Abstract
Objectives:
To assess the methodological quality of radiomic studies based on positron emission tomography/computed tomography (PET/CT) images predicting epidermal growth factor receptor (EGFR) mutation status in patients with non-small cell lung cancer (NSCLC).
Methods:
We systematically searched for eligible studies in the PubMed and Web of Science datasets using the terms “radiomics”, “PET/CT”, “NSCLC”, and “EGFR”. The included studies were screened by two reviewers independently. The quality of the radiomic workflow of studies was assessed using the Radiomics Quality Score (RQS). Interclass correlation coefficient (ICC) was used to determine inter rater agreement for the RQS. An overview of the methodologies used in steps of the radiomics workflow and current results are presented.
Results:
Six studies were included with sample sizes of 973 ranging from 115 to 248 patients. Methodologies in the radiomic workflow varied greatly. The first-order statistics were the most reproducible features. The RQS scores varied from 13.9 to 47.2%. All studies were scored below 50% due to defects on multiple segmentations, phantom study on all scanners, imaging at multiple time points, cut-off analyses, calibration statistics, prospective study, potential clinical utility, and cost-effectiveness analysis. The ICC results for majority of RQS items were excellent. The ICC for summed RQS was 0.986 [95% confidence interval (CI): 0.898–0.998].
Conclusions:
The PET/CT-based radiomics signature could serve as a diagnostic indicator of EGFR mutation status in NSCLC patients. However, the current conclusions should be interpreted with care due to the suboptimal quality of the studies. Consensus for standardization of PET/CT-based radiomic workflow for EGFR mutation status in NSCLC patients is warranted to further improve research.
Advances in knowledge:
Radiomics can offer clinicians better insight into the prediction of EGFR mutation status in NSCLC patients, whereas the quality of relative studies should be improved before application to the clinical setting.
Introduction
Lung cancer is the leading cause of cancer-related deaths around the world, and more than 26% of cancer deaths would be caused by lung cancer. Non-small cell lung cancer (NSCLC) accounts for over 85% of lung cancer, of which, lung adenocarcinoma being the most common pathological type. 1 In recent years, targeted therapy has attracted extensive attention in NSCLC due to the development of molecular biology. In particular, epidermal growth factor receptor (EGFR) targeting tyrosine kinase inhibitors (TKIs) has been widely used in the treatment of NSCLC. 2,3 Targeting EGFR is a promising strategy to effectively improve the prognosis of patients with lung adenocarcinoma. Thus, it is of great importance to identify whether a patient has EGFR mutations before TKIs targeted therapy.
Currently, tumor tissue or cytological specimens are commonly used in clinical practice for gene testing, however, they are mostly invasive and unavailable to a number of NSCLC patients due to technical challenges, medical cost, patient health status, and tumor heterogeneity. 4 Accordingly, it is necessary to develop non-invasive and accurate methods to detect gene mutations. Some studies used 18F-FDG PET/CT to predict the EGFR mutation status, usually based on visual analysis or conventional quantitative indicators such as standard uptake value (SUV), leading to unsatisfactory predictive ability. 5–7
Recent progress in EGFR mutation prediction demonstrate promising results in objectively interpreting PET/CT images using quantitative imaging analysis (QIA), such as an emerging field of radiomics. 8,9 Radiomics refers to a high-throughput QIA method that extracts a huge number of quantitative features from standard-of-care medical images. 10 It is hypothesized that the features capture underlying information invisible to the naked eyes in which the intratumoral heterogeneity of the entire lung tumor can be detected and quantified. 11,12 Recently, 18F-FDG PET/CT radiomics, which extracts textural features from PET/CT images, has demonstrated certain potential in predicting EGFR mutation. 13–18
The radiomic workflow consists of five phases: data selection, medical imaging, feature extraction, exploratory analysis, and modeling. 19 The methodology used in all steps would affect the quality of a radiomic work. Various methodologies may lead to heterogeneous findings that make the comparison across studies difficult. To the best of our knowledge, the variation in methodologies applied in PET/CT-based radiomics for predicting EGFR mutation status in NSCLC patients has not yet been evaluated. This systematic review aims to assess the quality of the radiomic workflow using the Radiomics Quality Score (RQS) as proposed by Lambin et al 20 and to report on the different methodologies used in all steps of radiomic workflow. The RQS assigns points to all steps in the radiomics workflow where the maximum number of points per item depends on the relevance to quality. In addition, we summarize and briefly evaluate the current results reported on the topic of PET/CT-based radiomics for the prediction of EGFR mutation in NSCLC patients. We intended to promote the quality of reporting of radiomics in NSCLC studies and increase the reliability of radiomics for the EGFR detection in the clinical setting.
Methods and materials
Literature search
For this systematic review, a structured search using the PubMed and Web of Science datasets from the conception to October 4, 2020, were performed. Two researchers with 10 and 15 years’ experience in lung cancer diagnosis independently conducted the literature search to identify the eligible studies. The search strategies are described in Supplementary Material 1.
Inclusion criteria and study selection
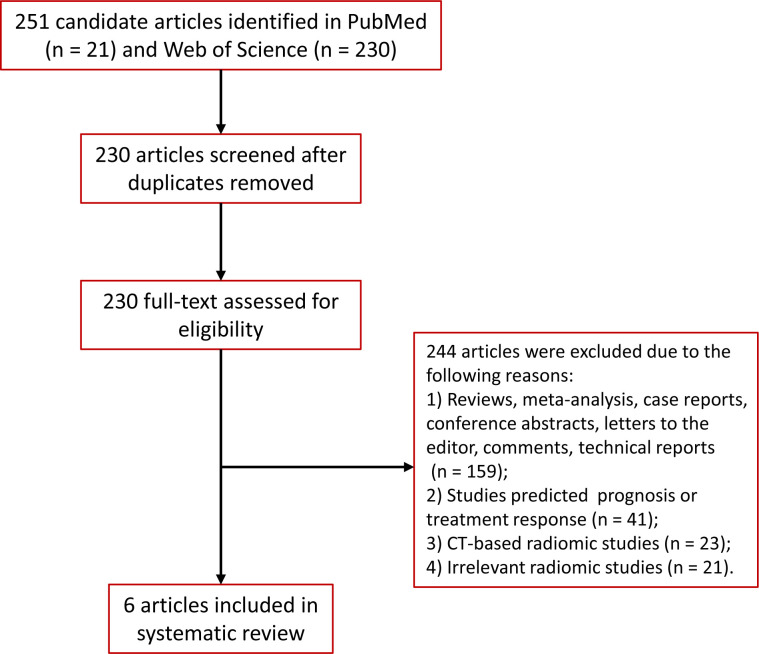
Studies were included if they met the following criteria: (1) The cohort consists of pathology-confirmed NSCLC patients who underwent pre-treatment 18F-FDG PET/CT scans before surgery or biopsy; (2) patients received no anti tumor prior to PET/CT; (3) patients had gene testing for EGFR mutation; (4) PET/CT-based radiomic analysis was used to predict the EGFR mutation status; and 5) studies were reported in English with available full-text. We excluded reviews, meta-analysis, case reports, conference abstracts, letters to the editor, comments, technical reports, and duplicate studies. Full details of screening and eligibility assessment are provided in Figure 1.
Figure 1.
Flow diagram of the study selection process
Two independent investigators first screened the titles and abstracts to determine whether the citation met the eligibility criteria. They screened the full text for potentially relevant radiomic studies when both agreed that a citation met the eligibility criteria. Disagreements between the investigators were resolved by consensus and, if necessary, consultation with a third investigator. For each included study, reference lists were searched manually for additional eligible studies.
Data extraction
The following data were extracted (if available): the first author, year of publication, study design, sample size, tumor stage, the proportion of EGFR-mutant patients, PET/CT imaging parameters, image processing, features extraction platform, number of features, the region of interest (ROI) segmentation method, feature selection method, modeling method, and prediction performance in terms of area under the curve (AUC).
Assessment of the quality of radiomic studies
To quantify the radiomic workflow quality and its reporting, the included studies were evaluated in consensus by the two initial reviewers using the RQS. This checklist is comprised of 16 components in the radiomic workflow, with a minimum score of −8 to 0 defined as 0% and a maximum score of 36 defined as 100%. 20 The selected studies were assessed for (1) Image protocol quality; (2) Multiple segmentations; (3) Phantom study on all scanners; (4) Imaging at multiple points; (5) Feature reduction or adjustment for multiple testing; (6) Multivariable analysis with non-radiomic features; (7) Detect and discuss biological correlates; (8) Cut-off analyses; (9) Discrimination statistics; (10) Calibration statistics; (11) Prospective study registered in a trial database; (12) Validation; (13) Comparison to “gold standard”; (14) Potential clinical utility; (15) Cost-effectiveness analysis; and (16) Open science and data. To reflect the importance of prospective studies, seven points are assigned when these are performed and published. Another important step is external validation on at least three datasets, which yielded five points, but if no external validation is performed at all, five points are deducted. Most items can receive zero, one, or two point(s). The detailed checklist is described in Supplementary Table 1.
Statistical analysis
Statistical analysis was performed with SPSS v.23.0 software (IBM Corporation, Armonk, NY). The summed RQS rating per study was calculated and the average rating of all raters is reported. The interclass correlation coefficient (ICC) was determined to describe inter rater agreement for the RQS.
Results
Study selection
A total of 251 records were identified through the searches in PubMed (21 records) and Web of Science (230 records). After removing duplicates, the titles and abstracts of records were screened, resulting in 230 records eligible for inclusion. The full text of these studies was read and the selection criteria were applied. Finally, a total of six studies were included for this systematic review.
Characteristics of the included studies
A total of 973 NSCLC patients were retrospectively included in the six studies presented in this review (Table 1). All studies were recently published, between 2019 and 2020. Sample size ranged from 115 to 248, with a largest validation cohort of 73 patients. Most studies were performed at a single center, only two were conducted in two centers. All studies reported the detection method of EGFR mutation with the exception of Koyasu et al 18 . The percentages of EGFR mutation ranged from 22.5 to 62.6%.
Table 1.
Characteristics of included studies
| First author | Zhang et al 16 | Shiri et al 15 | Liu et al 14 | Koyasu et al 18 | Yang et al 13 | Li et al 17 |
|---|---|---|---|---|---|---|
| Publication date | 2019 | 2020 | 2020 | 2019 | 2020 | 2019 |
| Study design | Retrospective | Retrospective | Retrospective | Retrospective | Retrospective | Retrospective |
| Sample size | 248 | 150 | 148 | 138 | 174 | 115 |
| Single center or multicenter | Single center | Unclear | Two centers | Two centers | Single center | Single center |
| PET/CT manufacturer | PHILIPS | GE and Siemens | Siemens | Unclear | Siemens | GE |
| Image processing | Unclear | Yes | Yes | No | Yes | No |
| ROI segmentation method | Semi-automatically | PET, manually CT, semi-automatically |
Manually | Semi-automatically | Semi-automatically | Semi-automatically |
| Feature classes | FOS, Shape, GLCM, GLZLM, GLRLM, NGLDM | FOS, GLCM, GLRLM, Shape, GLSZM, GLDM, NGTDM | FOS, Shape, GLCM, GLRLM, GLSZM, GLDM, NGTDM | Histogram, GLCM, LBP | FOS, Shape, GLCM, GLDM, GLRLM, GLSZM, NGTDM | morphological features, grayscale statistic features, GLCM, GGCM, GLDS |
| Feature extraction software | LIFEx package | Python with package Pyradiomics | Python 3.6.2 with package Pyradiomics |
Unclear | Radiomics prototype (Radiomics, Frontier, Siemens) |
MATLAB |
| Feature selection method | LASSO | SKB, VT, SP, SM, VT-SM, VT-KB |
VIF, RF, LR | Unclear | LASSO | LASSO |
| No. of total features per patient | 92 (47 from PET and 45 from CT) |
109 from PET | 1570 (1470 from CT and 100 from PET) |
261 (173 from CT and 88 from PET) | 3344 (1672 from CT and 1672 from PET) | 38 |
| No. of selected features | 9 | 3 | 10 | 6 | 11 | 9 |
| Modeling algorithm | LR | SVM, KNN, DT, QDA, MLP, SGD, LR, NB, GNB, RF, AB, BAG | XGBoost | RF and XGBoost | RF | Ensembles for boosting machine learning scheme |
| No. EGFR mutation (%) | 53.6 | 22.5 | 50.6 | 27.5 | 62.6 | 56.0 |
| Statistical findings: radiomic model performance | The model showed a significant ability to discriminate between EGFR mutation and EGFR wild type, with AUC of 0.79 in the training set, and 0.85 in the validation set, compared with 0.75 and 0.69 for the clinical model. When clinical variables and radiomics signature were combined, the AUC increased to 0.86 (95% CI: 0.80–0.91) in the training set and 0.87 (95% CI: 0.79–0.95) in the validation set, thus showing better performance in the prediction of EGFR mutations. |
Multivariate machine learning-based AUC performances were significantly improved to 0.82 for EGFR (LOG preprocessed image of PET with sigma three with variance threshold feature selector and stochastic gradient descent classifier (q-value = 4.86E-05). . |
The corresponding radiomic predictors achieved the prediction accuracies of 0.77 and 0.92 in terms of AUC, respectively. Combing these two predictors, the overall model for predicting EGFR mutation positivity was also constructed, and the AUC was 0.87. | In the classification of EGFR mutation status, the AUC values were: RF, single type: 0.625; XGBoost, single type: 0.617; RF, multiple types: 0.577; XGBoost, multiple types: 0.659. | The mutant/wild-type model was identified in the training (AUC, 0.77) and validation (AUC, 0.71) groups. The 19/21 mutation site model had an AUC of 0.82 and 0.73 in the training and validation groups, respectively. | A radiomic signature based on both PET and CT radiomic features outperformed individual radiomic features, the PET or CT radiomic signature, and the conventional PET parameters including the maximum standardized uptake value (SUVmax), SUVmean, SUVpeak, metabolic tumor volume (MTV), and total lesion glycolysis (TLG), in discriminating between mutant-type of EGFR and wild-type of EGFR- cases with an AUC of 0.805, an accuracy of 80.798%, a sensitivity of 0.826 and a specificity of 0.783. |
| Conclusions | The PET/CT-based radiomic features showed good performance in predicting EGFR mutation in NSCLC, providing a useful method for the choice of targeted therapy in a clinical setting. | Our work demonstrated that non-invasive and reliable radiomics analysis can be successfully used to predict EGFR and KRAS mutation status in NSCLC patients. We demonstrated that radiomic features extracted from different image-feature sets could be used for EGFR and KRAS mutation status prediction in NSCLC patients and showed improved predictive power relative to conventional image-derived metrics. | We established predictive models based on radiomic analysis of 18F-FDG PET/CT images. And it achieved a satisfying prediction power in the identification of EGFR mutation status as well as the certain EGFR mutation subtypes in lung cancer. | The radiomics approach to PET/CT images, together with XGBoost and Bayesian optimization, is useful for classifying histological subtypes and EGFR mutation status in lung cancer. | Radiomic features based on (18F)-FDG PET/CT combined with clinicopathological factors could reflect genetic differences and predict EGFR mutation type. | Tumor imaging phenotypes that are driven by somatic mutations may be predicted by radiomics based on PET/CT images. |
AB, Adaptive boosting; AUC, area under the curve; BAG, Bagging; CI, Confidence interval; DT, Decision tree; EGFR, Epidermal growth factor receptor;FOS, First-order statistics; GLCM, Gray-level co-occurrence matrix; GLDM, Gray-level dependence matrix; GLRLM, Gray-level run length matrix; GLSZM, Gray-Level Size Zone Matrix; GNB, Gaussian naive Bayes; KNN, K-nearest neighbors; KRAS, Kirsten rat sarcoma viral oncogene; LASSO, Least absolute shrinkage and selection operator; LR, Logistic regression; MLP, Multilayer perceptron; NB, Naive Bayes; NGTDM, Neighboring gray tone difference matrix; NSCLC, Non-small cell lung cancer; PET/CT, Positron emission tomography/Computed tomography; QDA, Quadratic discriminant analysis; RF, Random forest; ROI, Region of interest; SGD, Stochastic gradient descent; SKB, Select K best; SM, Select from model; SP, Select percentile; SVM, Support vector machine; VIF, Variance inflation factor; VT, Variance threshold; VT-KB, Variance threshold and select K best; VT-SM, Variance threshold and select from model.
Quality analysis of the included studies
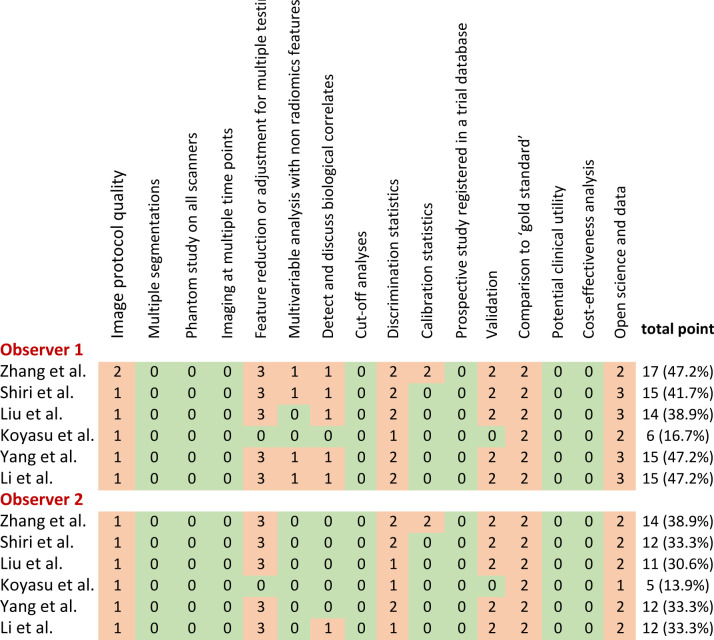
The RQS heatmap (Figure 2) presents the score of each item and the total score for each study. The mean RQS of all studies evaluated by two raters was 12.7 (35.2%) points, ranging from 5 (13.9%) to 17 (47.2%) points. All studies were scored below 50% due to defects on multiple segmentations, phantom study on all scanners, imaging at multiple time points, cut-off analyses, calibration statistics, prospective study registered in a trial database, potential clinical utility, and cost-effectiveness analysis. The ICC results for the assessment of major RQS items were good (Table 2). The ICC for summed RQS between two raters was 0.986 (95% CI: 0.898–0.998), demonstrating good agreement (Table 2).
Figure 2.
Heat map of radiomics quality score (RQS) per component of all included studies.
Table 2.
Average rating and interrater agreement per component of QRS
| No. | RQS scoring item | Range | Average | ICC (95% CI) |
|---|---|---|---|---|
| 1 | Image protocol quality | 0 to 2 | 1.17 | 1.00 (1.00–1.00) |
| 2 | Multiple segmentations | 0 to 1 | 0 | 1.00 (1.00–1.00) |
| 3 | Phantom study on all scanners | 0 to 1 | 0 | 1.00 (1.00–1.00) |
| 4 | Imaging at multiple time points | 0 to 1 | 0 | 1.00 (1.00–1.00) |
| 5 | Feature reduction or adjustment for multiple testing | −3 to 3 | 2.50 | 1.00 (1.00–1.00) |
| 6 | Multivariable analysis with non-radiomics features | 0 to 1 | 0.58 | 0.83 (-0.23–0.98) |
| 7 | Detect and discuss biological correlates | 0 to 1 | 0.50 | 0.33 (-3.76–0.91) |
| 8 | Cut-off analyses | 0 to 1 | 0 | 1.00 (1.00–1.00) |
| 9 | Discrimination statistics | 0 to 2 | 1.67 | 0.60 (-1.86–−0.94) |
| 10 | Calibration statistics | 0 to 2 | 0.33 | 1.00 (1.00–1.00) |
| 11 | Prospective study registered in a trial database | 0 to 7 | 0 | 1.00 (1.00–1.00) |
| 12 | Validation | −5 to 5 | 1.67 | 1.00 (1.00–1.00) |
| 13 | Comparison to “gold standard” | 0 to 2 | 2.00 | 1.00 (1.00–1.00) |
| 14 | Potential clinical utility | 0 to 2 | 0 | 1.00 (1.00–1.00) |
| 15 | Cost-effectiveness analysis | 0 to 1 | 0 | 1.00 (1.00–1.00) |
| 16 | Open science and data | 0 to 4 | 2.25 | 0.76 (-0.70–0.97) |
| Total points: −8 to 0 = 0%, 36 = 100% | 0–100% |
CI, Confidence interval; ICC, Interclass correlation coefficient; RQS, Radiomics quality score.
Radiomics workflow
Image acquisition and pre-processing: One study established the imaging acquisition protocol following the Image Biomarker Standardization Initiative (IBSI) reporting guidelines. 16 The authors of all studies performed their analyses on the pre-treatment PET/CT images. None of the studies analyzed the differences between PET/CT exams at least two time points. Various PET/CT manufacturers were used, including GE, PHILIPS, Siemens, or both. Half of the studies reported on image pre-processing performed by interpolation (sitkBSpline algorithm, B-spline of order three interpolation), Wavelet transform +Laplacian of Gaussian (Fine, Median, Coarse)+Bin discretization, resample +discretization + rescale to reduce the bias caused by different scanners and imaging protocols. None of the studies performed feature normalization.
Volume of interest segmentation: All studies performed three dimensional (3D) segmentation, four performed semi-automatically, one manually, and one both semi-automatically (CT images) and manually (PET images). For semi-automatic segmentation by methods including region growing or drawing a line along the boundary of the tumor with a Radiomics prototype, the region of interest (or tumor) used a threshold of 40–42% of the maximum standardized uptake value (SUVmax). The manual segmentations were conducted with OSIRIX® or ITK-SNAP or ImageJ 1.50i software. The radiologists were blinded to the pathologic and EGFR mutation test results.
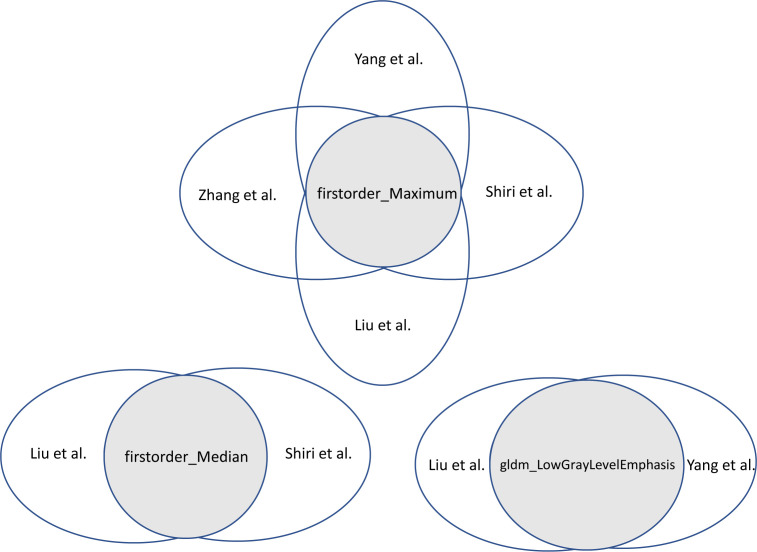
Feature extraction and selection: The number of features extracted from 38 to 3344, mostly extracted with in-house built software, such as LIFEx, Pyradiomics, and MATLAB. The studies extracted similar classes of features: first-order statistics, shape-based features, and texture features. Feature selection only applied to the studies developing multivariate prediction models, where these studies opted for a different approach, with the least absolute shrinkage and selection operator (LASSO) most often. Two studies used more than one feature selection method. 14,15 The study of Koyasu et al 18 did not report on the feature selection method. The number of features included in the models varied between 3 and 11 features (Supplementary Table 2). Figure 3 shows the overlapped features among the included features. The first-order statistics (e.g. maximum and median) were the most reproducible features that significantly related to EGFR mutations.
Radiomics analysis and modeling: All studies presented individual features significant for EGFR mutation status. Three studies developed clinical, radiomic, and combined models. 13,16,17 The clinical models usually consisted of sex and smoking history, other variables included age, tumor stage, and location. The combined models were superior to clinical model and radiomic model alone. Two studies analyzed the predictive value of PET/CT metabolic parameters alone or together with radiomic signature and/or clinical variables. 13,15 One study compared the predictive value of radiomic features derived from PET/CT, PET, and CT, respectively, and found that the combined PET/CT radiomic features outperformed radiomics signature based on PET alone and CT alone. 17 All studies predicted general EGFR mutation except one study 14 predicted EGFR mutation subtypes (EGFR exon 19 deletions and exon 21 L858R missense). Three studies 15,17,18 compared at least two machine learning methods in predicting EGFR mutation status, especially Shiri et al 15 . To validate the models, the majority of studies performed 10-fold cross-validation. All studies divided their data into a training and a validation cohort. No study externally validated their models.
Prediction performance evaluation: The prediction performance of models was distinctly different. The AUCs results of the multivariate analysis in differentiating EGFR mutation status, for the validation cohort, ranged from 0.659 to 0.870. The performance of models based on clinical or PET/CT or radiomic signature alone was relatively low and could be significantly improved when incorporated, highlighting the necessity of combining multisource data to develop prediction models prior to clinical application.
Figure 3.
The overlap features among the included studies.
Discussion
The systematic review provides an overview of the recent literature on PET/CT-based radiomics for EGFR mutation status in NSCLC patients. Overall, the studies indicated large methodologies heterogeneity in each step of radiomic workflow, such as differences in image acquisition, feature extraction, feature selection, and modeling strategy. Despite the radiomic workflow heterogeneities, the results seem promising. The AUCs of the models in differentiating EGFR mutation status, for the validation cohort, ranged from 0.659 to 0.870.
18F-FDG PET/CT cannot only depict anatomical structure, but also reflect the biological characteristics of tumors. However, the PET/CT parameters in predicting EGFR mutation status remain controversial. 21–24 Shiri et al performed a univariate analysis of conventional PET metrics for EGFR mutation prediction showed that SUVpeak had the highest value, with an AUC of 0.69, whereas the AUCs of other parameters were below 0.60. 15 Li et al 17 found that the AUCs of conventional PET parameters to predict EGFR mutational status in NSCLC ranged from 0.615 to 0.621. Previous studies demonstrated that the predictive performance of radiomic features extracted from PET/CT images was significantly higher than conventional PET/CT parameters. 13,15 The addition of clinical variables and radiomic features into PET/CT parameters could further improve the discrimination of models, achieving an AUC of around 0.80 in the validation cohorts. 13,16,17 The radiomic features incorporated in the prediction models greatly varied across studies, the overall robustness of features was relatively poor, however, the susceptibility of those features of variation was significantly different. The findings showed that first-order features (e.g. maximum, median) were potentially more related to EGFR mutations in NSCLC. Variations in scanners, imaging protocols, segmentation strategies, feature selection, and feature-intrinsic characteristics pose a threat to robustness and reproducibility of features. Identification of robust radiomics features may improve the generalizability of radiomic models in future studies and fasten the clinical application.
In this review, we applied the RQS checklist to assess the quality of included studies and reported the radiomic workflow. The inter rater agreement was good for most items, as indicated by some previous reviews. 25,26 The studies employing multivariate analysis achieved a mean RQS of 12.7 (35.2%) points, ranging from 5 (13.9%) to 17 (47.2%) points. The RQS is largely affected by the items prospective study registered in a trial database and validation because they account for 7 and 5 points, respectively (i.e. 33.3% of the maximum score). In this review, all studies were retrospective and internally validated. Besides, none of the studies conducted multiple segmentations, phantom study, imaging at multiple time points, cut-off analyses, clinical utility, and cost-effectiveness analysis. Some previous systematic reviews have evaluated the quality of radiomic studies across various tumors (e.g. bone, soft-tissues and visceral sarcomas, prostate cancer, NSCLC, lymphoma, renal cell carcinoma, neuro-oncology, breast cancer, rectal cancer, liver cancer, head and neck cancer). 25–40 The authors found that the mean RQS scores of all studies were less than 50%, ranging from 9.4 to 41.2%. Substantial quality limitations were found in study design, radiomic workflow methodology, validation, prospective study, and open science and data. Most of radiomic studies were retrospective and performed in single center or two centers. Few studies shared their code, images, and features. As radiomics is still a very young field, radiomic research has not yet been translated into clinical use. Current promising radiomic models can be externally validated to demonstrate the generalizability of findings and their impact assessed within the clinical pathway prior to they can be implemented as a clinical decision-making tool to facilitate targeted treatment for patients with NSCLC.
Some limitations in this systematic review should be acknowledged. Firstly, PET/CT-based radiomics studies for the prediction of EGFR mutation status are limited. Hence, only six studies with approximately 300 patients were analyzed. Secondly, the RQS has limitations, several components are not always applicable to every study, for example, the clinical utility for pre-clinical studies or “comparison to gold standard” for outcomes in which no gold standard exists for the assessment of outcome (e.g. intratumoral heterogeneity). Other components are not described in detail in the scoring table (e.g. what should be specifically be mentioned on clinical utility) or are relatively ambiguous (e.g. in case that only a subgroup of features has been extracted that does not feature selection in strict sense). Moreover, the interpretability of the RQS score is left to a large extent to the raters, since it is not meant to report on the overall quality of a manuscript or the research presents, but rather to guide the reader on a radiomic workflow appraisal. Therefore, widespread application of RQS, pre-trained RQS scoring procedure, and modification of RQS in response to actual clinical practice needs are necessary considering radiomics is a developing field. Thirdly, a meta-analysis could not be performed because most studies did not report the necessary data. In addition, the heterogeneity across studies were substantial, so it would be better to conduct systemic review. However, a network meta-analysis may relieve the limitation caused by missing data and increase the possibility of conducting a meta-analysis of radiomic studies on the same topic. Thus, future works can use this network meta-analysis approach to accelerate the clinical translation of predicting EGFR mutation using radiomics. Finally, this review did not investigate the role of CT- or PET-based radiomics for EGFR detection or survival prediction in patients with EGFR mutations. 41–43 However, radiomics using PET/CT may provide more information than that using CT or PET alone.
In conclusion, the quality of PET/CT-based radiomic studies for predicting EGFR mutation status in NSCLC was somewhat poor. Methodologies in the radiomic workflow varied greatly. Although these scores are relatively low, and radiomics has not yet reached clinical utility in NSCLC, it is important to underscore the fact that these early studies pave the way for the radiomic field with a focus on NSCLC. Further efforts are needed to make PET/CT-based radiomic studies in NSCLC useful and reproducible with an acceptable level of evidence. A better knowledge of the RQS and the reporting guidelines of radiomics could improve the quality of NSCLC radiomic studies. Increasing education about the particular radiomics’ methodology and quality metrics are necessary to fasten and accelerate clinical applications. Overall, our review stresses the urgent need for improving the quality and reproducibility of radiomic studies. This review will be beneficial for the construct of a more consistent or reproducible research in the field of radiomics.
Contributor Information
Meilinuer Abdurixiti, Email: mlnr3355@sina.cn.
Mayila Nijiati, Email: 1581103983@qq.com.
Rongfang Shen, Email: shenrongfang0723@sina.com.
Qiu Ya, Email: 1254695790@qq.com.
Naibijiang Abuduxiku, Email: 591330377@qq.com.
Mayidili Nijiati, Email: 1376906729@qq.com.
REFERENCES
- 1. Hsu W-H, Yang JC-H, Mok TS, Loong HH. Overview of current systemic management of EGFR-mutant NSCLC. Ann Oncol 2018; 29(suppl_1): i3–9. doi: 10.1093/annonc/mdx702 [DOI] [PubMed] [Google Scholar]
- 2. Cheng H, Li X-J, Wang X-J, Chen Z-W, Wang R-Q, Zhong H-C, et al. A meta-analysis of adjuvant EGFR-TKIs for patients with resected non-small cell lung cancer. Lung Cancer 2019; 137: 7–13. doi: 10.1016/j.lungcan.2019.08.002 [DOI] [PubMed] [Google Scholar]
- 3. Tang W, Li X, Xie X, Sun X, Liu J, Zhang J, et al. Egfr inhibitors as adjuvant therapy for resected non-small cell lung cancer harboring EGFR mutations. Lung Cancer 2019; 136: 6–14. doi: 10.1016/j.lungcan.2019.08.001 [DOI] [PubMed] [Google Scholar]
- 4. Wang S, Shi J, Ye Z, Dong D, Yu D, Zhou M, et al. Predicting EGFR mutation status in lung adenocarcinoma on computed tomography image using deep learning. Eur Respir J 2019; 53: 1800986. doi: 10.1183/13993003.00986-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cook GJR, Azad G, Owczarczyk K, Siddique M, Goh V. Challenges and promises of PET Radiomics. Int J Radiat Oncol Biol Phys 2018; 102: 1083–9. doi: 10.1016/j.ijrobp.2017.12.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee SM, Bae SK, Jung SJ, Kim CK. Fdg uptake in non-small cell lung cancer is not an independent predictor of EGFR or KRAS mutation status: a retrospective analysis of 206 patients. Clin Nucl Med 2015; 40: 950–8. doi: 10.1097/RLU.0000000000000975 [DOI] [PubMed] [Google Scholar]
- 7. Takamochi K, Mogushi K, Kawaji H, Imashimizu K, Fukui M, Oh S, et al. Correlation of EGFR or KRAS mutation status with 18F-FDG uptake on PET-CT scan in lung adenocarcinoma. PLoS One 2017; 12: e0175622. doi: 10.1371/journal.pone.0175622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whi W, Ha S, Bae S, Choi H, Paeng JC, Cheon GJ, et al. Relationship of EGFR mutation to glucose metabolic activity and Asphericity of metabolic tumor volume in lung adenocarcinoma. Nucl Med Mol Imaging 2020; 54: 175–82. doi: 10.1007/s13139-020-00646-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ninatti G, Kirienko M, Neri E, Sollini M, Chiti A, Gaia N. Imaging-based prediction of molecular therapy targets in NSCLC by radiogenomics and AI approaches: a systematic review. Diagnostics 2020; 10: 359: E359 30 05 2020. doi: 10.3390/diagnostics10060359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gillies RJ, Kinahan PE, Hricak H, Gillies Robert J, Hedvig H. Radiomics: images are more than pictures, they are data. Radiology 2016; 278: 563–77. doi: 10.1148/radiol.2015151169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ha S, Choi H, Paeng JC, Cheon GJ. Radiomics in oncological PET/CT: a methodological overview. Nucl Med Mol Imaging 2019; 53: 14–29. doi: 10.1007/s13139-019-00571-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee JW, Lee SM, Won LJ, Mi LS. Radiomics in oncological PET/CT: clinical applications. Nucl Med Mol Imaging 2018; 52: 170–89. doi: 10.1007/s13139-017-0500-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang B, Ji H-S, Zhou C-S, Dong H, Ma L, Ge Y-Q, et al. 18F-fluorodeoxyglucose positron emission tomography/computed tomography-based radiomic features for prediction of epidermal growth factor receptor mutation status and prognosis in patients with lung adenocarcinoma. Transl Lung Cancer Res 2020; 9: 563–74. doi: 10.21037/tlcr-19-592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu Q, Sun D, Li N, Kim J, Feng D, Huang G, et al. Predicting EGFR mutation subtypes in lung adenocarcinoma using 18F-FDG PET/CT radiomic features. Transl Lung Cancer Res 2020; 9: 549–62. doi: 10.21037/tlcr.2020.04.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shiri I, Maleki H, Hajianfar G, Abdollahi H, Ashrafinia S, Hatt M, et al. Next-Generation Radiogenomics sequencing for prediction of EGFR and KRAS mutation status in NSCLC patients using multimodal imaging and machine learning algorithms. Mol Imaging Biol 2020; 22: 1132–48. doi: 10.1007/s11307-020-01487-8 [DOI] [PubMed] [Google Scholar]
- 16. Zhang J, Zhao X, Zhao Y, Zhang J, Zhang Z, Wang J, et al. Value of pre-therapy 18F-FDG PET/CT radiomics in predicting EGFR mutation status in patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging 2020; 47: 1137–46. doi: 10.1007/s00259-019-04592-1 [DOI] [PubMed] [Google Scholar]
- 17. Li X, Yin G, Zhang Y, Dai D, Liu J, Chen P, et al. Predictive power of a radiomic signature based on 18F-FDG PET/CT images for EGFR mutational status in NSCLC. Front Oncol 2019; 9: 1062. doi: 10.3389/fonc.2019.01062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koyasu S, Nishio M, Isoda H, Nakamoto Y, Togashi K. Usefulness of gradient tree boosting for predicting histological subtype and EGFR mutation status of non-small cell lung cancer on 18F FDG-PET/CT. Ann Nucl Med 2020; 34: 49–57. doi: 10.1007/s12149-019-01414-0 [DOI] [PubMed] [Google Scholar]
- 19. Fornacon-Wood I, Faivre-Finn C, O'Connor JPB, Price GJ. Radiomics as a personalized medicine tool in lung cancer: separating the hope from the hype. Lung Cancer 2020; 146: 197–208. doi: 10.1016/j.lungcan.2020.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 2017; 14: 749–62. doi: 10.1038/nrclinonc.2017.141 [DOI] [PubMed] [Google Scholar]
- 21. Putora PM, Szentesi K, Glatzer M, Rodriguez R, Müller J, Baty F, et al. SUVmax and tumour location in PET-CT predict oncogene status in lung cancer. Oncol Res Treat 2016; 39: 681–6. doi: 10.1159/000450622 [DOI] [PubMed] [Google Scholar]
- 22. Desseroit M-C, Visvikis D, Tixier F, Majdoub M, Perdrisot R, Guillevin R, et al. Development of a nomogram combining clinical staging with (18)F-FDG PET/CT image features in non-small-cell lung cancer stage I-III. Eur J Nucl Med Mol Imaging 2016; 43: 1477–85. doi: 10.1007/s00259-016-3325-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang D, Zhang M, Gao X, Yu L. Prognostic value of baseline 18F-FDG PET/CT functional parameters in patients with advanced lung adenocarcinoma stratified by EGFR mutation status. PLoS One 2016; 11: e0158307. doi: 10.1371/journal.pone.0158307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miao Z, Ren G, Liu H, Qi S, Wu S, Cheng Z. Pet of EGFR expression with an 18F-labeled affibody molecule. J Nucl Med 2012; 53: 1110–8. doi: 10.2967/jnumed.111.100842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stanzione A, Gambardella M, Cuocolo R, Ponsiglione A, Romeo V, Imbriaco M. Prostate MRI radiomics: a systematic review and radiomic quality score assessment. Eur J Radiol 2020; 129: 109095. doi: 10.1016/j.ejrad.2020.109095 [DOI] [PubMed] [Google Scholar]
- 26. Ursprung S, Beer L, Bruining A, Woitek R, Stewart GD, Gallagher FA, et al. Radiomics of computed tomography and magnetic resonance imaging in renal cell carcinoma-a systematic review and meta-analysis. Eur Radiol 2020; 30: 3558–66. doi: 10.1007/s00330-020-06666-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Crombé A, Fadli D, Italiano A, Saut O, Buy X, Kind M, Amandine C, David F, Antoine I. Systematic review of sarcomas radiomics studies: bridging the gap between concepts and clinical applications? Eur J Radiol 2020; 132: 109283. doi: 10.1016/j.ejrad.2020.109283 [DOI] [PubMed] [Google Scholar]
- 28. Sunoqrot MRS, Selnæs KM, Sandsmark E, Nketiah GA, Zavala-Romero O, Stoyanova R, et al. A quality control system for automated prostate segmentation on T2-weighted MRI. Diagnostics 2020; 10: 714: E714. doi: 10.3390/diagnostics10090714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhong J, Hu Y, Si L, Jia G, Xing Y, Zhang H, et al. A systematic review of radiomics in osteosarcoma: utilizing radiomics quality score as a tool promoting clinical translation. Eur Radiol 2021; 31: 1526–35. doi: 10.1007/s00330-020-07221-w [DOI] [PubMed] [Google Scholar]
- 30. Chetan MR, Gleeson FV, Chetan Madhurima R, Fergus G V. Radiomics in predicting treatment response in non-small-cell lung cancer: current status, challenges and future perspectives. Eur Radiol 2021; 31: 1049-1058. doi: 10.1007/s00330-020-07141-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. T JMC, Arif M, Niessen WJ, Schoots IG, Veenland JF. Automated classification of significant prostate cancer on MRI: a systematic review on the performance of machine learning applications. Cancers 2020; 12: E1606 10.3390/cancers12061606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang H, Zhou Y, Li L, Hou W, Ma X, Tian R. Current status and quality of radiomics studies in lymphoma: a systematic review. Eur Radiol 2020; 30: 6228–40. doi: 10.1007/s00330-020-06927-1 [DOI] [PubMed] [Google Scholar]
- 33. Sollini M, Gelardi F, Matassa G, Delgado Bolton RC, Chiti A, Kirienko M. Interdisciplinarity: an essential requirement for translation of radiomics research into clinical practice -A systematic review focused on thoracic oncology. Rev Esp Med Nucl Imagen Mol 2020; 39: 146–56. doi: 10.1016/j.remnie.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 34. Park JE, Kim HS, Kim D, Park SY, Kim JY, Cho SJ, et al. A systematic review reporting quality of radiomics research in neuro-oncology: toward clinical utility and quality improvement using high-dimensional imaging features. BMC Cancer 2020; 20: 29. doi: 10.1186/s12885-019-6504-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Granzier RWY, van Nijnatten TJA, Woodruff HC, Smidt ML, Lobbes MBI. Exploring breast cancer response prediction to neoadjuvant systemic therapy using MRI-based radiomics: a systematic review. Eur J Radiol 2019; 121: 108736. doi: 10.1016/j.ejrad.2019.108736 [DOI] [PubMed] [Google Scholar]
- 36. Hamerla G, Meyer H-J, Hambsch P, Wolf U, Kuhnt T, Hoffmann K-T, et al. Radiomics model based on Non-Contrast CT shows no predictive power for complete pathological response in locally advanced rectal cancer. Cancers 2019; 11: E1680. doi: 10.3390/cancers11111680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wakabayashi T, Ouhmich F, Gonzalez-Cabrera C, Felli E, Saviano A, Agnus V, et al. Radiomics in hepatocellular carcinoma: a quantitative review. Hepatol Int 2019; 13: 546–59. doi: 10.1007/s12072-019-09973-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park JE, Kim D, Kim HS, Park SY, Kim JY, Cho SJ, et al. Quality of science and reporting of radiomics in oncologic studies: room for improvement according to radiomics quality score and TRIPOD statement. Eur Radiol 2020; 30: 523–36. doi: 10.1007/s00330-019-06360-z [DOI] [PubMed] [Google Scholar]
- 39. Sanduleanu S, Woodruff HC, de Jong EEC, van Timmeren JE, Jochems A, Dubois L, et al. Tracking tumor biology with radiomics: a systematic review utilizing a radiomics quality score. Radiother Oncol 2018; 127: 349–60. doi: 10.1016/j.radonc.2018.03.033 [DOI] [PubMed] [Google Scholar]
- 40. Valdora F, Houssami N, Rossi F, Calabrese M, Tagliafico AS, Francesca V. Rapid review: radiomics and breast cancer. Breast Cancer Res Treat 2018; 169: 217–29. doi: 10.1007/s10549-018-4675-4 [DOI] [PubMed] [Google Scholar]
- 41. Rossi G, Barabino E, Fedeli A, Ficarra G, Coco S, Russo A, et al. Radiomic detection of EGFR mutations in NSCLC. Cancer Res 2021; 81: 724–31. doi: 10.1158/0008-5472.CAN-20-0999 [DOI] [PubMed] [Google Scholar]
- 42. Chen Y-H, Wang T-F, Chu S-C, Lin C-B, Wang L-Y, Lue K-H, et al. Incorporating radiomic feature of pretreatment 18F-FDG PET improves survival stratification in patients with EGFR-mutated lung adenocarcinoma. PLoS One 2020; 15: e0244502. doi: 10.1371/journal.pone.0244502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ninomiya K, Arimura H, Chan WY, Tanaka K, Mizuno S, Muhammad Gowdh NF, et al. Robust radiogenomics approach to the identification of EGFR mutations among patients with NSCLC from three different countries using topologically invariant Betti numbers. PLoS One 2021; 16: e0244354. doi: 10.1371/journal.pone.0244354 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.