Summary
Maternal–fetal transmission of cytomegalovirus (CMV) represents the most common infectious cause of long-term neurodevelopmental disability in children. Congenital CMV (cCMV) infection is associated with microcephaly, seizure disorders, cognitive disability, developmental delay, and sensorineural hearing loss (SNHL). Of these disabilities, SNHL is the most common, affecting approximately 10% of infants with cCMV. Although the sequelae of cCMV are well recognized, it is much less clear what long-term morbidities may occur in neonates that acquire post-natal CMV infection. Post-natal CMV (pCMV) infection is most commonly transmitted by breast-feeding, and in full-term infants is of little consequence. However, in preterm, very-low birthweight (VLBW) infants (<1500 g), pCMV can result in a severe sepsis-like syndrome, with wide-ranging end-organ disease manifestations. Although such short-term complications are well recognized among clinicians caring for premature infants, the long-term risks with respect to adverse neurodevelopmental outcomes remain controversial. In this review, we provide an overview of the clinical manifestations of breast milk-acquired pCMV infection. In particular, we summarize studies that have examined—sometimes with conflicting conclusions—the risks of long-term adverse neurodevelopmental outcome in VLBW infants that acquire pCMV from breast milk. We highlight proposed preventive strategies and antiviral interventions, and offer recommendations for high-priority areas for future basic science and clinical research.
Keywords: breast milk, cytomegalovirus infection, cytomegalovirus vaccine, hearing loss, neurological sequelae, viral transmission
1 |. INTRODUCTION TO NEONATAL CMV INFECTIONS
Human cytomegalovirus (HCMV) is one of the most commonly encountered viral pathogens in newborn infants.1 Infection may be accquired in utero, congenital CMV (cCMV), or in the immediate postnatal period (pCMV infection). It is estimated that cCMV infection occurs in 0.5% to 0.65% of all deliveries in the developed world, although there is considerable geographic variation.2,3 In the United States, this corresponds to approximately 40 000 infected newborn infants annually. There are substantial racial and ethnic differences in the prevalence of cCMV.4 Globally, the seroprevalence of CMV antibodies is estimated to be ∼80%.5 The prevalence of cCMV infection is directly proportional to the overall prevalence of CMV antibodies in women of child-bearing age in the population being studied.2 Preconception seropositivity does not protect against maternal reinfection with new strains of virus during pregnancy or reactivation of latent maternal infection. Hence, the biggest burden engendered by cCMV infection appears to be in infants born to women with preconception immunity, likely due to maternal re-infections during pregnancy with new, novel strains of virus. Approximately 10% of infants with cCMV have clinically evident disease at birth, including visceral organomegaly, microcephaly with intracranial calcifications, chorioretinitis, and skin manifestations including petechiae and purpura. This complex of findings, characterized as the syndrome of cytomegalic inclusion disease (CID) of the newborn, predicts a poor prognosis with respect to neurodevelopmental sequelae.6–10
Neurological sequelae caused by cCMV include microcephaly, neuronal migration defects, intracranial calcifications, developmental delay, seizure disorders, and mental retardation.11–13 Long-term deficits are more common in infants who are symptomatic at birth.14,15 However, even among those infected infants who appear normal at birth, there is a risk of cCMV-related sequelae. The risk of neurological sequelae appears to be highly dependent on the timing of infection in utero, with first trimester infections presenting a particularly high risk for fetal brain infection and injury.16 Sensorineural hearing loss (SNHL) is the most important and common long-term disability occurring in the setting of cCMV. Approximately 22% to 65% of children with symptomatic and 6% to 23% of children with asymptomatic cCMV infection will develop SNHL.17 Although controversial,8 there is evidence to suggest that cCMV infections that occur in the context of preconception maternal immunity are less likely to be associated with long-term neurodevelopmental sequelae than are those congenital infections that occur in the context of primary maternal infection during pregnancy.18
In contrast to cCMV, pCMV infection has generally been considered to be of little clinical consequence. Typically such infections are acquired by breast-feeding, and this form of transmission is so ubiquitous and (generally) innocuous that some reviews have referred to it as a form of “natural immunization”.19,20 However, even the initial studies demonstrating transmission of CMV from mother-to-infant by breast milk did offer a note of caution19 that the possibility that acquisition of CMV infection from human milk might produce serious disease in premature infants was “very disturbing, and needs immediate resolution before embarking on widespread milk-banking programs for premature infants”. This cautionary note was prescient, and the ensuing 40 years have confirmed the concerns raised about the risks of acquisition of pCMV infection in the VLBW infant. In the following sections of this review, we summarize the findings from this body of work, with a particular emphasis on recent research regarding the potential for long-term neurodevelopmental consequences of pCMV infections that might occur in VLBW infants.
2 |. BREAST MILK-ACQUIRED POST-NATAL CMV INFECTIONS
2.1 |. Epidemiology and pathogenesis
In contrast to cCMV, which is generally believed to occur due to maternal viremia with subsequent transplacental spread to the fetus,21 pCMV acquisition from breast-feeding probably occurs from a transmucosal route. What is unclear is where CMV transmission occurs anatomically. Since most VLBW infants are not developmentally ready for full oral feedings, administration of nutrition often requires use of gastric or jejunal routes of enteral nutrition. This implies that some (or even most) breast-milk-associated pCMV infections may be transmitted trans-mucosally at the level of the small bowel mucosa. This route of transmission is supported by studies demonstrating that polarized Caco-2 intestinal epithelial cells are infected by CMV at the baso-lateral surface.22,23 On the other hand, pCMV infection may initiate at the level of the oropharyngeal or nasopharyngeal epithelium, with subsequent infection of regional lymph nodes, viremic spread to the reticuloendothelial system, and widespread end-organ infection24; this transmucosal route in the nasopharynx/oropharynx may be the most common route of infection in term babies. Although infection has been postulated to begin in epithelial cells at the site of initial replication, another potential route by which CMV might enter the susceptible host is by a trans-olfactory route of infection. Studies using the murine CMV (MCMV) virus have demonstrated a nasal/transolfactory (as opposed to oropharyngeal) route of infection is employed by the virus, both after experimental virus uptake and during natural transmission in littermates. Subsequent to this initial spread by the trans-olfactory route, long-term, transmissible infection is then maintained in the salivary glands.25 Other studies of MCMV infection in neonatal mice have demonstrated that intraperitoneal injection of MCMV-positive breast milk is sufficient to initiate infection, indicating that transmission at a mucosal surface is not strictly required for milk to be infectious, at least not in murine models.26 These potential routes of entry of CMV via breast milk-mediated transmission are outlined in Figure 1.
FIGURE 1.
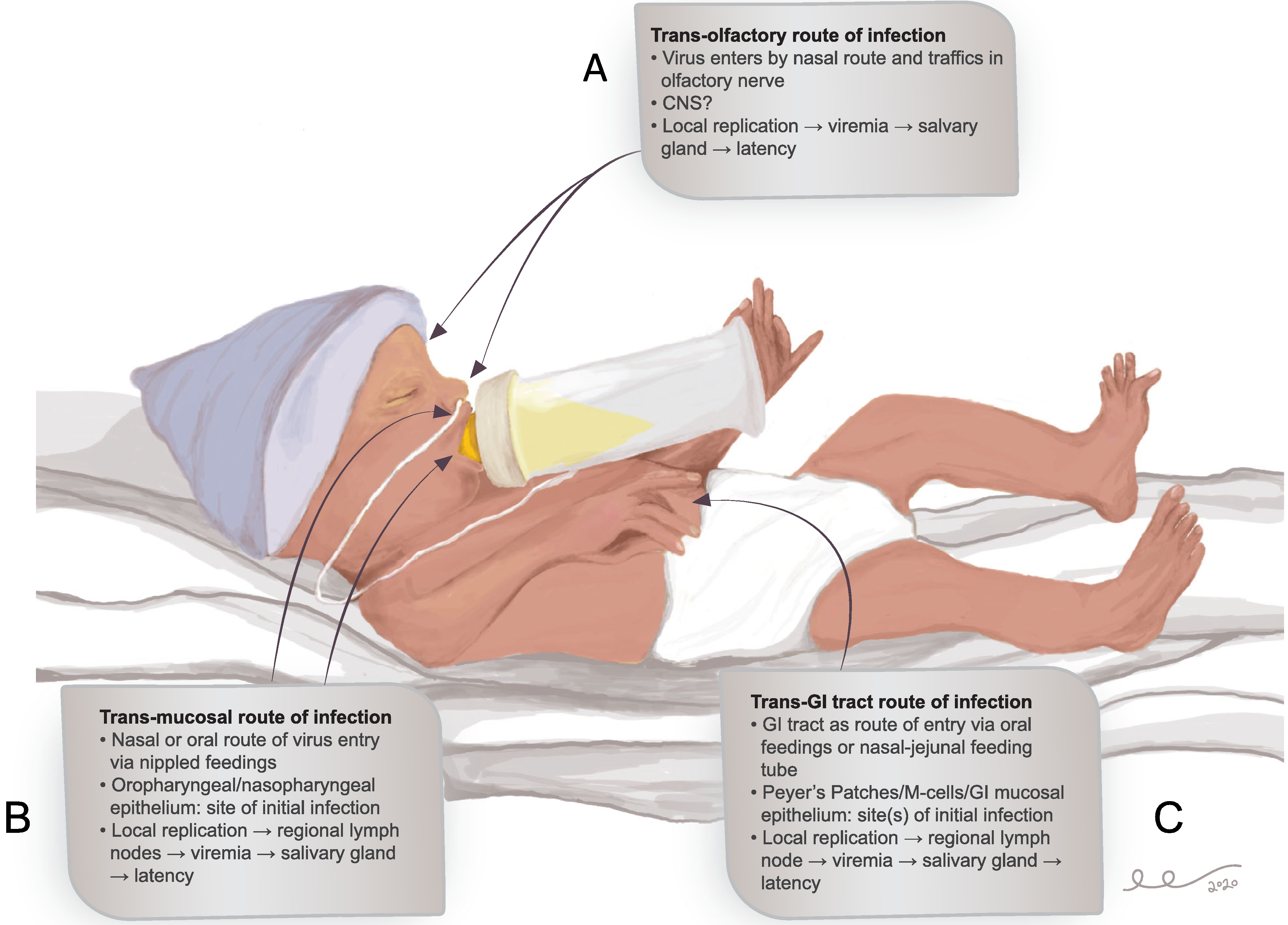
Potential and postulated routes of entry for CMV infection in the VLBW premature infant. Possible routes of entry include trans-olfactory (Panel A), trans-mucosal in the oropharynx and/or nasopharynx (Panel B), and trans-jejunal via intestinal epithelial cells, Peyer’s patches, or M-cells (Panel C). These routes of entry are not mutually exclusive. The trans-olfactory route has been identified in murine models25 but not validated in human infants. Unresolved questions include whether cell-free or cell-associated virus in milk is infectious. It is presumed that viremia ensues irrespective of the route(s) involved, followed by salivary gland seeding and establishment of latent/persistent infection
Irrespective of the route of entry of the virus, it is intriguing that not all infants exposed to CMV-positive breast milk become infected. Previous studies have shown that CMV reactivation in breast milk occurs in ∼90% of seropositive lactating women. Yet, not all infants receiving CMV-positive breast milk become infected; indeed, published estimates suggest that only ∼20% of exposed infants will acquire infection in the newborn intensive care unit (NICU) setting.27,28 Studies have examined whether those infants that acquire infection have specific deficits in adaptive immunity. One study investigated the relationship of the magnitude of CMV-specific cellular and humoral immune responses in milk of 30 seropositive mothers of VLWB preterm infants to milk CMV load and symptomatic pCMV transmission. Although IgG avidity was inversely correlated to milk CMV load (P = .009), there were no avidity-dependent differences on the likelihood of symptomatic pCMV infection.29 Another study in Ugandan infants of mothers with and without concurrent HIV infection examined associations between CMV-specific Ig responses in mothers at the time of delivery and their infants’ CMV status at 6 months of age, and found no associations between antibody titer and breast milk transmission.30 However, in the Ugandan study, when the IgG titers specific for individual CMV proteins (in particular the major envelope protein glycoprotein B [gB]) were examined, antibody levels to gB were higher in infants without CMV infection and were associated with delayed CMV acquisition, although the results did not achieve statistical significance. A similar lack of correlation between antibody titer and breast milk-mediated transmission was reported in a study in The Netherlands.31 In this study, the anti-CMV IgG levels of 79 CMV seropositive mothers and their 94 infants were examined in the context of postnatal CMV infection, which was detected in 39/94 (41%) infants in the perinatal period. Maternal and/or infant anti-CMV IgG levels were not significantly different between infants with and without pCMV infection.
More information is needed about what benefit, if any, maternal IgG provides against pCMV transmission. At present, it is unclear if any putative benefit of maternal antibody is due to partitioning of IgG into the breast milk, where it could serve to directly inactivate virus infectivity, or if it potentially functions in the infant compartment to block infection of the neonate. In a study of functional anti-CMV antibody in a group of 18 CMV-seropositive women who had milk and serum available for assessment post-partum, anti-gB IgG-antibodies were variably expressed in whey, but continuously in plasma, and at higher levels. Virus neutralization assays demonstrated substantially higher values in plasma compared to whey at 2 months postpartum. No newborn transmission correlates were provided, but the authors concluded that, during reactivation in the post-partum period, CMV-specific-IgG reactivities and neutralizing capacities are much lower in whey than in plasma.32 These findings suggest that any beneficial effect of maternal antibody on modulating breast milk-associated transmission may be mediated by protection against infection in the systemic, not mucosal, compartment.
Other studies have examined innate immune responses and their potential capacity to prevent pCMV transmission in some infants. One innate antiviral factor of interest in breast milk is lactoferrin. Lactoferrin is well recognized to have activity against CMV in cell culture models,33 and in vivo, in a MCMV challenge model, lactoferrin was found to protect against disease in virally challenged mice.34 However, in VLBW infants in the NICU setting, although lactoferrin is known to neutralize HCMV in vitro, no correlation between levels of lactoferrin in breast milk and the likelihood of mother-to-infant pCMV transmission has been demonstrated.35,36
In addition to examining correlates of transmission and protection in the newborn, it is of interest to explore the factors that modulate reactivation and cessation of viral shedding in the maternal compartment. Reactivation of HCMV in post-partum women has been mostly found to be restricted to the lactating breast, although the sites of latency and virus production, as well as the mechanism of reactivation, remain unclear.37 Most of the virus in the breast milk has been found to be free virus in the whey fraction, rather than cell-associated virus.38 CMV shedding seems to follow a defined pattern, with peak levels noted in breast milk at around 1 month post-delivery.39,40 The phenotype of T cells resident in the mammary gland during lactation was examined in HIV-positive and HIV-negative women.41 This study demonstrated that specific CD8(+) T cells, as measured by the interferon-gamma (IFN-γ) ELISPOT and MHC class I tetramer staining, were all present at greater frequencies in breast milk than they were in blood. The phenotype of the extra-lymphoid CD8(+) T cells in the breast milk was CD45RO(+)/HLADR(+), and cells expressed CD45RA, CD62L, and CCR7, consistent with an effector memory population (and different from those in peripheral blood). The local cellular immunological milieu in lactating CMV-seropositive mothers was further investigated in a study of CMV T-cell responses specific for the tegument phosphoprotein pp65 (ppUL83).42 The frequency of pp65-specific CD8(+) T cells IFN-γ alone or dual IFN-γ/granzyme B producers was increased in breast milk compared with PBMCs. Intriguingly, there was a positive correlation between breast milk CMV viral load and the CD8(+) pp65-specific T-cell response, suggesting that local virus replication drives antigen-specific CD8(+) T cells into the breast. The authors suggested that the presence of these CD8(+) T cells may be of benefit to the breast-feeding infant, and this question deserves further experimental scrutiny. Finally, another recent study has examined the impact of CMV reactivation on the cytokine profile in breast milk.43 This study reported that reactivating mothers demonstrated higher levels of both CC-chemokines (MCP-2, CCL19, and CCL20), as well as CXC-chemokines (IL-8, CXCL9, CXCL10, and CXCL11). The proinflammatory cytokine IL-17C, glycoprotein CD5, and TNFSF14 were also upregulated. Further exploration of how cytokines modulate the kinetics of reactivation and (possibly) transmission are warranted.
2.2 |. Short-term complications of pCMV infection
Although breast-milk pCMV transmission was first proposed in 1972,44 little attention was paid to this route of CMV infection until the late 1990s, when a series of groundbreaking studies in Tübingen, Germany, examined in depth the clinical impact of breast milk-acquired pCMV infection in premature infants.45 These studies demonstrated that VLBW (<1500 g; <32 weeks gestational age at birth) infants were at high risk of acquiring CMV from breast milk. CMV status in mothers and infants was examined by serology, virus culture, and PCR of milk. Among CMV-seropositive, lactating mothers, 73 of 76 (93%; corresponding to 87 premature infants) reactivated the virus in breast milk, as assessed by PCR. Early appearance of virus in milk whey and the presence of infectious virus (by culture) in milk whey were predictive of transmission. The overall rate of transmission was 37% (27 of the 73 mothers with reactivation of CMV in milk, corresponding to 33 infants), and the mean time to infection of the infant was 42 days. Of the 33 preterm infants who acquired CMV infection, 16 (48%) were symptomatic, as determined by clinical and/or laboratory abnormalities. Clinical manifestations included hepatopathy, neutropenia, thrombocytopenia, and sepsis syndrome. No infants were treated with antiviral therapy in this report, and no CMV-related mortality was observed. Subsequent studies have confirmed the clinical importance of pCMV transmission in the NICU setting, although transmission rates have been lower (in the ∼20% range) in most studies.27,28 The reported range for breast milk associated pCMV transmission is variable: one analysis of the literature found, after review of 26 studies, that the reported percentage of transmission to infants ranged from 5.7% to 58.6%.46 This review also noted that symptomatic CMV disease occurred in 0% to 34.5% (median 3.7%) and severe sepsis-like syndrome in 0% to 13.8% (median 0.7%). The variability and wide ranges in rates of transmission and disease may reflect some heterogeneity in the definition of “prematurity” in the studies reviewed. Notably, the authors commented that published recommendations for prevention, therapy, and follow-up varied “markedly” among experts (discussed below). Another meta-analysis predicted that, among 299 infants fed untreated breast milk, there would be an estimated 19% (11%-32%) of VLBW infants that would acquire pCMV infection, and that 4% (2%-7%) would develop CMV sepsis-like syndrome.47
2.3 |. Potential long-term neurodevelopmental complications of pCMV infection
Although some VLBW infants have significant short-term disease manifestations due to pCMV infection, the risks associated with pCMV acquisition remain largely unknown. As noted above, for full-term infants, the risk for impaired development is not any higher than children without CMV infection. Transmission of protective maternal antibodies, starting about the 29th week of gestation, may help explain why full-term infants rarely develop symptomatic CMV disease without some alternate reason for immune compromise.48 There are few existing studies that address the long-term outcomes of preterm infants who acquire CMV during the newborn period. Of the studies available, there remain conflicting results, thus highlighting several unresolved questions about long-term neurodevelopmental outcome after acquired pCMV infection.
Early studies of outcome in preterm children with post-natal CMV infection in the late 1970s and early 1980s suggested that these infants may have increased risk for developmental problems including hearing loss,49,50 but these studies included infants that acquired pCMV infection from nonbreast milk sources, including transfusion-acquired CMV, a problem which has essentially been solved by leukofiltration of blood products.51 Similarly, nosocomial CMV transmission, though described in the pediatric hospital setting,52 probably plays at best a minimal role in acquisition of pCMV infection. More recent work, focusing on breast milk-acquired pCMV infection, has yielded mixed findings about the impact of infection on neurodevelopmental outcomes in the VLBW premature infant. A systematic literature review published in 2010 provided an overview of neurodevelopmental outcomes associated with pCMV acquisition.46 The authors reported an analysis of 26 studies of post-natally acquired CMV via breast milk. Of those trials, five reported on long-term outcomes of acquired CMV infection in the preterm infant.50,53–57 There was no association with SNHL documented in any trial. Moreover, there were no differences in motor or speech development in the CMV-infected infants and controls. In one of these studies,5322 preterm infants with a median gestational age of 27.6 weeks and median birth weight of 1020 g, with early pCMV infections, were compared to 22 CMV-negative matched controls. Controls were matched for gestational age, birth weight, gender, intracranial hemorrhage, and duration of mechanical ventilation. At 2 to 4.5 years of age, follow-up assessments including neurologic examination, neurodevelopmental assessment based on the concept of essential developmental milestones, and detailed audiologic evaluations were completed. This study, although consisting of a relatively small sample size, showed no differences between the groups with regard to neurologic, speech and language, or motor development. Therefore, these authors concluded that in preterm infants with symptomatic CMV disease, even severe sepsis-like syndrome, that there was a low risk of neurologic and cognitive sequelae and no increased risk for hearing impairment.
These reassuring findings notwithstanding, recently the question of long-term neurodevelopment after acquired CMV infection in preterm infants has been examined with respect to a heightened detection of more subtle adverse neurologic outcomes. Additional analyses by the Tübingen research group have highlighted more subtle adverse neurologic outcomes associated with the acquisition of CMV. Additional longitudinal analyses of their study cohorts over time demonstrated significant differences between the CMV-positive and CMV-negative groups.58 Forty-one from the original cohort of 44 children were examined at school age to investigate neurodevelopmental outcome. Assessments included neurologic examination by skilled examiners, assessment of motor function using the Movement Assessment Battery for Children (M-ABC), cognitive function using the Kaufman Assessment Battery for Children (K-ABC), and audiologic tests. In both groups, irrespective of CMV status, performance in motor and cognitive assessments fell within the normal range. Significant differences, however, were seen between the CMV-positive and CMV-negative group in both motor and cognitive function, with poorer performance in the CMV-positive group. All had normal hearing function. Further analysis of these patients again noted no difference in the prevalence of cerebral palsy, but following pCMV infection, infants had significantly lower results in the simultaneous processing scales of the K-ABC. Since simultaneous processing tasks represent more complex cognitive function, these findings could be of major concern. The authors concluded that in this study, cognitive and motor function in preterm children with pCMV infection transmitted via breast milk was within the normal range, but their outcomes were poorer than those in preterm children without CMV infection.58 Another follow-up study of 42 VLBW infants from this group examined long-term cognitive outcome and prevalence of cerebral palsy (CP), as well as neurological and neurocognitive assessment using the K-ABC at the age of >4 years.59 The study was performed in blinded fashion, using a controlled matched-pairs design with gestational age, gender, and date of birth as matching criteria. The prevalence of cerebral palsy was the same in cases and controls. Infants with pCMV infection were found to have significantly lower results in the simultaneous processing scale of the K-ABC (P = .029) after correction for additional risk factors like socioeconomic status (SES). However, results for the sequential and achievement scales of the K-ABC were only slightly reduced (P > .05). The authors concluded from this study that pCMV infection from breast milk might have a detrimental effect on long-term cognitive development.
More data linking pCMV infection from a breast milk source to sequelae was reported in another follow-up study from the Tübingen group.60 In this study, adolescents born very preterm (n = 42), with and without a history of pCMV infection, were compared to full-term controls using both the German version of the Wechsler Intelligence Scale and the Developmental Test for Visual Perception. Consistent with other studies of neurodevelopmental outcome in VLBW infants, adolescents born preterm had significantly lower scores than term-born controls on IQ and visual perception testing. Notably, the adolescents born preterm with pCMV infection scored significantly lower than those without the infection regarding overall cognitive abilities (P = .03), although visuoperceptive abilities were not significantly impaired. These studies were then extended to functional magnetic resonance imaging (fMRI) evaluation of these subjects. Premature infants with (15 adolescents) and without (19 adolescents) a history of pCMV acquisition were compared to healthy controls (37 subjects). A “dual use” fMRI investigated language and visuospatial functions. The investigators found significant activation differences in the left hippocampus and the right anterior cingulate cortex while performing the language task and a small region of the occipital cortex while performing the visuospatial task.61 There were also differences in gray matter volume in several brain regions. The authors suggest that early postnatal CMV infection in former preterm infants may have long-term neurological consequences that can be detected via fMRI in older children when performing higher-level cognitive tasks, and that intervention strategies aimed at preventing pCMV infection may be warranted.
Another important recent study that may have major implications for our understanding of the neurodevelopmental consequences of pCMV infection was recently reported by Weimer and colleagues.62 As noted, cCMV infection is well recognized to be a major cause of SNHL,17 but this association had not been noted in the context of pCMV infection.53 This group examined the prevalence of failed hearing screens, the calculated postnatal age, and somatic growth parameters at discharge in VLBW infants with pCMV infections (and uninfected controls) at the time of NICU discharge. A compelling aspect of this study was the size of the database that was examined: it spanned 302 NICUs managed by the Pediatrix Medical Group (https://www.mednax.com/hospitals/solutions/neonatal/) from years 2002 to 2016, and over 75 000 infants met the inclusion criteria and had data available for analysis. A total of 304 infants with pCMV infection were identified, and 273 (90%) of these infants were matched to controls without pCMV. Notably, newborn hearing screen (NHS) failure occurred in 45/273 (16%) infants with pCMV compared to 25/273 (9%) infants without pCMV (P = .01). Although a failed NHS does not establish with certainty that an infant has SNHL, there are strong correlations between a failed hearing screen, subsequent confirmation of SNHL, and the presence of CMV infection. Hence, these data from this large VLBW infant database support the need for comprehensive audiological and neurodevelopmental follow-up in infants with pCMV infection, and this should help resolve the question of risk of long-term CMV-related disability in this population. Table 1 summarizes the reported studies to date that have assessed for potential adverse long-term neurodevelopmental and hearing outcomes in VLBW infants acquiring pCMV from breast milk.
TABLE 1.
Summary of studies evaluating hearing and neurodevelopmental outcomes following pCMV infection in premature infants
| Study | Increased risk of SNHL | Neurocognitive Deficits | Comments | Reference |
|---|---|---|---|---|
| Study of 22 VLBW infants pCMV and controls | No | No | Studies at 2 to 4.5 years; neurologic examination, neurodevelopmental assessment and audiology | 53 |
| VLBW infants (n = 55); 14 with pCMV, 41 without pCMV | No | No (Bayley scale) | Long-term outcomes: audiologic tests, gross motor quotient, Infant International Battery; at 12 and 24 months | 54 |
| Birth weight < 2000 g; 55 pCMV infants and controls | No (4/43 pCMV and 2/43 controls) | Possibly; abnormal EEG in 4/23 pCMV v. 1/23 controls; severe handicaps in 4/29 pCMV v. 2/29 controls | Clinical evaluation performed at 3 years of age; hearing assessment, physical examination, EEG | 50 |
| VLBW infants; 17 cCMV, 10 pCMV, 21 controls | Yes in cCMV (4/17); not pCMV (0/10) | No | Cognitive, behavioral, neurologic, audiometric, speech/language evaluation; no difference in mean IQ | 57 |
| VLBW infants; 20 pCMV, 21 controls | No | Yes; significant differences between pCMV and controls in motor/cognitive function | Neurological examination, Movement Assessment Battery for Children, Kaufman Assessment Battery for Children | 58 |
| VLBW infants; 42 pCMV infants and matched controls | Not reported | Yes; pCMV infants with lower results in the simultaneous processing scale of the K-ABC (p = 0.029) | Kaufman Assessment Battery for Children (K-ABC) at the age of >4 years | 59 |
| VLBW infants (n = 42; 19 with, 23 without pCMV); controls (n = 23) | Not reported | Yes; pCMV subjects significantly lower in cognitive (P = .03), but not visuoperceptive abilities (P > .05) | Wechsler Intelligence Scale and Developmental Test for Visual Perception; assessed in adolescents (11.3–16.6 years) | 60 |
| VLBW infants; 304 with pCMV and 273 controls | Yes; inferred by newborn hearing screen (NHS) | Not reported | Pediatrix data base; NHS failure in 45/273 (16%) with pCMV compared to 25/273 (9%) infants without pCMV (p = 0.01) | 62 |
2.4 |. Other long-term complications associated with pCMV infection
In addition to the evolving body of knowledge about potential neurodevelopmental sequelae (including SNHL) associated with pCMV infection, there are other emerging concepts about complications of prematurity that are likely impacted by this infection. Bronchopulmonary dysplasia (BPD) is a common complication of prematurity, and studies of pCMV suggest a role for CMV infection as a risk factor. In a retrospective study of >100 000 VLBW infants, pCMV infection was associated with an increased risk for BPD (risk ratio, 1.33; 95% CI, 1.19–1.50), and other changes in cardiorespiratory status were associated with pCMV infection, including a requirement for vasopressor medications, intubation for mechanical ventilation, a new oxygen requirement, and death.63 In another single-center, retrospective study of 2132 VLBW infants pCMV-infected infants were noted to have significantly more exposure to mechanical ventilation and longer duration of hospitalization than controls. BPD was significantly more common in the pCMV group (odds ratio, 4.0 [95% confidence interval, 1.3–12.4); P = .02).64 In addition to BPD, it has been suggested that another complication of premature birth, necrotizing enterocolitis (NEC) may be associated with pCMV infection.65 However, in the Weimer study, NEC did not stand out as a complication of pCMV infection, although an association between pCMV infection and BPD was confirmed. Weimer et al. also observed that viral infection was associated with an increased postnatal age at discharge of nearly 12 days and a lower weight-for-age z-score, suggesting that CMV infection prolonged hospitalization and had a negative impact on infant growth.62 An impact of delayed hospital discharge on the overall cost of care was not investigated, but this issue should be examined in future cost–benefit analyses.66
3 |. MANAGEMENT STRATEGIES
3.1 |. Prevention efforts
As summarized above, there is a rapidly evolving body of information that suggests that pCMV has both significant short-term and long-term consequences. There is currently no consensus about what measures, if any, should be undertaken in an attempt to prevent pCMV infections. Some experts have expressed uncertainty about the importance of preventing these infections.67,68 However, with the accumulating evidence that pCMV poses short-term and (probably) long-term health risks to the VLBW infant, some systematic efforts toward prevention seem warranted. The Red Book Committee of the American Academy of Pediatrics recommends that, for premature infants born at <32 weeks estimated gestational age (EGA), consideration should be given to serological screening of mothers and, for CMV-seropositive women, short-term pasteurization of breast milk.69 Pasteurization is highly effective at inactivating CMV and is recommended in France for VLBW infants born at <32 weeks EGA and < 1500 birth weight,70 but pasteurization may carry a negative impact by inactivating many of the salutary components of breast milk, including lactoferrin, lysozyme, immunoglobulins, erythropoietin, cytokines, and growth factors.71–73 Short-term pasteurization seems to minimize the impact on the salutary components of breast milk, while still retaining the ability to inactivate virus.74 The impact of short-term pasteurization was studied in a NICU population in which an intervention group of infants (receiving pasteurized milk) was compared to historical controls administered unpasteurized milk.75 Two of 87 (2.3%) study infants had a pCMV transmission, in contrast to 17 of 83 (20.5%) historical controls. The risk ratio of controls compared to study infants was 8.3 (95% CI, 2.4–52.4) by Cox proportional hazard model (P = .0003). Thus, short-term pasteurization does appear to be a viable option for prevention of pCMV transmission.
An alternative to pasteurization is freezing milk at −20°C for 24 to 72 hours. The Red Book committee notes that this intervention can decrease, but not eliminate, the risk of infectivity.69 However, it should be noted that a recent controlled study failed to demonstrate any benefit of freezing breast milk on pCMV transmission in the NICU setting.76 In this study, the CMV transmission rate in infants fed with CMV-DNA positive milk was 8% (3 of 37) in the intervention (freezing) group and 6% (2 of 33) in controls. Thus, there is insufficient data to warrant freezing of breast milk as a means to prevent CMV transmission in the NICU. Other innovative modalities to inactivate CMV in breast milk have been proposed, such as microwave irradiation,77,78 but this intervention has not yet been evaluated in a clinical study. Bedside, point-of-care CMV testing of breast milk might identify, in real-time, high-risk situations where pasteurization is warranted. Application of other strategies to render milk noninfectious is also worth considering, including treatment with anti-CMV immunoglobulins and/or leukofiltration of milk. Consideration might be given to monitoring VLBW infants for CMV infection while receiving breast milk. It has been proposed that routine monitoring for DNAemia in this setting may herald the onset of symptomatic disease,65 and identify infants who might benefit from “pre-emptive” therapy that could be administered, much as it is to an immunocompromised patient,79 to prevent development of CMV end-organ disease. Such surveillance PCR testing could become a part of routine laboratory testing protocols for VLBW infants in the NICU. Preemptive screening for pCMV acquisition could also be considered through assays such as weekly saliva-based PCR for at-risk infants in the NICU setting. The recent licensure of point-of-care diagnostic assays for saliva-based screening could be useful in identifying VLBW infants that acquire CMV infection in this setting, and such infants could be managed and monitored expectantly for emergence of CMV disease and/or sequelae.80 Other strategies for management, such as ensuring exclusion of cCMV by newborn dried blood spot analysis and monitoring infants with clearcut pCMV acquisition for end-organ complications, deserve future evaluation.81
3.2 |. Antiviral therapy
Another aspect of control of pCMV that remains even less clear is the role of antiviral therapy with ganciclovir or immune globulin for the prevention or therapy of breast milk-acquired infection. Although there is limited experience with antivirals targeting CMV with the nucleoside analog, ganciclovir, there may be a role for its use (by parenteral administration, or by oral administration of the prodrug, valganciclovir) in selected infants with CMV end-organ disease or severe sepsis-like syndrome following CMV acquisition. Most experience with cytomegalovirus antivirals has come from the study of congenital infection.1,15 Since recent reports have emerged in cCMV that long-term oral nucleoside therapy improves neurodevelopmental outcomes, the use of these agents may be of considerable therapeutic importance for pCMV infection, if the impact on neurodevelopmental outcomes can be confirmed in future prospective studies. In breast milk-acquired pCMV, antiviral therapy in symptomatic infants may be useful in reduction of the severity of symptoms and the duration of illness.81 Other potential interventions that could be conceived of would include administration of high-titer anti-CMV immune globulin to high-risk infants, or therapeutic vaccination of lactating mothers of VLBW premature infants, toward the goal of augmenting CMV immunity in milk. This approach would require licensure of a CMV vaccine, and elucidation of the aspects of protective immunity that prevent pCMV transmission to VLBW infants. Potential preventative and/or therapeutic interventions are summarized in Table 2.
TABLE 2.
Potential preventative and therapeutic interventions for addressing/preventing pCMV infections acquired from breast milk
| Intervention | Pros | Cons | Comments |
|---|---|---|---|
| Freezing breast milk(−20°C, minimum of 24 h) | Convenience; minimal inactivation of beneficial components of milk | Ineffective; reduces titre of CMV but does not eliminate infectivity | Formerly recommended by RedBook Committee of American Academy of Pediatrics |
| Pasteurization of breast milk (62.5°C, minimum of 30 min) | Highly effective at eliminating infectivity of CMV | Inactivates beneficial immunological components of human milk (lactoferrin, defensins, leukocytes, immunoglobulin) | Recommended for consideration by RedBook Committee of American Academy of Pediatrics, depending on maternal CMV serological status |
| Short-term pasteurization of breast milk (62.5°C, 5 s) | Appears effective at inactivating CMV75 | May be less damaging to immune components of milk | Substantially reduced, but did not eliminate risk of pCMV transmission from breast milk in clinical trial75 |
| Agents to inactivate CMV in milk (monoclonal antibodies, exogenous lactoferrin) | Could eliminate CMV infectivity of breast milk following addition of agent(s) to milk | Would preserve immunological components of milk | No correlation between levels of antibody or lactoferrin35,36 in breast milk30,31 and pCMV transmission has been demonstrated |
| Antibody or antiviral-based therapies administered to susceptible infants | Could prevent acquisition of pCMV in infant receiving CMV-positive milk | Immunological correlates of prevention of neonatal infection not defined for antibody therapies; unacceptable risk/benefit ratio for nucleoside prophylaxis | Concept of “pre-emptive therapy” with nucleosides, based on plasma PCR monitoring of asymptomatic infants, has been proposed for potential clinical study65 |
| Maternal vaccination | Immune response induced by vaccine: potential to prevent maternal re-activation and/or breast milk transmission? | Immunological correlates of protection unknown; no data on protection | Timing of maternal vaccination (once vaccine is licensed) needs to be defined; vaccination to prevent re-activation in breast milk would require immune responses higher than those in natural infection |
4 |. PRIORITIES FOR FUTURE RESEARCH
As there remains no consensus among neonatologists or pediatric infectious disease specialists on whether efforts should be made to prevent or treat breast milk-acquired CMV infections, there are some clear knowledge deficits requiring future research that may provide insight into advancing clinical care. First, better understanding of the incidence, immunological, and virological correlates of maternal infection and infant disease should be explored. Unresolved questions, not only of short-term risks but also potentially of the long-term neurodevelopmental sequelae of post-natal acquisition of CMV need additional answers. These studies assessing the magnitude of impact of CMV on neurodevelopment will help lay the groundwork for future research to clarify whether preventative or therapeutic antivirals for CMV are warranted in VLBW infants at risk for CMV. Finally, elucidation of the basic science and cellular biology of pCMV breast milk-mediated transmission is a high priority. Of particular importance are the questions of how CMV infects the infant (portal of entry and pathways of dissemination); is the infection mediated by cell-associated or cell free virus; what are the immunological correlates and/or deficits associated with infection; and how does pCMV exacerbate processes such as BPD and contribute to the pathogenesis of SNHL and neurodevelopmental injury? These critical areas of knowledge deficit are summarized in Table 3. As future research finds answers to these questions, a more consistent and cogent approach to the prevention and management of breast milk-acquired pCMV infections should emerge, and the risks of long-term sequelae for this fragile group of newborns should be minimized in the future.
TABLE 3.
High priority areas for future research
| Research Question | Rationale |
|---|---|
| Correlates of protective immunity against pCMV transmission | Transmission by breast milk is common and ubiquitous but not inevitable - either in term infants or VLBW premature infants. What are the maternal/infant immune responses that block transmission in some (but not all) cases? |
| Virological correlates of transmission | Are there differences in viral strains/genotypes that either augment or deter pCMV transmission? Is transmission more common in setting of maternal re-infection than reactivation? What is the impact of magnitude and/or duration of viral load during lactation? |
| How does CMV infect the nursing infant? | Where does virus enter the breast-fed baby (oropharyngeal epithelium, small bowel mucosal, lymphoid vs epithelial cells)? Is CMV transmitted as free virus or cell-associated virus? What are the relative roles of fusion vs endocytosis in virus access? Animal models should be developed to assess. |
| Can CMV be inactivated prior to breast feeding? | Could eliminate CMV infectivity of breast milk following addition of agent(s) to milk. |
| Should women be screened for CMV antibodies prior to administration of breast milk? | Could identify “high-risk” situations in which pasteurization, immune based therapies, or anti-virals would be warranted. |
| Should breast milk be tested by PCR or rapid, point-of-care assays prior to administration to VLBW infant? | CMV-positive breast milk could be treated/pasteurized prior to feeding. |
| Should VLBW infants have periodic CMV surveillance PCRs in course of clinical care to monitor for DNAemia or viral shedding? | Identify babies at risk for CMV end-organ disease; inform and direct decision-making by clinicians regarding other NICU therapies (eg, steroids for BPD). Identify infants that might benefit from pre-emptive antiviral therapy that in turn could prevent CMV end-organ disease (lung disease, CMV sepsis syndrome) and, possibly, neurodevelopmental sequelae. |
| What is the long-term neurodevelopmental impact of pCMV infection in VLBW infants? | Studies to date are inconclusive; data on increased numbers of newborn hearing screen referrals in VLBW infants with pCMV infection do not necessarily translate to SNHL; impact on neurodevelopmental outcomes still unresolved; controlled, prospective clinical trials are needed. |
ACKNOWLEDGEMENTS
The authors acknowledge a UMN Department of Pediatrics “cross-divisional” grant to support these studies. This work was also supported by the University of South Carolina’s Disability Research and Dissemination Center (DRDC) through its Cooperative Agreement (Number 6U19DD001218) with the Centers for Disease Control and Prevention (CDC). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the DRDC or CDC. Additional grant support: R01 HD079918 and R01 HD098866. We thank Emily Eck designs (www.instagram.com/emilyeckdesigns) for assistance with illustration.
Funding information
Centers for Disease Control and Prevention, Grant/Award Numbers: 6U19DD001218, R01 HD098866, R01 HD079918; Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Number: HD079918; University of South Carolina’s Disability Research and Dissemination Center, Grant/Award Number: 6U19DD001218
Abbreviations:
- BPD
bronchopulmonary dysplasia
- cCMV
congenital cytomegalovirus infection
- CID
cytomegalic inclusion disease
- CMV
cytomegalovirus
- EGA
estimated gestational age
- ELISPOT
enzyme-linked immune absorbent spot
- fMRI
functional magnetic resonance imaging
- gB
glycoprotein B
- HCMV
human cytomegalovirus
- HIV
human immunodeficiency virus
- IFN-γ
interferon gamma
- IgG
immunoglobulin G
- K-ABC
Kaufman assessment battery for children
- M-ABC
movement assessment battery for children
- MCMV
murine cytomegalovirus
- MHC
major histocompatibility complex
- NEC
necrotizing enterocolitis
- NHS
newborn hearing screening
- NICU
newborn intensive care unit
- ppUL83
phosphoprotein unique long 83
- SNHL
sensorineural hearing loss
- VLBW
very-low birthweight
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest relevant to this work.
REFERENCES
- 1.Swanson EC, Schleiss MR. Congenital cytomegalovirus infection: new prospects for prevention and therapy. Pediatr Clin North Am. 2013;60: 335–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17:253–276. [DOI] [PubMed] [Google Scholar]
- 3.Boppana SB, Ross SA, Shimamura M, et al. Saliva polymerase-chain-reaction assay for cytomegalovirus screening in newborns. N Engl J Med. 2011;364:2111–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowler KB, Ross SA, Shimamura M, et al. Racial and ethnic differences in the prevalence of congenital cytomegalovirus infection. J Pediatr. 2018;200:196–201. [DOI] [PubMed] [Google Scholar]
- 5.Zuhair M, Smit GSA, Wallis G, et al. Estimation of the worldwide seroprevalence of cytomegalovirus: a systematic review and meta-analysis. Rev Med Virol. 2019;29:e2034. 10.1002/rmv.2034. [DOI] [PubMed] [Google Scholar]
- 6.Britt W. Controversies in the natural history of congenital human cytomegalovirus infection: the paradox of infection and disease in offspring of women with immunity prior to pregnancy. Med Microbiol Immunol. 2015;204:263–271. [DOI] [PubMed] [Google Scholar]
- 7.Britt WJ. Maternal immunity and the natural history of congenital human cytomegalovirus infection. Viruses. 2018;10(8):1–18. 10.3390/v10080405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Britt WJ. Congenital human cytomegalovirus infection and the enigma of maternal immunity. J Virol. 2017;91(15):1–7. 10.1128/JVI.02392-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbosa NG, Yamamoto AY, Duarte G, et al. Cytomegalovirus shedding in seropositive pregnant women from a high-seroprevalence population: the Brazilian cytomegalovirus hearing and maternal secondary infection study. Clin Infect Dis. 2018;67:743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mussi-Pinhata MM, Yamamoto AY, Aragon DC, et al. Seroconversion for cytomegalovirus infection during pregnancy and fetal infection in a highly seropositive population: "the BraCHS study". J Infect Dis. 2018;218:1200–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheeran MC, Lokensgard JR, Schleiss MR. Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin Microbiol Rev. 2009;22:99–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teissier N, Fallet-Bianco C, Delezoide AL, et al. Cytomegalovirus-induced brain malformations in fetuses. J Neuropathol Exp Neurol. 2014;73:143–158. [DOI] [PubMed] [Google Scholar]
- 13.Gabrielli L, Bonasoni MP, Lazzarotto T, et al. Histological findings in foetuses congenitally infected by cytomegalovirus. J Clin Virol. 2009; 46(Suppl 4):S16–S21. [DOI] [PubMed] [Google Scholar]
- 14.Townsend CL, Forsgren M, Ahlfors K, Ivarsson SA, Tookey PA, Peckham CS. Long-term outcomes of congenital cytomegalovirus infection in Sweden and the United Kingdom. Clin Infect Dis. 2013;56: 1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rawlinson WD, Boppana SB, Fowler KB, et al. Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect Dis. 2017;17(6):e177–e188. 10.1016/S1473-3099(17)30143-3. [DOI] [PubMed] [Google Scholar]
- 16.Faure-Bardon V, Magny JF, Parodi M, et al. Sequelae of congenital cytomegalovirus following maternal primary infections are limited to those acquired in the first trimester of pregnancy. Clin Infect Dis. 2019;69:1526–1532. [DOI] [PubMed] [Google Scholar]
- 17.Fowler KB, Boppana SB. Congenital cytomegalovirus (CMV) infection and hearing deficit. J Clin Virol. 2006;35:226–231. [DOI] [PubMed] [Google Scholar]
- 18.Permar SR, Schleiss MR, Plotkin SA. Advancing our understanding of protective maternal immunity as a guide for development of vaccines to reduce congenital cytomegalovirus infections. J Virol. 2018;92(7): 00030–00018. 10.1128/JVI.00030-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stagno S, Reynolds DW, Pass RF, Alford CA. Breast milk and the risk of cytomegalovirus infection. N Engl J Med. 1980;302:1073–1076. [DOI] [PubMed] [Google Scholar]
- 20.Schleiss MR. Acquisition of human cytomegalovirus infection in infants via breast milk: natural immunization or cause for concern? Rev Med Virol. 2006;16:73–82. [DOI] [PubMed] [Google Scholar]
- 21.McDonagh S, Maidji E, Chang HT, Pereira L. Patterns of human cytomegalovirus infection in term placentas: a preliminary analysis. J Clin Virol. 2006;35:210–215. [DOI] [PubMed] [Google Scholar]
- 22.Esclatine A, Lemullois M, Servin AL, Quero AM, Geniteau-Legendre M. Human cytomegalovirus infects Caco-2 intestinal epithelial cells basolaterally regardless of the differentiation state. J Virol. 2000;74:513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarvis MA, Wang CE, Meyers HL, et al. Human cytomegalovirus infection of caco-2 cells occurs at the basolateral membrane and is differentiation state dependent. J Virol. 1999;73:4552–4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson JW, Sparer T. There is always another way! Cytomegalovirus’ multifaceted dissemination schemes. Viruses. 2018;10(7):383. 10.3390/v10070383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farrell HE, Lawler C, Tan CS, et al. Murine cytomegalovirus exploits olfaction to enter new hosts. mBio. 2016;7(2):e00251–e00216. 10.1128/mBio.00251-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu CA, Paveglio SA, Lingenheld EG, Zhu L, Lefrançois L, Puddington L. Transmission of murine cytomegalovirus in breast milk: a model of natural infection in neonates. J Virol. 2011;85: 5115–5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Josephson CD, Caliendo AM, Easley KA, et al. Blood transfusion and breast milk transmission of cytomegalovirus in very low-birth-weight infants: a prospective cohort study. JAMA Pediatr. 2014;168:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright CJ, Permar SR. Preventing postnatal cytomegalovirus infection in the preterm infant: should it be done, can it be done, and at what cost? J Pediatr. 2015;166:795–798. [DOI] [PubMed] [Google Scholar]
- 29.Ehlinger EP, Webster EM, Kang HH, et al. Maternal cytomegalovirus-specific immune responses and symptomatic postnatal cytomegalovirus transmission in very low-birth-weight preterm infants. J Infect Dis. 2011;204:1672–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saccoccio FM, Jenks JA, Itell HL, et al. Humoral immune correlates for prevention of postnatal cytomegalovirus acquisition. J Infect Dis. 2019;220:772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nijman J, van Loon AM, Krediet TG, Verboon-Maciolek MA. Maternal and neonatal anti-cytomegalovirus IgG level and risk of postnatal cytomegalovirus transmission in preterm infants. J Med Virol. 2013; 85:689–695. [DOI] [PubMed] [Google Scholar]
- 32.Lazar K, Rabe T, Goelz R, Hamprecht K. Human cytomegalovirus reactivation during lactation: impact of antibody kinetics and neutralization in blood and breast milk. Nutrients. 2020;12(2):338. 10.3390/nu12020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harmsen MC, Swart PJ, de Béthune MP, et al. Antiviral effects of plasma and milk proteins: lactoferrin shows potent activity against both human immunodeficiency virus and human cytomegalovirus replication in vitro. J Infect Dis. 1995;172:380–388. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu K, Matsuzawa H, Okada K, et al. Lactoferrin-mediated protection of the host from murine cytomegalovirus infection by a T-cell dependent augmentation of natural killer cell activity. Arch Virol. 1996;141:1875–1889. [DOI] [PubMed] [Google Scholar]
- 35.Weimer KED, Roark H, Fisher K, et al. Breast milk and saliva lactoferrin levels and postnatal cytomegalovirus infection. Am J Perinatol. 2020. 10.1055/s-0040-1701609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Strate BW, Harmsen MC, Schäfer P, et al. Viral load in breast milk correlates with transmission of human cytomegalovirus to preterm neonates, but lactoferrin concentrations do not. Clin Diagn Lab Immunol. 2001;8:818–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meier J, Lienicke U, Tschirch E, Krüger DH, Wauer RR, Prösch S. Human cytomegalovirus reactivation during lactation and mother-to-child transmission in preterm infants. J Clin Microbiol. 2005;43: 1318–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asanuma H, Numazaki K, Nagata N, Hotsubo T, Horino K, Chiba S. Role of milk whey in the transmission of human cytomegalovirus infection by breast milk. Microbiol Immunol. 1996;40: 201–204. [DOI] [PubMed] [Google Scholar]
- 39.Hamprecht K, Maschmann J, Vochem M, Dietz K, Speer CP, Jahn G. Epidemiology of transmission of cytomegalovirus from mother to preterm infant by breastfeeding. Lancet. 1998;357:513–518. [DOI] [PubMed] [Google Scholar]
- 40.Vochem M, Hamprecht K, Jahn G, Speer CP. Transmission of cytomegalovirus to preterm infants through breast milk. Pediatr Infect Dis J. 1998;17:53–58. [DOI] [PubMed] [Google Scholar]
- 41.Sabbaj S, Ghosh MK, Edwards BH, et al. Breast milk-derived antigen-specific CD8+ T cells: an extralymphoid effector memory cell population in humans. J Immunol. 2005;174:2951–2956. [DOI] [PubMed] [Google Scholar]
- 42.Moylan DC, Pati SK, Ross SA, Fowler KB, Boppana SB, Sabbaj S. Breast milk human cytomegalovirus (CMV) viral load and the establishment of breast milk CMV-pp65-specific CD8 T cells in human CMV infected mothers. J Infect Dis. 2017;216: 1176–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rabe T, Lazar K, Cambronero C, Goelz R, Hamprecht K. Human cytomegalovirus (HCMV) reactivation in the mammary gland induces a proinflammatory cytokine shift in breast milk. Microorganisms. 2020;8 (2):289. 10.3390/microorganisms8020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayes K, Danks DM, Gibas H, Jack I. Cytomegalovirus in human milk. N Engl J Med. 1972;287:177–178. [DOI] [PubMed] [Google Scholar]
- 45.Maschmann J, Hamprecht K, Dietz K, Jahn G, Speer CP. Cytomegalovirus infection of extremely low-birth weight infants via breast milk. Clin Infect Dis. 2001;33:1998–2003. [DOI] [PubMed] [Google Scholar]
- 46.Kurath S, Halwachs-Baumann G, Müller W, Resch B. Transmission of cytomegalovirus via breast milk to the prematurely born infant: a systematic review. Clin Microbiol Infect. 2010;16:1172–1178. [DOI] [PubMed] [Google Scholar]
- 47.Lanzieri TM, Dollard SC, Josephson CD, Schmid DS, Bialek SR. Breast milk-acquired cytomegalovirus infection and disease in VLBW and premature infants. Pediatrics. 2013;131:e1937–e1945. 10.1542/peds.2013-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fouda GG, Martinez DR, Swamy GK, Permar SR. The impact of IgG transplacental transfer on early life immunity. Immunohorizons. 2018; 2:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Granström ML. Development of children with early cytomegalovirus infection. Eur J Pediatr. 1979;132:277–287. [DOI] [PubMed] [Google Scholar]
- 50.Paryani SG, Yeager AS, Hosford-Dunn H, et al. Sequelae of acquired cytomegalovirus infection in premature and sick term infants. J Pediatr. 1985;107:451–456. [DOI] [PubMed] [Google Scholar]
- 51.Delaney M, Mayock D, Knezevic A, et al. Postnatal cytomegalovirus infection: a pilot comparative effectiveness study of transfusion safety using leukoreduced-only transfusion strategy. Transfusion. 2016;56:1945–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Demmler GJ, Yow MD, Spector SA, et al. Nosocomial cytomegalovirus infections within two hospitals caring for infants and children. J Infect Dis. 1987;156:9–16. [DOI] [PubMed] [Google Scholar]
- 53.Vollmer B, Seibold-Weiger K, Schmitz-Salue C, et al. Postnatally acquired cytomegalovirus infection via breast milk: effects on hearing and development in preterm infants. Pediatr Infect Dis J. 2004;23: 322–327. [DOI] [PubMed] [Google Scholar]
- 54.Jim WT, Chiu NC, Ho CS, et al. Outcome of preterm infants with postnatal cytomegalovirus infection via breast milk: a two-year prospective follow-up study. Medicine. 2015;94(43):e1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miron D, Brosilow S, Freiszer K, et al. Incidence and clinical manifestations of breast milk-acquired cytomegalovirus infection in low birth weight infants. J Perinatol. 2005;25:299–303. [DOI] [PubMed] [Google Scholar]
- 56.Kumar ML, Nankervis GA, Jacobs IB, et al. Congenital and postnatally acquired cytomegalovirus infection: long-term follow-up. J Pediatr. 1984;104:674–679. [DOI] [PubMed] [Google Scholar]
- 57.Kumar ML, Nankervis GA, Cooper AR, Gold E. Postnatally acquired cytomegalovirus infections in infants of CMV-excreting mothers. J Pediatr. 1984;104:669–673. [DOI] [PubMed] [Google Scholar]
- 58.Bevot A, Hamprecht K, Krägeloh-Mann I, Brosch S, Goelz R, Vollmer B. Long-term outcome in preterm children with human cytomegalovirus infection transmitted via breast milk. Acta Paediatr. 2012;101:e167–e172. [DOI] [PubMed] [Google Scholar]
- 59.Goelz R, Meisner C, Bevot A, Hamprecht K, Kraegeloh-Mann I, Poets CF. Long-term cognitive and neurological outcome of preterm infants with postnatally acquired CMV infection through breast milk. Arch Dis Child Fetal Neonatal Ed. 2013;98:F430–F433. 10.1136/archdischild-2012-303384. [DOI] [PubMed] [Google Scholar]
- 60.Brecht KF, Goelz R, Bevot A, Krägeloh-Mann I, Wilke M, Lidzba K. Postnatal human cytomegalovirus infection in preterm infants has long-term neuropsychological sequelae. J Pediatr. 2015;166:834–839. [DOI] [PubMed] [Google Scholar]
- 61.Dorn M, Lidzba K, Bevot A, Goelz R, Till-Karsten H, Marko W. Long-term neurobiological consequences of early postnatal hCMV-infection in former preterms. Human Brain Mapp. 2014;35(6):2594–2606. 10.1002/hbm.22352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weimer KED, Kelly MS, Permar SR, Clark RH, Greenberg RG. Association of adverse hearing, growth, and discharge age outcomes with postnatal cytomegalovirus infection in infants with very low birth weight. JAMA Pediatr. 2020;174(2):133. 10.1001/jamapediatrics.2019.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kelly MS, Benjamin DK, Puopolo KM, et al. Postnatal cytomegalovirus infection and the risk for bronchopulmonary dysplasia. JAMA Pediatr. 2015;169(12):e153785. 10.1001/jamapediatrics.2015.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mukhopadhyay S, Meyer SA, Permar SR, Puopolo KM. Symptomatic postnatal cytomegalovirus testing among very low-birth-weight infants: indications and outcomes. Am J Perinatol. 2016;33(09):894–902. 10.1055/s-0036-1581080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tengsupakul S, Birge ND, Bendel CM, et al. Asymptomatic DNAemia heralds CMV-associated NEC: case report, review, and rationale for preemption. Pediatrics. 2013;132(5):e1428–e1434. 10.1542/peds.2013-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schleiss MR. Breast milk-acquired cytomegalovirus in premature infants: uncertain consequences and unsolved biological questions. JAMA Pediatr. 2019;174:121. 10.1001/jamapediatrics.2019.4538. [DOI] [PubMed] [Google Scholar]
- 67.Schanler RJ. CMV acquisition in premature infants fed human milk: reason to worry? J Perinatol. 2005;25:297–298. [DOI] [PubMed] [Google Scholar]
- 68.Luck S, Sharland M. Postnatal cytomegalovirus: innocent bystander or hidden problem? Arch Dis Child Fetal Neonatal Ed. 2009;94:F58–F64. 10.1136/adc.2007.131623. [DOI] [PubMed] [Google Scholar]
- 69.American Academy of Pediatrics Human Milk. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. Red Book: 2018 Report of the Committee on Infectious Diseases. 31st ed. Itasca, IL: American Academy of Pediatrics; 2018:113–121. [Google Scholar]
- 70.Lopes AA, Champion V, Mitanchez D. Nutrition of preterm infants and raw breast milk-acquired cytomegalovirus infection: French national audit of clinical practices and diagnostic approach. Nutrients. 2018;10(8):1119. 10.3390/nu10081119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peila C, Moro GE, Bertino E, et al. The effect of Holder pasteurization on nutrients and biologically-active components in donor human milk: a review. Nutrients. 2016;8(8):477. 10.3390/nu8080477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamprecht K, Maschmann J, Müller D, et al. Cytomegalovirus (CMV) inactivation in breast milk: reassessment of pasteurization and freeze-thawing. Pediatr Res. 2004;56:529–535. [DOI] [PubMed] [Google Scholar]
- 73.Goelz R, Hihn E, Hamprecht K, et al. Effects of different CMV-heat-inactivation-methods on growth factors in human breast milk. Pediatr Res. 2009;65:458–461. [DOI] [PubMed] [Google Scholar]
- 74.Maschmann J, Müller D, Lazar K, Goelz R, Hamprecht K. New short-term heat inactivation method of cytomegalovirus (CMV) in breast milk: impact on CMV inactivation, CMV antibodies and enzyme activities. Arch Dis Child Fetal Neonatal Ed. 2019;104:F604–F608. 10.1136/archdischild-2018-316117. [DOI] [PubMed] [Google Scholar]
- 75.Bapistella S, Hamprecht K, Thomas W, et al. Short-term pasteurization of breast milk to prevent postnatal cytomegalovirus transmission in very preterm infants. Clin Infect Dis. 2019;69:438–444. [DOI] [PubMed] [Google Scholar]
- 76.Omarsdottir S, Casper C, Navér L, et al. Cytomegalovirus infection and neonatal outcome in extremely preterm infants after freezing of maternal milk. Pediatr Infect Dis J. 2015;34:482–489. 10.1097/INF.0000000000000619. [DOI] [PubMed] [Google Scholar]
- 77.Ben-Shoshan M, Mandel D, Lubetzky R, Dollberg S, Mimouni FB. Eradication of cytomegalovirus from human milk by microwave irradiation: a pilot study. Breastfeed Med. 2016;11:186–187. [DOI] [PubMed] [Google Scholar]
- 78.Lloyd ML, Hod N, Jayaraman J, et al. Inactivation of cytomegalovirus in breast milk using ultraviolet-C Irradiation: Opportunities for a new treatment option in breast milk banking. PloS One. 2016;11(8): e0161116. 10.1371/journal.pone.0161116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients-guidelines of the American Society of Transplantation infectious diseases Community of Practice. Clin Transplant. 2019;33: e13512. 10.1111/ctr.13512. [DOI] [PubMed] [Google Scholar]
- 80.Gantt S, Goldfarb DM, Park A, et al. Performance of the Alethia CMV assay for detection of cytomegalovirus by use of neonatal saliva swabs. J Clin Microbiol. 2020;58:01951–01919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kadambari S, Whittaker E, Lyall H. Postnatally acquired cytomegalovirus infection in extremely premature infants: how best to manage? Arch Dis Child Fetal Neonatal Ed. 2020;105:334–339. [DOI] [PubMed] [Google Scholar]


