Abstract
The left ventricle (LV) is affected in 20–25% of patients with sarcoidosis and its involvement is associated with morbidity and mortality. However, effects of sarcoidosis on the right ventricle (RV) are not well documented. Our aims were to investigate the prevalence of RV dysfunction in patients with sarcoidosis and determine whether it is predominantly associated with direct cardiac involvement, severity of lung disease, or pulmonary hypertension (PH). We identified 50 patients with biopsy-proven extra-cardiac sarcoidosis and preserved LV function, who underwent echocardiography, pulmonary function (PF) testing, and cardiovascular magnetic resonance. RV function was quantified by free wall longitudinal strain. Tricuspid valve Doppler and estimated right atrial pressure were used to estimate systolic pulmonary artery pressure. Myocardial late gadolinium enhancement was considered diagnostic for cardiac sarcoidosis and assumed to involve both ventricles. Of the 50 patients, 28 (56%) had RV dysfunction, 4 with poorly defined PF status. Of the remaining 24 patients, 16 (67%) had lung disease, 8 (33%) had PH, and 10 (42%) had LV involvement. Ten patients had greater than one of these findings, and 4 had all 3. In contrast, in 4/24 patients (17%), RV dysfunction could not be explained by these mechanisms, despite severely reduced RV strain. In conclusion, RV dysfunction is common in patients with sarcoidosis and is usually associated with either direct LV involvement, lung disease, or PH, but may occur in the absence of these mechanisms, suggesting the possibility of isolated RV involvement and underscoring the need for imaging protocols that would include RV strain analysis.
Keywords: cardiac sarcoidosis, right ventricle, magnetic resonance imaging, delayed gadolinium enhancement, echocardiography, myocardial strain
Sarcoidosis is a systemic disorder characterized by noncaseating granulomatous infiltration that most commonly affects the lungs and lymph nodes. However, autopsy data suggest that a major cause of death is cardiac arrhythmia and heart failure due to myocardial infiltration.1 Manifestations of cardiac involvement can range from no symptoms to advanced heart failure or even sudden cardiac death. Regions of granulomatous infiltration are thought to evolve into scar tissue that serves as a substrate for reentry ventricular tachycardia2 and atrial arrhythmias.3,4 Cardiovascular magnetic resonance (CMR) with late gadolinium enhancement (LGE) imaging has been shown to readily identify cardiac involvement that is not otherwise clinically recognized, because of its ability to detect even small areas of myocardial damage.5 Recent studies have shown that even in the absence of left ventricular (LV) dysfunction, over 15% of patients with sarcoidosis have LGE, indicating cardiac involvement,6 and are at significantly increased risk of death and sustained ventricular arrhythmia. It is also known that cardiac sarcoidosis (CS) can directly involve the right ventricle in addition to the left ventricle and that the presence of right ventricular (RV) dysfunction is associated with worse prognosis.7 However, the prevalence or underlying mechanisms of RV involvement in patients with CS have not been well established. Furthermore, the assessment of RV function or free wall infiltration is challenging and commonly is not part of the clinical paradigm used to evaluate patients with sarcoidosis for cardiac involvement.
Despite the clinical utility of CMR for the detection and risk stratification of CS, the 2014 Heart Rhythm Society consensus document8 does not recommend its routine use as a screening test for identifying individuals with CS, given the lack of availability and cost of a CMR examination. Rather they recommend that patients with sarcoidosis undergo a thorough medical history and physical evaluation, as well as electrocardiogram and echocardiogram to screen for cardiac involvement. However, it is well known that these examinations in their current implementation have a poor diagnostic sensitivity for the detection of CS.9 There is a clinical need to identify other variables from these screening tests that might increase the detection rate of CS. We hypothesized that echocardiographic assessment of RV function using novel deformation indices, such as RV free wall longitudinal strain, might aid in the diagnosis of CS. Accordingly, the aims of our study were as follows: (1) to investigate the prevalence of RV dysfunction in patients with biopsy-proven extra-cardiac sarcoidosis and preserved LV function; (2) to better define the prevalence of the different underlying mechanisms of the RV dysfunction in this patient population; and (3) to use this information to identify patients who would benefit from further evaluation.
Methods:
Study Population and Design:
We retrospectively identified 50 patients (41 women, age 58 ± 11 year) with biopsy-proven extra-cardiac sarcoidosis and preserved LV function (ejection fraction >50% on CMR) who were referred for three tests, including echocardiography, pulmonary function, and CMR to evaluate for suspected CS. Echocardiography findings were used to identify patients with RV dysfunction (defined as abnormal RV free wall strain) and those with PH (defined as elevated pulmonary artery pressure). CMR findings were used to identify patients with CS (defined by LGE in the left ventricle). Results of pulmonary function testing were used to identify patients with lung disease. None of the study patients had implanted defibrillators.
This information was used to determine the proportion of patients with RV dysfunction among those with extra-cardiac sarcoidosis, as well as the distribution of the potential common mechanisms of RV involvement in cardiac sarcoidosis, that is, pulmonary hypertension, lung disease, LV sarcoid involvement (as evidenced by LV LGE), to better understand the etiology of RV dysfunction in this disease.
Demographics, clinical characteristics, and CMR-derived parameters of LV and RV size and function of the study patients are shown in Table I. Institutional Review Board approval was obtained for this study.
TABLE I.
Demographics, Clinical Characteristics, and CMR-Derived Parameters of Left and Right Ventricular Size and Function of the 50 Study Patients
| Gender (F) | 41 (82) |
| Age (years) | 54 ± 11 |
| African American | 34 (68) |
| Caucasian | 11 (22) |
| Duration of disease (years) | 15 ± 11 |
| Number of organs involved | 1.9 ± 1.1 |
| AVB/LBBB/RBBB on ECG | 8 (16) |
| Immunomodulators | 24 (48) |
| Steroids | 26 (52) |
| Hypertension | 29 (58) |
| Hyperlipidemia | 10 (20) |
| Diabetes | 9 (18) |
| LV EDVi (mL/m2) | 68 ± 14 |
| LV ESVi (mL/m2) | 27 ± 7 |
| LV EF (%) | 60 ± 5 |
| RV EDVi (mL/m2) | 74 ± 17 |
| RV ESVi (mL/m2) | 36 ± 13 |
| RV EF (%) | 54 ± 9 |
Numbers (and percentages) are listed for categorical variables, while means ± SD are shown for continuous variables. LV = left ventricular; RV = right ventricular; EF = ejection fraction; EDVi, ESVi = end-diastolic and end-systolic volume index, respectively; F = female; AVB = atrioventricular block; LBBB = left bundle branch block; RBBB = right bundle branch block.
Echocardiographic Evaluation of RV Function and Pulmonary Artery Pressure:
Comprehensive transthoracic 2D and Doppler evaluation was performed using the iE33 imaging system equipped with an S5 transducer (Philips Healthcare, Andover, MA, USA). Digital cine loops of standard echocardiographic views plus an RV-focused apical 4-chamber view were used. This view allows the visualization of the RV free wall throughout the cardiac cycle, which is crucial for accurate assessment of RV free wall strain analysis using speckle tracking, which is considerably more difficult in the standard apical 4-chamber view. Images were digitally stored for offline analysis (Xcelera, Philips).
Conventional echocardiographic parameters of RV systolic function, including tricuspid annular plane systolic excursion (TAPSE), RV fractional area change (FAC), tissue Doppler tricuspid annular peak systolic velocity (S’), were measured. FAC was measured in the RV-focused apical 4-chamber view as: (end-diastolic area − end-systolic area)/end-diastolic area × 100. In addition, the RV-focused views were used to measure global free wall strain using dedicated software (EchoInsight, Epsilon Imaging, Ann Arbor, MI, USA). To this effect, RV endocardial border was manually traced at end systole. A region of interest was then automatically generated and wall thickness was adjusted to optimize tracking. An automated speckle tracking algorithm was then applied throughout the cardiac cycle. Longitudinal strain curves were generated for the RV free wall and the peak value was measured. Strain above −20% (i.e., <20% in magnitude) was considered abnormal and used as indicative of RV dysfunction.10
Tricuspid valve Doppler and estimated right atrial pressure by inferior vena cava size and collapsibility were used to calculate peak systolic pulmonary artery pressure (SPAP) using standard methodology.10 SPAP > 35 mmHg was used as a cutoff for the presence of PH.
CMR Determination of CS:
CMR was performed using a 1.5T scanner (Achieva, Philips Healthcare, Best, The Netherlands) with a five-channel flexible surface coil. Standard imaging protocol included steady-state free precession cines in 3 long-axis (2-, 3-, and 4-chamber views) planes, along with a stack of short-axis slices spanning the ventricles from base to apex (retrospectively gated, repetition time 2.9 ms, echo time 1.5 ms, flip angle 60°, temporal resolution 25–40 ms). LGE images of the same views were obtained 10 minutes after infusion of gadodiamide or gadobenate dimeglumine (0.1–0.2 mmol/kg) using a T1-weighted gradient echo pulse sequence with a phase-sensitive inversion recovery reconstruction (repetition time 4.5 ms, echo time 2.2 ms, inversion time 200–300 ms, flip angle 30°, phase-sensitive inversion recovery flip angle 5°, voxel size 2 × 2 × 10 mm, sense factor 1–2).
Philips Extended MR Workspace software was used to review the images and detect LV LGE. CS was defined as the presence of any LGE, that is, if any LV myocardium had a signal intensity >5 SD above the mean signal intensity of normal remote myocardium. No attempt was made to determine the presence of RV LGE because the voxel size used in the imaging protocol was not adequate to reliably differentiate increase in RV signal from partial volume effects caused by epicardial fat from actual LGE.
Pulmonary Function Testing:
The presence and severity of lung disease was determined by a pulmonologist using the following parameters: distribution of pulmonary sarcoidosis (using Scadding stage11) and pulmonary function test (PFT) parameters (%predicted total lung capacity, forced expiratory volume [FEV1], forced vital capacity [FVC], FEV1/FVC ratio, and diffusing capacity for carbon monoxide). For the purposes of this study, lung disease was defined as FEV1 and DLCO <80%.
Data Analysis:
Patients with RV dysfunction were counted and proportion of the total study group was calculated. Of these, the proportions of patients with the commonly implicated mechanisms of RV dysfunction, including CS, PH, and lung disease, were calculated. In addition, the number and proportion of patients who had more than one of these findings was calculated for each combination of these findings. Finally, patients with RV dysfunction but without any of the above mechanisms of RV dysfunction were identified. All echocardiographic measures of RV systolic function, including TAPSE, RV FAC, S’ velocity, as well as RV strain, were averaged for each subgroup of patients. Significance of difference in these mean values was tested using two-tailed Student’s t-tests.
Reproducibility Analysis:
Longitudinal free wall strain measurements were repeated in a randomly selected group of 30 patients by two experienced readers for purposes of reproducibility analysis. This included repeated measurements by the same observer, at least 1 month later, as well as measurements by a second independent observer, both blinded to all prior measurements. Inter-observer variability and intra-observer variability were calculated as an absolute difference between the corresponding pair of repeated measurements as a percentage of their mean in each patient and then averaged over the entire group.
Results:
Figure 1 shows examples of CMR images obtained in two patients with sarcoidosis and RV dysfunction: one without cardiac involvement and the other with LGE in the LV myocardium, indicative of CS. Of the 50 patients, 16 (32%) had myocardial LGE, while the remaining 34 (68%) patients did not. Figure 2 shows examples of RV free wall longitudinal strain time curves obtained in two patients with CS (namely LGE): one with normal RV function and the other with RV dysfunction. RV dysfunction as reflected by reduced peak longitudinal free wall strain was noted in 28/50 patients (56%), of whom four patients did not have conclusive PFT studies to allow for definitive determination of their pulmonary status.
Figure 1.
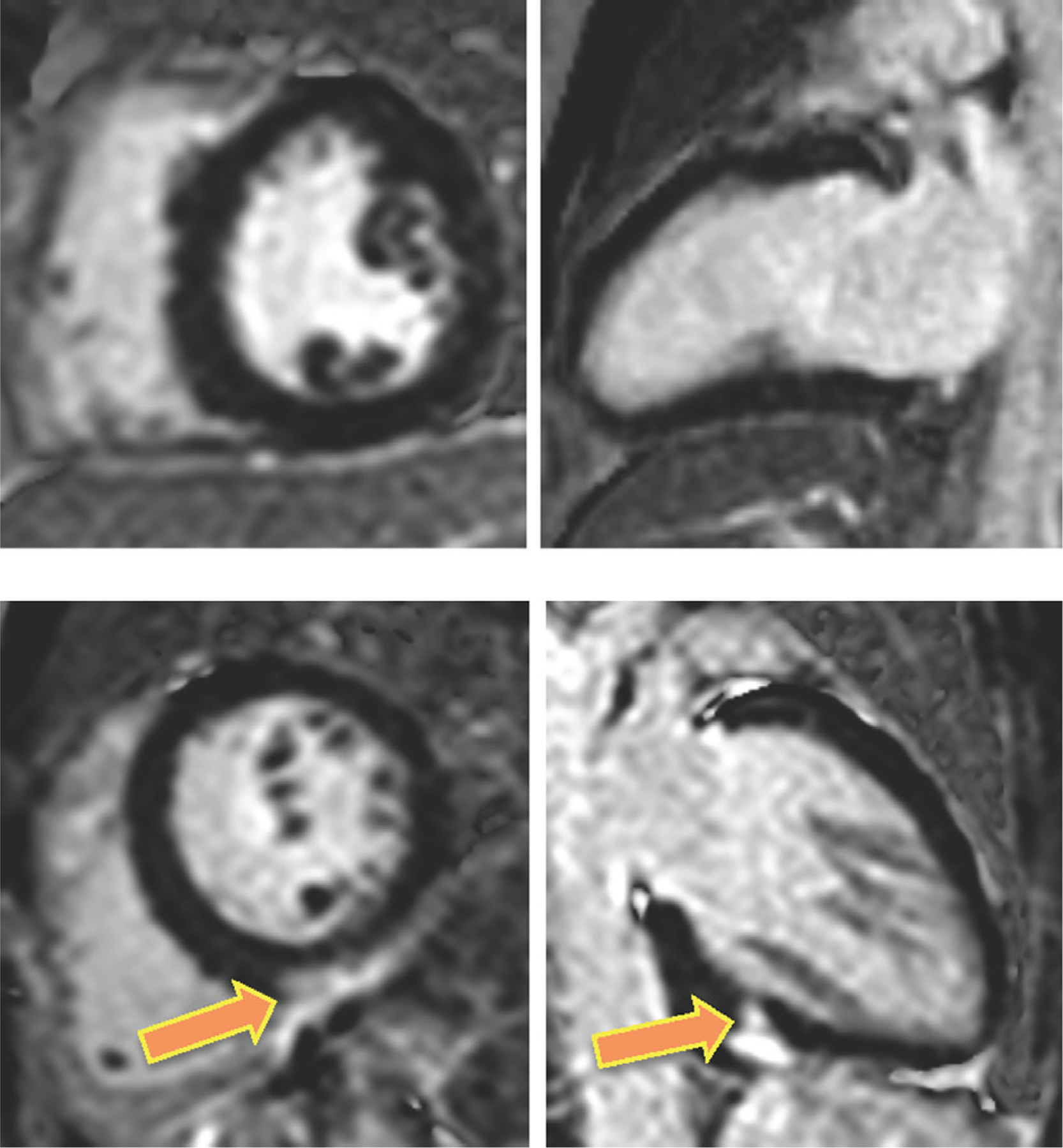
CMR images obtained in two patients with extra-cardiac sarcoidosis and RV dysfunction: one without cardiac involvement (top) and the other with LGE in the LV myocardium (bottom), indicative of CS. Note atypical mid-inferior pattern of LGE in this latter patient (arrows).
Figure 2.
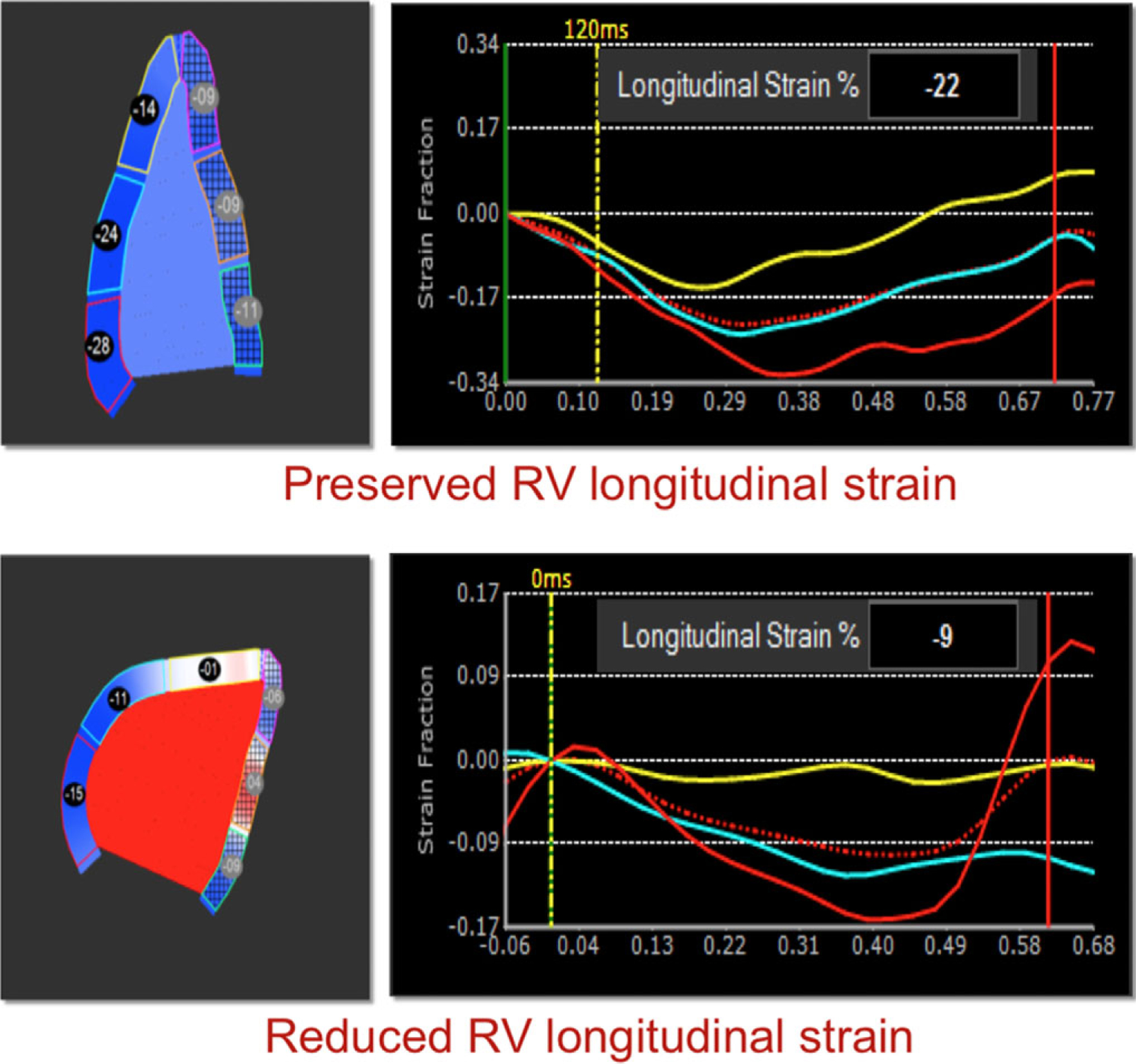
Examples of RV free wall longitudinal strain analysis in two patients with CS (namely LGE): one with normal RV function (top) and the other with RV dysfunction (bottom). RV dysfunction is reflected by reduced peak strain derived from the time curves (right).
Of the remaining 24 patients with reduced peak longitudinal free wall strain, 16 (67%) had lung disease, 8 (33%) had PH, and 10 (42%) had cardiac involvement. Ten patients (42%) had more than one of these findings, and 4 (17%) had all three (Fig. 3). Four of the 24 patients (17%) had RV dysfunction that could not be explained by any of the three mechanisms studied, that is, did not have either lung disease, PH, or LGE in the LV myocardium.
Figure 3.
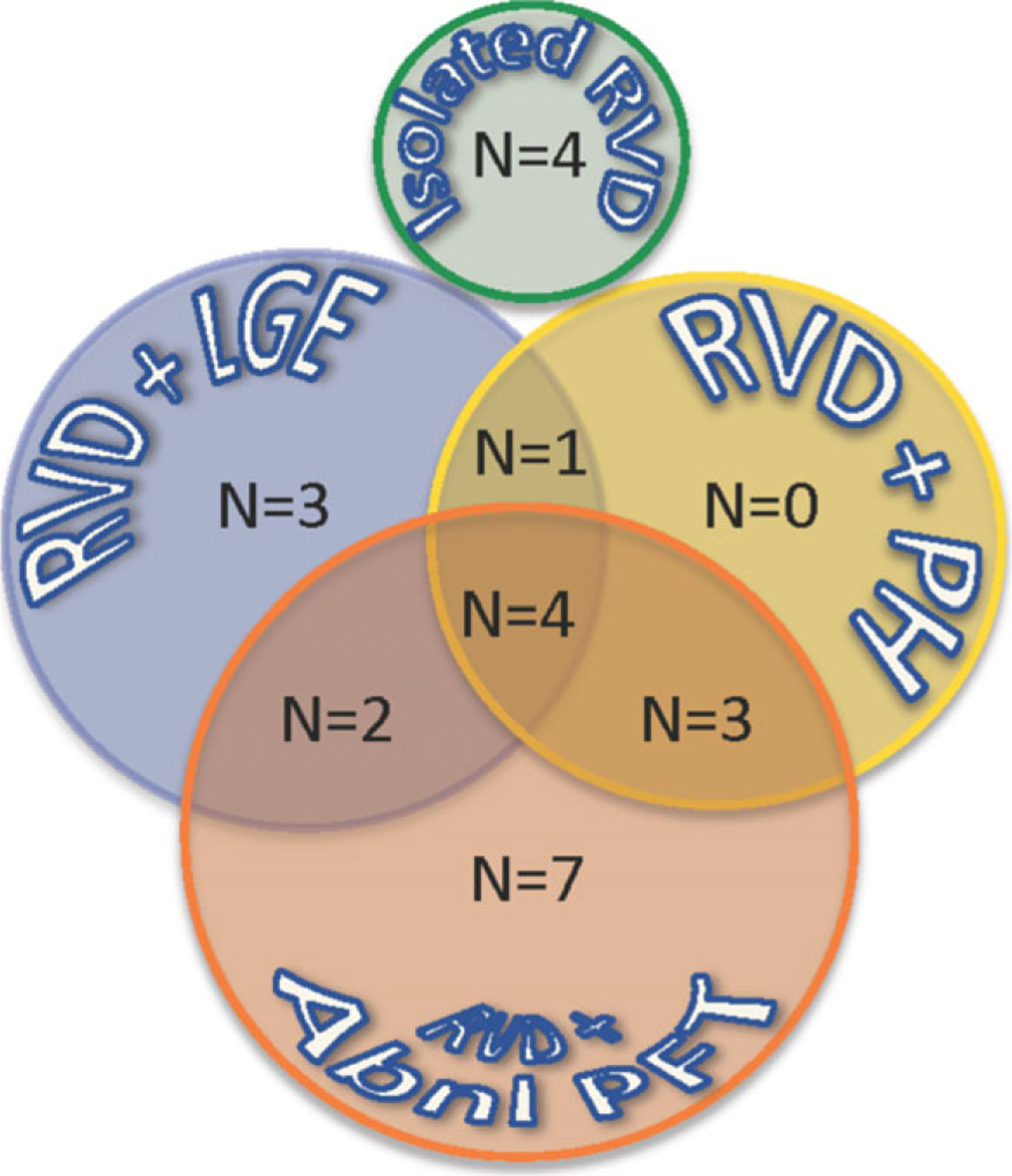
Bubble diagram depicting associated findings in 28 patients with sarcoidosis and RV dysfunction (RVD): late gadolinium enhancement (LGE), pulmonary hypertension (PH), and abnormal pulmonary function tests (PFTs). Numbers in the bubble indicate the number of patients in each subcategory, including those who had more than one of the findings shown in the overlapping areas.
Determination of RV dysfunction based on additional echocardiographic parameters, including fractional area change, TAPSE, and RV S’ according to the potential mechanisms of RV dysfunction, is shown in Table II. The values of all three conventional parameters of RV function were similar, irrespective of the mechanism of RV dysfunction. Similarly, RV strain was almost identical in patients with lung disease, PH, and LV sarcoid involvement. In contrast, in small number of patients, RV strain was significantly reduced, despite the absence of all aforementioned mechanisms of RV dysfunction.
TABLE II.
Echocardiographic Measurements of Right Ventricular (RV) Function Obtained in Patients with Sarcoidosis and Abnormal RV Function as Defined by Reduction in Magnitude of Peak Longitudinal Free Wall Strain. Data are Summarized Separately for Patients with Lung Disease, Pulmonary Hypertension, and Left Ventricular Sarcoid Involvement Evidenced by Late Gadolinium Enhancement, as Well as Patients with None of the Above Findings (See Text for Detail)
| N | TAPSE (mm) | RV S’ (cm/sec) | RV FAC (%) | Free Wall Strain (%) | |
|---|---|---|---|---|---|
| Lung disease | 16 | 14 ± 5 | 7.5 ± 2 | 25 ± 8 | −14.3 ± 3.8 |
| Pulmonary hypertension | 8 | 16 ± 4 | 7.8 ± 1.7 | 26 ± 9 | −14.1 ± 3.2 |
| LV sarcoid involvement | 10 | 17 ± 2 | 8.2 ± 1.3 | 25 ± 10 | −14.5 ± 3.5 |
| None of the above | 4 | 16 ± 6 | 13.3 ± 5.1 | 22 ± 10 | −6.0 ± 11.9* |
LV = left ventricular; RV = right ventricular; TAPSE = tricuspid annular plane systolic excursion, FAC = fractional area change, S’ = tissue Doppler tricuspid annular peak systolic velocity.
P < 0.02 versus all three other categories.
Reproducibility analysis for RV free wall strain resulted in inter-observer variability of 9.4 ± 8.3% and intra-observer variability of 8.6 ± 7.3%.
Discussion:
Cardiac sarcoidosis is a leading cause of death in patients with sarcoidosis. Studies looking at the nature of cardiac involvement have largely implicated LV dysfunction and LGE burden in the morbidity and mortality of these patients. More recent work, however, has provided some initial insight in regard to RV effects on prognosis and outcomes.
We hypothesized that individuals with sarcoidosis may commonly have echocardiographic evidence of RV dysfunction due to a variety of mechanisms, even in the presence of preserved LV function. To test this hypothesis, we studied patients with biopsy-proven extra-cardiac sarcoidosis who had normal LV EF and had been referred for additional testing including echocardiography, pulmonary function testing, and CMR. Patients with EF < 50% were excluded, as these patients would clearly warrant further evaluation for CS. RV dysfunction was diagnosed utilizing RV free wall longitudinal strain by echocardiography but also characterized using conventional echocardiographic parameters. Ultimately, these patients were categorized into those having RV dysfunction secondary to pulmonary hypertension, lung disease, or LV LGE, to better understand the etiology of RV dysfunction.
We found that in the majority of patients with extra-cardiac sarcoidosis and preserved LV function, RV dysfunction could be attributed to either lung disease, PH, or cardiac involvement as defined by LV LGE, or a combination of these mechanisms. Interestingly, however, we found that in 17% of these patients, RV dysfunction could not be explained by any of the above mechanisms. This finding raises the question of the prevalence and clinical significance of isolated RV dysfunction.
Sarcoidosis, a multiorgan disorder, involving granulomatous infiltration of various tissues, is a complex disease from diagnosis to management. More specifically, CS has a myriad of clinical complications that span from arrhythmias to heart failure and sudden cardiac death.12–14 Autopsy findings have shown myocardial involvement in at least 25% of patients, which could be underestimated due to the microscopic nature of the involvement.1 Conclusive findings on endomyocardial biopsy can be pathognomonic for CS; however, most often have low diagnostic yield, largely due to its patchy distribution.15 Therefore, noninvasive tools have been extensively studied to identify sensitive parameters reflecting cardiac infiltration. On echocardiography, classic features of CS include regional wall motion abnormalities and wall thinning, usually of the basal anterior septum. While regional wall thinning may be highly suggestive in the clinical scenario appropriate for CS, it is a relatively uncommon finding. The modified guidelines for the diagnosis of CS based on the Japanese Ministry of Health and Welfare (JMHW) criteria include echo findings that show abnormal wall motion, regional wall thinning, or dilation of the left ventricle.5 Kim et al reported the diagnostic yield of myocardial abnormalities based on late gadolinium enhancement CMR was twice as sensitive as that based on consensus guidelines of the JMHW criteria.5 Similarly, our group has looked at the risk of death and significant ventricular arrhythmias in patients with CS and preserved EF and found that none of the patients with adverse events in the cohort we studied met the JMHW criteria for CS.16 These observations have led to the use of alternative, more sensitive imaging-based parameters for the detection of CS.
Consequently, evaluation of the RV involvement has become increasingly important in its potential role in patients with CS. A recent study focusing on RV LGE in patients with CS and LV EF > 35% found that RV involvement was predictive of adverse events and arrhythmogenesis.7 In that study, increased extent of RV LGE was also associated with the greatest risk of arrhythmias. Similarly, an analysis by our group has shown that increased LV LGE burden in patients with CS and preserved EF correlates well with adverse events, for which every 1% increase of LGE burden is associated with a 16% increase in death or significant ventricular arrhythmia. In patients with increasing LGE burden, there was a reduction in RV EF and increase in RV end-systolic volume.16
As echocardiography is often times the initial study of choice in patients suspected to have CS, we hoped to identify an echocardiography-derived parameter that would allow early detection of RV dysfunction in these patients. As previously described, RV dysfunction has been found to independently have adverse affects in patients with CS and is additive to LV LGE in its ability to risk stratify these individuals. In our study, we found that RV dysfunction identified by reduced free wall longitudinal strain was present in more than half of the patients with extra-cardiac sarcoidosis. When present, reduced RV strain was almost always associated with PH, abnormal pulmonary function, or LV LGE. Importantly, in the presence of RV dysfunction, more than 1/3 of the patients had LV LGE. Ultimately, the presence of abnormal RV longitudinal strain on echocardiography in patients with extra-cardiac sarcoidosis should be closely evaluated, as this finding is often associated with MRI predictors of poor outcomes, namely LV LGE.
Interestingly, several patients had isolated RV dysfunction that could not be explained by the commonly known mechanisms. Of note, in these patients, RV strain was severely reduced. Although statistically significant, this finding needs to be interpreted cautiously because of the small number of patients in this category. Nevertheless, this finding may be a signal that isolated RV dysfunction may indeed exist in sarcoidosis, and may perhaps signify subclinical disease that could potentially predict future granulomatous infiltration and fibrosis of the LV and RV myocardium. Improvements in current CMR protocols are needed to confidently identify individuals with RV LGE (Fig. 4), to visually confirm myocardial sarcoid infiltration in the RV free wall. Our findings in this study along with recent work on the effects of LV LGE and RV dysfunction on prognosis support the use of early echocardiographic testing, including RV strain analysis in patients with extra-cardiac sarcoidosis and preserved LV EF. Reduced RV free wall strain on echocardiography should prompt referral to CMR even when other etiologies for RV dysfunction are present.
Figure 4.
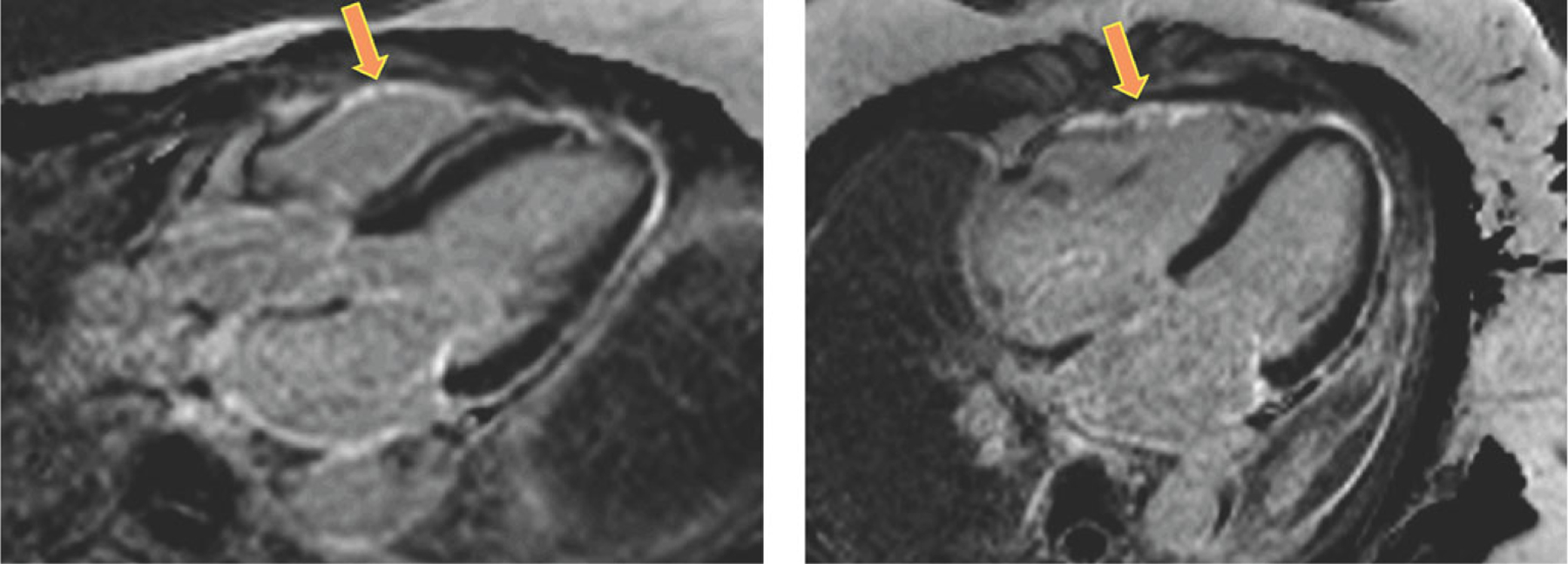
CMR images obtained in a patient with extra-cardiac sarcoidosis and RV dysfunction, without LGE in the left ventricular myocardium, who had LGE in the right ventricular free wall (arrows). Of note, this finding could not be used to diagnose RV involvement with a similar level of confidence in other study patients.
Limitations:
One limitation of our study is its retrospective nature. Indeed, our findings should be considered hypothesis generating, because the data were not specifically collected to address the study goals. However, in this case, if the study had been conducted prospectively, CMR imaging would have been performed using the same protocol and the same standard settings, and the images would not have been any different. Moreover, the quantitative analysis of these images was performed specifically for the purposes of the research study by a trained investigator, who did not rely on any existing data.
Another limitation is the challenge in identifying LGE of the RV myocardium, which is likely secondary to the fact that the RV free wall is thin and to the limited in-plane and through-plane resolution of the LGE images. For this reason, we did not include RV LGE assessment in this study as a way to increase our own confidence in our results.
Conclusions:
RV dysfunction, as defined by RV longitudinal strain, is a common entity in patients with sarcoidosis and preserved LV function, and is often associated with the presence of LV LGE. Because echocardiography is recommended as one of the initial screening tests in patients with extra-cardiac sarcoidosis and clinically suspected cardiac involvement, RV strain analysis may be useful. Specifically, abnormal RV strain should prompt a referral to CMR LGE imaging even when other mechanisms of RV dysfunction are present. Furthermore, severely reduced RV strain in patients without PH or lung disease may indicate isolated RV involvement, which warrants further investigation, even when no LV LGE is detected.
References
- 1.Silverman KJ, Hutchins GM, Bulkley BH: Cardiac sarcoid: A clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 1978;58:1204–1211. [DOI] [PubMed] [Google Scholar]
- 2.Banba K, Kusano KF, Nakamura K, et al. : Relationship between arrhythmogenesis and disease activity in cardiac sarcoidosis. Heart Rhythm 2007;4:1292–1299. [DOI] [PubMed] [Google Scholar]
- 3.Cain MA, Metzl MD, Patel AR, et al. : Cardiac sarcoidosis detected by late gadolinium enhancement and prevalence of atrial arrhythmias. Am J Cardiol 2014;113:1556–1560. [DOI] [PubMed] [Google Scholar]
- 4.Viles-Gonzalez JF, Pastori L, Fischer A, et al. : Supraventricular arrhythmias in patients with cardiac sarcoidosis prevalence, predictors, and clinical implications. Chest 2013;143:1085–1090. [DOI] [PubMed] [Google Scholar]
- 5.Patel MR, Cawley PJ, Heitner JF, et al. : Detection of myocardial damage in patients with sarcoidosis. Circulation 2009;120:1969–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel AR, Klein MR, Chandra S, et al. : Myocardial damage in patients with sarcoidosis and preserved left ventricular systolic function: An observational study. Eur J Heart Fail 2011;13:1231–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crawford T, Mueller G, Sarsam S, et al. : Magnetic resonance imaging for identifying patients with cardiac sarcoidosis and preserved or mildly reduced left ventricular function at risk of ventricular arrhythmias. Circ Arrhythm Electrophysiol 2014;7:1109–1115. [DOI] [PubMed] [Google Scholar]
- 8.Birnie DH, Sauer WH, Bogun F, et al. : HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 2014;11:1305–1323. [DOI] [PubMed] [Google Scholar]
- 9.Freeman AM, Curran-Everett D, Weinberger HD, et al. : Predictors of cardiac sarcoidosis using commonly available cardiac studies. Am J Cardiol 2013;112:280–285. [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Badano LP, Mor-Avi V, et al. : Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39. e14. [DOI] [PubMed] [Google Scholar]
- 11.Scadding JG: Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Br Med J 1961;2:1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koplan BA, Soejima K, Baughman K, et al. : Refractory ventricular tachycardia secondary to cardiac sarcoid: Electrophysiologic characteristics, mapping, and ablation. Heart Rhythm 2006;3:924–929. [DOI] [PubMed] [Google Scholar]
- 13.Mehta D, Mori N, Goldbarg SH, et al. : Primary prevention of sudden cardiac death in silent cardiac sarcoidosis: Role of programmed ventricular stimulation. Circ Arrhythm Electrophysiol 2011;4:43–48. [DOI] [PubMed] [Google Scholar]
- 14.Uusimaa P, Ylitalo K, Anttonen O, et al. : Ventricular tachyarrhythmia as a primary presentation of sarcoidosis. Europace 2008;10:760–766. [DOI] [PubMed] [Google Scholar]
- 15.Orii M, Imanishi T, Akasaka T: Assessment of cardiac sarcoidosis with advanced imaging modalities. Biomed Res Int 2014;2014:897956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel K, Murtagh G, Lafin L, et al. : Global longitudinal strain can risk stratify patients with suspected cardiac sarcoidosis. J Am Soc Echocardiogr 2015;28:B18–B19 (Abstract). [Google Scholar]


