Abstract
Tubal and peritoneal disease are the main causes of infertility. Tubal pathology can be either congenital malformation or acquired, proximal or distal, unilateral or bilateral and transient or permanent. Several imaging methods such as laparoscopy, fluoroscopy, saline infusion sonography, and hysterosalpingography (HSG) have been used in the assessment of tubal and peritoneal pathology. Although laparoscopy is the modality of choice for investigating tubal patency and pelvic structure in many infertility centers, HSG is usually the initial diagnostic method for infertility workup because of its ease of performance, accuracy, and minimal risk of complications. This method provides useful information about size, contour, and anatomy of the inner surface of the fallopian tubes and is the gold standard for evaluation of tubal lumen. Tubal and peritubal pathology show various imaging manifestations on HSG. This review illustrates the radiographic features of congenital and acquired structural abnormalities of the proximal tubal pathology and along with etiology of proximal obstruction or occlusion will be described.
Introduction
Evaluation of fallopian tubal patency is a very important part of a routine infertility workup. Tubal and peritoneal disease are the main causes of infertility and account for 40% of all infertility problems. 1
Normal anatomy and physiology of the fallopian tube are necessary for many reproductive functions such as sperm transport and capacitation, ovum pick up and transport, fertilization, nourishment, transport and the first stages of embryo development.
A wide spectrum of disorders may interfere with appropriate function of the tubes. Tubal blockage can be either congenital malformation or acquired abnormalities that include spasms, polyp, mucus plugs, infection, scarring, endometriosis, or other factors. 2 Tubal pathology may occur in any part of the tube – the proximal, mid portion, distal, or multiple sites – and may be transient (obstruction) or permanent (occlusion), unilateral or bilateral. 3
Obstruction implies a time-limited process, which may be reversible. Mechanical causes of tubal obstruction include tubal spasm or plugging by amorphous material.
Tubal occlusion is a permanent organic damage, such as salpingitis isthemica nodosa (SIN), tuberculosis and hydrosalpinx. 3
Imaging plays a significant role in the diagnostic evaluation of infertility. The current methods of tubal patency assessment include laparoscopy with chromopertubation, fluoroscopy, saline infusion sonography, and hysterosalpingography (HSG). Laparoscopy is widely considered the ‘‘gold standard’’ method for investigating tubal patency and pelvic structure in many infertility centers. 4 However, laparoscopy is an expensive, invasive procedure that is associated with operative risks. Moreover, the procedure requires special surgical skills and equipment.
HSG has frequently been used as an alternative for evaluation of intrauterine disorders and tubal patency. The procedure is usually the initial diagnostic method for the evaluation of infertile females with suspected tubal disease because of its ease of performance, accuracy, and minimal risk of complications. 5 This method provides useful information about size, contour, and anatomy of the inner surface of the fallopian tubes, allowing detection of tubal patency, tubal occlusion, and peritubal disorders due to peritubal adhesion or deep infiltrating endometriosis. 6 HSG is still the golden standard for evaluation of tubal lumen. 7
HSG is considered to have a sensitivity of 72–85% and a specificity of 68–89% in the diagnosis of tubal patency compared to laparoscopy as the gold standard. 8
The specificity of HSG in the diagnosis of pelvic inflammatory disease (PID)-related tubal occlusion achieves 90%. 9
False-negative diagnoses are mainly the result of the inability of the radiographic examination to diagnose peritubal disease, especially adhesions. 10 False-positive diagnosis results largely from misinterpreting tubal caliber and shape as a sign of adnexal disease and partial filling of the tube as obstruction.
We retrospectively reviewed 41 407 hysterosalpingograms performed over a 31-year period (January 1985–December 2015) by one author (GS). The indications for HSG included abnormal uterine bleeding, infertility, and symptoms related to uterine fibroids.
This review illustrates the radiographic features of congenital and acquired structural abnormalities of the proximal tubal pathology and describes the etiology of proximal obstruction or occlusion.
The normal fallopian tube
Normal gross anatomy
At six weeks of development, the paired paramesonephric ducts (Müllerian ducts) form from the coelomic epithelium. The cranial portions of the paramesonephric ducts develop into the fallopian tubes, and the caudal portions fuse to form the uterus.
The normal fallopian tubes are approximately 10–12 cm in length and usually less than 1 cm in diameter. They arise from the uterus at the fundal cornua and extend laterally. Fallopian tubes are situated in the mesosalpinx, which is the upper free border of the broad ligament of the uterus. 11 Each tube consists of three layers – the mucosa, a muscular layer, and the serosa. 11 The muscularis layer consists of two layers of smooth muscle, an outer longitudinal and an inner circular layer. The mucosa joins directly on the muscularis and has many folds and papillae. The tube can be divided into four segments with different functional and anatomic distinctions: interstitial (uterotubal), isthmus, ampulla, and fimbriated.
The interstitial segment is the narrowest portion of the tube. It measures from 1.5 to 2.5 cm in length, with an average luminal diameter of 100 µm. 12 Each tube passes through the uterine wall and forms the tubal opening at the endometrial cavity, called the ostium. The interstitial segment begins at the uterotubal ostium and ends at the uterotubal junction (UTJ). The arrangement of the muscles at the UTJ works like a sphincter, which relaxes or contracts to tubal or uterine distension, respectively.
The isthmic portion measures 2–3 cm in length and the narrow lumen is about 1 mm in diameter. 11 It is a rounded part of the fallopian tube.
The ampulla is thin-walled and the widest portion of the tube. It is about 5 cm long and is where fertilization of the ovum takes place. Diameter of the larger ampulla varies from 2 to 12 mm (mean: 5 mm). 11 The fimbriated (infundibulum), which is the end of the tube that is funnel-shaped, terminates at a small orifice surrounded by numerous fimbriae.
Radiographic anatomy
Each fallopian tube is radiographically divided into three segments. The interstitial (intramural or corneal region) is the short segment and its lumen shows a thread-like appearance. The UTJ may show a shadow-free zone or focal constriction that corresponds to the tubal sphincter (Figure 1).
Figure 1.

Different parts of normal fallopian tubes. 1. Intramural, 2. Isthmus, 3. Ampulla (arrows). The pear-shaped zone (black arrow) that corresponds to tubal sphincters are seen at the uterotubal junction (UTJ).
The isthmic portion has a thin lumen with a diameter of 1–2 mm and may assume various directions. It is the cordlike portion of the tube. The shadow of this part of the tube is usually narrow and straight, but the outlines are somewhat curled.
The ampullary portion is the dilated region next to the ovary. The shadow of the ampullary portion is broad and tortuous. Diameter of the larger ampulla varies from 2 to 12 mm. 11 Rugal folds are often seen in the ampulla.
The fimbriated part is the distal portion of the tube is funnel-shaped and not usually seen on HSG. Tubal patency is defined radiographically as the spillage of the contrast medium into the pelvic peritoneum.
Congenital tubal abnormalities
Congenital anomalies of the fallopian tube are rare but may include agenesis (fail to form of tube), hypoplasia accessory ostia, paratubal cysts, accessory tube, accessory ampulla, multiple luminal, congenital diverticulae, unilateral absence of an fallopian tube, total or partial duplication of fallopian tube, tubal dislocation, and complete and segmental absence of a portion of the fallopian tubes. 13,14 A few of these are representable on HSG.
Fallopian tube accessory ostium
Fallopian tube accessory ostium is a rare Müllerian duct anomaly, with a prevalence of 1.9% in infertile patients who undergo laparoscopy. 15 This condition is characterized by an ectopic fimbria, which is located at a distance from the primary ostium (Figure 2a). 15 The accessory ostium can be associated with endometriois or primary infertility. 16 The pathogenesis is completely not understood; however, it is stated that multiple secondary invagination of coelomic epithelium in the initial embryonic stage may lead to formation of an accessory ostium. 17
Figure 2.

Unicornuate uterus. Arrested unilateral development of Müllerian ducts results in a uterus unicornis, unicollis with one tube at that side.
In addition to the ampullary ostium, an ectopic opening may bilaterally form at a distance from the primary endometrial ostium and is visualized on HSG. 15 The result of a study has shown that laparoscopic reconstructive surgery led to favorable pregnancy outcomes. 15
Accessory fallopian tubes
Accessory fallopian tubes are rare developmental malformations that results from bifurcation of the cranial ends of Müllerian ducts, which normally develop into fallopian tubes (Figure 2b). 18
It is sometimes seen in surgery for elective sterilization, ectopic pregnancy, or torsion of the accessory fallopian tube. 19
The accessory fallopian tube can be associated with complications such as ectopic pregnancy, cystic swelling, and pyosalpinx.
Unilateral absence of a tube
This is a common finding in cases of unicornuate uterus. 20 Patients with a unicornuate uterus generally have normal fertility, although the risk of premature labor is higher and the term delivery rate approaches 45%. 21
On HSG images, a unicornuate uterus has a fusiform shape with a single fallopian tube. The uterus is usually shifted off of the developed side (banana-shaped) because the contralateral uterine segment is absent or rudimentary in development (Figure 3). A small communicating rudimentary horn may be seen on HSG.
Figure 3.

Schematic picture of: (a) Accessory ampullary ostium (arrow) and (b) accessory tube (ampulla). This developmental malformation results from bifurcation of the cranial ends of Müllerian ducts, which normally develop into fallopian tubes (open arrow).
Congenital segmental absence of fallopian the tube
This rare and usually asymptomatic Müllerian anomaly is commonly discovered during diagnostic laparoscopy. 22
In this condition, tubal torsion or twisting with interruption of the blood supply may lead to avascular necrosis and tissue reabsorption.
Duplication of partial or total portion of the fallopian tube
Duplication of partial or total portion of the fallopian tube has been reported. 13,23 Here, we present the first case of bilateral duplication of the interstitial segment of the fallopian tube with a radiographically normal configuration of the uterus (Figure 4). Defects in canalization of the Müllerian system may result in a double ostium of the fallopian tube. 18
Figure 4.

Duplication of a partial segment of a fallopian tube. Hysterosalpingography (HSG) shows bilateral duplication of the interstitial portion of a fallopian tube. Defects in canalization of the Müllerian system may result in this malformation (arrows).
Diverticula
Tubal diverticula are present in approximately 6% of infertility cases, and it is associated with abnormal reproductive functions such as infertility or ectopic pregnancy. 24
Diverticulae are small, concentric, localized, multiple or solitary, thin-walled outpouching seen along the isthmus or ampullary region. 24 Development of the sacculations may be related to weakening of the local muscle layer of the fallopian tube, increased internal pressure of the tube, external traction and inflammatory adhesion of the fallopian tube.
Diverticulum of the ampullary portion should be differentiated from the hydrosalpinx. In tubal diverticula, the residual contrast medium is finally drained into the pelvic cavity, whereas in hydrosalpinx, there is no accumulation of contrast medium in the pelvic cavity. 24
On HSG, diverticula is typically differentiated with salpingitis isthmica nodosa, internal tubal endometriosis, and genital tuberculosis with normal shape and no rigidity of the fallopian tube (Figure 5a and b). Enlarged ampulla and normal spillage of contrast are present and the pattern of dissemination of contrast to the peritoneum is cloudy and patchy.
Figure 5.

Tubal diverticulum. (a) Diverticulum located at the isthmic portion of the left tube and is similar to a thread passing through a single bead. Note the accumulation of contrast medium in the right ampullary portion, which is suggestive of hyderosalpinx in the right tube (open arrow). (b) Multiple diverticulae around the left tube (arrows). The right tube has a normal size and shape.
Pathophysiology of a proximal tubal blockage
Proximal tubal obstruction (PTO) is a common finding on HSG and accounts for approximately 20% of tubal factor cases. 25 Lack of filling of tubes during HSG may result from either tubal obstruction (transient) or tubal occlusion (permanent). 3
Obstruction can be secondary to tubal spasm, thickened mucus plugging of the proximal segment, technical problems, immediate evacuation of contrast from tubes, insufficient injection pressure, and air bubbles. The small caliber, the presence of a thick muscular wall, narrow lumen in the interstitial portion, and reduced the number of ciliated cells in the epithelium potentially provide a site for spasm or obstruction by uterine debris. 12
The main causes of PTO include fibrosis secondary to pelvic infection or tubal ligation, SIN, intramuscosal endometriosis and chronic tubal inflammation. Tuberculosis is an important infectious agent in this part of the tube. The uterine lesion located in the UTJ such as a uterine polyp, a submucous myoma, endometrial adhesions, or a tumor could rarely obstruct the proximal tube.
Proximal tubal blockage can be classified into three different types – nodular (salpinigitis isthemica nodosa or endometriosis), non-nodular (true fibrotic occlusion), and so-called pseudo occlusion (mucus plugs, detritus, polyps, and hypoplastic tubes). 26
This classification is useful to assist physicians and the patient to differentiate tubal obstruction from occlusion and decide upon proper treatment catheterization or in vitro fertilization.
Cornual spasm
Cornual spasm, produced by transient muscular spasm of the interstitial segment, must be differentiated from true organic obstruction of the proximal fallopian tube. On HSG, a rounded “breast like” shape of the uterine horn suggests cornual spasm (Figure 6). Cornual spasm has been postulated as a more common etiology when the PTO is unilateral.
Figure 6.

Both cornual spasms produced by transient muscular spasm of the interstitial segment give a rounded “breast like” appearance (arrow).
In this condition, a single fallopian tube fills with contrast while the contralateral tube appears to be an interstitial obstruction. This condition is associated with a combination of patient anxiety, painful procedure, and rapid contrast injection.
Some drugs such as atropine, antispasmodic drugs and narcotics have been used to suppress tubal muscular spasms. In a setting where the emphasis is on patient comfort and gentle techniques, tubal spasms rarely occur.
Often tubal filling is easily completed by allowing a small interval of rest before injection of additional contrast into the uterine cavity, and turning the patient toward the non-filling side-or placing her in the prone position while introducing more contrast. If, after these maneuvers the tube remains occluded, it is likely that true mechanical cornual obstruction exists. Interstitial obstruction may extend into the isthmic segment but seldom involves the ampulla.
Tubal polyps
Tubal polyps most often occur in the proximal segment (commonly interstitial portion) of the tube and may be seen in 1 to 3% of HSGs. 27 It consists of a benign proliferation of endometrial epithelium and stroma, which may be unilateral or bilateral. Tubal polyps appear as smooth oval filling defects, which are less than 1 cm in length and located just beyond the uterine horn (Figure 7a and b).
Figure 7.

Bilateral tubal polyp. (a) An oval-shaped filling defect in the interstitial (intramural) segment of the right tube (arrow), which is distinguished from an air bubble by its fixed position after injection of additional medium. (b) Bilateral tubal polyp (arrows).
Tubal fistulas
Tubal fistulas manifest as a contrast agent that exits from the distal isthmic or proximal ampullary portion of the tube into the peritoneal cavity, usually from the site of tubal ligation or salpingectomy. Fallopian tube fistulas most often communicate with the intestine and in more than one-half of the reported cases are secondary to tuberculous salpingities. 28 Since the incidence of genital tuberculosis is declining, bowel disease and surgery are becoming the more common sources of tubal fistulas. 29 Colonic diverticula, appendicitis, Crohn’s disease, and ruptured ectopic pregnancies have produced enterotubal fistulas.
HSG usually shows a dilated, fixed fallopian tube, and spill of contrast into an abnormal site (Figure 8). Delayed films help to confirm flow into the intestine, perineum or ureter.
Figure 8.

Left fallopian tube fistula secondary to tuberculosis salpingities. Hysterosalpingogram demonstrates passing of contrast material through a narrow channel (arrow), which is connected to the left tube.
Pelvic inflammatory disease (PID)
PID caused by a polymicrobial infection in the upper genital tract is responsible for 5–20% of gynecologic disorders that lead to hospital admissions. 30 In most cases, a bacterial infection spreads from the cervix into the uterus, fallopian tubes, or peritoneal cavity.
Epithelial damage caused by salpingitis is the most common reason for fibrosis, agglutination of folds and luminal obliteration of the tube usually occur at the corneal region, which may then lead to tubal occlusion and infertility.
Bacteria are the usual source of infection and common causal agents are Chlamydia trachomatis, Neisseria gonorrhoeae, and aerobic and anaerobic vaginal flora. Tuberculosis is now an uncommon cause of chronic salpingitis 31 and gonococcal infection accounts for only a minority of the current cases of PID. 32 The causative factors include vaginal or cervical infections that lead to salpingitis, the presence of an intrauterine device, cervical, or uterine instrumentation, and postpartum or post-abortion complications. Patients with PID may complain of lower abdominal pain, dyspareunia, fever, back pain, abnormal vaginal discharge, and postcoital bleeding. Other symptoms may include nausea, vomiting, dysuria, and cramping.
Several studies in females with PID diagnosed by laparoscopy have shown that the risk of subsequent tubal infertility increases with the number and severity of pelvic infections. 33
Radiographic findings of PID depend on the severity and localization of the infection. 34 Tubal obstruction can be unilateral or bilateral and can affect any portion of the tube (Figure 9a and b). HSG enables one to localize tubal obstruction but gives no specific information regarding the nature of the pathologic process that causes the obstruction. Differential diagnosis is based on the radiologic image, the patient’s history, symptoms, physical findings, the results of previous ultrasounds, and laparoscopy.
Figure 9.

Tubal occlusion. (a) Hysterosalpingogram shows unilateral tubal occlusion in the interstitial portion of the left tube (arrow). The right fallopian tube has preferentially been filled by spillage of contrast medium into the peritoneal cavity. Introduction of an air bubble into the uterine cavity produced a large filling defect (open arrow) (b) Bilateral tubal occlusion in the distal portion of the tubes owing to hydrosalpinx (open arrows).
Salpingitis isthemica nodosa (SIN)
SIN was described more than 100 years ago as irregular benign extensions of the tubal epithelium into the myosalpinx associated with reactive myohypertrophy and sometimes inflammation. 35 The etiology is unclear. Possible etiologies include congenital causes, hormonal factors, and degenerative and post-inflammatory causes. 36 The name reflects its inflammatory origin (salpingitis), location (tubal isthmus), and gross nodular appearance of the tube. SIN is important clinically due to its association with PID, ectopic pregnancy, and infertility 36 ; bilateral blockage is present in more than 50% of cases. 36
SIN is characterized pathologically by glandular mucosal proliferation and muscular hyperplasia.
Mucosal invasion into the muscularies and the formation of glandular islands that often communicate with the lumen of the tube lead to the formation of diverticulosis. The lesion should not be confused with tubal endometriosis, which shows invasion of the wall by foci of the endometrial glands and stroma. On gross inspection, SIN appears as thickening in the isthmic section of the tube.
The typical radiologic features of the SIN are tubal irregularities that are usually restricted to the isthmic portion, and the presence of multiple, small diverticula (Figure 10a). 37 The diverticula measure up to 2 mm in diameter and are clustered together in the proximal one-third of the tube. 37 The disease may be minimal and affect a short segment of the tube with only a few tiny diverticula or there may be more severe involvement. Tubal occlusion is not characteristic and most cases of SIN have tubal patency with or without associated hydrosalpinx.
Figure 10.
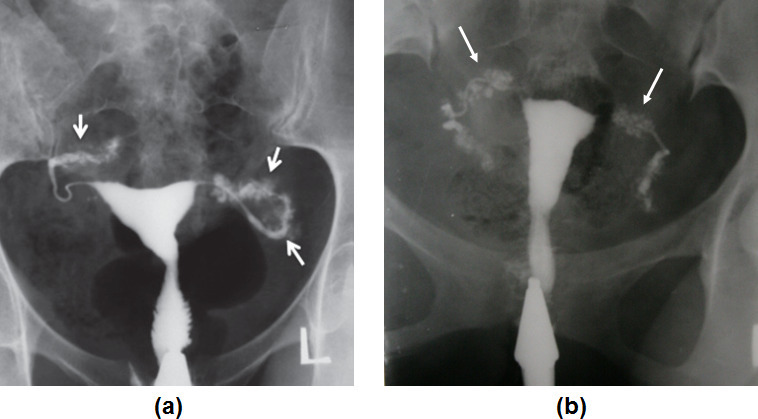
(a) Bilateral classic salpingities isthemica nodosa (SIN) in a patient with a history of pelvic inflammatory disease (PID). Numerous small diverticula-like structures are present in the isthmic segment of both tubes (arrows). (b) TB-SIN like. Penetration of contrast medium between the mucosal folds produces small diverticular-like outpouchings with a bizarre pattern. Both tubes are completely involved (arrows). Diverticular outpouching of tuberculosis are larger with a more bizarre pattern (in size and number) compared to classic SIN.
In tuberculosis, penetration of contrast medium between the mucosal folds produces small diverticular-like structures with a bizarre pattern called “TB-SIN like appearance’’.
TB-SIN-like appearances can be differentiated from classic SIN by the size and pattern of the outpouchings. TB-SIN diverticular outpouchings are larger, asymmetric, and have a more bizarre pattern (in size and number). They are not usually restricted to the isthmic portion of the tube compared with those of SIN (Figure 10b).
Endometriosis
Endometriosis is a cause of proximal tubal blockage and found in 7–14% of patients with isthmic blockage and infertility. 38 Endometriosis most often localizes in the interstitial and isthemic portions, although extension into other sites can occur. 39 Endometrial nodules of variable size cause polypoid protrusion that may lead to luminal compression and narrowing. The nodules are less than 1 cm in diameter, but can be larger in more severe cases. 38 Tubal endometriosis is associated with adhesions, distortion of pelvic anatomy, scarred fallopian tubes, and infertility. 40
HSG is limited in the evaluation of endometriosis because the tube is rarely involved and pelvic endometriosis does not have a characteristic picture on salpingography. 41 Tubal occlusions, the presence of a cystic ovarian tumor, and pelvic adhesions are suggestive of associated pelvic endometriosis.
The characteristic appearance of tubal endometriosis results when contrast medium passes from the tubal lumen into the delicate paratubal spaces, which constitute the typical patho-anatomical lesions of endometriosis. 41 A fine network of shadows is seen outside the tubal lumen and the pattern depends on the size and number of the paratubal channels (Figure 11). Tubal endometriosis may show a beaded appearance produced by multiple constrictions from the endometrial deposits, which can cause occlusion. 41
Figure 11.

Tubal endometriosis confirmed at laparoscopy. The isthmic and ampullary portion of both fallopian tubes show a network of fine channels (arrows).
Laparoscopy is known as the gold standard for definitive diagnosis of endometriosis. Previous studies have demonstrated that HSG identifies only one-third of the patients with pelvic endometriosis and a high number of patients with normal HSG have pelvic endometriosis and adhesions. 42,43 Although transvaginal ultrasonography assists in identifying and ruling out the retroperitoneal and uterosacral lesions, it does not accurately diagnose peritoneal or small endometriomas lesions. 44
Tuberculous salpingitis
Female genital tuberculosis is an important cause of tubal pathology leading to infertility, especially in developing countries. Tuberculosis manifests various appearances on HSG from non-specific changes such as hydrosalpinx to specific pattern such as “beaded tube”, “golf club tube”, “pipestem tube”, “cobble stone tube” and the “leopard skin tube”. These radiographic features of tube have been previously illustrated in a comprehensive review. 45
Conclusion
Tubal and peritubal pathology is a potential cause of infertility. HSG is the initial diagnostic test used to assess tubal patency because of its high sensitivity in the diagnosis of tubal occlusions. 8 This method is a less invasive procedure than laparoscopy and reveals valuable information about inner anatomy and internal architecture of the fallopian tubes, which are considered in differential diagnosis of pathological conditions, proper intervention, and appropriate treatment.
Footnotes
The authors Firoozeh Ahmadi and Malek Soleimani Mehranjani contributed equally to the work.
Contributor Information
Fatemeh Zafarani, Email: fzafarani@royaninstitute.org, fzafarani1391@gmail.com.
Firouzeh Ghaffari, Email: ghafaryf@yahoo.com.
Firoozeh Ahmadi, Email: dr.ahmadi1390@gmail.com.
Malek Soleimani Mehranjani, Email: m-Soleimani@araku.ac.ir.
Golam Shahrzad, Email: dr.gh.shahrzad@gmail.com.
REFERENCES
- 1. Lavy Y, Lev-Sagie A, Holtzer H, Revel A, Hurwitz A. Should laparoscopy be a mandatory component of the infertility evaluation in infertile women with normal hysterosalpingogram or suspected unilateral distal tubal pathology? Eur J Obstet Gynecol Reprod Biol 2004; 114: 64–8. doi: 10.1016/j.ejogrb.2003.09.035 [DOI] [PubMed] [Google Scholar]
- 2. Al-Jaroudi D, Aldughayyim AA, Alshamry WS, Alrashidi AS, Bahnassy AA. Hysterosalpingogram findings among subfertile women undergoing assisted reproductive technology. Int J Womens Health 2018; 10: 431–6. doi: 10.2147/IJWH.S156157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Honoré G, Holden AE, Schenken RS. Pathophysiology and management of proximal tubal blockage. Fertil Steril 1999; 71: 785–95. doi: 10.1016/S0015-0282(99)00014-X [DOI] [PubMed] [Google Scholar]
- 4. Saunders RD, Shwayder JM, Nakajima ST. Current methods of tubal patency assessment. Fertil Steril 2011; 95: 2171–9. doi: 10.1016/j.fertnstert.2011.02.054 [DOI] [PubMed] [Google Scholar]
- 5. Fayez JA, Mutie G, Schneider PJ. The diagnostic value of hysterosalpingography and laparoscopy in infertility investigation. Int J Fertil 1988; 33: 98–101. [PubMed] [Google Scholar]
- 6. Simpson WL, Beitia LG, Mester J. Hysterosalpingography: a reemerging study. RadioGraphics 2006; 26: 419–31. doi: 10.1148/rg.262055109 [DOI] [PubMed] [Google Scholar]
- 7. Merchant SA, Bharati AH, Badhe PB. Female genital tract tuberculosis: a review of hysterosalpingographic appearances part 1-the tube. J Women’s Imaging 2004; 6: 146–52. [Google Scholar]
- 8. Exacoustos C, Zupi E, Carusotti C, Lanzi G, Marconi D, Arduini D. Hysterosalpingo-Contrast sonography compared with hysterosalpingography and laparoscopic dye pertubation to evaluate tubal patency. J Am Assoc Gynecol Laparosc 2003; 10: 367–72. doi: 10.1016/S1074-3804(05)60264-2 [DOI] [PubMed] [Google Scholar]
- 9. Krynicki E, Kaminski P, Szymanski R, Gasior W, Marianowski L. Comparison of hysterosalpingography with laparoscopy and chromopertubation. J Am Assoc Gynecol Laparosc 1996; 3(4,Supplement): S22–3. doi: 10.1016/S1074-3804(96)80209-X [DOI] [PubMed] [Google Scholar]
- 10. Swart P, Mol BWJ, van der Veen F, van Beurden M, Redekop WK, Bossuyt PMM. The accuracy of hysterosalpingography in the diagnosis of tubal pathology: a meta-analysis. Fertil Steril 1995; 64: 486–91. doi: 10.1016/S0015-0282(16)57781-4 [DOI] [PubMed] [Google Scholar]
- 11. Fawcett DW. A textbook of histology. 12th ed. New York: Chapman & Hall; 1994. pp. 816–60. [Google Scholar]
- 12. Sweeney W. The interstitial portion of the uterine tube—its gross anatomy, course, and length. Obstet Gynecol 1962; 19: 3–8. [Google Scholar]
- 13. Beyth Y, Kopolovic J. Accessory tubes: a possible contributing factor in infertility. Fertil Steril 1982; 38: 382–3. [PubMed] [Google Scholar]
- 14. Daw E. Duplication of the uterine tube. Obstet Gynecol 1973; 42: 137–8. [PubMed] [Google Scholar]
- 15. Zheng X, Han H, Guan J. Clinical features of fallopian tube accessory Ostium and outcomes after laparoscopic treatment. Int J of Obstet 2015; 129: 260–3. doi: 10.1016/j.ijgo.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 16. Pereira N, Kligman I. Clinical implications of accessory fallopian tube Ostium in endometriosis and primary infertility. Women's Health 2016; 12: 404–6. doi: 10.1177/1745505716658897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gardner GH, Greene RR, Peckham BM. Normal and cystic structures of the broad ligament. Am J Obstet Gynecol 1948; 55: 917–39. doi: 10.1016/0002-9378(48)90570-5 [DOI] [PubMed] [Google Scholar]
- 18. Guan J, Watrelot A. Fallopian tube subtle pathology. Best Pract Res Clin Obstet Gynaecol 2019; 59: 25–40. doi: 10.1016/j.bpobgyn.2018.12.012 [DOI] [PubMed] [Google Scholar]
- 19. Skandalakes JE. Female genital system. In: Skandalakis’ Surgical Anatomy: the embryologic and anatomic basis of modern surgery. 1st ed. vol 2. Athens, Greece: Paschalides Medical Publication; 2004. pp. 1488–93. [Google Scholar]
- 20. The American Fertility Society classifications of adnexal adhesions, Distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, Müllerian anomalies and intrauterine adhesions. Fertil Steril 1988; 49: 944–55. [DOI] [PubMed] [Google Scholar]
- 21. Grimbizis GF, Camus M, Tarlatzis BC, Bontis JN, Devroey P. Clinical implications of uterine malformations and hysteroscopic treatment results. Hum Reprod Update 2001; 7: 161–74. doi: 10.1093/humupd/7.2.161 [DOI] [PubMed] [Google Scholar]
- 22. Nawroth F, Nugent W, Ludwig M. Congenital partial atresia of the fallopian tube. Reprod Biomed Online 2006; 12: 205–8. doi: 10.1016/S1472-6483(10)60862-0 [DOI] [PubMed] [Google Scholar]
- 23. Yablonski M, Sarge T, Wild RA. Subtle variations in tubal anatomy in infertile women. Fertil Steril 1990; 54: 455–8. [PubMed] [Google Scholar]
- 24. Han H, Guan J, Wang Y, Zhang Q, Shen H. Diagnosis and treatment of tubal diverticula: report of 13 cases. J Minim Invasive Gynecol 2014; 21: 142–6. doi: 10.1016/j.jmig.2013.05.022 [DOI] [PubMed] [Google Scholar]
- 25. Wiedemann R, Sterzik K, Gombisch V, Stuckensen J, Montag M. Beyond recanalizing proximal tubal occlusion: the argument for further diagnosis and classification. Hum Reprod 1996; 11: 986–91. doi: 10.1093/oxfordjournals.humrep.a019336 [DOI] [PubMed] [Google Scholar]
- 26. Letterie GS. Sakas El: histology of proximal tubal obstruction in cases of unsuccessful tubal canalization. Fertil. Steril 1991; 56: 831–5. [PubMed] [Google Scholar]
- 27. Gordts S, Boeckx W, Vasquez G, Brosens I. Microsurgical resection of intramural tubal polyps. Fertil Steril 1983; 40: 258–9. doi: 10.1016/S0015-0282(16)47247-X [DOI] [PubMed] [Google Scholar]
- 28. Kumar A, Bhargava SK, Mehrotra G, Pushkarna R. Enterotubal fistulae secondary to tuberculosis: report of three cases and review of literature. Clin Radiol 2001; 56: 858–60. doi: 10.1053/crad.2001.0774 [DOI] [PubMed] [Google Scholar]
- 29. Steckel J, Badillo F, Waldbaum RS. Uretero-fallopian tube fistula secondary to laparoscopic Fulguration of pelvic endometriosis. Urol Radiol 1992; 14: 191–3. [DOI] [PubMed] [Google Scholar]
- 30. Burnakis TG, Hildebrandt NB. Pelvic inflammatory disease: a review with emphasis on antimicrobial therapy. Clinical Infectious Diseases 1986; 8: 86–116. doi: 10.1093/clinids/8.1.86 [DOI] [PubMed] [Google Scholar]
- 31. Gompel C, Silverberg SG. The fallopian tube. In: Gompel C, Sliverberg S. G, eds. Pathology in gynecology and obstetrics. 4th ed. Philadelphia: JB Lippincott; 1994. pp. 284–312. [Google Scholar]
- 32. Weström LV. Sexually transmitted diseases and infertility. Sex Transm Dis 1994; 21(2 Suppl): S32–7. [PubMed] [Google Scholar]
- 33. Westrom L. Effect of pelvic inflammatory disease on fertility. Venereology 1995; 8: 219–22. [PubMed] [Google Scholar]
- 34. Collins JI, Woodward PJ. Radiological evaluation of infertility. Seminars in Ultrasound, CT and MRI 1995; 16: 304–16. doi: 10.1016/0887-2171(95)90035-7 [DOI] [PubMed] [Google Scholar]
- 35. Thurmond AS, Burry KA, Novy MJ. Salpingitis isthmica nodosa: results of transcervical fluoroscopic catheter recanalization. Fertil Steril 1995; 63: 715–22. [PubMed] [Google Scholar]
- 36. Honoré LH. Salpingitis Isthmica nodosa in female infertility and ectopic tubal pregnancy. Fertil Steril 1978; 29: 164–8. doi: 10.1016/S0015-0282(16)43094-3 [DOI] [PubMed] [Google Scholar]
- 37. Creasy JL, Clark RL, Cuttino JT, Groff TR. Salpingitis isthmica nodosa: radiologic and clinical correlates. Radiology 1985; 154: 597–600. doi: 10.1148/radiology.154.3.3969459 [DOI] [PubMed] [Google Scholar]
- 38. Punnonen R, Soederstroem K, Alanen A. Isthmic tubal occlusion: etiology and histology. Acta Eur Fertil 1984; 15: 39–42. [PubMed] [Google Scholar]
- 39. Seibel MM, Berger MJ, Weinstein FG, Taymor ML. The effectiveness of danazol on subsequent fertility in minimal endometriosis. Fertil Steril 1982; 38: 534–7. doi: 10.1016/S0015-0282(16)46630-6 [DOI] [PubMed] [Google Scholar]
- 40. Bulletti C, Coccia ME, Battistoni S, Borini A. Endometriosis and infertility. J Assist Reprod Genet 2010; 27: 441–7. doi: 10.1007/s10815-010-9436-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Johnson WK, Ott DJ, Chen MY, Fayez JA, Gelfand DW. Efficacy of hysterosalpingography in evaluating endometriosis. Abdom Imaging 1994; 19: 278–80. doi: 10.1007/BF00203528 [DOI] [PubMed] [Google Scholar]
- 42. Coimbra H, Pereira HS, Real FC, Sampaio MG, Lagarto R, Falcão F. Hysterosalpingography in the diagnosis of pelvic endometriosis. Acta Med Por 2000; 13: 255–8. [PubMed] [Google Scholar]
- 43. Jedrzejczak P, Serdyńska M, Brazert M, Pelesz M, Pawelczyk L. Laparoscopic assessment following failure to achieve pregnancy after intrauterine inseminations in patients with normal hysterosalpingograms. Ginekol Pol 2006; 77: 582–8. [PubMed] [Google Scholar]
- 44. Brosens I, Puttemans P, Campo R, Gordts S, Brosens J. Non-Invasive methods of diagnosis of endometriosis. Curr Opin Obstet Gynecol 2003; 15: 519–22. doi: 10.1097/00001703-200312000-00011 [DOI] [PubMed] [Google Scholar]
- 45. Ahmadi F, Zafarani F, Shahrzad G, Gh S. Hysterosalpingographic appearances of female genital tract tuberculosis: Part I. fallopian tube. Int J Fertil Steril 2014; 7: 245–52. [PMC free article] [PubMed] [Google Scholar]


