESRD 2019 | Incidence 2019a (p.m.p.) | Incidence 2019b (p.m.p.) | No. of ESRD prevalent | Prevalencea (p.m.p.) | Prevalenceb (p.m.p.) |
|---|---|---|---|---|---|---|---|
| 0–19 | 522 625 | 15 | 29 | 10.6 | 47 | 90 | 33.3 |
| 20–39 | 459 330 | 162 | 353 | 114.8 | 1300 | 2830 | 921 |
| 40–59 | 300 334 | 149 | 496 | 105.6 | 729 | 2427 | 509 |
| 60–79 | 115 793 | 140 | 1209 | 99.2 | 686 | 5924 | 486 |
| >80 | 17 339 | 8 | 162 | 5.6 | 65 | 3748 | 46 |
Age-adjusted incidence and prevalence.
Incidence and prevalence unadjusted.
The most frequent dialysis modality in the state is hemodialysis (n = 1216), representing 65% of cases, while peritoneal dialysis is used in 661 patients (35%).
We detected 950 transplanted patients, representing 33% of all patients on some modality of replacement therapy. The current prevalence of kidney transplant patients in the state is 673 p.m.p. and its incidence in 2019 was 48.2 p.m.p. (n = 68).
Mortality
A total of 327 deaths have been reported in 20 months of follow-up: 310 were on dialysis (hemodialysis: 207, peritoneal dialysis: 103) and 17 were transplant recipients. The main cause of death was of infectious origin (n = 111), followed by cardiovascular causes (n = 93). There are 88 cases in which the cause of death was unknown (unspecific description in the death certificate). The remaining records referred to gastrointestinal bleeding (n = 14), malignancies (n = 6) and other causes (n = 15).
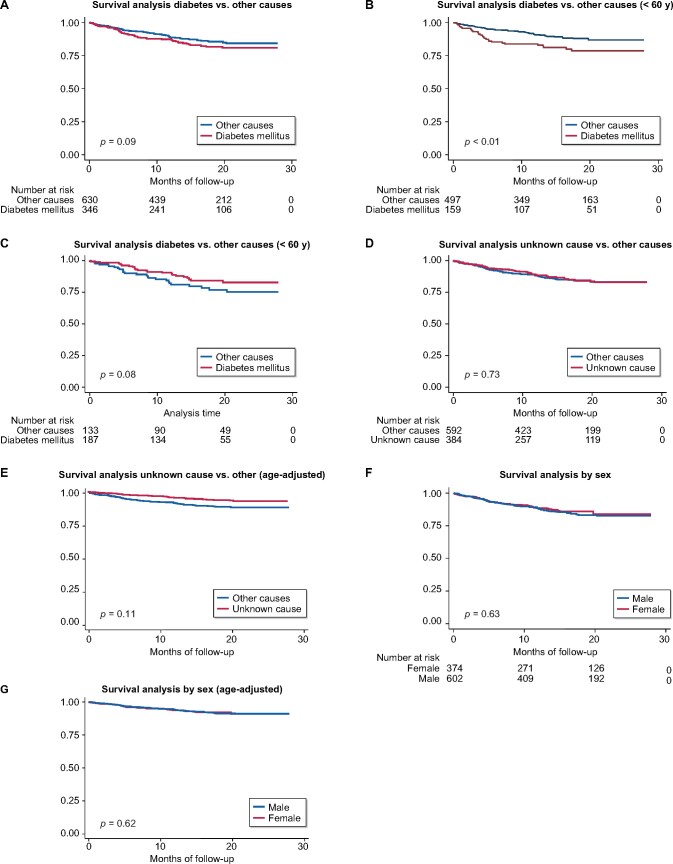
The mortality in incident patients since 2018 is 13.2%. Patients with DM had numerically higher mortality, but the difference was not statistically significant (15.6 versus 11.9, P = 0.1) after a median follow-up of 15.1 months (IQR 8.3–23.3). In the group of patients ˂60 years of age, DM represented a significant risk factor for mortality (log-rank <0.01) (Figure 5).
FIGURE 5: Incident patient survival since 2018. Median follow-up 15.1 months.
(A) Survival analysis diabetes versus other causes, (B) survival analysis diabetes versus other causes (<60 years), (C) survival analysis diabetes versus other causes (<60 years), (D) survival analysis unknown causes versus other causes, (E) survival analysis unknown cause versus other (age-adjusted), (F) survival analysis by sex and (G) survival analysis by sex (sex-adjusted).
Renal biopsy state registry
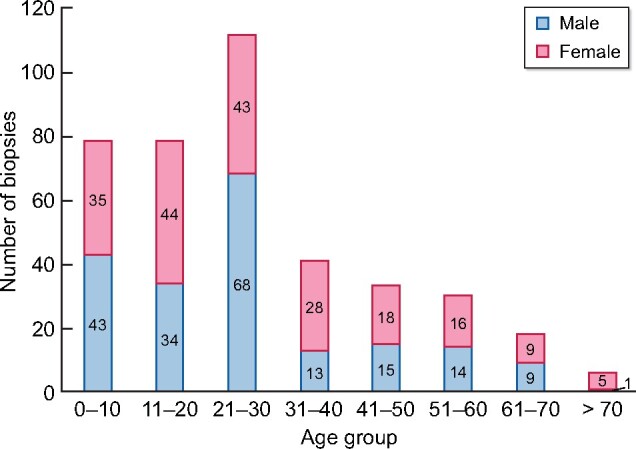
Since 2012, 395 native kidney biopsies have been performed in the state. The age group with the greatest number of biopsies is between 21 and 30 years old (Figure 6).
FIGURE 6: Total number of kidney biopsies registered from 2012 to 2019, distributed by age and sex.
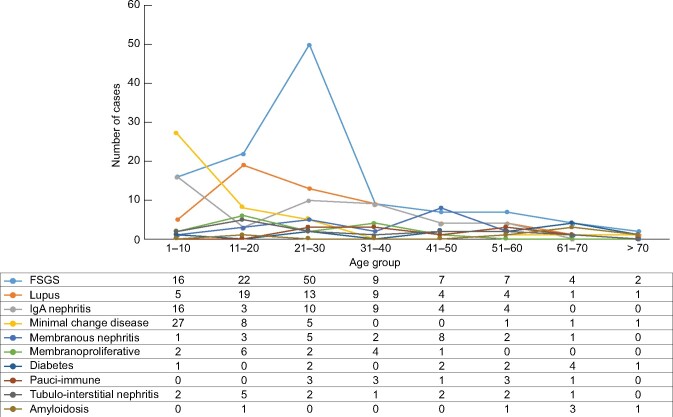
The etiology of the disease differs in terms of the age group; the group ˂10 years of age has a higher proportion of minimal change disease. In the group between 10 and 20 years, the most prevalent cause is FSGS followed by lupus nephritis. Between the ages of 21–30 years (age group with the highest number of biopsies), the main reported glomerulopathy is FSGS, which is also the most prevalent glomerulopathy in the state (Figure 7).
FIGURE 7: Main histopathological diagnoses and age distribution.
Fifty-nine biopsies with FSGS were obtained in the age group between 21 and 40 years. The clinical diagnosis in 15 patients was nephrotic syndrome and the rest had sub-nephrotic proteinuria with a reduced glomerular filtration rate. Forty-two biopsies could be reanalyzed, all with sub-nephrotic proteinuria. Segmental sclerosis was observed in 36 (85%). The histologic categories were perihilar (43%, n = 18), classic (43%, n = 18), and 6 biopsies only showed glomerulomegaly. The median value of interstitial fibrosis was 20% (IQR 5–30) and tubular atrophy, also in 20% (IQR 10–30). Twenty-eight biopsies (66%) had some interstitial inflammation, with a median of 10% (IQR 0–20). Twenty-six biopsies had glomerulomegaly (62%) and 24 showed global sclerosis (57%). (Table 2).
Table 2.
Histologic characteristics of patients with FSGS without nephrotic syndrome and between 20 and 40 years old
| Variables | n = 42 |
|---|---|
| Number of glomerulus, median (IQR) | 10 (5–11) |
| Global glomerular sclerosis | |
| Median (IQR), % | 11 (0–36) |
| 0, n (%) | 18 (43) |
| 1–10, n (%) | 3 (7) |
| 11–25, n (%) | 9 (21) |
| 26–50, n (%) | 7 (17) |
| >50, n (%) | 5 (12) |
| Segmental glomerular sclerosis (patients), n (%) | 36 (85) |
| Perihilar, n (%) | 18 (43) |
| Classic pattern, n (%) | 18 (43) |
| Glomerulomegaly, n (%) | 26 (62) |
| Interstitial fibrosis | |
| Median (IQR) | 20 (10–40) |
| 0, n (%) | 9 (21) |
| 1–10, n (%) | 4 (9) |
| 11–25, n (%) | 13 (31) |
| 26–50, n (%) | 16 (39) |
| >50, n (%) | 0 |
| Tubular atrophy | |
| Median (IQR) | 20 (10–30) |
| 0, n (%) | 8 (19) |
| 1–10, n (%) | 6 (14) |
| 11–25, n (%) | 12 (28) |
| 26–50, n (%) | 16 (39) |
| >50, n (%) | 0 |
| Interstitial inflammation | |
| Median (IQR) | 10 (0–20) |
| 0, n (%) | 14 (33) |
| 1–10, n (%) | 8 (19) |
| 11–25, n (%) | 13 (31) |
| 26–50, n (%) | 7 (17) |
| >50, n (%) | 0 |
| Tubulitis, | |
| Without tubulitis, n (%) | 24 (57) |
| Mild, n (%) | 13 (31) |
| Moderate, n (%) | 5 (12) |
| Severe, n (%) | 0 |
| Mesangial proliferation, n (%) | 11 (26) |
| Arteriolar changes, n (%) | 27 (64) |
| Median hyperplasia, n (%) | 17 (40) |
| Mild subintimal fibrosis, n (%) | 13 (31) |
| Arteriolar hyalinosis, n (%) | 2 (4.7) |
Information on arterial blood pressure was obtained in 51 patients with FSGS. None of the patients had a SAH diagnosis prior to the biopsy. During follow-up, the mean systolic blood pressure was 119 mmHg (±12.9), and the mean diastolic pressure was 74.5 mmHg (±9.9). Fifty-eight percent (n = 30) now have a SAH diagnosis, 11 cases are on ARA2 and 19 ACEI antihypertensive therapy, and only 4 patients required two or more antihypertensive drugs.
Access to the public website
As of April 2020, there is an online domain to access the updated data in the registry in both Spanish and English. Every year, a report will be generated and contributions will be made to international bases such as USRDS. The domain is accessed through the following website: https://www.issea.gob.mx/ercpub/en/RepPrevalenciaErcPbiPub.aspx
DISCUSSION
The prevalence and incidence of treated ESRD in the state of Aguascalientes are among the highest worldwide [6]. The most affected age range is between 20 and 40 years, with 45% of cases (n = 1299), followed by patients between 50 and 70, accounting for 29% (n = 844) of cases. The prevalence and incidence in young adults are especially high. The American CKD report (USRDS) takes into account the age range between 20 and 44 years, and the incidence in Aguascalientes in that age range is 333 p.m.p., which is the highest in comparison with the incidence reported by other countries in the USRDS [6].
Mexico lacks an official CKD registry and the only consistently reported data over the past few years is from Jalisco, which has been submitted to the USRDS [6]. Disease prevalence between Jalisco and Aguascalientes differs significantly, being higher in Aguascalientes (1975 versus 1447 p.m.p.); this could be explained by geographic differences since there is easier access to renal replacement therapies in Aguascalientes. In terms of the etiology of CKD, it is striking that more than half of CKD patients have a diagnosis of CKD of unknown origin and only 24% are secondary to DM. This differs from the reports from Jalisco in which DM2 is responsible for 65% of cases of CKD, although the reported proportion in Aguascalientes is also high [6].
We lack a precise record of whether this phenomenon is new to the state, since dialysis and transplant coverage has only been available in the past 25 years, and complete health coverage will remain unavailable for some time. Based on the Jalisco registries in the USRDS and the ‘burden of disease study’, we have observed a constant increase in the prevalence and incidence of ESRD in Mexico in the last 20 years. Specifically in terms of the impact of the disease (disability-adjusted life years), Mexico is one of the countries with the highest annual increase and Aguascalientes has followed the same trend [3, 6].
CKD etiology differs significantly between age ranges. In the range between 20 and 40 years, the main cause of CKD is of unknown origin (n = 961, 73%), while in patients between 50 and 70 years, chronic degenerative diseases (DM and SAH) are the most common etiologies (n = 502, 59.6%).
Only 24% of prevalent patients and 34.5% of incident cases have a history of DM, while 54% of prevalent patients and 44% of incident cases are listed as having an unknown cause of CKD. Although the categorization of unknown origin was based on secondary information (clinical records), it follows the definition suggested by recent consensus [7]. It also shares some similarities with the demographic pattern reported in other countries with a high prevalence of CKD of unknown cause: patients tend to be younger (average 39 years) and there is a higher proportion of males [8, 9].
There is a very low proportion of glomerulopathies, most likely due to the late diagnosis of CKD and also, the result of an insufficient number of renal biopsies. During the last 8 years, due to the arrival of specialists in nephropathology to Aguascalientes, this has substantially changed. In biopsies, the highest proportion of FSGS is in patients between the ages of 10 and 40 years. Although most of the patients in the renal biopsy registry are not included in the CKD registry, we do have an approximation on what may be causing the high proportion of CKD in young people in our state. The characterization of these biopsies mostly shows glomerular involvement with some tubulointerstitial compromise. In the absence of electronic microscopy in this series, we can only hypothesize that the clinical presentation of sub-nephrotic proteinuria without other characteristics of the nephrotic syndrome in most FSGS cases, suggests secondary or genetic causes.
CKD of unknown origin is a globally described entity and it has been recognized as a major health problem in various countries. It is the main cause of kidney disease in high-prevalence CKD areas (hot spots), which have been reported in countries such as El Salvador, Nicaragua, Sri Lanka and Udaan [10]. Despite multiple efforts, the specific causes have not been clarified, although studies have not been conducted following the best methodology [8–12]. The histologic reports of CKD in these areas differ substantially from our cohort. Reports from Central America and Sri Lanka show interstitial involvement with almost no involvement of the glomeruli [12–15].
Due to the high prevalence of CKD of unknown origin in our state, two approaches have been proposed. The first and probably the most important is the timely detection of CKD, since this entity has no associated comorbidity and since frequently, the patient is diagnosed with CKD in advanced stages, screening maneuvers based on risk factors are difficult to implement. Based on the peak prevalence between the ages of 20 and 30 years, screening is currently conducted in high school students since March 2020. This is an exploratory maneuver to attempt to determine the prevalence of CKD in the early stages in this group of patients, hence establishing a timely diagnosis and fostering a delay in disease progression. Also, a follow-up cohort will begin to be recruited to understand the natural history of the disease and establish what factors are associated with this pathological entity.
The second approach is causal and will require more time. Based on the renal biopsy registry, we have observed that the most frequent diagnosis in patients between 20 and 30 years of age is FSGS. Most of the clinically characterized patients do not develop nephrotic syndrome, which is highly suggestive of secondary or genetic causes. Secondary FSGS is usually associated with a history of an obvious trigger, such as obesity, drug exposure, surgery or recurrent infections. Although we did not mention it in this report, patients with this condition in Aguascalientes do not refer to suggestive histories that could have led to secondary renal injury. For this reason, genetic causes of adult-onset CKD should be considered. Currently, 17 genes related to FSGS have been described in adults and it is believed that most are still unknown [16]. Recently, typing of allelic and haplotypic frequencies and their linkage disequilibrium were reported in 95 Mexicans from the state of Aguascalientes. The main genetic components are Native American [54.53 ± 3.22% by maximum likelihood (ML); 44.21% of Native American haplotypes] and European (44.34 ± 0.45% by ML; 40.53% of European haplotypes), and a relatively low African genetic component (1.13 ± 2.33% by ML; 5.26% of African haplotypes). Based on this report, African-American ancestry genes related to FSGS (APOL1) are an unlikely pathogenic factor. Other risk factors, such as inbreeding, are not common in this area; a recent pilot study conducted with 187 surveys found only five participants with this history [17].
Another likely hypothesis pertains to environmental triggers. In countries with a high prevalence of CKD of unknown origin, the patient has been suspected of having one or more risk factors, such as dehydration, fieldwork, exposure to heavy metals, etc. However, none of these hypotheses has demonstrated causality [7]. Specifically, in Calvillo (municipality with the highest prevalence of ESRD in Aguascalientes), the water sanitation records have shown a high proportion of wells with high levels of fluoride. Of the 89 wells analyzed, 39 (44%) have levels above international standards (<1.5 mg/L); there were no abnormalities in the rest of the metals analyzed, including lead, arsenic, aluminum, barium, cadmium, copper, total chromium, iron, manganese, mercury and zinc [18], Supplementary data, Table S1). In terms of fluoride exposure, nephropathies associated with its chronic ingestion have recently been described [19–21]. It is necessary to address this issue in future research studies, specifically determining urinary fluoride levels as part of our approach. Although the association between poverty, marginalization and CKD is not explored in our registry, it is obvious and will be fully analyzed in future studies. This certainly suggests a possible association with an environmental factor.
There are several limitations to this study. The most important is the representativeness of this registry in terms of the entire country. Aguascalientes represents just ˃1% of the country’s population. The complexity of the health system in Mexico must be taken into account, since it is characterized by multifragmentation, with different levels of access to renal replacement therapies. This report represents the first inter-institutional effort to understand the causes leading to CKD in our country. More importantly, the concerning high prevalence of kidney disease in young adults that we have described is based on a confident report, at least in this region, and the same phenomenon may be occurring in other regions of the country.
CONCLUSION
CKD in Aguascalientes is highly prevalent. Male patients between the ages of 20 and 40 years are the most affected. The most frequently diagnosed entity is CKD of unknown origin. Patients between the ages of 20 and 30 years with a renal biopsy are mainly diagnosed with FSGS. Substantial long-term efforts should be continued for the timely detection of CKD and research on related environmental and genetic factors must be undertaken. It is essential to strengthen the state’s CKD registry since the information it provides is invaluable for both the state and the country.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this article have not been published previously in whole or in part, except as an abstract presented at the American Society of Nephrology Kidney Week Meeting 2019 (Washington, DC).
Supplementary Material
REFERENCES
- 1.International Diabetes Federation. IDF Diabetes Atlas, 9th edn. Brussels, Belgium: 2019
- 2.Prevalence of Obesity and Overweight, Global Health Observatory (GHO). 2020. Available from: www.who.int/gho/ncd/risk_factors/overweight/en/ (4 March 2020, date last accessed)
- 3.Institute of Health Metrics and Evaluation. Seattle, WA. IHME, University of Washington. 2017. Available from: http://www.healthdata.org/data-visualization/gbd-compare. (4 March 2020, date last accessed) [Google Scholar]
- 4. Alegre-Díaz J, Herrington W, López-Cervantes M. et al. Diabetes and cause-specific mortality in Mexico city. N Engl J Med 2016; 375: 1961–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Informe al Ejecutivo Federal y al congreso de la unión sobre la situación financiera y los riesgos del Instituto Mexicano del Seguro Social 2017-2018 Official Webside of Mexican Institute of Sotial Security (Instituto Mexicano del Seguro Social). Available from: http://www.imss.gob.mx/sites/all/statics/pdf/informes/20172018/21-InformeCompleto.pdf (4 March 2020, date last accessed)
- 6.United States Renal Data Sytem. Chapter 11 International Comparisons.https://www.usrds.org/2018/view/v2_11.aspx (4 March 2020, date last accessed)
- 7. Obrador GT, Levin A.. CKD hotspots: challenges and areas of opportunity. Semin Nephrol 2019; 39: 308–314 [DOI] [PubMed] [Google Scholar]
- 8. Stanifer JW, Muiru A, Jafar TH. et al. Chronic kidney disease in low- and middle-income countries. Nephrol Dial Transplant 2016; 31: 868–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weaver VM, Fadrowski JJ, Jaar BG.. Global dimensions of chronic kidney disease of unknown etiology (CKDu): a modern era environmental and/or occupational nephropathy? BMC Nephrol 2015; 16: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martín-Cleary C, Ortiz A.. CKD hotspots around the world: where, why and what the lessons are. A CKJ review series. Clin Kidney J 2014; 7: 519–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ordunez P, Saenz C, Martinez R. et al. The epidemic of chronic kidney disease in Central America. Lancet Glob Health 2014; 2: e440–e441 [DOI] [PubMed] [Google Scholar]
- 12. Fischer R, Vangala C, Truong L. et al. Early detection of acute tubulointerstitial nephritis in the genesis of Mesoamerican nephropathy. Kidney Int 2018; 93: 681–690 [DOI] [PubMed] [Google Scholar]
- 13. Wijkström J, González-Quiroz M, Hernandez M. et al. Renal morphology, clinical findings, and progression rate in Mesoamerican nephropathy. Am J Kidney Dis 2017; 69: 626–636 [DOI] [PubMed] [Google Scholar]
- 14. Wijkström J, Leiva R, Elinder CG. et al. Clinical and pathological characterization of Mesoamerican nephropathy: a new kidney disease in Central America. Am J Kidney Dis 2013; 62: 908–918 [DOI] [PubMed] [Google Scholar]
- 15. Wijkström J, Jayasumana J, Dassanayake R. et al. Morphological and clinical findings in Sri Lankan patients with chronic kidney disease of unknown cause (CKDu): similarities and differences with Mesoamerican Nephropathy. PLoS One 2018; 13: e0193056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lepori N, Zand L, Sethi S. et al. Clinical and pathological phenotype of genetic causes of focal segmental glomerulosclerosis in adults. Clin Kidney J 2018; 11: 179–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arreola-Guerra JM, Gutierrez CM, Macias MJ. et al. Factors related to significant albuminuria or low glomerular filtration rate in adolescents from a population with a high prevalence of chronic kidney disease of unknown origin. Abstract accepted in American Society of Nephrology, Kidney Week Congress 2020
- 18.Official Mexican Standard NOM-127-SSA1-1994, Environmental Health. Water for Human Use and Consumption. Permissible Quality Limits and Treatments to Which the Water Must be Subjected for its Purification. Available from: http://www.salud.gob.mx/unidades/cdi/nom/m127ssa14.html (3 September 2020, date last accessed)
- 19. Quadri JA, Sarwar S, Sinha A. et al. Fluoride-associated ultrastructural changes and apoptosis in human renal tubule: a pilot study. Hum Exp Toxicol 2018; 37: 1199–1206 [DOI] [PubMed] [Google Scholar]
- 20. Alhusaini AM, Faddah LM, El Orabi NF. et al. Role of some natural antioxidants in the modulation of some proteins expressions against sodium fluoride-induced renal injury. Biomed Res Int 2018; 2018: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Song C, Fu B, Zhang J. et al. Sodium fluoride induces nephrotoxicity via oxidative stress regulated mitochondrial SIRT3 signaling pathway. Sci Rep 2017; 7: 672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.