Abstract
Objective:
To explore the utility of first-order MRI-texture analysis (TA) parameters in predicting histologic grade and muscle invasion in urinary bladder cancer (UBC).
Methods:
After ethical clearance, 40 patients with UBC, who were imaged on a 3.0-Tesla scanner, were retrospectively included. Using the TexRADTM platform, two readers placed freehand ROI on the sections demonstrating the largest dimension of the tumor, evaluating only one tumor per patient. Interobserver reproducibility was assessed using the intraclass correlation coefficient (ICC). Mann–Whitney U test and ROC curve analysis were used to identify statistical significance and select parameters with high class separation capacity (AUC >0.8), respectively. Pearson’s test was used to identify redundancy in the results.
Results:
All texture parameters showed excellent ICC. The best parameters in differentiating high and low-grade tumors were mean/ mean of positive pixels (MPP) at SSF 0 (AUC: 0.897) and kurtosis at SSF 5 (AUC: 0.828) on the ADC images. In differentiating muscle invasive from non-muscle invasive tumors, mean/ MPP at SSF 0 on the ADC images showed AUC >0.8; however, this finding resulted from the confounding effect of high-grade histology on the ADC values of muscle invasive tumors.
Conclusion:
MRI-TA generated few parameters which were reproducible and useful in predicting histologic grade. No independent parameters predicted muscle invasion.
Advances in knowledge:
There is lacuna in the literature concerning the role of MRI-TA in the prediction of histologic grade and muscle invasion in UBC. Our study generated a few first-order parameters which were useful in predicting high-grade histology.
Introduction
Urinary bladder cancer (UBC) has high prevalence, largely attributable to the high 5 year survival rate of 60–80%. 1 Typically, these patients undergo multiple treatment sessions and long-term follow-up with cystoscopy. These factors result in high medical costs along with physical and emotional toll on the patients. 2,3
The treatment of UBC occurs in multiple stages. 4 Once a neoplastic bladder mass is suspected on imaging, a transurethral resection of the bladder tumor (TURBT) is performed along with deep muscle biopsy to assess histologic grade and presence of muscle invasion. This treatment would generally suffice for patients with low-grade non-muscle invasive disease, who are then kept on follow-up cystoscopy. Patients with high-grade non-muscle invasive tumors are subjected to restage TURBT for ensuring absence of residual tumor. If muscle invasion is present at the time of the initial TURBT, then the patient proceeds to radical cystectomy, 6–8 weeks after the TURBT. Both TURBT and cystoscopy, being invasive procedures, entail risks of bleeding, perforation and sepsis in addition to anesthesia-related complications. 5
Prediction of muscle invasion and histologic grade on imaging could help in streamlining treatment protocols so that patients can directly proceed to definitive surgery (TURBT or radical cystectomy). At present, the Vesical Imaging-Reporting and Data System (VI-RADS) provides a scoring system on multiparametric MRI to help differentiate muscle-invasive from non-muscle tumors based on morphologic parameters. VI-RADS has been validated and found to have good diagnostic performance. 6 Simple ADC quantification on diffusion-weighted imaging (DWI) is a useful tool in predicting histologic grade. 7
There exists an unmet need for additional quantitative tools which could improve the diagnostic performance of MRI in predicting muscle invasion and histologic grade. Texture analysis (TA) is one such tool which can be retrospectively applied to medical images for the assessment of tumor heterogeneity at the pixel level. TA has been hypothesized to predict histologic grade and early treatment response. 8 Several studies have assessed TA on CT images to predict muscle invasion and histologic grade in UBC. 9–11 However, MRI, with its higher intrinsic tissue contrast, enables better differentiation of cancerous tissue from the bladder wall than CT, and provides several vistas for the assessment of tumor morphology and physiology. Hence, TA on MRI is expected to yield more robust results than CT. Few studies have assessed radiomics on MRI in predicting tumor stage and muscle invasion. 12–15 However, these studies assessed several higher-order texture parameters which involve analysis of large volumes of data that are too complex and computationally intensive for routine purposes, apart from the question of reproducibility across different scanners and acquisition parameters. Also, beyond big data, the biological basis for these higher-order parameters is largely unknown. Hence, this study explored the potential of first-order MRI-TA parameters in predicting histologic grade and muscle invasion in UBC.
Methods
Patient selection
After approval from the institutional ethics committee, this retrospective study included 52 consecutive patients who were subjected to dedicated pelvic MRI for suspected UBC between January 2014 and April 2016 and had urothelial cancer on subsequent histopathology. Since all the MRI were acquired several years prior to the study, informed consent was waived. Six patients, whose surgery was delayed beyond 1 month from the MRI, were excluded since the delay potentially compromised comparability between MRI and histopathology. Another six patients were excluded since their tumors were too small (<1 cm in maximum dimension) to draw a satisfactory ROI. Thus, 40 patients were available for TA. Among these, 34 patients were also part of a previous published study that assessed the utility of UBC stalk morphology and quantitative ADC on DWI to predict muscle invasion and histologic grade, respectively. 16 Although there is some overlap in the patient population between the two studies, the techniques used (TA vs simple mean ADC estimation) and the methods of ROI placement were completely different.
MRI protocol
Prior to the MRI, patients were asked to fast for 6 h and refrain from voiding for 2 h to ensure distension of the urinary bladder. The MRI were acquired on a 3.0-Tesla scanner (Achieva, Philips Healthcare, Best, The Netherlands), using a 16-channel phased array torso coil. The acquired sequences included turbo spin echo T 1 weighted imaging (T 1WI), fat-suppressed T 2 weighted imaging (T 2WI) and diffusion-weighted imaging (DWI) in the standard axial plane. T 2WI and DWI were also acquired in an additional orthogonal plane depending on the tumor location (coronal plane for tumors based on the superior, inferior and lateral walls; sagittal plane for tumors arising from the anterior and posterior walls). DWI was acquired using fat-suppressed echoplanar imaging under navigator-triggered respiratory gating and b-values of 0, 500, 1000 and 1500 s/mm2 were used. After acquisition, the corresponding ADC maps were autogenerated on a pixel-by-pixel basis. Contrast-enhanced imaging was not performed as a significant number of patients had deranged renal function. The acquisition parameters are detailed in Table 1. Two sample cases are shown in Figures 1 and 2.
Table 1.
MRI acquisition protocol
| Sequence | TR (ms) | TE (ms) | Slice thickness (mm) | No of averages | FOV (mm) | Matrix |
|---|---|---|---|---|---|---|
| T1 Ax | 452 | 10 | 4 | 2 | 294 × 200 | 239 × 204 |
| T2 FS Ax | 5700 | 90 | 4 | 2 | 295 × 200 | 188 × 168 |
| T2 FS Cor | 5250 | 90 | 4 | 2 | 383 × 220 | 242 × 184 |
| T2 FS Sag | 4510 | 90 | 4 | 2 | 258 × 220 | 184 × 162 |
| DWI Ax | 2177 | 61 | 5 | 3 | 375 × 278 | 124 × 92 |
| DWI Cor | 2683 | 60 | 4 | 2 | 258 × 220 | 184 × 162 |
| DWI Sag | 2372 | 59 | 5 | 3 | 220 × 220 | 73 × 72 |
Ax, axial; Cor, coronal; DWI, diffusion-weighted imaging; FOV, field-of-view; FS, fat-suppressed; Sag, sagittal; TE, echo time; TR, repetition time; mm, millimeters; ms, milliseconds.
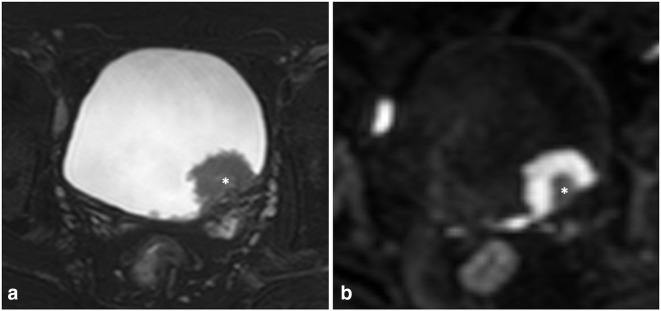
Figure 1.
67-year-old male with high-grade, non-muscle invasive urothelial carcinoma. (A) Axial T 2 weighted images showing a polypoidal, T2-intermediate mass arising from the left posterolateral wall of the urinary bladder, in close proximity to the vesicoureteric junction. The mass has a central T2-hyperintense stalk (asterisk), which was excluded while placing the ROI. (B) Axial DWI b1500 images showing intense diffusion restriction within the tumor, whereas the central stalk is hypointense (asterisk). DWI, diffusion-weighted imaging; ROI, region of interest.
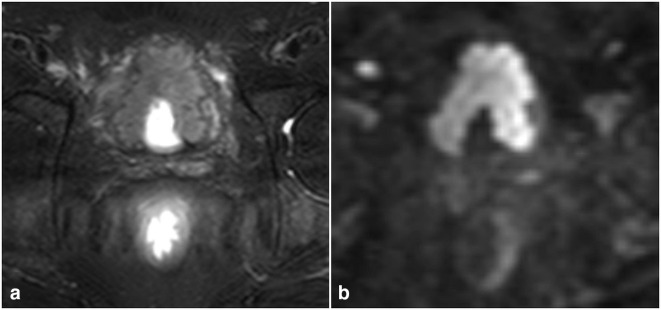
Figure 2.
60-year-old male with high-grade, muscle invasive urothelial cancer. (A) Axial T 2 weighted images showing an irregular plaque-like mass arising from the anterior and both lateral walls of the urinary bladder. The bladder is poorly distended secondary to the tethering effect of the tumor on the bladder wall. (B) Axial DWI b1500 images showing marked diffusion restriction of the tumor. DWI, diffusion-weighted imaging.
Texture analysis
Two radiologists (A.R. and S.M., both with 6 years of experience in general radiology) performed TA independently. Both were blinded to each other’s TA-derived data as well the histopathologic reports. For TA, the images used were axial fat-suppressed T 2WI, DWI b1500 s/mm2 and the corresponding ADC maps. All images were loaded onto the commercially available TexRADTM software (Feedback Plc.). The image slice showing the largest dimension of the tumor was selected for each MRI sequence and segmentation was performed using freehand 2D ROI to cover the entire tumor except the peripheral 1–2 mm. This was done to prevent erroneous results arising from inclusion of peritumoral tissue and volume averaging (Figure 3A). The central stalk (hyperintense on T 2WI and hypointense on DWI), if present, was not included while drawing the ROI, since it represented the reactive component of the tumor and not the tumor itself. If multiple tumors were present in a patient, only the largest tumor was assessed. Care was taken to avoid sections showing necrosis or hemorrhage, and in such cases, the next best slice was considered for ROI placement. We did not use 3D volumetric (whole-tumor) ROI or analysis of the adjacent healthy bladder wall in this study.
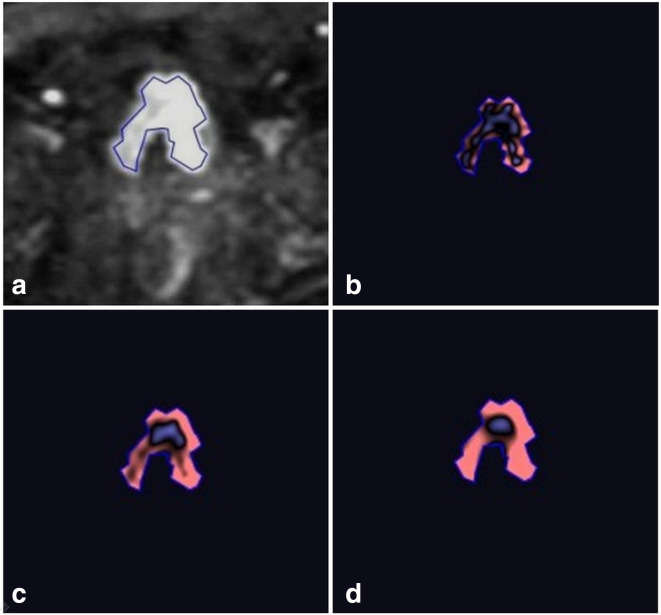
Figure 3.
Texture analysis in a 60-year-old male with high-grade, muscle invasive urothelial cancer (same patient as in Figure 2). (A) Placement of a free-hand polygonal 2D ROI (outlined in blue colour) on the axial DWI b1500 slice containing the maximum viable component of the tumor, avoiding the peripheral 1–2 mm. (B, C and D) Display of the filtered images at spatial scale factors of 2 mm, 4 mm and 6 mm, respectively. DWI, diffusion-weighted imaging; ROI, region of interest
After confirmation of the ROI placement, the TexRADTM software used a Laplacian of Gaussian spatial band-pass filter to mitigate photon noise and enhance features corresponding to the spatial scale factors (SSF) applied. Five such SSF were utilized, ranging from 2 to 6 mm (hereafter labeled as SSF 2–6). The unfiltered base images (SSF 0) also underwent TA. Following this, for each SSF, the software generated a histogram representing the distribution of signal intensities across pixels and derived the following first-order texture features: mean (average grayscale intensity), standard deviation (SD; dispersion or spread of intensities relative to the mean), entropy (disorder in the distribution of the intensities), mean of positive pixels (MPP), skewness (asymmetry of the histogram), and kurtosis (peakedness of the histogram). This resulted in 36 texture parameters being generated for each ROI (Figure ).
Statistical analysis
Statistical analysis was performed using XLSTAT Premium, v. 2019.1.2 for windows (Addinsoft, NY). To ensure reproducibility of all the six TA features derived by the two readers, intraclass correlation coefficient (ICC) was used. Only the texture features with ICC >0.8, suggestive of excellent interobserver agreement, qualified for the subsequent analysis.
Histopathology was used as the reference standard to classify tumors as muscle invasive or non-muscle invasive and high-grade or low-grade. To assess for any difference between the texture parameters of high-grade from low-grade tumors and muscle-invasive from non-muscle-invasive tumors, the Mann–Whitney U test was applied and a p-value < 0.05 was defined as significant. Following this, ROC curve analysis was performed for all the parameters that showed significant p-value and AUC values were derived. Only the parameters that showed AUC >0.8, suggestive of high class separation capacity, were listed out in the final results. In addition, since some of the texture parameters are known to correlate with each other and introduce redundancy in the results, all the features with AUC >0.8 underwent the Pearson’s product–moment correlation test to look for any mutual correlation. The Pearson’s test was also used to assess any correlation between the texture parameters of high-grade tumors and muscle invasive tumors in order to negate the confounding effect of high-grade histology in the prediction of muscle invasion.
Results
Patient demographics
The study population included 40 patients, among whom a large majority were males (n: 35/40, 87.5%). The group aged between 31 and 81 years, with the mean age being 57.6 ± 11.8 years. Most patients belonged to the seventh decade of life (n: 15/40; 37.5%). 22 patients (55%) were initial presenters and 18 had recurrent disease after prior treatment for UBC. The most common presenting symptom was hematuria (n: 31/40; 77.5%), followed by increased frequency of micturition (n: 2/40). Two patients had both symptoms, whereas the remaining five were asymptomatic and the disease was picked up on follow-up cystoscopy after prior treatment for UBC. The mean duration of symptoms at presentation was 16.8 ± 14.8 weeks (range: 5 days–4 years). The study group of 40 patients had a total of 86 tumors, with the majority having single tumor (n: 25/40; 62.5%). The remaining 15 patients had multifocal disease, with a mean of 2.2 tumors (range: 2–11 tumors) per person. However, for the purpose of the study, only the largest lesion was taken into account for TA. Thus, 40 tumors were evaluated. The mean size of the evaluated tumors was 3.2 ± 1.8 cm (range: 1.0–10.6 cm). After histopathological examination, all the tumors were proven to be urothelial cancer. 29 patients (72.5%) had high-grade tumors and 11 (27.5%) had low-grade tumors. 25 patients (62.5%) had non-muscle invasive disease and 15 (37.5%) showed muscle invasion. All the patients with muscle invasive disease had high-grade tumors and underwent radical cystectomy.
Interobserver correlation of texture values
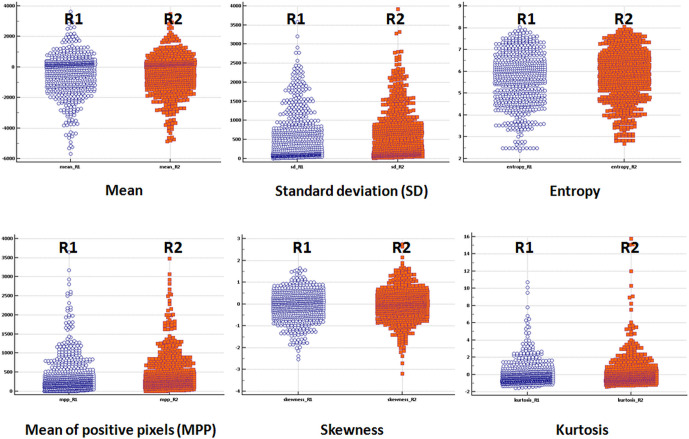
All the measured texture parameters showed excellent interobserver correlation (ICC >0.8) between the two readers, with the highest correlation seen for entropy (ICC: 0.9573) ( Table 2, Figure 4 ). This ensured reproducibility of the TA-derived parameters. The subsequent discussion is based on the texture parameters generated by the first reader (A.R., chosen randomly).
Table 2.
ICC for the measurements extracted by the two readers (R1 and R2) for each of the six texture features
| Parameter | Correlation coefficient |
|---|---|
| Mean | 0.9428 |
| SD | 0.9558 |
| Entropy | 0.9573 |
| MPP | 0.9513 |
| Skewness | 0.8920 |
| Kurtosis | 0.8177 |
ICC, intraclass correlation coefficient; MPP, mean of positive pixels; SD, standard deviation.
Figure 4.
Dots plots representing intraclass correlation between the measurements extracted by the two readers (R1 and R2) for each of the six texture features (mean, standard deviation, entropy, mean of positive pixels, skewness and kurtosis).
Prediction of histologic grade
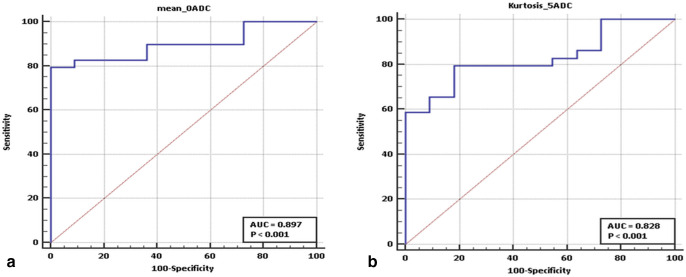
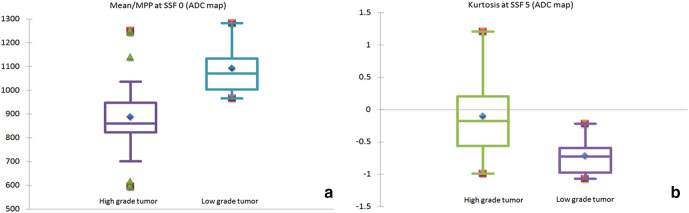
A total of 13 parameters (four on T 2WI, two on DWI b1500 and 7 on the ADC images) showed statistical significance (p-value < 0.05) in differentiating high-grade from low-grade tumors on the Mann–Whitney U test (Table 3). On ROC curve analysis, only three among these showed AUC >0.8 (Table 4). These parameters were (a) mean/ MPP at SSF 0 (AUC: 0.897; 95% CI: 0.759–0.970; optimal cut-off:<963.479 x 10−3 mm2/s), and (b) kurtosis at SSF 5 (AUC: 0.828; 95% CI: 0.675–0.928; optimal cut-off:>−0.579); both on the ADC images. Among these, mean and MPP had exactly the same values since ADC maps contain only positive pixels. This was proven by perfect linear correlation (r: 1.0) between these parameters. On the other hand, no correlation was observed between mean/ MPP at SSF 0 and kurtosis at SSF 5 on the ADC images (r: −0.293). ROC curve analysis and box plots for the above parameters are given in Figures 5 and 6, respectively.
Table 3.
Mann–Whitney U test in the differentiation of high-grade from low-grade urinary bladder cancer: p-values of all the parameters, listed MR sequence-wise
| SSF | Mean | SD | Entropy | MPP | Skewness | Kurtosis |
|---|---|---|---|---|---|---|
| T2W | ||||||
| 0 | 0.272 | 0.807 | 0.713 | 0.272 | 0.196 | 0.518 |
| 2 | 0.205 | 0.503 | 0.878 | 0.101 | 0.012 | 0.017 |
| 3 | 0.257 | 0.546 | 0.899 | 0.791 | 0.014 | 0.031 |
| 4 | 0.286 | 0.807 | 0.708 | 0.381 | 0.296 | 0.409 |
| 5 | 0.351 | 0.858 | 0.574 | 0.518 | 0.596 | 0.679 |
| 6 | 0.568 | 0.858 | 0.487 | 0.994 | 0.960 | 0.728 |
| DWI b 1500 | ||||||
| 0 | 0.462 | 0.218 | 0.381 | 0.462 | 0.407 | 0.665 |
| 2 | 0.782 | 0.317 | 0.827 | 0.987 | 0.339 | 0.996 |
| 3 | 0.782 | 0.419 | 0.987 | 0.935 | 0.573 | 0.776 |
| 4 | 0.909 | 0.546 | 0.934 | 0.987 | 0.942 | 0.127 |
| 5 | 0.987 | 0.524 | 0.935 | 0.935 | 0.540 | 0.005 |
| 6 | 0.909 | 0.462 | 0.974 | 0.782 | 0.765 | 0.004 |
| ADC | ||||||
| 0 | 0.000 | 0.961 | 0.791 | 0.000 | 0.573 | 0.398 |
| 2 | 0.832 | 0.334 | 1.000 | 0.289 | 0.047 | 0.363 |
| 3 | 0.807 | 0.482 | 0.960 | 0.398 | 0.041 | 0.246 |
| 4 | 0.909 | 0.636 | 0.960 | 0.707 | 0.104 | 0.034 |
| 5 | 0.590 | 0.757 | 0.960 | 0.807 | 0.426 | 0.001 |
| 6 | 0.286 | 0.961 | 0.948 | 0.988 | 0.202 | 0.046 |
MPP, mean of positive pixels; SD, standard deviation; SSF, spatial scale factor.
The statistically significant parameters are shown in bold text.
Table 4.
AUC and optimal cut-off values of the statistically significant parameters (p < 0.05) in the differentiation of high-grade from low-grade urinary bladder cancer, listed MR sequence-wise
| Parameter | SSF | AUC | 95% CI | Cut-off | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| T 2W | ||||||
| Skewness | 2 | 0.748 | 0.585–0.871 | <−0.930 | 68.97 | 81.82 |
| Skewness | 3 | 0.727 | 0.564–0.856 | <−0.479 | 75.86 | 72.73 |
| Kurtosis | 2 | 0.712 | 0.547–0.844 | >2.009 | 41.38 | 100 |
| Kurtosis | 3 | 0.693 | 0.527–0.829 | >0.270 | 41.38 | 100 |
| DWI b1500 | ||||||
| Kurtosis | 5 | 0.779 | 0.620–0.895 | >−0.379 | 58.62 | 100 |
| Kurtosis | 6 | 0.752 | 0.591–0.875 | >−0.560 | 65.52 | 81.82 |
| ADC | ||||||
| Mean | 0 | 0.897 | 0.759–0.970 | <963.479 | 79.31 | 100 |
| MPP | 0 | 0.897 | 0.759–0.970 | <963.479 | 79.31 | 100 |
| Skewness | 2 | 0.702 | 0.537–0.836 | <−0.519 | 65.52 | 81.82 |
| Skewness | 3 | 0.721 | 0.557–0.851 | <−0.460 | 65.52 | 90.91 |
| Kurtosis | 4 | 0.704 | 0.539–0.837 | >−0.280 | 48.28 | 90.91 |
| Kurtosis | 5 | 0.828 | 0.675–0.928 | >−0.579 | 79.31 | 81.82 |
| Kurtosis | 6 | 0.712 | 0.547–0.844 | >−0.730 | 72.41 | 72.73 |
AUC, area under curve; CI, confidence interval; MPP, mean of positive pixels; SSF, spatial scale factor.
Parameters with AUC >0.8 are shown in bold text.
Figure 5.
ROC curve analysis results for the two texture parameters which showed high class separation capacity (AUC >0.8) in differentiating high-grade from low-grade tumors: (A) Mean/ MPP at SSF 0 and (B) Kurtosis at SSF 5 on the ADC images. ADC, apparent diffusion coefficient; AUC, area under the curve; MPP, mean of positive pixels; ROC, receiver operating characteristic; SSF, spatial scale factor.
Figure 6.
Box and whisker plots of the two texture parameters which showed high class separation capacity (AUC >0.8) in differentiating high-grade from low-grade tumors: (A) Mean/ MPP at SSF 0 and (B) Kurtosis at SSF 5 on the ADC images. The Y-axis represents mean ADC (x 10−6 mm2/s) in panel A and kurtosis in panel B. ADC, apparent diffusion coefficient; AUC, area under the curve; MPP, mean of positive pixels; ROC, receiver operating characteristic; SSF, spatial scale factor.
Prediction of muscle invasion
p-values generated from the Mann–Whitney U test were statistically significant in distinguishing muscle invasive from non-muscle invasive tumors for 19 parameters (five on T 2WI, six on DWI b1500 and 8 on the ADC images) (Table 5). However, on ROC curve analysis, the only parameters with AUC >0.8 in predicting muscle invasion were mean and MPP at SSF 0 on the ADC images (AUC: 0.819; 95% CI: 0.665–0.922; optimal cut-off:<930.5 x 10−3 mm2/s) (Table 6). As expected, Pearson’s test showed perfect linear correlation between these two parameters (r: 1.0). However, since all the muscle invasive tumors in our population were high-grade, it was possible that these texture parameters merely reflected the confounding effect of the high-grade histology of these tumors. Confirming this suspicion, Pearson’s test showed perfect correlation (r: 1.0) between the mean/ MPP values (at SSF 0 on the ADC images) of high-grade tumors and those of the muscle invasive tumors. Hence, it was concluded that no texture parameters independent of those which reflected high histologic grade, showed high class separation capacity in differentiating muscle invasive from non-muscle invasive tumors.
Table 5.
Mann–Whitney U test in the differentiation of muscle invasive from non-muscle invasive urinary bladder cancer: p-values of all the parameters, listed MR sequence-wise
| SSF | Mean | SD | Entropy | MPP | Skewness | Kurtosis |
|---|---|---|---|---|---|---|
| T 2W | ||||||
| 0 | 0.315 | 0.940 | 0.423 | 0.315 | 0.095 | 0.076 |
| 2 | 0.021 | 0.223 | 0.904 | 0.361 | 0.080 | 0.077 |
| 3 | 0.050 | 0.235 | 0.820 | 0.785 | 0.006 | 0.028 |
| 4 | 0.100 | 0.665 | 0.988 | 0.964 | 0.023 | 0.276 |
| 5 | 0.287 | 0.870 | 0.868 | 0.844 | 0.083 | 0.372 |
| 6 | 0.463 | 0.893 | 0.809 | 0.515 | 0.270 | 0.389 |
| DWI b 1500 | ||||||
| 0 | 0.058 | 0.006 | 0.082 | 0.058 | 0.397 | 0.586 |
| 2 | 0.754 | 0.027 | 0.204 | 1.000 | 0.079 | 0.250 |
| 3 | 0.893 | 0.030 | 0.276 | 0.777 | 0.112 | 0.832 |
| 4 | 0.870 | 0.043 | 0.289 | 0.800 | 0.150 | 0.762 |
| 5 | 0.893 | 0.034 | 0.260 | 0.709 | 0.372 | 0.468 |
| 6 | 0.754 | 0.016 | 0.215 | 0.520 | 0.364 | 0.639 |
| ADC | ||||||
| 0 | 0.001 | 0.777 | 0.303 | 0.001 | 0.762 | 0.535 |
| 2 | 0.135 | 0.601 | 0.458 | 0.880 | 0.020 | 0.035 |
| 3 | 0.171 | 0.315 | 0.380 | 0.739 | 0.008 | 0.178 |
| 4 | 0.287 | 0.361 | 0.364 | 0.621 | 0.003 | 0.397 |
| 5 | 0.665 | 0.482 | 0.325 | 0.820 | 0.009 | 0.348 |
| 6 | 0.560 | 0.410 | 0.296 | 0.559 | 0.013 | 0.596 |
MPP, mean of positive pixels; SD, standard deviation; SSF, spatial scale factor.
The statistically significant parameters are shown in bold text.
Table 6.
AUC and optimal cut-off values of the statistically significant parameters (p < 0.05) in the differentiation of muscle invasive from non-muscle invasive urinary bladder cancer, listed MR sequence-wise
| Parameter | SSF | AUC | 95% CI | Cut-off | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| T2W | ||||||
| Mean | 2 | 0.701 | 0.536–0.835 | >−273.109 | 60.00 | 80.00 |
| Mean | 3 | 0.669 | 0.503–0.810 | >−617.830 | 66.67 | 80.00 |
| Skewness | 3 | 0.733 | 0.570–0.860 | <−0.709 | 66.67 | 80.00 |
| Skewness | 4 | 0.696 | 0.530–0.831 | <−0.259 | 73.33 | 68.00 |
| Kurtosis | 3 | 0.675 | 0.508–0.814 | >0.270 | 60.00 | 88.00 |
| DWI b1500 | ||||||
| SD | 0 | 0.789 | 0.631–0.902 | >25.729 | 93.33 | 56.00 |
| SD | 2 | 0.741 | 0.579–0.867 | >101.309 | 60.00 | 88.00 |
| SD | 3 | 0.739 | 0.576–0.864 | >149.639 | 60.00 | 88.00 |
| SD | 4 | 0.725 | 0.561–0.854 | >193.979 | 53.33 | 92.00 |
| SD | 5 | 0.736 | 0.573–0.862 | >137.990 | 80.00 | 68.00 |
| SD | 6 | 0.76 | 0.599–0.881 | >145.130 | 80.00 | 72.00 |
| ADC | ||||||
| Mean | 0 | 0.819 | 0.665–0.922 | <930.5 | 86.67 | 72.00 |
| MPP | 0 | 0.819 | 0.665–0.922 | <930.5 | 86.67 | 72.00 |
| Skewness | 2 | 0.729 | 0.566–0.857 | <−0.519 | 80 | 64.00 |
| Skewness | 3 | 0.757 | 0.596–0.879 | <−0.589 | 66.67 | 84.00 |
| Skewness | 4 | 0.773 | 0.614–0.890 | <−0.270 | 80 | 80.00 |
| Skewness | 5 | 0.744 | 0.582–0.869 | <−0.129 | 73.33 | 80.00 |
| Skewness | 6 | 0.751 | 0.589–0.874 | <0.079 | 73.33 | 84.00 |
| Kurtosis | 2 | 0.688 | 0.522–0.825 | >−0.289 | 80.00 | 60.00 |
AUC, area under curve; CI, confidence interval; MPP, mean of positive pixels; SD, standard deviation; SSF, spatial scale factor.
Parameters with AUC >0.8 are shown in bold text.
Discussion
High histologic grade and presence of muscle invasion are important prognostic factors which guide the treatment of UBC. At present, this information is obtained through TURBT, which is invasive. There exists an unmet need for non-invasive biomarkers capable of providing this information accurately. Among the novel tools used in quantitative imaging, radiomics and TA have attracted significant interest with several studies having used TA on both CT and MRI images to predict histologic grade and muscle invasion in UBC. 9–15
We investigated the role of first-order TA parameters on MRI in predicting histologic grade and presence of muscle invasion. MRI was preferred over CT since the higher intrinsic tissue contrast of MRI enables better differentiation of tumor from the bladder wall. MRI also possesses multiple paradigms for the morphologic (e.g. T 1WI, T 2WI) and functional (e.g., DWI) assessment of tumors. Hence, TA on MRI may provide more robust results in tumor characterization than CT. All the studies which evaluated TA on MRI till date for staging and grading of UBC assessed numerous higher-order statistical parameters. 12–15 Although these higher-order parameters may have better discriminative capability, the large dimensions of data involved make calculations too complex and computationally intensive for practical and clinical purposes. First-order parameters have also been observed to be more reproducible than higher order parameters, with entropy being the most stable feature. 17 This is relevant since the question of reproducibility across different scanners, acquisition parameters and segmentation techniques is the most significant barrier in the development of prediction models for routine clinical implementation. The generation of numerous texture features from a single data set can result in significant inflation of type-I error, which may be overlooked unless a validation data set is used. 18 Also, the biological basis and pathologic correlates of first-order parameters are better understood than those of higher-order features, the causality of which are difficult to infer. 19–21 For these reasons, only first-order parameters were assessed in this study. We also confirmed the reproducibility of our segmentation technique by observing excellent interobserver correlation across the two readers who extracted the data.
In this study, two parameters (mean/MPP at SSF 0 and kurtosis at SSF 5 on the ADC images) showed excellent class separation capacity in the differentiation of high-grade from low-grade tumors. High-grade tumors showed significantly lower mean/MPP values on the unfiltered ADC images. This finding is attributable to the marked cellularity of high-grade tumors, which restricts free diffusion of water molecules in the interstitial space and results in low signal intensity on the ADC map images. The presence of a significant difference between the ADC values of low-grade and high-grade tumors is a well-established fact. 7,22,23 A similar observation of significantly lower ADC in high-grade tumors was also made in our previously published study. 16 Although there is some overlap in the patient population between the two studies, the techniques used (TA vs simple mean ADC estimation) and the methods of ROI placement were completely different. However, no comparable results were observed on the corresponding DWI b1500 images. This could have occurred since ADC value, which is a measure of the slope of signal intensity interpolated across multiple b-values, is a more precise and quantifiable representation of true diffusion restriction than a single b-value DWI trace image. In addition, TA results on the filtered ADC maps (SSF 2–6) failed to show similar results as the unfiltered images. This raises the possibility that filtration, which was originally intended to enhance features and mitigate photon noise, could in turn introduce a type II error when applied to parametric maps. High-grade tumors also showed higher kurtosis values, suggestive of higher peakedness of the histogram. This implies the presence of an identical pattern of restricted diffusion across pixels within the measured ROI.
In the prediction of muscle invasion, only one parameter (mean/MPP at SSF 0 on the ADC images) showed high class separation capacity. However, this resulted from the confounding effect of high-grade tumor histology since all the muscle invasive tumors were of high-grade. This possibility was confirmed by the perfect correlation observed between the texture parameters of high-grade and muscle invasive tumors. Hence, no texture features independent of those which reflected high histologic grade, showed high class separation capacity in the differentiation of muscle invasive from non-muscle invasive tumors.
There are a few limitations of this study. Our sample size of 40 tumors was small and a larger number of patients would be required to produce more robust results. Our small sample size also limited the incorporation of machine learning techniques which could have helped in generating better classifiers. Intuitively, although whole tumor assessment would have provided more representative data, we limited ourselves to 2D (single slice) ROI placement since volumetric assessment is cumbersome, results in confounding errors from volume averaging and inclusion of hemorrhage/ necrosis, and is less likely to be reproducible across observers. 19 Thirdly, although we accounted for interobserver variability in ROI placement by confirming excellent ICC between the two readers, reproducibility across different scanners and imaging acquisition protocols were not assessed. This could limit the generalizability of our findings. We also did not perform TA of the normal bladder wall, which could have served as an internal reference. Lastly, the reference standard in a significant number of our patients was the TURBT specimen. Since TURBT is known to undersample the tumor and miss muscle invasion, this reference standard may have been imperfect. 24
In conclusion, our study revealed two first-order texture parameters (mean/MPP at SSF 0 and kurtosis at SSF 5 on the ADC images) which showed excellent class separation capacity in differentiating high-grade from low-grade tumors. Similar diagnostic performance was not seen with the filtered images. No texture parameters independent of those which predicted histologic grade were observed to be useful in the differentiation of muscle invasive from non-muscle invasive tumors. All the texture parameters were reproducible across the two readers. Larger validation studies are required and uniform acquisition protocols need to be adopted before TA can be used in the routine management of UBC patients.
Footnotes
Author contributions: Study concepts and design: A.R., C.J.D., R.S.; Data acquisition: A.R., S.M.; Data analysis and interpretation: A.R., C.J.D., R.S., S.M., S.S., A.S., D.N.S.; Literature research: A.R., C.J.D., R.S.; Manuscript drafting: A.R.; Manuscript revision for important intellectual content: A.R., C.J.D., R.S.; Approval of the final version of the submitted manuscript: all authors.
Contributor Information
Abdul Razik, Email: razik89@gmail.com.
Chandan J Das, Email: dascj@yahoo.com.
Raju Sharma, Email: raju152@yahoo.com.
Sundeep Malla, Email: sundeepmalla1047@gmail.com.
Sanjay Sharma, Email: drssharma@hotmail.com.
Amlesh Seth, Email: amlesh.seth@gmail.com.
Deep Narayan Srivastava, Email: drdeepsrivastava@rediffmail.com.
REFERENCES
- 1. Richters A, Aben KKH, Kiemeney LALM. The global burden of urinary bladder cancer: an update. World J Urol 2020; 38: 1895–904. doi: 10.1007/s00345-019-02984-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Svatek RS, Hollenbeck BK, Holmäng S, Lee R, Kim SP, Stenzl A, et al. The economics of bladder cancer: costs and considerations of caring for this disease. Eur Urol 2014; 66: 253–62. doi: 10.1016/j.eururo.2014.01.006 [DOI] [PubMed] [Google Scholar]
- 3. Pham H, Torres H, Sharma P. Mental health implications in bladder cancer patients: a review. Urol Oncol 2019; 37: 97–107. doi: 10.1016/j.urolonc.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 4. Cheung G, Sahai A, Billia M, Dasgupta P, Khan MS. Recent advances in the diagnosis and treatment of bladder cancer. BMC Med 2013; 11: 13. doi: 10.1186/1741-7015-11-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Traxer O, Pasqui F, Gattegno B, Pearle MS. Technique and complications of transurethral surgery for bladder tumours. BJU Int 2004; 94: 492–6. doi: 10.1111/j.1464-410X.2004.04990.x [DOI] [PubMed] [Google Scholar]
- 6. Wang H, Luo C, Zhang F, Guan J, Li S, Yao H, et al. Multiparametric MRI for bladder cancer: validation of VI-RADS for the detection of detrusor muscle invasion. Radiology 2019; 291: 668–74. doi: 10.1148/radiol.2019182506 [DOI] [PubMed] [Google Scholar]
- 7. Takeuchi M, Sasaki S, Ito M, Okada S, Takahashi S, Kawai T, et al. Urinary bladder cancer: diffusion-weighted MR imaging--accuracy for diagnosing T stage and estimating histologic grade. Radiology 2009; 251: 112–21. doi: 10.1148/radiol.2511080873 [DOI] [PubMed] [Google Scholar]
- 8. Ganeshan B, Miles KA. Quantifying tumour heterogeneity with CT. Cancer Imaging. 2013; 13. :: 140–9 Published 2013;13(1). doi: 10.1102/1470-7330.2013.0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang G-M-Y, Sun H, Shi B, Jin Z-Y, Xue H-D. Quantitative CT texture analysis for evaluating histologic grade of urothelial carcinoma. Abdom Radiol 2017; 42: 561–8. doi: 10.1007/s00261-016-0897-2 [DOI] [PubMed] [Google Scholar]
- 10. Liu ZH, Shi JY, Wang HY, Ye HY, Wang ZB, Yang T, et al. Ct texture analysis in bladder carcinoma: histologic grade characterization. Zhonghua Zhong Liu Za Zhi 2018; 40: 379–83. doi: 10.3760/cma.j.issn.0253-3766.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 11. Wang Z, He Y, Wang N, Zhang T, Wu H, Jiang X, et al. Clinical value of texture analysis in differentiation of urothelial carcinoma based on multiphase computed tomography images. Medicine 2020; 99: e20093. doi: 10.1097/MD.0000000000020093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu X, Liu Y, Zhang X, Tian Q, Wu Y, Zhang G, et al. Preoperative prediction of muscular invasiveness of bladder cancer with radiomic features on conventional MRI and its high-order derivative maps. Abdom Radiol 2017; 42: 1896–905. doi: 10.1007/s00261-017-1079-6 [DOI] [PubMed] [Google Scholar]
- 13. Zhang X, Xu X, Tian Q, Li B, Wu Y, Yang Z, et al. Radiomics assessment of bladder cancer grade using texture features from diffusion-weighted imaging. J Magn Reson Imaging 2017; 46: 1281–8. doi: 10.1002/jmri.25669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu X, Zhang X, Tian Q, Wang H, Cui L-B, Li S, et al. Quantitative identification of Nonmuscle-Invasive and muscle-invasive bladder carcinomas: a multiparametric MRI Radiomics analysis. J Magn Reson Imaging 2019; 49: 1489–98. doi: 10.1002/jmri.26327 [DOI] [PubMed] [Google Scholar]
- 15. Xu S, Yao Q, Liu G, Jin D, Chen H, Xu J, et al. Combining DWI radiomics features with transurethral resection promotes the differentiation between muscle-invasive bladder cancer and non-muscle-invasive bladder cancer. Eur Radiol 2020; 30: 1804–12. doi: 10.1007/s00330-019-06484-2 [DOI] [PubMed] [Google Scholar]
- 16. Razik A, Das CJ, Sharma S, Seth A, Srivastava DN, Mathur S, et al. Diagnostic performance of diffusion-weighted MR imaging at 3.0 T in predicting muscle invasion in urinary bladder cancer: utility of evaluating the morphology of the reactive tumor stalk. Abdom Radiol 2018; 43: 2431–41. doi: 10.1007/s00261-018-1458-7 [DOI] [PubMed] [Google Scholar]
- 17. Traverso A, Wee L, Dekker A, Gillies R. Repeatability and reproducibility of radiomic features: a systematic review. Int J Radiat Oncol Biol Phys 2018; 102: 1143–58. doi: 10.1016/j.ijrobp.2018.05.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chalkidou A, O'Doherty MJ, Marsden PK. False discovery rates in PET and CT studies with texture features: a systematic review. PLoS One 2015; 10: e0124165. doi: 10.1371/journal.pone.0124165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miles KA, Ganeshan B, Hayball MP. Ct texture analysis using the filtration-histogram method: what do the measurements mean? Cancer Imaging 2013; 13: 400–6. doi: 10.1102/1470-7330.2013.9045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lubner MG, Smith AD, Sandrasegaran K, Sahani DV, Pickhardt PJ. Ct texture analysis: definitions, applications, biologic correlates, and challenges. Radiographics 2017; 37: 1483–503. doi: 10.1148/rg.2017170056 [DOI] [PubMed] [Google Scholar]
- 21. Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology 2016; 278: 563–77. doi: 10.1148/radiol.2015151169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang H-jun, Pui MH, Guo Y, Li S-rong, Guan J, Zhang X-ling, Wang H, Li S, Zhang X, et al. Multiparametric 3-T MRI for differentiating Low-Versus high-grade and category T1 versus T2 bladder urothelial carcinoma. American Journal of Roentgenology 2015; 204: 330–4. doi: 10.2214/AJR.14.13147 [DOI] [PubMed] [Google Scholar]
- 23. Yoshida S, Takahara T, Kwee TC, Waseda Y, Kobayashi S, Fujii Y. Dwi as an imaging biomarker for bladder cancer. American Journal of Roentgenology 2017; 208: 1218–28. doi: 10.2214/AJR.17.17798 [DOI] [PubMed] [Google Scholar]
- 24. Herr HW. The value of a second transurethral resection in evaluating patients with bladder tumors. J Urol 1999; 162: 74–6. doi: 10.1097/00005392-199907000-00018 [DOI] [PubMed] [Google Scholar]