Abstract
Background
The efficacy and safety of rituximab (RTX) in adult frequent-relapsing (FR) or steroid-dependent (SD) nephrotic syndrome (NS), including minimal change disease (MCD) or focal segmental glomerulosclerosis (FSGS), are still inconclusive.
Methods
We performed a systematic review and meta-analysis registered in PROSPERO (CRD42019148102) by pooling data of cohort studies or case series on adult patients with difficult-to-treat NS. Steroid-resistant NS was excluded. The primary outcomes were the complete remission (CR) rate and the relapse rate. Partial remission (PR) rate, no response (NR) rate and adverse events were the secondary outcomes. A random-effects model was performed for all the outcomes.
Results
We included 21 studies involving 382 adult MCD/FSGS subjects with a median follow-up duration from 12 to 43 months. RTX treatment induced a pooled 84.2% CR rate [95% confidence interval (CI): 67.7–96.3%], while MCD patients had a high 91.6% CR rate and FSGS patients a moderate 43% CR rate. However, 27.4% (95% CI 20.7–34.5%) of the patients relapsed during the follow-up. The pooled PR and NR rates were 5.8% (95% CI 1.2–12.5%) and 5.2% (95% CI 0.0–15.0%), respectively. RTX was associated with trivial adverse events and good tolerance.
Conclusions
In summary, by pooling results of current pilot studies, RTX may be an effective and relatively safe alternative for most adult FR or SD MCD/FSGS to displace calcineurin inhibitors or prednisone in the hierarchy of treatment. More clinical trials comparing RTX with other immunosuppressants and concerning the long-term adverse events are needed.
Keywords: adult, focal and segmental glomerulosclerosis, minimal change disease, nephrotic syndrome, rituximab
INTRODUCTION
The majority of adult nephrotic syndrome (NS) is controllable, but treatment for frequent-relapsing (FR) or steroid-dependent (SD) NS is still challenging. Minimal change disease (MCD) and focal and segmental glomerulosclerosis (FSGS) are two leading causes of FR or SD NS in adults [1]. Although MCD and FSGS are immunologic disorders, the exact mechanism of their pathophysiology is still unclear [2, 3]. To avoid constant renal damage, and subsequent renal function decrease, active treatment should be performed. According to the Kidney Disease: Improving Global Outcomes (KDIGO) guideline, the management of adult MCD and FSGS should include corticosteroids (or add other immunosuppressive agents) and antiproteinuric drugs [4, 5]. The commonly used immunosuppressive agents include cyclophosphamide, calcineurin inhibitors and mycophenolate mofetil (MMF). Although adding immunosuppressive agents leads to better efficacy, the total remission rate and relapse rate are not that satisfactory [6]. Patient compliance is usually poor due to toxicity, adverse effects or the annoying blood concentration monitoring. Therefore, agents with good efficacy and safety are highly desirable. Although the mechanism underlying MCD and FSGS is not fully understood, current research suggests that not only T lymphocytes but also B lymphocytes are involved in the pathogenesis. In addition, the discovery of several antibodies indicated that B-cell suppression may be a target of immunotherapy for membranous nephropathy [7]. Due to the shared pathogenesis between membranous nephropathy and MCD/FSGS, B-cell suppression may be effective in MCD/FSGS.
Rituximab (RTX), a monoclonal antibody against CD20-bearing cells that can deplete CD20+ B lymphocytes selectively, has already been used in lymphoma and autoimmune diseases [8–10]. It showed satisfactory safety and efficacy profile in pediatric NS (including FSGS and MCD) patients [11–14]. RTX also offered an alternative to therapies for membranous nephropathy in adults. Besides, RTX was reported to be a sparing agent to reduce the long-term toxicity of immunosuppressive agents. Recently, a meta-analysis [15] assessed RTX use in adult patients with MCD/FSGS, but the study did not differentiate FR or SD NS from the steroid-resistant (SR) NS. SR NS includes patients with several hereditary kidney diseases, which may be resistant to RTX as well. Besides, the currently available results of RTX in FR or SD MCD/FSGS are controversial [11–14, 16]. Therefore, we carried out a systematic review and meta-analysis to summarize the available evidence of RTX in adult patients with FR or SD MCD and FSGS.
MATERIALS AND METHODS
This systematic review was registered in PROSPERO (CRD42019148102) and was performed following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline [17]. A search for published studies was performed using the PubMed database, EMBASE and the Cochrane library. We used ‘rituximab or Mabthera’ AND ‘minimal change disease or focal segmental glomerulosclerosis’ as the keywords. The search strategy may be slightly different across the database. We did not apply any language or publication date limitations. We did not collect any unpublished data. The searching date was December 2019. Publications that addressed anti-CD20 antibody and proteinuria were deemed relevant and were further reviewed. Additional relevant articles were then gained by using citations in publications identified by the initial search.
Publications that met the following inclusion criteria were selected: (i) randomized controlled trials, cohort studies or case series; (ii) enrolled more than five adult patients with FR and SD MCD or FSGS or NS; (iii) RTX in any dosage administrated in the treatment group while the control group received immunosuppressive agents including glucocorticoids, calcineurin inhibitors, MMF, etc.; and (iv) patients were followed up for >12 months, and at least one of the following endpoints was reported: remission rate, relapse rate and adverse events. We excluded studies with following properties: (i) children or adolescents at age <16 years; (ii) patients with SR MCD or FSGS; (iii) patients with other pathological kidney diseases except for MCD or FSGS; (iv) studies using other anti-CD20 antibodies other than RTX such as ofatumumab or using RTX prior to the study; and (v) reviews, animal research or studies with data not available for analysis. We contacted the original authors to obtain further data if necessary.
The following information was extracted from the included studies by B.Y. and C.X. independently: first author, publication year, study design, country, gender, the age, the follow-up time, the number of patients, estimated glomerular filtration rate (eGFR), serum creatinine (Scr), serum albumin, proteinuria, RTX doses, adverse events, B-cell repletion during relapse, the relapse time (RT), numbers of complete remission (CR), partial remission (PR), no response (NR) and relapse. Any discrepancies between authors were resolved by discussion. PR was defined as a 50% reduction in the urinary protein:creatinine ratio (UPCR) from baseline and a UPCR of <3.5 g/g. CR was defined as a UPCR <0.3 g/g. NS was defined as a UPCR of ≥3.5 g/g and serum albumin of <3.0 g/dL with edema or hyperlipidemia. Relapse was defined as a recurrence of massive UPCR >3.5 g/g or >3+ of the urine albumin dipstick test. CR and relapse rates were the primary outcomes. PR rates, NR rates, relapse rate change, steroid dose change, proteinuria change, the RT and risk factors for relapse were the secondary outcomes. The rates were pooled by evaluating the reported data with its 95% confidence interval (CI) in each study and calculating the weight according to the sample size [17, 18].
Assessment of risk of bias was performed by two authors (J.X. and C.Z.) independently using the NOS [19]. J.X. and C.Z. were blinded to the authors and institution of the studies. Any discrepancies between authors were resolved by discussion. Studies scored a maximum of 9 points by Newcastle-Ottawa Quality Assessment Scale (NOS). Random-effects meta-analyses were performed for the outcomes. Rates for dichotomous data, and mean difference or standardized mean difference (SMD) for continuous data were analyzed using Stata 16.0 (Stata Corp. LP, TX, USA) metaprop package [18]. Statistical heterogeneity of studies was evaluated using I2 statistics. I2 < 25% was recognized as low heterogeneity, 25% < I2 < 75% was recognized as moderate heterogeneity, and I2 > 75% was recognized as high heterogeneity [18]. The sources of heterogeneity were explored by subgroup analysis or meta-regression. Sensitivity analysis was performed by changing models (random-effects model to fixed-effect model) and excluding studies with low NOS scores (<6). Subgroup analysis was performed according to different study design, histologic diagnosis, NS onset age, sample sizes, follow-up time and ethnicities to provide estimates of treatment effect for clinically relevant subgroups of patients. If the number of studies was >10, publication bias would be investigated by funnel plots, Begg’s test and Egger’s test [20]. A two-sided P < 0.05 was considered statistically significant.
RESULTS
Literature search
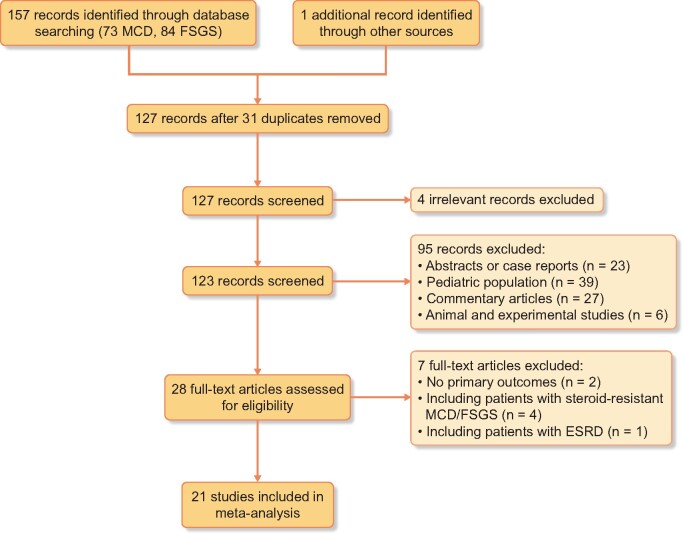
The process of selecting relevant studies initially found 158 publications from databases (Figure 1). After exclusion of irrelevant studies and 31 duplicates, 28 potentially eligible studies were deeply screened. Eventually, 21 studies involving 382 adult patients with FR or SD MCD/FSGS were included in the systematic review [16, 21–40].
FIGURE 1.
Flow of study identification, inclusion and exclusion. ESRD, end-stage renal disease.
Study characteristics
Characteristics of the included studies were presented in Table 1 [16, 21–39, 41]. There were 7 prospective cohort studies, 10 retrospective cohort studies and 4 case series. Three studies [30, 35, 38] were performed in multiple centers, while the others were in a single center. Sample sizes of included studies ranged from 5 to 54 [16, 21–26, 28–30, 32, 34–41]. The median follow-up duration ranged from 12 to 43 months [16, 21–26, 28–30, 32, 34–41]. The mean age of included patients ranged from 19 to 63.9 years old at RTX treatment. Two studies [21, 25] were performed in America, 13 studies [16, 23–25, 27, 28, 30, 32, 33, 35–38] in Europe and the remaining 6 studies [22, 26, 29, 31, 39, 40] were in Asia. Fourteen studies [21–23, 26–28, 31–33, 36–40) reported RTX used in biopsy-proven MCD patients, six studies [24, 25, 29, 30, 34, 35] in both MCD and FSGS and one study in FSGS [16]. Five studies [22, 30, 35, 37, 39] included only adult-onset patients and seven studies [23, 25, 27, 28, 32, 36, 38] included both child- and adult-onset patients. All studies which mentioned whether the type of NS were SD or FR types. Nine studies reported an eGFR (median range, 76.7–128 mL/min/1.73 m2). Seventeen studies reported Scr levels (median range, 0.7–2.6 mg/dL). Seven studies reported the renal function of included patients, which was between chronic kidney disease Stages 1 and 2. Eighteen studies reported serum albumin levels (median range, 1.9–4.2 g/dL). Nearly, all the studies reported previous immunosuppressive treatments in MCD/FSGS patients, which included azathioprine, cyclosporine, cyclophosphamide, tacrolimus, prednisone, levamisole, MMF, mizoribine, chlorambucil, rapamycin and sirolimus (Table 2) [16, 21–40]. RTX administrations varied in different studies, ranging from 1 × 375 mg/m2 to 11 g. The most common administration was 4 × 375 mg/m2 (1-week or 6-month interval) or 2 × 1 g (2-week or 6-month interval).
Table 1.
Characteristics of included studies
| Author | Year | Country | SD | Setting | Treatment | Sample size | Histologic diagnosis (n) | Gender (F/M) | Age at treatment (years) | Onset age (years) | Follow-up (months) | NS type (n) | eGFR (mL/min/1.73 m2) | Scr (mg/dL) | Albumin (g/dL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cortazar | 2019 | USA | R | S | RTX | 13 | MCD/FSGS 8/5 | 4/9 | 22–72 | NA | 35 (19–57) | SD/FR 12/1 | NA | 1.1 (0.8–1.3) | 2.7 (2.1–3.2) |
| Fenoglio | 2018 | Italy | R | S | RTX | 6 | MCD | 4/2 | 61 (59–72) | 45–73 | 8–36 | NA | NA | 0.8 (0.6–1.6) | 2.1 (1.7–2.3) |
| Iwabuchi | 2018 | Japan | R | S | RTX | 19 | MCD | 7/12 | 36.0 ± 11.4 | 30.6 ± 9.9 | 24 | NA | 85.2 ± 20.8 | 0.80 ± 0.20 | 3.60 ± 0.95 |
| Katsuno | 2018 | Japan | R | S | RTX | 8 | MCD | 5/3 | 43 (30–49.5) | 23 (22–41.5) | 13.9 (11.6–20) | SD 8 | 76.7 (68.2–87.8) | 0.69 (0.61–0.99) | 3.8 (3.3–4.0) |
| DaSilva | 2017 | Spain | R | M | RTX | 28 | MCD/FSGS 24/4 | 12/16 | 37 ± 15 | 25 ± 19 | 31 ± 26 | SD/FR 26/2 | 86 ± 21 | 0.84 ± 0.36 | 2.0 ± 0.5 |
| Steroid ± IS | 22 | MCD/FSGS 18/4 | 8/14 | NA | 27 ± 22 | 13 ± 8.3 (years) | SD/FR 20/2 | 89 ± 27 | 0.75 ± 0.19 | 1.9 ± 0.5 | |||||
| King | 2017 | UK | R | S | RTX | 13 | MCD | 3/10 | 23 (19–83) | 4 (1–80) | 20 (6–85) | SD/FR 10/13 | 90 | 0.75 (0.51–1.71) | 4.2 (3.1–5.2) |
| Ren | 2017 | China | P | S | RTX | 15 | MCD/FSGS 9/6 | 5/10 | 25 (16–54) | NA | 8 (3–36) | FR 15 | 128 (93–135) | 0.71 (0.48–0.88) | 3.7 (1.4–4.5) |
| Roccatello | 2017 | Italy | R | S | RTX | 8 | FSGS | 4/4 | 63.9 ± 14.0 | NA | 29.1 ± 8.8 | NA | NA | 2.6 ± 1.2 | 2.7 ± 0.15 |
| Brown | 2017 | USA | R | S | RTX | 5 | MCD | 0/5 | 57 (50–59) | NA | 39.5 (20–80) | SD 5 | NA | 0.98 (0.66–4.72) | 2.5 (1.9–4) |
| Papakrivopoulou | 2016 | UK | P | S | RTX | 15 | MCD | 6/9 | 27 (18–46) | 60% <18 years | 43 | SD/FR 8/7 | NA | 0.95 ± 0.24 | 4.14 ± 0.13 |
| Dekkers | 2015 | Netherlands | R | S | RTX | 10 | MCD | 2/8 | 26.4 ± 13.21 | 15.7 ± 14.4 | 43 ± 23.5 | NA | NA | NA | NA |
| Miyabe | 2015 | Japan | R | S | RTX | 54 | MCD | 13/41 | 28.2 ± 10.4 | NA | 24 | SD 54 | NA | 0.7 ± 0.02 | 3.7 ± 0.08 |
| Bruchfeld | 2014 | Sweden | R | S | RTX | 16 | MCD | 9/7 | 19–73 | 2–60 | 44 (12–70) | SD/FR 13/3 | NA | 0.92 (0.75–1.21) | 2.9–3.6 |
| Guitard | 2014 | France | P | M | RTX | 41 | MCD | 30/11 | 26 (15–83) | 6 (0.8–67) | 39 (6–71) | SD 8 | 93 ± 25 | 1.04 ± 0.29 | 3.2 ± 0.9 |
| Iwabuchi | 2014 | Japan | P | S | RTX | 25 | MCD | 4/21 | 30.1 ± 11.9 | NA | 24 | NA | NA | NA | 3.6 ± 0.8 |
| Ruggenenti | 2014 | Italy | P | M | RTX | 20 | MCD/FSGS 15/5 | 10/10 | 34.3 (22.7–47.4) | 20.6 (4.3–39.8) | 12 | SD/FR 15/5 | 105.0 ± 24.9 | 0.81 ± 0.19 | 3.86 ± 0.56 |
| Takei | 2013 | Japan | P | S | RTX | 25 | MCD | 6/19 | 30 ± 12 | NA | 12 | NA | NA | 0.7 ± 0.2 | 3.4 ± 0.8 |
| Munyentwali | 2013 | France | R | S | RTX | 17 | MCD | 4/13 | 29.4 (18.5–65) | 16 (1–63.2) | 29.5 (5.1–82.2) | SD 17 | 108 (61–175) | NA | 3.3 (2.2–3.8) |
| Kronbichler | 2013 | Austria | R | S | RTX | 5 | MCD/FSGS 2/3 | 1/4 | 29.4 ± 3.6 | 13.6 ± 8.4 | 22.6 ± 7.1 | SD 5 | NA | 0.93 ± 0.22 | 2.6 ± 0.9 |
| Kong | 2012 | Australia | R | S | RTX | 11 | MCD/FSGS 7/4 | 6/5 | 19 (13–44) | NA | 31.5 (15–44) | NA | NA | NA | NA |
| Hoxha | 2011 | Germany | P | S | RTX | 6 | MCD | 1/5 | 24.8 ± 6.3 | NA | 17.2 ± 4.8 | SD/FR | NA | 0.5–1.2 | NA |
SD, study design; M, male; F, female; R, retrospective; P, prospective; S, single center; M, multicenter; n, number; NA, not available; IS, immunosuppression.
Table 2.
Previous immunosuppressive therapies, RTX administration and its adverse events
| Author | Year | Previous immunosuppressive therapies | RTX administration | Adverse events |
|---|---|---|---|---|
| Cortazar | 2019 | Pred, CsA, FK, MMF, Abat, Cyc, Aza | 9 g (7.5–11 g), 1 g IV dose, 4-month interval | 1 cellulitis, 1 Clostridium difficile colitis |
| Fenoglio | 2018 | NA | 4 × 375 mg/m2, 1-week interval | None |
| Iwabuchi | 2018 | Pred, CsA, FK, MMF, Cyc, MZ | 4 × 375 mg/m2, 6-month interval | 19.7% infusion reactions, 1 neutropenia |
| Katsuno | 2018 | Pred, CsA, FK, MMF, Cyc, MZ | 1 × 500 mg (3), 2 × 500 mg (3), 3 × 500 mg (1), 3100 mg (1, seven times) | 1 hypotension |
| DaSilva | 2017 | Pred, CsA, FK, MMF, Cyc | 1 × 1 g (10), 2 × 1 g (9), 3 × 1 g (5), 4 × 1 g (4), 1788 ± 704 mg | 1 chills, 1 skin rash |
| King | 2017 | Pred, CsA, FK, MMF, Cyc, Lev, rapamycin, sirolimus | 2 × 1 g, 2 weeks apart | None |
| Ren | 2017 | Pred, CsA, FK, MMF, Cyc, Aza | 4 × 375 mg/m2, 1-week interval | None |
| Roccatello | 2017 | NA | 8 × 375 mg/m2, 1-week interval | NA |
| Brown | 2017 | Pred, CsA, FK, MMF, Cyc | 2 × 1g 2–3 weeks interval | Upper respiratory infection (3), neutropenia, disorientation and confusion 2 weeks after infusion |
| Papakrivopoulou | 2016 | Pred, CsA, FK, MMF, Cyc, Aza, Lev | 2 × 1 g, 6 months apart, 1–3 g | 1 lower respiratory tract infection, 1 gastroenteritis, 7 Type I hypersensitivity reactions |
| Dekkers | 2015 | Pred, CsA, FK, MMF, Cyc | 2 × 375 mg/m2 | 1 allergic reaction |
| Miyabe | 2015 | Pred, CsA, FK, MMF, Cyc, MZ | 4 × 375 mg/m2 (25), 6-month interval | 1 neutropenia, 1 agranulocytosis mild infusion reactions occurred in 31 patients (57 %): itching, exanthema and pharyngalgia |
| Bruchfeld | 2014 | Pred, CsA, FK, MMF, Cyc, MZ | 2 × 500 mg (8), 2 × 1 g (3), 3 × 375 mg/m2 (1), 4 × 375 mg/m2 (4) | 1 hypotension, 1 itchy and red eye |
| Guitard | 2014 | Pred, CsA, FK, MMF, Cyc, Aza | 1 × 1 g (1), 2 × 1 g (21), 2 × 1 g (5), 3 × 375 mg/m2 (2), 4 × 375 mg/m2 (12) | 2 dyspnea, 1 thoracic pain, 2 urinary tract infections |
| Iwabuchi | 2014 | Pred, CsA, FK, MMF, Cyc, MZ | 4 × 375 mg/m2, 6-month interval | 9 infusions reactions (36%) such as cough and hiccups; 1 exanthema, 1 leukopenia |
| Ruggenenti | 2014 | Pred, CsA, Cyc, Aza, MMF | 1 × 375 mg/m2 or 2 × 375 mg/m2 | 5 infections, 1 seizure, 1 biliary colic, 1 melanoma |
| Takei | 2013 | Pred, MMF, CsA, MZ | 2 × 375 mg/m2 (25), 6-month interval | 5 infusion reactions such as cough and hiccough, 1 leukopenia, 1 exanthema |
| Munyentwali | 2013 | Pred, CsA, FK, MMF, Cyc, Aza, Lev, chlorambucil | 1 × 375 mg/m2 (1), 2 × 375 mg/m2 (7), 3 × 375 mg/m2 (4), 4 × 375 mg/m2 (3) | None |
| Kronbichler | 2013 | Pred, CsA, MMF | 4 × 375 mg/m2 | NA |
| Kong | 2012 | Pred, CsA, FK, MMF, Cyc, Aza, chlorambucil | 1 × 500 mg, 1 × 600 mg, 4 × 600 mg, 1 × 700 mg, 2 × 700 mg | 1 bronchopneumonia, infusion reactions (skin rash, throat irritation, chest tightness, difficulty in breathing, hypotension, bradycardia and body ache) |
| Hoxha | 2011 | Pred, CsA, FK, MMF, Cyc, Aza, Lev | 1 × 375 mg/m2 | None |
Abat, abatacept; Aza, azathioprine; CsA cyclosporine; Cyc, cyclophosphamide; FK, tacrolimus; Pred, prednisone; Lev, levamisole; MZ, mizoribine; NA, not available; IV, intravenous.
Qualities of included studies
Quality rating for each study was evaluated by the NOS scale (Supplementary data, Table S1). More than half of the included studies scored seven stars or more, which indicated moderate qualities [16, 21–26, 28–30, 32, 34–41]. In domains of selection and comparability, all studies with single arms did not fulfill the selection of the nonexposed cohort except for DaSilva et al.’s [35] study. The comparability was satisfied in most studies due to study controls for comparisons before and after. In domains of outcome assessment, all included studies [16, 21–26, 28–30, 32, 34–41] were awarded full stars.
Complete remission rate to RTX treatment
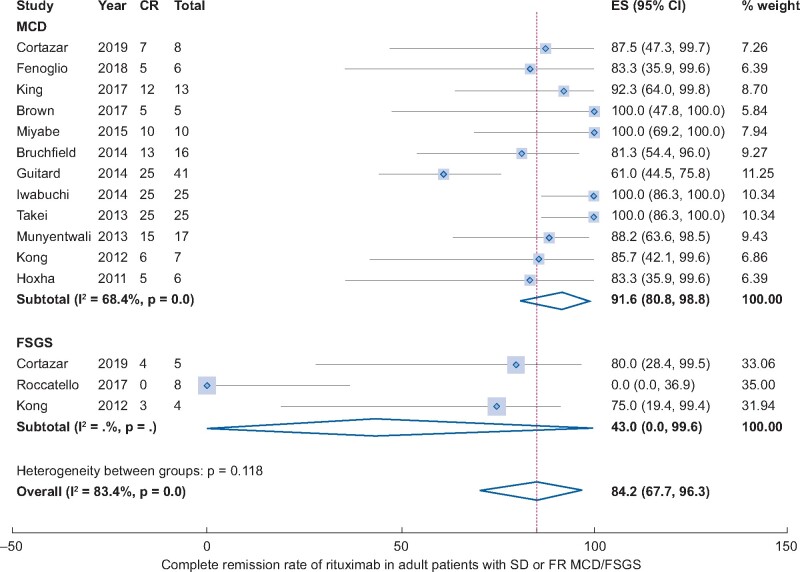
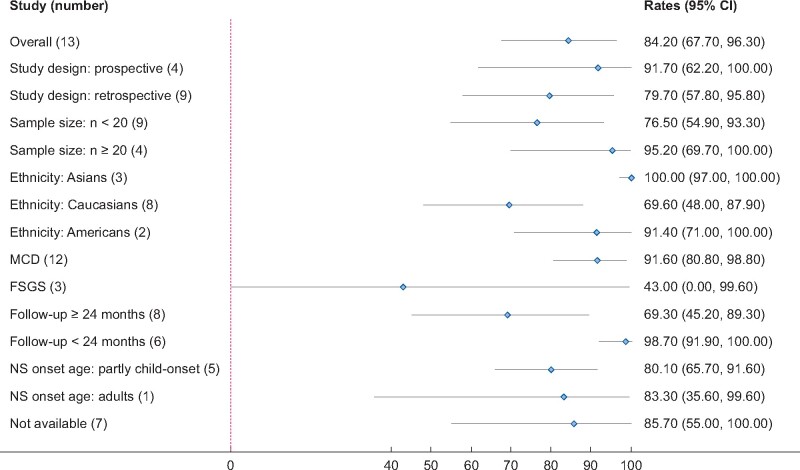
All included studies [16, 21–26, 28–30, 32, 34–41] addressed the rate of CR in adult MCD/FSGS patients. However, eight studies with patients who were already in remission could not be included in the meta-analysis. The pooled rate of CR after RTX treatment was 84.2% (13 studies, 95% CI 67.7–96.3%, Figure 2) adjusted by sample size. Heterogeneity was high, with I2 = 83.4%. There was no significant publication bias (Begg’s test: P = 0.625 and Egger’s test: P = 0.94, Supplementary data, Figure S1). Results of subgroup analyses were shown in Figure 3. Subgroup analysis by histologic diagnosis found different results MCD had a much higher CR rate compared with FSGS (91.6% versus 43%), although there was no statistical significance due to the large 95% CI in FSGS group. The CR rates were 100.0% in Asians (95% CI 97.0–100.0%), 69.6% in Caucasians (95% CI 48–87.9%) and 91.4% in Americans (95% CI 71.0–100.0%). Prospective studies were associated with a higher CR rate compared with retrospective studies (91.7% versus 79.7%). Studies with longer follow-up time (>24 months) were associated with a lower CR rate compared with studies with follow-up time <24 months (69.3% versus 98.7%, P < 0.01). Studies with different sample sizes and NS onset-age showed similar CR rates. Sensitivity analysis found similar results.
FIGURE 2.
CR rate of RTX in adult patients with FR or SD MCD/FSGS.
FIGURE 3.
Subgroup analysis of CR rate of RTX in adult patients with FR or SD MCD/FSGS.
Relapse rate post-RTX treatment
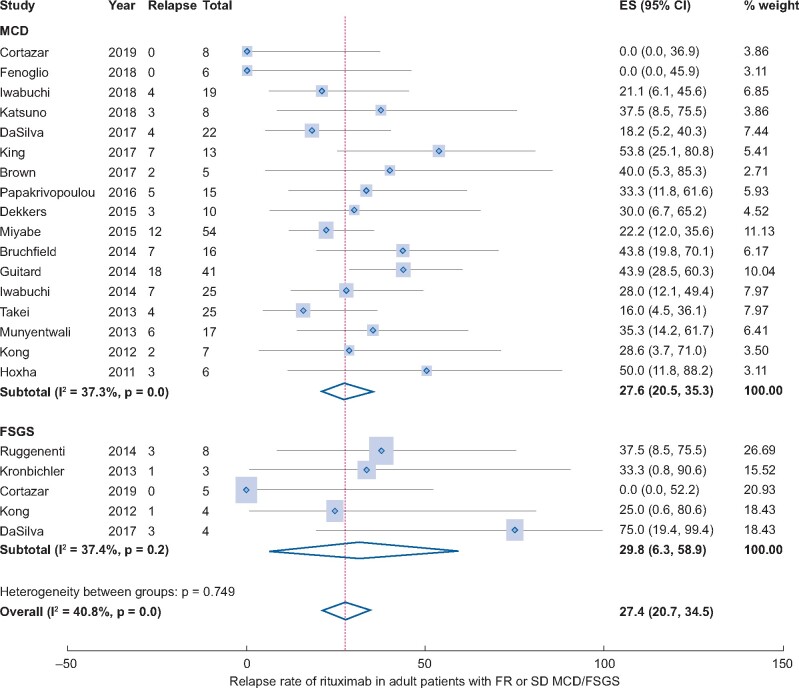
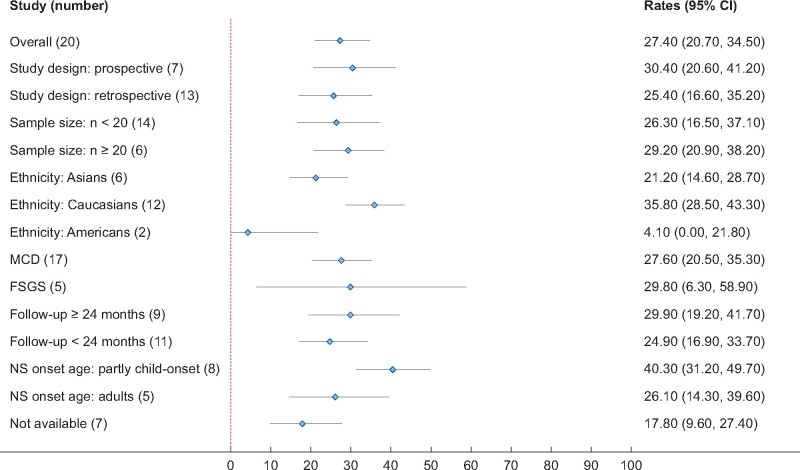
All studies [16, 21–26, 28–30, 32, 34–41] addressed the rate of relapse post-RTX treatment except the study of Roccatello et al. [16]. The pooled rate of relapse was 27.4% (20 studies, 95% CI 20.7–34.5%, Figure 4) adjusted by sample size. Heterogeneity was moderate, with I2 = 40.8%. There were no significant publication bias (Begg’s test: P = 1.00 and Egger’s test: P = 0.995, Supplementary data, Figure S2). Results of subgroup analyses were shown in Figure 5. Subgroup analysis by histologic diagnosis found similar results (MCD: 27.6% versus FSGS: 29.8%). The relapse rates were 21.2% in Asians (95% CI 14.6–28.7%), 35.8% in Caucasians (95% CI 28.5–43.3%) and 4.1% in Americans (95% CI 0.0–21.8%). The relapse rate in prospective studies was similar to retrospective studies (30.4% versus 25.4%). Studies with longer follow-up time (>24 months) were associated with a higher relapse rate (29.9% versus 24.9%). Studies with different sample sizes showed similar CR rates. Studies including the child- and adult-onset NS had a higher relapse rate compared with studies including only adult-onset NS (40.3% versus 26.1%). Results were stable in sensitivity analysis.
FIGURE 4.
Relapse rate of RTX in adult patients with FR or SD MCD/FSGS.
FIGURE 5.
Subgroup analysis of relapse rate of RTX in adult patients with FR or SD MCD/FSGS.
PR rate and NR rate to RTX treatment
We further addressed the rate of PR post-RTX treatment. The pooled rate of PR was 5.8% (13 studies, 95% CI 1.2–12.5%, Supplementary data, Figure S3) adjusted by sample size. Heterogeneity was moderate, with I2 = 44.7%. There was no significant publication bias by Begg’s test: P = 0.392 or by Egger’s test: P = 0.848, Supplementary data, Figure S4. The pooled rate of NR was 5.2% (13 studies, 95% CI 0.0–15.0%, Supplementary data, Figure S5). Heterogeneity was moderate, with I2 = 73.1%. There was no significant publication bias by Begg’s test: P = 0.542 or by Egger’s test: P = 0.998, Supplementary data, Figure S6.
Yearly relapse rate change, proteinuria change and steroid dose change post-RTX treatment
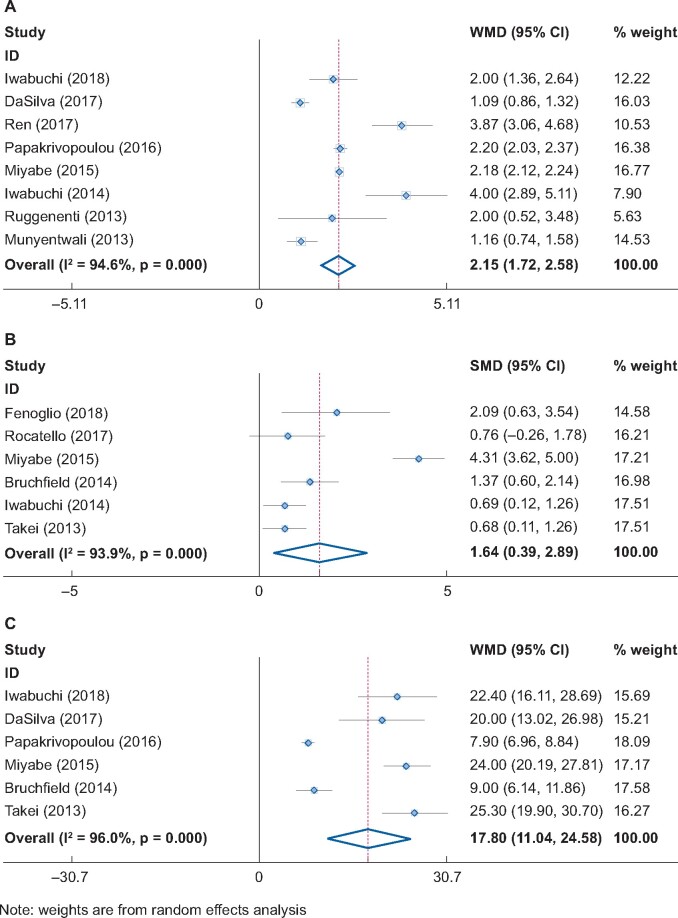
Pooled comparison of the number of relapses per year before and after RTX treatment showed that the relapse rate significantly decreased by 2.15 times/year (eight studies, 95% CI 1.72–2.58 times/year, P < 0.001, Figure 6A). RTX also significantly reduced the steroid dose by 17.8 mg/day (six studies, 95% CI 11.04–24.56 mg/day, P < 0.001, Figure 6B). Moreover, RTX significantly decreased the level of proteinuria or albuminuria in MCD/FSGS patients (six studies, SMD = 1.64, 95% CI 0.39–2.89, P < 0.01, Figure 6C).
FIGURE 6.
Yearly relapse rate change, proteinuria change and steroid dose change post-RTX treatment.
Relapse time and CD20+ B-cell repletion during relapse
Fourteen studies reported the relapse month post-RTX treatment with a wide range (1–74 months). Most of the relapses happened after 6 months in European studies (Table 3). However, relapses often occurred within the first 6 months in Asian studies that used 500 mg RTX 6 months apart. Seven studies addressed the CD20+ B-cell repletion during relapse post-RTX treatment (Table 3). Most of them found an association between CD20+ B-cell recovery and the relapse. CD19+ B-cell reappeared preceding the relapse with a median time 2 months (0–14) in Guitard et al.’s study [38]. However, several patients did not relapse on the reappearance of CD19+ cells [38]. More trials are needed to confirm this finding.
Table 3.
Remission, relapse and benefits of RTX treatment
| Author | Year | Sample size | R (n) | RT | Relapse time (months) | B-cell repletion during R, yes/no | Pre-relapse rate (times/year) | Post-relapse rate (times/year) | Pred dose pre-RTX (mg/day) | Pred dose post-RTX (mg/day) | Proteinuria/ACR pre-RTX (g/day) | Proteinuria/ACR post-RTX (g/day) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cortazar | 2019 | 13 | 0 | NA | NA | NA | NA | NA | 60 (40–60) | 3.75 (0–5) | 9.0 (4.9–12.1) | 0.1 (0.1–0.6) |
| Fenoglio | 2018 | 6 | 0 | NA | NA | NA | NA | NA | NA | NA | 11.75 ± 7.76 | 0.28 ± 0.46 |
| Iwabuchi | 2018 | 19 | 4 | 6 | NA | NA | 2.15 ± 1.4 | 0.15 ± 0.3 | 23.2 ± 13.9 | 0.8 ± 1.6 | 0.09 (0–0.78) | 0 (0–0.03) |
| Katsuno | 2018 | 8 | 3 | 3 | 8.1, 10, 13.6 | Yes (2/3) | 1 (1–3) | 0 (0–1) | 20 (10–27.5) | 5.5 (2.5–7) | 0.10 (0.02–0.42) | NA |
| DaSilva | 2017 | 28 | 8 | 15 | 15 (8–26) | NA | 1.1 ± 0.63 | 0.01 ± 0.02 | 24 ± 18 | 4.0 ± 5.6 | 7.1 ± 3.5 | NA |
| King | 2017 | 13 | 7 | NA | 10 (1–11) | NA | 4 (2–6) | 0.4 (0–0.9) | NA | NA | 0.06 (0–0.4) | NA |
| Ren | 2017 | 15 | 2 | 2 | 8,10 | NA | 4 ± 1.6 | 0.13 | NA | NA | 1.6 (0.05–5.76) | NA |
| Roccatello | 2017 | 8 | NA | NA | NA | NA | NA | NA | No use | NA | 5.3 ± 1.9 | 3.9 ± 1.8 |
| Brown | 2017 | 5 | 2 | 4 | 8, 33, 36, 74 | NA | NA | NA | 37 ± 19.4 | NA | 3.93 ± 2.78 | NA |
| Papakrivopoulou | 2016 | 15 | 5 | 7 | 4, 12, 19, 25 | Yes (5/7) | 2.60 ± 0.28 | 0.4 ± 0.19 | 8 ± 1.85 | 0 ± 0 | 0.043 ± 0.023 | NA |
| Dekkers | 2015 | 10 | 3 | 4 | 21, 21, 24, 36 | NA | NA | NA | NA | NA | NA | NA |
| Miyabe | 2015 | 54 | 12 | 12 | <6 (7), 6–12 (4), 12–24 (1) | Yes (12/12) | 2.2 ± 0.2 | 0.02 ± 0.1 | 24.7 ± 14.1 | 0.7 ± 2.2 | 1.3 ± 0.3 | 0.008 ± 0.3 |
| Bruchfeld | 2014 | 16 | 7 | NA | 9, 12, 13, 18, 20, 28 | Yes (2/5) | NA | NA | 10 ± 5 | 1 ± 3 | 0.43 ± 0.39 | 0.04 ± 0.1 |
| Guitard | 2014 | 41 | 18 | NA | 18 (3–36) | Yes (16/18) | NA | NA | NA | NA | 1.3 (0–23) | NA |
| Iwabuchi | 2014 | 25 | 7 | 8 | <6 (6), 6–12 (2) | NA | 4.3 ± 2.8 | 0.3 ± 0.5 | 24 (5–60) | 0.6 (0–5) | 2.5 ± 4.9 | 0 ± 0 |
| Ruggenenti | 2014 | 20 | 8 | NA | 1–12 | NA | 2.5 (2–4) | 0.5 (0–1) | 16 (11.4–36) | 0 (0–13.8) | 0.13 (0.08–0.30) | 0.15 (0.10–0.22) |
| Takei | 2013 | 25 | 4 | 4 | <6 (3), 6–12 (1) | Yes (4/4) | 1 | 0.16 | 26.4 ± 13.5 | 1.1 ± 2.8 | 2.5 ± 3.5 | 0.5 ± 2.2 |
| Munyentwali | 2013 | 17 | 6 | 9 | 11.9 (4.8–16.3) | Yes (5/6) | 1.32 ± 0.85 | 0.16 ± 0.21 | 40 (10–70) | 5.2 (0–30) | 0.06 (0.002–0.6) | NA |
| Kronbichler | 2013 | 5 | 1 | 1 | 23 | NA | NA | NA | 30 ± 17.9 | NA | 3.4 ± 3.4 | NA |
| Kong | 2012 | 11 | 3 | 4 | NA | NA | 0.44 | 0.24 | NA | NA | NA | NA |
| Hoxha | 2011 | 6 | 3 | 3 | 4–12 | Yes | NA | NA | NA | NA | 1.5 (0.7–7.3) | 0 |
Y, yes; n, no; OR, overall remission; R, relapse; NA, not available; ACR, Urinary microalbumin creatinine ratio.
Comparison with other immunosuppressive drugs
One study [35] compared RTX with other immunosuppressive drugs in multiple centers retrospectively. Twenty-eight patients received RTX as an additional treatment, while the other 22 patients did not receive RTX as the control group [35]. After a median of 31 month follow-up, RTX effectively reduced the relapse rate and the need for maintenance immunosuppressive drugs compared with the control group.
Adverse events
Eighteen studies reported adverse events of RTX treatment (Table 2). Tolerance of RTX seemed well, and the most common complications were infusion reactions such as chills, skin rash, allergic reaction, hypotension, thoracic pain, dyspnea, bradycardia and body ache. Infectious episodes or hematological toxicity were the main adverse events during follow-ups, such as respiratory infections, urinary tract infections and neutropenia.
DISCUSSION
In this systemic review and meta-analysis, we included 21 studies involving 382 adults with SD or FR MCD/FSGS according to the predesigned criteria. RTX treatment induced a pooled 84.2% CR rate overall in adult patients with SD or FR MCD/FSGS, while MCD patients had a high 91.6% CR rate and FSGS patients a moderate 43% CR rate. Several studies have suggested that MCD patients were more likely to respond to RTX than FSGS. This is in concurrence with the effects of other immunosuppressants such as prednisone in MCD and FSGS. In FSGS it is harder to achieve CR than in MCD because of the more severe damage of glomeruli than MCD.
In terms of the rate of relapse after RTX treatment, there were about 27.4% of patients with at least a relapse during the follow-up (MCD: 27.6% versus FSGS: 29.8%). Therefore, additional RTX treatment was needed while the patients had a relapse. Detailed dose and time of RTX reuse is determined by the situation of each patient. The pooled PR and NR rates were 5.8% and 5.2%, respectively, treated with RTX, which were both relatively low. The pre- and post-RTX treatment comparison suggested that RTX can significantly decrease the yearly relapse rate, steroid dose and proteinuria level. Relapse time after RTX treatment is various in different races. Safety analysis indicated that RTX was associated with good tolerance.
The mechanism of RTX in treating MCD and FSGS remains unclear; there may be a correlation between B-cell recovery and disease replapsing. There are not enough data supporting the assumption that relapse rate is B-cell count dependent. The main hypothesis for the development of podocyte injury in MCD and FSGS is the abnormally increased circulating permeability factors produced by dysregulated T cells. RTX may disrupt the B cell–T cell interactions that ultimately lead to the production of permeability factors. Furthermore, in our experience, we found that the numbers of CD4+ or CD8+ T cells and natural killer (NK) cells might change as an immune compensation. Therefore, RTX may not only delete most B cells but also change the immune system and environment, which creates a buffer period for kidney repair in MCD/FSGS patients. In addition, the podocyte cytoskeleton may be a direct target for RTX through a B-lymphocyte-independent mechanism [42]. The different effects of RTX observed in patients with SD and SR NS suggested the possibility of distinct mechanisms. Giving the role of B cells and T helper (Th) cells interaction, in the future, new therapeutics on MCD or FSGS may be developed targeting Th cells.
The subgroup results of CR rate were discrepant. When followed up for >1 year, the CR rate decreased significantly. That is reasonable, since the long-term CR rate is naturally lower than short-term CR. We also found that CR in adult-onset patients was a little higher; that may be because the morphological damage in these patients was not as severe as in the child-onset ones. In the subgroup analysis with study design, we revealed that CR in prospective studies was higher compared with the retrospective ones. We posited that in retrospective study design, patients with CR were more likely lost to follow-up. Furthermore, CRs in different ethnicities tended to be higher in Asians compared with Caucasians. Perhaps the sensibility and usage of RTX in different ethnicities could partly explain the result. More appropriate usage of RTX for different ethnicities is needed, as is the case for usages of tacrolimus and cyclosporine.
Observation studies revealed that cyclosporine may induce 10–75% remission rate and high relapse rate (60–80%) [5]. With our searching strategy, there was little research containing data comparing RTX with other immune suppressers directly. More studies comparing RTX with other immune suppressers are needed in the future. Moreover, it could be inferred that multitarget therapy with RTX and immune suppressers combination may lead to a better effect. In this case, the dose and subsequent toxicity of the combined-used immune suppressers may also decrease. However, further evidence is needed to prove our hypothesis.
Our study has several strengths. First, most of the studies included were of good methodological quality, which makes the pooled results unlikely to be affected by bias. Second, in most studies, the subjects were followed up for long enough to examine the relapse. In addition, appropriate subgroup analyses were performed, which means the sources of heterogeneity were examined. In addition, we used sensitivity analyses to test the robustness of the results. However, this research has several limitations. First, most of the studies were retrospective, which was not the preferred study design for examining the efficacy and safety of RTX, and due to the short-term observation period, the long-term adverse events could not be observed. Second, the sample size of included studies was limited. Although we performed subgroup analysis according to the sample size, the results still need to be validated in the future. Third, we are also interested in the comparison of RTX with other immunosuppressants; however, the lack of the original data do not support the analyses. Fourth, it is hard to discuss safety without evidence of adverse events for a long-term period. For example, progressive multifocal leukoencephalopathy may be a rare adverse event by RTX [43]. Last but not the least, the definition of the relapse or PR was slightly different according to the different studies. That may be one of the sources of heterogeneity which could not be detected by subgroup analyses.
A recently published network meta-analysis examined the efficacy and acceptability of immunosuppressants (including RTX) in pediatric NS patients [44]. The included subjects were not necessarily MCD or FSGS patients. The investigators revealed that RTX may be acceptable medications for children with FR or SD NS. For the pediatric SR NS patients, RTX also showed a favorable effect, according to another meta-analysis [14]. For adult FSGS/MCD patients, Kronbichler and Bruchfeld systematically reviewed the effect of RTX in 2014, but no safe conclusion was drawn due to the lack of evidence [45]. After that, more clinical studies were published and included in our study. Compared with Kronbichler and Hansrivijit’s systematic reviews [15, 46], we included more updated publications and also synthesized more comprehensive results. Not only in MCD and FSGS, but also another high profile clinical trial investigating RTX on membranous nephropathy, also suggested that RTX was noninferior to cyclosporine [47].
In summary, by pooling the results of pilot studies, RTX may be an effective and safe choice in most adult patients with FR or SD FSGS/MCD, although about 27% of patients may suffer from relapses. More clinical trials comparing the RTX with other immunosuppressants are needed.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
FUNDING
National Natural Science Foundation of China (81700579, 81873595, 81970640 and 81670612). Shanghai Natural Science Foundation (19ZR1400100), Shanghai Top Priority Key Clinical Disciplines Construction Project (2017ZZ02009), Shanghai Science and Technology Talents Program (19YF1450300), Research Projects of Shanghai Traditional Medicine (ZHYY-ZXYJHZX-2-201713) and Research Projects of Shanghai Science and Technology Committee (17411972100). Funders and their institution had no role in study design; collection, analysis and interpretation of data; writing the report and the decision to submit the report for publication.
AUTHORS’ CONTRIBUTIONS
Research idea and study design was by J.X. and C.-G.X.; data acquisition was performed by C.X. and B.Y.; data analysis/interpretation was done by L.Z., Z.C., C.X. and B.Y.; statistical analysis was carried out by X.G. and B.D.; and supervision or mentorship was provided by C.M., Z.M., S.Y., J.X. and C.-G.X. Each author contributed important intellectual content during manuscript drafting or revision, accepts personal accountability for the author’s contributions and agrees to ensure that questions about the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST STATEMENT
None declared.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
REFERENCES
- 1. Siligato R, Cernaro V, Nardi C. et al. Emerging therapeutic strategies for minimal change disease and focal and segmental glomerulosclerosis. Expert Opin Investig Drugs 2018; 27: 839–879 [DOI] [PubMed] [Google Scholar]
- 2. Kronbichler A, Leierer J, Oh J. et al. Immunologic changes implicated in the pathogenesis of focal segmental glomerulosclerosis. BioMed Res Int 2016; 2016: 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bertelli R, Bonanni A, Di Donato A. et al. Regulatory T cells and minimal change nephropathy: in the midst of a complex network. Clin Exp Immunol 2016; 183: 166–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chapter 5: minimal-change disease in adults. Kidney Int Supp l 2012; 2: 177–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chapter 6: idiopathic focal segmental glomerulosclerosis in adults. Kidney Int Suppl 2012; 2: 181–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beer A, Mayer G, Kronbichler A.. Treatment strategies of adult primary focal segmental glomerulosclerosis: a systematic review focusing on the last two decades. BioMed Res Int 2016; 2016: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beck LH, Bonegio RGB, Lambeau G. et al. M-type phospholipase A 2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 2009; 361: 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jayne D. Role of rituximab therapy in glomerulonephritis. J Am Soc Nephrol 2010; 21: 14–17 [DOI] [PubMed] [Google Scholar]
- 9. Edwards JCW, Szczepański L, Szechiński J. et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 2004; 350: 2572–2581 [DOI] [PubMed] [Google Scholar]
- 10. Smith KGC, Jones RB, Burns SM. et al. Long-term comparison of rituximab treatment for refractory systemic lupus erythematosus and vasculitis: remission, relapse, and re-treatment. Arthritis Rheum 2006; 54: 2970–2982 [DOI] [PubMed] [Google Scholar]
- 11. Pravitsitthikul N, Willis NS, Hodson EM. et al. Non-corticosteroid immunosuppressive medications for steroid-sensitive nephrotic syndrome in children. Cochrane Database Syst Rev 2013; 10: CD002290. [DOI] [PubMed] [Google Scholar]
- 12. Tan L, Li S, Yang H. et al. Efficacy and acceptability of immunosuppressive agents for pediatric frequently-relapsing and steroid-dependent nephrotic syndrome. Medicine 2019; 98: e15927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iijima K, Sako M, Kamei K. et al. Rituximab in steroid-sensitive nephrotic syndrome: lessons from clinical trials. Pediatr Nephrol 2018; 33: 1449–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jellouli M, Charfi R, Maalej B. et al. Rituximab in the management of pediatric steroid-resistant nephrotic syndrome: a systematic review. J Pediatr 2018; 197: 191–197.e1 [DOI] [PubMed] [Google Scholar]
- 15. Hansrivijit P, Cheungpasitporn W, Thongprayoon C. et al. Rituximab therapy for focal segmental glomerulosclerosis and minimal change disease in adults: a systematic review and meta-analysis. BMC Nephrol 2020; 21: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roccatello D, Sciascia S, Rossi D. et al. High-dose rituximab ineffective for focal segmental glomerulosclerosis: a long-term observation study. Am J Nephrol 2017; 46: 108–113 [DOI] [PubMed] [Google Scholar]
- 17. de Jong MC, Kors WA, de Graaf P. et al. The incidence of trilateral retinoblastoma: a systematic review and meta-analysis. Am J Ophthalmol 2015; 160: 1116–1126.e5 [DOI] [PubMed] [Google Scholar]
- 18. Ampt FH, Willenberg L, Agius PA. et al. Incidence of unintended pregnancy among female sex workers in low-income and middle-income countries: a systematic review and meta-analysis. BMJ Open 2018; 8: e021779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605 [DOI] [PubMed] [Google Scholar]
- 20. Zhou C, Gu Y, Mei C. et al. Dialysis modality and mortality in polycystic kidney disease. Hemodial Int 2018; 22: 515–523 [DOI] [PubMed] [Google Scholar]
- 21. Brown LC, Jobson MA, Payan Schober F. et al. The evolving role of rituximab in adult minimal change glomerulopathy. Am J Nephrol 2017; 45: 365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Katsuno T, Masuda T, Saito S. et al. Therapeutic efficacy of rituximab for the management of adult-onset steroid-dependent nephrotic syndrome: a retrospective study. Clin Exp Nephrol 2019; 23: 207–214 [DOI] [PubMed] [Google Scholar]
- 23. King C, Logan S, Smith SW. et al. The efficacy of rituximab in adult frequently relapsing minimal change disease. Clin Kidney J 2017; 10: 16–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kong WY, Swaminathan R, Irish A.. Our experience with rituximab therapy for adult-onset primary glomerulonephritis and review of literature. Int Urol Nephrol 2013; 45: 795–802 [DOI] [PubMed] [Google Scholar]
- 25. Kronbichler A, König P, Busch M. et al. Rituximab in adult patients with multi-relapsing/steroid-dependent minimal change disease and focal segmental glomerulosclerosis: a report of 5 cases. Wien Klin Wochenschr 2013; 125: 328–333 [DOI] [PubMed] [Google Scholar]
- 26. Miyabe Y, Takei T, Iwabuchi Y. et al. Amelioration of the adverse effects of prednisolone by rituximab treatment in adults with steroid-dependent minimal-change nephrotic syndrome. Clin Exp Nephrol 2016; 20: 103–110 [DOI] [PubMed] [Google Scholar]
- 27. Munyentwali H, Bouachi K, Audard V. et al. Rituximab is an efficient and safe treatment in adults with steroid-dependent minimal change disease. Kidney Int 2013; 83: 511–516 [DOI] [PubMed] [Google Scholar]
- 28. Papakrivopoulou E, Shendi AM, Salama AD. et al. Effective treatment with rituximab for the maintenance of remission in frequently relapsing minimal change disease. Nephrology 2016; 21: 893–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ren H, Lin L, Shen P. et al. Rituximab treatment in adults with refractory minimal change disease or focal segmental glomerulosclerosis. Oncotarget 2017; 8: 93438–93443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ruggenenti P, Ruggiero B, Cravedi P. et al. Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol 2014; 25: 850–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takei T, Itabashi M, Moriyama T. et al. Effect of single-dose rituximab on steroid-dependent minimal-change nephrotic syndrome in adults. Nephrol Dial Transplant 2013; 28: 1225–1232 [DOI] [PubMed] [Google Scholar]
- 32. Bruchfeld A, Benedek S, Hilderman M. et al. Rituximab for minimal change disease in adults: long-term follow-up. Nephrol Dial Transplant 2014; 29: 851–856 [DOI] [PubMed] [Google Scholar]
- 33. Hoxha E, Stahl RAK, Harendza S.. Rituximab in adult patients with immunosuppressive-dependent minimal change disease. Clin Nephrol 2011; 76: 151–158 [DOI] [PubMed] [Google Scholar]
- 34. Cortazar FB, Rosenthal J, Laliberte K. et al. Continuous B-cell depletion in frequently relapsing, steroid-dependent and steroid-resistant nephrotic syndrome. Clin Kidney J 2019; 12: 224–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. DaSilva I, Huerta A, Quintana L et al.; Spanish Group for the Study of Glomerular Diseases (GLOSEN) et al. Rituximab for steroid-dependent or frequently relapsing idiopathic nephrotic syndrome in adults: a retrospective, multicenter study in Spain. BioDrugs 2017; 31: 239–249 [DOI] [PubMed] [Google Scholar]
- 36. Dekkers MJ, Groothoff JW, Zietse R. et al. A series of patients with minimal change nephropathy treated with rituximab during adolescence and adulthood. BMC Res Notes 2015; 8: 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fenoglio R, Sciascia S, Beltrame G. et al. Rituximab as a front-line therapy for adult-onset minimal change disease with nephrotic syndrome. Oncotarget 2018; 9: 28799–28804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guitard J, Hebral A-L, Fakhouri F. et al. Rituximab for minimal-change nephrotic syndrome in adulthood: predictive factors for response, long-term outcomes and tolerance. Nephrol Dial Transplant 2014; 29: 2084–2091 [DOI] [PubMed] [Google Scholar]
- 39. Iwabuchi Y, Miyabe Y, Makabe S. et al. Comparison of the response of frequently relapsing steroid-dependent minimal change nephrotic syndrome to rituximab therapy between childhood-onset and adult-onset disease. Medicine 2018; 97: e12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Iwabuchi Y, Takei T, Moriyama T. et al. Long-term prognosis of adult patients with steroid-dependent minimal change nephrotic syndrome following rituximab treatment. Medicine 2014; 93: e300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sugiura H, Takei T, Itabashi M. et al. Effect of single-dose rituximab on primary glomerular diseases. Nephron Clin Pract 2011; 117: c98–c105 [DOI] [PubMed] [Google Scholar]
- 42. Fornoni A, Sageshima J, Wei C. et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med 2011; 3: 85ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Berger JR, Malik V, Lacey S. et al. Progressive multifocal leukoencephalopathy in rituximab-treated rheumatic diseases: a rare event. J Neurovirol 2018; 24: 323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kamei K, Ogura M, Sato M. et al. Infusion reactions associated with rituximab treatment for childhood-onset complicated nephrotic syndrome. Pediatr Nephrol 2018; 33: 1013–1018 [DOI] [PubMed] [Google Scholar]
- 45. Kronbichler A, Bruchfeld A.. Rituximab in adult minimal change disease and focal segmental glomerulosclerosis. Nephron Clin Pract 2014; 128: 277–282 [DOI] [PubMed] [Google Scholar]
- 46. Kronbichler A, Kerschbaum J, Fernandez-Fresnedo G. et al. Rituximab treatment for relapsing minimal change disease and focal segmental glomerulosclerosis: a systematic review. Am J Nephrol 2014; 39: 322–330 [DOI] [PubMed] [Google Scholar]
- 47. Fervenza FC, Appel GB, Barbour SJ. et al. Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med 2019; 381: 36–46 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.