Abstract
Artificial intelligence (AI) applications, in the form of machine learning and deep learning, are being incorporated into practice in various aspects of medicine, including radiation oncology. Ample evidence from recent publications explores its utility and future use in external beam radiotherapy. However, the discussion on its role in brachytherapy is sparse. This article summarizes available current literature and discusses potential uses of AI in brachytherapy, including future directions. AI has been applied for brachytherapy procedures during almost all steps, starting from decision-making till treatment completion. AI use has led to improvement in efficiency and accuracy by reducing the human errors and saving time in certain aspects. Apart from direct use in brachytherapy, AI also contributes to contemporary advancements in radiology and associated sciences that can affect brachytherapy decisions and treatment. There is a renewal of interest in brachytherapy as a technique in recent years, contributed largely by the understanding that contemporary advances such as intensity modulated radiotherapy and stereotactic external beam radiotherapy cannot match the geometric gains and conformality of brachytherapy, and the integrated efforts of international brachytherapy societies to promote brachytherapy training and awareness. Use of AI technologies may consolidate it further by reducing human effort and time. Prospective validation over larger studies and incorporation of AI technologies for a larger patient population would help improve the efficiency and acceptance of brachytherapy. The enthusiasm favoring AI needs to be balanced against the short duration and quantum of experience with AI in limited patient subsets, need for constant learning and re-learning to train the AI algorithms, and the inevitability of humans having to take responsibility for the correctness and safety of treatments.
Introduction
The advancing footprint of artificial intelligence (AI) in medicine and more so in modern radiation oncology (RO) indicates that it stands to impact all aspects of RO in near future, mandating that we are adequately prepared to interact with AI-driven RO. 1 Last few years have seen many deliberations on the topic at great length. 1–4 While it is believed that akin to its projected role in external beam radiotherapy (EBRT), AI will also influence the process of brachytherapy (BT), the data and discussions on its role in BT have been limited. The authors have tried to summarize the contemporary research on AI in BT and its potential utility across diverse sites and indications. We understand that machine learning (ML) and deep learning (DL) are incrementally more advanced and effective modalities of AI; however, to keep things simple, we have refrained from delving into technical details of complicated algorithms and limited the discussion to clinical domains.
Through this manuscript, the reader will navigate through several studies where AI or ML has been used in the field of BT and get a sense of how purposefully the available data and potential applications thereof can fall in line toward the common goal of improving treatment quality.
Methods
A PubMed search was performed for English language publications (till March 2020) in humans using the MeSH terms “artificial intelligence,” “machine learning”, or “deep learning” in combination with “neoplasms” and “brachytherapy”. The retrieved abstracts were hand-sorted for relevance. Cross-references from the relevant articles were also retrieved from non-PubMed sources after eliminating duplicates. Full texts of all selected publications were screened for inclusion. In case of multiple similar publications pertaining to a particular disease site, sub-topic or modality, the most recent publications or the ones with the largest number of subjects were chosen for discussion. The available data were summarized in the form of a narrative review with focus on developments related to role of AI pertinent to each step of brachytherapy process.
Results
Decision-making regarding use of BT at initiation of cancer treatment
Clinicians are using AI with excellent results in qualitative analysis of oncological imaging. AI holds substantial promise in the field of radiomics, using radiographical features of tumors for volumetric delineation, and determining association of the tumor genotype and predicted biological path. Prediction of clinical outcome, and assessment of the impact of disease and treatment on adjacent organs by use of AI tools (such as clinical response and risk of pneumonitis from baseline imaging in lung cancer) is now being validated. 5 AI-assisted automatic segmentation helps improve efficiency, reproducibility and quality of tumor measurements. Whole-body imaging data can be evaluated rapidly, allowing identification of subclinical disease and organ dysfunction. Diagnostic modalities such as gastrointestinal endoscopy, cystoscopy and laboratory settings are adopting AI to improve diagnostic yield. 6,7 It helps in more accurate tumor identification and delineation, especially in identification of skip lesions or multicentric disease. Factors such as tumor size, extent, multicentricity, radiobiological behavior, radiation sensitivity, cure rates and toxicities arising from radiotherapy can be gleaned from AI-driven algorithms. 5,8,9 These factors have a direct bearing on the role and scope of AI in clinical BT; at present, their role is limited to research but they may potentially drive or guide tumor board discussions and decisions in future. Prostate cancer is one of the earliest malignancies where AI algorithms were used for risk categorization, treatment optimization, toxicity prediction and follow-up. DL can help treatment decision-making in prostate cancer treatment where BT can be one of the therapeutic options. 10,11 Determination and validation of the risk of lymph nodal involvement and prediction of capsular invasion through artificial neural networks in large databases have helped select candidates who would benefit from radical BT as the sole treatment modality. Similar applications in breast cancer to identify low risk early disease may help more efficient selection of candidates for accelerated partial breast irradiation. 12 However, it needs to be understood that while AI improves efficiency of the process, its benefits have been studied only in small cohorts. It is not fool-proof and needs constant learning, re-learning and validation across larger population subsets. In the aforementioned prostate cancer study, AI had a 16% false-negative rate for prediction of capsular invasion. 11 For toxicity prediction in a cohort of 321 prostate cancer patients, the overall accuracy was only 70% with both false-negatives and false-positives being reported. The AI algorithm demonstrated a decent sensitivity of 84.6% but the specificity was low at 58.8%. 8
AI in pre-planning
Pre-planning is an essential component in BT that includes review of the clinical situation, assessment of volume to be treated, approach and technique to be used for implanting the tumor, choice of applicator and planning a prescription depending on the surrounding vital structures. AI can play a significant role here. We know that DL can perform fully automatic segmentation of healthy and neuropathic sciatic nerves from standard magnetic resonance neurography (MRN) images with good accuracy and in a clinically feasible timespan. 13 Similarly, muscle compartments can be precisely delineated to aid planning of orthopedic and general surgical interventions. 14 In a not-too-far foreseeable future, the physicians will have this information upfront and approach an intraoperative BT catheter placement with far more confidence about their intended regions of implantation and avoidance for dose prescription. AI found one of its earliest uses in low dose rate (LDR) seed BT of prostate cancer. Nicole and colleagues used ML to extract dosimetrically optimal pre-plans which were comparable in quality to those by expert planners, but with a significant reduction in planning time (0.84 ± 0.57 minutes vs 17.88 ± 8.76 minutes, p = 0.020). 15 There are preliminary reports that DL methods using previous experiences can guide selection of suitable applicators for high dose rate (HDR) BT in cancer cervix. This has been validated in the choice of interstitial over intracavitary applicators based on geometric characteristics of data such as shape and volume of high-risk clinical target volume. ML algorithms can help in decision-making and augmenting a physician’s judgment leading to more consistency, obviating many logistic issues and last-minute unwanted plan changes in the operating room, with no compromise in plan quality. 16 Clinical experience tells us that reirradiation can salvage nearly 50% prostate cancer patients with post-irradiation recurrences; ML algorithms may help segregate and select patients with a better chance of control with salvage radiotherapy, thus sparing the other half from unnecessary re-exposure to radiation. 17 ML-based algorithms have helped yield prediction models of rectovaginal fistula formation in patients undergoing interstitial BT for advanced gynecological malignancies. 18 The information generated, at best, can be considered hypothesis-generating and need to be validated for clinical utility across large datasets (exceeding thousands of patients) over a long period or multiple institutions. This is a challenge since it requires universal standardization of data recording and reporting. Also, the model predictive accuracy is limited not only by small sample sizes but also a small number of events under investigation. The model is only able to predict based on factors incorporated and validated; information such as genetic susceptibility, if unavailable, is not used despite having a possible impact on outcome or number of events.
AI in assisting procedure in BT operating room
Diagnostic ultrasonography (USG) is a powerful tool in real-time BT for guiding interstitial needle insertion, but its use by RO professionals is limited by lack of experience and training. 19 Three-dimensional (3D) USG image analysis has shown great potential in USG-based clinical application of BT. 19 Application of novel DL in automated imaging analysis tasks (lesion/ nodule classification, organ segmentation, object detection, registration, measurements, quality assessment) and image-guided interventions, specific to BT in sites such as prostate, breast, tongue, etc., are areas of potential future research. 20
DL has proven itself useful in tasks such as classification, segmentation, detection, registration, image-guided interventions, and therapy in real time. USG is being experimented and validated for use in real time interventions. Deep convolutional network may help identify critical nerves and blood vessels in USG images. 21 This application in sites such as neck (due to complex neural and vascular architecture) may expand the indications of interstitial BT as well as instil confidence in the physician for complex procedures. AI application of USG in future may provide real-time guidance in operating room to identify target as well as avoid juxtaposed critical structures in patients undergoing implants for BT.
Besides USG, endoscopy is an underexplored modality for seed or catheter placement. There are case reports of using AI-driven navigation system for real-time localization of the airways and lung nodules using fluoroscopic images. The navigation catheter guided by AI could reach the suspicious nodule endoscopically where a computed tomography (CT)-guided biopsy had failed. 22 LDR seeds may be placed via endoscopic routes in several sites such as pancreas, esophagus, and lungs; usage of AI here may help in more accurate placement of radioactive seeds with greater therapeutic efficacy. Whether these anecdotal scenarios translate into real-world applications of AI in guiding BT procedures will become clear only with wider availability and time. At present, the limited experience with USG and endoscopy in the hands of RO professionals indicates that this AI approach, although promising, would be slow to be adopted.
AI in imaging with applicator
Almost all BT procedures require post-procedure imaging, and while most departments have their own protocols for imaging, the quality of images may vary with patient, site of application, and type and orientation of applicators. Advanced imaging techniques and personalized protocols for imaging acquisition, supported by ML can help us to acquire better images in this era of image-guided brachytherapy (IGBT); this in turn will aid in better delineation of targets and organs at risk. 23
AI in applicator/ catheter reconstruction
Reconstruction of applicator, needles, and catheters needs experience, and is a time-consuming and tedious job; the digitization process often takes more than half the treatment planning time. 24 It also delays the first treatment session, thus necessitating prolongation of the patient’s hospital stay. For HDR IGBT of cervical cancer, applicator digitization errors have a considerable effect on dosimetric parameters of treatment planning. 25 DL can reliably help in automated applicator (tandem and ovoid) reconstruction in a computation time of about 25 seconds, while reducing observer-related errors and reducing planning time. 26 DL-enabled automation for digitization of interstitial needles has yielded a reconstruction time of under 5 minutes for an average of 20.7 interstitial needles. 27 Furthermore, DL methods show promise in intraoperative magnetic resonance imaging (MRI)-based catheter reconstruction in complicated interstitial gynecological BT procedures with 10–35 catheters with computational time under 3 min. 28 There was, however, the problem of identification due to similarity of intensity with blood vessels and plastic tubing such as urinary catheters in MRI leading to a false-positivity rate of 13.8% in this study; reconstruction was discontinuous since all voxels were defined as catheter or non-catheter instead of the system attempting linear reconstruction in physical space according to shape and length of catheter. Also, at its current stage of development, the learning–relearning process involves only one MRI scanner at a time and a significant computational load. The authors believe that with more widespread implementation across centers, this problem may be overcome in the future.
AI in target delineation/ image registration/ radiomics
Dose escalation to dominant intraprostatic nodules identified on multiparametric MRI is possible in prostate HDR BT if this information can be incorporated into real-time USG to guide catheter placement. Reliable registration of MRI-USG images is a challenging task due to different gray-level intensity and image field size between MRI and USG. Zeng et al 29 devised a weakly supervised learning DL-based model for performing automatic MRI-US registration. This system suffered, however, from inability to verify if the system-generated prostate deformations for matching indeed matched the actual deformability of prostate, in addition to the limited dataset size. An algorithm of AI, residual learning of convoluted neural network (CNN) appreciably decreases metal artefacts in cervical cancer CT images, thus improving critical organ visualization and confidence of treating physician in delineation of target. 30 Similar AI applications have been explored in prostate BT for target volume and normal tissue segmentation on transrectal USG images. 31 The available results are encouraging, but need larger volume of training datasets to have more generalizability. The accuracy is lower in prostate apical and base regions.
AI in planning/ dose prescription
Extraction and analysis of rigidly defined radiomic features can transform medical imaging data into quantifiable variables to predict survival, other failure modes, and response to therapeutic agents. 9 A recent study on 142 patients with locally advanced cervical cancer shows a DL model can assist early prediction of local and distant failures from positron emission tomography-CT (PET-CT) and diffusion-weighted MRI data. 32 These data when widely available may help individualize treatment and follow-up; those predicted for distant failures may need to incorporate some form of systemic therapy or undergo more rigorous follow-up, while those with higher chances of local failure may merit dose escalation with advanced BT techniques. 33 Preliminary reports indicate that deep CNN can be used to predict rectal toxicity in patients with locally advanced cervical cancer. 34 This information may help in dose prescription and plan evaluation when we need to trade-off between toxicity and cure. AI use is likely to change the paradigm of radiotherapy planning practice, including BT, in next two decades. 35 Deep reinforcement learning-based iterative weight-tuned inverse planning algorithms in cervical cancer BT are able to give inputs mimicking weight adjustments by a human planner with equivalent plan quality albeit in a much shorter planning time. 36 Most available interpretations are from single center retrospective studies evaluating small patient numbers, similar scanning equipment (PET or MRI), and their inherent bias. Capability of interpretation or computing may be affected by the interslice gap in MR images, leading to potential misreading of data.
AI in adaptive planning/ treatment monitoring
AI is increasingly assuming a greater role in EBRT planning process. BT is, in our opinion, the most accurate form of treatment delivery due to direct contact of radiation source with target; however, catheter displacement may happen between treatments and there may be movement of surrounding critical organs. Ideally, the treatment (source position and dwell time) should adapt to catheter movements and the adaptive anatomy of surrounding organs. Electromagnetic tracking (EMT) may determine the spatial position and dwell time of a radiation source within the implanted volume, in reference to planning CT data. Automated analysis of EMT datasets has helped to ensure concordance of the source movement with treatment plan after elimination of movement artifacts in breast BT. This tool combines ML techniques to precisely detect and quantify mismatches between the treatment plan and actual EMT measurements and can quantify deviations before a treatment session is started. 37 We need fast and practical models to correct the treatment plan for new anatomical positions or shapes of organ at risk before dose delivery. After determining the deformation-related intra-fractional rectum and bladder dose variations, CNNs help in plan optimization in gynecological cancers. 38
AI in routine clinical physics workflow and quality checks
AI may assist clinical physicists in treatment planning, scheduled quality assurance (QA) procedures and periodic chart verification, considerably reducing time spent by them in these activities. However, several non-routine activities which require interface with other professionals such as dosimetrists and clinicians, cannot be adequately handled by AI technology yet and continue to be performed solely by physicists. 39
As experience with AI in BT grows, we will have ways to incorporate it in several other steps. AI is already showing promise in assessment of implant quality in prostate seed BT. Typically, post-implant dosimetry is performed at day 30 of the procedure to evaluate the implant quality. Traditionally, CT with its limited soft tissue resolution is used; MRI can give better organ delineation, but has its own uncertainties. To circumvent this problem, Nosrati et al 40 devised a technique that utilizes unsupervised ML in specialized MRI sequencing. Their findings suggest that the technique is accurate and robust for localizing seeds’ position and orientation, and can replace the current widely practiced CT-based workflow.
Discussion
BT has the geometric advantage of better conformity compared to even stereotactic EBRT techniques, owing to the proximity of radiation source to target, and rapid peripheral dose fall-off. 41 Akin to surgical techniques, BT skills are also physician-dependent and require a long-learning curve before confident applications. Within BT techniques as well, interstitial procedures demand more practice and precision than luminal or surface applications. For these reasons, it is likened to an art form that needs passion for precision in addition to being a skill-based technical field. Since acquisition of skill is an ongoing process on individual basis, technological advancements have had a lesser impact in BT than in other branches of medicine and EBRT. There are institutional innovations and modifications of the art to adjust with logistics leading to differing practices across institutions. 42 Such variable practice will delay and defer incorporation of AI in BT. Although AI technologies may help in the pre-planning steps, the eventual plan will depend heavily on how applicators, interstitial needles or catheters are inserted by the clinician rather than all other technical factors combined. A higher focus on technological advancements such as intensity modulation (IMRT), image guidance and stereotactic body radiotherapy (SBRT) in EBRT, in addition to economic (better reimbursement for EBRT over BT) and logistic (higher personnel time per treatment session for BT over EBRT, lesser training opportunities) factors, has led to decreased enthusiasm and willingness to spend time to gain BT skills; this is evident in the declining trend of BT in the last decades. 43,44 In prostate cancer, despite better outcomes with BT boost in high-risk disease, dose escalated EBRT is more widely practiced. 45 Combined with decreased overall utilization of RT in prostate cancer due to decreased prostate cancer screening, this has led to decrease in number of high volume centers for BT as well as reduction in annual BT procedures per center. The decline in BT in carcinoma cervix (96.7% to 86.1% from 2004 to 2011) was accompanied by a proportionate increase in use of IMRT and SBRT (3.3% to 13.9% over the same duration), and resulted in a survival detriment with a hazard ratio of 1.86, according to National Cancer Database. 46,47 These findings have strengthened the realization that BT is irreplaceable by EBRT in a variety of clinical situations and led to a renewal of interest in BT, and several national brachytherapy societies are now enhancing their focus on improving incorporation of BT into national treatment guidelines, increasing awareness and opportunities for training among young radiation oncologists, and well as promoting inter-institutional and inter-societal collaborations, besides advocacy for cost-effectiveness and equal reimbursements as an incentive to practicing physicians. 48 BT stands a chance of benefiting from advancements in radiology, modern procedural techniques, treatment planning, QA and delivery to survive and rather flourish in this era of conformal radiotherapy and particle therapy. The global BT seeds market (HDR and LDR) is set to post a compound annual growth rate of 9% during 2019–2023. This growth is propelled by several technological innovations, which include the recent advances in pulsed dose rate BT, IGBT, advancement in real-time imaging for procedures and. 49 Seed BT with innovations of robotic handling for added safety, 3D templates for accurate positioning, and lower cost has yielded tumor coverage and normal tissue sparing similar to SBRT in sites such as lung tumors. 50 Modern BT procedures with increasing complexities and steps will require more technical resources and skilled manpower. It is imperative that we encourage novel BT technologies that will enhance speed, popularity, and acceptance of BT both for patient and physician, simultaneously retaining its safety, accuracy, and evidence-based efficacy. Current research discussed here underlines the need of encouragement for new technologies involving AI platforms.
Researchers from Sunnybrook cancer hospital have published a phase 1 trial for prostate LDR BT day-30 dosimetry comparing ML-based treatment planning system with conventional planning by experts, concluding that the ML module produces non-inferior postoperative dosimetry but offers significant gains in procedure time and efficiency. 51 Investigators at University of Texas have used AI techniques to automatically segment organs, perform a fully automated planning process, including applicator digitization, radioactive source placement, and dwell time determination; the whole process completed in significantly lesser time than a conventional manual planning process with desired safety and efficacy, and better time efficiency. 52
Figure 1 illustrates a hypothetical case of cancer cervix where AI assists each step from decision making to treatment (Figure 1).
Figure 1.
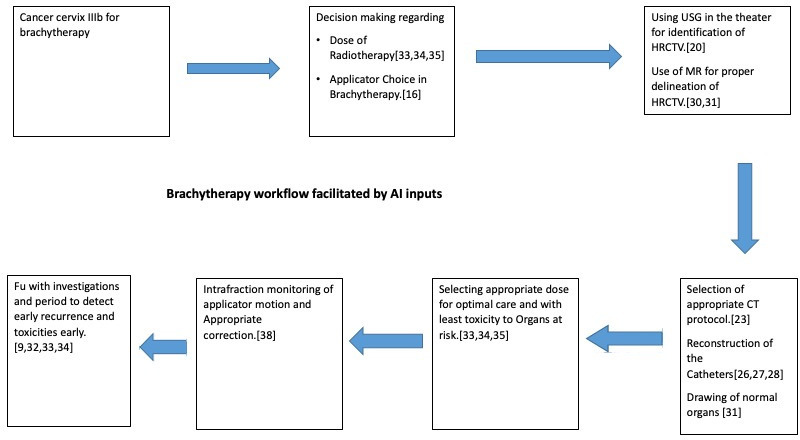
A conceptual block diagram showing possible use of AI in every step of cancer cervix brachytherapy. ( in last block of the diagram “FU with investigations and period....and toxicities early” be replaced by “Deciding investigations and period of Fu to detect early recurrence and toxicities”
As discussed earlier, this enthusiasm in advocating AI for BT stemming from the success of pioneering efforts is counter-balanced by low BT patient volume, as well as the fact that the current literature for AI practices is limited to small datasets in mono-institutional retrospective studies that need process standardization and validation across larger patient populations and multiple institutions. ML capacity and development of such algorithms depend on correlations of the input and outcome data. Hence, the use of such algorithms becomes restrictive in clinical science as the data itself has many constraints and observational errors, as well as biases that creep in due to age-old practices and prejudices of clinicians. 53 Besides the inherent limitations of data quality, the quantity of data also matters. RO datasets are smaller and more limited compared to what other professions are using to tune their predictive algorithms. BT suffers from even more scarcity of data. Treatment decisions and practices are customized and physician-specific. BT practices vary widely across institutions and even among practitioners in same institution. All these factors pose a barrier in formulating predictive AI algorithms. The resultant faulty outputs can lead to clinical catastrophe especially in HDR BT where treatment precision is paramount and side effects of these errors potentially debilitating; this is owing to the fact that HDR BT traditionally employs higher dose per fractions and lower number of fractions, leading to higher impact of dose errors per fraction compared to conventionally fractionated EBRT.
There is an effort by responsible organizations to introduce uniformity in practice across the world; standard guidelines for common sites such as prostate, cervix and soft tissue sarcomas are now in place. 54–56 Hopefully, generation of more standardized and carefully complied data in future practices of BT will ensure inter-institutional homogeneity and this will generate more consistent inputs for AI algorithms and thus more reliable outputs as well. The ensuing long term results and their applications eventually may be incorporated to aid and strengthen routine clinical practice. In addition to strengthening of training for BT, familiarity with basics of AI will also need to be incorporated into resident training programs, so that clinicians may work more fruitfully as a team with physics and engineering peers for generation of clinically useful algorithms.
Conclusion
In contrast to EBRT and its technological advancements including contribution of AI, BT is still dependent more on the skills and technique of the physician than technological advances. That said, there is a huge potential for incorporation of AI in BT technology; it may be used to refine skills and well as save time and effort in applying already defined rules and variations thereof. Just like in EBRT, AI-driven planning in BT is likely to improve process efficiency, consistency, and quality but human intervention and quality checks for validation would still take the central place for quite a while as they will need to shoulder the responsibility for plan approval and treatment safety despite any number of automation inputs. We are still at the nascent stage of AI application to add value to the existing clinical workflow; it will require a great deal of understanding, input of large patient databases (imaging, treatment plans, genetic information, follow up imaging and clinical data) from varied institutions and imaging/treatment sources to improve the accuracy of the learning and training processes as well as possibility of generalization across different populations. This also means relatively unrestricted access to patient data which may interfere with privacy, data security, and regulatory issues. Prospective studies with multicenter collaborations and standardization of nomenclature as well as QA, planning, and reporting processes are an idealist but humongous undertaking. There needs to be a great deal of interaction between clinical users and industrial developers/engineers to find solutions that drive future research. Attempts to explore and adopt the contemporary developments in BT with the added advantages of efficiency and cost-effectiveness may make it more attractive for young radiation oncologists, thereby enhancing its use and acknowledging its equal or greater utility vis-a-vis other modalities, including particle therapy.
Footnotes
Acknowledgements: None
Competing interests: None of the authors have any conflicts of interest to disclose
Funding: None
Patient consent: Not applicable
Ethics approval: Not applicable
Disclosure: None
Contributor Information
Susovan Banerjee, Email: susovan.banerjee@medanta.org.
Shikha Goyal, Email: drshikhagoyal@gmail.com.
Saumyaranjan Mishra, Email: saumyaranjan86@gmail.com.
Deepak Gupta, Email: deepakonco@gmail.com.
Shyam Singh Bisht, Email: shyam.bisht@medanta.org.
Venketesan K, Email: venkphysics@gmail.com.
Kushal Narang, Email: kushal.narang@medanta.org.
Tejinder Kataria, Email: tejinder.kataria@medanta.org.
REFERENCES
- 1. Thompson RF, Valdes G, Fuller CD, Carpenter CM, Morin O, Aneja S, et al. Artificial intelligence in radiation oncology: a specialty-wide disruptive transformation? Radiother Oncol 2018; 129: 421–6. doi: 10.1016/j.radonc.2018.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feng M, Valdes G, Dixit N, Solberg TD. Machine learning in radiation oncology: opportunities, requirements, and needs. Front Oncol 2018; 8: 110. doi: 10.3389/fonc.2018.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jarrett D, Stride E, Vallis K, Gooding MJ. Applications and limitations of machine learning in radiation oncology. Br J Radiol 2019; 92: 20190001. doi: 10.1259/bjr.20190001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rattan R, Kataria T, Banerjee S, Goyal S, Gupta D, Pandita A, et al. Artificial intelligence in oncology, its scope and future prospects with specific reference to radiation oncology. BJR Open 2019; 1: 20180031. doi: 10.1259/bjro.20180031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bi WL, Hosny A, Schabath MB, Giger ML, Birkbak NJ, Mehrtash A, et al. Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J Clin 2019; 69: 127–57. doi: 10.3322/caac.21552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shkolyar E, Jia X, Chang TC, Trivedi D, Mach KE, Meng MQ-H, et al. Augmented bladder tumor detection using deep learning. Eur Urol 2019; 76: 714–8. doi: 10.1016/j.eururo.2019.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abadir AP, Ali MF, Karnes W, Samarasena JB. Artificial intelligence in gastrointestinal endoscopy. Clin Endosc 2020; 53: 132–41. doi: 10.5946/ce.2020.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pella A, Cambria R, Riboldi M, Jereczek-Fossa BA, Fodor C, Zerini D, et al. Use of machine learning methods for prediction of acute toxicity in organs at risk following prostate radiotherapy. Med Phys 2011; 38: 2859–67. doi: 10.1118/1.3582947 [DOI] [PubMed] [Google Scholar]
- 9. Lou B, Doken S, Zhuang T, Wingerter D, Gidwani M, Mistry N, et al. An image-based deep learning framework for individualizing radiotherapy dose. Lancet Digit Health 2019; 1: e136–47. doi: 10.1016/S2589-7500(19)30058-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu X, Cammann H, Meyer H-A, Miller K, Jung K, Stephan C. Artificial neural networks and prostate cancer--tools for diagnosis and management. Nat Rev Urol 2013; 10: 174–82. doi: 10.1038/nrurol.2013.9 [DOI] [PubMed] [Google Scholar]
- 11. Gamito EJ, Stone NN, Batuello JT, Crawford ED. Use of artificial neural networks in the clinical staging of prostate cancer: implications for prostate brachytherapy. Tech Urol 2000; 6: 60–3. [PubMed] [Google Scholar]
- 12. Polgár C, Van Limbergen E, Pötter R, Kovács G, Polo A, Lyczek J, et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the Groupe Européen de Curiethérapie-European Society for therapeutic radiology and oncology (GEC-ESTRO) breast cancer Working group based on clinical evidence (2009. Radiother Oncol 2010; 94: 264–73. doi: 10.1016/j.radonc.2010.01.014 [DOI] [PubMed] [Google Scholar]
- 13. Balsiger F, Steindel C, Arn M, Wagner B, Grunder L, El-Koussy M, et al. Segmentation of peripheral nerves from magnetic resonance neurography: a Fully-Automatic, deep Learning-Based approach. Front Neurol 2018; 9: 777. doi: 10.3389/fneur.2018.00777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kamiya N. Muscle segmentation for orthopedic interventions. Adv Exp Med Biol 2018; 1093: 81–91. doi: 10.1007/978-981-13-1396-7_7 [DOI] [PubMed] [Google Scholar]
- 15. Nicolae A, Morton G, Chung H, Loblaw A, Jain S, Mitchell D, et al. Evaluation of a Machine-Learning algorithm for treatment planning in prostate low-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys 2017; 97: 822–9. doi: 10.1016/j.ijrobp.2016.11.036 [DOI] [PubMed] [Google Scholar]
- 16. Stenhouse K, Ciunkiewicz P, Roumeliotis M, McGeachy P. Use of machine learning algorithms to identify predictive geometric features for optimal applicator selection in high dose-rate (HDR) cervical brachytherapy: scientific session 5B: Brachytherapy–04. Medical Physics 2019; 46: 5397–8. [Google Scholar]
- 17. Valdes G, Chang AJ, Interian Y, Owen K, Jensen ST, Ungar LH, et al. Salvage HDR brachytherapy: multiple hypothesis testing versus machine learning analysis. Int J Radiat Oncol Biol Phys 2018; 101: 694–703. doi: 10.1016/j.ijrobp.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 18. Tian Z, Yen A, Zhou Z, Shen C, Albuquerque K, Hrycushko B. A machine-learning-based prediction model of fistula formation after interstitial brachytherapy for locally advanced gynecological malignancies. Brachytherapy 2019; 18: 530–8. doi: 10.1016/j.brachy.2019.04.004 [DOI] [PubMed] [Google Scholar]
- 19. Banerjee S, Kataria T, Gupta D, Goyal S, Bisht SS, Basu T, et al. Use of ultrasound in image-guided high-dose-rate brachytherapy: enumerations and arguments. J Contemp Brachytherapy 2017; 9: 146–50. doi: 10.5114/jcb.2017.67456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu S, Wang Y, Yang X, Lei B, Liu L, SX L, et al. Deep learning in medical ultrasound analysis: a review. Engineering 2019; 5: 261–75. [Google Scholar]
- 21. Smistad E, Johansen KF, Iversen DH, Reinertsen I. Highlighting nerves and blood vessels for ultrasound-guided axillary nerve block procedures using neural networks. J Med Imaging 2018; 5: 044004. doi: 10.1117/1.JMI.5.4.044004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Whitten P. Artificial intelligence driven diagnosis of lung cancer in patients with multiple pulmonary nodules. Chest 2019; 156: A534. [Google Scholar]
- 23. European Society of Radiology (ESR). What the radiologist should know about artificial intelligence - an ESR white paper. Insights Imaging 2019; 10: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Michaud AL, Benedict S, Montemayor E, Hunt JP, Wright C, Mathai M, et al. Workflow efficiency for the treatment planning process in CT-guided high-dose-rate brachytherapy for cervical cancer. Brachytherapy 2016; 15: 578–83. doi: 10.1016/j.brachy.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 25. Schindel J, Zhang W, Bhatia SK, Sun W, Kim Y. Dosimetric impacts of applicator displacements and applicator reconstruction-uncertainties on 3D image-guided brachytherapy for cervical cancer. J Contemp Brachytherapy 2013; 5: 250–7. doi: 10.5114/jcb.2013.39453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deufel CL, Tian S, Yan BB, Vaishnav BD, Haddock MG, Petersen IA. Automated applicator digitization for high-dose-rate cervix brachytherapy using image thresholding and density-based clustering. Brachytherapy 2020; 19: 111–8. doi: 10.1016/j.brachy.2019.09.002 [DOI] [PubMed] [Google Scholar]
- 27. Jung H, Shen C, Gonzalez Y, Albuquerque K, Jia X. Deep-learning assisted automatic digitization of interstitial needles in 3D CT image based high dose-rate brachytherapy of gynecological cancer. Phys Med Biol 2019; 64: 215003. doi: 10.1088/1361-6560/ab3fcb [DOI] [PubMed] [Google Scholar]
- 28. Zaffino P, Pernelle G, Mastmeyer A, Mehrtash A, Zhang H, Kikinis R, et al. Fully automatic catheter segmentation in MRI with 3D convolutional neural networks: application to MRI-guided gynecologic brachytherapy. Phys Med Biol 2019; 64: 165008. doi: 10.1088/1361-6560/ab2f47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zeng Q, Fu Y, Tian Z, Lei Y, Zhang Y, Wang T, et al. Label-driven magnetic resonance imaging (MRI)-transrectal ultrasound (TRUS) registration using weakly supervised learning for MRI-guided prostate radiotherapy. Phys Med Biol 2020; 65: 135002. doi: 10.1088/1361-6560/ab8cd6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang X, Wang J, Tang F, Zhong T, Zhang Y. Metal artifact reduction on cervical CT images by deep residual learning. Biomed Eng Online 2018; 17: 175. doi: 10.1186/s12938-018-0609-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anas EM, Nouranian S, Mahdavi SS, Spadinger I, Morris WJ, Salcudean SE. Clinical target-volume delineation in prostate brachytherapy using residual neural networks. In: Descoteaux D, Maier-Hein L, Franz A, Jannin P, Collins D. L, Duchesne S, eds. Medical image computing and computer assisted Intervention − MICCAI 2017. Cham, Switzerland: Springer; 2017. pp. 365–73. [Google Scholar]
- 32. Lucia F, Visvikis D, Desseroit M-C, Miranda O, Malhaire J-P, Robin P, et al. Prediction of outcome using pretreatment 18F-FDG PET/CT and MRI radiomics in locally advanced cervical cancer treated with chemoradiotherapy. Eur J Nucl Med Mol Imaging 2018; 45: 768–86. doi: 10.1007/s00259-017-3898-7 [DOI] [PubMed] [Google Scholar]
- 33. Pötter R, Tanderup K, Kirisits C, de Leeuw A, Kirchheiner K, Nout R, et al. The embrace II study: the outcome and prospect of two decades of evolution within the GEC-ESTRO GYN Working group and the embrace studies. Clin Transl Radiat Oncol 2018; 9: 48–60. doi: 10.1016/j.ctro.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhen X, Chen J, Zhong Z, Hrycushko B, Zhou L, Jiang S, et al. Deep convolutional neural network with transfer learning for rectum toxicity prediction in cervical cancer radiotherapy: a feasibility study. Phys Med Biol 2017; 62: 8246–63. doi: 10.1088/1361-6560/aa8d09 [DOI] [PubMed] [Google Scholar]
- 35. Wang C, Zhu X, Hong JC, Zheng D. Artificial intelligence in radiotherapy treatment planning: present and future. Technol Cancer Res Treat 2019; 18: 1533033819873922. doi: 10.1177/1533033819873922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shen C, Gonzalez Y, Klages P, Qin N, Jung H, Chen L, et al. Intelligent inverse treatment planning via deep reinforcement learning, a proof-of-principle study in high dose-rate brachytherapy for cervical cancer. Phys Med Biol 2019; 64: 115013. doi: 10.1088/1361-6560/ab18bf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Götz TI, Lahmer G, Strnad V, Bert C, Hensel B, Tomé AM, et al. A tool to automatically analyze electromagnetic tracking data from high dose rate brachytherapy of breast cancer patients. PLoS One 2017; 12: e0183608. doi: 10.1371/journal.pone.0183608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jaberi R, Siavashpour Z, Aghamiri MR, Kirisits C, Ghaderi R. Artificial neural network based gynaecological image-guided adaptive brachytherapy treatment planning correction of intra-fractional organs at risk dose variation. J Contemp Brachytherapy 2017; 9: 508–18. doi: 10.5114/jcb.2017.72567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tang X, Wang B, Rong Y. Artificial intelligence will reduce the need for clinical medical physicists. J Appl Clin Med Phys 2018; 19: 6–9. doi: 10.1002/acm2.12244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nosrati R, Soliman A, Safigholi H, Hashemi M, Wronski M, Morton G, et al. Mri-Based automated detection of implanted low dose rate (LDR) brachytherapy seeds using quantitative susceptibility mapping (QSM) and unsupervised machine learning (ML. Radiother Oncol 2018; 129: 540–7. doi: 10.1016/j.radonc.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 41. Banerjee S, Mahantshetty U, Shrivastava S. Brachytherapy in India - a long road ahead. J Contemp Brachytherapy 2014; 6: 331–5. doi: 10.5114/jcb.2014.45761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Banerjee S, Kaliyaperumal V, Kataria T, Kamaraj D. The Medanta AOLO template for locally advanced cancer cervix brachytherapy: design and clinical implementation. J Contemp Brachytherapy 2020; 12: 44–7. doi: 10.5114/jcb.2020.92528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Petereit DG, Frank SJ, Viswanathan AN, Erickson B, Eifel P, Nguyen PL, et al. Brachytherapy: where has it gone? J Clin Oncol 2015; 33: 980–2. doi: 10.1200/JCO.2014.59.8128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Han K, Milosevic M, Fyles A, Pintilie M, Viswanathan AN. Trends in the utilization of brachytherapy in cervical cancer in the United States. Int J Radiat Oncol Biol Phys 2013; 87: 111–9. doi: 10.1016/j.ijrobp.2013.05.033 [DOI] [PubMed] [Google Scholar]
- 45. Morton G. The best method for dose escalation: prostate brachytherapy. Can Urol Assoc J 2012; 6: 196–8. doi: 10.5489/cuaj.12121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gill BS, Lin JF, Krivak TC, Sukumvanich P, Laskey RA, Ross MS, et al. National cancer data base analysis of radiation therapy consolidation modality for cervical cancer: the impact of new technological advancements. Int J Radiat Oncol Biol Phys 2014; 90: 1083–90. doi: 10.1016/j.ijrobp.2014.07.017 [DOI] [PubMed] [Google Scholar]
- 47. Holschneider CH, Petereit DG, Chu C, Hsu I-C, Ioffe YJ, Klopp AH, et al. Brachytherapy: a critical component of primary radiation therapy for cervical cancer: from the Society of gynecologic oncology (SGO) and the American brachytherapy Society (ABS. Brachytherapy 2019; 18: 123–32. doi: 10.1016/j.brachy.2018.11.009 [DOI] [PubMed] [Google Scholar]
- 48. Tagliaferri L, Vavassori A, Lancellotta V, De Sanctis V, Barbera F, Fusco V, et al. Can brachytherapy be properly considered in the clinical practice? trilogy project: the vision of the AIRO (Italian association of radiotherapy and clinical oncology) interventional radiotherapy Study Group. J Contemp Brachytherapy 2020; 12: 84–9. doi: 10.5114/jcb.2020.92765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Global brachytherapy seeds market worth USD 39.73 million, at 9% CAGR during 2019-2023. 2019. Available from: https://www.businesswire.com/news/home/20190611005572/en/Global-Brachytherapy-Seeds-Market-Worth-USD-39.73.
- 50. Banerjee S, Kataria T, Goyal S, Gupta D, Bisht SS, Narang K, et al. Low dose rate permanent seed brachytherapy: tracing its evolution and current status. Prec Radiat Oncol 2020; 4: 89–98. [Google Scholar]
- 51. Nicolae A, Semple M, Lu L, Smith M, Chung H, Loblaw A, et al. Conventional vs machine learning-based treatment planning in prostate brachytherapy: results of a phase I randomized controlled trial. Brachytherapy 2020; 19: 470–6. doi: 10.1016/j.brachy.2020.03.004 [DOI] [PubMed] [Google Scholar]
- 52. Shen C, Gonzalez Y, Chen L, Nguyen D, Jia X. Automatic treatment planning in a human-like manner: operating treatment planning systems by a deep reinforcement learning based virtual treatment Planner. Int J Radiat Oncol Biol Phys 2019; 105: S256. [Google Scholar]
- 53. Jepsen P, Johnsen SP, Gillman MW, Sørensen HT. Interpretation of observational studies. Heart 2004; 90: 956–60. doi: 10.1136/hrt.2003.017269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pötter R, Haie-Meder C, Van Limbergen E, Barillot I, De Brabandere M, Dimopoulos J, et al. Recommendations from gynaecological (GYN) GEC ESTRO Working Group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol 2006; 78: 67–77. doi: 10.1016/j.radonc.2005.11.014 [DOI] [PubMed] [Google Scholar]
- 55. Naghavi AO, Fernandez DC, Mesko N, Juloori A, Martinez A, Scott JG, et al. American brachytherapy Society consensus statement for soft tissue sarcoma brachytherapy. Brachytherapy 2017; 16: 466–89. doi: 10.1016/j.brachy.2017.02.004 [DOI] [PubMed] [Google Scholar]
- 56. Kovács G, Martinez-Monge R, Budrukkar A, Guinot JL, Johansson B, Strnad V, et al. GEC-ESTRO ACROP recommendations for head & neck brachytherapy in squamous cell carcinomas: 1st update - Improvement by cross sectional imaging based treatment planning and stepping source technology. Radiother Oncol 2017; 122: 248–54. doi: 10.1016/j.radonc.2016.10.008 [DOI] [PubMed] [Google Scholar]


