Abstract
Aim/Hypothesis: The complexity and heterogeneity of multiple pathological features make Alzheimer’s disease (AD) a major culprit to global health. Drug repurposing is an inexpensive and reliable approach to redirect the existing drugs for new indications. The current study aims to study the possibility of repurposing approved anticancer drugs for AD treatment. We proposed an in silico pipeline based on “omics” data mining that combines genomics, transcriptomics, and metabolomics studies. We aimed to validate the neuroprotective properties of repurposed drugs and to identify the possible mechanism of action of the proposed drugs in AD. Results: We generated a list of AD-related genes and then searched DrugBank database and Therapeutic Target Database to find anticancer drugs related to potential AD targets. Specifically, we researched the available approved anticancer drugs and excluded the information of investigational and experimental drugs. We developed a computational pipeline to prioritize the anticancer drugs having a close association with AD targets. From data mining, we generated a list of 2914 AD-related genes and obtained 49 potential druggable targets by functional enrichment analysis. The protein–protein interaction (PPI) studies for these genes revealed 641 interactions. We found that 15 AD risk/direct PPI genes were associated with 30 approved oncology drugs. The computational validation of candidate drug–target interactions, structural and functional analysis, investigation of related molecular mechanisms, and literature-based analysis resulted in four repurposing candidates, of which three drugs were epidermal growth factor receptor (EGFR) inhibitors. Conclusion: Our computational drug repurposing approach proposed EGFR inhibitors as potential repurposing drugs for AD. Consequently, our proposed framework could be used for drug repurposing for different indications in an economical and efficient way.
1. Introduction
The alarming progression rate, limited therapeutics, and the slow pace of new drug development for Alzheimer’s disease (AD) draw the attention of research groups and pharmaceutical companies toward exploring new alternatives. Conventionally, AD is denoted as a central nervous system (CNS) disorder characterized by abnormal amyloid-β (Aβ) aggregation, tangle formation of hyperphosphorylated tau, oxidative stress, and hyperactivity glial and microglial cells.1 The latest reports by the Alzheimer’s association suggested that five FDA-approved drugs are currently marketed for AD.2 The failure rate of AD therapeutics is more than 99%, and for the disease-modifying therapies, it is 100%. It has been a matter of more than 20 years; no new drug is licensed for AD. The research community is continuously involved in developing new drug discovery strategies; one of the examples is drug repurposing. To encourage the use of repurposed drugs, the National Institute of Aging grants $2.8 million to Case Western Reserve University School of Medicine to identify potential FDA-approved medicines as repurposed agents for AD. The major classes of drugs investigated for AD as repurposed agents are antihypertensive, antidiabetic, antiasthmatic, retinoid receptors, anticancer agents, antiepileptic, antidepressive, and antimicrobial agents.3 In addition to omics analysis, the concept of pharmacogenomics has gained significant attention in drug repurposing. Studies have suggested that drugs can regulate the expression of small noncoding RNAs such as micro RNAs (miRNAs) and their precursors. For instance, miravirsen is the first miRNA-targeted small molecule that has come in clinical trials and can inhibit miR-122 expression required to replicate hepatitis C virus.4 In a study by Yu et al., potential repurposing drugs were identified for breast cancer based on miRNA–disease–drug tripartite relationships.5 Likewise, in a recent study, Aydin et al. reported miRNA-mediated repurposed drugs for Prolactinoma treatment via in vitro experimentation.6
Drug repurposing is an opportunistic strategy of identifying new indications of the drugs already approved in the market. A review of different repurposing examples suggested that about 46 drugs have already been repurposed for various indications, and encouraging studies are consistently publishing.7 A recent study has revealed that pharmaceutical companies have placed the market for repositioned drugs at $31.3 billion in 2020, generating about 25% of this industry’s annual revenue. Recent estimates suggested that about 30% of the FDA-approved drugs are actually the repurposed drugs.8
To date, most of the repurposing studies have been published for parasitic diseases, multiple cancers, tuberculosis, and malaria.9 This drug discovery strategy is gaining continuous appreciation as it bypasses the efforts and cost input required for the early stages of drug development. The repurposing of drugs involves two different approaches, computational and experimental.10 Computational approaches are the combination of systematic steps taken for the initial identification of promising repurposable compounds. The primary methods used for the computational approach are network-based, text mining-based, and semantics-based.11
In the last few years, omics sciences accelerated the drug discovery process by overcoming the challenges associated with it. Recent technological advancements enabled scientists to develop genomics-, transcriptomics-, proteomics-, and metabolomics-based databases. Genomics studies helped us to understand the genetic basis of complex diseases.12 In the past decade, the genome-wide association studies (GWAS) catalog has revolutionized the area of genomics to identify complex genotype–phenotype associations.13 The transcriptomics studies help us to understand the effect of drugs on different cellular states. The expression profiling and genomics studies give the right directionality to gene–phenotype associations.14,15 The proteomics studies are extensively used to understand the mechanistic basis of disease.16 Similarly, the analysis of metabolome provides knowledge of associations of biochemical events with phenotypes.17
An exciting interplay between cancer and AD gives a direction to use anticancer drugs as repurposed therapeutics. Accumulating evidence has suggested that cancer and AD share some familiar biological hallmarks, and a significant link exists between cancer history and AD neuropathology.18,19 In a recent study, Lee et al. established an interrelationship between cancer and AD at the transcription level. They compared differentially expressed genes between AD and nine different cancers and found that glioblastoma multiforme shared a strong correlation with AD.20 The repurposing of oncology drugs for AD is underway, and many drugs, for instance, bosutinib, dasatinib, nilotinib, bexarotene, tamibarotene, and thalidomide (ClinicalTrials.gov identifier: NCT02921477, NCT04063124, NCT02947893, NCT01782742, NCT01120002, and NCT01094340, respectively), are in clinical trials for AD.21 A study by Lonskaya et al. confirmed the therapeutic relevance of tyrosine kinase inhibitors nilotinib and bosutinib in AD, where the drugs facilitated amyloid clearance and reduced neuroinflammation.22 A drug repurposing study by the neuroinformatics approach has proposed that the anticancer drug bexarotene could reduce Aβ aggregation by interacting with receptors for advanced glycation end products (RAGE) and beta-secretase (BACE-1).23 A drug repurposing study by Madepalli Lakshmana and the group found that anticancer drug carmustine (BiCNU) could regulate amyloid precursor protein (APP) processing and trafficking to reduce Aβ aggregation in the brain.24 Likewise, a study targeting vascular activation in AD has proposed that the anticancer drug sunitinib could reduce vascular activation of various proteins such as amyloid-beta, tumor necrosis factor-alpha (TNFα), interleukin-6 (IL-6), interleukin-1 beta, thrombin, and matrix metalloproteinase 9 and ameliorated cognitive dysfunction in AD transgenic mice. Additionally, a study on the antimitotic drug, paclitaxel, has revealed the drug’s potential in reducing tau-associated pathologies by preventing tau-induced axonal swelling, reversal of microtubule polar orientation, prevention of neurite degeneration, and inhibition of impaired organelle transport and accumulation.25 In parallel, a study on the tyrosine kinase inhibitor, pazopanib, in the AD mouse model has identified the potential of the drug in reducing tau pathology and astrocytic activity. The study has proposed that the drug could not alter microglial activity; however, it could modulate the activity of inflammatory markers and thus provide neuroprotection.26
The motivation of this study is to uncover the hidden neuroprotective potential of anticancer drugs. We adopted an integrated omics data-based repurposing strategy, including genomics, transcriptomics, and metabolomics, and validated our results by different computational methods. Our study was concentrated on FDA-approved anticancer drugs and their repurposing for AD. We developed a bioinformatic pipeline to assign a ranking of the repurposed drugs based on the computational drug repurposing score (CoDReS) validated by network and structural similarity analysis with approved AD drugs. The study also aims to combine the physicochemical analysis, drug-likeness, pathway analysis, and microRNA (miRNA) analysis of repurposing anticancer drugs to understand better the mechanisms involved. The study helped to identify the significant pathways and cancer-related genes associated with the pathogenesis of AD. The study also set a new direction to understand the complex relationship between AD and cancer that would be considered for other neurodegenerative diseases.
2. Methodology
2.1. Data Extraction
To obtain information on AD-associated genetic variations, we analyzed GWAS studies for AD from NHGRI-EBI GWAS catalog (http://www.ebi.ac.uk/gwas).27 The database provides a consistent knowledge of single-nucleotide polymorphism (SNP)-trait associations for various diseases. We extracted GWAS data for (1) PUBMED ID, (2) study accession, (3) genes, (5) SNPs, (6) P-value, and (7) OR (odds ratio). Genes are considered significant, which fall under the genomic regions associated with SNPs (r2 > 0.6). For transcriptomics data, NCBI Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) database that contains microarray and next-generation sequence functional genomic data sets was used.28 The collected expression profile of the AD series GSE1297 was analyzed by GEO2R. The GSE1297 series contains microarray analysis data of the hippocampal region of 9 control and 22 AD subjects. The metabolomics data were collected from the Human Metabolome Database (HMDB, http://www.hmdb.ca), which contains 114,187 metabolite entries.29 The database was searched for (1) AD linked metabolites, (2) protein name, (3) Uniprot ID, (4) type of metabolite, and (5) gene name.
2.2. Prioritization of Candidate Genes
We utilized two different computational tools to identify the most significant genes associated with AD. The genes obtained from various omics approaches were then subjected to enrichment analysis by online DAVID functional annotation tool and gene set to diseases (GS2D) tool. DAVID (https://david.ncifcrf.gov) provides an integrated platform to extract meaningful biological information from the list of genes enriched in genome-scale studies.30 GS2D (http://cbdm.uni-mainz.de/geneset2diseases) is a web tool that performs enrichment analysis based on significant biomedical citations from PubMed.31 The gene–disease associations were filtered by a minimum number of citations found (default = 5), the minimum number of gene–disease associations (default = 2), and the maximum false discovery rate (FDR = 0.05). The FDR is used as a matric in drug repurposing to measure significance of drug-indication scores.32
The enriched genes were then analyzed for protein–protein interaction (PPI) using the Molecular Interaction Search Tool (MIST) database. MIST (http://fgrtools.hms.harvard.edu/MIST/) database can be used to devise significant protein–protein and genetic interactions for different species.33
2.3. Drug Target Mapping
We have combined the information from genomics, transcriptomics, and metabolomics approaches and had a list of genes associated with AD. To develop a link between AD-related genes with currently available drug projects, we tracked two different databases. DrugBank (www.drugbank.com) (version 5.1.5) contains around 13,554 drug entries incorporating various approved and experimental small molecules and biologics.34 Similarly, the Therapeutic Target Database (TTD) (http://db.idrblab.net/ttd/) accommodates 3419 targets and 37316 drug projects.35 We included only those targets for which anticancer drugs are available and excluded the others. All the drugs with clinical, experimental, or withdrawn status were excluded, and only FDA-approved drugs were considered for this study. The information about drugs such as drug name, DrugBank ID, current indication, and drug mode of action was collected.
2.4. Validation of Candidate Drugs
The PPIs from the previous steps were then analyzed by the STRING database (string-db.org) that covers known and predicted interactions for different organisms.36 The experimentally significant interactions (with high interaction scores) were selected, and the others were excluded from the study. The drug–target interactions were evaluated using the STITCH (search tool for interactions of chemicals) (http://stitch.embl.de/) database that integrates interactions of 300,000 chemicals and 2.6 million proteins.37 In a complex system, two interacting genes are represented as nodes connected by an edge. The interaction networks were further analyzed, and networks were generated using Cytoscape software v3.3.0 (www.cytoscape.org).
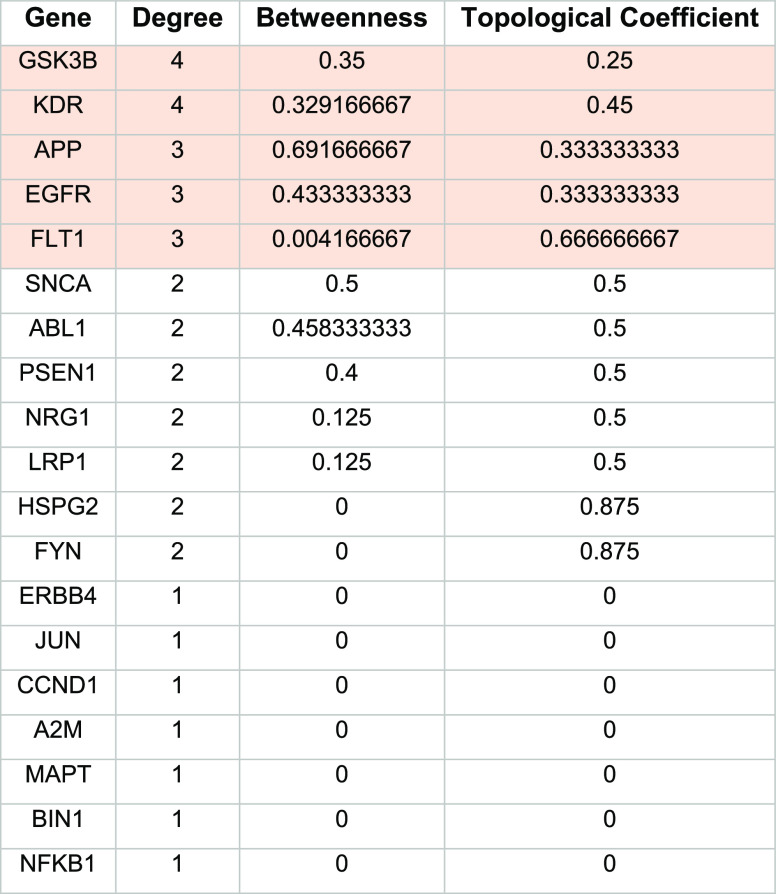
For validation of promising drug candidates on the validation network, we measured network topology parameters such as degree centrality, betweenness, and topological coefficients using the CentiScaPe app on Cytoscape software. A degree is a topological parameter that corresponds to the number of interactions or connections for a given node. Betweenness corresponds to the centrality index of a given node. It represents the shortest path between two adjacent nodes. In biological networks, only a few nodes (hub nodes) have a high degree centrality and the nodes having the shortest path distance are designated as bottlenecks. Both hub nodes and bottlenecks are considered topologically important and biologically significant.38 The topological coefficient is a relative measure that denotes the extent to which a node shares neighbors with other nodes in the network. The nodes that share no neighbor are assigned a topological coefficient value of 0. The candidate drugs were given ranks based on different topological parameters. The drugs having a higher degree centrality value were considered as topologically important and biologically significant. In short, the drugs (nodes) with higher degree centrality values are regarded as hub nodes with considerable importance in the network.
2.5. Drug Repurposing
The candidate drugs obtained from the previous studies were analyzed for their repurposing potential for AD using the CoDReS tool. CoDReS (http://bioinformatics.cing.ac.cy/codres) is a web-based tool that integrates information from the biologically available data sets, calculates affinity scores of protein and ligand pairs, and evaluates drug-likeness and structural similarities.39 The candidate drugs with good repositioning scores were then presented by the hierarchical clustering algorithm of the ChemMine server.40 Hierarchical clustering is a powerful approach to find structural and physicochemical similarities of compounds based on atom pair similarity measures. The similarity scores were calculated based on the Z-score values. Also, we calculated the structural similarity with the approved Alzheimer’s drugs, namely, donepezil, rivastigmine, galantamine, and memantine. The similarity workbench tool of the ChemMine server was used, and similarity scores were represented as the Tanimoto coefficient, the most widely used metric to compare the molecular structure similarities in medicinal chemistry.41 The tool utilizes the maximum common substructure (MCS) fingerprint method to find the largest substructures two compounds have in common and present it as the Tanimoto coefficient.
2.6. Literature Validation of the Drug–Disease Relationship
To obtain the information related to neuroprotective functions of anticancer drugs, we have searched the PubMed database using the keywords “anticancer drugs and neuroprotection,” “anticancer drugs and AD,” and anticancer drugs and neurodegenerative disorders. We collected information on whether the proposed repurposing drugs have any neuroprotective mechanism associated with them.
2.7. Swiss ADMET Analysis of Candidate Drugs
The development of drugs for the CNS disorders poses a challenge due to the blood–brain barrier (BBB). While designing a drug for brain diseases, physicochemical properties and brain permeation properties should be optimized. In consideration of this challenge, we analyzed our candidate repurposed drugs for physicochemical properties using the SwissADME analysis tool. SwissADME (http://www.swissadme.ch/) is a user-friendly web tool to predict physiochemical properties, pharmacokinetics, and drug-likeness of small molecules.42 We collected information about physiochemical properties such as molecular weight, number of rotatable bonds, number of H-bond donor and acceptors present, partition coefficient (M log P), and topological polar surface area (TPSA) and blood–brain permeation, where M log P was the measure of lipophilicity and TPSA was the measure of the sum of the surfaces of polar atoms present.
2.8. Functional Similarity with MicroRNAs
To further validate our results, we identified miRNAs related to AD from Human microRNA Disease Database (HMDD) (https://www.cuilab.cn/hmdd).43 HMDD contains information regarding experimentally validated microRNA–disease associations. We also retrieved information of miRNAs associated with the identified repurposed drugs and then constructed a network that combines miRNAs that share common targets between the repurposed drugs and AD. We considered only the miRNAs that were neuroprotective in nature. The disease–miRNA–drug and miRNA–drug relationships were presented in the form of a network using Cytoscape software. The information of AD-related miRNAs, repurposed drugs, and their targets was given as the input.
2.9. Pathway Analysis
To establish a connection of AD-related genes with cancer, we compare the expression pattern of genes with AD and the most common 13 types of cancers prescribed by the National Cancer Institute (NIH).44 To discover the molecular mechanisms regulated by the identified genes, we performed pathway analysis (KEGG,45 Bioplanet,46 and WikiPathways47) using the Enrichr tool. Enrichr (http://amp.pharm.mssm.edu/Enrichr/) is a web-based enrichment analysis tool that accumulates biological knowledge (genes, diseases, pathways, and drugs) of more than 102 gene set libraries.48 The tool has provided information about biologically relevant pathways or enriched pathways for the set of the given genes. These enriched pathways were associated with the given gene list more than would be expected by chance. We also extracted the information of disease signatures (DisGeNET and OMIM-based information) related to the given genes using the Enrichr tool. The output of Enrichr is ranked list terms, and ranking is provided based on p-value scores. Enrichr calculates the p-value based on Fisher’s exact test that assumes binomial distribution and independence for the probability of the given input gene.
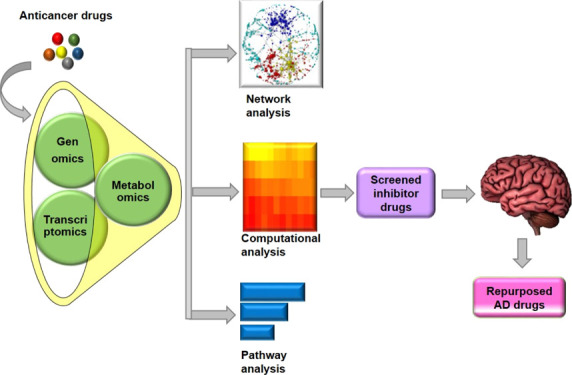
An overview of the complete pipeline is shown in Figure 1.
Figure 1.
Flow chart of drug repurposing by omics data mining: We retrieved information on AD risk genes from GWAS, transcriptomics, and metabolomics approaches. We found 2914 AD risk genes from which 58 genes were extracted from GWAS, 229 genes were extracted from GEO transcriptomics data, and 2627 genes were related to 128 metabolites from the HMDB database. After functional enrichment analysis, we filtered out 49 AD-associated targets. The PPI network analysis resulted in 641 PPI interactions. We performed drug target mapping to find candidate drugs from DrugBank and TTD databases. Out of 641, 25 PPI interactions were found to be associated with 36 approved anticancer drugs. We excluded the information related to investigational and experimental drugs. We analyzed gene–gene and gene–drug interactions and selected the top 10 PPI interactions that correspond to 30 anticancer compounds. These 30 drugs were then analyzed by the CoDReS web tool that proposes 10 candidate drugs for AD. These drugs were then compared with the available Alzheimer’s therapeutics for structural and functional similarities, where six drugs have shown to be hierarchically clustered. ADMET analysis, pathway analysis, and functional similarity with miRNAs resulted in potential repurposing anticancer drugs against AD.
3. Results
3.1. Omics Data Mining and Enrichment Analysis Revealed AD-Related Genes
The omics data approach enabled us to identify AD-related genes. We collected information about 58 unique genes from 37 GWAS studies. The P-value of the identified genes varies from 8 × 10–189 (minimum) to 8 × 10–6 (maximum). We identified 229 genes in the form of differentially coexpressed genes from transcriptomics studies. The data obtained from the HMDB database reported 128 AD-related metabolites that correspond to 2627 genes from metabolomics data. Most of the proteins associated with the retrieved metabolites had unknown functions, while some were enzymes or transporters. We combined the information from different omics approaches, and finally, 2914 genes were found to be associated with AD.
DAVID functional enrichment analysis of 2914 genes revealed that 13 genes from GWAS studies, 18 genes from the transcriptomics approach, and 239 genes from the metabolomics approach have significant associations with AD. Similarly, GS2D functional enrichment analysis revealed that 12 genes from GWAS studies, 4 genes from the transcriptomics approach, and 62 genes from the metabolomics approach were significantly linked with AD.
When we compared the two enrichment analysis methods, 49 AD-related genes were shared in the two enrichment methods (Table S1).
3.2. PPI Network Analysis Revealed Potential Interactors of AD-Risk Genes
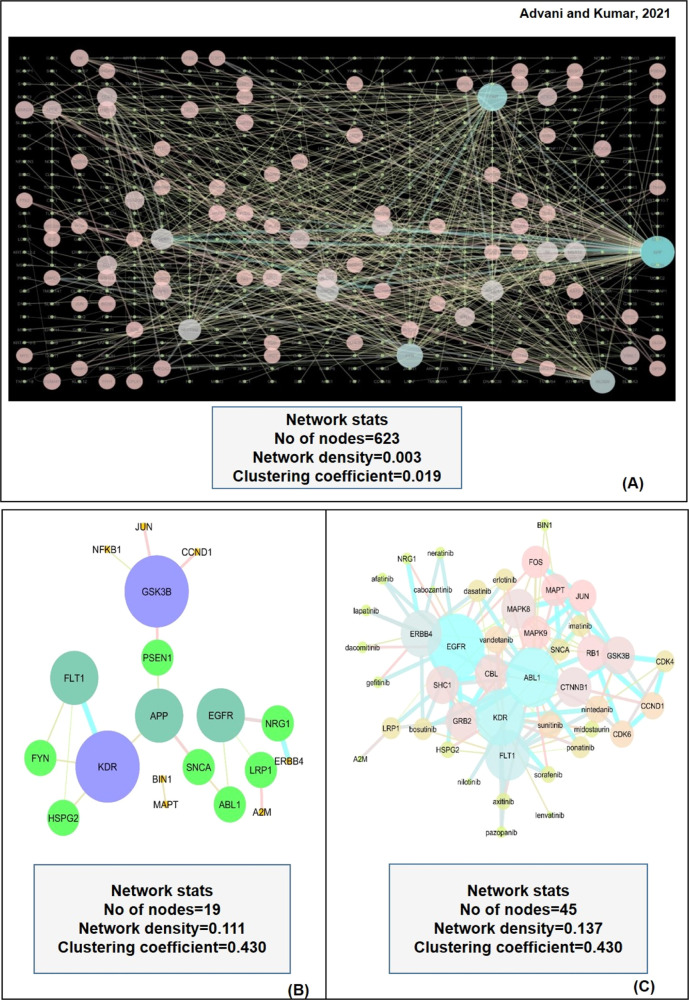
We evaluated the PPI network of the 49 AD-risk genes to explore the possibility of any of the genes from the PPI network that serve as a target for approved anticancer drugs. We selected PPI interactions with a high confidence score and excluded the interactions with medium to low confidence. We found 641 PPI interactions from the MIST database results, as shown in Figure 2A. All the PPI genes of 641 interactions, along with 49 AD-risk genes, were searched in the DrugBank database and TTD to find the association with known anticancer drugs. Among the PPI interactors, 17 genes were reported to have approved anticancer medications available in the considered drug repositories. We found that the epidermal growth receptor (EGFR) is the most frequently appeared PPI interactor interacting with four different AD-associated targets APP, alpha-synuclein (SNCA), neuregulin 1 (NRG1), and LDL receptor related protein 1 (LRP1). These PPI interactions were then evaluated by the STRING database and presented on the validation network, as shown in Figure 2B. The topological parameters of genes in STRING, such as degree centrality, betweenness, and topological coefficients, were analyzed by Cytoscape and are presented in Table 1.
Figure 2.
(A) Network is showing PPI interactions for AD-related genes. (B) STRING network of experimentally significant interactions. Glycogen synthase kinase 3 beta (GSK3B), vascular endothelial growth factor receptor 2 (KDR), APP, vascular endothelial growth factor receptor 1 (FLT1), and epidermal growth factor receptor (EGFR) were identified as the hub nodes. (C) STITCH network of drug-gene interactions. Nintedanib, sunitinib, vandetanib, dasatinib, erlotinib, imatinib, ponatinib, and bosutinib were reported as hub nodes as drugs. The size of individual nodes and the thickness of edges correspond to the significance and strength of interactions, respectively.
Table 1. Topological Parameters of Genes (Nodes) on the STRING Validation Network Using CentiScaPe App on Cytoscape Softwarea.
Genes with significant values are highlighted.
The topological parameters were used to identify the hub nodes in the validation network. We identified glycogen synthase kinase beta (GSK3B), kinase insert domain receptor (KDR), APP, EGFR, and Fms-related receptor tyrosine kinase 1 (FLT1) as the top five nodes. GSK3B and KDR had the highest degree centrality values of 4.0 and betweenness values of 0.35 and 0.32, respectively, while APP, EGFR, and FLT1 had degree centrality values of 4 and betweenness values of 0.69, 0.43, and 0.004, respectively. Among the identified genes, GSK3B is a multifunctional protein kinase regulating various cellular processes and is implicated in several diseases. In AD, GSK3 is considered a regulator of the two pathological hallmarks, senile plaques and neurofibrillary tangles.49,50 The other identified target APP is a single transmembrane protein that acts as a multifunctional cell surface receptor. APP plays a major role in AD pathogenesis as it is associated with Aβ production, synaptic function, and neuronal homeostasis.51,52 The EGFR is a transmembrane molecule that belongs to the HER/ERBb superfamily of receptors. The binding of ligands to this receptor triggers several signaling pathways that promote cell proliferation and cell survival. The other two genes, vascular endothelial growth factor receptor (VEGFR1) or FLT1 and VEGFR2 or KDR, are the two receptors playing a significant role in the signal transduction pathways mediated by the VEGF.53 Some studies have suggested that both FLT1 and KDR are associated with AD neuropathology by inhibiting pro-angiogenic signaling mediated by the VEGF.54,55
3.3. Drug Mapping Identified Potential Repurposing Candidates for AD
Drug target mapping from DrugBank and TTD has shown that 28 direct PPI/AD risk genes were associated with 36 FDA-approved anticancer drugs (Table S2). We omitted the targets related to any investigational, experimental, or withdrawn anticancer drugs. From 36 drugs, 11 drugs were associated with only one direct PPI gene/AD risk gene, while 25 drugs were those that interacted with more than one gene. The retrieved drugs were related to diverse modes of actions, such as inhibitors, antagonists, substrates, and some had unknown functions. The experimentally significant interactions obtained from STRING analysis corresponded to 30 drugs from which 4 drugs (brigatinib, zanubrutinib, osimertinib, and erdafitinib) were not identified by the STITCH database and were excluded from the study. Of the 26 candidate repurposing drugs, six drugs (cisplatin, encorafenib, vinblastine, paclitaxel, docetaxel, and regorafenib) had not shown any interaction.
Additionally, three drugs (bosutinib, nilotinib, and dasatinib) were in clinical trials for AD or related dementias and were not included in this study. Therefore, the remaining 17 drugs were considered novel candidate repurposing drugs for AD. The candidate drugs with their AD-related targets and PPI targets are summarized in Figure 3.
Figure 3.

Summary of AD risk genes, genes in direct PPI, and targeted anticancer drugs. Drugs shown in yellow boxes were known in clinical studies as AD therapeutics, and drugs in green boxes were considered as potential repurposing candidates. Some drugs such as afatinib, axitinib, lenvatinib, nintedanib, pazopanib, sorafenib, and ponatinib interact with more than one target. NRG1: neuregulin 1; ERBB4: Erb-B2 receptor tyrosine kinase 4; LRP1: LDL receptor-related protein 1; EGFR: epidermal growth factor receptor; HSPG2: heparan sulfate proteoglycan 2; FLT1: Fms-related receptor tyrosine kinase 1; KDR: kinase insert domain receptor; SNCA: synuclein alpha; ABL1: ABL proto-oncogene 1, nonreceptor tyrosine kinase, NSCLC: nonsmall cell lung cancer, PC: pancreatic cancer, HBC: HER-positive breast cancer, RCC: renal cell carcinoma, STS: soft-tissue sarcoma, HC: hepatocellular carcinoma, GIST: gastrointestinal tumors, MTC: medullary thyroid cancer, AML: acute myelogenous leukemia, and CML: chronic myelogenous leukemia.
3.4. Computational Validation of Candidate Repurposed Drugs
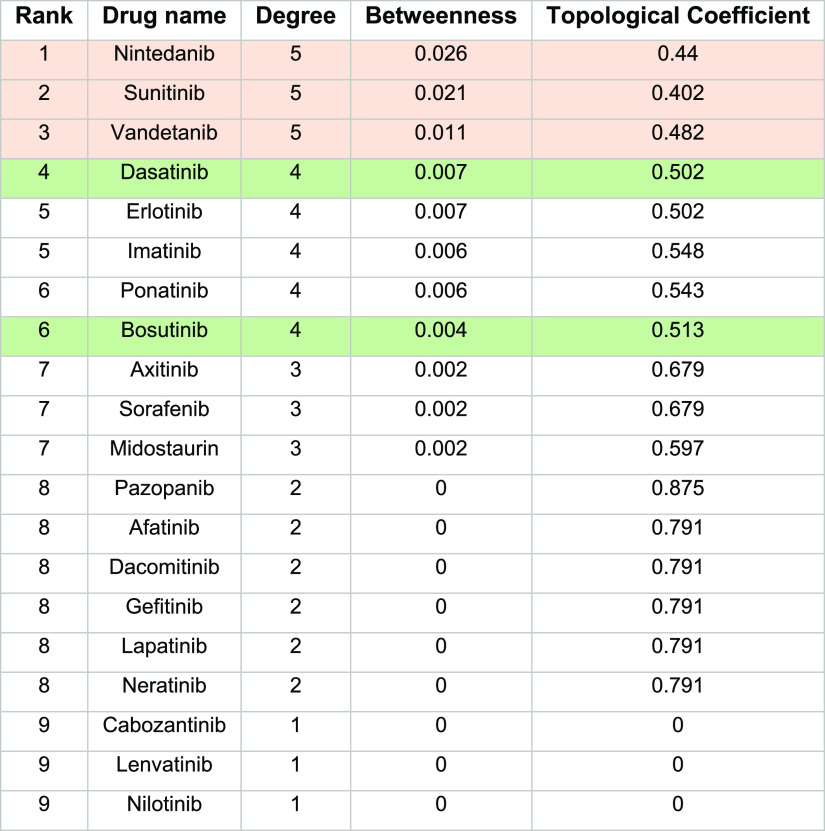
The drug-gene validation network was constructed using the STITCH database (Figure 2C) and analyzed using Cytoscape software, and drugs were ranked based on the degree centrality and betweenness values. The results shown in Table 2 have indicated that the known anticancer drugs, dasatinib and bosutinib, were the hub nodes among known neuroprotective anticancer drugs with the highest value of degree centrality of 4.0 and betweenness values of 0.007 and 0.004, respectively. Similarly, nintedanib, sunitinib, and vandetanib were identified as the important hub nodes among promising drug candidates with a degree centrality of 5.0 and betweenness values of 0.026, 0.021, and 0.011, respectively. We also identified the interactive targets of the topologically important drugs. The most considerable node nintedanib had a strong relationship with the genes KDR, FLT1, GSK3B, cyclin-dependent kinase 4 (CDK4), and ABL proto-oncogene 1 (ABL1). Similarly, sunitinib interacted on the validation network with FLT1, KDR, EGFR, CDK6, and ABL1, while vandetanib had close interactions with ABL1, EGFR, KDR, and FLT1.
Table 2. Topological Parameters of Drugs on the Validation Networka.
Promising drugs with the highest ranks are highlighted in pink, and known neuroprotective anticancer drugs are highlighted in green.
3.5. Functional and Structural Analysis Validated the Repurposing Potential of Candidate Drugs
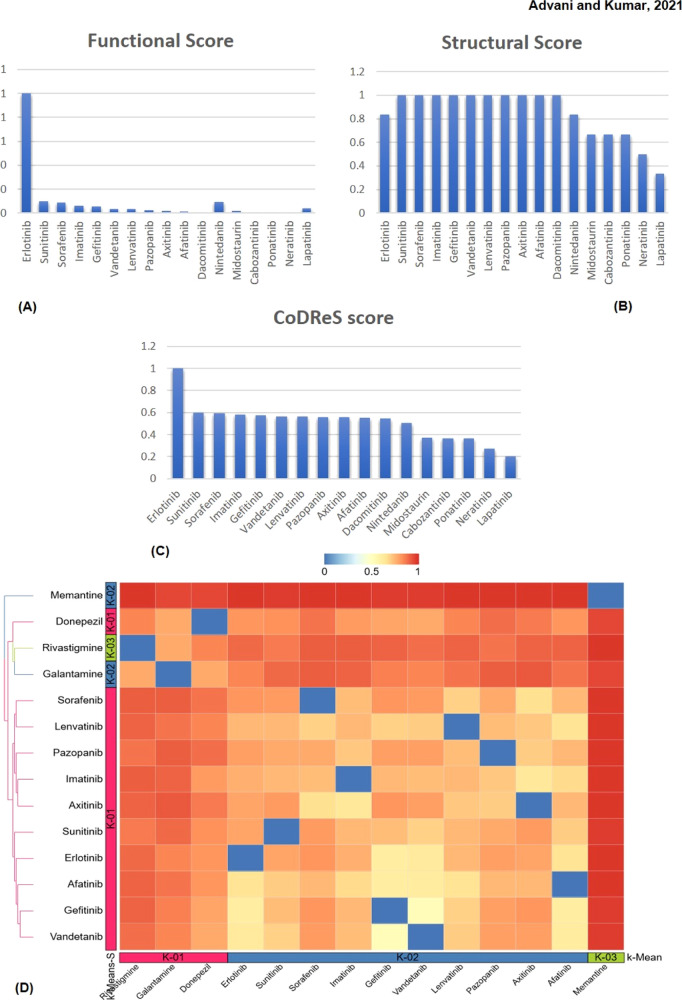
The potential repurposing candidates from the previous steps were evaluated for their functional and structural properties by the CoDReS tool. The tool is based on a disease-specific approach to compare drug–disease relationships concerning a training set of drugs approved or investigated for a disease. We have incorporated this tool to rerank the candidate drugs based on their repurposing scores. The comparative values for different drugs have been provided in (Table S3). Figure 4A–C has illustrated the comparative functional, structural, and CoDReS scores of the candidate drugs, respectively. The values have suggested that most of the drugs have good structural scores, but functional scores have shown significant variations. We found that erlotinib had the highest functional score (1.0), while dacomitinib had the lowest value (0.001). Similarly, sunitinib, sorafenib, imatinib, gefitinib, vandetanib, lenvatinib, pazopanib, axitinib, afatinib, and dacomitinib had the highest values (1.0) in terms of structural score, and lapatinib had the lowest score (0.33). Moreover, erlotinib had the highest CoDReS value (1.0), and lapatinib had the lowest value (0.20). We have selected the top 10 drugs with the highest CoDReS scores for further study. The CoDReS results have indicated that erlotinib would be a good repurposing drug having the highest functional and structural scores.
Figure 4.
(A) Functional scores of different candidate repurposing drugs as calculated using the CoDReS tool. (B) Structural scores of different candidate repurposing drugs as calculated using the CoDReS tool. (C) CoDReS scores of candidate repurposing drugs. Erlotinib is shown as the most promising repurposing drug with good structural and functional scores. The structural scores of the drugs are more or less similar, while the functional scores have shown great variations. (D) Clustered heat map of candidate repurposing drugs with known Alzheimer’s drugs donepezil, rivastigmine, galantamine, and memantine. The heat map is generated using a distance matrix as the input generated by subtracting the similarity coefficient from 1. The colors from blue to red represent the correlation intensities of drugs where blue represents complete correlation and red represents no correlation.
Additionally, we exploited the ChemMine server to investigate anti-Alzheimer’s properties of candidate drugs and compared their clinical potential with donepezil, rivastigmine, galantamine, and memantine. The hierarchical clustering was performed using a clustering threshold of 1. We noticed no drug clusters with typical anti-Alzheimer drugs. We have selected the closest neighbors to Donepezil such as vandetanib, gefitinib, erlotinib, imatinib, afatinib, and sunitinib. Similarly, for another anti-Alzheimer drug rivastigmine, we found sunitinib as the closest match. Likewise, for galantamine, we found vandetanib, erlotinib, and gefitinib as the closest neighbors. We have found no nearest neighbor to memantine. The results are presented in Table 3. The best candidates obtained from clustering analysis have also demonstrated good structural similarity values, as highlighted in red in the table. Finally, we have selected 6 out of 10 drugs for supplementary analysis. The clustered groups were represented in the form of a heat map, as shown in Figure 4D.
Table 3. Similarity Scores (Tanimoto Coefficient) of Repurposed Drugs with Known Alzheimer’s Drugsa.
Highlighted drugs have more or less similar scores to known AD drugs.
3.6. Literature Studies and ADMET Analysis Evaluated the Neuroprotective Potential of Repurposed Drugs
To further validate our results, we have searched for the available information regarding the neuroprotective properties of the drugs proposed from the previous steps. A few bibliographic studies were available regarding neuroprotective functions of anticancer drugs, as summarized in Table 4. Based on these results, we confirmed that all six drugs have repurposing potential for AD. ADMET analysis of the six drugs has confirmed that four drugs (erlotinib, gefitinib, vandetanib, and sunitinib) have good physicochemical properties (molecular weight, no of rotatable bonds, no of H-bond donors, no of H-bond acceptors, TPSA, and M log P) and were able to cross the BBB, as shown in (Table S4). Two drugs, afatinib and imatinib, would not be able to cross the BBB and thus were excluded from the study.
Table 4. Literature Studies for Neuroprotective Functions of Potential Repurposing Candidates.
| drug | neuroprotective function | references |
|---|---|---|
| afatinib | inhibition of oxygen/glucose-induced neuroinflammation and EGFR activation | (56) |
| erlotinib | reduction in Aβ-induced memory loss in AD | (57) |
| gefitinib | improvement in cognition and memory functions | (57) |
| may improve AD pathogenesis by inhibiting the β-secretase activity | (58) | |
| imatinib | inhibition of Aβ accumulation by the selective inhibition of BACE activity | (59) |
| promotes degradation of Aβ by inducing the activity of Aβ-degrading enzyme neprilysin | (60) | |
| inhibition of brain c-Abl, reduction in circulating levels of Aβ, shifts APP processing to non-amyloidogenic pathway | (61) | |
| sunitinib | provides neuroprotection by inhibiting NO production | (62) |
| inhibition of acetylcholinesterase activity and attenuation of cognitive impairments in scopolamine-induced AD mice | (63) | |
| vandetanib | may inhibit acetylcholinesterase activity in AD | (64) |
3.7. Functional Similarity Analysis with MicroRNAs
To further validate our results, we extracted the list of AD-related miRNAs and also searched for the miRNAs related to the repurposed drugs (Table S5). After comparison, we found that erlotinib and gefitinib shared three miRNAs with AD where only one miRNA has neuroprotective functions, while vandetanib shared 33 different miRNAs with AD, as shown in the network in Figure 5. Of the 33 miRNAs, 11 miRNAs have neuroprotective functions. We found that miRNA-200a is the only AD-related miRNA with a neuroprotective function associated with all three drugs. miRNA-200a targets the EGFR gene, and a literature survey has confirmed its neuroprotective role in attenuating amyloid-beta overproduction by downregulating BACE1 expression and tau hyperphosphorylation by reducing the expression of protein kinase A (PKA).65
Figure 5.
(A) Network is showing the interrelationship of miRNAs associated with AD and those associated with repurposed anticancer drugs erlotinib, gefitinib, and vandetanib. The network shows that vandetanib shares many common targets such as EGFR, PTK6, RET, TEK, and VEGFA with AD-related miRNAs, while both erlotinib and gefitinib share functional similarity through the EGFR gene. (B–D) Association of erlotinib, gefitinb, and vandetanib with miRNAs, respectively, where miRNAs shown in green are neuroprotective, while miRNAs shown in purple are neurodegenerative as identified through literature analysis. miRNA-200a is the only one that shows association with all three repurposed drugs.
3.8. Pathway Analysis Confirmed the Repurposing Potential of EGFR Inhibitors
The significant AD-related genes were searched in the DisGeNET database to develop an expression pattern among AD and various types of cancers. The results are presented in the form of a heat map shown in Table 5 where the blue color represents high expression values, while the red color represents low expression values. We found that CCND1, EGFR, and KDR are among the top genes which are commonly expressed in AD and in a different type of cancer. Furthermore, the experimentally significant gene interactions obtained from the STRING database were considered for pathway analysis by the Enrichr tool. We used KEGG, BioPlanet, and WikiPathway databases for pathway analysis (Table 6).
Table 5. Heat Map Showing the Expression Pattern of Shared Genes between AD and 13 Most Common Cancer Typesa.

AD: Alzheimer’s disease; NHL: non-Hodgkin lymphoma.
Table 6. Pathway Analysis of STRING Interactions Based on p-Valuesa.
Genes in red are the most frequently appeared genes in the enriched pathways.
Here, the p-value represents the probability of any gene belonging to a biological pathway.
The most frequently appeared genes in the enriched pathways (biologically relevant) were the EGFR, JUN, and GSK3B. The ERBb signaling pathway, focal adhesion, mitogen-activated protein kinase (MAPK) signaling, Cu homeostasis, and phosphatidylinositol-3-kinase (PI3-Akt) pathways were the top signaling pathways associated with AD pathogenesis. There were many pieces of evidence available for the pathways identified by our study with AD. The pathological role of ErBb4 activity in AD is confirmed by Woo et al., where ErBb4 was accompanied by AD progression.66 The role of focal adhesion signaling in AD pathology is established because Aβ upregulates many proteins related to focal adhesion signaling that induce re-entry of neurons into the cell cycle.67 Aberrant activation of focal adhesion kinases is associated with synaptic loss and neuronal dystrophy in AD.68 Many studies have proposed that MAPK signaling plays an essential role in AD pathogenesis by regulating tau phosphorylation, APP processing, and neuronal apoptosis.69 Several MAPKs interact with AD-related proteins such as tau, APP, presenilin (PS), and apolipoprotein E (ApoE).70 The role of Cu in AD pathogenesis is controversial. Some studies have demonstrated that Cu overload is responsible for neurotoxicity in AD brains, while other studies have proposed Cu deficiency as a contributing factor to AD pathogenesis.71 Likewise, the role of the PI3K pathway is confirmed by studies where abnormal activities of the pathway were responsible for Aβ production and sequestration.72 The PI3K pathway activation has therapeutic potential to treat AD as some of the drugs such as donepezil, coenzyme Q10, and human telomerase reverse transcriptase (hTERT) are known to treat AD by GSK3B inhibition and PI3K activation.73
DisGeNET and OMIM databases were used to find the most closely associated diseases with the identified genes (Table 7). The DisGeNET results reported that out of 15 genes, 13 genes were associated with AD (P-value 7.44 × 10–12), while OMIM disease analysis identified 3 genes (P-value 5.77 × 10–5) related to AD. Functional classification of identified genes from STRING interactions and their associated drugs retrieved from the STITCH network has revealed that kinases and their inhibitors are the major class of targets and targeted drugs associated with AD, respectively (Figure 6).
Table 7. Disease-Based Analysis of STRING Interactions Based on p-Values.
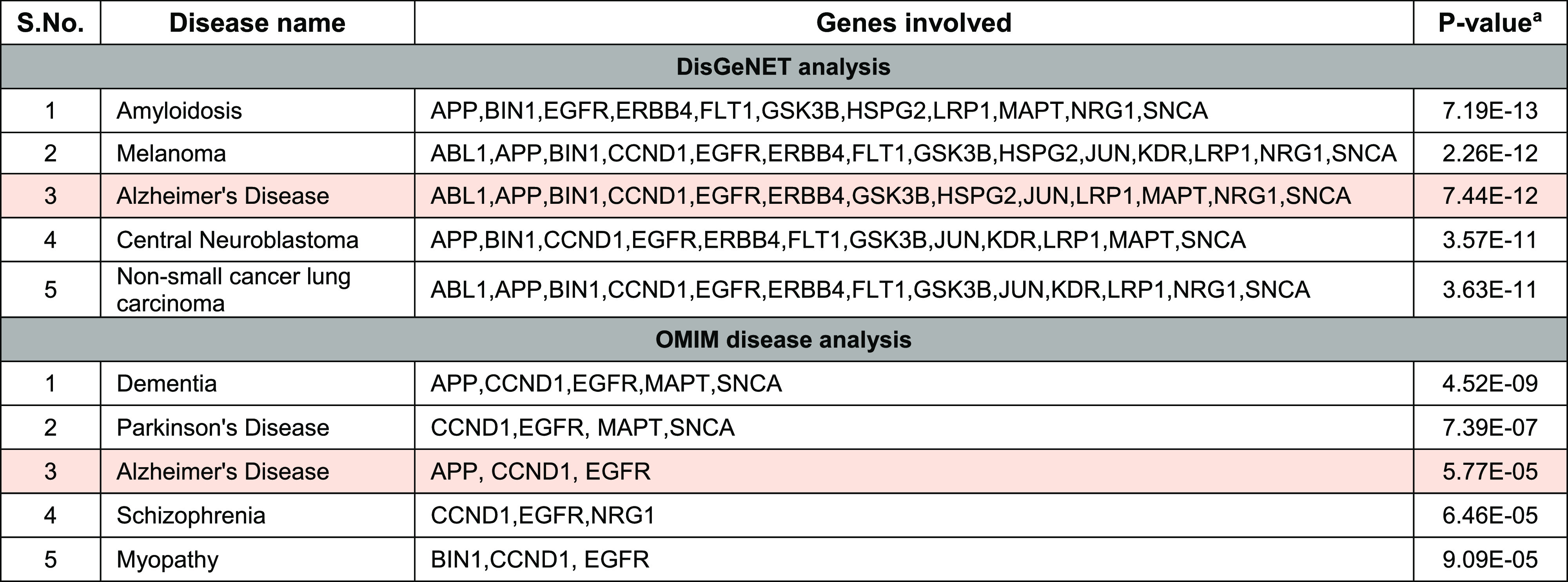
Here, the p-value represents the probability of any gene belonging to a biological disease.
Figure 6.
(A) Figure showing the functional categories of AD-related genes/PPI genes. The relative area of each segment corresponds to the relative fraction of a particular target class. As shown, protein kinases represent the major functional target protein class. (B) Functional classification of candidate repurposing anticancer drugs for AD. As expected, kinase inhibitors are the most prevalent drugs having neuroprotective functions.
4. Discussion
Drug repurposing is a productive approach to identify novel therapeutic uses of available drugs. The common biological pathways of different diseases and the advancements in system biology tools open up new horizons to analyze the off-target effects of approved drugs for various indications. Over the last decade, several studies have been published, emphasizing the shared molecular mechanism of cancer and AD. Indeed, drug repurposing of anticancer drugs as neuroprotective agents has been applied to overcome AD-related clinical consequences. However, the complexity of different neuropathological states and limited understanding of different cellular signaling mechanisms in AD posed a big challenge to develop repurpose therapeutics. In the present study, we used an integrated approach to reveal potential AD-related targets. We opted for a comprehensive data analysis approach to identify neuroprotective anticancer drugs and analyzed the data with network-based and pathway-based tools. We identified 49 AD-related genes by combining GWAS, transcriptomics, and metabolomics studies. We reported 17 cancer-related genes that have direct interactions with the identified AD-related targets. We identified 36 approved anticancer drugs that have associations with these targeting genes. For further study, we selected the experimentally significant genes with the highest interaction scores, as shown in the STRING network. We found 30 anticancer drugs as respective targets of the experimentally significant genes.
Computational validation by CoDReS ranked the repurposing drugs based on their functional and structural properties. Among the proposed drugs, dasatinib (phase I/II), nilotinib (phase II), and bosutinib (phase I) are in clinical trials as repurposed therapeutics for AD, thus validating the authenticity of our drug repurposing approach. The top 10 drugs obtained from CoDReS scoring were analyzed for their similarities with the known AD drugs and clustered based on their similarity scores. We selected the closest neighbors, vandetanib, erlotinib, gefitinib, afatinib, imatinib, and sunitinib. The literature studies have confirmed the repurposing potential of these anticancer drugs. The ADMET analysis of these six drugs revealed that afatinib and imatinib did not possess good physicochemical properties and were not BBB-penetrant. Thus, we proposed vandetanib, erlotinib, gefitinib, and sunitinib as potential repurposing drugs.
The pathway analysis identified the EGFR and GSK3B as the most frequently appeared genes in AD-associated pathways. The CCND1, EGFR, and KDR are found as the most commonly expressed genes in AD and in 13 most common types of cancers. Network analysis of PPI interactions revealed that GSK3B, KDR, APP, EGFR, and FLT1 were the hub genes in the PPI network. Literature studies have supported the neuroprotective potential of these targets and their associated drugs. In short, our integrated omics analysis with computational validation tools had prioritized the role of GSK3B and EGFR in AD pathogenesis. ErBb signaling, focal adhesion, MAPK pathway, Cu homeostasis, and PI3-Akt were the over-representative pathways targeted by these genes that we prioritized by pathway analysis using different databases. However, the therapeutic relevance of targeting the EGFR in AD is not well established. Still, some studies have supported the fact that the EGFR prevents Aβ and ApoE-induced cognitive deficits and considered a preferred target for treating AD.57,74 We also established a new connection of the EGFR with AD-related targets such as APP, SNCA, LRP1, and NRG. Many bibliographic mentions also supported this finding. A recently published study has identified that APP-EGFR interaction promoted extracellular signal-regulated kinase (ERK) signaling and contributed to neuritogenesis and neuronal differentiation.75 Some studies have reported that the EGFR has structural and expression similarities with ErBb4, the primary receptor of NRG1, in several brain regions. Some studies have found that the EGFR was coexpressed with ErBb4 in several GABAergic neurons.76,77 This finding would be helpful to establish new connections of EGFR inhibitors with NRG1. Although the role of the EGFR in SNCA gene polymorphisms in AD brains is not explored, a study by Yan et al. confirmed that SNCA plays a significant role in EGFR signaling in lung adenocarcinoma cells.78
Our proposed repurposed drug list had three EGFR inhibitors—vandetanib, erlotinib, and gefitinib. Among the proposed drugs, vandetanib, a tyrosine kinase inhibitor, is currently marketed to treat tumors of the thyroid gland. Likewise, erlotinib, an EGFR inhibitor, is used for treating nonsmall cell lung cancer (NSCLC) and pancreatic cancer. Similarly, gefitinib, an inhibitor of EGFR tyrosine kinase, is approved to treat locally advanced or metastatic NSCLC. Structural similarities of these drugs with approved AD drugs and physicochemical and BBB analyses also supported the therapeutic potential of these drugs. Earlier studies have proposed that erlotinib and gefitinib rescued EGFR-induced Aβ toxicity and memory loss in Drosophila and mouse models,57 but the exact molecular mechanism and affected signaling pathways are yet to be elucidated.
Furthermore, some recent computational studies have predicted the potential drug–disease relations based on miRNA data. Based on this fact, we searched for miRNAs that were related to AD and correlated the gene targets of these miRNAs with the gene targets of the proposed repurposed drugs. From this analysis, we identified some neuroprotective microRNAs and established their relationship with the repurposed drugs. We identified miRNA-200a as a potential neuroprotective candidate that shares targets with all three repurposed EGFR inhibitors. In such a way, miRNA–disease–drug relations helped us to establish a link between repurposed drugs and AD concerning the miRNA axis.
To find out the significance of the results, we curated the available literature and proposed the potential neuroprotective functions of the repurposing drugs in AD pathogenesis, as shown in Figure 7. We suggested that tau phosphorylation, autophagy, and neuroinflammation were the significant AD-related biological mechanisms regulated by the proposed EGFR inhibitor drugs. PI3-Akt signaling, NF-kappa B pathway, and Ca2+ signaling were the significant pathways targeted by the proposed drugs.
Figure 7.
Schematic representation of the proposed mechanism of neuroprotective functions of EGFR inhibitors in AD. The binding of a ligand to the EGFR causes conformational changes in the receptor and activates various signaling cascades. Activation of the PI3K/Akt axis activates mTOR that is a major inhibitor of the autophagic process. The inhibition of autophagy leads to neuronal death. Activated mTOR is responsible for tau phosphorylation and Aβ production, the two major pathological hallmarks of AD. Activated Akt further induces endothelial nitric oxide synthase (eNOS) that generates nitric oxide (NO), a neurotoxin. The activated Akt instigates inflammatory cytokine production by inducing NF-κB production. The activated EGFR induces Ca2+ release from the endoplasmic reticulum by inducing phospholipase C gamma (PLC-γ) production. Excessive release of Ca2+ causes synaptic dysfunction and Aβ production from APP. All the events trigger neuroinflammation and neurodegeneration. Pharmacological inhibition of the EGFR by inhibitors, erlotinib, gefitinib, and vandetanib, may reverse the downstream signaling cascades of the EGFR and provide neuroprotection, a reduction in synaptic dysfunction, reduced tau phosphorylation, inhibition of neuronal death, and inhibition of neuroinflammatory processes. Dotted arrows represent the proposed neuroprotective functions of the repurposed drugs.
5. Conclusions
Repurposed drugs can be a promising way of treating complex diseases such as AD. Our study has proposed an integrated omics-based data mining approach to identify the possible relationship of anticancer drugs with AD-associated genes. We further integrated network-based and pathway-based analysis methods to validate the overlap of anticancer drugs with AD-related pathways. The resulting drugs were validated based on computational repurposing tools, similarity scores, and physicochemical analysis. Additionally, literature validation, the functional similarity with miRNAs, and pathway analysis supported the hypothesis that EGFR inhibitors vandetanib, erlotinib, and gefitinib might play therapeutic roles by targeting AD-related proteins. Furthermore, we elucidated the mechanistic basis of these drugs in ameliorating AD-associated neurotoxicity and neuroinflammation. Additionally, our comprehensive approach also proposed a connection between AD-related targets and the reported repurposing drugs. As far as experimental aspects are concerned, in vitro and animal studies are warranted to confirm their neuroprotective potential.
Acknowledgments
We would like to thank the senior management of Delhi Technological University (DTU) and the Department of Biotechnology (DBT), Indian Government, for their constant support and financial assistance.
Glossary
Abbreviations
- AD
Alzheimer’s disease
- ABL1
ABL proto-oncogene 1
- Aβ
amyloid-β
- ApoE
apolipoprotein E
- APP
amyloid precursor protein
- BBB
blood–brain barrier
- BACE-1
beta-secretase
- CDK4
cyclin-dependent kinase 4
- CNS
central nervous system
- CoDReS
computational drug repositioning score
- EGFR
epidermal growth factor receptor
- ERK
extracellular signal-regulated kinase
- FDR
false discovery rate
- FLT1
Fms-related receptor tyrosine kinase 1
- GEO
Gene Expression Omnibus
- GS2D
gene set to diseases
- GWAS
genome-wide association studies
- GSK3B
glycogen synthase kinase 3 beta
- HMDB
Human Metabolome Database
- HMDD
Human microRNA Disease Database
- hTERT
human telomerase reverse transcriptase
- IL-6
interleukin-6
- KDR
kinase insert domain receptor
- LRP1
LDL receptor-related protein 1
- MAPK
mitogen-activated protein kinase
- MCS
maximum common substructure
- MIST
Molecular Interaction Search Tool
- M log P
partition coefficient
- NIH
National Cancer Institute
- NRG1
neuregulin 1
- NSCLC
nonsmall cell lung cancer
- OR
odds ratio
- PI3K-Akt
phosphatidylinositol-3-kinase
- PPI
protein–protein interaction
- PKA
protein kinase A
- PS
presenilin
- RAGE
receptors for advanced glycation end products
- SNCA
alpha-synuclein
- SNP
single-nucleotide polymorphism
- STITCH
search tool for interactions of chemicals
- TNF-α
tumor necrosis factor-alpha
- TPSA
topological polar surface area
- TTD
Therapeutic Target Database
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c01526.
Functional enrichment analysis of AD-associated genes, list of candidate repurposing anticancer drugs, computational drug repositioning scores, physiochemical properties of repurposed drugs, and AD-related miRNAs, drugs, and targets (PDF)
Author Contributions
P.K. conceived and designed the manuscript. D.A. collected and analyzed data. D.A. and P.K. wrote the manuscript, discussed the results, and analyzed the entire data.
D.A. has received Senior Research Fellowship (SRF) by Department of Biotechnology (DBT), Govt. of India (Fellow ID: DBT/2018/DTU/1067).
The authors declare no competing financial interest.
Supplementary Material
References
- Wang J.; Gu B. J.; Masters C. L.; Wang Y.-J. A systemic view of Alzheimer disease - insights from amyloid-β metabolism beyond the brain. Nat. Rev. Neurol. 2017, 13, 612. 10.1038/nrneurol.2017.111. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Schmitt-Ulms G.; Sato C.; Xi Z.; Zhang Y.; Zhou Y.; St George-Hyslop P.; Rogaeva E. Drug Repositioning for Alzheimer’s Disease Based on Systematic “omics” Data Mining. PLoS One 2016, 11, e0168812 10.1371/journal.pone.0168812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durães F.; Pinto M.; Sousa E. Old Drugs as New Treatments for Neurodegenerative Diseases. Pharmaceuticals 2018, 11, 44. 10.3390/ph11020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford R. E.; Hildebrandt-Eriksen E. S.; Petri A.; Persson R.; Lindow M.; Munk M. E.; Kauppinen S.; Ørum H. Therapeutic Silencing of MicroRNA-122 in Primates with Chronic Hepatitis C Virus Infection. Science 2010, 327, 198. 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L.; Zhao J.; Gao L. Predicting Potential Drugs for Breast Cancer Based on MiRNA and Tissue Specificity. Int. J. Biol. Sci. 2018, 14, 971. 10.7150/ijbs.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin B.; Arslan S.; Bayraklı F.; Karademir B.; Arga K. Y. MiRNA-Mediated Drug Repurposing Unveiled Potential Candidate Drugs for Prolactinoma Treatment. Neuroendocrinology 2021, 10.1159/000515801. [DOI] [PubMed] [Google Scholar]
- Ashburn T. T.; Thor K. B. Drug Repositioning: Identifying and Developing New Uses for Existing Drugs. Nat. Rev. Drug Discovery 2004, 3, 673. 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- Rudrapal M.; J. Khairnar S.; G. Jadhav A.. Drug Repurposing (DR): An Emerging Approach in Drug Discovery. Drug Repurposing—Hypothesis, Molecular Aspects and Therapeutic Applications; IntechOpen, 2020. [Google Scholar]
- Karatzas E.; Bourdakou M. M.; Kolios G.; Spyrou G. M. Drug Repurposing in Idiopathic Pulmonary Fibrosis Filtered by a Bioinformatics-Derived Composite Score. Sci. Rep. 2017, 7, 12569. 10.1038/s41598-017-12849-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushpakom S.; Iorio F.; Eyers P. A.; Escott K. J.; Hopper S.; Wells A.; Doig A.; Guilliams T.; Latimer J.; McNamee C.; Norris A.; Sanseau P.; Cavalla D.; Pirmohamed M. Drug Repurposing: Progress, Challenges and Recommendations. Nat. Rev. Drug Discovery 2019, 18, 41. 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- Xue H.; Li J.; Xie H.; Wang Y. Review of Drug Repositioning Approaches and Resources. Int. J. Biol. Sci. 2018, 14, 1232. 10.7150/ijbs.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paananen J.; Fortino V. An Omics Perspective on Drug Target Discovery Platforms. Briefings Bioinf. 2019, 21, 1937. 10.1093/bib/bbz122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam V.; Patel N.; Turcotte M.; Bossé Y.; Paré G.; Meyre D. Benefits and Limitations of Genome-Wide Association Studies. Nat. Rev. Genet. 2019, 20, 467. 10.1038/s41576-019-0127-1. [DOI] [PubMed] [Google Scholar]
- Alexander-Dann B.; Pruteanu L. L.; Oerton E.; Sharma N.; Berindan-Neagoe I.; Módos D.; Bender A. Developments in Toxicogenomics: Understanding and Predicting Compound-Induced Toxicity from Gene Expression Data. Mol. Omics 2018, 14, 218. 10.1039/c8mo00042e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard J.-L. E.; O’Mara T. A.; Glubb D. M. Enhancing the Promise of Drug Repositioning through Genetics. Front. Pharmacol. 2017, 8, 896. 10.3389/fphar.2017.00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirle M.; Bantscheff M.; Kuster B. Mass Spectrometry-Based Proteomics in Preclinical Drug Discovery. Chem. Biol. 2012, 19, 72. 10.1016/j.chembiol.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Patti G. J.; Yanes O.; Siuzdak G. Metabolomics: the apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263. 10.1038/nrm3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudelman K. N. H.; McDonald B. C.; Lahiri D. K.; Saykin A. J. Biological Hallmarks of Cancer in Alzheimer ’ s Disease. Mol. Neurobiol. 2019, 56, 7173. 10.1007/s12035-019-1591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monacelli F.; Cea M.; Borghi R.; Odetti P.; Nencioni A. Do Cancer Drugs Counteract Neurodegeneration? Repurposing for Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 55, 1295. 10.3233/JAD-160840. [DOI] [PubMed] [Google Scholar]
- Lee S. Y.; Song M.-Y.; Kim D.; Park C.; Park D. K.; Kim D. G.; Yoo J. S.; Kim Y. H. A Proteotranscriptomic-Based Computational Drug-Repositioning Method for Alzheimer’s Disease. Front. Pharmacol. 2020, 10, 1653. 10.3389/fphar.2019.01653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Advani D.; Gupta R.; Tripathi R.; Sharma S.; Ambasta R. K.; Kumar P. Protective Role of Anticancer Drugs in Neurodegenerative Disorders: A Drug Repurposing Approach. Neurochem. Int. 2020, 140, 104841. 10.1016/j.neuint.2020.104841. [DOI] [PubMed] [Google Scholar]
- Lonskaya I.; Hebron M. L.; Selby S. T.; Turner R. S.; Moussa C. E.-H. Nilotinib and Bosutinib Modulate Pre-Plaque Alterations of Blood Immune Markers and Neuro-Inflammation in Alzheimer’s Disease Models. Neuroscience 2015, 304, 316. 10.1016/j.neuroscience.2015.07.070. [DOI] [PubMed] [Google Scholar]
- Bibi N.; Rizvi S. M. D.; Batool A.; Kamal M. A. Inhibitory Mechanism of An Anticancer Drug, Bexarotene Against Amyloid β Peptide Aggregation: Repurposing Via Neuroinformatics Approach. Curr. Pharm. Des. 2019, 25, 2989. 10.2174/1381612825666190801123235. [DOI] [PubMed] [Google Scholar]
- Hayes C. D.; Dey D.; Palavicini J. P.; Wang H.; Patkar K. A.; Minond D.; Nefzi A.; Lakshmana M. K. Striking Reduction of Amyloid Plaque Burden in an Alzheimer’s Mouse Model after Chronic Administration of Carmustine. BMC Med. 2013, 11, 81. 10.1186/1741-7015-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemesh O. A.; Spira M. E. Rescue of Neurons from Undergoing Hallmark Tau-Induced Alzheimer’s Disease Cell Pathologies by the Antimitotic Drug Paclitaxel. Neurobiol. Dis. 2011, 43, 163. 10.1016/j.nbd.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Javidnia M.; Hebron M. L.; Xin Y.; Kinney N. G.; Moussa C. E.-H. Pazopanib Reduces Phosphorylated Tau Levels and Alters Astrocytes in a Mouse Model of Tauopathy. J. Alzheimer’s Dis. 2017, 60, 461. 10.3233/JAD-170429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buniello A.; Macarthur J. A. L.; Cerezo M.; Harris L. W.; Hayhurst J.; Malangone C.; McMahon A.; Morales J.; Mountjoy E.; Sollis E.; Suveges D.; Vrousgou O.; Whetzel P. L.; Amode R.; Guillen J. A.; Riat H. S.; Trevanion S. J.; Hall P.; Junkins H.; Flicek P.; Burdett T.; Hindorff L. A.; Cunningham F.; Parkinson H. The NHGRI-EBI GWAS Catalog of Published Genome-Wide Association Studies, Targeted Arrays and Summary Statistics 2019. Nucleic Acids Res. 2019, 47, D1005. 10.1093/nar/gky1120. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://www.ebi.ac.uk/gwas
- Barrett T.; Wilhite S. E.; Ledoux P.; Evangelista C.; Kim I. F.; Tomashevsky M.; Marshall K. A.; Phillippy K. H.; Sherman P. M.; Holko M.; Yefanov A.; Lee H.; Zhang N.; Robertson C. L.; Serova N.; Davis S.; Soboleva A. NCBI GEO: archive for functional genomics data sets-update. Nucleic Acids Res. 2013, 41, D991. 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://www.ncbi.nlm.nih.gov/geo/
- Wishart D. S.; Feunang Y. D.; Marcu A.; Guo A. C.; Liang K.; Vázquez-Fresno R.; Sajed T.; Johnson D.; Li C.; Karu N.; Sayeeda Z.; Lo E.; Assempour N.; Berjanskii M.; Singhal S.; Arndt D.; Liang Y.; Badran H.; Grant J.; Serra-Cayuela A.; Liu Y.; Mandal R.; Neveu V.; Pon A.; Knox C.; Wilson M.; Manach C.; Scalbert A. HMDB 4.0: The Human Metabolome Database for 2018. Nucleic Acids Res. 2018, 46, D608. 10.1093/nar/gkx1089. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://www.hmdb.ca
- Huang D. W.; Sherman B. T.; Lempicki R. A. Systematic and Integrative Analysis of Large Gene Lists Using DAVID Bioinformatics Resources. Nat. Protoc. 2009, 4, 44. 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]; https://david.ncifcrf.gov
- Andrade-Navarro M. A.; Fontaine J. F. Gene Set to Diseases (GS2D): Disease Enrichment Analysis on Human Gene Sets with Literature Data. Genom. Comput. Biol. 2016, 2, e33 10.18547/gcb.2016.vol2.iss1.e33. [DOI] [Google Scholar]; http://cbdm.uni-mainz.de/geneset2diseases
- Cavalla D. Predictive Methods in Drug Repurposing: Gold Mine or Just a Bigger Haystack?. Drug Discov. Today 2013, 18, 523–532. 10.1016/j.drudis.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Vinayagam A.; Nand A.; Comjean A.; Chung V.; Hao T.; Mohr S. E.; Perrimon N. Molecular Interaction Search Tool (MIST): An Integrated Resource for Mining Gene and Protein Interaction Data. Nucleic Acids Res. 2018, 46, D567–D574. 10.1093/nar/gkx1116. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://fgrtools.hms.harvard.edu/MIST/
- Wishart D. S.; Feunang Y. D.; Guo A. C.; Lo E. J.; Marcu A.; Grant J. R.; Sajed T.; Johnson D.; Li C.; Sayeeda Z.; Assempour N.; Iynkkaran I.; Liu Y.; MacIejewski A.; Gale N.; Wilson A.; Chin L.; Cummings R.; Le D.; Pon A.; Knox C.; Wilson M. DrugBank 5.0: A Major Update to the DrugBank Database for 2018. Nucleic Acids Res. 2018, 46, D1074. 10.1093/nar/gkx1037. [DOI] [PMC free article] [PubMed] [Google Scholar]; www.drugbank.com
- Wang Y.; Zhang S.; Li F.; Zhou Y.; Zhang Y.; Wang Z.; Zhang R.; Zhu J.; Ren Y.; Tan Y.; Qin C.; Li Y.; Li X.; Chen Y.; Zhu F. Therapeutic Target Database 2020: Enriched Resource for Facilitating Research and Early Development of Targeted Therapeutics. Nucleic Acids Res. 2020, 48, D1031. 10.1093/nar/gkz981. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://db.idrblab.net/ttd/
- Szklarczyk D.; Gable A. L.; Lyon D.; Junge A.; Wyder S.; Huerta-Cepas J.; Simonovic M.; Doncheva N. T.; Morris J. H.; Bork P.; Jensen L. J.; Mering C. v. STRING V11: Protein-Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607. 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]; string-db.org
- Szklarczyk D.; Santos A.; von Mering C.; Jensen L. J.; Bork P.; Kuhn M. STITCH 5: Augmenting Protein-Chemical Interaction Networks with Tissue and Affinity Data. Nucleic Acids Res. 2016, 44, D380. 10.1093/nar/gkv1277. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://stitch.embl.de/
- Jadamba E.; Shin M. A Systematic Framework for Drug Repositioning from Integrated Omics and Drug Phenotype Profiles Using Pathway-Drug Network. BioMed Res. Int. 2016, 2016, 7147039. 10.1155/2016/7147039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatzas E.; Minadakis G.; Kolios G.; Delis A.; Spyrou G. M. A Web Tool for Ranking Candidate Drugs Against a Selected Disease Based on a Combination of Functional and Structural Criteria. Comput. Struct. Biotechnol. J. 2019, 17, 939. 10.1016/j.csbj.2019.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://bioinformatics.cing.ac.cy/codres
- Backman T. W. H.; Cao Y.; Girke T. ChemMine Tools: An Online Service for Analyzing and Clustering Small Molecules. Nucleic Acids Res. 2011, 39, W486. 10.1093/nar/gkr320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggiora G.; Vogt M.; Stumpfe D.; Bajorath J. Molecular Similarity in Medicinal Chemistry. J. Med. Chem. 2014, 57, 3186. 10.1021/jm401411z. [DOI] [PubMed] [Google Scholar]
- Daina A.; Michielin O.; Zoete V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://www.swissadme.ch/
- Huang Z.; Shi J.; Gao Y.; Cui C.; Zhang S.; Li J.; Zhou Y.; Cui Q. HMDD v3.0: A Database for Experimentally Supported Human MicroRNA-Disease Associations. Nucleic Acids Res. 2019, 47, D1013–D1017. 10.1093/nar/gky1010. [DOI] [PMC free article] [PubMed] [Google Scholar]; https://www.cuilab.cn/hmdd
- American Cancer Society . Cancer Facts & Figures 2020; American Cancer Society, 2020. [Google Scholar]
- Kanehisa M.; Sato Y.; Furumichi M.; Morishima K.; Tanabe M. New Approach for Understanding Genome Variations in KEGG. Nucleic Acids Res. 2019, 47, D590. 10.1093/nar/gky962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R.; Grishagin I.; Wang Y.; Zhao T.; Greene J.; Obenauer J. C.; Ngan D.; Nguyen D.-T.; Guha R.; Jadhav A.; Southall N.; Simeonov A.; Austin C. P. The NCATS BioPlanet - An Integrated Platform for Exploring the Universe of Cellular Signaling Pathways for Toxicology, Systems Biology, and Chemical Genomics. Front. Pharmacol. 2019, 10, 445. 10.3389/fphar.2019.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slenter D. N.; Kutmon M.; Hanspers K.; Riutta A.; Windsor J.; Nunes N.; Mélius J.; Cirillo E.; Coort S. L.; DIgles D.; Ehrhart F.; Giesbertz P.; Kalafati M.; Martens M.; Miller R.; Nishida K.; Rieswijk L.; Waagmeester A.; Eijssen L. M. T.; Evelo C. T.; Pico A. R.; Willighagen E. L. WikiPathways: A Multifaceted Pathway Database Bridging Metabolomics to Other Omics Research. Nucleic Acids Res. 2018, 46, D661. 10.1093/nar/gkx1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuleshov M. V.; Jones M. R.; Rouillard A. D.; Fernandez N. F.; Duan Q.; Wang Z.; Koplev S.; Jenkins S. L.; Jagodnik K. M.; Lachmann A.; McDermott M. G.; Monteiro C. D.; Gundersen G. W.; Ma’ayan A. Enrichr: A Comprehensive Gene Set Enrichment Analysis Web Server 2016 Update. Nucleic Acids Res. 2016, 44, W90. 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://amp.pharm.mssm.edu/Enrichr/
- Llorens-Martín M.; Jurado J.; Hernández F.; Ávila J. GSK-3β, a Pivotal Kinase in Alzheimer Disease. Front. Mol. Neurosci. 2014, 7, 46. 10.3389/fnmol.2014.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima A. GSK-3 Is Essential in the Pathogenesis of Alzheimer’s Disease. J. Alzheimer’s Dis. 2006, 9, 309. 10.3233/jad-2006-9s335. [DOI] [PubMed] [Google Scholar]
- Müller U. C.; Deller T.; Korte M. Not Just Amyloid: Physiological Functions of the Amyloid Precursor Protein Family. Nat. Rev. Neurosci. 2017, 18, 281. 10.1038/nrn.2017.29. [DOI] [PubMed] [Google Scholar]
- Murphy M. P.; Levine H. Alzheimer’s Disease and the Amyloid-β Peptide. J. Alzheimer’s Dis. 2010, 19, 311. 10.3233/JAD-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno S.; Oda N.; Abe M.; Terai Y.; Ito M.; Shitara K.; Tabayashi K.; Shibuya M.; Sato Y. Roles of Two VEGF Receptors, Flt-1 and KDR, in the Signal Transduction of VEGF Effects in Human Vascular Endothelial Cells. Oncogene 2000, 19, 2138. 10.1038/sj.onc.1203533. [DOI] [PubMed] [Google Scholar]
- Harris R.; Miners J. S.; Allen S.; Love S. VEGFR1 and VEGFR2 in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 61, 741. 10.3233/JAD-170745. [DOI] [PubMed] [Google Scholar]
- Mahoney E. R.; Dumitrescu L.; Moore A. M.; Cambronero F. E.; De Jager P. L.; Koran M. E. I.; Petyuk V. A.; Robinson R. A. S.; Goyal S.; Schneider J. A.; Bennett D. A.; Jefferson A. L.; Hohman T. J. Brain Expression of the Vascular Endothelial Growth Factor Gene Family in Cognitive Aging and Alzheimer’s Disease. Mol. Psychiatr. 2019, 26, 888. 10.1038/s41380-019-0458-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.-J.; Hsu C.-C.; Shiao Y.-J.; Wang H.-T.; Lo Y.-L.; Lin A. M. Y. Anti-Inflammatory Effect of Afatinib (an EGFR-TKI) on OGD-Induced Neuroinflammation. Sci. Rep. 2019, 9, 2516. 10.1038/s41598-019-38676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Chiang H.-C.; Wu W.; Liang B.; Xie Z.; Yao X.; Ma W.; Du S.; Zhong Y. Epidermal growth factor receptor is a preferred target for treating Amyloid- -induced memory loss. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 16743. 10.1073/pnas.1208011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu M.; Hu J.; Wu S.; Zhang X.; Xu H.; Zhang Y.; Zhang J.; Yang Y. Structural Bioinformatics-Based Identification of EGFR Inhibitor Gefitinib as a Putative Lead Compound for BACE. Chem. Biol. Drug Des. 2014, 83, 81. 10.1111/cbdd.12200. [DOI] [PubMed] [Google Scholar]
- Netzer W. J.; Bettayeb K.; Sinha S. C.; Flajolet M.; Greengard P.; Bustos V. Gleevec shifts APP processing from a β-cleavage to a nonamyloidogenic cleavage. Proc. Natl. Acad. Sci. 2017, 114, 1389. 10.1073/pnas.1620963114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele Y. S.; Baumann M.; Klebl B.; Nordhammer C.; Jucker M.; Kilger E. Gleevec Increases Levels of the Amyloid Precursor Protein Intracellular Domain and of the Amyloid-β-degrading Enzyme Neprilysin. Mol. Biol. Cell 2007, 18, 3591. 10.1091/mbc.E07-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada L. D.; Chamorro D.; Yañez M. J.; Gonzalez M.; Leal N.; Von Bernhardi R.; Dulcey A. E.; Marugan J.; Ferrer M.; Soto C.; Zanlungo S.; Inestrosa N. C.; Alvarez A. R. Reduction of Blood Amyloid-β Oligomers in Alzheimer’s Disease Transgenic Mice by c-Abl Kinase Inhibition. J. Alzheimer’s Dis. 2016, 54, 1193. 10.3233/JAD-151087. [DOI] [PubMed] [Google Scholar]
- Sanchez A.; Tripathy D.; Yin X.; Luo J.; Martinez J. M.; Grammas P. Sunitinib enhances neuronal survival in vitro via NF-κB-mediated signaling and expression of cyclooxygenase-2 and inducible nitric oxide synthase. J. Neuroinflammation 2013, 10, 857. 10.1186/1742-2094-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L.; Lin J.; Xiang S.; Zhao K.; Yu J.; Zheng J.; Xu D.; Mak S.; Hu S.; Nirasha S.; Wang C.; Chen X.; Zhang J.; Xu S.; Wei X.; Zhang Z.; Zhou D.; Zhou W.; Cui W.; Han Y.; Hu Z.; Wang Q. Sunitinib, a Clinically Used Anticancer Drug, Is a Potent AChE Inhibitor and Attenuates Cognitive Impairments in Mice. ACS Chem. Neurosci. 2016, 7, 1047. 10.1021/acschemneuro.5b00329. [DOI] [PubMed] [Google Scholar]
- Hassan M.; Raza H.; Abbasi M. A.; Moustafa A. A.; Seo S.-Y. The Exploration of Novel Alzheimer’s Therapeutic Agents from the Pool of FDA Approved Medicines Using Drug Repositioning, Enzyme Inhibition and Kinetic Mechanism Approaches. Biomed. Pharmacother. 2019, 109, 2513–2526. 10.1016/j.biopha.2018.11.115. [DOI] [PubMed] [Google Scholar]
- Wang L.; Liu J.; Wang Q.; Jiang H.; Zeng L.; Li Z.; Liu R. MicroRNA-200a-3p Mediates Neuroprotection in Alzheimer-Related Deficits and Attenuates Amyloid-Beta Overproduction and Tau Hyperphosphorylation via Coregulating BACE1 and PRKACB. Front. Pharmacol. 2019, 10, 806. 10.3389/fphar.2019.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo R.-S.; Lee J.-H.; Yu H.-N.; Song D.-Y.; Baik T.-K. Expression of ErbB4 in the Neurons of Alzheimer’s Disease Brain and APP/PS1 Mice, a Model of Alzheimer’s Disease. Anat. Cell Biol. 2011, 44, 116. 10.5115/acb.2011.44.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caltagarone J.; Jing Z.; Bowser R. Focal Adhesions Regulate Aβ Signaling and Cell Death in Alzheimer’s Disease. Biochim. Biophys. Acta, Mol. Basis Dis. 2007, 1772, 438. 10.1016/j.bbadis.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace E. A.; Busciglio J. Aberrant Activation of Focal Adhesion Proteins Mediates Fibrillar Amyloid β-Induced Neuronal Dystrophy. J. Neurosci. 2003, 23, 493. 10.1523/jneurosci.23-02-00493.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E. K.; Choi E.-J. Pathological Roles of MAPK Signaling Pathways in Human Diseases. Biochim. Biophys. Acta, Mol. Basis Dis. 2010, 1802, 396. 10.1016/j.bbadis.2009.12.009. [DOI] [Google Scholar]
- Zhu X.; Lee H.-g.; Raina A. K.; Perry G.; Smith M. A. The Role of Mitogen-Activated Protein Kinase Pathways in Alzheimer ’ s Disease. Neurosignals 2002, 11, 270–281. 10.1159/000067426. [DOI] [PubMed] [Google Scholar]
- Bagheri S.; Squitti R.; Haertlé T.; Siotto M.; Saboury A. A. Role of Copper in the Onset of Alzheimer’s Disease Compared to Other Metals. Front. Aging Neurosci. 2018, 9, 446. 10.3389/fnagi.2017.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H.; Ho Koh S. Interaction between Amyloid Beta Toxicity and the PI3K Pathway in Alzheimer’s Disease. J. Alzheimer’s Dis. Park. 2016, 6, 269. 10.4172/2161-0460.1000269. [DOI] [Google Scholar]
- Yu H.-J.; Koh S.-H. The Role of PI3K/AKT Pathway and Its Therapeutic Possibility in Alzheimer’s Disease. Hanyang Med. Rev. 2017, 37, 18. 10.7599/hmr.2017.37.1.18. [DOI] [Google Scholar]
- Thomas R.; Zuchowska P.; Morris A. W. J.; Marottoli F. M.; Sunny S.; Deaton R.; Gann P. H.; Tai L. M. Epidermal Growth Factor Prevents APOE4 and Amyloid-Beta-Induced Cognitive and Cerebrovascular Deficits in Female Mice. Acta Neuropathol. Commun. 2016, 4, 111. 10.1186/s40478-016-0387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Rocha J. F.; Bastos L.; Domingues S. C.; Bento A. R.; Konietzko U.; da Cruz e Silva O. A. B.; Vieira S. I. APP Binds to the EGFR Ligands HB-EGF and EGF, Acting Synergistically with EGF to Promote ERK Signaling and Neuritogenesis. Mol. Neurobiol. 2021, 58, 668. 10.1007/s12035-020-02139-2. [DOI] [PubMed] [Google Scholar]
- Fox I. J.; Kornblum H. I. Developmental Profile of ErbB Receptors in Murine Central Nervous System: Implications for Functional Interactions. J. Neurosci. Res. 2005, 79, 584. 10.1002/jnr.20381. [DOI] [PubMed] [Google Scholar]
- Iwakura Y.; Nawa H. ErbB1-4-Dependent EGF/Neuregulin Signals and Their Cross Talk in the Central Nervous System: Pathological Implications in Schizophrenia and Parkinson’s Disease. Front. Cell. Neurosci. 2013, 7, 4. 10.3389/fncel.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y.; Xu Z.; Hu X.; Qian L.; Li Z.; Zhou Y.; Dai S.; Zeng S.; Gong Z. SNCA Is a Functionally Low-Expressed Gene in Lung Adenocarcinoma. Genes 2018, 9, 16. 10.3390/genes9010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.